Summary
Background
α-fetoprotein (AFP) response has been proven a key tumor marker for hepatocellular carcinoma (HCC), but its definition remains controversial. This study aims to characterize AFP trajectories after transarterial chemoembolization (TACE) and examine its impact on clinical outcomes.
Methods
This longitudinal, multicenter, retrospective, cohort study examined data from the electronic medical record system of four hospitals in China between January 1, 2007 to December 31, 2016. A latent class growth mixed model was applied to distinguish potential AFP dynamic changing trajectories. The multivariable Cox models were used to calculate adjusted hazard ratios (aHRs) and 95% CIs for overall survival. Inverse-probability-of-treatment weighted analyses were performed to eliminate unmeasured confounders through marginal structural models.
Findings
A total of 881 patients, who had intermediate-stage HCC with AFP repeatedly measured 3 to 10 times, were included in the study. Three distinct trajectories were identified using the latent class growth mixture model: high-rising (25.7%; n = 226), low-stable (58.7%; n = 517), and sharp-falling (AFP serological response, 15.6%; n = 138). Compared with the low-stable class, the aHRs for death were 5.13 (3.71, 7.10) and 0.52 (0.33, 0.81) for the high-rising and sharp-falling class, adjusted by gender, baseline major tumor size, intrahepatic lesions number, and logAFP(smooth). Furthermore, high-rising class had a significantly higher HR in the subgroup of female patients (10.60, 95%CI: 6.29, 17.86), age<55 (6.78, 95%CI: 4.79, 9.59) and Child-Pugh class B (23.01, 95%CI:8.07, 65.63) (P = 0.014, 0.046 and 0.033 for interaction, respectively). Trajectories of AFP had the highest relative importance of each parameter to survival, including largest tumor size, intrahepatic lesions number, Child-Pugh class, and baseline AFP.
Interpretation
AFP trajectories were associated with overall survival for intermediate-stage HCC after TACE.
Funding
The Natural Science Foundation of Fujian Province (Nos. 2018J01352, 2016J01576 and 2016J01586); the Science and Technology Innovation Joint Foundation of Fujian Province (Nos. 2017Y9125).
Keywords: Hepatocellular carcinoma, Transarterial chemoembolization, AFP trajectory, AFP serological response, Hit-differentiation hypothesis
Research in context.
Evidence before this study
α-fetoprotein (AFP) is the most important biomarker for hepatocellular carcinoma (HCC) in the clinical setting. Although AFP response has been proven a key tumor marker for HCC, its definition remains controversial. The role of dynamic serum AFP trajectories after transarterial chemoembolization (TACE) is ignored. We screened MEDLINE, Web of Science for relevant articles on Aug 1, 2021, without language or date restrictions using the terms (“α-fetoprotein” OR “α-fetoprotein change” OR “α-fetoprotein response”) AND (“hepatocellular carcinoma” OR “liver cancer”). There was no study to explore the association between AFP trajectories and clinical outcomes for intermediate-stage HCC after TACE.
Added value of this study
Three distinct trajectories were identified using the latent class growth mixture model: high-rising, low-stable, and sharp-falling. Of note, we found that about 10-fold hazard ratios of mortality exists between AFP high-rising and sharp-falling group, which had the highest relative importance of each parameter to overall survival. We define AFP sharp-falling as AFP serological response. Furthermore, the hit-differentiation hypothesis is developed to explain AFP serological response curve.
Implications of all the available evidence
To our knowledge, this is the first study to characterize latent trajectories of AFP change, and the results help to clarify the controversies in the AFP response definition. It may be a new easy-to-use method for exploring the prognostic value of multiple AFP measurements. In the future, all predictive models for HCC containing AFP may be updated based on our findings.
Alt-text: Unlabelled box
Introduction
Hepatocellular carcinoma (HCC) ranks the third leading cause of cancer-related death worldwide, with chronic hepatitis B virus (HBV) infection for key determinants in China.1 α-fetoprotein (AFP), the first identified oncofetal biomarker in HCC patients, is the most commonly used for detecting and clinical follow-up of patients with HCC. Compared with the general HCC population, higher AFP is linked with worse prognosis in different clinical settings.2, 3, 4, 5, 6 It is a valuable biomarker to predict the risk of tumor recurrence after hepatic resection and identify the best candidates for liver transplantation.2,3 In the nonsurgical setting, baseline AFP levels have been proven to predict survival prognosis with locoregional and systemic therapy.4, 5, 6 To further explore the new utility of this old marker, AFP response (over 20% decrease after therapy) is employed to predict radiologic response and survival among the HCC patients undergoing systemic chemotherapy7 and receiving sorafenib,8, 9, 10 cabozantinib,11 ramucirumab,12 and immune checkpoint inhibitors.13 However, AFP response is also identified as a > 50% AFP decline during the treatment of thalidomide,14 transarterial locoregional therapies,15, 16, 17 and radiofrequency ablation.18 Moreover, the identifications of time intervals are various among these researches.
The intermediate-stage HCC is a highly heterogeneous disease. It contains a population with a wide range of tumor burden (>3 nodules or ≥2 nodules if any >3 cm) and liver functions (Child-Pugh score 5–9). For the unresectable HCC of Barcelona Clinic Liver Cancer (BCLC) stage B, transarterial chemoembolization (TACE) is the mainstay of first-line treatment.19 In this clinical setting, AFP response (>20% decrease after a TACE session) has been demonstrated as an independent factor for the enhanced survival after TACE.20 In another study, serum AFP changes are divided into four subclasses according to the AFP change rate, which moderately correlates with EASL criteria and predicts the clinical outcome.21 However, the role of AFP change, including change rate and time interval, is still unclear and poorly defined, with an urgent need to identify the latent trajectories of AFP for intermediate-stage HCC after TACE. In this longitudinal, multicenter, retrospective, cohort study, we aim to characterize trajectories of AFP and examine its impact on clinical outcomes.
Methods
We present the following article following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.22
Patients and follow-up plan
Between January 1, 2007 to December 31, 2016, all consecutive HCC patients in the BCLC stage B treated with TACE were retrospectively retrieved from the electronic medical record system of four hospitals in Guangzhou, China. Details of this longitudinal, multicenter, retrospective, cohort study were previously described in full.23,24 Patients were included if preoperative serum AFP data and at least two postoperative serum AFP measurements were available. The study flowchart was shown in Figure 1.
Figure 1.
Flowchart for the patients with intermediate-stage HCC after TACE. HCC=hepatocellular carcinoma; TACE=transarterial chemoembolization; BCLC= Barcelona Clinic Liver Cancer. Between January 2007 and May 2012, 5005 consecutive patients with newly diagnosed HCC at Sun Yat-sen University Cancer Center (SYSUCC) were retrospectively reviewed to develop the derivation cohort. Another consecutive independent series of 3843 HCC patients (2012.6–2015.12) for internal testing cohort. Besides, between January 2010 and December 2016, 843 patients from Fifth Affiliated Hospital of Sun Yat-sen University, 415 patients from the Third Affiliated Hospital of Sun Yat-sen University, and 437 patients from the Second Hospital of Guangzhou Medical University were reviewed to develop the multicenter testing cohort.
For the first 2 years, HCC patients were followed up every 2 or 3 months to check whether complete remission was achieved. After 2-year remission, the frequency gradually decreased to every 3–6 months.
The ethics committee (2017-FXY-129) approved this multicenter retrospective study of each participating hospital. All the patient data in the survey were anonymized, and the requirement for informed consent was waived, owing to the study's retrospective nature.
AFP measuring, covariates, and outcome definition
The serum AFP level was measured by electrochemiluminescence immunoassay using the Roche Cobas E602 system (Roche Diagnostics GmbH, Mannheim, Germany) of the manufacturer's instructions.25 The cut-off value of AFP for HCC was set at 25 ng/mL.23,25 Preoperative serum AFP was defined as the AFP value closest to the first TACE treatment within the first follow-up record. Postoperative serum AFP included the AFP value before any treatment at each follow-up record after the first TACE. Repeat AFP tests at each follow-up record were excluded. The baseline covariates included age, gender, largest tumor size, intrahepatic lesions number, Child-Pugh class, and preoperative serum AFP. Those were afforded before the first TACE without any treatment at the first follow-up record.
The primary outcome was overall survival (OS), which was the time from the first TACE to death for any cause. Besides, the secondary outcomes included stage progression-free survival (SPFS) and intrahepatic recurrence-free survival (RFS). SPFS was defined as the time between the first TACE and tumor stage progression to BCLC stage C or D, and RFS was the time from the first TACE to the appearance of new intrahepatic tumors but not the residual lesions of main lesion within six months.
Statistical analysis
Unsupervised cluster analysis was performed to explore the trajectories of serum AFP level using a latent class growth mixed model (LCGMM). Log transformation was applied for serum AFP levels because of its left skewness. The R package lcmm (version 1.9.2)26 in R 3.6.3 was used to perform LCGMM, setting the log AFP as a function of time with a class number ranging from 2 to 6 with the same starting values calculated from the 1-group model.
When the LCGMM model was fitted, we assessed the polynomial function of linear, quadratic, and cubic and tried the grouping number from 1 to 6 in each function form. To avoid convergence towards local maxima, LCGMM models with 2 to 6 classes were performed several times with different sets of random starting values based on the 1-class model. The criteria for the choice of a best-fit model together with the study-specific requirements were as followed27: (1) significant improvement of the model in Bayesian information criterion (at least 10 points reduction); (2) a posterior probability > 0.7 for all latent classes; and (3) ≥ 5% participants in any single trajectory class. Finally, cubic trajectories of the three groups were the optimal fit model based on the above criteria.
Characteristics across different groups were compared using Student's t-test or Kruskal–Wallis tests for continuous variables and χ2 statistics or Fisher's exact test for categorical variables. Kaplan-Meier method was firstly used to estimate the OS, SPFS, and RFS for each trajectory group, with the differences compared by the log-rank test. Cox proportional hazard models were used to explore the association between AFP trajectories and clinical outcome, which was adjusted of gender, major tumor size (≤5, >5), intrahepatic lesions number(≤3, >3), and AFP (<25, ≥25). To address the non-linearity of confounding factors, we set up a final model adjusted for logAFP (smooth) through restricted cubic spline and other baseline confounders. The relative importance of each parameter to survival risk was assessed using the χ² from Harrell's rms R package.
Sensitivity analysis
Finally, we applied three approaches to evaluate the risk estimates' robustness in a sensitivity analysis. To eliminate the unmeasured confounding factors, inverse-probability-of-treatment weighted analysis (IPTW) was performed through marginal structural models. In this model, the predicted probabilities, which were calculated by gender, largest tumor size (≤5, >5), intrahepatic lesions number (≤3, >3), and logAFP(Smooth), were used to evaluate the stabilized inverse-probability-of-treatment weight. To search for potential heterogeneity sources, subgroup analyses were performed by participating cohort, age, sex, baseline Child-Pugh class, major tumor size, and intrahepatic tumor number, with tests for interaction by the Cox regression model. To account for potential biases of the various following-up times, sequential landmark analyses evaluating survival with distinct AFP trajectories were performed for patients with overall survival of fewer than 3 years, 4 years, and 5 years.
Role of the funding sources
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. CX and SLJ have accessed and verified the data. CX is responsible for the decision to submit the manuscript.
Results
Clinical features of cohorts
The baseline characteristics of patients with inclusion and exclusion criteria are shown in Table S1. 881 patients were finally included in this study, with the 5.8 (range, 3–10) times of AFP measurements. The median follow-up time was 23.7 (range, 3.8–115.3) months, including 16.9 (range, 10.9–26.2) months for the death and 27.8 (range, 18.8–44.4) months for those who were censored. During the follow-up period, 361 patients died. All the patients were in good performance status (ECOG PS 0), whose leading cause of HCC was HBV/HCV infection.23 Figure 1 presented the flowchart of enrollment, and a summary of baseline characteristics of patients in each cohort was shown in Table S2.
Identification of number of trajectories
Table S3 summarizes the fitting process for 2 through 6 classes by the latent class growth mixed model. Specifically, a model of cubic parameters with three classes provided the optimal fit according to the criteria mentioned above. Detailed parameter estimates of the best fitting 3-class cubic trajectory model are shown in Table S4.
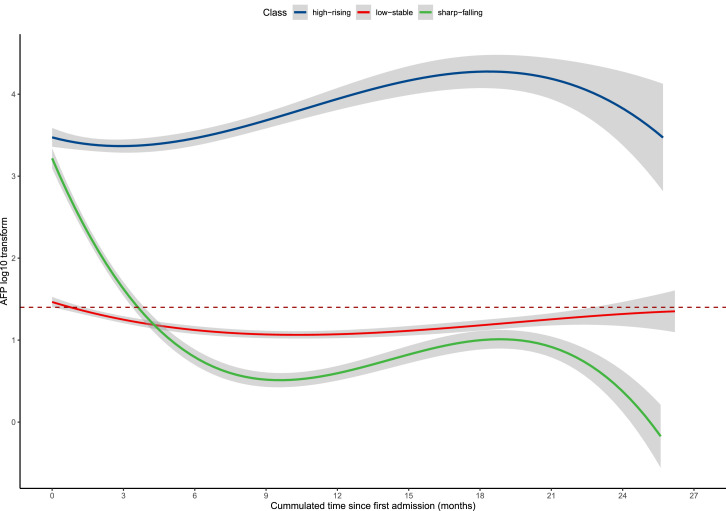
Figure 2 shows the predicted mean trajectory of serum AFP. Three distinct trajectories were identified, labeled as: high-rising (25.7%; n = 226), low-stable (58.7%; n = 517), and sharp-falling (15.6%; n = 138). The AFP remained within the range (0–25 ng/mL) in the low-stable group after the first TACE treatment. In the sharp-falling group, AFP declined rapidly from elevated preoperative level (>10^2.5 ng/mL) toward the range (0–25 ng/mL) within four months of TACE and then kept stable. It was defined as AFP serological response curve. In the high-rising group, AFP increased slowly from an elevated preoperative level toward to higher level.
Figure 2.
Trajectories of serum AFP in intermediate-stage HCC patients after TACE. Red dashed line = AFP value equaled to 25 ng/mL. Shadows = 95% confidence intervals. HCC=hepatocellular carcinoma; TACE=transarterial chemoembolization; AFP=α-fetoprotein.
Table 1 summarizes the baseline characteristics of the study population by AFP trajectory classes. Compared with the low-stable class, the high-rising and sharp-falling class had higher age values, largest tumor size, and baseline AFP.
Table 1.
Baseline characteristics of patients stratified by trajectory classes of AFP.
| low-stable | sharp-falling | high-rising | P-value | |
|---|---|---|---|---|
| N | 517 | 138 | 226 | |
| Age (years) | 54.1 ± 11.1 | 51.6 ± 10.9 | 50.6 ± 13.5 | <0.0001 |
| Gender | 0.64 | |||
| male | 364 (70.4%) | 92 (66.7%) | 154 (68.1%) | |
| female | 153 (29.6%) | 46 (33.3%) | 72 (31.9%) | |
| Child-Pugh class | 0.98 | |||
| A | 431 (90.2%) | 115 (89.8%) | 192 (89.7%) | |
| B | 47 (9.8%) | 13 (10.2%) | 22 (10.3%) | |
| Diameter of main tumor(cm) | <0.0001 | |||
| Mean ± SD | 5.9 ± 3.1 | 6.8 ± 3.0 | 7.9 ± 3.5 | |
| <5 | 234 (45.3%) | 49 (35.5%) | 48 (21.2%) | |
| ≥5 | 283 (54.7%) | 89 (64.5%) | 178 (78.8%) | |
| Intrahepatic lesions number | <0.0001 | |||
| <3 | 235 (45.5%) | 63 (45.7%) | 69 (30.5%) | |
| ≥3 | 282 (54.5%) | 75 (54.3%) | 157 (69.5%) | |
| AFP (ng/mL) | <0.0001 | |||
| Log AFP | 1.6 ± 0.9 | 3.5 ± 0.7 | 3.6 ± 1.0 | |
| <25 | 252 (48.7%) | 0 (0.0%) | 0 (0.0%) | |
| ≥25 | 265 (51.3%) | 138 (100.0%) | 226 (100.0%) |
Differences are compared using the chi-square test (or Fisher's exact test) for categorical measures and Kruskal–Wallis test for continuous measures. Numbers that do not add up to 881 are attributable to missing data. AFP=α-fetoprotein.
Association between phenotype and clinical outcomes
We next estimated the OS, RFS and SP-free survival for each trajectory group. As demonstrated in Figure 3, the 3-year OS rate in the low-stable group was 61.9% (95%CI: 60.0%, 67.2%), which was significantly higher than high-rising group (7.7%, 95%CI: 3.7%, 16.2%), but lower than sharp-falling group (80.0%, 95%CI: 73.0%, 87.7%). The median OS was 48.6 (95%CI: 42.7, NA) months, 13.8 (95%CI: 12.4, 16.3) months, and NA (95%CI: 73.9, NA) months for the low-stable, high-rising, and sharp-falling group, respectively. Similar difference of the 1-year SPFS rate among three groups was observed, as shown in Figure S1A (the low-stable group: 75.9%, 95% CI: 72.0%, 80.0%; the high-rising group: 28.5%, 95% CI: 21.8%, 37.2%; the sharp-falling group, 85.1%, 95% CI: 79.1%, 91.5%; P < 0.0001), and 1-year RFS rate in Figure S1B (the low-stable group: 56.5%, 95% CI: 52.0%, 61.4%; the high-rising group: 16.6%, 95% CI: 11.5%, 24.0%; the sharp-falling group, 76.6%, 95%CI: 69.3%, 84.7%; P < 0.0001)
Figure 3.
Kaplan-Meier curves of overall survival in patients with intermediate-stage HCC after TACE. Shadows = 95% confidence intervals. HCC=hepatocellular carcinoma; TACE=transarterial chemoembolization; AFP=α-fetoprotein.
The effect sizes of the relationship between AFP trajectory and clinical outcome were displayed in Table 2. Compared with the low-stable class, the high-rising class had a higher risk of death (HR:5.64, 95%CI:4.48, 7.10), but a lower risk of death in the sharp-falling class (HR: 0.52, 95%CI: 0.35, 0.76) in the unadjusted model. After adjusting the confounding factors gender, largest tumor size (≤5, >5), intrahepatic lesions number (≤3, >3) and baseline logAFP(smooth), the high-rising class was associated with 4.13 times (HR:5.13, 95%CI: 3.71, 7.10) risk of death increase and 48% (HR:0.52, 95%CI: 0.33, 0.81) risk decrease for the sharp-falling group. Similar associations were observed between AFP trajectory groups and SPFS/RFS (Figure S1, Tables 2).
Table 2.
Trajectory classes of AFP and multivariate hazard ratios of overall survival with 95% confidence intervals.
| Event/N | Non-adjusted | Adjust I0 | Adjust II# | |
|---|---|---|---|---|
| Overall Survival | ||||
| low-stable | 180/517 | 1 | 1 | 1 |
| sharp-falling | 31/138 | 0.52 (0.35, 0.76) | 0.47 (0.30, 0.72) | 0.52 (0.33, 0.81) |
| high-rising | 150/226 | 5.64 (4.48, 7.10) | 4.70 (3.39, 6.52) | 5.13 (3.71, 7.10) |
| Stage progression-free survival | ||||
| low-stable | 145/517 | 1 | 1 | 1 |
| sharp-falling | 25/138 | 0.55 (0.36, 0.84) | 0.49 (0.31, 0.77) | 0.40 (0.24, 0.66) |
| high-rising | 136/226 | 3.89 (3.05, 4.95) | 3.48 (2.60, 4.65) | 2.86 (1.99, 4.10) |
| Recurrence-free Survival | ||||
| low-stable | 246/517 | 1 | 1 | 1 |
| sharp-falling | 41/138 | 0.49 (0.35, 0.68) | 0.47 (0.33, 0.67) | 0.56 (0.38, 0.84) |
| high-rising | 164/226 | 2.97 (2.42, 3.65) | 2.71 (2.13, 3.45) | 3.32 (2.47, 4.46) |
Low-stable group: first AFP measuring <10^2.5 ng/mL, and not increasing within 4 months. Sharp-falling group: first AFP measuring ≥10^2.5 ng/mL, and declining toward at least <10^2 ng/mL within 4 months; else belonging to the high-rising group.
HR(95%CI)= hazard ratio(95% confidence intervals). AFP=α-fetoprotein.
This model was adjusted of gender, largest tumor size (≤5, >5), intrahepatic lesions number (≤3, >3), AFP (<25, ≥25).
This model was adjusted of gender, largest tumor size (≤5, >5), intrahepatic lesions number (≤3, >3), log AFP (Smooth). Restricted cubic spline was applied.
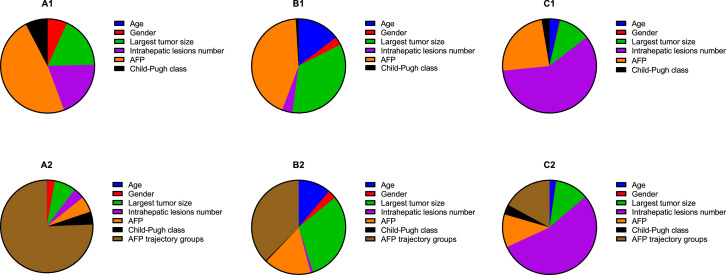
Furthermore, we analyzed the relative contribution of each parameter to predict clinical outcome, including age, sex, largest tumor size, intrahepatic lesions number, Child-Pugh class, and baseline AFP (Figure 4 A1–C1). After plus the AFP trajectory class, we could find that it was stronger than all these clinical parameters for OS (Figure 4 A2) and SPFS (Figure 4 B2), except for RFS (Figure 4 C2).
Figure 4.
Relative importance of each risk factor for overall survival (A), Stage progression-free survival (B) and recurrence-free survival (C). AFP=α-fetoprotein. A2, B2, and C2 show the relative importance of risk factors plus AFP trajectory groups. A1: Age, 0.05%; Gender, 6.77%; Largest tumor size, 17.77%; Intrahepatic lesions number, 19.7%; serum AFP, 48.03%; Child-Pugh class, 7.65%. A2: Age, 0.16%; Gender, 2.69%; Largest tumor size, 7.52%; Intrahepatic lesions number, 3.91%; serum AFP, 5.58%; Child-Pugh class, 4.52%; AFP trajectory groups, 75.62%. B1: Age, 14.70%; Gender, 2.82%; Largest tumor size, 34.63%; Intrahepatic lesions number, 3.59%; AFP, 43.31%; Child-Pugh class, 0.95%. B2: Age,11.19%; Gender, 2.97%; Largest tumor size, 30.89%; Intrahepatic lesions number, 0.87%; AFP, 16.20%; Child-Pugh class, 0.39%; AFP trajectory groups, 37.49%. C1: Age, 3.52%; Gender, 0.21%; Largest tumor size, 10.9%; Intrahepatic lesions number, 58.77%; AFP, 23.90%; Child-Pugh class, 2.70%. C2: Age, 2.24%; Gender, 0.17%; Largest tumor size, 11.40%; Intrahepatic lesions number, 54.34%; AFP, 11.34%; Child-Pugh class, 3.43%; AFP trajectory groups, 17.08%.
Sensitivity analyses
In this section, we used three additional sensitivity analyses to verify the robustness of risk estimates. In the IPTW model, HRs were 4.48 (95%CI: 2.40, 8.39) for death risk in the high-rising group and 0.66 (95%CI: 0.42, 1.03) in the sharp-falling group, which was consistent with the core results. Finally, we performed an exploratory subgroup analysis of OS according to baseline patients' characteristics. Compared with low-stable class, high-rising class had a significantly higher HR in the subgroup of female patients (10.60, 95%CI: 6.29, 17.86), age<55 (6.78, 95%CI: 4.79, 9.59) and Child-Pugh class B (23.01, 95%CI:8.07, 65.63) (P = 0.014, 0.046 and 0.033 for interaction, respectively). This stratified analysis of OS found similar results for the overall population, as shown in Table 3. Figure S2 displayed the landmark analyses evaluating the impact of three AFP trajectories for survivors of ≤ 3, ≤ 4, and ≤ 5 years. Overall, the association between AFP trajectories and overall survival was still robust for the survivors at each sequential landmark (all P < 0.0001).
Table 3.
Subgroup analysis of overall survival for serum AFP trajectories stratified by clinical features.
| Event/N | high-rising vs. low-stable |
sharp-falling vs. low-stable |
|||
|---|---|---|---|---|---|
| HR (95%CI) | P-value* | HR (95%CI) | P-value* | ||
| Gender | 0.014 | 0.46(0.29,0.72) | 0.23 | ||
| male | 281/610 | 4.75 (3.65, 6.18) | 0.76(0.36,1.61) | ||
| female | 80/271 | 10.60 (6.29, 17.86) | |||
| Age (years) | 0.046 | 0.097 | |||
| <55 | 179/454 | 6.78 (4.79, 9.59) | 0.70(0.43,1.15) | ||
| ≥55 | 182/427 | 4.67 (3.39, 6.42) | 0.35(0.19, 0.64) | ||
| Child-Pugh class | 0.033 | 0.52 | |||
| A | 307/738 | 5.58 (4.33, 7.17) | 0.52(0.35,0.79) | ||
| B | 34/82 | 23.01 (8.07, 65.63) | 0.65(0.19,2.28) | ||
| Intrahepatic lesions number | 0.12 | 0.26 | |||
| <3 | 134/367 | 7.73 (5.22, 11.44) | 0.38(0.20, 0.73) | ||
| ≥3 | 227/514 | 4.67 (3.49, 6.25) | 0.62(0.39, 0.99) | ||
| Diameter of main tumor(cm) | 0.69 | 0.77 | |||
| <5 | 101/331 | 6.21(3.75, 10.26) | 0.51(0.25,1.06) | ||
| ≥5 | 260/550 | 4.68 (3.57, 6.12) | 0.47(0.30,0.74) | ||
| Derivation cohort | 0.27 | 0.88 | |||
| No | 180/526 | 6.37 (4.57, 8.87) | 0.50(0.30, 0.86) | ||
| Yes | 181/355 | 4.81(3.47, 6.67) | 0.53(0.31,0.92) | ||
P value for interaction. HR(95%CI) = hazard ratio(95% confidence intervals). AFP=α-fetoprotein.
Discussion
In this longitudinal multicenter study, we used time-series data to identify three distinct AFP trajectories of HCC undergoing TACE treatment and found significant associations between AFP trajectory groups with clinical outcomes. Although the response to TACE is highly heterogeneous, three AFP trajectories were still robust during the follow-up time after first-line treatment. We further calculated the relative importance of each covariate and found AFP trajectories were the maximum-weight parameter to predict both OS and SPFS. To the best of our knowledge, this was the first study to characterize the latent trajectories of AFP change, and the results help to clarify the controversy for AFP response definition.
Numerous prior studies have established a subgroup of HCC patients with AFP level decrease, including early-stage HCC treated with surgery or liver transplantation,28, 29, 30 intermediate-stage HCC with TACE,20,21 advanced HCC with systemic therapies.8, 9, 10, 11, 12, 13 Besides, decreasing APF level predicted reduced incidence of HCC in patients receiving antiviral therapy.31,32 This evidence suggested that AFP decline was the robust subclass across all stages of HCC, which supported the finding of AFP serological response and implied a specified pathophysiological process. On the other hand, a low-stable group was observed with the AFP level remaining within 25 ng/mL after TACE for the AFP-negative HCC patients. Those belong to the non-AFP–producing population, accounting for approximately 31% of HCC patients with significantly better clinical outcomes undergoing liver transplant.33 Some studies also demonstrated that its less aggressive tumor phenotype and postoperative serum AFP change provided poor sensitivity for clinical outcome after radiofrequency ablation34 and hepatectomy.35 To further discuss the potential pathophysiological process of AFP trajectories, our sightlines were focused on the molecular classification of HCC.36 Did the microenvironment dysregulated subgroup of HBV-related HCC, with an intermediate expression of metabolic and proliferative proteins, have directed differentiation potential to the metabolism subgroup or proliferation subgroup after therapy? Furthermore, was there a specified process of "hit-differentiation" for AFP serological response?
In this research, we took advantage of the time-series data of AFP to find an AFP serological response curve. It may be a novel and easy-to-use method by which doctors only need to observe AFP changes in clinical settings rather than calculating AFP change. Of note, we found that the hazard ratio of death had reached about 10-fold between the high-rising and sharp-falling group, suggesting its promising future in clinical and scientific research.
This study also had limitations. Firstly, not every patient meets all the features of AFP trajectory groups. For instance, the baseline of AFP level over 25 ng/mL (logAFP, mean±SD:1.6 ± 0.9) held nearly half of patients in the low-stable group (Figure S3). Nevertheless, they shared similar characteristics of AFP change and clinical outcomes with AFP-negative HCC. Secondly, the molecular subtypes of HCC have been grouped into the proliferation and nonproliferation class.37 The current three AFP trajectories were based on the Chinese population with prevalent hepatitis B infection and cirrhosis. The nonproliferation class of HCC, more commonly with HCV infection and alcohol abuse, may have different AFP trajectories. Thirdly, patients in the current study had relatively less tumor load (Table S1); thus, our conclusions might not be suitable for HCC intermediate-stage with high tumor load. Moreover, bias could be caused by residual and unmeasured confounders. Our findings must also be verified by a prospective randomized controlled trial and larger-scale population.
In summary, three distinct AFP trajectories are identified for intermediate-stage HCC after TACE treatment, and it has a significant impact on clinical outcomes. We provide new insights into the prognostic significance of AFP serological response. It implies that patients with the sharp-falling AFP have the best survival and may experience a specified process of "hit-differentiation."
Contributors
Conception and design: LLB, SLJ, WZX; Data analysis and interpretation: LLB, SLJ; Resources: SYH, HPF, XZF, LC; Funding acquisition: XZF, CX; Writing-original draft: LLB, SLJ; Writing-review & editing: All authors. Final approval of manuscript: All authors. LLB (doctorxiaolin@fjmu.edu.cn) is responsible for the concept of AFP serological response and hit-differentiation hypothesis. CX and SLJ have accessed and verified the data. CX is responsible for the decision to submit the manuscript.
Data sharing statement
All data generated or analyzed during this study are included in this article and its supplementary material files. Further inquiries can be directed to the corresponding author.
Funding
The Natural Science Foundation of Fujian Province (Nos 2018J01352, 2016J01576 and 2016J01586); the Science and Technology Innovation Joint Foundation of Fujian Province (Nos2017Y9125).
Declaration of interests
All authors declare no competing interests.
Acknowledgments
We are grateful for the statistical support after the peer review stage from Empower U team of the Department of Epidemiology and Biostatistics, X&Y solutions Inc. in Boston.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101391.
Appendix. Supplementary materials
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Mazzaferro V., Sposito C., Zhou J., et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology. 2018;154(1):128–139. doi: 10.1053/j.gastro.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Lai Q., Nicolini D., Inostroza Nunez M., et al. A novel prognostic index in patients with hepatocellular cancer waiting for liver transplantation: time-radiological-response-alpha-fetoprotein-inflammation (TRAIN) score. Ann Surg. 2016;264(5):787–796. doi: 10.1097/SLA.0000000000001881. [DOI] [PubMed] [Google Scholar]
- 4.Zhu A.X., Park J.O., Ryoo B.Y., et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859–870. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J., Qin S., Merle P., et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 6.Lee D.H., Lee J.M., Lee J.Y., et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270(3):900–909. doi: 10.1148/radiol.13130940. [DOI] [PubMed] [Google Scholar]
- 7.Chan S.L., Mo F.K., Johnson P.J., et al. New utility of an old marker: serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol. 2009;27(3):446–452. doi: 10.1200/JCO.2008.18.8151. official journal of the American Society of Clinical Oncology. [DOI] [PubMed] [Google Scholar]
- 8.Yau T., Yao T., Chan P., et al. The significance of early alpha-fetoprotein level changes in predicting clinical and survival benefits in advanced hepatocellular carcinoma patients receiving sorafenib. Oncologist. 2011;16(9):1270–1279. doi: 10.1634/theoncologist.2011-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Personeni N., Bozzarelli S., Pressiani T., et al. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2012;57(1):101–107. doi: 10.1016/j.jhep.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Lee S., Kim B.K., Kim S.U., et al. Early alpha-fetoprotein response predicts survival in patients with advanced hepatocellular carcinoma treated with sorafenib. J Hepatocell Carcinoma. 2015;2:39–47. doi: 10.2147/JHC.S79353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley R., Meyer T., Rimassa L., et al. Serum alpha-fetoprotein levels and clinical outcomes in the phase III CELESTIAL Study of Cabozantinib versus placebo in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2020;26(18):4795–4804. doi: 10.1158/1078-0432.CCR-19-3884. an official journal of the American Association for Cancer Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu A., Finn R., Kang Y., et al. Serum alpha-fetoprotein and clinical outcomes in patients with advanced hepatocellular carcinoma treated with ramucirumab. Br J Cancer. 2021;124(8):1388–1397. doi: 10.1038/s41416-021-01260-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao Y., Liu T., Hsu C., et al. Early alpha-foetoprotein response associated with treatment efficacy of immune checkpoint inhibitors for advanced hepatocellular carcinoma. Liver Int. 2019;39(11):2184–2189. doi: 10.1111/liv.14210. official journal of the International Association for the Study of the Liver. [DOI] [PubMed] [Google Scholar]
- 14.Chen L.T., Liu T.W., Chao Y., et al. alpha-fetoprotein response predicts survival benefits of thalidomide in advanced hepatocellular carcinoma. Aliment Pharmacol Ther. 2005;22(3):217–226. doi: 10.1111/j.1365-2036.2005.02547.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim B.K., Ahn S.H., Seong J.S., et al. Early alpha-fetoprotein response as a predictor for clinical outcome after localized concurrent chemoradiotherapy for advanced hepatocellular carcinoma. Liver Int. 2011;31(3):369–376. doi: 10.1111/j.1478-3231.2010.02368.x. [DOI] [PubMed] [Google Scholar]
- 16.Memon K., Kulik L., Lewandowski R.J., et al. Alpha-fetoprotein response correlates with EASL response and survival in solitary hepatocellular carcinoma treated with transarterial therapies: a subgroup analysis. J Hepatol. 2012;56(5):1112–1120. doi: 10.1016/j.jhep.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riaz A., Ryu R.K., Kulik L.M., et al. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. J Clin Oncol. 2009;27(34):5734–5742. doi: 10.1200/JCO.2009.23.1282. official journal of the American Society of Clinical Oncology. [DOI] [PubMed] [Google Scholar]
- 18.Yu S.J., Kwon J.H., Kim W., et al. Initial alpha-fetoprotein response predicts prognosis in hepatitis B-related solitary HCC patients after radiofrequency ablation. J Clin Gastroenterol. 2018;52(3):e18–e26. doi: 10.1097/MCG.0000000000000841. [DOI] [PubMed] [Google Scholar]
- 19.European Association for the Study of the Liver Electronic address EEE, European Association for the Study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Liu G., Ouyang Q., Xia F., et al. Alpha-fetoprotein response following transarterial chemoembolization indicates improved survival for intermediate-stage hepatocellular carcinoma. HPB. 2019;21(1):107–113. doi: 10.1016/j.hpb.2018.06.1800. the official journal of the International Hepato Pancreato Biliary Association. [DOI] [PubMed] [Google Scholar]
- 21.Tian M., Zhang X., Huang G., Fan W., Li J., Zhang Y. Alpha-fetoprotein assessment for hepatocellular carcinoma after transarterial chemoembolization. Abdom Radiol. 2019;44(10):3304–3311. doi: 10.1007/s00261-019-02116-x. (New York) [DOI] [PubMed] [Google Scholar]
- 22.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Shen L., Zeng Q., Guo P., et al. Dynamically prognosticating patients with hepatocellular carcinoma through survival paths mapping based on time-series data. Nat Commun. 2018;9(1):2230. doi: 10.1038/s41467-018-04633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu L., Zhang Y., Zheng P., et al. Elevated platelet count is associated with poor survival after transarterial chemoembolization treatment in patients with hepatocellular carcinoma: a cohort study. J Hepatocell Carcinoma. 2020;7:191–199. doi: 10.2147/JHC.S274349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan Y., Sun X., Hu Z., et al. Prognostic values of alpha-fetoprotein and des-gamma-carboxyprothrombin in hepatocellular carcinoma in china: an analysis of 4792 patients. J Hepatocell Carcinoma. 2021;8:657–670. doi: 10.2147/JHC.S316223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Proust-Lima C., Philipps V., Liquet B. Estimation of extended mixed models using latent classes and latent processes: the R Package lcmm. J Stat Softw. 2017;78(2):1–56. [Google Scholar]
- 27.Li Z., Li C., Pu H., et al. Trajectories of perioperative serum carcinoembryonic antigen and colorectal cancer outcome: a retrospective, multicenter longitudinal cohort study. Clin Transl Med. 2021;11(2):e293. doi: 10.1002/ctm2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halazun K., Rosenblatt R., Mehta N., et al. Dynamic α-fetoprotein response and outcomes after liver transplant for hepatocellular carcinoma. JAMA Surg. 2021;156(6):559–567. doi: 10.1001/jamasurg.2021.0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X.L., Zhu X.D., Cai H., et al. Postoperative alpha-fetoprotein response predicts tumor recurrence and survival after hepatectomy for hepatocellular carcinoma: a propensity score matching analysis. Surgery. 2019;165(6):1161–1167. doi: 10.1016/j.surg.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Rungsakulkij N., Suragul W., Mingphruedhi S., Tangtawee P., Muangkaew P., Aeesoa S. Prognostic role of alpha-fetoprotein response after hepatocellular carcinoma resection. World J Clin Cases. 2018;6(6):110–120. doi: 10.12998/wjcc.v6.i6.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osaki Y., Ueda Y., Marusawa H., et al. Decrease in alpha-fetoprotein levels predicts reduced incidence of hepatocellular carcinoma in patients with hepatitis C virus infection receiving interferon therapy: a single center study. J Gastroenterol. 2012;47(4):444–451. doi: 10.1007/s00535-011-0505-8. [DOI] [PubMed] [Google Scholar]
- 32.Oze T., Hiramatsu N., Yakushijin T., et al. Post-treatment levels of α-fetoprotein predict incidence of hepatocellular carcinoma after interferon therapy. Clin Gastroenterol Hepatol. 2014;12(7):1186–1195. doi: 10.1016/j.cgh.2013.11.033. the official clinical practice journal of the American Gastroenterological Association. [DOI] [PubMed] [Google Scholar]
- 33.Agopian V.G., Harlander-Locke M.P., Markovic D., et al. Evaluation of patients with hepatocellular carcinomas that do not produce alpha-fetoprotein. JAMA Surg. 2017;152(1):55–64. doi: 10.1001/jamasurg.2016.3310. [DOI] [PubMed] [Google Scholar]
- 34.Siripongsakun S., Wei S.H., Lin S., et al. Evaluation of alpha-fetoprotein in detecting hepatocellular carcinoma recurrence after radiofrequency ablation. J Gastroenterol Hepatol. 2014;29(1):157–164. doi: 10.1111/jgh.12438. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X.F., Qi X., Meng B., et al. Prognosis evaluation in alpha-fetoprotein negative hepatocellular carcinoma after hepatectomy: comparison of five staging systems. Eur J Surg Oncol. 2010;36(8):718–724. doi: 10.1016/j.ejso.2010.05.022. the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. [DOI] [PubMed] [Google Scholar]
- 36.Gao Q., Zhu H., Dong L., et al. Integrated proteogenomic characterization of HBV-related hepatocellular carcinoma. Cell. 2019;179(2):561–577. doi: 10.1016/j.cell.2019.08.052. e22. [DOI] [PubMed] [Google Scholar]
- 37.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.