Abstract
Although capsaicin has been studied extensively as an activator of the transient receptor potential vanilloid cation channel subtype 1 (TRPV1) channels in sensory neurons, little is known about its TRPV1-independent actions in gastrointestinal health and disease. Here, we aimed to investigate the pharmacological actions of capsaicin as a food additive and medication on intestinal ion transporters in mouse models of ulcerative colitis (UC). The short-circuit current (Isc) of the intestine from WT, TRPV1-, and TRPV4-KO mice were measured in Ussing chambers, and Ca2+ imaging was performed on small intestinal epithelial cells. We also performed Western blots, immunohistochemistry, and immunofluorescence on intestinal epithelial cells and on intestinal tissues following UC induction with dextran sodium sulfate. We found that capsaicin did not affect basal intestinal Isc but significantly inhibited carbachol- and caffeine-induced intestinal Isc in WT mice. Capsaicin similarly inhibited the intestinal Isc in TRPV1 KO mice, but this inhibition was absent in TRPV4 KO mice. We also determined that Ca2+ influx via TRPV4 was required for cholinergic signaling–mediated intestinal anion secretion, which was inhibited by capsaicin. Moreover, the glucose-induced jejunal Iscvia Na+/glucose cotransporter was suppressed by TRPV4 activation, which could be relieved by capsaicin. Capsaicin also stimulated ouabain- and amiloride-sensitive colonic Isc. Finally, we found that dietary capsaicin ameliorated the UC phenotype, suppressed hyperaction of TRPV4 channels, and rescued the reduced ouabain- and amiloride-sensitive Isc. We therefore conclude that capsaicin inhibits intestinal Cl- secretion and promotes Na+ absorption predominantly by blocking TRPV4 channels to exert its beneficial anti-colitic action.
Keywords: TRPV4 channels, Na+/K+-ATPase, epithelial Na+ channels, ulcerative colitis, short-circuit current
Abbreviations: AA, arachidonic acid; [Ca2+]cyt, cytosolic Ca2+ concentrations; Caf, caffeine; CCh, carbachol; CD, Crohn's disease; CYP450, cytochrome P450; DSS, dextran sodium sulfate; ENaC, epithelial Na+ channels; ER, endoplasmic reticulum; GI, gastrointestinal tract; IBD, inflammatory bowel disease; IECs, intestinal epithelial cells; Isc, short-circuit current; MPO, myeloperoxidase; NKA, Na+/K+-ATPase; PIP2, phosphatidylinositol 4,5-bisphosphate; PLA, phospholipase A; PLC, phospholipase C; PSS, physiological salt solution; TRPV, transient receptor potential vanilloid; UC, ulcerative colitis
Inflammatory bowel disease (IBD) is a group of chronic inflammatory intestinal disorders, including Crohn’s disease (CD) and ulcerative colitis (UC). CD can affect any part of the digestive tract characterized by transmural inflammation, but UC is limited to the colon. Ulcerations and bloody diarrhea are the characteristic symptoms in UC (1). IBD is thought to be triggered by environmental factors in genetically susceptible individuals (2). Hallmarks of the disease include immune cell infiltration and activation in the mucosa leading to inflammation and ulceration of the intestinal wall (3, 4). So far, there are limited clinical therapies for IBD, but currently available therapies (such as anti-TNF-α biologics) cannot cure IBD due to the lack of in-depth understanding of its pathogenesis (5). Therefore, it is urgent to elucidate the detailed pathogenesis and develop new cures for IBD patients.
Chili pepper has a long history of flavoring, preserving food, as well as medication worldwide (6). As an active compound from chili pepper, capsaicin has numerous beneficial roles outside of human gastrointestinal (GI) tract. Capsaicin has been used for the prevention/treatment of pain, hypertension, and inflammation (7, 8). Even more interestingly as reported recently, dietary capsaicin could significantly enhance hematopoietic stem cells mobilization to improve their yield for stem cell-based therapeutic agents (9). Although dietary capsaicin is absorbed with a great efficiency in GI tract, its exact pharmacological actions have not been well studied in this organ. After capsaicin is passively absorbed in the upper GI tract (10), it stimulates gut mucosal afferent nerves and blood flow rates (11) and also ameliorates abnormal glucose homeostasis (12).
Numerous studies have revealed that capsaicin has both transient receptor potential vanilloid 1 (TRPV1)-dependent and -independent actions in mammals. The former includes such as capsaicin triggering a painful and burning sensation throughout human GI tract (13, 14) and increasing glucagon-like peptide-1 levels in the plasma and the ileum (12), but the latter includes such as capsaicin inhibiting intestinal epithelial anion secretion and inducing apoptosis of gastric cancer cells (15, 16). While the TRPV1-dependent action of capsaicin has been studied extensively on sensory neurons mostly, little is known about its TRPV1-independent actions on epithelial cells in GI health and disease. Moreover, growing evidence suggests that TRPV4 channels, another important member of TRPV family, play a critical role in the pathogenesis of UC (17, 18, 19); however, it is unknown if capsaicin acts on aberrant TRPV4 channels to affect UC outcome. In addition, although pathological roles of TRPV4 channels have attracted more attention in GI disease, their physiological roles in intestinal epithelial ion transports are still obscure.
Since capsaicin has long been used as daily flavoring worldwide and is orally delivered to GI tract, it is therefore important to investigate its actions in GI health and disease. In particular, the effects of dietary capsaicin on IBD and the underlying mechanisms need to be verified since it is still elusive if capsaicin is a friend or foe to UC. Capsaicin was reported previously to alleviate (20), exacerbate (21), or not affect the severity of experimental UC (22). However, clinical epidemiological studies indicate that the incidence rate of IBD, including UC and CD, is much lower in the high chili consuming regions in China (such as Chengdu and Xian) than the light/no chili consuming regions (such as Guangzhou and Hong Kong) (23, 24), suggesting that dietary capsaicin might prevent/treat human IBD. Consistently, in a previous study, we demonstrated that capsaicin ameliorated experimental colitis through vasorelaxation of submucosal arteries to likely increase blood perfusion to the colonic mucosae (25); however, it is largely unknown for the direct actions of capsaicin on intestinal epithelia in health and UC and the underlying molecular mechanisms. Therefore, this fellow-up study sought to further investigate these issues. Using multidisciplinary approaches, including electrophysiology, pharmacology, molecular biology, and transgenic mouse models (TRPV1 and TRPV4 KO mice) in vitro and in vivo approaches, we uncover that capsaicin inhibits intestinal Cl- secretion and promotes Na+ absorption predominately by blocking TRPV4 channels to exert its beneficial anti-colitic action.
Results
Capsaicin inhibition of epithelial anion secretion in the jejunum and distal colon
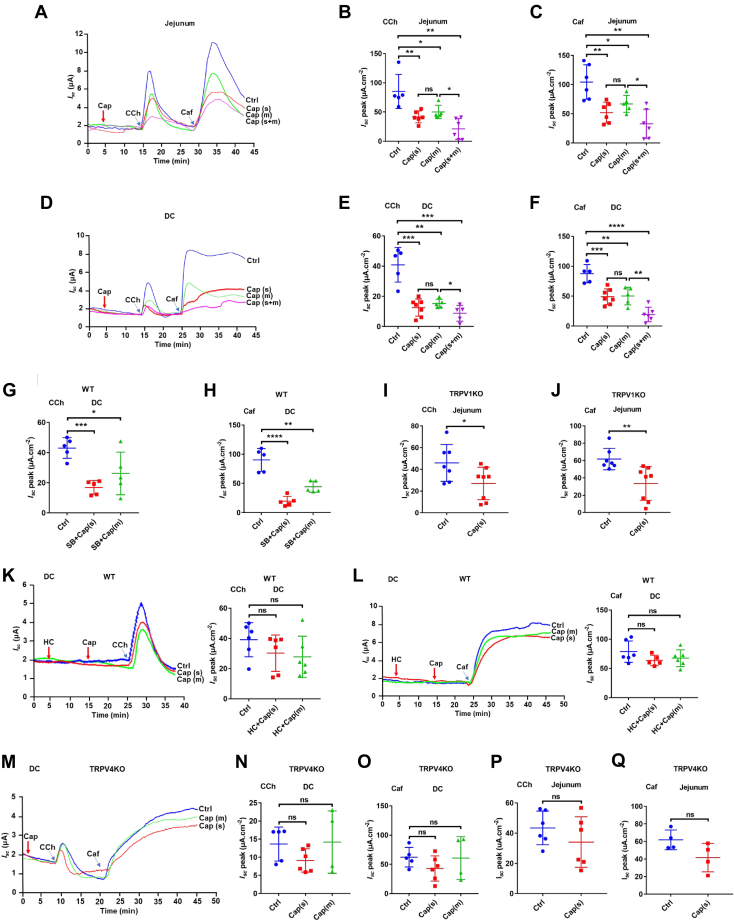
Since epithelial ion transport is a defining physiological process of the intestine, we performed Ussing chamber experiments to examine the effect of capsaicin on epithelial ion transport in the jejunum and distal colon in mice. In the first series of experiments, we tested the effect of capsaicin on jejunal Isc. In the present study, we applied capsaicin at 30 μM (IC50 value on T84 cell monolayer Isc) (15) to native intestinal tissues. Both mucosal and serosal additions of capsaicin (30 μM) did not affect basal jejunal Isc (Fig. 1A). However, either mucosal or serosal addition of capsaicin significantly attenuated carbachol (CCh)-induced jejunal Isc, with bilateral additions further attenuating CCh-induced jejunal Isc (Fig. 1, A and B), indicating that capsaicin inhibits CCh-induced Isc from both apical and basolateral sides of the jejunum via Ca2+ signaling because CCh is a well-known cell Ca2+ mobilizer in the epithelia. We also examined if capsaicin affects caffeine (Caf)-induced jejunal Isc via Ca2+ signaling predominately (26). Similarly, capsaicin also inhibited Caf-induced Isc from each side of the jejunum (Fig. 1, A and C). Therefore, capsaicin inhibits the jejunal anion secretion likely triggered by Ca2+ signaling. In the second series of experiments, we tested if there is any regional heterogeneity between the jejunum and distal colon for the inhibitory effect of capsaicin. Like in the jejunum, capsaicin (30 μM) did not affect basal colonic Isc (Fig. 1D). (Z)-capsaicin and dihydrocapsaicin (30 μM), the other two selective activators of TRPV1 channels, did not affect basal colonic Isc neither (data not shown). However, capsaicin significantly attenuated CCh- and Caf-induced Isc after its addition to each side or both sides of the distal colon (Fig. 1, E and F). Thus, capsaicin as a well-known activator of TRPV1 channels inhibited intestinal Isc without altering its baseline. Moreover, there is no regional heterogeneity between the jejunum and distal colon for the inhibitory effect of capsaicin on CCh- or Caf-induced epithelial ion transports. Given these findings, the jejunum and distal colon were used in the subsequent experiments.
Figure 1.
Capsaicin inhibition of secretagogues-induced intestinal Iscvia a TRPV4-dependent but TRPV1-independent manner.A–C, representative time courses and summary data showing the inhibitory effect of capsaicin (Cap) on carbachol (CCh, 100 μM, n = 6)- or caffeine (Caf, 10 mM, n = 6)-stimulated jejunal Isc after mucosal (m), serosal (s) addition, and mucosal plus serosal (m+s) addition of capsaicin (30 μM). D–F, representative time courses and summary data showing the inhibitory effect of Cap on CCh (n = 6)- or Caf (n = 6)-stimulated distal colonic Isc after capsaicin addition as in A–C. G and H, summary data showing the inhibitory effect of capsaicin (Cap, 30 μM) on carbachol (CCh, 100 μM, n = 5)- or caffeine (Caf, 10 mM, n = 5)-stimulated distal colonic Isc in the absence or the presence of SB705498 (SB, 5 μM) to both sides in WT mice. I and J, summary data showing the inhibitory effect of capsaicin on CCh (n = 7)- or Caf (n = 7)-stimulated jejunal Isc in TRPV1 KO mice. K and L, representative time courses and summary data showing the inhibitory effect of capsaicin on CCh (n = 5)- or Caf (n = 5)-stimulated distal colonic Isc in the absence or the presence of HC067047 (HC, 10 μM) to both sides in WT mice. M–O, representative time courses and summary data showing the inhibitory effect of capsaicin on CCh (n = 5)- or Caf (n = 5)-stimulated distal colonic Isc in TRPV4 KO mice. P and Q, summary data showing the inhibitory effect of capsaicin on CCh (n = 5)- or Caf (n = 5)-stimulated jejunal Isc in TRPV4 KO mice. Ctrl represents as the control without capsaicin treatment, and m or s in parentheses represents mucosal or serosal addition of capsaicin, respectively. CCh and Caf both add to serosal side. The data are presented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 were performed by Student’s t test. ns, no significant differences. Caf, caffeine; CCh, carbachol; Isc, short-circuit current; TRPV, transient receptor potential vanilloid.
Capsaicin inhibition of anion secretion through TRPV4 rather than TRPV1 channels
Since capsaicin can act in both TRPV1-dependent and -independent manners, we utilized pharmacological blockade and genetic KO of TRPV1 channels to study the detailed role of capsaicin on intestinal ion transport. In WT mice, either mucosal or serosal addition of capsaicin still attenuated CCh- or Caf-induced colonic Isc in the presence of SB705498 (5 μM), a selective TRPV1 blocker (Fig. 1, G and H). Similarly, in TRPV1 KO mice, serosal addition of capsaicin also attenuated CCh- or Caf-induced jejunal Isc (Fig. 1,I and J). These data indicate that capsaicin inhibits intestinal anion secretion independently of TRPV1 channels.
After excluding the well-known TRPV1-dependent actions of capsaicin, we examined its TRPV1-independent actions on intestinal anion secretion. Since cholinergic signaling can increase Ca2+ entry through TRPV4 channels (27), we tested if capsaicin-induced inhibition of intestinal anion secretion occurs via TRPV4 channels. In WT mice, either mucosal or serosal addition of capsaicin failed to attenuate CCh- or Caf-induced colonic Isc in the presence of HC067047 (10 μM), a selective TRPV4 blocker (Fig. 1, K and L). Similarly, in TRPV4 KO mice, capsaicin lost its inhibitory action on CCh- or Caf-induced colonic Isc (Fig. 1, M–O) and jejunal Isc (Fig. 1, P and Q). Taken together, these data suggest that TRPV4 channels are responsible for capsaicin inhibition of intestinal anion secretion.
Capsaicin inhibition of anion secretion by blocking TRPV4 channels
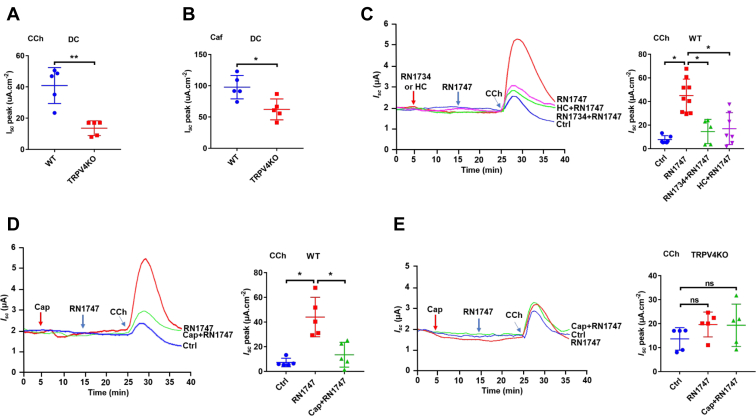
After demonstrating the involvements of TRPV4 channels in capsaicin-induced inhibition, we focused on regulatory role of these channels in anion secretion. First, the CCh- and Caf-induced colonic Isc was significantly reduced in TRPV4 KO mice compared to WT mice (Fig. 2, A and B). Second, TRPV4 activator RN1747 (40 μM) significantly potentiated the CCh (50 μM)-induced colonic Isc, which was prevented by RN1734 (50 μM) and HC067047 (10 μM), two selective TRPV4 blockers with different chemical structures (Fig. 2C). Similarly, TRPV4 activator RN1747 potentiated the CCh (50 μM)-induced colonic Isc in WT mice, which was attenuated by capsaicin (30 μM) (Fig. 2D). Third, these phenomena occurred in WT mice were abolished in TRPV4 mice (Fig. 2E). Together, these data confirm capsaicin inhibition of intestinal secretion by blocking TRPV4 channels.
Figure 2.
Capsaicin inhibition of CCh-induced colonic Cl-secretion by selective blockade of TRPV4 channels.A and B, summary data comparing carbachol (CCh, 100 μM, n = 5)- or caffeine (Caf, 10 mM, n = 5)-stimulated distal colonic Isc between WT mice and TRPV4 KO mice. C, representative time courses and summary data showing the inhibitory effect of TRPV4 blockers RN1734 (50 μM, n = 5) and HC067047 (HC, 10 μM, n = 7) on CCh (50 μM, n = 6) -stimulated distal colonic Isc in the absence or the presence of TRPV4 activator RN1747 (40 μM) in WT mice. D, representative time courses and summary data showing the inhibitory effect of capsaicin (Cap, 30 uM, n = 5) on CCh (50 μM, n = 5) -stimulated distal colonic Isc in the absence or the presence of TRPV4 activator RN1747 (40 μM) in WT mice. E, representative time courses and summary data showing the effect of Cap (30 μM, n = 5) on CCh (100 μM, n = 5)-stimulated distal colonic Isc in the absence or the presence of TRPV4 activator RN1747 (40 μM, n = 5) in TRPV4 KO mice. Ctrl represents as the control with CCh treatment only. CCh adds to serosal side, RN1747, RN1734, and HC add to both sides. The data are presented as mean ± SD. ∗p < 0.05 and ∗∗p < 0.01 were performed by Student’s t test. ns, no significant differences. Caf, caffeine; CCh, carbachol; Isc, short-circuit current; TRPV, transient receptor potential vanilloid.
Role of TRPV4 channels in cholinergic signaling–mediated anion secretion
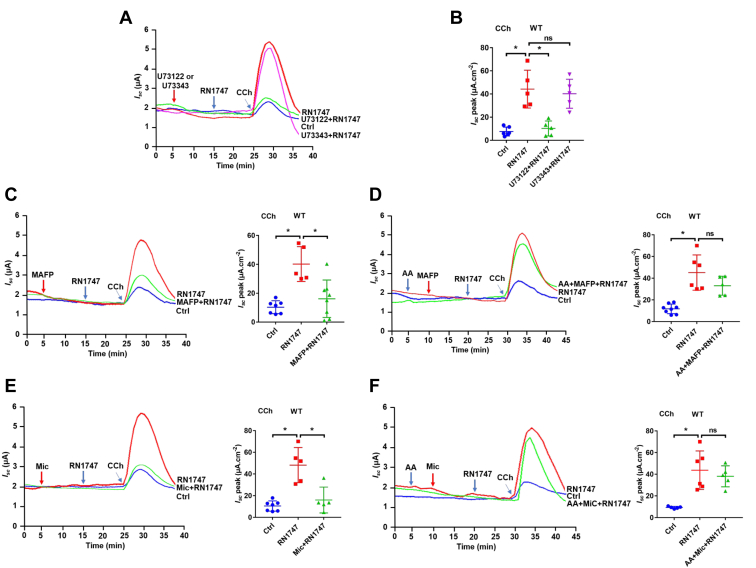
Although capsaicin inhibits anion secretion by blocking TRPV4 channels, their role in regulating secretion has not been explored. We initially applied RN1747, GSK1016790A, and 4α-PPD, three selective TRPV4 activators with different chemical structures, but all of them did not alter basal colonic Isc in WT mice (data not shown, n = 6). Since TRPV4 channels could be suppressed by phosphatidylinositol 4,5-bisphosphate (PIP2) under the resting state (28), we tested if this also happens in the intestine by hydrolyzing PIP2 via activation of phospholipase C (PLC) and phospholipase A2 (PLA2) during cholinergic signaling (29, 30, 31). The active PLC inhibitor U73122 (30 μM), but not its inactive form U73343 (10 μM), attenuated TRPV4 activator RN1747-potentiated Isc induced by CCh (50 μM) (Fig. 3, A and B). Similar findings were seen with methyl arachidonyl fluorophosphonate (10 μM), a selective PLA2 inhibitor (Fig. 3C), However, arachidonic acid (AA) pretreatment could rescue this process (Fig. 3D). These data suggest that PIP2 suppresses intestinal TRPV4 channels, which can be relieved after hydrolysis of PIP2 by PLC and PLA2. Finally, we tested if cytochrome P450 (CYP450) enzyme is involved since CYP450 metabolites have been considered as the endogenous activators of TRPV4 channels. Indeed, the CCh (50 μM)-induced Isc potentiated by TRPV4 activator RN1747 was attenuated by miconazole (10 μM), the CYP450 inhibitor (Fig. 3E); however, this process could be rescued by AA, the substrate for CYP450 enzyme (Fig. 3F). Therefore, we reveal a novel role of TRPV4 channels in cholinergic signaling–mediated intestinal secretion, in which PLC, PLA2, and CYP450 enzymes are involved in the relief of TRPV4 channels suppressed by PIP2.
Figure 3.
Contribution of TRPV4 channels to the PLC, PLA2, and CYP450 pathway-mediated colonic epithelial Cl-secretion.A and B, representative time courses and summary data showing the effect of TRPV4 activator RN1747 (40 μM) on CCh (50 μM)-stimulated distal colonic Isc (n = 5) in the absence or the presence of U73122 (30 μM) or U73343 (10 μM). C and D, representative time courses and summary data showing the effect of RN1747 on CCh-stimulated distal colonic Isc (n = 6) in the absence or the presence of MAFP (10 μM) or MAFP plus arachidonic acid (AA, 50 μM). E and F, representative time courses and summary data showing the effect of RN1747 on CCh-stimulated distal colonic Isc (n = 5) in the absence or the presence of miconazole (Mic, 10 μM) or Mic plus AA. Ctrl represents as the control with CCh treatment only. CCh adds to serosal side. RN1747, U73122, U73343, MAFP, AA, and Mic add to both sides. The data are presented as mean ± SD. ∗p < 0.05 was performed by Student’s t test. ns, no significant differences. CCh, carbachol; Isc, short-circuit current; MAFP, methyl arachidonyl fluorophosphonate; PLA, phospholipid A; PLC, phospholipid C; TRPV, transient receptor potential vanilloid.
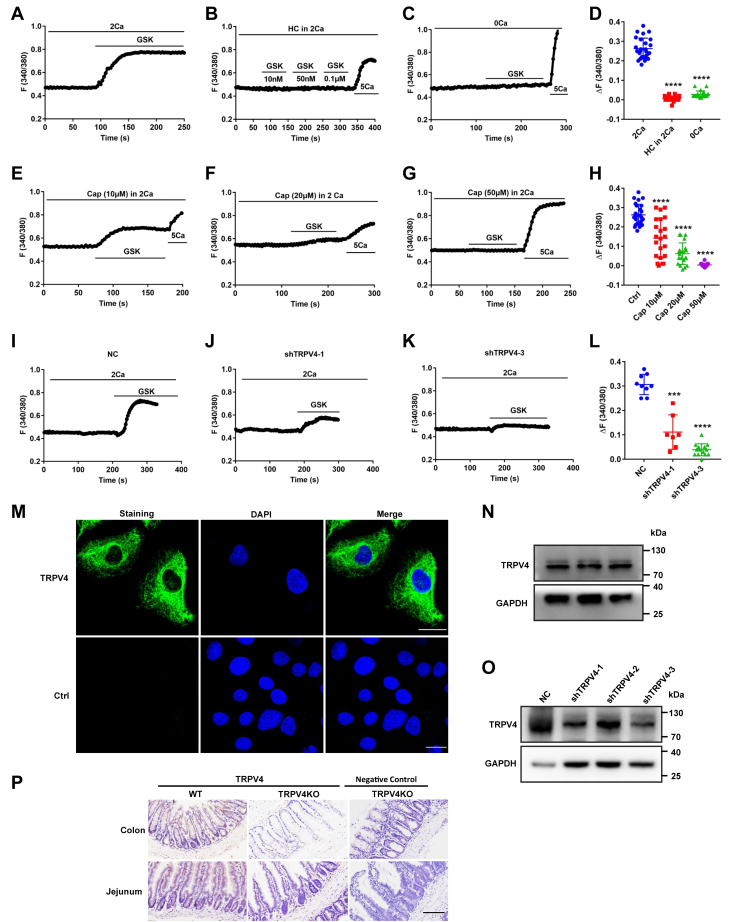
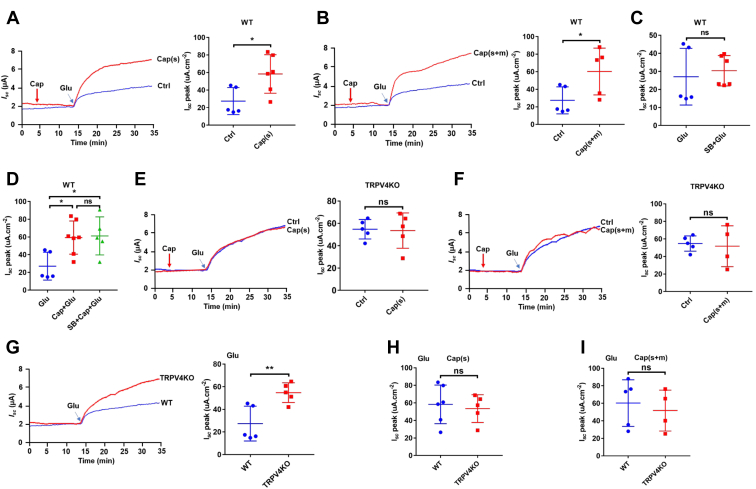
Capsaicin blockade of Ca2+ entry via TRPV4 channels in IEC-6 cells
After verifying capsaicin blockade of the Ca2+-permeable TRPV4 channels in native intestinal tissues, we applied single cell Ca2+ imaging to further study if capsaicin indeed blocks Ca2+ entry via TRPV4 channels in intestinal epithelial cell (IEC)-6 cells. First, GSK1016790A (10 nM), a selective TRPV4 agonist, significantly stimulated cytosolic Ca2+ concentrations ([Ca2+]cyt) signaling in Ca2+-containing solutions (Fig. 4A), but not in Ca2+-free solutions (Fig. 4C). Moreover, GSK-induced [Ca2+]cyt signaling in Ca2+-containing solutions could be abolished by a selective TRPV4 antagonist HC067047 (5 μM) (Fig. 4B). Figure 4D summarizes the GSK-induced changes in [Ca2+]cyt peak in the absence or the presence of Ca2+ or HC067047, which is consistent with previous reports on the functional expression of TRPV4 channels in IEC-6 (19). Second, capsaicin as a well-known TRPV1 agonist at high concentrations of 10 to 50 μM did not alter basal [Ca2+]cyt signaling in IEC-6 (Fig. 4, E–G), indicating undetectable activity of TRPV1 channels in these cells. However, capsaicin dose-dependently blocked Ca2+ entry via TRPV4 channels (Fig. 4, E–H). Capsaicin at 50 μM totally abolished GSK-induced [Ca2+]cyt signaling without altering 5 mM Ca2+-induced [Ca2+]cyt signaling, suggesting its selectivity for TRPV4 channels. Moreover, after shTRPV4 was applied to successfully knock down the protein expression of TRPV4 in IEC-6 cells (Fig. 4O), GSK-induced [Ca2+]cyt signaling in Ca2+-containing solutions was almost abolished as well (Fig. 4,I–L). These data obtained from IEC-6 cells verify our previous notion that capsaicin blocks Ca2+ entry via epithelial TRPV4 channels predominately.
Figure 4.
Capsaicin blockade of Ca2+entry via TRPV4 channels and the protein expression of TRPV4 in IEC-6 cells and mouse intestinal tissues.A−C, summary tracings of [Ca2+]cyt time course in response to GSK1016790A (GSK, 10–100 nM) and calcium (5 mM) in the absence (0Ca2+, n = 26) or the presence (2Ca2+, n = 23) of calcium and calcium plus HC067047 (HC, 5 μM, n = 19). D, summary data showing the peaks of GSK-increased [Ca2+]cyt signaling as in (A−C). E−G, summary tracings of [Ca2+]cyt time course in response to GSK (10 nM) in the absence or the presence of different dose of capsaicin (Cap, n = 26). H, summary data showing the peaks of GSK-increased [Ca2+]cyt signaling described as in (E−G). I−K, summary tracings of [Ca2+]cyt time course in response to GSK (10 nM) of NC and shTRPV4-1 or shTRPV4-3. L, summary data showing the peaks of GSK-increased [Ca2+]cyt signaling described as in (I−K). The data are presented as mean ± SD. ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001 were performed by Student’s t test. M, immunostaining of TRPV4 proteins to confirm their expression in IEC-6 cells. The upper panels: the specific staining of TRPV4 proteins (in green) and merge with the nuclei of the cells stained with DAPI. The lower panel: the nuclei of the cells stained with DAPI (in blue) without primary antibodies against TRPV4 as a negative control. The scale bar represents 20 μm for each image. N and O, Western blotting analysis of TRPV4 proteins expression in IEC-6 cells (N) and shTRPV4 in IEC-6 cells (O). GAPDH was used as a loading control. Each one is the representative of all images taken from 3 independent experiments with similar results. P, immunohistological analysis on TRPV4 proteins in jejunal and colonic tissues from WT mice (the left panels) and TRPV4 KO mice (the middle panels). The right panels were without primary antibodies against TRPV4 in the intestinal tissues from TRPV4 KO mice as negative controls. The scale bar represents 100 μm for each image. [Ca2+]cyt, cytosolic Ca2+ concentrations; IECs, intestinal epithelial cells; TRPV, transient receptor potential vanilloid.
To identify the protein expression of TRPV4 channels in IEC-6 cells, we first performed immunofluorescence to stain for TRPV4 channels. As shown in Figure 4M, TRPV4 proteins were expressed in the cells, however, the immunofluorescence staining was not observed without the primary antibodies against TRPV4 in the control, indicating specific staining on these proteins in IEC-6 cells. Afterward, we applied Western blot to further confirm the protein expression of TRPV4 channels in these cells (Fig. 4N). Finally, we applied immunohistological analysis on TRPV4 protein expression in jejunal and colonic tissues from TRPV4 KO and WT mice. As shown in Figure 4P, TRPV4 expression was slightly detected in the tissues from WT mice (the left panels) but not detected in the tissues from TRPV4 KO mice with (the middle panels) or without primary antibody against TRPV4 (the right panels), indicating the specificity of TRPV4 antibody.
Capsaicin enhancement of jejunal Na+ absorption by blocking TRPV4 channels
Since intestinal Na+ absorption is also important for GI physiology and the reduced Na+ absorption would cause diarrhea, we further examined if capsaicin affects intestinal Na+ absorption. Because Na+-glucose cotransporter Na+-glucose cotransporter is critical for intestinal Na+ absorption, we tested the effect of capsaicin on jejunal Na+-glucose cotransporter. Glucose (5 mM) induced jejunal Isc in WT mice, which was significantly enhanced by either serosal addition of capsaicin (30 μM) (Fig. 5A) or both side additions (Fig. 5B). However, SB705498 (5 μM), a selective TRPV1 antagonist, affected neither glucose-induced Isc (Fig. 5C) nor capsaicin-enhanced Isc (Fig. 5D), excluding the involvement of TRPV1 channels.
Figure 5.
Capsaicin promotion of jejunal Na+absorption by rather blocking TRPV4 channels than activating TRPV1 channels.A and B, representative time courses and summary data showing the effect of capsaicin (Cap, 30 μM, n = 5) on glucose-induced jejunal Isc after its serosal (s) addition or serosal plus mucosal (s+m) addition in WT mice. C and D, summary data showing the effect of SB705498 (SB, 5 μM, n = 5) on glucose-induced jejunal Isc and the effect of Cap on the Isc (n = 5) in the absence or the presence of SB in WT mice. E and F, representative time courses and summary data showing the effect of Cap on glucose-induced jejunal Isc (n = 5) after its serosal (s) addition or serosal plus mucosal (s+m) addition in TRTPV4 KO mice. G, representative time courses and summary data comparing the glucose-induced jejunal Isc (n = 5) between WT mice and TRPV4 KO mice. H and I, summary data comparing the effect of Cap on glucose-induced jejunal Isc (n = 5) between WT mice and TRPV4 KO mice after its serosal (s) addition or serosal plus mucosal (s+m) addition. The data are presented as mean ± SD. ∗p < 0.05 and ∗∗p < 0.01 were performed by Student’s t test. ns, no significant differences. Isc, short-circuit current; TRPV, transient receptor potential vanilloid.
In contrast, capsaicin-enhanced jejunal Na+ absorption disappeared in TRPV4 KO mice after its addition to either serosal side (Fig. 5E) or both sides (Fig. 5F), indicating the role of TRPV4 channels in capsaicin-enhanced Na+ absorption. Surprisingly, glucose-induced jejunal Isc was about 2-fold greater in TRPV4 KO mice than in WT mice (Fig. 5G), revealing the TRPV4 suppression on jejunal Na+ absorption. Finally, capsaicin-enhanced Na+ absorption was comparable between TRPV4 KO and WT mice after its addition to either serosal side (Fig. 5H) or both sides (Fig. 5I), suggesting that capsaicin enhances Na+ absorption by blocking TRPV4 channels to relieve its suppression on the absorption in WT mice. The loss of TRPV4 channels results in the failure of capsaicin to enhance Na+ absorption in KO mice. Taken together, these data reveal a novel suppressive role of TRPV4 channels in jejunal Na+ absorption, which can be relieved by capsaicin blockade of the channels.
Anti-colitic effect of capsaicin in the murine colitic model
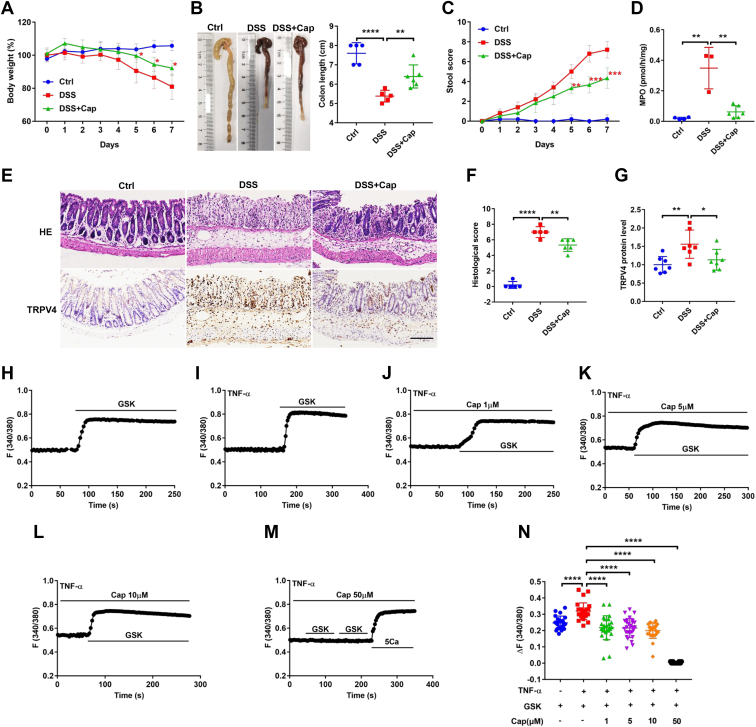
Since the therapeutic actions of capsaicin on colitis are still elusive, we examined the effect of capsaicin in dextran sodium sulfate (DSS)-induced mouse colitis, a commonly used experimental model of UC. First, after mice were treated with DSS for 7 days, their body weight and colon length were reduced (Fig. 6, A and B), but the stool score and myeloperoxidase (MPO) were increased (Fig. 6, C and D), indicating successful establishment of colitis. Second, capsaicin (intragastrically 10 mg/kg, once per day for 7 days) rescued not only the DSS-induced decrease in body weight and colon length (Fig. 6, A and B), but also the increase in stool score and MPO (Fig. 6, C and D). Third, histological examination showed that compared to normal colon, the colitic colon had significant epithelial damage, inflammatory cell infiltration, all of which were rescued by capsaicin (Fig. 6, E and F). Finally, immunohistological analysis revealed that compared to normal colon, TRPV4 expression was upregulated in colitic colon, which is consistent with a previous report on the contribution of TRPV4 to UC exacerbation (18). Likewise, capsaicin attenuated TRPV4 upregulation in mouse colitis (Fig. 6, E and G). These data reveal that capsaicin ameliorates UC likely via TRPV4 suppression, consistently with the previous reports (17, 18, 19).
Figure 6.
Anti-coliticeffect of capsaicin in mice and its inhibition on Ca2+signaling in IEC-6 cells pretreated with TNF-α.A and B, summary data showing the time course of body weight and mouse colon length after different treatments without (Ctrl) or with capsaicin (intragastrically (10 mg/kg) once per day for 7 days), DSS, or DSS + Cap (n = 5). C and D, summary data showing the time courses of stool score and MPO after different treatments as in A and B. E, histological examination and immunohistological analysis showing colonic micro-structure (the upper panels) and TRPV4 immunostaining (the lower panels) after different treatments as in A and B. The scale bar represents 100 μm for each image. F and G, summary data showing histological score and TRPV4 protein level after different treatments as in A and B. H and I, summary tracings of [Ca2+]cyt time course in response to GSK (10 nM) in IEC-6 cells without (n = 22) or with (n = 28) TNF-α (40 ng/ml) pretreatment. J–M, summary tracings of [Ca2+]cyt time course showing the inhibitory effect of Cap at different doses on GSK-induced [Ca2+]cyt signaling in IEC-6 cells pretreated with TNF-α. N, summary data showing the inhibitory effect of Cap at different doses on peaks of GSK-increased [Ca2+]cyt signaling described as in H–M. The data are presented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001 were performed by Student’s t test. [Ca2+]cyt, cytosolic Ca2+ concentrations; DSS, dextran sodium sulfate; IECs, intestinal epithelial cells; MPO, myeloperoxidase; TRPV, transient receptor potential vanilloid.
Capsaicin attenuation of TNF-α-enhanced TRPV4 activity in IEC-6 cells
Since the enhanced expression and activity of TRPV4 channels play pathogenesis role during colitis (17, 18, 19), we further elucidated if capsaicin exerts anti-colitic action by blocking the aberrant TRPV4 channels in intestinal epithelial cells. To this end, IEC-6 cells were pretreated for 12 h with TNF-α (40 ng/ml), a well-known inflammatory factor and marker of intestinal inflammation (32). As shown in Figure 6,H and I, TNF-α indeed increased the TRPV4 activity in IEC-6, consistently with the previous reports on UC (17, 18, 19). Importantly, capsaicin could attenuate the TNF-α-enhanced TRPV4 activity (Fig. 6, J–M). In Figure 6M, GSK1016790A was applied twice to double check no response to it and then followed by 5 mM Ca to make sure the cells were still alive. Figure 6N summarizes the GSK-induced changes in [Ca2+]cyt peak in the presence of TNF-α with or without different doses of capsaicin (1–50 μM), showing that TNF-α enhanced Ca2+ entry via TRPV4 channels, which was markedly blocked by capsaicin at 1 to 10 μM but completely abolished at 50 μM. TNF-α pretreatment may contribute to the biphasic dose-dependence response of the cells shown in Figure 6N. Taken together with our results obtained from mouse colitic model, capsaicin inhibition of the aberrant TRPV4/Ca2+ signaling, at least in part, contributes to its anti-colitic action.
Capsaicin enhancement of colonic Na+ absorption in health and colitis
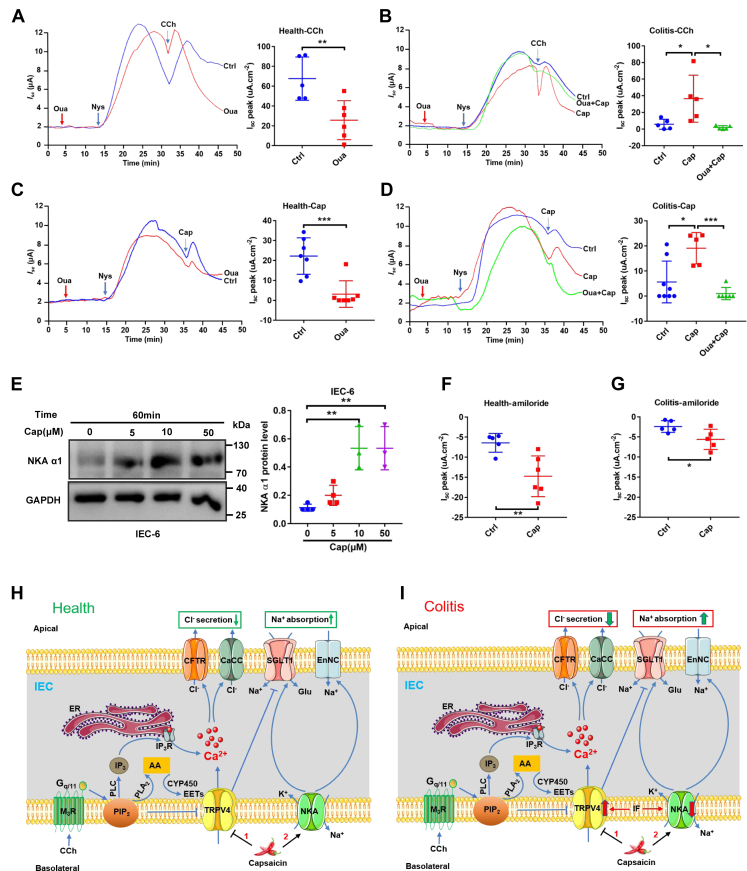
Since colonic Na+ absorption was reduced in the pathogenesis of colitis (33), we examined the effects of capsaicin on colonic Na+ absorption in health and colitis. Because Na+-K+-ATPase (NKA) plays a critical role in the regulation of colonic Na+ absorption and cholinergic signaling could stimulate NKA activity, apically permeabilized colonic tissues were used to measure NKA currents across the basolateral membrane (34). First, after the apical membrane was permeabilized by the ionophore nystatin (100 μM) at the mucosal side of normal colon tissues, in the presence of mucosal Na+, but in the absence of a driving force for K+ flux across K+ channels, CCh (100 μM at the serosal side) induced a strong increase in Isc across the basolateral membrane in apically permeabilized epithelia, which was suppressed by pretreatment with ouabain (100 μM), a selective NKA inhibitor (Fig. 7A). Second, the CCh-induced colonic Isc across the basolateral membrane was attenuated in colitis, which was rescued by pretreatment with dietary capsaicin for 1 week but was suppressed again by ouabain (Fig. 7B). These data are not only consistent with the previous report of cholinergic stimulation of basolateral NKA activity in colon (35) but also suggest the anti-colitic role of capsaicin via NKA stimulation.
Figure 7.
Capsaicin stimulation of NKA and ENaC activities in the colonic epithelia of healthy and colitis mice and the proposed mechanisms of capsaicin action on intestinal epithelial ion transports.A, representative time courses and summary data showing the effect of CCh (100 μM, n = 5) on distal colonic Isc across the basolateral membrane in apically permeabilized epithelia in the absence or the presence of ouabain (Oua, 100 μM, n = 5) in healthy mice. B, representative time courses and summary data showing the effect of CCh on distal colonic Isc (n = 5) across the basolateral membrane in apically permeabilized epithelia in the absence or the presence of ouabain in colitic mice with or without capsaicin pretreated. C, representative time courses and summary data showing the effect of capsaicin (Cap, 30 μM, n = 7) on distal colonic Isc across the basolateral membrane in apically permeabilized epithelia in the absence or the presence of Oua (n = 7) in healthy mice. D, representative time courses and summary data showing the effect of Cap on distal colonic Isc across the basolateral membrane in apically permeabilized epithelia in the absence or the presence of Oua (n = 6) in colitic mice with or without capsaicin pretreated. E, Western blot showing that Cap induced NKAα1 in IEC-6 cells (n = 3 independent experiments). F, summary data showing the distal colonic Isc in response to amiloride (100 μM, n = 5) in the absence or the presence of capsaicin (30 μM, n = 5) in healthy mice. G, summary data showing the colonic Isc (n = 5) in response to amiloride in colitic mice with or without capsaicin pretreated. CCh and ouabain were added to serosal side. Amiloride was added to mucosal side, but capsaicin was added to both sides. The data are presented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 were performed by Student’s t test. H, in health, after the PIP2 suppression on basolateral TRPV4 channels is relieved by the activation of PLC and PLA2 in cholinergic signaling pathway, Ca2+ enters via TRPV4 channels and ER/Ca2+ releases via IP3R. Cytosolic Ca2+ stimulates Ca2+-dependent Cl- secretion through apical CFTR and CaCC. TRPV4 channels could also be activated by their endogenous ligands CYP450 metabolites produced from AA/CYP450 pathway. Capsaicin attenuates Cl- secretion but stimulates Na+ absorption by blocking TRPV4 channels but stimulating NKA activity on the basolateral side of intestinal epithelial cells (IEC). I, in colitis, the expression and function of TRPV4 channels are enhanced but NKA activities are reduced by inflammatory factors, the Cl- secretion are increased, and Na+ absorption are decreased. Capsaicin ameliorates colitis and reduces diarrhea by: (1) suppressing hyperactivation of basolateral TRPV4 channels to reduce intestinal Cl- secretion and (2) blocking basolateral TRPV4 channels and stimulating NKA activity to increase intestinal Na+ absorption. AA, arachidonic acid; CaCC, Ca2+-activated Cl- channels; CFTR, cystic fibrosis transmembrane conductance regulator; CYP450, cytochrome P450; [Ca2+]cyt, cytosolic Ca2+ concentrations; ENaC, epithelial Na+ channels; ER, endoplasmic reticulum; IP3R, IP3 receptor; IF, inflammatory factors; NKA, Na+/K+-ATPase; SGLT1, Na+-glucose cotransporter 1; TRPV, transient receptor potential vanilloid.
Next, we confirmed if capsaicin like CCh could directly stimulate basolateral NKA activity in the colon. Indeed, capsaicin (30 μM) induced a significant increase in Isc across the basolateral membrane in apically permeabilized epithelia of normal colon, which was suppressed by pretreatment with ouabain (100 μM) (Fig. 7C). The capsaicin-induced Isc was attenuated in colitic colon tissues, which was rescued by pretreatment with dietary capsaicin for 1 week but was suppressed again by ouabain (Fig. 7D). Since NKAα1 subunit is essential for the functioning of NKA (36), we verified that capsaicin dose-dependently increased NKAα1 level in IEC-6 cells (Fig. 7E). These data indicate that capsaicin can directly stimulate NKA activity in both normal and colitic colon.
Since apical epithelial Na+ channels (ENaC) also play a critical role in colonic Na+ absorption, we recorded the changes in Isc in response to amiloride, a selective ENaC inhibitor, as a direct measure of ENaC activity (37). In normal colon tissues, amiloride (100 μM) reduced basal Isc, indicating colonic Na+ absorption via the ENaC, which was enhanced by capsaicin (30 μM) (Fig. 7F). However, in colitic colon tissues, although the amiloride-sensitive ENaC activity was reduced, capsaicin still significantly promoted colonic Na+ absorption via ENaC (Fig. 7G). These data suggest that capsaicin can enhance Na+ absorption via the ENaC in both normal and colitic colon.
Discussion
Chili pepper and its active compound capsaicin is a daily food additive worldwide; however, the actions of capsaicin on GI tract as its most delivery pathway have not been well addressed. Moreover, although the expression and function of TRPV channels in sensory neurons of GI tract have been intensively studied, their functions in intestinal epithelial cells (IECs) remain to be elucidated. Since capsaicin is a well-known agonist for TRPV1 channels, the previous studies on its GI action mostly focused on sensory afferents and visceral hypersensitivity in a TRPV1-dependent manner (6); however, emerging evidence points the capsaicin acting independently of TRPV1 channels (16), which is largely unknown in GI tract. Moreover, although we previously demonstrated the beneficial effect of capsaicin on colitic mice through blood perfusion increase to the colonic mucosae (25); it is largely unknown for the direct actions of capsaicin on intestinal epithelia in health and UC and molecular mechanisms. Therefore, the major findings of our study are the novel actions of capsaicin on intestinal epithelial ion transports in GI health and colitis: (1) capsaicin modulates Ca2+-dependent intestinal epithelial ion transports in TRPV4-dependent but TRPV1-independent manner; (2) it inhibits intestinal Cl- secretion by blocking epithelial TRPV4/Ca2+ signaling; (3) it promotes intestinal Na+ absorption by blocking TRPV4 channels but stimulating NKA activity; and (4) since the expression and activity of TRPV4 channels are enhanced during colitis (17, 18, 19), but the expression and activity of NKA are reduced (35, 36), capsaicin ameliorates experimental UC likely by suppressing hyperactivation of TRPV4 channels to reduce Cl- secretion but by activating NKA to increase Na+ absorption.
Up to current knowledge, capsaicin inhibition of intestinal epithelial Cl- secretion can be accomplished mainly by blocking: (1) apical Cl- channels, (2) basolateral K+ channels, and (3) basolateral NKCC1. It was reported previously that in mouse colon and T84 cells, capsaicin activated apical Cl- conductance without effect on basolateral K+ conductance, but induced basolateral NKCC1 internalization in a TRPV1-independent mechanism, explaining in part the inhibition of colonic Cl- secretion (15). The Ussing chamber is a valuable and time-proved physiological system that measures ion transports across epithelial tissues. Active transport or the capacity of epithelium to move ions (such as Cl- and Na+) against an electrical and/or concentration gradient had been well demonstrated by isotopic tracer experiments (38). Therefore, using this well-recognized system, we have provided the following strong evidence for a novel action of capsaicin inhibition of intestinal Cl- secretion by blocking epithelial TRPV4 channels rather than stimulating TRPV1 channels as a well-known selective activator of the channels (Fig. 7H): (1) capsaicin did not affect basal intestinal Isc, but inhibited secretagogues-induced Isc; (2) capsaicin similarly inhibited secretagogues–induced intestinal Isc either in the presence of selective TRPV1 blocker in WT mice or in TRPV1 KO mice, but it could not inhibit secretagogues–induced intestinal Isc either in the presence of selective TRPV4 blocker in WT mice or in TRPV4 KO mice; (3) selective TRPV4 activator potentiated CCh-induced intestinal Isc via Ca2+ signaling, which could be attenuated by either selective TRPV4 blocker or capsaicin; (4) like selective blockers of TRPV4 channels, capsaicin dose-dependently inhibited Ca2+ entry via TRPV4 channels in IEC-6 cells. Therefore, capsaicin may inhibit epithelial TRPV4 channels/Ca2+ signaling-mediated intestinal Cl- secretion via apical cystic fibrosis transmembrane conductance regulator and Ca2+-activated Cl- channels, as previously reported by us (26).
TRPV4 expression was polarized and concentrated on the basolateral surface of Caco-2 cells and IEC-6 cells (17, 19). They are moderately Ca2+-permeable channels which have a homo-dimeric tetramer structure with the TRPV family standard of six transmembrane segments (39). TRPV4 channels are activated by a range of stimuli, including hypotonicity, stretch, and endogenous ligands, such as CYP450 metabolites (5,6-EET and 8,9-EET). Moreover, TRPV4 channels contain six ankyrin repeats and the phospholipid, PIP2, can bind to this site inhibiting the channels (40). Although TRPV4 channels are known to express throughout GI tract (14), their physiological roles in IECs are largely unknown since previous studies mostly focused on their pathological roles in GI tract, such as IBS and IBD (19). Therefore, we initially examined if TRPV4 channels are involved in regulating intestinal ion transports. Interestingly, although TRPV4 channels were functionally expressed in IEC-6 cells, selective TRPV4 activators per se could not induce any intestinal Isc, which prompted us to test if TRPV4 channels in the intestine are suppressed by PIP2 as in other tissues (28). Indeed, PIP2 suppression on epithelial TRPV4 channels could be relieved after PIP2 hydrolysis by either PLC or PLA2 during cholinergic signaling. Furthermore, the AA/CYP450 pathway that produces CYP450 metabolites as the endogenous ligands for TRPV4 channels is also involved in cholinergic signaling–mediated intestinal anion secretion (41). Therefore, we demonstrate for the first time that epithelial TRPV4 channels may play a physiological role in the regulation of intestinal anion secretion during cholinergic signaling.
It was previously reported that capsaicin increased glucose absorption in a TRPV1-dependent manner (42). Surprisingly, we found that capsaicin enhanced jejunal Na+-glucose cotransport in a TRPV4-dependent but TRPV1-independent manner. Moreover, the jejunal Na+ absorption is significantly increased in TRPV4 KO mice compared to WT mice, indicating that TRPV4 inhibition on Na+ absorption is suppressed by capsaicin to finally potentiate the absorption. Therefore, we also demonstrate for the first time that (1) epithelial TRPV4 channels inhibits intestinal Na+ absorption via Na+-glucose cotransporter and (2) capsaicin potentiates intestinal Na+ absorption by blocking epithelial TRPV4 channels (Fig. 7H).
Since NKA establishes the Na+ and K+ gradients used for epithelial ion transports and electrical signaling in the intestine (43), the regulatory mechanisms that control NKA activity are of high biological significance. In the present study, we reveal that like the cholinergic stimulation of basolateral NKA activity in colonic epithelia (35), capsaicin can also stimulate colonic NKA activity, which is confirmed by its direct induction of NKAα1 in IEC-6 cells. Capsaicin stimulation of basolateral NKA activity would establish the ion gradient and driving force used for apical Na+ absorption via jejunal Na+-glucose cotransport and via colonic epithelial amiloride-sensitive ENaC. Therefore, capsaicin promotes intestinal Na+ absorption not only by blocking TRPV4 channels but also by stimulating NKA (Fig. 7H).
Most previous studies suggest that capsaicin might be harmful to GI tract because they provoke a burning sensation, but an epidemiological study revealed that the population who consumes more chili has three times lower peptic ulcer incidence than those who consumes less (44), suggesting beneficial effect of chili/capsaicin in GI disease. Capsaicin also markedly reduced alcohol- and aspirin-induced gastric mucosal injury (45). Here, we have provided strong evidence for the anti-colitic action of capsaicin (Fig. 7I), and it may ameliorate experimental UC by (1) suppressing the hyperactivation of epithelial TRPV4 channels to increase intestinal Na+ absorption but to reduce Cl- secretion, leading to a decrease in colitic diarrhea; (2) stimulating the reduced epithelial NKA activity to increase Na+ absorption via jejunal Na+-glucose cotransports and colonic ENaC. Although capsaicin has multiple pharmacological actions (such as anti-inflammation, anti-oxidant, and anti-cancer) (16), our novel findings herein of its anti-colitic action expend the pharmacological spectrums of capsaicin and the mechanisms of its action.
It has been reported that TRPV family is involved in the pathogenesis of UC (17, 18, 19). Since TRPV1 expression was markedly decreased in the colonic epithelium of UC patients compared to healthy subjects (46), it is hard to presume the beneficial anti-colitic effect of capsaicin is by targeting the decreased TRPV1 channels in the epithelium. Indeed, using TRPV1 KO mice, we have excluded the role of TRPV1 channels in capsaicin inhibition of epithelial anion secretion. However, TRPV4 expression was markedly increased in the colonic epithelium of UC patients (46) and TRPV4 activators increased [Ca2+]cyt but decreased transepithelial resistance of IEC-6 cell monolayer (19). Moreover, Matsumoto K et al (18) showed that TRPV4 activator exacerbated the severity of DSS-induced colitis in WT mice, but colitis was significantly attenuated in TRPV4 KO mice, verifying a critical role of TRPV4 channels in the pathogenesis of colitis. Finally, it is known that the expression and activity of TRPV4 are enhanced with the reduced Na+ absorption but increased Cl- secretion in UC, leading to colitis diarrhea. Therefore, it has been established that TRPV4 channels activate inflammatory signals by IECs and colitis in humans and mice (17, 18, 19). Our findings are in line with the previous notion that small numbers of TRPV4 channels lead to local Ca2+ increase that may play physiological roles, but excessive activation of TRPV4 channels lead to rapid global Ca2+ overload that may cause pathological disorders (27). Since TRPV4 channels are an attractive target for the prevention/treatment of IBD, capsaicin ameliorates experimental UC likely by targeting the enhanced TRPV4 channels as demonstrated herein by us.
So far, the exact actions of capsaicin in colitis and the underling mechanisms remain to be elucidated, except a few studies, including increased visceral sensitivity (47) and ameliorated colitis through submucosal arterial relaxation to increase colonic blood perfusion (25). Here, we reveal the beneficial anti-colitic actions of dietary capsaicin and elucidate, at least partially, the mechanism of its action. Our finding that capsaicin ameliorates experimental colitis is not only consistent with clinical epidemiological investigations that the incidence rate of UC is much lower in high chili consuming regions in China (23, 24) but also with the experimental studies that capsaicin could alleviate abdominal pain and improve UC symptomatology (48, 49, 50). Capsaicin may reach to the colon by bloodstream after absorption in the upper GI tract (∼85% of the orally administered capsaicin) and directly getting into the colon (∼25% of the remaining) (51). From a mouse study (52), 10 mg/kg capsaicin by oral gavage (same dose to our mouse study in vivo) can reach ∼4 μM of serum concentration, which is at the similar range mostly used in our study (1 ∼ 10 μM). Although it needs further confirmation by extensive human study, based on the clinical epidemiological studies showing low UC incidence with high chili consumption (23, 24), our previous and present animal studies strongly support that dietary capsaicin has potential to become a promising and safe drug for the prevention/treatment of UC (25).
In conclusion, TRPV4 channels play a role in regulating intestinal epithelial ion transports in healthy mice, but their expression and function are enhanced in the pathogenesis of UC. Dietary capsaicin inhibits intestinal epithelial Cl- secretion via TRPV4 channel blockade but promotes epithelial Na+ absorption via stimulation of both NKA and ENaC. Capsaicin ameliorates diarrheal effects of experimental UC by blocking the hyperaction of epithelial TRPV4 channels and rescuing the impaired activities of both NKA and ENaC. Therefore, capsaicin is potentially a safe drug for the prevention/treatment of UC.
Experimental procedures
Cell culture
IEC-6 (ATCC Cat# CRL-1592, RRID: CVCL_0343) rat small intestinal cells were purchased from ATCC and were maintained in DMEM-HIGH GLUCOSE medium (Gibco), containing 10% fetal bovine serum and 1% penicillin/streptomycin (Gibco) in a humidified 5% CO2 atmosphere at 37 °C. After the cells had grown to confluence, they were replated onto 12-mm round coverslips (Warner Instruments Inc) and incubated for at least 24 h before use for intracellular Ca2+ measurement or immunostaining.
Animal studies and ethics
All animal care and experimental procedures complied with the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health, USA, and were approved by the Ethics Committee of Chongqing Medical University and AMU. Animal studies are reported in compliance with the ARRIVE guidelines (53). Animal experiments were conducted on male C57BL/6 mice (6–8 weeks old; 18–22 g), purchased from Chongqing Tengxin Biotechnology Co Ltd. TRPV1 and TRPV4 KO mice were purchased from The Jackson Laboratory. Animals were housed in a temperature-controlled room (21 ± 1 °C) with a 12:12-h light-dark cycle in polypropylene plastic cages with an internal area of 672 cm2 × 16.0 cm depth, with wood shavings bedding, up to a maximum of five animals per cage. The mice were fed with normal chow with unlimited access to tap water. Following the Experimental procedures, mice were euthanized by 100% CO2 inhalation and then cervical dislocation. Animals were assigned randomly to different experimental groups for all studies. Data collection and evaluation of all experiments were performed blindly, and the experimenters were unaware of group treatments. All animal studies, only male mice were used to minimize possible variation between sexes.
Ussing chamber experiments
Ussing chamber experiments were performed as previously described (54). The jejuna and distal colonic tissues from each mouse were stripped of seromuscular layers, divided, and examined in chambers (window area, 0.1 cm2). Experiments were performed under continuous short-circuited conditions (Voltage-Current Clamp, VCC MC6; Physiologic Instruments). The transepithelial short-circuit currents (Isc) were measured via an automatic voltage clamp, in which μA was used for the original recordings, but μA·cm-2 was used for summary data. After 30 min equilibration, different agents were added to the mucosal, serosal side, or both sides of the tissues. When it was studied for intestinal Na+ absorption and Cl- secretion, the mucosal solution contained the following (mM): 115 NaCl, 25 sodium-D-gluconate, 5.2 potassium-D-gluconate, 1.2 CaCl2, 1.2 MgCl2, and 10 mannitol. The serosal solution contained the following (mM): 115 NaCl, 25 NaHCO3, 2.2 K2HPO4, 1.2 CaCl2, 1.2 MgCl2, 0.8 KH2PO4, 10 glucose, and 0.01 indomethacin to inhibit possible endogenous PGE2 production resulted from mucosal injury during experiments. The osmolalities for both solutions were ∼300 mosmol·kg−1 of H2O. When NKA currents across the basolateral membrane were measured, the solutions for both sides of the chamber contained the following (mM): 107 NaCl, 4.5 KCl, 25 NaHCO3, 1.8 Na2HPO4, 0.2 NaH2PO4, 1.25 CaCl2, 1 MgSO4, and 12.2 glucose (34).
Infection of lentiviruses
Lentiviruses were purchased from Genechem Co, Ltd. The sequences for TRPV4 shRNA and NC were as follows: shRNA-1 (5′-GGAAGGCTCCTCTTCTCTTTC-3′), shRNA-2 (5′-GCAACATGCGGGAGTTCATCA-3′), shRNA-3 (5′-GGGTCTTTCAGCACATCATCC-3′), and NC shGFP (5′-GAAGCAGCACGACTTCTTC-3′). IEC-6 cells were infected with lentiviruses according to the protocol of the manufacturer, and puromycin was used to screen the stable cells.
Immunohistochemistry
The slides with jejunal and colonic tissues from C57BL/6 and TRPV4 KO mice were incubated with an anti-TRPV4 antibody (Abcam, ab39260; 1:400) overnight at 4 °C. Then, the primary antibodies were detected with biotinylated goat anti-rabbit IgG (Vector Laboratories) secondary antibody at room temperature for 1 h. Immunoreactivity was detected using a 3′-,3′-diaminobenzidine kit (BioGenex) followed by counterstaining with hematoxylin, dehydration, and mounting.
Immunofluorescence staining
The IEC-6 cells grown on coverslips were fixed with 4% paraformaldehyde, blocked with bovine serum albumin, and incubated with anti-TRPV4 (Abcam, ab39260; 1:200) antibody overnight at 4 °C. Then cells were incubated with Alexa Fluor 488 labeled anti-rabbit (Abcam, ab150077; 1:500) secondary antibody for 1 h at room temperature and nuclei were stained with DAPI for 5 min. Fluorescent images were captured in a blinded manner and analyzed with a laser scanning confocal microscope (Olympus). To demonstrate TRPV4 specificity of the antibody labeling, a control experiment was performed in which the primary antibody was omitted.
Measurement of [Ca2+]cyt by single-cell imaging
[Ca2+]cyt imaging experiments were performed as previously described (26, 55). Cells were grown on glass coverslips (Warner Instruments) in 12-well cell culture plates (Corning Incorporated) for 24 h. [Ca2+]cyt was measured using Ca2+-sensitive fluorescent indicator fura-2/AM (Invitrogen). Cells were incubated with 5 μM fura-2/AM for 1 h in physiological salt solution (PSS) at 22 °C in the dark and then washed in PSS for 30 min. Thereafter, cells on coverslips were mounted in a standard perfusion chamber on the stage of an inverted fluorescence microscope (Leica). Fluorescence signals were imaged using an intensified CCD camera (ICCD200) attached to an inverted fluorescence microscope (Leica) and recorded with MetaFluor software (Universal Imaging Corporation). Images were acquired every 3 s. The dual wavelength excitation method for the measurement of fura-2 fluorescence was used. The excitation wavelengths were 340 and 380 nm, and the emitted fluorescence was collected at 510 nm. [Ca2+]cyt was presented as fluorescence ratios (F340/F380) after background subtraction. The PSS used in digital measurement contained the following: 140 mM Na+, 5 mM K+, 2 mM Ca2+, 147 mM Cl-, 10 mM Hepes, and 10 mM glucose (pH 7.4). The osmolality for the solution was ∼300 mosmol.kg−1 of H2O.
Western blot analysis
To detect alpha 1 Sodium Potassium ATPase (phospho Y10) (NKAα1) or TRPV4, the protein sample and MW marker (Thermo Fisher Scientific) were separated using 10% SDS-PAGE and transferred to a PVDF membrane (Millipore). Following blocking with 5% nonfat milk for 2 h at room temperature, the membranes were incubated with the following primary antibodies: Anti-alpha 1 Sodium Potassium ATPase (phospho Y10) (Abcam, ab124677; 1:10,000), anti-TRPV4 (Abcam, ab39260; 1:1000), and anti-GAPDH (Proteintech, 60004-1-Ig, 1:10,000). After washing three times (5 min each) with Tris-buffered saline with 0.05% Tween 20, the membranes were incubated with corresponding secondary antibodies for 2 h at room temperature. After washing another three times with Tris-buffered saline with 0.05% Tween 20, the signals were visualized using enhanced chemiluminescence (Millipore) in an ImageQuant LAS 400 digital biomolecular imaging system.
Mouse model of ulcerative colitis
DSS was used for induction of ulcerative colitis in the mice (56, 57, 58). Eight-week-old male C57BL/6 mice were randomly divided into three groups. The control group received drinking water, the DSS-induced colitis group was administered with drinking water with 2.5% DSS for 7 days, and the DSS plus capsaicin group was given 2.5% DSS plus capsaicin that was administered intragastrically (10 mg/kg) once per day for 7 days of experimental period. This dose of capsaicin was chosen based on its pharmacokinetic properties reported previously (51). Mice were monitored daily using the measures of body weight, stool consistency, and occult/gross blood. Furthermore, the DSS water consumption by mice was tracked throughout treatment period, and no significant differences were found between DSS group and DSS plus capsaicin group. At the end of the protocol, animals were euthanized by 100% CO2 inhalation and cervical dislocation. The colons of both groups were collected and their lengths were measured. The stool score was performed based on two parameters as described previously (18, 59). The stool characteristic was scored as follows: normal (score 0), soft with well-formed pellets (score 1), very soft (score 2), diarrhea (score 3), and severe diarrhea (score 4). The occult blood was scored as follows: no blood (score 0), occult blood in the stool (score 1), traces of blood visible in the stool (score 2), gross bleeding (score 3), and rectal bleeding (score 4). These scores were added to obtain the stool score (maximum score of 8). The determination of MPO activity was according to MPO Assay Kit (Fluorometric) (Abcam, ab111749) as previously described (60).
Histological score
The resected mice colon tissues were stained with H&E. The histological scoring was evaluated by two double-blinded pathologists and performed based on the severity of inflammation, crypt damage, and ulceration as described previously (18, 59). The inflammation was scored as follows: rare inflammatory cells in the lamina propria (score 0), increased numbers of granulocytes in the lamina propria (score 1), confluence of inflammatory cells extending into the submucosa (score 2), and transmural extension of the inflammatory infiltrate (score 3). The crypt damage was scored as follows: intact crypts (score 0), loss of the basal 1/3 (score 1), loss of the basal 2/3 (score 2), entire crypt loss (score 3), a change in the epithelial surface with erosion (score 4), and confluent erosion (score 5). Ulceration was scored as follows: absence of ulcer (score 0), one or two foci of ulcerations (score 1), three or four foci of ulcerations (score 2), and extensive ulceration (score 3). These scores were added to obtain the histological score.
Blinding and randomization
Three segments of distal colon tissues obtained from each mouse were assigned randomly to different treatments. One segment was used as control and the others were used as treatments with different drugs. N is the number of intestinal tissues in Ussing chambers, and each group of n was obtained from at least three mice. All samples were analyzed in a blinded manner.
Materials
All salts were supplied by Sangon Biotech. Glucose, caffeine, carbachol, and indomethacin were purchased from Sigma-Aldrich. Capsaicin, ouabain, SB705498, RN1734, RN1747, U73343, HC067047, GSK1016790A, methyl arachidonyl fluorophosphonate, AA, miconazole, and nystatin were purchased from MedChemExpress (MCE; Monmouth Junction). DSS was from MP Biomedicals (MP Bio). U73122 was from Tocris Bioscience (Tocris). Amiloride hydrochloride was from Molecular Probes, Inc (Molecular Probes). TNF-α and Fura-2/AM (Cat# F1221, Invitrogen) was from Invitrogen. TRPV4 (Abcam Cat# ab39260, RRID: AB_1143677), alpha 1 Sodium Potassium ATPase (phospho Y10) (Abcam Cat# ab124677, RRID: AB_10974461), Alexa Fluor 488 labeled anti-rabbit (Abcam Cat# ab150077, RRID: AB_2630356) antibodies, and MPO Assay Kit were from Abcam. Anti-GAPDH (Proteintech Cat# 60004-1-Ig, RRID: AB_2107436) was from Proteintech.
Data and statistical analysis
All results shown are means ± SD. The number of biological repeats (n) in the figures is the number of individual tissues or cells obtained from at least three mice or three independent experiments. The statistical significance of differences in the means of experimental groups was determined using Student's t test. Posthoc tests were run only if F achieved p < 0.05 (GraphPad Prism 7.0, GraphPad Software, Inc, RRID:SCR_002798), and there was no significant variance inhomogeneity. A probability value ∗p < 0.05 was considered statistically significant.
Data availability
The data supporting the findings of this study are available within the article. The data not shown can be available from the corresponding author Hui Dong upon request.
Conflicts of interests
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We are grateful to Dr Xuefeng Tang for helping with histological score.
Author contributions
H. W., X. Y. C., F. Z., J. C., and F. C. investigation; H. W., X. Y. C., F. Z., J. C., and F. C. data curation; H. W. validation; H. W. formal analysis; H. W. and H. D. funding acquisition; H. W., X. Y. C., F. Z., J. C., F. C., Z. M. S., F. X., and H. D. writing–review and editing; F. X. and H. D. supervision; H. D. conceptualization; H. D. and F. X. methodology; H. D. writing–original draft.
Funding and additional information
These studies were supported by research grants from the National Natural Science Foundation of China (81972328 to X. H. W and No. 81873544 to H. D).
Edited by Mike Shipston
Contributor Information
Feng Xu, Email: xufeng9899@163.com.
Hui Dong, Email: h2dong@ucsd.edu.
References
- 1.Chang J.T. Pathophysiology of inflammatory bowel diseases. N. Engl. J. Med. 2020;383:2652–2664. doi: 10.1056/NEJMra2002697. [DOI] [PubMed] [Google Scholar]
- 2.de Mattos B.R., Garcia M.P., Nogueira J.B., Paiatto L.N., Albuquerque C.G., Souza C.L., Fernandes L.G., Tamashiro W.M., Simioni P.U. Inflammatory bowel disease: An overview of immune mechanisms and biological treatments. Mediators Inflamm. 2015;2015:493012. doi: 10.1155/2015/493012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keita Å V., Lindqvist C.M., Öst Å., Magana C.D.L., Schoultz I., Halfvarson J. Gut barrier dysfunction-a primary defect in twins with Crohn's disease predominantly caused by genetic predisposition. J. Crohn's colitis. 2018;12:1200–1209. doi: 10.1093/ecco-jcc/jjy045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michielan A., D'Incà R. Intestinal permeability in inflammatory bowel disease: Pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm. 2015;2015:628157. doi: 10.1155/2015/628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumgart D.C., Le Berre C. Newer biologic and small-molecule therapies for inflammatory bowel disease. N. Engl. J. Med. 2021;385:1302–1315. doi: 10.1056/NEJMra1907607. [DOI] [PubMed] [Google Scholar]
- 6.Kraft K.H., Brown C.H., Nabhan G.P., Luedeling E., Luna Ruiz Jde J., Coppens d'Eeckenbrugge G., Hijmans R.J., Gepts P. Multiple lines of evidence for the origin of domesticated chili pepper, Capsicum annuum, in Mexico. Proc. Natl. Acad. Sci. U. S. A. 2014;111:6165–6170. doi: 10.1073/pnas.1308933111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richards B.L., Whittle S.L., Buchbinder R. Neuromodulators for pain management in rheumatoid arthritis. Cochrane database Syst. Rev. 2012;1:Cd008921. doi: 10.1002/14651858.CD008921.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh U., Bernstein J.A. Intranasal capsaicin in management of nonallergic (vasomotor) rhinitis. Prog. Drug Res. Fortschritte der Arzneimittelforschung. Progres des recherches pharmaceutiques. 2014;68:147–170. doi: 10.1007/978-3-0348-0828-6_6. [DOI] [PubMed] [Google Scholar]
- 9.Gao X., Zhang D., Xu C., Li H., Caron K.M., Frenette P.S. Nociceptive nerves regulate haematopoietic stem cell mobilization. Nature. 2021;589:591–596. doi: 10.1038/s41586-020-03057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawada T., Suzuki T., Takahashi M., Iwai K. Gastrointestinal absorption and metabolism of capsaicin and dihydrocapsaicin in rats. Toxicol. Appl. Pharmacol. 1984;72:449–456. doi: 10.1016/0041-008x(84)90121-2. [DOI] [PubMed] [Google Scholar]
- 11.Leung F.W. Capsaicin-sensitive intestinal mucosal afferent mechanism and body fat distribution. Life Sci. 2008;83:1–5. doi: 10.1016/j.lfs.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Wang P., Yan Z., Zhong J., Chen J., Ni Y., Li L., Ma L., Zhao Z., Liu D., Zhu Z. Transient receptor potential vanilloid 1 activation enhances gut glucagon-like peptide-1 secretion and improves glucose homeostasis. Diabetes. 2012;61:2155–2165. doi: 10.2337/db11-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domotor A., Peidl Z., Vincze A., Hunyady B., Szolcsanyi J., Kereskay L., Szekeres G., Mozsik G. Immunohistochemical distribution of vanilloid receptor, calcitonin-gene related peptide and substance P in gastrointestinal mucosa of patients with different gastrointestinal disorders. Inflammopharmacology. 2005;13:161–177. doi: 10.1163/156856005774423737. [DOI] [PubMed] [Google Scholar]
- 14.Holzer P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol. Ther. 2011;131:142–170. doi: 10.1016/j.pharmthera.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouyer P.G., Tang X., Weber C.R., Shen L., Turner J.R., Matthews J.B. Capsaicin induces NKCC1 internalization and inhibits chloride secretion in colonic epithelial cells independently of TRPV1. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;304:G142–G156. doi: 10.1152/ajpgi.00483.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braga Ferreira L.G., Faria J.V., Dos Santos J.P.S., Faria R.X. Capsaicin: TRPV1-independent mechanisms and novel therapeutic possibilities. Eur. J. Pharmacol. 2020;887:173356. doi: 10.1016/j.ejphar.2020.173356. [DOI] [PubMed] [Google Scholar]
- 17.D'Aldebert E., Cenac N., Rousset P., Martin L., Rolland C., Chapman K., Selves J., Alric L., Vinel J.P., Vergnolle N. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology. 2011;140:275–285. doi: 10.1053/j.gastro.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto K., Yamaba R., Inoue K., Utsumi D., Tsukahara T., Amagase K., Tominaga M., Kato S. Transient receptor potential vanilloid 4 channel regulates vascular endothelial permeability during colonic inflammation in dextran sulphate sodium-induced murine colitis. Br. J. Pharmacol. 2018;175:84–99. doi: 10.1111/bph.14072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamawaki H., Mihara H., Suzuki N., Nishizono H., Uchida K., Watanabe S., Tominaga M., Sugiyama T. Role of transient receptor potential vanilloid 4 activation in indomethacin-induced intestinal damage. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;307:G33–G40. doi: 10.1152/ajpgi.00105.2013. [DOI] [PubMed] [Google Scholar]
- 20.Kihara N., de la Fuente S.G., Fujino K., Takahashi T., Pappas T.N., Mantyh C.R. Vanilloid receptor-1 containing primary sensory neurones mediate dextran sulphate sodium induced colitis in rats. Gut. 2003;52:713–719. doi: 10.1136/gut.52.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engel M.A., Khalil M., Mueller-Tribbensee S.M., Becker C., Neuhuber W.L., Neurath M.F., Reeh P.W. The proximodistal aggravation of colitis depends on substance P released from TRPV1-expressing sensory neurons. J. Gastroenterol. 2012;47:256–265. doi: 10.1007/s00535-011-0495-6. [DOI] [PubMed] [Google Scholar]
- 22.Lee J., Yamamoto T., Kuramoto H., Kadowaki M. TRPV1 expressing extrinsic primary sensory neurons play a protective role in mouse oxazolone-induced colitis. Auton. Neurosci. 2012;166:72–76. doi: 10.1016/j.autneu.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Ng W.K., Wong S.H., Ng S.C. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016;14:111–119. doi: 10.5217/ir.2016.14.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng S.C., Kaplan G.G., Tang W., Banerjee R., Adigopula B., Underwood F.E., Tanyingoh D., Wei S.C., Lin W.C., Lin H.H., Li J., Bell S., Niewiadomski O., Kamm M.A., Zeng Z., et al. Population density and risk of inflammatory bowel disease: A prospective population-based study in 13 countries or regions in asia-pacific. Am. J. Gastroenterol. 2019;114:107–115. doi: 10.1038/s41395-018-0233-2. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L., Lu W., Lu C., Guo Y., Chen X., Chen J., Xu F., Wan H., Dong H. Beneficial effect of capsaicin via TRPV4/EDH signals on mesenteric arterioles of normal and colitis mice. J. Adv. Res. 2021 doi: 10.1016/j.jare.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang F., Wan H., Yang X., He J., Lu C., Yang S., Tuo B., Dong H. Molecular mechanisms of caffeine-mediated intestinal epithelial ion transports. Br. J. Pharmacol. 2019;176:1700–1716. doi: 10.1111/bph.14640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonkusare S.K., Bonev A.D., Ledoux J., Liedtke W., Kotlikoff M.I., Heppner T.J., Hill-Eubanks D.C., Nelson M.T. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harraz O.F., Longden T.A., Hill-Eubanks D., Nelson M.T. PIP2 depletion promotes TRPV4 channel activity in mouse brain capillary endothelial cells. Elife. 2018;7 doi: 10.7554/eLife.38689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kankanamge D., Ubeysinghe S., Tennakoon M., Pantula P.D., Mitra K., Giri L., Karunarathne A. Dissociation of the G protein betagamma from the Gq-PLCbeta complex partially attenuates PIP2 hydrolysis. J. Biol. Chem. 2021;296:100702. doi: 10.1016/j.jbc.2021.100702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sulpice J.C., Zachowski A., Devaux P.F., Giraud F. Requirement for phosphatidylinositol 4,5-bisphosphate in the Ca(2+)-induced phospholipid redistribution in the human erythrocyte membrane. J. Biol. Chem. 1994;269:6347–6354. [PubMed] [Google Scholar]
- 31.Rogalski S.L., Chavkin C. Eicosanoids inhibit the G-protein-gated inwardly rectifying potassium channel (Kir3) at the Na+/PIP2 gating site. J. Biol. Chem. 2001;276:14855–14860. doi: 10.1074/jbc.M010097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braegger C.P., Nicholls S., Murch S.H., Stephens S., MacDonald T.T. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992;339:89–91. doi: 10.1016/0140-6736(92)90999-j. [DOI] [PubMed] [Google Scholar]
- 33.Rajendran V.M., Nanda Kumar N.S., Tse C.M., Binder H.J. Na-H exchanger isoform-2 (NHE2) mediates butyrate-dependent Na+ absorption in dextran sulfate sodium (DSS)-induced colitis. J. Biol. Chem. 2015;290:25487–25496. doi: 10.1074/jbc.M115.654277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bader S., Lottig L., Diener M. Stimulation of Na(+) -K(+) -pump currents by epithelial nicotinic receptors in rat colon. Br. J. Pharmacol. 2017;174:880–892. doi: 10.1111/bph.13761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirota C.L., McKay D.M. Loss of Ca2+-mediated ion transport during colitis correlates with reduced ion transport responses to a Ca2+-activated K+ channel opener. Br. J Pharmacol. 2009;156:1085–1097. doi: 10.1111/j.1476-5381.2009.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saha P., Manoharan P., Arthur S., Sundaram S., Kekuda R., Sundaram U. Molecular mechanism of regulation of villus cell Na-K-ATPase in the chronically inflamed mammalian small intestine. Biochim. Biophys. Acta. 2015;1848:702–711. doi: 10.1016/j.bbamem.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Goodman M.B., Ernstrom G.G., Chelur D.S., O'Hagan R., Yao C.A., Chalfie M. MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature. 2002;415:1039–1042. doi: 10.1038/4151039a. [DOI] [PubMed] [Google Scholar]
- 38.Clarke L.L. A guide to Ussing chamber studies of mouse intestine. Am J. Physiol. Gastrointest. Liver Physiol. 2009;296:G1151–G1166. doi: 10.1152/ajpgi.90649.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shigematsu H., Sokabe T., Danev R., Tominaga M., Nagayama K. A 3.5-nm structure of rat TRPV4 cation channel revealed by Zernike phase-contrast cryoelectron microscopy. J. Biol. Chem. 2010;285:11210–11218. doi: 10.1074/jbc.M109.090712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu S., Huang S., Ding Y., Wang W., Wang A., Lu Y. Transient receptor potential ion-channel subfamily V member 4: A potential target for cancer treatment. Cell Death Dis. 2019;10:497. doi: 10.1038/s41419-019-1708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe H., Vriens J., Prenen J., Droogmans G., Voets T., Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 42.Domotor A., Szolcsanyi J., Mozsik G. Capsaicin and glucose absorption and utilization in healthy human subjects. Eur. J. Pharmacol. 2006;534:280–283. doi: 10.1016/j.ejphar.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 43.Skou J.C. The fourth Datta lecture. The energy coupled exchange of Na+ for K+ across the cell membrane. The Na+, K(+)-pump. FEBS Lett. 1990;268:314–324. doi: 10.1016/0014-5793(90)81278-v. [DOI] [PubMed] [Google Scholar]
- 44.Abdel-Salam O.M., Szolcsanyi J., Mozsik G. Capsaicin and the stomach. A review of experimental and clinical data. J. Physiol. Paris. 1997;91:151–171. doi: 10.1016/s0928-4257(97)89479-x. [DOI] [PubMed] [Google Scholar]
- 45.Holzer P., Pabst M.A., Lippe I.T. Intragastric capsaicin protects against aspirin-induced lesion formation and bleeding in the rat gastric mucosa. Gastroenterology. 1989;96:1425–1433. doi: 10.1016/0016-5085(89)90508-8. [DOI] [PubMed] [Google Scholar]
- 46.Rizopoulos T., Papadaki-Petrou H., Assimakopoulou M. Expression profiling of the transient receptor potential vanilloid (TRPV) channels 1, 2, 3 and 4 in mucosal epithelium of human ulcerative colitis. Cells. 2018;7:61. doi: 10.3390/cells7060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eijkelkamp N., Kavelaars A., Elsenbruch S., Schedlowski M., Holtmann G., Heijnen C.J. Increased visceral sensitivity to capsaicin after DSS-induced colitis in mice: Spinal cord c-Fos expression and behavior. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G749–G757. doi: 10.1152/ajpgi.00114.2007. [DOI] [PubMed] [Google Scholar]
- 48.Goso C., Evangelista S., Tramontana M., Manzini S., Blumberg P.M., Szallasi A. Topical capsaicin administration protects against trinitrobenzene sulfonic acid-induced colitis in the rat. Eur. J. Pharmacol. 1993;249:185–190. doi: 10.1016/0014-2999(93)90431-g. [DOI] [PubMed] [Google Scholar]
- 49.Motte J., Ambrosius B., Gruter T., Bachir H., Sgodzai M., Pedreiturria X., Pitarokoili K., Gold R. Capsaicin-enriched diet ameliorates autoimmune neuritis in rats. J. Neuroinflam. 2018;15:122. doi: 10.1186/s12974-018-1165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosca A.E., Iesanu M.I., Zahiu C.D.M., Voiculescu S.E., Paslaru A.C., Zagrean A.M. Capsaicin and gut microbiota in health and disease. Molecules. 2020;25:5681. doi: 10.3390/molecules25235681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rollyson W.D., Stover C.A., Brown K.C., Perry H.E., Stevenson C.D., McNees C.A., Ball J.G., Valentovic M.A., Dasgupta P. Bioavailability of capsaicin and its implications for drug delivery. J. Control Release. 2014;196:96–105. doi: 10.1016/j.jconrel.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rollyson W., Stover C., Brown K., Perry H., Stevenson C., Crabtree C., Dom A., Lau J., Witte T., Hardman W., Dasgupta P. The anti-cancer dietary compound capsaicin shows higher bioavailability in the lung than other organs. Faseb J. 2014;281(Suppl. 644.2) [Google Scholar]
- 53.Percie du Sert N., Hurst V., Ahluwalia A., Alam S., Avey M.T., Baker M., Browne W.J., Clark A., Cuthill I.C., Dirnagl U., Emerson M., Garner P., Holgate S.T., Howells D.W., Karp N.A., et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tuo B., Wen G., Wang X., Xu J., Xie R., Liu X., Dong H. Estrogen potentiates prostaglandin E(2)-stimulated duodenal mucosal HCO(3)(-) secretion in mice. Am. J. Physiol. Endocrinol. Metab. 2012;303:E111–E121. doi: 10.1152/ajpendo.00575.2011. [DOI] [PubMed] [Google Scholar]
- 55.Wan H., Xie R., Xu J., He J., Tang B., Liu Q., Wang S., Guo Y., Yang X., Dong T.X., Carethers J.M., Yang S., Dong H. Anti-proliferative effects of nucleotides on gastric cancer via a novel P2Y6/SOCE/Ca(2+)/beta-catenin pathway. Sci. Rep. 2017;7:2459. doi: 10.1038/s41598-017-02562-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meir M., Burkard N., Ungewiss H., Diefenbacher M., Flemming S., Kannapin F., Germer C.T., Schweinlin M., Metzger M., Waschke J., Schlegel N. Neurotrophic factor GDNF regulates intestinal barrier function in inflammatory bowel disease. J. Clin. Invest. 2019;129:2824–2840. doi: 10.1172/JCI120261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okayasu I., Hatakeyama S., Yamada M., Ohkusa T., Inagaki Y., Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 58.Wirtz S., Popp V., Kindermann M., Gerlach K., Weigmann B., Fichtner-Feigl S., Neurath M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017;12:1295–1309. doi: 10.1038/nprot.2017.044. [DOI] [PubMed] [Google Scholar]
- 59.Zheng W., Song H., Luo Z., Wu H., Chen L., Wang Y., Cui H., Zhang Y., Wang B., Li W., Liu Y., Zhang J., Chu Y., Luo F., Liu J. Acetylcholine ameliorates colitis by promoting IL-10 secretion of monocytic myeloid-derived suppressor cells through the nAChR/ERK pathway. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2017762118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yasuda M., Kawahara R., Hashimura H., Yamanaka N., Iimori M., Amagase K., Kato S., Takeuchi K. Dopamine D(2)-receptor antagonists ameliorate indomethacin-induced small intestinal ulceration in mice by activating alpha7 nicotinic acetylcholine receptors. J. Pharmacol. Sci. 2011;116:274–282. doi: 10.1254/jphs.11037fp. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available within the article. The data not shown can be available from the corresponding author Hui Dong upon request.