Abstract
Objective
Although it is well established that urocortin 2 (Ucn2), a peptide member of the corticotrophin releasing factor (CRF) family, and its specific corticotrophin-releasing factor 2 receptor (CRF2R) are highly expressed in skeletal muscle, the role of this peptide in the regulation of skeletal muscle mass and protein metabolism remains elusive.
Methods
To elucidate the mechanisms how Ucn2 directly controls protein metabolism in skeletal muscles of normal mice, we carried out genetic tools, physiological and molecular analyses of muscles in vivo and in vitro.
Results
Here, we demonstrated that Ucn2 overexpression activated cAMP signaling and promoted an expressive muscle hypertrophy associated with higher rates of protein synthesis and activation of Akt/mTOR and ERK1/2 signaling pathways. Furthermore, Ucn2 induced a decrease in mRNA levels of atrogin-1 and in autophagic flux inferred by an increase in the protein content of LC3-I, LC3-II and p62. Accordingly, Ucn2 reduced both the transcriptional activity of FoxO in vivo and the overall protein degradation in vitro through an inhibition of lysosomal proteolytic activity. In addition, we demonstrated that Ucn2 induced a fast-to-slow fiber type shift and improved fatigue muscle resistance, an effect that was completely blocked in muscles co-transfected with mitogen-activated protein kinase phosphatase 1 (MKP-1), but not with dominant-negative Akt mutant (Aktmt).
Conclusions
These data suggest that Ucn2 triggers an anabolic and anti-catabolic response in skeletal muscle of normal mice probably through the activation of cAMP cascade and participation of Akt and ERK1/2 signaling. These findings open new perspectives in the development of therapeutic strategies to cope with the loss of muscle mass.
Keywords: Urocortin 2, Hypertrophy, Fatigue resistance, cAMP
Highlights
-
•
Ucn2 overexpression promotes muscle growth due to an increase in protein synthesis.
-
•
Ucn2 inhibits FoxO activity and autophagic-lysosomal system.
-
•
Ucn2-induced skeletal muscle phenotype is dependent on Akt and ERK1/2.
-
•
Ucn2 induces a fast-to-slow fiber type shift and improves fatigue resistance.
-
•
The increase in muscle fatigue resistance is dependent on ERK1/2.
1. Introduction
The peptide urocortin 2 (Ucn2), also known as stresscopine-related peptide (SRP), is a member of the corticotrophin-releasing factor (CRF) neuropeptide family, which also includes CRF homologues such as urotensin 1, sauvagin, urocortin 1, and urocortin 3 [1,2]. Ucn2 is a selective agonist of corticotrophin-releasing factor 2 receptor (CRF2R) [[3], [4], [5]], which expression was initially detected in a few regions of the central nervous system of rats, such as arched and paraventricular nuclei of hypothalamus and locus coeruleus [1]. More recently, Ucn2 expression has been identified in lower levels in several peripheral tissues such as lung, stomach, adrenal, testes, ovary, brown adipose tissue (BAT), thymus and spleen and in high levels in skin and skeletal muscle [6]. It has been reported that Ucn2 can regulates hypothalamic–pituitary–adrenal axis (HPA) [[7], [8], [9], [10]] which suggests that this peptide is an endogenous moderator of the actions triggered by the HPA axis under stress. Ucn2 has been shown to promote satiety [2], cardioprotection in a rat model of ischaemia/reperfusion injury [11] and peripheral vasodilation [12]. However, the physiological role of Ucn2 is far from being completely established.
Ucn2 is a myokine since it is produced, expressed, and released by muscle fibers and exerts either autocrine, paracrine or endocrine effects [1,2,13]. It has been demonstrated that acute treatment of Ucn2 increases muscle mass and strength [14], and prevents atrophy in rodent muscles submitted to models of sciatic motor denervation and treatment with dexamethasone [14,15]. A recent study demonstrated that modified Ucn2 peptide systemically administered enhances skeletal muscle mass and function in high-fat diet-induced obesity in mice [16]. Although the growth-promoting and anti-atrophy actions of Ucn2 are known, the underlying mechanisms and related intracellular mediators of these actions are not completely established. CRF2R is a Gαs protein-coupled receptor (GPCR) that can activate adenylyl cyclase (AC), which enhances intracellular cAMP levels, and consequently activates cAMP-dependent protein kinase (PKA) [5,13]. Indeed, Ucn2 has been shown to stimulate the in vitro production of cAMP in C2C12 myotubes [17] and in isolated mouse muscles [14,15]. Cyclic AMP enhancers such as the nonspecific phosphodiesterase (PDE) inhibitor isobutylmethylxanthine and the selective PDE4 inhibitor rolipram have shown to suppress muscle protein degradation [18]. β2-adrenergic agonists may also increase cAMP levels and promote muscle growth by stimulating protein synthesis via a cross-talking with insulin/IGF-1 pathways [19]. Thus, these findings open a possibility that the growth-promoting effects of Ucn2 involve similar mechanisms.
Insulin/IGF-1 signaling is the major intracellular pathway controlling protein metabolism in skeletal muscle by activating the kinases Akt and ERK1/2 [[20], [21], [22], [23]]. Both kinases regulate muscle mass by two possible downstream targets: 1) by activating the mechanistic target of rapamycin (mTOR), the main responsible for the stimulation of muscle protein synthesis [[24], [25], [26]], and 2) by inhibiting Forkhead box class O (FoxO), the main transcriptional factor that regulates atrophy-related genes, also known as atrogenes [27,28]. Several atrogenes code components of the ubiquitin-proteasome system (UPS) [e.g., the ubiquitin-ligases atrogin-1 and muscle RING finger-1 (Murf-1)] and autophagic-lysosomal [e.g., microtubule-associated protein light chain 3 beta (Map1lc3b), gamma-aminobutyric acid (GABA) A receptor-associated protein-like 1 (Gabarapl1) and cathepsin L (Ctsl)] system, which degrade the most cellular proteins and organelles in skeletal muscle during physiological and pathological states [[29], [30], [31], [32]].
In addition to muscle size, cAMP/PKA and ERK1/2 signaling may also regulate fiber type specification and function. PKA signaling induces a slow-oxidative phenotype by upregulating the expression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), a master regulator of mitochondrial biogenesis [[33], [34], [35]]. It has been demonstrated that the transcriptional factor cAMP response element-binding protein (CREB) once stimulated by PKA mediates the induction of PGC-1α in C2C12 cells [36]. Murgia et al. [37] reported that ERK1/2 activity is increased by low-frequency electrical nerve stimulation, and a dominant-negative mutant of Ras, an upstream of ERK1/2, inhibited these effects. These findings indicate that Ras-ERK1/2 signaling promotes nerve-activity-dependent differentiation of slow-twitch muscle fibers. It is noteworthy that slow oxidative fibers are more resistant than fast glycolytic fibers under various atrophic conditions caused by FoxO [38]. Moreover, Ucn2 treatment activates ERK1/2 inducing cardioprotection in isolated hearts from rats subjected to protocol of ischemia/reperfusion injury [39]. However, it is unknown whether or not Ucn2 can induce a shift toward slow fiber type and fatigue resistance mediated by ERK1/2.
Therefore, we hypothesized that the effects of Ucn2 on skeletal muscle involves cAMP signaling and insulin/IGF-1 downstream targets. The current study was undertaken to clarify the muscle phenotype, the molecular mechanisms and the signaling pathways regulated by Ucn2 in controlling muscle protein metabolism. Since previous literature data available reported systemic treatment to study Ucn2-induced skeletal muscle effects, in order to rule out the indirect peripheral effects of Ucn2 that could interfere on its hypertrophic action, skeletal muscles were transfected in vivo with Ucn2 by electroporation or incubated in vitro with the peptide. Here we show that the in vivo overexpression of Ucn2 promotes substantial muscle hypertrophy in normal mice, which was attenuated by genetic blockage of either Akt or ERK1/2. Ucn2 also stimulated the downstream targets of these kinases favoring protein synthesis (i.e., mTOR signaling) and inhibited atrophy-related proteins suppressing proteolysis (i.e., FoxO signaling). Ucn2-induced muscle hypertrophy was also accompanied by a shifting toward a slow oxidative phenotype and fatigue resistance, an effect that was prevented by the overexpression of mitogen-activated protein kinase phosphatase 1 (MKP-1), an inhibitor of ERK1/2. Identifying mechanisms behind a peptide with such muscle-growth potential provide therapeutic targets for combating muscle loss of several diseases.
2. Material and methods
2.1. Animals
In vitro experiments were performed in fed 4-week-old male Wistar rats (∼70 g) since the incubation procedure requires intact muscles of a sufficient thinness to allow the adequate diffusion of metabolites and oxygen [40]. All in vivo experiments were performed in fed 8-week-old male C57Bl6/J mice (∼30 g). All animals were housed in a room with a 12 h–12 h light–dark cycle and were given free access to water and a normal lab chow diet until the start of the experiment. All experiments and protocols were performed in accordance with the ethical principles for animal research adopted by Brazilian College of Animal Experimentation and were approved by the Ribeirão Preto Medical School of the University of São Paulo – Ethical Commission of Ethics in Animal Research (no. 063/2014).
2.2. Adult skeletal muscle in vivo transfection
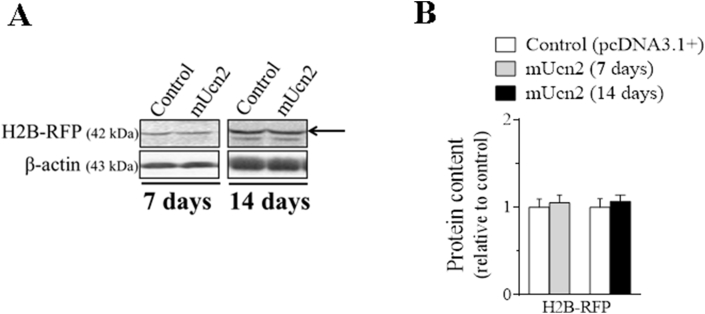
Mice were anaesthetized by intraperitoneal injection of 85 mg.kg−1 ketamine and 10 mg.kg−1 xylazine. A minor incision was performed on mouse hindlimb to expose tibialis anterior (TA) muscles in order to inject along its length 30 μl of 0.9% saline containing purified plasmid DNA (PureLink HiPure Plasmid Filter Purification Kit, Invitrogen). For evaluation of the direct Ucn2 effect, 30 μg of mouse (m) Ucn2 and 10 μg of green fluorescent protein (GFP) were used. For evaluation of FoxO activity, 10 μg of DBE-FoxO luciferase and 5 μg of pRL-null-Renilla luciferase were used. Electric pulses were then applied using an electroporator (CUY21; Tokiwa Science, Fukuoka, Japan) and two stainless steel spatula electrodes placed on each side of the isolated muscle. Four positives and four negatives square-wave pulses with a pulse length of 20- and 200- ms intervals between each pulse were delivered at 21 V. Seven and fourteen days after transfection, animals were killed by cervical dislocation and muscles were removed to molecular analysis. Transfection efficiency of muscle fibers was also evidenced by the co-transfection of a plasmid expressing a GFP gene in both groups as can be observed in TA muscles cross-sections (Figure 1B). In order to demonstrate the homogeneity of the in vivo electroporation process, TA were co-transfected with a plasmid expressing histone 2 B fused to red fluorescent protein (H2B-RFP) plus an empty vector or mUcn2 for 7 and 14 days. There was no difference in the protein content of RFP (Fig. S1), demonstrating that both muscles of control and experimental groups had the same transfection efficiency pattern in the analyzed periods. In addition, there were neither injury signs nor cellular regeneration in cross-section of TA stained with hematoxylin and eosin (data not shown), demonstrating the integrity of muscle fibers after Ucn2 overexpression for 14 days through the in vivo electroporation technique.
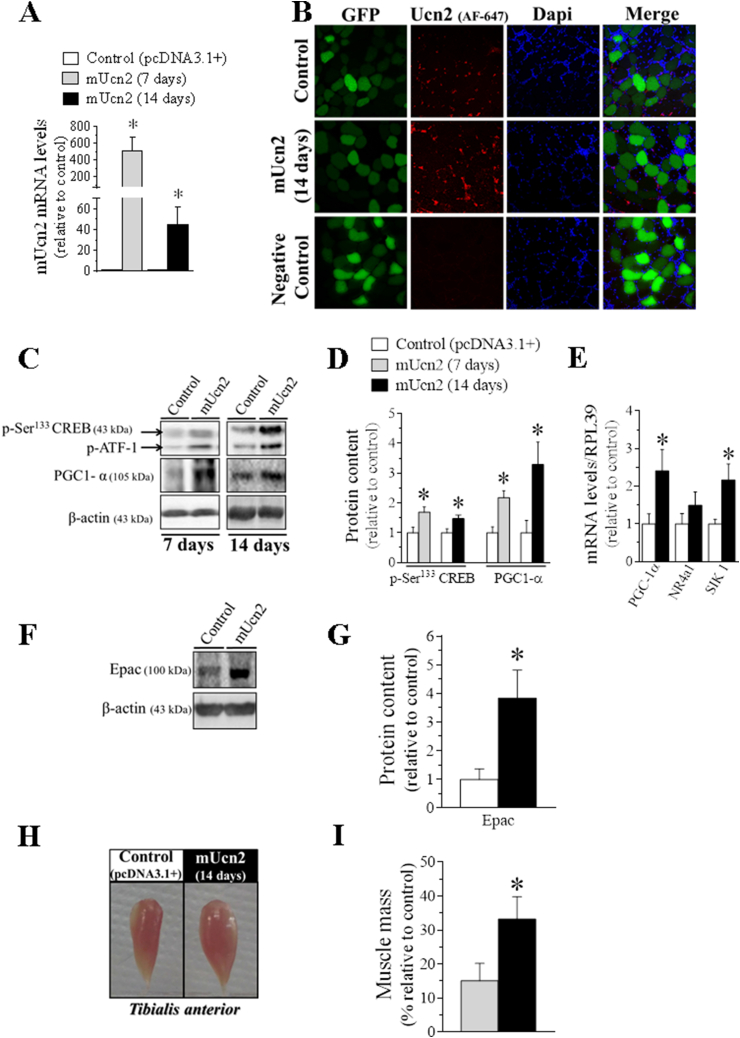
Figure 1.
Characterization of Ucn2 overexpression in vivo in mice skeletal muscle phenotype. The expression of Ucn2 mRNA (A) and Ucn2 protein (B) of normal mice transfected in vivo with the plasmid expressing pcDNA 3.1+ empty vector + GFP or mUcn2 + GFP for 7 and 14 days. The effect of Ucn2 overexpression for 7 and 14 days in the protein content of p-Ser133 CREB and PGC1-α (C, D), in CREB target genes (E) and in the protein content of Epac (F, G). The temporal effect of Ucn2 overexpression in vivo for 7 and 14 days in TA muscle mass of normal mice (H, I). P: phospho; AF: Alexa Fluor; GFP: Green Fluorescent Protein. Microscopy images with 40x of magnification. Negative control was obtained by omission of primary antibody. The nuclei were stained with Dapi. Results are expressed as mean ± SEM. (∗P ≤ 0.05 vs control) (n = 4–5).
2.3. Luciferase reporter assay
TA muscles were homogenized in Passive Lysis Buffer on the TissueLyzerII (Qiagen) and processed to quantify the activity of luciferase reporter according to kit instructions (Promega #E1910). The samples were read in a luminometer (Glomax 20/20 Luminometer, Promega, Model: 2031–002) and FoxO activity was determined by normalizing firefly luciferase activity (DBE-FoxO reporter) to pRL-null-Renilla luciferase activity.
2.4. In vivo muscle function experiments
Considering that Ucn2 was able to modulate muscle mass the investigation whether Ucn2 hypertrophic effect impacts on skeletal muscle function was performed. Plasmids encoding mUcn2, dominant-negative Akt mutant (Aktmt) and MKP-1 were co-transfected in TA of normal mice for 14 days. After this period, animals were anaesthetized with tribromoethanol (20 mg.100 g−1 body weight, i.p.). The sciatic nerve was then exposed through an incision 1 cm above the popliteal fossa and connected to an electrode. Then, mice were placed on an acrylic platform with a metallic bar crossing the knee in order to immobilize the limb. The TA tendon was connected to a force transducer coupled to a computer that was used in order to collect and analyze data related to the strength generated by the muscle contraction. Muscle twitch strength and tetanic force were recorded using a data acquisition system (Biopac Systems, USA). Muscle strength was analyzed using the AcqKnowledge program, version 3.9.1.6 (Biopac Systems, USA). Mice were submitted to external warming in order to maintain core temperature throughout the procedure. At the start of the experiment, the muscle was set to the optimum length (L0, defined as the length resulting in maximum twitch strength). There was a 2 min rest period between stimuli [41]. To achieve the maximal plateau strength with the minimal frequency, we chose to use stimuli of 350 Hz for measuring the maximum isometric tetanic strength and 200 Hz for measuring fatigue [42]. Based on Chan & Head [42], isolated twitches (0.2 Hz) were generated over a 2 min period, followed by a pre-fatigue maximum tetanic contraction (induced at 350 Hz for 2s) in each TA muscle. We then performed a fatigue protocol, which consisted of ten tetanic stimulations (2s each) at 200 Hz, each followed by a 4s rest. At the end of the fatigue protocol, a 2 min rest-period was given to the muscle by stimulating it at 0.2 Hz, followed by a post-fatigue maximum tetanic contraction (induced at 350 Hz for 2s). Muscle force was normalized by the mass of TA muscle. Tetanic specific strength is expressed in millinewtons per milligram of muscle. The maximum tetanic strength loss was measured through difference between the pre-fatigue- and the post-fatigue maximum tetanic contraction.
2.5. In vitro measurements of protein degradation
Rats were killed by cervical dislocation and extensor digitorum longus (EDL) and soleus muscles were rapidly dissected, weighed and maintained at approximately its resting length by securing the tendons in specific supports. Tissues were incubated at 37 °C in Krebs–Ringer bicarbonate buffer (pH 7.4) equilibrated with 95% oxygen and 5% carbon dioxide. Isolated muscles were incubated in the presence or in the absence of Ucn2 (10−7 M, 5.10−7 M, 10−6 M) for 2 h. Briefly, overall proteolysis was measured by following the tyrosine released into the medium in the presence of cycloheximide (0.5 mM), which prevented protein synthesis and reincorporation of tyrosine back into proteins. Tissues were preincubated for 1 h and then incubated for 2 h in fresh medium with the identical composition. For measurement of lysosomal proteolysis, muscles from ipsilateral limb were incubated in the absence of methylamine and branched-chain amino acids, a condition in which the lysosomal system is activated. Contralateral muscles were incubated in the presence of leucine (170 μM), isoleucine (100 μM), valine (200 μM) and methylamine (10 mM), a weak base that increases pH and inhibits lysosomal proteolysis. The overall proteolysis was evaluated by measuring the tyrosine released into the medium, using a fluorometric method [43] and the difference of tyrosine values between the two muscles estimated the contribution of lysosomal proteolytic process.
2.6. Measurements of in vivo protein synthesis with SUnSET
In vivo measurements of protein synthesis was performed by using the SUnSET technique [44]. Mice previously transfected with Ucn2 for 14 days received one single intraperitoneal injection of 0.040 μmol.g-1 puromycin dissolved in phosphate buffered saline 30 min before euthanasia. Then, TA muscles were harvested and frozen in liquid nitrogen to be processed for immunodetection of puromycin-labeled peptides. In this case, the magnitude of the protein synthesis is directly proportional to the intensity of the bands marked with anti-puromycin antibody.
2.7. Autophagic flux quantification
The autophagic flux was monitored in animals using a modified colchicine protocol (C9754, Sigma–Aldrich) [45]. Briefly, mice were treated with one injection of vehicle (saline) or 0.4 mg.kg−1 of colchicine (i.p.) three times (48, 24 and 1 h) before the euthanasia.
2.8. Quantitative PCR
TA muscles were harvested and promptly frozen in liquid nitrogen. Total RNA was extracted from individual muscles using TRIzol (Invitrogen, Carlsbad, California), then reverse transcribed into cDNA using SuperScript IV First-Strand Synthesis System (Invitrogen, Carlsbad, California) according to the manufacturer's protocols. Quantitative PCR was carried out using PowerUp SYBR Green Master Mix (ThermoFisher) with primers as detailed in Table 1, and normalized to RPL39.
Table 1.
Oligonucleotide primers used for qPCR analysis.
| Forward | Reverse | |
|---|---|---|
| Ucn2 | 5′ CAGCTTCCTGGAGCAACTCT 3′ | 5′ GATTCCTGGCAGCCTTGTAA 3′ |
| PGC-1α | 5′ AATCCAGCGGTCTTAGCACT 3′ | 5′ TTTCTGTGGGTTTGGTGTGA 3′ |
| NR4a1 | 5′ AGCTTGGGTGTTGATGTTCC 3′ | 5′ AGAACCGCATTGCTAGCTGT 3′ |
| SIK1 | 5′ TCCACCACCAAATCTCACCG 3′ | 5′ GTTTCGGCGCTGCCTCTTC 3′ |
| Atrogin-1 | 5′ CAGGTCGGTGATCGTGAG 3′ | 5′ GCAGAGAGTCGGCAAGTC 3′ |
| Murf1 | 5′ TGTGCAAGGAACACGAAG 3′ | 5′ TGAGAGATGATCGTCTGC 3′ |
| Mapllc3b | 5′ CGTCCTGGACAAGACCAAGT 3′ | 5′ ATTGCTGTCCCGAATGTCTC 3′ |
| Gabarapl1 | 5′ CATCGTGGAGAAGGCTCCTA 3′ | 5′ ATACAGCTGGCCCATGGTAG 3′ |
| p62 | 5′ AGAATGTGGGGGAGAGCGTGGC 3′ | 5′ GGGTGTCAGGCGGCTTCTCTT 3′ |
| RPL39 | 5′ CAAAATCGCCCTATTCCTCA 3′ | 5′ AGACCCAGCTTCGTTCTCCT 3′ |
2.9. Western blot analysis
TA muscles were homogenized in RIPA buffer containing 10 mM sodium pyrophosphate, 100 mM sodium fluoride, 10 mM sodium orthovanadate, 5 μg.mL-1 of aprotinin, 1 mg.mL−1 of leupeptin, and 1 mM phenylmethyl-sulfonyl fluoride. Lysates were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunoblotted with anti-phospho-Ser133 CREB (1:1000, Cell Signaling #9198), anti-PGC1-α (1:500, Cell Signaling #2178), anti-β-actin (1:2000, Santa Cruz sc - 81,178), anti-Epac1 (1:500, Cell Signaling #4155), anti-puromycin (1:500, Kerafast EQ 0001), anti-phospho-Ser2448 mTOR (1:500, Cell Signaling #2971), anti-phospho-Ser235/236S6 (1:1000, Cell Signaling #2211), anti-phospho-Thr37/46 4 E-BP1 (1:1000, Cell Signaling #2855), anti-phospho-Ser209 eIF4E (1:1000, Cell Signaling #9741), anti-mTOR (1:500, Cell Signaling #2972), anti-S6 (1:500, Cell Signaling #2217), anti-4E-BP1 (1:1000, Cell Signaling #9452), anti-eIF4E (1:1000, Cell Signaling #9742), anti-LC3 (1:1000, Cell Signaling #2775), anti-p62 (1:1000, Cell Signaling #5114), anti-phospho-Ser256 FoxO1 (1:500, Cell Signaling #9461), anti-phospho-Thr24 FoxO1 and anti-phospho-Thr32 FoxO3a (1:500, Cell Signaling #9464), anti-FoxO1 (1:500, Cell Signaling #9454), anti-phospho-Ser473 Akt (1:1000, Cell Signaling #9271), anti-phospho-Thr308 Akt (1:500, Cell Signaling #9275), anti-phospho-Thr202/Tyr204 ERK1 and anti-phospho-Thr185/Tyr187 ERK2 (1:1000, Cell Signaling #9101), anti-phospho-Thr180/Tyr182 p38 MAPK (1:1000, Cell Signaling #9211), anti-Akt (1:500, Cell Signaling #9272), anti-ERK1/2 (1:1000, Cell Signaling #9102), anti-slow myosin (1:1000, Sigma–Aldrich M8421), anti-fast myosin (1:2000, Sigma–Aldrich M4276), anti-GFP (1:1000, Santa Cruz sc - 8334), anti-phospho-Tyr1135/1136 IGF-I receptor β and anti-phospho-Tyr1150/1151 Insulin Receptor β (1:500, Cell Signaling #3024). Primary antibodies were detected using peroxidase-conjugated secondary antibodies (1:5000 for β-actin, slow- and fast-myosin and 1:1000 for the other primary antibodies) and visualized using enhanced chemiluminescence (ECL) reagents on ChemiDoc XRS + System (Bio-Rad). Band intensities were quantified using the software ImageJ/Fiji (version 1.52 d, National Institutes of Health, USA).
2.10. Plasmids, antibodies and reagents
The pcDNA3.1+ and pEGFP-N1 plasmids were obtained from Addgene Vector Database. The mUcn2-pcDNA3.1+ was a gift from Dr. Paul Sawchenko (Salk Institute, San Diego, CA) and has been previously described [46]. The dominant-negative Akt mutant (Aktmt) Thr308/Ser473 was a gift from Dr. S. Dimmeler (University of Frankfurt, Germany) and has been previously described [47]. The pWay21-EGFP and pWay21-EGFP-MKP-1 plasmids were a gift from Dr. Anton Bennett (Yale University School of Medicine, New Haven, CT) and have been previously described [48]. The DBE-FoxO reporter containing six forkhead (DAF-16) binding sites was previously described [30], pRL-null Renilla was obtained from Promega. The pDest-mCherry-EGFP-p62 was a gift from Dr. Terje Johansen (University of Tromsø, Oslo, NO) and has been previously described [49].
The Aktmt plasmid expresses a mutant isoform of Akt in Ser473 and Thr308 residues and therefore cannot be activated to exert its catalytic activity, and thus, by a competitive binding mechanism to the endogenous Akt substrates, it inhibits Akt downstream signaling pathway [47]. The suppression of endogenous ERK1/2 activity occurs through MKP-1 phosphatase, which dephosphorylates and inhibits mitogen-activated protein kinases (MAPKs), amongst then ERK1/2 [48]. TA muscles of normal mice were co-transfected with the control vector pWay21-EGFP and/or Aktmt or pWay21-EGFP and/or MKP-1 (control groups) or co-transfected with plasmids expressing mUcn2 and/or Aktmt, or mUcn2 and/or MKP-1 (experimental groups).
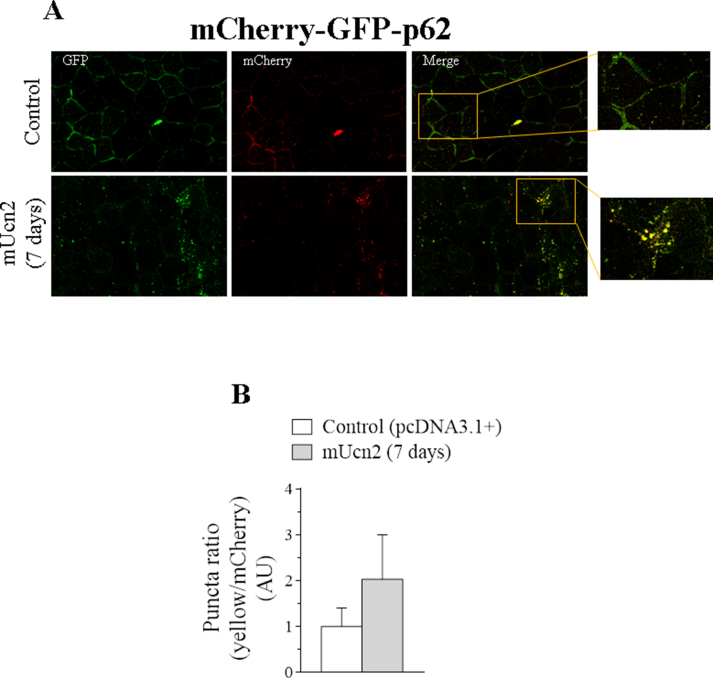
The double-tagged pDest-mCherry-EGFP-p62 plasmid is a pH-sensitive sensor used to assess different steps of autophagy. The GFP is acid-sensitive and mCherry is acid-insensitive. So, in the autophagosome, the junction of both tags results in a yellow fluorescent. However, the green fluorescent from GFP is lost when autophagosome is fused with lysosome but, the red fluorescent from mCherry remains until the substrates and the double tagged protein are degraded in the autolysosome.
Reagents, primary and secondary antibodies were purchased from Sigma–Aldrich (St Louis, Missouri), Cell Signaling Technology (Danvers, Massachusetts), Santa Cruz Biotechnology (Dallas, Texas), KPL (Gaithersburg, Maryland) and Jackson ImmunoResearch Laboratories (West Grove, Pennsylvania).
2.11. Immunofluorescence assay and confocal microscopy analyses
TA muscles were carefully harvested, snap-frozen in isopentane, and stored in liquid nitrogen at −80 °C. Muscles were mounted in optimal cutting temperature (OCT) medium (Sakura Finetek, USA) and cryosections of 10 μm thick of muscles were performed through a Leica CM1850 UV cryostat at −25 °C (Leica Microsystems, Wetzlar, Germany) then placed on 76-mm slides in order to be processed for immunofluorescence and confocal microscopy analyses using standard conditions. Immunofluorescence for Ucn2 staining was performed on fixed muscle cryosections with paraformaldehyde 4%, 10 min at room temperature. Sections were permeabilized with 0.1% Triton X-100 for 2 min at room temperature. Primary antibody was incubated overnight at 4 °C and secondary antibody was incubated 1 h at room temperature. Images were captured and examined with a confocal microscope (Leica TCS SP5; Software LAS AF). Transfected fibers and their myonuclei were identified by GFP and DAPI staining, respectively. Images were processed using Image J 1.53c (Fiji is Just) and approximately 400 fibers were analyzed per muscle.
2.12. Statistical analysis
Data are presented as mean ± standard error of mean (SEM). The means from different groups were analyzed using paired or unpaired Student's t-test. P ≤ 0.05 was taken as the criterion of significance. Animals were randomly allocated to the different experimental groups.
3. Results
3.1. Ucn2 overexpression activates cAMP signaling and promotes skeletal muscle growth
The efficacy of Ucn2 overexpression was attested by an increase in Ucn2 mRNA levels in TA muscles after 7 (∼500-fold) and 14 days (∼40-fold) of the transfection compared with muscles of control group, which received the plasmid expressing the empty vector (pcDNA3.1+) (Figure 1A). The Ucn2 mRNA increase was accompanied by a higher content of Ucn2 protein (Figure 1B), which mainly remained around myofibers as compared with the intracellular medium, suggesting that this peptide may be synthesized and secreted by myocytes and exerts its autocrine/paracrine effects on muscle cells. Since it is well known that Ucn2 increases cAMP levels in vitro [14,15,17] we confirmed that in vivo in Ucn2-transfected TA muscles for 14 days (1508 ± 109∗ vs 1082 ± 100 cAMP fmol/mg of muscle, control group; n = 3). Then, the involvement of PKA, the canonical downstream effector of cAMP, was investigated. Ucn2 overexpression for 7 and 14 days increased the phosphorylation levels of CREB, a well-known PKA substrate (Figure 1C–D), and also CREB target genes (Figure 1E; 14 days only), highlighted for a substantial increase in the protein content of PGC1-α (Figure 1C–D). In addition, Ucn2 overexpression drastically increased the protein content of exchange protein directly activated by cAMP (Epac) (Figure 1F–G), a non-canonical direct target for cAMP [50]. Although Ucn2 overexpression for 7 days did not cause significant changes in muscle mass, the transfection for 14 days was able to induce a striking hypertrophy in TA (33%) as compared to control muscles (Figure 1H, I).
3.2. The hypertrophy promoted by Ucn2 overexpression is associated with an increase in protein synthesis and mTOR signaling
To investigate the mechanisms underlying the Ucn2-induced muscle hypertrophy, protein synthesis was investigated by measuring the labeling of proteins bound to puromycin. Ucn2 overexpression increased by 39% the content of puromycin-labeled peptides in TA (Figure 2B, D) indicating an enhancement of protein synthesis.
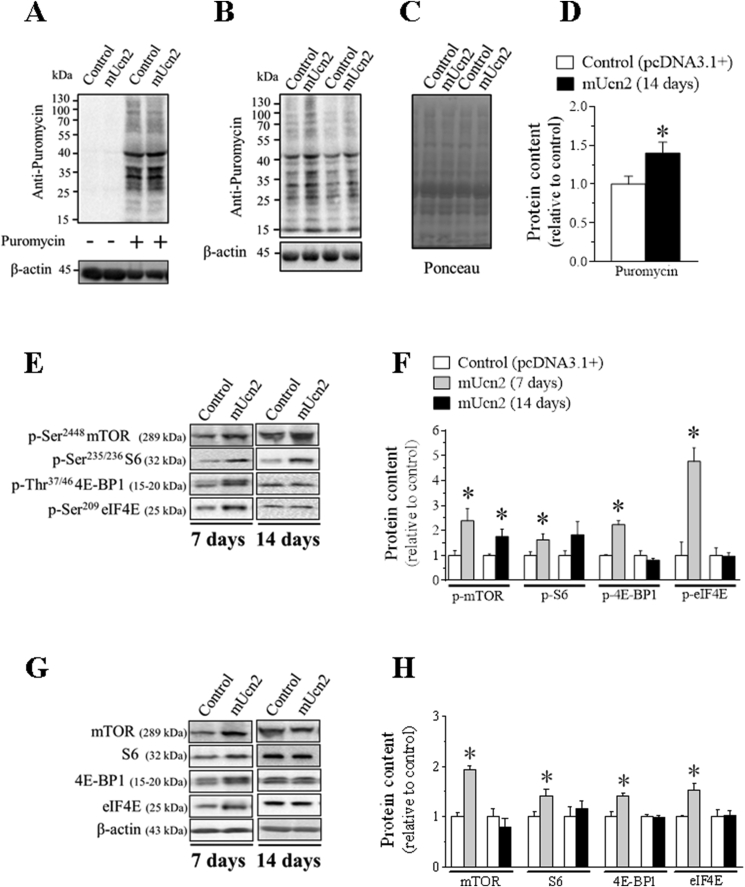
Figure 2.
The protein synthesis induced by Ucn2 overexpression in vivo is mediated by mTOR signaling in mice skeletal muscles. Demonstration of the anti-puromycin antibody specificity (A) which labeling was only identified in the TA muscles samples of puromycin-treated mice. Effect of Ucn2 overexpression in vivo for 14 days on the content of proteins that incorporated puromycin in TA muscles of normal mice and its respective Ponceau (B–D). The effect of Ucn2 overexpression in vivo for 7 and 14 days in the phosphorylation levels (E, F) and in the total protein content of mTOR, S6, 4 E-BP1 and eIF4E in TA of normal mice (G, H). Results are expressed as mean ± SEM. (∗P ≤ 0.05 vs control) (n = 5–7).
Next, the activation status of mTOR kinase and its downstream targets was evaluated in Ucn2 transfected muscles because this signaling pathway has been considered the major regulator of protein synthesis [24,26]. The in vivo transfection of Ucn2 caused an increase in the phosphorylation levels of mTOR after 7 (∼1.5-fold) and 14 (76%) days (Figure 2E–F). Consistently, Ucn2 overexpression for 7 days increased the phosphorylation of S6 (62%) and 4E-BP1 (∼1.2 fold) (Figure 2E–F), the two downstream targets of mTOR, suggesting an increased activity of mTOR. When hyperphosphorylated, 4E-BP1 protein becomes inactive, thus releasing eIF4E from its repressive action [51]. Indeed, at the same period, Ucn2 increased the phosphorylation levels of eIF4E (∼4 fold) (Figure 2E–F), the downstream target of MAP kinase-interacting serine/threonine-protein kinase 1 (Mnk1), a well-known MAPKs substrate [52]. The protein content of mTOR (∼1.9 fold), S6 (40%), 4E-BP1 (42%) and eIF4E (53%) was also increased by Ucn2 overexpression at day 7 (Figure 2G–H). However, all these effects were no more observed after 14 days of transfection (Figure 2G–H).
Collectively, these data suggest that the stimulation of protein synthesis via mTOR signaling may mediate Ucn2-induced muscle growth in vivo.
3.3. Ucn2 reduces skeletal muscle protein degradation in vitro and exerts a negative modulatory effect on FoxO transcription factors in vivo
In order to investigate whether or not muscle protein degradation could be directly modulated by Ucn2, rates of protein degradation were estimated in isolated skeletal muscles in vitro. As shown in Figure 3A, the addition of 10−7, 5.10−7 and 10−6 M Ucn2 to the incubation medium of EDL muscles for 2 h reduced the rate of overall proteolysis (nmol tyrosine.mg−1.2 h−1) by 17%. A similar anti-proteolytic effect was observed when soleus muscles were incubated with 10−6 M Ucn2 (Figure 3A). In attempt to investigate which intracellular proteolytic systems might be regulated by Ucn2, the proteolytic activity of UPS, lysosomal and Ca+2-dependent was evaluated. Ucn2 (5.10−7 M) in vitro reduced by 41% the activity of lysosomal system but did not affect the activity of Ca+2-dependent system (Figure 3B). There was a tendency (P = 0.06) towards reduced activity of the UPS in Ucn2-transfected muscles, but this did not reach statistical significance (Figure 3B). Moreover, Ucn2 overexpression for 7 days reduced by 25% atrogin-1 expression suggesting a suppression of the process of ubiquitination in vivo (Figure 3E).
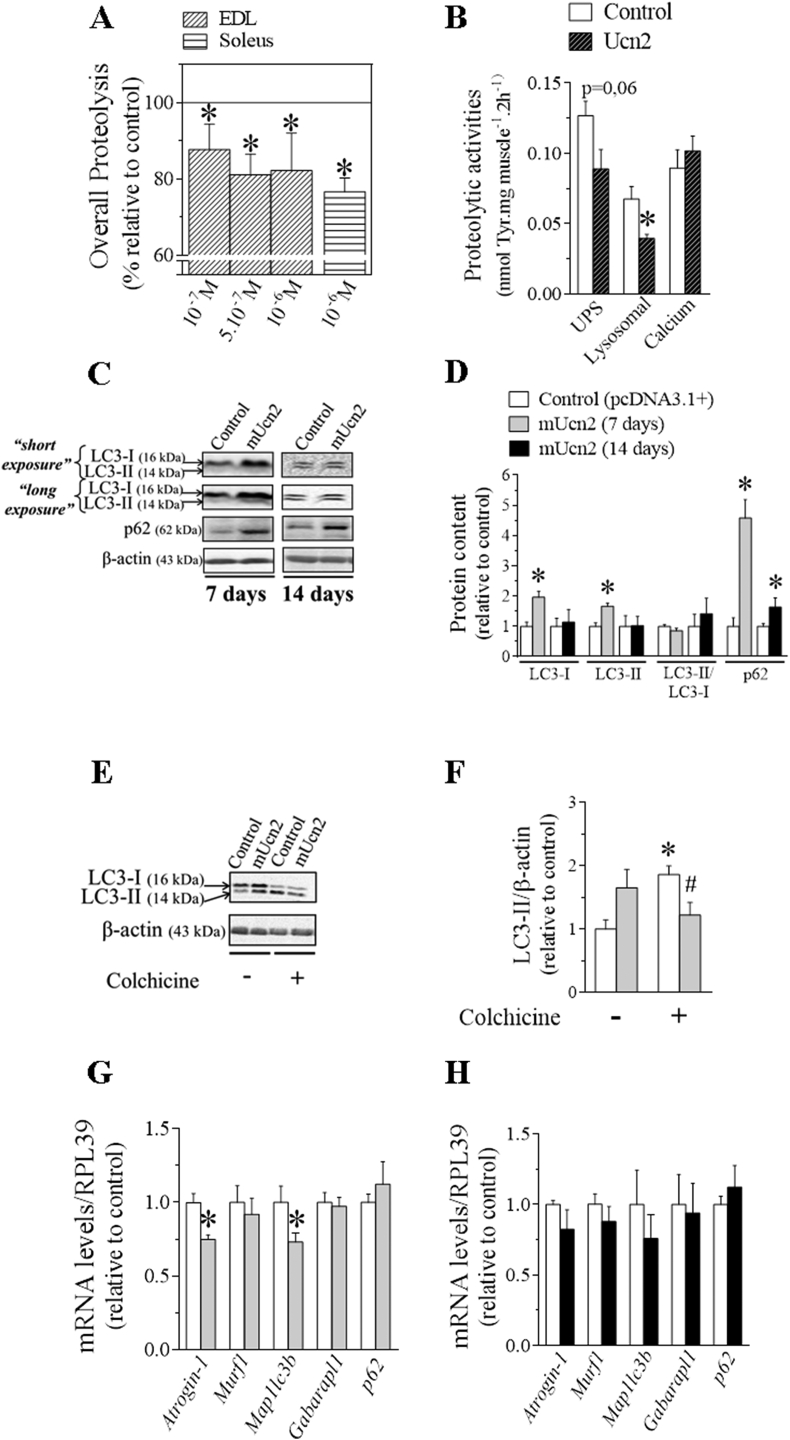
Figure 3.
The antiproteolytic effect of Ucn2 is due to an inhibition of lysosomal system in rats and mice skeletal muscles. The effect of Ucn2 in overall proteolysis (A) and in the activity of the main proteolytic systems involved in the loss of muscle mass (B). Values are expressed relative to control, taken as 100%. Effect of Ucn2 overexpression in vivo for 7 and 14 days in the protein content of autophagy markers LC3-I, LC3-II and p62 without (C, D) or after the treatment with colchicine (E,F) and also in the mRNA levels of atrophic genes in TA muscles of normal mice (G, H). Results are expressed as mean ± SEM. (∗P ≤ 0.05 vs control; #P ≤ 0.05 vs colchicine control pcDNA3-1+) (n = 4–7).
To further investigate the inhibitory effect of Ucn2 in the lysosomal system, the protein content of autophagy markers was measured to infer whether this peptide could also control autophagic flow in vivo. It is known that the lipidated form of LC3-II is responsible for the formation and the expansion of the autophagosome double membrane and is considered a marker of autophagic flow [53]. In addition to the LC3-II measurement, we also evaluated another lysosomal marker known as p62/SQTM1 (sequestosome 1), which in turn, binds to LC3-II and is therefore preferentially degraded by lysosome [54]. Thus, the accumulation of LC3-II and p62 proteins occurs under conditions characterized by the inhibition of the autophagic-lysosomal process. Ucn2 overexpression in vivo for 7 days increased the protein content of LC3-I (96%), LC3-II (70%), p62 (6-fold) (Figure 3C, D) as well as reduced by 27% levels of mapllc3b (27%) without altering p62 mRNA (Figure 3G) as compared to controls. After 14 days of Ucn2 transfection no change was observed in the protein content of LC3-I and LC3-II, however, an increase in the protein content of p62 (64%) could still be observed (Figure 3C, D), with no change in their mRNA levels (Figure 3H). These results suggest that the accumulation of LC3-II and p62 is due to an inhibition of its degradation by lysosome and Ucn2 in vivo exerts an inhibitory modulation on the autophagic-lysosomal system in skeletal muscle. To confirm the inhibition of autophagy by Unc2, we treated animals with colchicine, a microtubule-disrupting agent that inhibits autophagosome-lysosome fusion. LC3-II accumulation after lysosomal inhibition reflects LC3-II flux or autophagy flux. Colchicine inhibited autophagosome degradation as identified by the increased protein content of LC3-II, and Ucn2 overexpression for 7 days decreased this effect (Figure 3E–F). We also monitored the formation of autophagic vesicles in TA muscles after the co-transfection of pcDNA3.1+ (or mUcn2) + pDest-mCherry-EGFP-p62 plasmids for 7 days (Figs. S3A–B). There was a tendency for Ucn2 to increase the ratio of yellow/mCherry puncta (Figs. S3A–B), indicating that Ucn2 might inhibit the fusion of autophagosome to lysosome vesicles.
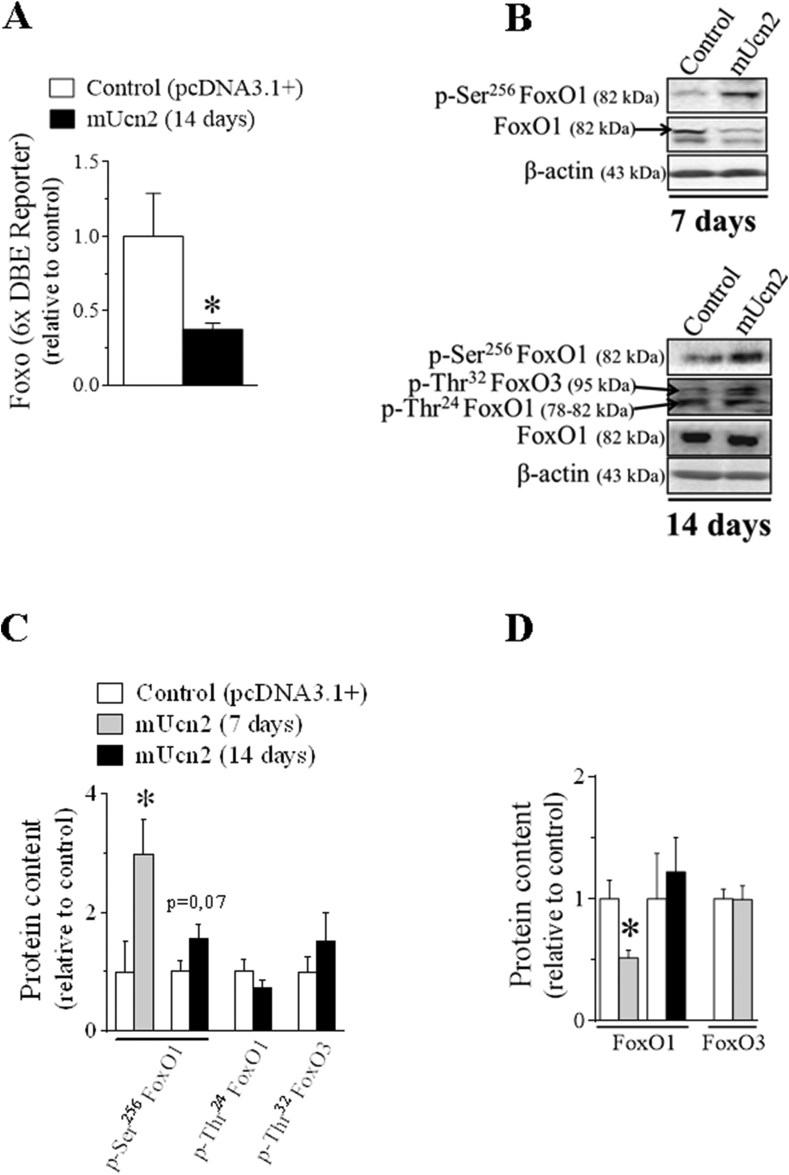
Because the atrogenes atrogin-1 and map1lc3b are downstream target genes of FoxO, the master regulator of the UPS and autophagic-lysosomal systems [55], respectively, the activity of this transcription factor was evaluated by means of FoxO-dependent reporter and western blot. Interestingly, the transcriptional activity of FoxO was reduced by 70% after 14 days of Ucn2 overexpression (Figure 4A) but this effect was not accompanied by any changes in the mRNA levels of atrogenes (Figure 3F). On the other hand, this reduction in FoxO activity was related to a tendency (P = 0.07) toward higher phosphorylation levels of FoxO1 (Ser256) without any change in the phosphorylation levels of FoxO3 and FoxO1 (Thr24) (Figure 4B–C). However, the phosphorylation levels and the protein content of FoxO1 were significantly increased (2-fold) and decreased (50%), respectively, in Ucn2-transfected muscles for 7 days (Figure 4B–C).
Figure 4.
Ucn2 overexpression in vivo inhibits FoxO activity in mice skeletal muscles. The effect of Ucn2 overexpression in vivo for 7 and 14 days in the activity of FoxO (A) and in the phosphorylation and total protein levels of different FoxO family members (B–D) in TA muscles of normal mice. Results are expressed as mean ± SEM. (∗P ≤ 0.05 vs control) (n = 5).
Taken together, these data demonstrate that Ucn2 exerts a direct inhibitory action on protein degradation probably through the suppression of lysosomal and UPS activities in rat isolated muscle, being the suppression of atrogenes a FoxO-dependent mechanism.
3.4. Akt and ERK signaling mediate growth-promoting effects induced by Ucn2
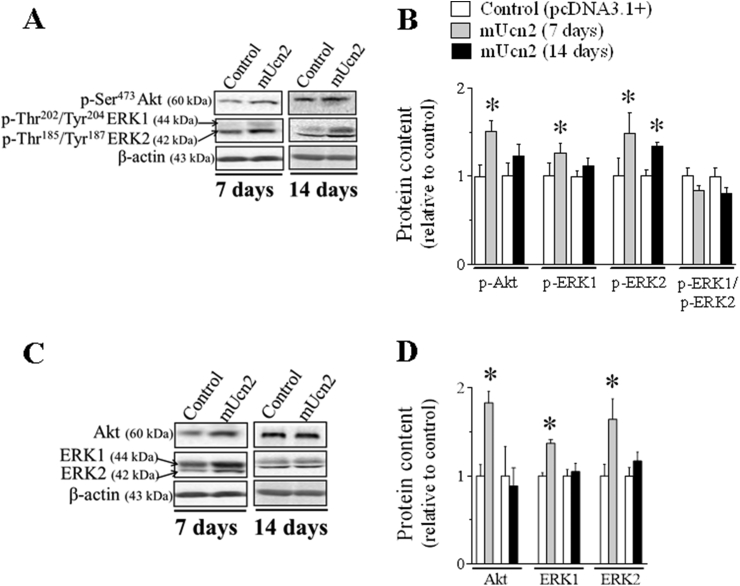
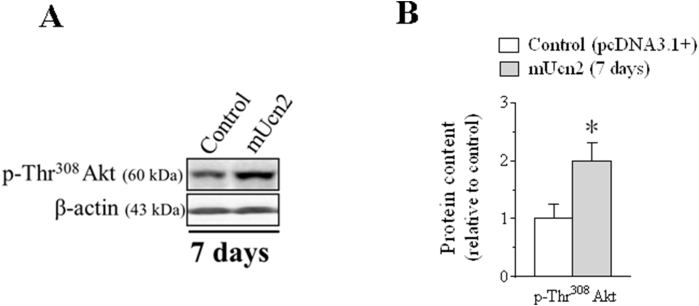
To investigate further the signaling pathways involved in the anabolic and anti-catabolic effects of Ucn2, Akt phosphorylation was assessed in Ucn2-transfected muscles for 7 days. In agreement with the activation of the phosphorylation status of FoxO, Ucn2 increased the phosphorylation of Akt in both residues of Ser473 (50%; Figure 5A–B) and Thr308 (2-fold; Figs. S2A–B). However, this effect disappeared 14 days after Ucn2 overexpression (Figure 5A–B). Following, different components of the MAPK pathway, a well-known signaling involved in the regulation of protein synthesis were also investigated [56,57]. At 7 days, Ucn2 overexpression increased both the protein content and phosphorylation levels of ERK1 (27%) and ERK2 (50%) with no alteration in ERK1/ERK2 ratio, but did not alter the phosphorylation levels of p38MAPK (Figs. S4A–B). At 14 days, Ucn2 also increased phospho-ERK2 (34%) indicating that Ucn2 selectively recruits ERK1/2.
Figure 5.
Ucn2 overexpression in vivo stimulates insulin/IGF-1 downstream targets. The effect of Ucn2 overexpression in vivo for 7 and 14 days in the phosphorylation levels (A, B) and in the total protein content (C, D) of Akt and ERK1/2 in TA muscles of normal mice. Results are expressed as mean ± SEM. (∗P ≤ 0.05 vs control) (n = 5).
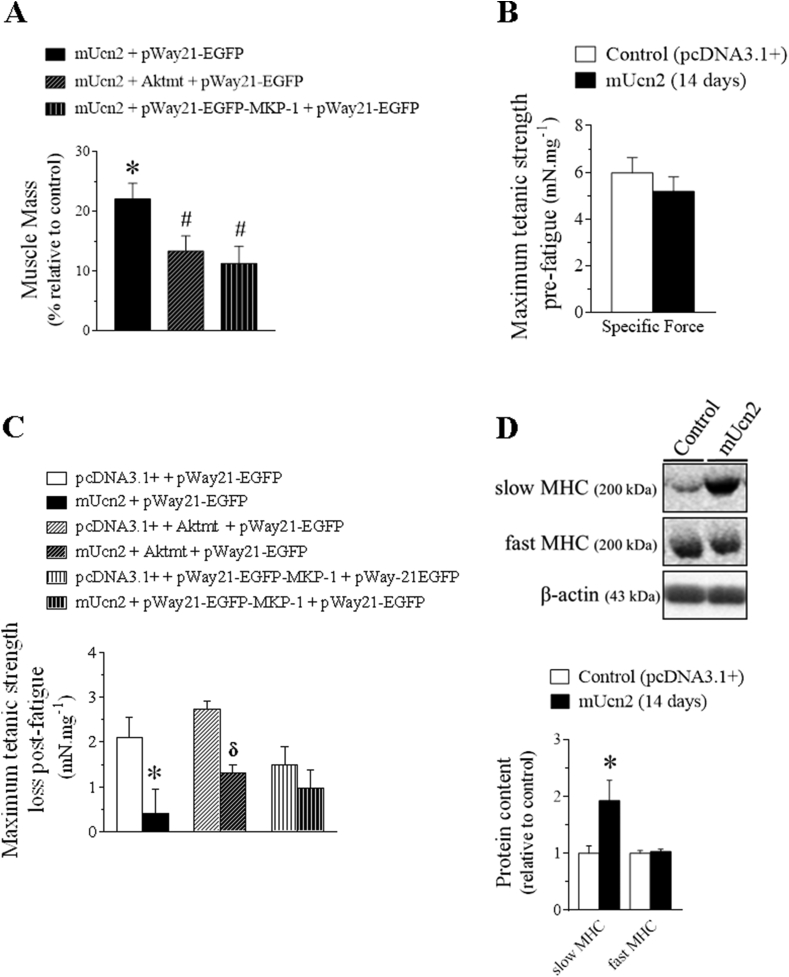
In order to investigate the involvement of Akt and ERK1/2 kinases in the growth-promoting effects induced by Ucn2, the endogenous activity of these kinases was indirectly blocked by means of a genetic approach. Thus, muscles were co-transfected with Ucn2 and/or Aktmt or MKP-1 genes. Overexpression of either Aktmt or MKP-1 plasmids significantly reduced by 38% and 48%, respectively, the hypertrophic effect induced by Ucn2 in vivo (Figure 6A), demonstrating that both Akt and ERK1/2 kinases mediate Ucn2-induced muscle growth.
Figure 6.
Akt and ERK1/2 mediate the hypertrophy promoted by Ucn2 and Ucn2-induced fast-to-slow fiber type shift increased fatigue resistance on an ERK1/2 dependent manner in mice skeletal muscles. The effect of Ucn2, Aktmt and MKP-1 overexpression for 14 days in TA muscle mass (A), in maximum tetanic strength pre-fatigue (B) and in maximum tetanic strength loss (C, difference between the pre-fatigue- and the post-fatigue maximum tetanic contraction) in TA of normal mice transfected with plasmids expressing the pcDNA3.1+ empty vector, mUcn2, mUcn2 + Aktmt or mUcn2 + MKP-1. The effect of Ucn2 overexpression in vivo for 14 days in the protein content of slow and fast MHC (D) in TA muscles of normal mice. Muscle strength is expressed in millinewtons (mN) per milligram (mg) of muscle. Results are expressed as mean ± SEM. (∗P ≤ 0.05 vs control; #P ≤ 0.05 vs mUcn2 + pWay21-EGFP; δ P ≤ 0.05 vs pcDNA3.1+ Aktmt + pWay21-EGFP) (n = 5). (mUcn2 = mouse urocortin 2, Aktmt = dominant-negative Akt mutant; MKP-1 = protein mitogen-activated kinase phosphatase 1).
3.5. The muscle growth induced by Ucn2 overexpression via ERK is associated with fatigue resistance enhancement
To investigate further whether or not muscle hypertrophy induced by Ucn2 overexpression is functionally relevant, we measured maximum tetanic strength and fatigue resistance in muscles from living animals co-transfected or not with Aktmt or MKP-1. Although Ucn2 overexpression for 14 days have not altered the maximum tetanic strength pre-fatigue (Figure 6B), the peptide reduced by 80% the maximum tetanic strength loss post-fatigue (Figure 6C), indicating an enhancement of muscle fatigue resistance in mice. These effects induced by Ucn2 overexpression were completely blocked in muscles co-transfected with MKP-1, but not with Aktmt (Figure 6C) demonstrating that they are probably mediated by activation of ERK signaling. Taken into account that Ucn2 increased PGC1-α protein content (Figure 1C–D) and also activates ERK1/2 (Figure 5A–D), well stablished components involved in the maintenance and expression of oxidative myofibrils [37,58], muscle fibers typing differences were investigated by means of western blot. Ucn2 increased the protein content of slow myosin heavy chain (MHC) (∼1,2 fold), but did not affect fast MHC fibers (Figure 6D). These findings suggest that Ucn2 induces a fast-to-slow fiber type shift, probably mediated by PGC1-α (Figure 1C–D), an effect that could contribute to the improvement in fatigue resistance induced by ERK signaling.
4. Discussion
The present study has provided several new insights concerning the role of Ucn2 in the regulation of muscle protein metabolism and its related signaling pathways. Using genetic, biochemical and functional approaches, we show, for the first time, that overexpression of Ucn2 promotes gain of muscle mass and resistance to fatigue in normal mice, a beneficial effect that persisted for up to 14 days and was probably due to direct actions of Ucn2 on muscle fiber. Similar phenotype has been reported in animals systemically treated with this peptide or its specific receptor CRF2R agonist in different catabolic situations, including denervation [15,59], corticosteroid treatment [15] and unloading [14,15]. Moreover, subcutaneous injections of Ucn2 for two weeks induced a slight increase in skeletal muscle mass [60,61] and improved muscle resistance [60] in dystrophic mdx mice. The present study complements and extends these findings and shows that Ucn2-induced muscle hypertrophy at baseline conditions was associated with an activation of cAMP cascade and an enhancement of protein synthesis. Indeed, in a recent study Borg et al. [16] have demonstrated that the treatment with a modified Ucn2 peptide analog of human Ucn2 increased lean mass and rates of skeletal muscle protein synthesis in ob/ob mice, even though cAMP intracellular signaling has not been investigated. The present finding that Ucn2 caused an upregulation of CREB phosphorylation, a well-established PKA target, and its target genes (i.e., PGC1-α and SIK1) suggests that Ucn2 activated cAMP/PKA/CREB signaling pathway. Recent reports have shown that compounds that act through the cAMP cascade, like catecholamines and β2-agonists, stimulate mTOR signaling in muscles [19,62], hearts [63] and BAT [64] from rodents. Consistently, we have shown that the physiological stimulation of sympathetic nervous system in the setting of cold plays a hypertrophic role in BAT by stimulating the cAMP and mTOR signaling [65]. Our data are in agreement with these previous reports and show that mTOR activity is highly induced by Ucn2 in transfected muscles in parallel to the induction of intracellular cAMP levels. Besides stimulating protein synthesis, our data show that Ucn2 in vitro decreased the overall proteolysis in different isolated muscles from rats and mice and this effect was due mainly to the suppression of lysosomal proteolytic system. Consistently, Ucn2 overexpression led to the accumulation of LC3-II and p62 as well as a reduction of mapllc3b, suggesting that Ucn2 genetic transfection negatively modulates the autophagic-lysosomal proteolytic pathway. In addition, Ucn2 in vivo transiently suppressed atrogin-1 and tended to decrease the UPS activity in isolated muscles suggesting an inhibitory effect of ubiquitination. Accordingly, in vivo overexpression of Ucn2 significantly increased the phosphorylation levels and suppressed the activity of FoxO, the major transcriptional activator of the autophagic-lysosomal and UPS systems. Thus, it is likely that the combined actions of both processes stimulation of protein synthesis and inhibition of proteolysis (i.e., autophagic-lysosomal and UPS systems) contributed to Ucn2-induced muscle hypertrophy. In agreement with this notion, we and others have previously demonstrated that similar mechanisms are involved in the gain of muscle mass promoted by treatment with β2-agonists in different species [19,41,[66], [67], [68], [69]]. The administration of formoterol [19] or clembuterol [70], selective β2-agonists, to mice increased muscle cAMP levels and in parallel inhibited the fasting-induced formation of autophagosome, activity of FoxO, and expression of atrogin-1 and MuRF-1. Furthermore, daily administration of cAMP PDE inhibitors that act through the increase of cAMP intracellular levels have been used to counteract UPS activity, atrophy and weakness in Yoshida sarcoma-bearing [71], septic rats [72] and diabetic rats [73]. Taken together, these findings suggest that cAMP signaling plays a critical role in the stimulation of protein synthesis and suppression of the autophagic-lysosomal and UPS systems promoted by Ucn2 in mice skeletal muscles.
Although it is well established that most metabolic actions of Ucn2 are mediated through the cAMP, like first postulated by Hinkle et al. [14], the involvement of its downstream intracellular effectors PKA and Epac on the control of muscle mass is still a focus of an intense investigation. Here, we show that in vivo overexpression of Ucn2 increased CREB phosphorylation and its target genes like PGC1-α [74,75]. Because the transcriptional coactivator PGC-1α, a master regulator of mitochondrial biogenesis that drives the formation of slow-twitch muscle fibers [58], prevents FoxO activation and protect muscles against atrophy [76,77], it is possible that Ucn2 overexpression may have inhibited FoxO activity and muscle proteolysis by activating PKA/CREB/PGC-1α axis. The present findings that Ucn2 overexpression increased the protein content of slow MHC and muscle resistance to fatigue are consistent with this possibility. Alternatively, FoxO activity could be negatively modulated by Ucn2 via a direct action of PKA. In a recent study, we demonstrated that the overexpression of catalytic subunit of PKA reduced the FoxO reporter activity in skeletal muscles. On the other hand, FoxO transcriptional activity was increased by the overexpression of PKI, an inhibitor of PKA [78].
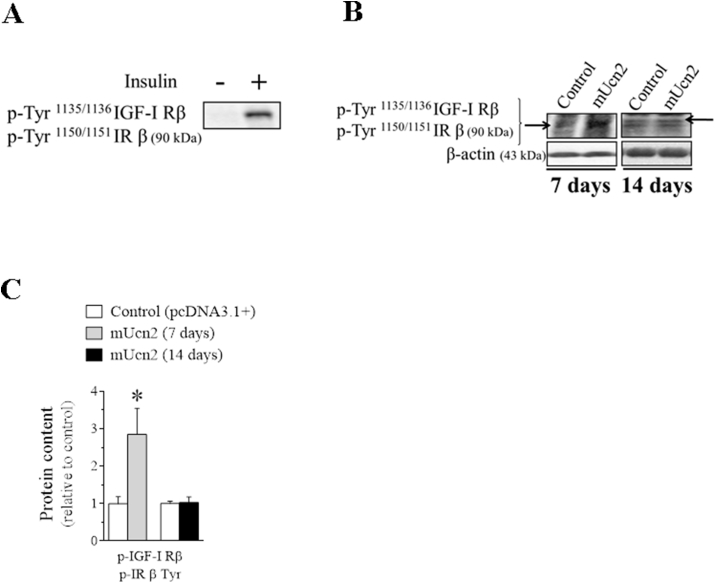
In addition to the canonical cAMP/PKA/CREB signaling pathway, Ucn2 also stimulated key components of the insulin/IGF-1 pathway. Indeed, the in vivo overexpression of Ucn2 positively modulated Akt and ERK1/2, as well as downstream targets of these kinases responsible for protein synthesis such as mTOR/S6 and eIF4E, respectively, suggesting an increase in insulin/IGF-1 sensitivity in TA muscles. The drastic increase in the phosphorylation levels of insulin/IGF-1 receptor (Fig. S5) and FoxO (Figure 4B–C), a well-known target of Akt, support this hypothesis. In agreement with this notion, the systemic administration of a modified Ucn2 peptide for 14 days enhanced glucose tolerance and concomitantly increased phosphorylation levels of Akt (Ser473 and Thr308) in skeletal muscles of obese mice [79]. In contrast, Chen et al. [13] have shown that Ucn2 knockout animals were more sensitive to insulin when compared to wild animals and in vitro studies [13,80] have reported that Ucn2 decreased the phosphorylation levels of Akt (Ser473), IRS-1 (Tyr608), and ERK1/2 (Thr202/204/Tyr185/187) in differentiated muscle cells incubated with insulin. These conflicting results may be due to differences in the experimental approaches including time of treatment, pharmacological doses and indirect effects of Ucn2.
Besides PKA, our data showed that Epac, a non-classical cAMP effector [50], is also upregulated by Ucn2. Accordingly, it has been shown that attenuation of necrosis in muscle from mdx mice systemically treated with Ucn2 was associated with the increased activity of Epac and PKA [60]. The finding that increases in Epac levels in different cell culture lineages [81] and in skeletal muscles [66,82] leads to the phosphorylation of Akt via PI3K highlights the possibility that the stimulation of Akt in response to Ucn2 overexpression may also occur via a Gs-AC-cAMP-Epac-PI3K signaling pathway independently of insulin. In addition to promoting Akt activation, Epac can activate ERK1/2 through Ras stimulation in HEK-293 cell culture [83]. Like Akt, ERK1/2 is a critical regulator of muscle metabolism by promoting differentiation and survival of C2C12 myotubes [84,85] as well as induction of muscle hypertrophy in mice [86,87]. Moreover, overexpression of MKP-1, an endogenous ERK1/2 suppressor, induced a robust atrophic phenotype in slow and fast muscles [88] and prevented the β-adrenergic agonist-induced hypertrophy in fast-twitch skeletal muscle in rats [89]. Using a similar genetic approach, the present work demonstrated that the inactivation of ERK1/2 partially attenuated the hypertrophic effect of Ucn2 in muscles of normal mice. Since a similar attenuation of hypertrophy was observed in muscles co-transfected with Ucn2 and Aktmt, these data suggest that both kinases ERK1/2 and Akt play a role in the gain of mass promoted by Ucn2 in vivo. Moreover ERK1/2 stimulation by Ucn2 overexpression could also explain the fast-to-slow fiber type shift, corroborating with Murgia et al. [37] that demonstrated the physiological relevance of Ras/ERK1/2 pathway in modulating the phenotype induced by slow motor neurons in skeletal muscle. Our functional studies show that Ucn2 overexpression did not affect maximum tetanic strength. In contrast, previous studies in pathological condition have shown that the systemic pharmacological activation of CRF2R in mdx animals increased the isometric contraction force in the triceps surae muscles [60,90]. Intriguingly, Ucn2 increased muscle resistance to fatigue, and this effect was associated with the activation of ERK1/2, but not Akt, because MKP-1 abolished the beneficial effect of Ucn2 on muscle fatigue resistance. Other studies have shown that the cardioprotective effects promoted by Ucn1 and Ucn2 are associated with ERK1/2 stimulation [91,92]. Brar et al. [93] have reported that astressin-2B, the selective antagonist of the CRF2R receptor, as well as the pharmacological inhibition of ERK1/2, abolished the cardioprotective effect promoted by Ucn2 and Ucn3. Although the underlying mechanisms involved in the role of ERK1/2 in controlling muscle mass and function are still focus of investigation, it is possible to speculate that such signaling is necessary for the force magnitude in Ucn2-transfected muscles. Further studies are needed to confirm this possibility.
In summary, we have demonstrated that 1) Ucn2 overexpression in vivo caused strikingly muscle hypertrophy, which was functional relevant and was probably due to the activation of protein synthesis, via mTOR, and inhibition of proteolysis (i.e., autophagic-lysosomal and UPS systems), via FoxO signaling; 2) the rise in intracellular cAMP levels and its downstream targets (PKA and Epac) promoted by Ucn2 may be one of the regulatory mechanisms stimulating anabolism and a fast-to-slow fiber type shift in skeletal muscle; and 3) while both Akt and ERK1/2 are involved in the signaling pathways of muscle growth induced by Ucn2, ERK/1/2 increases the muscle fatigue resistance. Thus, the present work opens new perspectives in the development of therapeutic strategies to improve muscle mass in the setting of catabolic diseases.
Author contributions
N. Lautherbach, D.A. Gonçalves and I.C. Kettelhut designed the research; N. Lautherbach and D.A. Gonçalves performed the laboratory analyses; N. Lautherbach, D.A. Gonçalves, W.S. Silveira, S. Paula-Gomes, R.R. Valentim, N.M. Zanon, M. Pereira performed the data collection. E.H. Miyabara provided critical reagents or analytic tools. N. Lautherbach and D.A. Gonçalves analyzed the data; N. Lautherbach drafted the manuscript and all authors edited and approved the final version. I.C. Kettelhut and L.C. Navegantes designed the study and helped write the manuscript.
Funding
This work was supported by grants to Natalia Lautherbach (CAPES/PROEX; CNPq 167033/2017-4); Dawit A. Gonçalves (FAPESP 2012/18861-0/ FAPEMIG APQ-01268-21); Wilian A. Silveira (PNPD/CAPES 20131672); Isis C. Kettelhut (FAPESP 2018/10089-2; 2012/24524-6; 2011/11003-5); Luiz C.C. Navegantes (FAPESP 2012/24524-6; 2011/11003-5).
Acknowledgments
We thank Elza Aparecida Filippin, Lilian C. Heck, Maria Antonieta R. Garófalo and Victor Diaz Galban for their constant technical support. We thank Dr. Paul Sawchenko, Dr. S. Dimmeler, Dr. Anton Bennett and Dr. Terje Johansen for kindly provided the plasmids used in this paper.
Conflict of interest
None declared.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2022.101492.
Contributor Information
Natalia Lautherbach, Email: nutriennes@yahoo.com.br.
Dawit A.P. Gonçalves, Email: dawit@ufmg.br.
Wilian A. Silveira, Email: wilian.silveira@uftm.edu.br.
Sílvia Paula-Gomes, Email: silvia.gomes@ufop.edu.br.
Rafael Rossi Valentim, Email: rafaelrossiphd@gmail.com.
Neusa M. Zanon, Email: neuzanon@yahoo.com.br.
Marcelo G. Pereira, Email: pereiramg@gmail.com.
Elen H. Miyabara, Email: elenm@usp.br.
Luiz C.C. Navegantes, Email: navegantes@fmrp.usp.br.
Isis C. Kettelhut, Email: idckette@fmrp.usp.br.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
figs5.
References
- 1.Reyes T.M., Lewis K., Perrin M.H., Kunitake K.S., Vaughan J., Arias C.A., et al. Urocortin II : a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(5):2–7. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu S.Y., Hsueh A.J. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nature Medicine. 2001;7(5):605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- 3.Kishimoto T., Ilt R.V.P., Lin C.R., Rosenfeldt M.G. A sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:1108–1112. doi: 10.1073/pnas.92.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovenberg T.W., Liaw C.W., Grigoriadist D.E., Clevenger W., Chalmerst D.T., Souzat E.B.D.E., et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perrin M.H., Vale W.W. Corticotropin releasing factor receptors and their ligand family. Annals of the New York Academy of Sciences. 1999;885:312–328. doi: 10.1111/j.1749-6632.1999.tb08687.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen A., Blount A.M.Y., Vaughan J., Brar B., Foundation C., Biology P., et al. Urocortin II gene is highly expressed in mouse skin and skeletal muscle tissues: localization, basal expression in corticotropin-releasing factor receptor (CRFR) 1- and CRFR2-null mice, and regulation by glucocorticoids. Endocrinology. 2004;145(5):2445–2457. doi: 10.1210/en.2003-1570. [DOI] [PubMed] [Google Scholar]
- 7.Coste S.C., Kesterson R.A., Heldwein K.A., Stevens S.L., Heard A.D., Hollis J.H., et al. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nature Genetics. 2000;24(4):403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- 8.Bale T.L., Contarino A., Smith G.W., Chan R., Gold L.H., Sawchenko P.E., et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nature Genetics. 2000;24(4):410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 9.Chen A., Vaughan J., Vale W.W. Glucocorticoids regulate the expression of the mouse urocortin II gene : a putative connection between the corticotropin-releasing factor receptor pathways. Molecular Endocrinology. 2003;17(8):1622–1639. doi: 10.1210/me.2003-0054. [DOI] [PubMed] [Google Scholar]
- 10.Neufeld-Cohen A., Tsoory M.M., Evans A.K., Getselter D., Gil S., Lowry C.A., et al. A triple urocortin knockout mouse model reveals an essential role for urocortins in stress recovery. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(44):19020–19025. doi: 10.1073/pnas.1013761107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bale T.L., Hoshijima M., Gu Y., Dalton N., Anderson K.R., Lee K.-F., et al. The cardiovascular physiologic actions of urocortin II: acute effects in murine heart failure. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(10):3697–3702. doi: 10.1073/pnas.0307324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiley K.E., Davenport A.P. CRF2 receptors are highly expressed in the human cardiovascular system and their cognate ligands urocortins 2 and 3 are potent vasodilators. British Journal of Pharmacology. 2004;143(4):508–514. doi: 10.1038/sj.bjp.0705985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen A., Brar B., Choi C.S., Rousso D., Vaughan J., Kuperman Y., et al. Urocortin 2 modulates glucose utilization and insulin sensitivity in skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(44):16580–16585. doi: 10.1073/pnas.0607337103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinkle R.T., Donnelly E., Cody D.B., Bauer M.B., Isfort R.J. Urocortin II treatment reduces skeletal muscle mass and function loss during atrophy and increases nonatrophying skeletal muscle mass and function. Endocrinology. 2003;144(11):4939–4946. doi: 10.1210/en.2003-0271. [DOI] [PubMed] [Google Scholar]
- 15.Hinkle R.T., Donnelly E., Cody D.B., Samuelsson S., Lange J.S., Bauer M.B., et al. Activation of the CRF 2 receptor modulates skeletal muscle mass under physiological and pathological conditions. American Journal of Physiology. Endocrinology and Metabolism. 2003;285(4):E889–E898. doi: 10.1152/ajpendo.00081.2003. [DOI] [PubMed] [Google Scholar]
- 16.Borg M.L., Massart J., De Castro Barbosa T., Archilla-Ortega A., Smith J.A.B., Lanner J.T., et al. Modified UCN2 peptide treatment improves skeletal muscle mass and function in mouse models of obesity-induced insulin resistance. J Cachexia Sarcopenia Muscle. 2021;12(5):1232–1248. doi: 10.1002/jcsm.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuperman Y., Issler O., Vaughan J., Bilezikjian L., Vale W., Chen A. Expression and regulation of corticotropin-releasing factor receptor type 2β in developing and mature mouse skeletal muscle. Molecular Endocrinology. 2011;25(1):157–169. doi: 10.1210/me.2010-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lira E.C., Gonçalves D.A.P., Parreiras-E-Silva L.T., Zanon N.M., Kettelhut I.C., Navegantes L.C.C. Phosphodiesterase-4 inhibition reduces proteolysis and atrogenes expression in rat skeletal muscles. Muscle & Nerve. 2011;44(3):371–381. doi: 10.1002/mus.22066. [DOI] [PubMed] [Google Scholar]
- 19.Gonçalves D.A., Silveira W.A., Manfredi L.H., Graça F.A., Armani A., Bertaggia E., et al. Insulin/IGF1 signalling mediates the effects of β2 -adrenergic agonist on muscle proteostasis and growth. J Cachexia Sarcopenia Muscle. 2019;10(2):455–475. doi: 10.1002/jcsm.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alessi D.R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., et al. Mechanism of activation of protein kinase B by insulin and IGF-1. The EMBO Journal. 1996;15(23):6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 21.Butler A.A., Yakar S., Gewolb I.H., Karas M., Okubo Y., LeRoith D. Insulin-like growth factor-I receptor signal transduction: at the interface between physiology and cell biology. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 1998;121(1):19–26. doi: 10.1016/s0305-0491(98)10106-2. [DOI] [PubMed] [Google Scholar]
- 22.LeRoith D., Werner H., Beitner-Johnson D., Roberts C.T. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocrine Reviews. 1995;16(2):143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- 23.Myers M.G., Grammer T.C., Wang L.M., Sun X.J., Pierce J.H., Blenis J., et al. Insulin receptor substrate-1 mediates phosphatidylinositol 3’-kinase and p70S6k signaling during insulin, insulin-like growth factor-1, and interleukin-4 stimulation. Journal of Biological Chemistry. 1994;269(46):28783–28789. [PubMed] [Google Scholar]
- 24.Bodine S.C., Stitt T.N., Gonzalez M., Kline W.O., Stover G.L., Bauerlein R., et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nature Cell Biology. 2001;3(11):1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 25.Inoki K., Zhu T., Guan K., Arbor A. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki M., Takemasa T. TSC2/Rheb signaling mediates ERK-dependent regulation of mTORC1 activity in C2C12 myoblasts. FEBS Open Bio. 2017;7(3):424–433. doi: 10.1002/2211-5463.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J.-Y., Zong C.S., Xia W., Yamaguchi H., Ding Q., Xie X., et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nature Cell Biology. 2008;10(2):138–148. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy S.K., Srivastava R.K., Shankar S. Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. Journal of Molecular Signaling. 2010;5(10) doi: 10.1186/1750-2187-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mammucari C., Milan G., Romanello V., Masiero E., Rudolf R., Del Piccolo P., et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metabolism. 2007;6(6):458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Sandri M., Sandri C., Gilbert A., Skurk C., Calabria E., Picard A., et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117(3):399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonaldo P., Sandri M. Cellular and molecular mechanisms of muscle atrophy. Disease Models & Mechanisms. 2013;6(1):25–39. doi: 10.1242/dmm.010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milan G., Romanello V., Pescatore F., Armani A., Paik J.-H., Frasson L., et al. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nature Communications. 2015;6(6670) doi: 10.1038/ncomms7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez-Marcos P.J., Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. American Journal of Clinical Nutrition. 2011;93(4) doi: 10.3945/ajcn.110.001917. 884S–890S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh S., Simpson R.L., Bennett R.G. Relaxin activates peroxisome proliferator-activated receptor γ (PPARγ) through a pathway involving PPARγ coactivator 1α (PGC1α) Journal of Biological Chemistry. 2015;290(2):950–959. doi: 10.1074/jbc.M114.589325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Handschin C., Kobayashi Y.M., Chin S., Seale P., Campbell K.P., Spiegelman B.M. PGC-1alpha regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes & Development. 2007;21(7):770–783. doi: 10.1101/gad.1525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang X.-Y., Tse M.C.L., Hu X., Jia W.-H., Du G.-H., Chan C.B. Interaction of CREB and PGC-1α induces fibronectin type III domain-containing protein 5 expression in C2C12 myotubes. Cellular Physiology and Biochemistry. 2018;50(4):1574–1584. doi: 10.1159/000494655. [DOI] [PubMed] [Google Scholar]
- 37.Murgia M., Serrano A.L., Calabria E., Pallafacchina G., Lomo T., Schiaffino S. Ras is involved in nerve-activity-dependent regulation of muscle genes. Nature Cell Biology. 2000;2(3):142–147. doi: 10.1038/35004013. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y., Pessin J.E. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Current Opinion in Clinical Nutrition and Metabolic Care. 2013;16(3):243–250. doi: 10.1097/MCO.0b013e328360272d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao X.-F., Zhou Y., Wang D.-Y., Lew K.-S., Richards A.M., Wang P. Urocortin-2 suppression of p38-MAPK signaling as an additional mechanism for ischemic cardioprotection. Molecular and Cellular Biochemistry. 2015;398(1–2):135–146. doi: 10.1007/s11010-014-2213-1. [DOI] [PubMed] [Google Scholar]
- 40.Navegantes L.C., Resano N.M., Migliorini R.H., Kettelhut I.C. Effect of guanethidine-induced adrenergic blockade on the different proteolytic systems in rat skeletal muscle. American Journal of Physiology. 1999;277(5):E883–E889. doi: 10.1152/ajpendo.1999.277.5.E883. [DOI] [PubMed] [Google Scholar]
- 41.Ryall J.G., Plant D.R., Gregorevic P., Sillence M.N., Lynch G.S. Beta 2-agonist administration reverses muscle wasting and improves muscle function in aged rats. The Journal of Physiology. 2004;555(Pt 1):175–188. doi: 10.1113/jphysiol.2003.056770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan S., Head S.I. Age- and gender-related changes in contractile properties of non-atrophied EDL muscle. PLoS One. 2010;5(8) doi: 10.1371/journal.pone.0012345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waalkes T.P., Udenfriend S. A fluorometric method for the estimation of tyrosine in plasma and tissues. The Journal of Laboratory and Clinical Medicine. 1957;50(5):733–736. [PubMed] [Google Scholar]
- 44.Goodman C.A., Mabrey D.M., Frey J.W., Miu M.H., Schmidt E.K., Pierre P., et al. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. Federation of American Societies for Experimental Biology Journal. 2011;25(3):1028–1039. doi: 10.1096/fj.10-168799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ju J., Varadhachary A.S., Miller S.E., Weihl C.C. Quantitation of “autophagic flux” in mature skeletal muscle. Autophagy. 2010;6(7):929–935. doi: 10.4161/auto.6.7.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaughan J.M., Donaldson C.J., Fischer W.H., Perrin M.H., Rivier J.E., Sawchenko P.E., et al. Posttranslational processing of human and mouse urocortin 2: characterization and bioactivity of gene products. Endocrinology. 2013;154(4):1553–1564. doi: 10.1210/en.2012-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R., Zeiher A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399(6736):601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 48.Wu J.J., Zhang L., Bennett A.M. The noncatalytic amino terminus of mitogen-activated protein kinase phosphatase 1 directs nuclear targeting and serum response element transcriptional regulation. Molecular and Cellular Biology. 2005;25(11):4792–4803. doi: 10.1128/MCB.25.11.4792-4803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pankiv S., Clausen T.H., Lamark T., Brech A., Bruun J.A., Outzen H., et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. Journal of Biological Chemistry. 2007;282(33):24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 50.de Rooij J., Zwartkruis F.J., Verheijen M.H., Cool R.H., Nijman S.M., Wittinghofer A., et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396(6710):474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 51.Zoncu R., Efeyan A., Sabatini D.M. mTOR: from growth signal integration to cancer, diabetes and ageing. Nature Reviews Molecular Cell Biology. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pyronnet S. Phosphorylation of the cap-binding protein eIF4E by the MAPK-activated protein kinase Mnk1. Biochemical Pharmacology. 2000;60(8):1237–1243. doi: 10.1016/s0006-2952(00)00429-9. [DOI] [PubMed] [Google Scholar]
- 53.Geng J., Klionsky D.J. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. “Protein modifications: beyond the usual suspects” review series. EMBO Reports. 2008;9(9):859–864. doi: 10.1038/embor.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bjørkøy G., Lamark T., Brech A., Outzen H., Perander M., Overvatn A., et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. The Journal of Cell Biology. 2005;171(4):603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao J., Brault J.J., Schild A., Cao P., Sandri M., Schiaffino S., et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metabolism. 2007;6(6):472–483. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Wang L., Proud C.G. Ras/Erk signaling is essential for activation of protein synthesis by Gq protein-coupled receptor agonists in adult cardiomyocytes. Circulation Research. 2002;91(9):821–829. doi: 10.1161/01.res.0000041029.97988.e9. [DOI] [PubMed] [Google Scholar]
- 57.Kelleher R.J., Govindarajan A., Jung H.-Y., Kang H., Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116(3):467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 58.Lin J., Wu H., Tarr P.T., Zhang C.-Y., Wu Z., Boss O., et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418(6899):797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 59.Hinkle R.T., Donnelly E., Cody D.B., Bauer M.B., Sheldon R.J., Isfort R.J. Corticotropin releasing factor 2 receptor agonists reduce the denervation-induced loss of rat skeletal muscle mass and force and increase non-atrophying skeletal muscle mass and force. Journal of Muscle Research & Cell Motility. 2004;25(7):539–547. doi: 10.1007/s10974-004-4088-3. [DOI] [PubMed] [Google Scholar]
- 60.Reutenauer-patte J., Boittin F., Patthey-vuadens O., Ruegg U.T., Dorchies O.M. Urocortins improve dystrophic skeletal muscle structure and function through both PKA- and epac-dependent pathways. AJPA. 2012;180(2):749–762. doi: 10.1016/j.ajpath.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 61.Hall J.E., Kaczor J.A.N.J., Hettinga B.P. Effects of a CRF2R agonist and exercise on mdx and wildtype skeletal muscle. Muscle & Nerve. 2007;36(3):336–341. doi: 10.1002/mus.20820. [DOI] [PubMed] [Google Scholar]
- 62.Jesinkey S.R., Korrapati M.C., Rasbach K.A., Beeson C.C., Schnellmann R.G. Atomoxetine prevents dexamethasone-induced skeletal muscle atrophy in mice. Journal of Pharmacology and Experimental Therapeutics. 2014;351(3):663–673. doi: 10.1124/jpet.114.217380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang W., Yano N., Deng M., Mao Q., Shaw S.K., Tseng Y.-T. β-Adrenergic receptor-PI3K signaling crosstalk in mouse heart: elucidation of immediate downstream signaling cascades. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0026581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu D., Bordicchia M., Zhang C., Fang H., Wei W., Li J.-L., et al. Activation of mTORC1 is essential for β-adrenergic stimulation of adipose browning. Journal of Clinical Investigation. 2016;126(5):1704–1716. doi: 10.1172/JCI83532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Przygodda F., Lautherbach N., Buzelle S.L., Goncalves D.A., Assis A.P., Paula-Gomes S., et al. Sympathetic innervation suppresses the autophagic-lysosomal system in brown adipose tissue under basal and cold-stimulated conditions. Journal of Applied Physiology. 2020;128(4):855–871. doi: 10.1152/japplphysiol.00065.2019. [DOI] [PubMed] [Google Scholar]
- 66.Baviera A.M., Zanon N.M., Navegantes L.C.C., Kettelhut I.C. Involvement of cAMP/Epac/PI3K-dependent pathway in the antiproteolytic effect of epinephrine on rat skeletal muscle. Molecular and Cellular Endocrinology. 2010;315(1–2):104–112. doi: 10.1016/j.mce.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 67.Navegantes L.C.C., Resano N.M.Z., Migliorini R.H., Kettelhut I.C. Catecholamines inhibit Ca(2+)-dependent proteolysis in rat skeletal muscle through beta(2)-adrenoceptors and cAMP. American Journal of Physiology. Endocrinology and Metabolism. 2001;281(3):E449–E454. doi: 10.1152/ajpendo.2001.281.3.E449. [DOI] [PubMed] [Google Scholar]
- 68.Navegantes L.C.C., Resano N.M.Z., Migliorini R.H. Role of adrenoceptors and cAMP on the catecholamine- induced inhibition of proteolysis in rat skeletal muscle. American Journal of Physiology. Endocrinology and Metabolism. 2000;279(3):E663–E668. doi: 10.1152/ajpendo.2000.279.3.E663. [DOI] [PubMed] [Google Scholar]
- 69.Ryall J.G., Sillence M.N., Lynch G.S. Systemic administration of beta2-adrenoceptor agonists, formoterol and salmeterol, elicit skeletal muscle hypertrophy in rats at micromolar doses. British Journal of Pharmacology. 2006;147(6):587–595. doi: 10.1038/sj.bjp.0706669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gonçalves D. a P., Silveira W.a., Lira E.C., Graça F.a., Paula-Gomes S., Zanon N.M., et al. Clenbuterol suppresses proteasomal and lysosomal proteolysis and atrophy-related genes in denervated rat soleus muscles independently of Akt. American Journal of Physiology. Endocrinology and Metabolism. 2012;302(1):E123–E133. doi: 10.1152/ajpendo.00188.2011. [DOI] [PubMed] [Google Scholar]
- 71.Costelli P., García-Martínez C., Llovera M., Carbó N., López-Soriano F.J., Agell N., et al. Muscle protein waste in tumor-bearing rats is effectively antagonized by a beta 2-adrenergic agonist (clenbuterol). Role of the ATP-ubiquitin-dependent proteolytic pathway. Journal of Clinical Investigation. 1995;95(5):2367–2372. doi: 10.1172/JCI117929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lira E.C., Graca F.A., Goncalves D.A.P., Zanon N.M., Baviera A.M., Strindberg L., et al. Cyclic adenosine monophosphate-phosphodiesterase inhibitors reduce skeletal muscle protein catabolism in septic rats. Shock. 2007;27(6):687–694. doi: 10.1097/SHK.0b013e31802e43a6. [DOI] [PubMed] [Google Scholar]
- 73.Baviera A.M., Zanon N.M., Carvalho Navegantes L.C., Migliorini R.H., do Carmo Kettelhut I. Pentoxifylline inhibits Ca2+-dependent and ATP proteasome-dependent proteolysis in skeletal muscle from acutely diabetic rats. American Journal of Physiology. Endocrinology and Metabolism. 2007;292(3):E702–E708. doi: 10.1152/ajpendo.00147.2006. [DOI] [PubMed] [Google Scholar]
- 74.Handschin C., Rhee J., Lin J., Tarr P.T., Spiegelman B.M. An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(12):7111–7116. doi: 10.1073/pnas.1232352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herzig S., Long F., Jhala U.S., Hedrick S., Quinn R., Bauer A., et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413(6852):179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 76.Sandri M., Lin J., Handschin C., Yang W., Arany Z.P., Lecker S.H., et al. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(44):16260–16265. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geng T., Li P., Yin X., Yan Z. PGC-1α promotes nitric oxide antioxidant defenses and inhibits FOXO signaling against cardiac cachexia in mice. American Journal Of Pathology. 2011;178(4):1738–1748. doi: 10.1016/j.ajpath.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Silveira W.A., Gonçalves D.A., Machado J., Lautherbach N., Lustrino D., Marcelo S.P., et al. cAMP-dependent protein kinase inhibits FoxO activity and regulates skeletal muscle plasticity in mice. The FASEB Journal. 2020;34(9):12946–12962. doi: 10.1096/fj.201902102RR. [DOI] [PubMed] [Google Scholar]
- 79.Borg M.L., Massart J., Schönke M., Barbosa T.D.C., Guo L., Wade M., et al. Modified UCN2 peptide acts as an insulin sensitizer in skeletal muscle of obese mice. Diabetes. 2019;68(7):1403–1414. doi: 10.2337/db18-1237. [DOI] [PubMed] [Google Scholar]
- 80.Chao H., Li H., Grande R., Lira V., Yan Z., Harris T.E., et al. Involvement of mTOR in type 2 CRF receptor inhibition of insulin signaling in muscle cells. Molecular Endocrinology. 2015;29(6):831–841. doi: 10.1210/me.2014-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mei F.C., Qiao J., Tsygankova O.M., Meinkoth J.L., Quilliam L.A., Cheng X. Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. Journal of Biological Chemistry. 2002;277(13):11497–11504. doi: 10.1074/jbc.M110856200. [DOI] [PubMed] [Google Scholar]
- 82.Brennesvik E.O., Ktori C., Ruzzin J., Jebens E., Shepherd P.R., Jensen J. Adrenaline potentiates insulin-stimulated PKB activation via cAMP and Epac: implications for cross talk between insulin and adrenaline. Cellular Signalling. 2005;17(12):1551–1559. doi: 10.1016/j.cellsig.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 83.Keiper M., Stope M.B., Szatkowski D., Böhm A., Tysack K., Vom Dorp F., et al. Epac- and Ca2+ -controlled activation of Ras and extracellular signal-regulated kinases by Gs-coupled receptors. Journal of Biological Chemistry. 2004;279(45):46497–46508. doi: 10.1074/jbc.M403604200. [DOI] [PubMed] [Google Scholar]
- 84.Jones N.C., Fedorov Y.V., Rosenthal R.S. ERK1/2 is required for myoblast proliferation but is dispensable for muscle gene expression and cell fusion. Journal of Cellular Physiology. 2001;186(1):104–115. doi: 10.1002/1097-4652(200101)186:1<104::AID-JCP1015>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 85.Yokoyama T., Takano K., Yoshida A., Katada F., Sun P., Takenawa T., et al. DA-Raf1, a competent intrinsic dominant-negative antagonist of the Ras-ERK pathway, is required for myogenic differentiation. The Journal of Cell Biology. 2007;177(5):781–793. doi: 10.1083/jcb.200703195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bueno O.F., De Windt L.J., Tymitz K.M., Witt S.a., Kimball T.R., Klevitsky R., et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. The EMBO Journal. 2000;19(23):6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ueyama T., Kawashima S., Sakoda T., Rikitake Y., Ishida T., Kawai M., et al. Requirement of activation of the extracellular signal-regulated kinase cascade in myocardial cell hypertrophy. Journal of Molecular and Cellular Cardiology. 2000;32(6):947–960. doi: 10.1006/jmcc.2000.1135. [DOI] [PubMed] [Google Scholar]
- 88.Shi H., Scheffler J.M., Zeng C., Pleitner J.M., Hannon K.M., Grant A.L., et al. Mitogen-activated protein kinase signaling is necessary for the maintenance of skeletal muscle mass. American Journal of Physiology - Cell Physiology. 2009;296(5):C1040–C1048. doi: 10.1152/ajpcell.00475.2008. [DOI] [PubMed] [Google Scholar]
- 89.Shi H., Zeng C., Ricome A., Hannon K.M., Grant A.L., Gerrard D.E. Extracellular signal-regulated kinase pathway is differentially involved in beta-agonist-induced hypertrophy in slow and fast muscles. American Journal of Physiology - Cell Physiology. 2007;292(5):C1681–C1689. doi: 10.1152/ajpcell.00466.2006. [DOI] [PubMed] [Google Scholar]
- 90.Hinkle R.T., Lefever F.R., Dolan E.T., Reichart D.L., Dietrich J.A., Gropp K.E., et al. Corticortophin releasing factor 2 receptor agonist treatment significantly slows disease progression in mdx mice. BMC Medicine. 2007;5(18) doi: 10.1186/1741-7015-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chanalaris A., Lawrence K.M., Stephanou A., Knight R.D., Hsu S.Y., Hsueh A.J.W., et al. Protective effects of the urocortin homologues stresscopin (SCP) and stresscopin-related peptide (SRP) against hypoxia/reoxygenation injury in rat neonatal cardiomyocytes. Journal of Molecular and Cellular Cardiology. 2003;35(10):1295–1305. doi: 10.1016/s0022-2828(03)00244-x. [DOI] [PubMed] [Google Scholar]
- 92.Roux P.P., Ballif B.A., Anjum R., Gygi S.P., Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(37):13489–13494. doi: 10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brar B.K., Jonassen A.K., Egorina E.M., Chen A., Negro A., Perrin M.H., et al. Urocortin-II and urocortin-III are cardioprotective against ischemia reperfusion injury: an essential endogenous cardioprotective role for corticotropin releasing factor receptor type 2 in the murine heart. Endocrinology. 2004;145(1):24–35. doi: 10.1210/en.2003-0689. [DOI] [PubMed] [Google Scholar]