Summary
During productive human cytomegalovirus (HCMV) infection, viral genes are expressed in a coordinated cascade that conventionally relies on the dependencies of viral genes on protein synthesis and viral DNA replication. By contrast, the transcriptional landscape of HCMV latency is poorly understood. Here, we examine viral gene expression dynamics during the establishment of both productive and latent HCMV infections. We redefine HCMV gene expression kinetics during productive infection and reveal that viral gene regulation does not represent a simple sequential cascade; many viral genes are regulated by multiple independent modules. Using our improved gene expression classification combined with transcriptome-wide measurements of the effects of a wide array of epigenetic inhibitors on viral gene expression during latency, we show that a defining feature of latency is the unique repression of immediate-early (IE) genes. Altogether, we recharacterize HCMV gene expression kinetics and reveal governing principles of lytic and latent gene expression.
Keywords: Human cytomegalovirus, herpesvirus, latency, gene expression
Graphical abstract

Highlights
-
•
Redefining HCMV gene expression cascade during productive infection
-
•
Many viral genes are regulated by multiple independent modules
-
•
Diverse inhibitors induce broad viral gene expression in monocytes
-
•
In monocytes, immediate-early (IE) genes are repressed compared to all other HCMV genes
Rozman et al. perform a transcriptome analysis along human cytomegalovirus (HCMV) lytic infection and redefine viral gene expression principles. They perform an epigenetic inhibitor screen on latent HCMV infection that reveals broad gene expression with repression of immediate-early genes, which may be a defining characteristic of latency.
Introduction
Human cytomegalovirus (HCMV) is a pervasive pathogen of the beta herpesvirus family that infects a majority of the world population (Staras et al., 2006). HCMV persists throughout the lifetime of the host by establishing latent infection from which the virus can later reactivate and cause life-threatening disease in immunocompromised individuals such as transplant recipients and HIV patients (Crough and Khanna, 2009; Pereira et al., 2011).
During productive infection, transcription from herpesvirus genomes is accomplished by the host RNA polymerase II and regulated by host and viral proteins, leading to a coordinated viral gene expression cascade resulting in the production of infectious progeny. Traditionally, viral genes are divided into three distinct and chronological expression groups, immediate-early (IE), early (E), and late (L) genes, which differ with respect to their regulation and kinetics. IE genes require no new cellular or viral protein synthesis for their expression and are necessary for the expression of E genes, whereas L gene expression is dependent on viral DNA synthesis (Knipe et al., 2002).
Historically, metabolic inhibitors such as cycloheximide (CHX), a protein synthesis inhibitor, and phosphonoformate (PFA), a viral DNA replication inhibitor, have been used to classify viral genes into IE, E, and L temporal expression profiles. CHX leads to the specific accumulation of IE transcripts, and these genes were assumed to be expressed at the onset of viral gene expression. PFA leads to the specific depletion of transcripts dependent on or enhanced by the onset of viral DNA synthesis, and these genes were assumed to be expressed with late kinetics. Early studies provided the framework for mapping viral gene expression kinetics using hybridization methodologies (DeMarchi et al., 1980; McDonough and Spector, 1983; Wathen and Stinski, 1982) and later on by using microarrays (Chambers et al., 1999). The reannotation of the HCMV transcriptional landscape has elucidated the crowded nature of its genome and its ability to encode many overlapping RNAs (Stern-Ginossar et al., 2012; Gatherer et al., 2011). Although some studies have provided detailed temporal protein profiling (Weekes et al., 2014), HCMV gene expression dynamics has not been extensively analyzed using unbiased transcriptomic methods.
Viral gene expression during latent infection has been extremely hard to define and is still a matter of controversy (Schwartz and Stern-Ginossar, 2019). In latently infected cells, the viral genome is repressed, and there is no production of new virions (Murphy et al., 2002; Reeves et al., 2005). Since the major immediate-early promoter (MIEP) drives the expression of IE1 and IE2, two potent transactivators of viral gene expression, its regulation has been extensively studied (Meier and Stinski, 1996; Collins-McMillen et al., 2019). It is well established that the latent genome is chromatinized and the MIEP is repressed in latent cells (Nitzsche et al., 2008; Reeves and Sinclair, 2010; Reeves et al., 2005), and this is considered a major mechanism dictating latent infection. However, whether the MIEP is uniquely regulated has not been addressed in a thorough manner. In parallel to these studies, extensive efforts were aimed at deciphering the latent HCMV transcriptome. The leading idea for many years was that in latency, viral gene expression is generally silenced and only a limited number of genes—the presumed latency genes—are expressed (Sinclair and Sissons, 2006; Slobedman et al., 2010). Surprisingly, recent transcriptomic studies on latent cells, including research from our lab, revealed that viral genes are broadly expressed, but at low levels (Shnayder et al., 2018; Cheng et al., 2017). These findings have complicated the characterization of latent viral gene expression and its distinction from lytic viral gene expression as well as the understanding of how the transcriptome reflects latency regulation.
Results
Temporal analysis of HCMV gene expression along lytic and latent infections
To comprehensively define viral gene expression kinetics, fibroblasts (lytic infection) and primary CD14+ monocytes (latent infection) were infected with the same TB40E HCMV strain and were harvested for RNA sequencing (strand-specific sequencing of full-length polyA selected transcripts) (Shishkin et al., 2015) over the time course of 72 h post infection (hpi). To determine IE genes, infected cells were treated with CHX and harvested for RNA-seq at 8 hpi. To define true L genes, infected cells were treated with PFA and harvested for RNA-seq at 24/48 and 72 hpi (Figure 1A). Latent infection was verified by the lack of viral progeny at several times post infection.
Figure 1.
RNA sequencing along HCMV lytic and latent infections
(A) Schematic representation of experimental setup. Fibroblasts and CD14+ monocytes infected with HCMV strain TB40E-GFP were harvested at 4, 8, 12, 24, 48, and 72 hpi for RNA sequencing. Infected fibroblasts and CD14+ monocytes were treated with PFA and harvested at 24/72 or 48/72 hpi, respectively, or treated with CHX and harvested at 8 hpi. All time points had two biological replicates.
(B) Heatmap depicting the Pearson correlations between RNA-seq samples based on viral and host reads for fibroblast samples (blue) and CD14+ monocyte samples (orange). Biological replicates are denoted by a/b.
(C and D) Percent of HCMV reads out of total mRNA reads in fibroblasts (C) and CD14+ monocytes (D). The plots include untreated (green), CHX (red), and PFA (purple) samples with both replicates of each sample.
We prepared two independent biological replicates for all time points, treatments, and cell types. When applying hierarchical clustering on the correlations of host and viral gene expression in each sample, biological replicates grouped together, supporting the reproducibility of our measurements (Figures 1B and S1A). Interestingly, replicates from all time points and treatments in latently infected CD14+ monocytes strongly correlate, indicating that there are few changes in the transcriptome along the establishment of latency (Figures 1B, S1A, and S1B).
In lytic infection, viral gene expression increases between 4 and 72 hpi, where viral reads compose up to half of the total mRNA reads by 72 hpi. As expected, at 72 hpi, viral reads from the PFA-treated fibroblast samples make up only around 10% of the total mRNA, likely due to less template (Figure 1C). However, viral transcripts compose less than 0.25% of the total mRNA reads in latently infected monocytes, and the percentage of viral reads continuously decreases through 72 hpi (Tables S1A and S1B). Surprisingly, PFA-treated latently infected monocytes expressed slightly higher levels of viral genes. At 72 hpi, this increase was statistically significant (p value = 0.0015) and reflected elevation in the majority of viral genes (Figures 1D and S1C). We speculate that this increase is due to changes in the host cell upon drug treatment.
HCMV gene expression kinetics along lytic infection reveals complex, multi-faced regulation
We first analyzed viral gene expression kinetics in lytically infected fibroblasts. After filtering for minimal expression, we were able to reliably quantify the majority of viral genes (134 genes). For each viral gene, we calculated its relative expression pattern out of total viral reads in a given time point or treatment. We performed hierarchical clustering based on the normalized viral gene expression, dividing viral genes into seven temporal classes (TCs) (Figure 2A). We term these clusters TC1 to TC7 (Figure 2B and Table S1A). There was overall good correspondence between classical TCs and our measurements. For example, the two canonical immediate-early genes UL123 and UL122 (Stenberg, 1996) were classified as TC1 and were highly expressed at 4 hpi. Their expression was not inhibited by translation inhibition with CHX, as seen with most other viral genes, so their relative level is higher upon treatment. The genes in clusters TC2 and TC3 have early and delayed early kinetics and contain many genes classically defined as early. The late clusters (TC5–TC7) contain many known late genes UL94 (Phillips and Bresnahan, 2012), UL75 (McWatters et al., 2002; Yurochko et al., 1997), and UL32 (AuCoin et al., 2006) with peak expression between 48 and 72 hpi, and their expression diminished when viral DNA replication was inhibited by PFA (Figure 2A). In general, there was high agreement between the mRNA TCs we defined and previously determined protein temporal classifications (Weekes et al., 2014) (Figure 2A, lower panel).
Figure 2.
Temporal classes of HCMV genes along productive infection
(A) Heatmap of normalized relative viral gene expression levels. Scaled normalized expression patterns of viral genes are shown including CHX and PFA samples (upper panel) and without the CHX and PFA samples (lower panel). The annotation bars show our defined temporal classes (top bar) and the protein temporal classes that were defined by Weekes et al. (2014).
(B) (Bottom bar) Expression profiles of all viral genes in each TC along infection of fibroblasts, averaged across replicates with description for each TC. Values are normalized to the maximal expression of each gene.
It is important to note that the 7 TCs reflect how each viral gene is expressed relative to all other viral genes in the same time point. Looking at the normalized expression levels of viral genes along infection in RPKM (reads per kilobase per million mapped reads), which more closely reflects their absolute quantities, almost all viral genes are most highly expressed at 72 hpi regardless of the TC in which they were classified (Figure S2A). This is expected due to viral DNA replication that leads to high levels of template DNA and a corresponding increase in viral transcription levels at later stages of infection. A unique outlier is UL123 (encoding for IE1) whose highest expression was measured at 8 hpi, and in absolute terms, it was hardly expressed at 72 hpi (Figure S2A). This unique expression pattern points to the critical role IE1 plays at early stages of the HCMV life cycle and to the distinct regulation to which it is subjected (Cherrington et al., 1991).
We noticed that for numerous viral genes, their expression along the time course of infection and their expression with CHX and PFA treatments do not fully align, revealing more than one regulatory mode of expression for these genes. For example, TC4 is composed of genes that show early expression kinetics, but these genes also show dependency on DNA replication because PFA treatment diminishes their expression, and consequently, they demonstrate a second wave of expression at 72 hpi. Interestingly, viral genes that are considered canonical late genes such as UL99 (encoding for pp28) and UL80 (encoding for capsid scaffold protein) are part of this TC. TC5 represents a group of genes with late expression kinetics that resembles the kinetics of TC6 and TC7, but the relative expression of genes in TC5 did not depend on DNA replication and was unaffected by PFA (Figure 2A, upper panel). Furthermore, genes in TC6, which includes UL83 (encoding for the major tegument protein pp65) (Browne and Shenk, 2003; Varnum et al., 2004) and UL86 (encoding for the major capsid protein) (Gibson, 1996), exhibit late kinetics and dependency on DNA replication. Surprisingly, however, these genes also exhibit expression that is independent of de novo protein synthesis upon CHX treatment (Figure 2A, upper panel). These results reveal a clear deviation from the classical definition of the herpesvirus gene expression cascade that traditionally relies on the dependence of protein synthesis and sensitivity of viral genes to viral DNA replication to define IE and L genes. The canonical naming is oversimplified and even misleading; for numerous viral genes, there are several modules that regulate their expression. We therefore propose a division into seven TCs that incorporates the canonical nomenclature but better reflects the complexity of viral gene expression: TC1, immediate-early; TC2, early; TC3, delayed early; TC4, early-late; TC5, late-replication-independent; TC6, late-translation-independent; and TC7, late (Figure 2B).
A group of late HCMV transcripts is also expressed in a protein-synthesis-independent manner
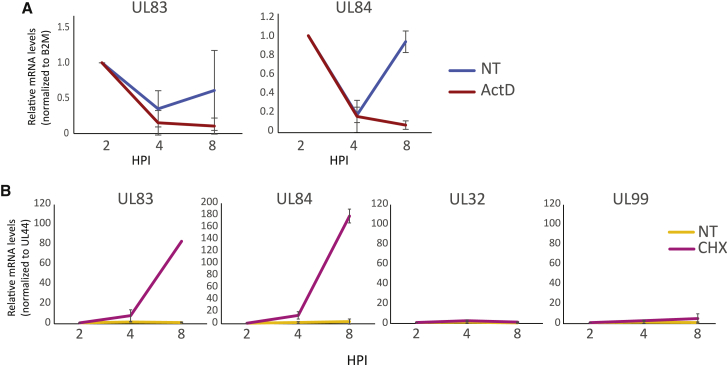
Analyzing IE gene expression in the presence of CHX can be hindered by the existence of virion-associated input RNA nonspecifically bound by or packaged within the virus particles and delivered to newly infected cells (Terhune et al., 2004). Indeed, many of the transcripts that presented late expression kinetics (TC5–TC7) are also relatively highly expressed at 4 hpi. Since TC6 presented such an unexpected expression profile composed of transcripts with late kinetics as well as expression that is independent of protein synthesis, we wanted to rule out the possibility that these transcripts might represent stable RNA species that entered the infected cell with the virus as “input” RNA and remained intact for 8 h, the time point at which we harvested the CHX samples. In order to assess if the expression of these genes at 8 hpi is independent of transcription, HCMV-infected fibroblasts were treated with actinomycin D (ActD), an RNA polymerase inhibitor. Using quantitative real-time PCR analysis for TC6 transcripts, we show that the expression of these transcripts, such as UL83 and UL84, is abolished with ActD treatment, indicating our measurements at 8 hpi represent true viral RNA transcription (Figure 3A). We also repeated this experiment and analyzed the expression of these genes at different time points following CHX treatment. Indeed, we detected a higher relative expression of TC6 transcripts (UL83 and UL84) upon CHX treatment, whereas the late transcripts UL32 and UL99 did not show an increase in their expression by quantitative real-time PCR (Figure 3B). Overall, these results illustrate de novo expression of traditional late genes in a protein-synthesis-independent manner and provide further support to the validity of the new TCs we have identified.
Figure 3.
Translation-independent transcription of a group of late transcripts
(A) Fibroblasts infected with HCMV and treated with actinomycin D (actD) or untreated (NT) at 2 hpi. mRNA levels of the viral transcripts UL83 and UL84 (TC6) were quantified by quantitative real-time PCR at 2, 4, and 8 hpi corresponding to 0, 2, and 6 h post actD treatment. Mean and standard deviation (SD) are shown.
(B) Fibroblasts infected with HCMV and treated with CHX or left untreated (NT) at 2 hpi. mRNA levels of viral transcripts UL83, UL84, UL32, and UL99 were quantified by quantitative real-time PCR at 2, 4, and 8 hpi. Mean and SD are shown.
High levels of input RNA at 4 hpi
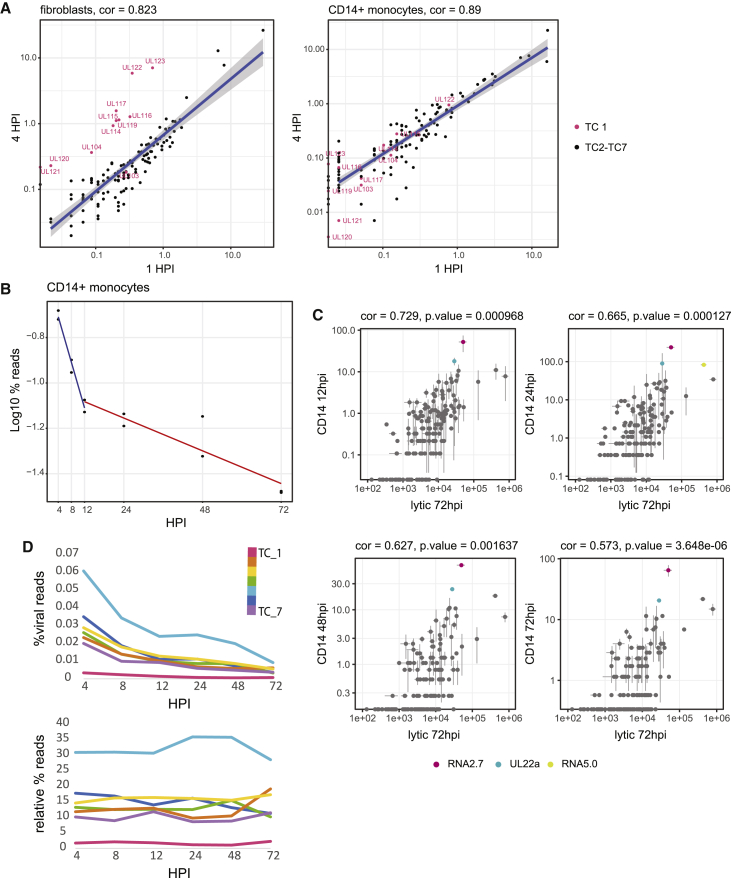
High levels of late transcripts in the RNA-seq samples at 4 hpi suggested a portion of the transcriptome at 4 hpi likely originates from virion-derived RNA, rather than RNA that had been synthesized following infection (Terhune et al., 2004). To better distinguish virion-associated input RNA from genuine transcription, we performed RNA-seq of both HCMV-infected fibroblasts and CD14+ monocytes at 1 hpi, a time point that likely precedes the onset of expression for most viral genes. The expression profiles between 1 and 4 hpi for each system are highly correlated (Figure 4A), indicating the considerable presence of input RNA even at 4 hpi. However, in lytic infection (Figure 4A, left panel), TC1 genes, which are expressed with IE kinetics, show significantly higher expression by 4 hpi, illustrating clear transcription of this set of genes above the background noise. By contrast, in CD14+ monocytes, there is no increased expression of specific transcripts at 4 hpi compared to 1 hpi. If transcription of viral IE genes occurs at 4 hpi, it is low and masked by a relatively high level of input RNA (Figure 4A, right panel). We examined whether the lack of increased transcript levels at early time points post infection, together with the gradual decrease in the percent of HCMV transcripts along infection in monocytes (Figure 1D), might indicate large quantities of input RNA and no de novo viral transcription in this system. Calculation of the decay rate of viral mRNA levels across infection in the infected CD14+ monocytes suggests that by 12 hpi, a substantial portion of the RNA originates from low levels of RNA synthesis (Figure 4B). Therefore, the source of viral reads in CD14+ monocytes is initially overwhelmed by input RNA, but early in infection, there is onset of low-level RNA synthesis, which eventually dominates the measured viral transcripts.
Figure 4.
Low-level transcription in latent HCMV infection
(A) Read number for each viral gene at 4 hpi versus 1 hpi in fibroblasts (left panel) and CD14+ monocytes (right panel). Viral IE genes (TC 1) marked in pink. Spearman correlations are indicated.
(B) Percentage of viral reads out of total mRNA reads along HCMV latent infection. The calculated overall decay (half-life) of viral transcripts at 1–12 hpi (0.68 h) is denoted by a blue regression line and at 12–72 hpi (8.93 h) is denoted by a red regression line and was calculated using log values for percent viral expression at each time point (A = A0e-Kt).
(C) Scatter plots showing normalized read number for viral genes at 72 hpi in fibroblasts versus 12, 24, 48, and 72 hpi in CD14+ monocytes. Spearman correlations and p values are indicated. Labeled in color are viral transcripts whose expression significantly deviated (p value < 0.05) from the correlation.
(D) Expression profile of all TCs along HCMV infection of CD14+ monocytes, as calculated by percentage of reads from all viral genes in a TC out of all reads (top) or out of total viral reads (bottom). Mean values of replicates are presented.
Latently infected CD14+ monocytes express a broad set of viral genes that resemble a late lytic profile
Although the latency transcriptome profile at early time points post infection was masked by input RNA, we examined if we could capture differential expression of our defined TCs along later time points in latent infection. We compared the expression profiles measured at 12, 24, 48, and 72 hpi in CD14+ monocytes to the expression measured in late lytic infection. We obtained good correlation between viral gene expression in infected monocytes with the late lytic expression in fibroblasts, and the correlation coefficient dropped over time due to the gradual reduction in the number of viral reads (Figure 4C). The only genes whose expression was significantly higher in monocytes compared to their relative abundance in fibroblasts at all time points were RNA2.7 and UL22a, both of which are highly expressed transcripts, and their relatively high levels are likely related to their high transcript stability. We further analyzed the expression kinetics of the 7 TCs we defined for lytic infection in the CD14+ dataset. As expected from the general decline in viral reads in latent monocytes, the expression of all TC classes declined with time (Figure 4D, upper panel). In contrast to the temporal dynamic that is clearly evident in lytic cells (Figure S2B), the proportion of reads from each TC remains relatively constant throughout the course of latent infection, (Figure 4D, lower panel) implying little to no changes in the overall expression profile. This fixed profile likely results from residual input RNA in combination with low-level transcription, which may reflect inherent susceptibilities of different loci of the viral genome to the host transcription machinery.
Diverse epigenetic inhibitors induce viral gene expression in infected monocytes
The cellular environment seems to be a key factor in determining the outcome of HCMV infection. Epigenetic regulation plays an important role in latent infection, where the viral genome is chromatinized and maintained as a repressed episome (Reeves, 2011; Sinclair, 2010). To verify that the expression we captured at 72 hpi in infected monocytes indeed reflects low-level transcription, we asked if we could enhance viral gene expression by interfering with host chromatin modifying factors. If so, we would expect to see increased expression of specific viral genes, with potenially variable effects between drugs that target different gene silencing pathways.
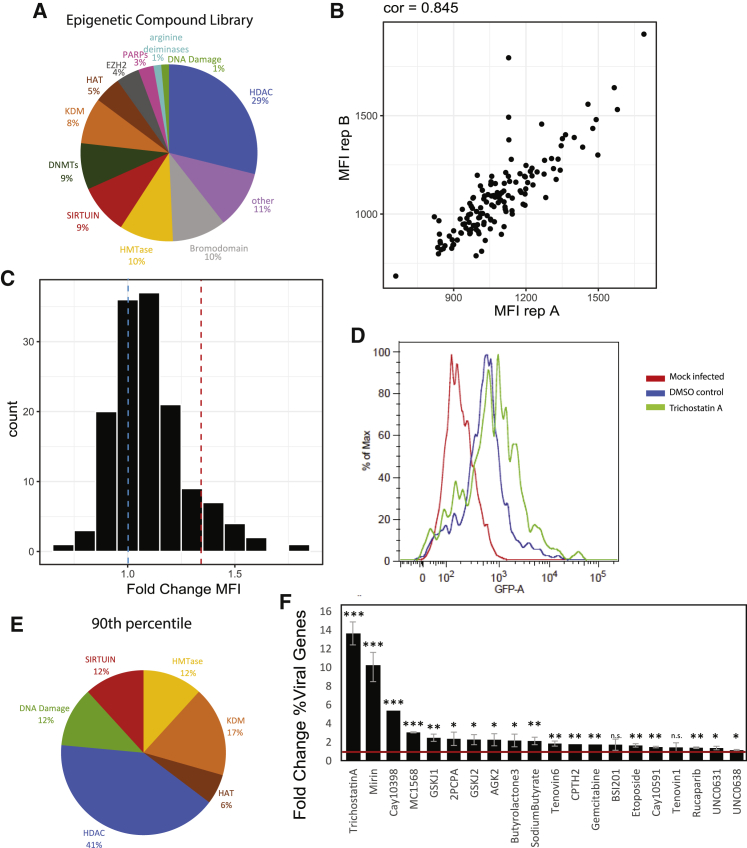
To probe a large range of molecules, we performed a high-throughput inhibitor screen for epigenetic regulators that affect HCMV gene expression. Primary CD14+ monocytes were latently infected with an HCMV strain TB40E virus encoding a green fluorescent protein (GFP) tag under a simian virus 40 (SV40) promoter (TB40-GFP) (Sinzger et al., 2008; O’Connor and Murphy, 2012). 48 hpi, at a time when much of the overload of input RNA has degraded and viral gene expression largely reflects low-level latent transcription, we incubated these cells with 140 different small molecule epigenetic inhibitors, in biological replicates. The inhibitors we tested fell within a broad range of categories, including inhibitors of bromodomains, histone deacetylases, histone methyltransferases, histone demethylases, and others (Figure 5A). The final concentration we used in this screen was set to 1 μM based on calibration of six different drugs targeting different functional categories with the aim to reduce drug cytotoxicity (Figure S3). 24 h following drug treatment (a time point we presumed is likely to reflect the immediate changes due to the drugs on viral gene expression), we screened by flow cytometry for bulk changes in GFP expression as a proxy for changes in viral gene expression compared to a DMSO-treated control. For the vast majority of epigenetic inhibitors, biological replicates showed little variation (Figure 5B), and treatments induced little to no changes in the GFP expression. The 15 drugs that fell within the top 10% for fold change in GFP expression (Figures 5C and 5D) were enriched in HDACs such as trichostatin A (TSA) (Park et al., 2007; Bigley et al., 2013), sodium butyrate (Radsak et al., 1989; Tanaka et al., 1991), and MC1568 (Krishna et al., 2016) and also included the sirtuin inhibitor AGK2 (He and Gao, 2014; Pei and Robertson, 2020), all of which have been reported to enhance herpesvirus gene expression. Additionally, these drugs included UNC0631, histone methyltransferase inhibitor, and etoposide and mirin, inhibitors of the DNA damage pathway (Hu et al., 2019) (Figure 5E).
Figure 5.
Epigenetic inhibitors induce viral gene transcription in HCMV-infected CD14+ monocytes
(A–F) TB40E-GFP-infected CD14+ monocytes were incubated with 1 μM of 140 compounds from an epigenetic inhibitor library for 24 h at 48 hpi, in biological replicates.
(A) A pie chart showing the distribution of drug targets in the epigenetic inhibitor library.
(B) GFP mean fluorescence intensity (MFI) values measured by flow cytometry for each treatment of biological replicates. Spearman correlation is indicated.
(C) Histogram depicting the distribution of MFI fold change, compared to a DMSO control, for all tested inhibitors. The blue dashed line shows the control sample (FC = 1), and the red dashed line pinpoints the 90th percentile.
(D) Flow cytometry analysis of GFP levels for a representative inhibitor, trichostatin A, whose FC MFI lies within the 90th percentile, compared to the control sample and uninfected cells.
(E) A pie chart showing the distribution of drug targets of the inhibitors in the 90th percentile for FC MFI.
(F) RNA-seq was performed in duplicates on HCMV-infected CD14+ monocytes treated with 20 different inhibitors and control. Barplot showing fold change of percent viral reads for each treatment. Mean and SD of duplicates are shown. Red line marks fold change of 1. p values representing significant increase in viral gene expression were calculated with t test: ∗∗∗ p value ≤ 0.001, ∗∗ p value ≤ 0.01, ∗ p value ≤ 0.05, n.s. p value > 0.05.
Since the GFP we used in our screen was driven by an exogenous promoter, we next verified that the epigenetic drugs identified in our screen based on GFP expression indeed correspond to the elevation in the expression of additional viral genes. To this end, we performed 3′ end RNA sequencing and analyzed the transcriptome of infected CD14+ monocytes following treatment with 20 drugs from the screen, most with biological replicates (Table S1C). The drugs that were used included the top 10% hits of our flow cytometry-based screen as well as several drugs from the top 20% hits that fall into additional functional categories. Reassuringly, treatment with most of the inhibitors elicited significant increase in total viral gene expression compared to the control (Figure 5F), with trichostatin A and Cay10398, two HDAC inhibitors, and mirin, an ATM pathway inhibitor inducing the most significant increases in viral gene expression (13.6-fold, 5.5-fold, and 10.3-fold correspondingly).
Transcription induction in infected monocytes reveals that the hallmark of HCMV latency is distinctive repression of IE genes
To examine which viral genes were affected by each of these drugs, we compared the gene expression profiles of HCMV-infected monocytes treated with epigenetic drugs to the infected control. Importantly, viral gene expression following all drug treatments, regardless of their specific target and the levels of viral gene induction, was highly correlated with the infected control sample, and statistical analysis showed almost no viral genes that were distinctively induced by each of these drugs (Figure 6A). This overall resemblance in viral gene expression between control latently infected monocytes and latent monocytes treated with diverse drugs that work by different mechanisms points to two critical conclusions: (1) Although there are substantial amounts of input RNA upon infection, viral RNA expression at 72 hpi mostly reflects low-level transcription that can be further enhanced. (2) Upon latent infection of monocytes, viral gene expression is repressed, and this repression is largely consistent throughout the viral genome, so treatment with diverse chromatin modifiers results in a uniformly enhanced expression profile.
Figure 6.
Unique repression of IE genes in HCMV-infected CD14+ monocytes
(A) Read number for viral genes in HCMV-infected CD14+ monocytes treated with DMSO as control versus treatment with 10 different inhibitors that elicited the strongest increase in viral gene expression. Spearman correlations are indicated. The red line marks 1:1 ratio. Only in the mirin sample, two viral genes (RNA5.0 and RL1) significantly deviated (p value <= 0.05 and FC => 2) from the correlation.
(B) Boxplot showing transcript level FC of viral genes from each TC in inhibitor-treated HCMV-infected CD14+ monocytes. p value calculated by t test comparing TC1 and all other TCs is indicated.
(C–E) RNA-seq was performed on HCMV-infected CD14+ monocytes treated with TSA and DMSO control, in biological replicates, at 0, 4, 12, and 24 h after treatment.
(C) Percentage of HCMV reads out of total mRNA reads at different time points following treatment. Error bars represent SD. p values were calculated with t test. ∗p value ≤ 0.05, ∗∗p value ≤ 0.01, ∗∗∗p value ≤ 0.001.
(D) Read number of viral genes in HCMV-infected CD14+ monocytes treated with TSA at 0, 4, 12, and 24 h after treatment, compared to control. Spearman correlations are indicated. The red line marks 1:1 ratio.
(E) Boxplot showing the transcript level FC of viral genes from each temporal class (TC) between TSA (0, 4, 12, 24 h) and infected control. p value = 0.0011 calculated by t test comparing TC1 and all other TCs at 24 h following treatment is indicated.
Since viral gene expression is overall very low (between 0.4% and 4% of expressed mRNAs are viral), small but significant changes in a limited number of genes might be difficult to detect and quantify. Therefore, we also analyzed the changes in expression of the TCs across the different drugs. This pooled analysis revealed an increase of all TCs (Figure 6B), but interestingly, a common signature of most drugs was the relatively low induction of TC1 expression (IE genes) (Figure S4), and this effect was statistically significant when analyzed across all drugs (Figure 6B). This finding indicates that a defining property of the latent transcriptome may be unique repression of IE transcripts that differs from the general repression of all other viral genes.
To verify that the low induction of TC1 genes is not related to the time point post drug treatment that we analyzed, we performed gene expression analysis at different time points after TSA treatment. We focused on this drug because it led to the most significant increase in viral gene expression, thereby providing us with a maximal dynamic range. 48 hpi, CD14+ monocytes were treated with TSA or DMSO as a control. Cells were harvested in replicates for RNA-seq at 0, 4, 12, and 24 h post TSA treatment. We observed a small (1.2-fold) but significant increase in viral expression already at 4 h post TSA treatment, and the induction in viral gene expression increased with time, reaching 14-fold by 24 h post treatment (Figures 6C and Table S1D). In agreement with our previous observations, viral gene expression patterns in infected monocytes treated with TSA were highly correlated with the infected control samples at all time points post treatment (Figure 6D). These measurements illustrate that also in the early time points post treatment, TSA does not lead to dynamic changes in viral gene expression but rather to enhancement of the same viral transcription profile that exists in latently infected monocytes. We next grouped viral transcripts according to their TC and analyzed the induction of different TC gene groups across the time points. Across all time points, expression of viral genes from all TCs increase. However, at 24 h post TSA treatment, when viral gene induction was the strongest, expression of TC1 genes was induced to a significantly less extent than genes from other TCs (p value < 0.001; Figure 6E). This analysis shows that the induction of viral gene expression in infected monocytes by epigenetic modifiers is associated with the relative unresponsiveness of IE genes, indicating an inherent difference in the repression or activation mechanism of IE genes compared with other viral genes during latent infection. As has been previously demonstrated (Danaher et al., 2005), we found that TSA, which also caused the highest induction of viral RNA expression, was able to eventually lead to reactivation from latency in a low proportion of infected monocytes (Figure S5). Overall, these results demonstrate that diverse epigenetic drugs lead to a broad increase in viral gene expression with weaker induction of IE genes, which appear to be under additional repressive regulation in latent cells. Nevertheless, when viral genes and IE genes are induced beyond a certain threshold, the virus can still reactivate.
HCMV gene expression induction in latent monocytes coincides with reduction in the expression of immune-related genes
To further probe the effects of these diverse epigenetic drugs, we analyzed the host gene expression landscapes following treatment with epigenetic modifiers. Gene set enrichment analysis (GSEA) shows that compared to infected control cells, the drug-treated infected monocytes (in which viral gene expression was induced by more than 2-fold) display significant reduction in many immune response-related pathways. These include TNFa signaling, IFN response, and inflammatory response as well as reduction in pathways related to cell cycle progression and apoptosis (Figure 7A). Remarkably, although the drugs target diverse chromatin modifiers, the changes in host gene expression are common between the different treatments. Monocytes that were treated with BSI201, which did not lead to a significant increase in viral gene expression, showed only marginal reduction in a limited number of these pathways (Figure 7A). Common changes in cellular gene expression likely reflect secondary effects that relate to the increase in viral RNA or protein expression. Furthermore, we observed an inverse correlation between the increase in viral gene expression and the reduction in host gene immune pathways such as IFNa/g responses and TNFa signaling (Figure 7B), supporting the notion that these changes are most likely driven by the increase in viral gene expression and not directly by the epigenetic drugs. Finally, we examined the kinetics of changes in host gene expression by analyzing the effects of TSA across the different time points (Figure S6). This analysis revealed that downregulation of IFNa and IFNg pathways coincides with the increase in viral gene expression (Figure 7C), further indicating that the enhancement in viral gene expression accounts for a major portion of the changes in cellular gene expression.
Figure 7.
Induction of viral gene expression in latent cells coincides with reduced expression of immune-related genes
(A) Enriched human hallmark pathways that are downregulated following inhibitor treatment of infected CD14+ monocytes for drugs that significantly induced more than 1.8-FC in viral gene expression. BSI201 (blue), did not produce a significant increase in viral gene expression.
(B) Enrichment scores for three host pathways: interferon alpha response (IFNa, red), interferon gamma response (IFNg, green) and TNF alpha signaling via NFKb (blue) versus FC (fold change) in viral transcript levels for different inhibitor treatments. Spearman correlations are indicated.
(C) Boxplot showing log2FC of transcript level of viral genes and IFNa/g induced host genes in different time points in TSA-treated samples.
Discussion
Using dense RNA-seq measurements, we discovered that in contrast to the traditional view of herpesvirus gene expression, the dependency of viral transcripts on metabolic drugs and viral transcripts’ temporal dynamics are often decoupled, implying that expression kinetics and the dependency on protein synthesis or viral DNA replication are frequently two independent properties in viral gene expression regulation. A recent study on alpha herpesvirus, varicella-zoster virus (VZV), which mapped VZV transcripts, also examined their expression kinetics and observed unexpected patterns of gene expression (Braspenning et al., 2020). In addition to the traditional IE, E, and L kinetic classes, they defined groups of genes as early-late and transactivated/true-late (TA/TL) (Braspenning et al., 2020). In their analysis, early-late genes show two waves of expression, one of which is dependent on de novo protein synthesis and a second, later wave of expression that depends on the onset of DNA replication. These genes are comparable to the HCMV genes characterized here in TC4, which we also name early-late. TA/TL genes correspond in expression dynamics to our TC6: late-translation-independent gene cluster. These genes are expressed in the absence of de novo protein production, but their predominant expression occurs at late time points post infection and is highly dependent on viral DNA replication. The authors hypothesized that the production of these transcripts in CHX-treated samples might be due to low-level transactivation by viral tegument proteins delivered with incoming virions. Whether our TC6 genes can be expressed independently of viral proteins or whether they in fact depend on incoming viral tegument proteins is an important question that warrants further research. Regardless, the ability of these genes to be expressed when infection starts suggests they play an additional important role in the early stages of infection or for reactivation.
More globally, in the herpesvirus literature, it has become commonplace to use the terms “leaky late” and “true late” to distinguish between genes whose expression is augmented by versus totally dependent on DNA replication, respectively. This terminology has hinted toward a more intricate expression regulation than initially presumed. The resemblance between the expression patterns we uncover here to the ones described for VZV (Braspenning et al., 2020) strongly points to complex expression regulation across diverse herpesviruses. We therefore propose that the clusters we describe, which are based on both regulation and temporal expression, may better capture the multiple modules herpesviruses utilize to tightly regulate their gene expression. A deeper understanding of herpesvirus gene regulation will be needed to decipher the connectivity between these different expression modules and how they functionally come into play throughout productive infection.
In order to clearly distinguish newly synthesized mRNAs from background input RNA noise during HCMV latency and to unambiguously determine transcription patterns at early time points during latency establishment, it would be necessary to perform metabolic labeling that enables the quantification of newly synthesized RNA species (Azarkh et al., 2011; Erhard et al., 2019; Marcinowski et al., 2012). However, since the levels of viral gene expression are incredibly low and metabolic labeling approaches facilitate labeling of only a small portion of the newly synthesized RNA pool, conducting these experiments will be technically challenging. Utilizing a combined approach between metabolic labeling and targeted enrichment (Cheng et al., 2017; Depledge et al., 2012) of viral genes could offer an interesting solution for studying low-abundance latent transcription.
We screened a wide array of chromatin modifiers during latency and tested their effects on viral gene expression, and we revealed compounds from several categories that enhanced viral gene expression, including inhibitors of HDACs, sirtuins, and DNA damage response, which were previously implicated in regulation of viral gene expression during herpesvirus latency (Groves et al., 2021; Hopcraft et al., 2018; Hu et al., 2019; Koyuncu et al., 2014; Ren et al., 2016). Although these drugs work by diverse mechanisms, causing different changes to the chromatin, characterization of the viral gene expression enhanced by these drugs uncovered induction of the same transcriptional program. Importantly, the only distinctive group was TC1-IE genes; induction of IE genes was significantly less prominent than the induction of viral genes from all other TC gene clusters. This implies that repression along the HCMV genome in latent cells is uniform with an additional and unique repression of IE genes. This unique regulation may still rely on epigenetics, but it likely involves additional mechanisms. The regulation of IE1 expression by PML-NB proteins in non-permissive cells has been studied by multiple groups with contradicting results (Saffert and Kalejta, 2007; Groves and Sinclair, 2007; Wagenknecht et al., 2015). We speculate that the absence of a particular transcription factor or, as recently suggested, the differing occupancy of polymerase II at the MIEP may be involved (Forte et al., 2021; Reeves et al., 2006). The unique downregulation of IE genes is well established in the context of lytic infection, where it was shown that repression is mediated through binding of a cis repression sequence and was thought to be important during viral replication (Cherrington et al., 1991). Our results suggest that in the context of latent infection, a similar or complementary regulatory mechanism might also exist in monocytes. The results from our epigenetic drug screen can also be interpreted to support the “stochastic transcription hypothesis,” which was shown to play a role in MCMV latency and proposes that viral genes become transiently de-silenced in latent viral genomes in a stochastic fashion and not following the canonical temporal cascade of reactivation (Seckert et al., 2012; Griessl et al., 2021). Enhanced, broad viral transcription by epigenetic drug treatments can eventually lead to reactivation of latent cells, and this may occur once IE gene levels surpass a given threshold.
When analyzing host gene expression after treatment with our diverse set of drugs, we observed recurrent downregulation of the host innate immune response pathway. In agreement with our previous findings (Shnayder et al., 2020), this indicates that viral gene expression during latent infection does have functional consequences and suggests a constant arms race between viral mRNA or viral protein expression and expression of host innate immune response genes during infection and latency (Linderman et al., 2017). In summary, our findings suggest that herpesvirus temporal gene expression cascade is dictated by a more intricate set of dependencies than was previously appreciated and that as envisioned for many years, the trademark of gene expression during HCMV latency is the unique repression of IE genes.
Limitations of the study
Although HCMV can infect many cell types, it has a limited host range and only infects humans. There are no high-quality in vivo models for researching HCMV latency. A major limitation of our research is the challenging in vitro system for investigating HCMV latency. Primary CD14+ monocytes are difficult to maintain in culture and produce extremely low levels of viral genes. This restricts our ability to analyze the dynamics of specific viral transcripts throughout the establishment of latency. However, using epigenetic drug treatments, we were able to obtain more robust measurements to determine that IE genes have an additional repressive regulation compared to all other viral genes during latency. In this study, we have assumed that the inhibitors used target the pathways indicated but off-target effects could also be relevant for increasing the viral gene expression observed in our results. Future work should seek to identify the regulatory mechanism of IE suppression during latency and aim to understand the physiological relevance of these findings for reactivation in immunocompromised individuals.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| HCMV TB40E-GFP | Gift from Eain A. Murphy (Upstate Medical University, Syracuse, New York) | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| T4 PNK | NEB | M0202 |
| SUPERase inhibitor | Thermo FIsher scientific | AM2696 |
| ActinomycinD | Sigma Aldrich | A1410 |
| DMEM | Sigma Aldrich | 01-052-1A |
| X-VIVO 15 Serum-free cell medium | Lonza | BE02-060F |
| Pen-strep solution | Biological Industries | 03-031-1B |
| Phosphonoformate | Sigma Aldrich | P6801 |
| Cycloheximide | Sigma Aldrich | C7698 |
| Fetal bovine Serum | gibco | 10270-106 |
| Affinity Script RT | Agilent | 600107-51 |
| Exonuclease I | NEB | M0293L |
| Second Strand cDNA Synthesis | NEB | E6112A |
| T7 RNA Pol Mix | NEB | M0255A |
| Turbo DNase I | Ambion | AM2222 |
| RNA fragmentation reagent 10x Zn2+ | Thermo Fisher scientific | AM8740 |
| T4 RNA ligase I | NEB | M0204S |
| EDTA, pH 8.0 | Sigma Aldrich | E7889-100ML |
| Superscript III enzyme | Invitrogen | 1973470 |
| Kapa HiFi MasterMix | Kapa biosystem | KM2605 |
| SYBR Green PCR master-mix | Applied Biosystems | 43-444-63 |
| FastAP | Thermo Scientific | 00794853 |
| Tri-Reagent | Sigma Aldrich | T9424-100ML |
| 96 well- Epigenetics Screening Library | Caymen Chemical | Item No. 11076 |
| L-glutamine Solution | Satorius | 03-020-1B |
| Lymphoprep | StemCell Technologies | 07851 |
| CD14 microbeads, human | Miltenyi Biotech | 130-050-201 |
| Critical Commercial Assays | ||
| Dynabeads mRNA direct purification kit | Thermo Fisher scientific | 61011 |
| qScript cDNA Synthesis Kit | Quanta Biosciences | 95049-100 |
| Deposited data | ||
| Next Generation sequencing data | GEO | GSE193467 |
| Experimental Models: Cell Lines | ||
| Human foreskin fibroblasts | ATCC | CRL-1634 |
| Oligonucleotides | ||
| UL123 - (TCCCGCTTATCCTCAGGTACA, TGAGCCTTTCGAGGACATGAA) | Sigma Aldrich | N/A |
| UL84 - (TCAAAGCATACGCTGAATCG, GCTTACAGTCTTGCGGTTCC) | Sigma Aldrich | N/A |
| UL83 - (CCAGCGTGACGTGCATAAAG, TCGTGTTTCCCACCAAGGAC) | Sigma Aldrich | N/A |
| UL44 - (AGCAAGGACCTGACCAAGTT, GCCGAGCTGAACTCCATATT) | Sigma Aldrich | N/A |
| UL99 - (GGGAGGATGACGATAACGAG, TGCCGCTACTACTGTCGTTT) | Sigma Aldrich | N/A |
| UL32 - (GGTTTCTGGCTCGTGGATGTCG, CACACAACACCGTCGTCCGATTAC) | Sigma Aldrich | N/A |
| RNA 2.7 - (TCCTACCTACCACGAATCGC, GTTGGGAATCGTCGACTTTG) | Sigma Aldrich | N/A |
| B2M - (TGCTGTCTCCATGTTTGATGTATCT, TCTCTGCTCCCCACCTCTAA | Sigma Aldrich | N/A |
| Software and Algorithms | ||
| Bowtie 1.1.2 | Langmead et al., 2009 | http://bowtie-bio.sourceforge.net/manual.shtml |
| FlowJo | TreeStar | Version 8 |
| Adobe illustrator | Adobe | 2021 |
| R 4.1.0 | N/A | https://www.r-project.org/ |
| Python 3.7.0 | N/A | https://python.org/ |
| DESeq2 (R package) | Love et al., 2014 | https://github.com/mikelove/DESeq2 |
| GSEA 4.0.3 | Subramanian et al., 2005 | https://www.gsea-msigdb.org/ |
Resource availability
Lead contact
Further information and requests for resources and reagents may be directed to and will be fulfilled by the lead contact, Noam Stern-Ginossar (noam.stern-ginossar@weizmann.ac.il).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Cells lines
Human foreskin fibroblasts (ATCC CRL-1634) were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, and 100 units/mL penicillin and streptomycin (Beit-Haemek, Israel) and maintained at 37°C in a 5% CO2 incubator.
Primary cell culture
Primary CD14+ monocytes were isolated from fresh venous blood, obtained from healthy human donors, using a Lymphoprep (StemCell Technologies) density gradient followed by magnetically activated cell sorting with CD14 magnetic beads (Miltenyi Biotec). CD14+ cells were cultured in X-Vivo 15 medium (Lonza) supplemented with 2.25 mM L-glutamine and maintained at 37°C in a 5% CO2 incubator.
Virus strains
The bacterial artificial chromosome (BAC) containing the clinical strain TB40E (Cobbs et al., 2014) with an SV40-GFP tag (TB40E-GFP) was described previously (O’Connor and Murphy, 2012; Sinzger et al., 2008). This strain lacks the US2-US6 region; therefore, these genes were not included in our analysis. Virus was propagated by adenofection of infectious BAC DNA into fibroblasts (Borst and Messerle, 2005). Viral stocks were concentrated by ultracentrifugation at 30,000xg at 4°C for 120 min. Infectious virus yields were assayed on fibroblasts and THP1 cells.
Method details
Infection procedures
For the time course analysis fibroblasts and CD14+ monocytes were infected with the same stock of HCMV strain TB40E-GFP at a multiplicity of infection (MOI) of 1 and 10, respectively. For all other experiments CD14+ monocytes were infected with HCMV strain TB40E-GFP at an MOI of 5. Cells were incubated with the virus for 2 h, washed, and supplemented with fresh medium. Infection was monitored 2–3 dpi by measurement of GFP expression on a BD accuri flow cytometer and analysis by FlowJo. Substantial shift in GFP levels, seen in most infected fibroblasts is indicative of productive infection, while a small shift in GFP levels, as seen in all CD14+ monocytes indicates that the cells were infected but the virus is repressed, as expected in latent infection. Latent monocytes were monitored to ensure no production of viral titers.
Cell treatments
CHX was added to infected fibroblasts and CD14+ monocytes immediately after infection at a final concentration of 100 ug/mL and 200 ug/mL (fibroblasts) or at 100 ug/mL (monocytes). PFA was added to fibroblasts and CD14 + monocytes immediately after infection at a final concentration of 400 ug/mL. ActD was added to infected fibroblasts at a final concentration of 5uM.
Inhibitor screen
The compounds used in the epigenetic inhibitor screen were purchased from Cayman Chemical as a complete Epigenetics Screening Library (96-Well, Item No. 11076). To calibrate cytotoxicity, HCMV-infected CD14+ monocytes were treated with six inhibitors at increasing concentrations (500 nM,1uM,10uM) for 48 h. Cells were washed, resuspended in PBS and stained with 0.5ug/mL of PI for 10 min prior to analysis by FACs- LSRII. The 1 M concentration was chosen for the complete screen as it caused minimal cytotoxicity. For the inhibitor screen at 48 hpi, TB40-infected CD14+ monocytes were divided into 1.2 mL tubes containing ∼100,000 cells per tube and inhibitors or DMSO for negative control were added to a final concentration of 1uM. After 24 h of incubation, Flow cytometry was performed using an LSRII, and analysis was performed using FlowJo software.
RNA library construction
For RNA-seq time course experiments in fibroblasts and CD14+ monocytes, cells were washed with PBS and then collected with Tri-Reagent (Sigma-Aldrich), total RNA was extracted by phase separation and poly(A) selection was performed using Dynabeads mRNA DIRECT Purification Kit (Invitrogen) according to the manufacturer’s protocol. RNA-seq libraries were generated as previously described (Shishkin et al., 2015). mRNA samples of ∼4 ng were subjected to DNaseI treatment and 3′ dephosphorylation using FastAP Thermosensitive Alkaline Phosphatase (Thermo Scientific) and T4 PNK (NEB) followed by 3′ adaptor ligation using T4 ligase (NEB). The ligated products were used for reverse transcription with SSIII (Invitrogen) for first-strand cDNA synthesis. The cDNA products were 3′ ligated with a second adaptor using T4 ligase and amplified with 8 cycles of PCR for final library products of 200–300 base pairs. For epigenetic drug-treated CD14+ monocyte samples, RNA libraries were generated according to the low input optimized 3′ end sequencing, MARS-seq protocol (Keren-Shaul et al., 2019). RNA was extracted directly from lysates by poly(A) selection using Dynabeads mRNA DIRECT Purification Kit (Invitrogen) according to the manufacturer’s protocol. 3′ ends of mRNAs were annealed to unique molecular identifiers (UMI) containing a T7 promoter. The mRNA was reverse transcribed by Affinity Script RT (Agilent) to generate the first cDNA strand and treated with exonuclease I to remove leftover RT primers. Next, the single stranded cDNA was converted to double-stranded cDNA using SSS master mix (NEB). The DNA strands were in vitro transcribed for linear amplification using T7 RNA Pol mix (NEB) and treated with Turbo DNase I (Ambion) to remove leftover DNA template in the mixture. The RNA strands were fragmented and annealed to sequencing adapters, followed by another round of RT to generate barcoded cDNA libraries that are ready for sequencing.
Quantitative real-time PCR
For analysis of RNA expression, total RNA was extracted using Tri-Reagent (Sigma) according to the manufacturer’s protocol. cDNA was prepared using the qScript cDNA Synthesis Kit (Quanta Biosciences) according to the manufacturer’s protocol. Real-time PCR was performed using the SYBR Green PCR master-mix (ABI) on the QuantStudio 12K Flex (ABI) with primers ordered from Sigma-Aldrich Israel Ltd. and listed in the Key Resources Table.
Quantification and statistical analysis
All computations and statistical analyses were carried out in the R computing environment. For boxplots appearing in this study, box boundaries represent interquartile ranges, whiskers extend to the most extreme data point which is no more than 1.5 times the interquartile range, and the line in the middle of the box represents the median. RNA expression data were log(x+1) transformed, where x represents the gene expression counts. Mean with SD values are reported in the figures and figure legends foe qPCR experiments. p values were calculated using log transformed expression data in a two-tailed student’s t-test in R with the built in function using default parameters (paired = F, var.equal = F). The following symbols are used to denote significance in the figures and figure legends: ∗∗∗ pval≤0.001, ∗∗ pval≤0.01, ∗ pval≤0.05, n.s. pval>0.05.
Next-generation sequencing alignments
All RNA-Seq libraries (pooled at equimolar concentration) were sequenced using Novaseq6000 (Illumina), with read parameters: Read1: 72 cycles and Read2: 15 cycles.
For time course libraries, raw sequences were first trimmed at their 3′ end, removing the illumina adapter and polyA tail. Alignment was performed using Bowtie (Langmead et al., 2009) (allowing up to 2 mismatches) and reads were aligned to concatenation of the human (hg19) and the viral genomes (NCBI EF999921.1). Reads aligned to ribosomal RNA were removed. Reads that were not aligned to the genome were then aligned to the transcriptome.
For the epigenetic drug screen libraries generated with the MARS-seq protocol, 37-bp reads were aligned using Bowtie (allowing up to 2 mismatches) to concatenation of the human (hg19) and the viral genomes (NCBI EF999921.1). Counting of reads per gene was done based on unique molecular identifiers (UMIs) (8 bp). The transcription units of the virus were based on NCBI annotations, with some changes, including merging several transcripts (considering that the library maps only the 3′ ends of transcripts). All analyses and figures were done using in-house R-scripts. Epigenetic drug treated CD14+ monocyte samples which had less than 100,000 UMIs were left out from further analysis leaving three drug treatments without biological duplicates- Cay10398, CPTH2, and Gemcitabine.
Differential expression and enrichment analysis
Differential expression analysis was done with DESeq2 (version 1.22.2) (Love et al., 2014) using default parameters, with the number of reads in each of the samples as an input. The normalized number of reads according to DESeq2 were used for enrichment analysis using GSEA (version 4.0.3) (Subramanian et al., 2005). The MSigDB hallmark (version 7.1) gene sets were used (Liberzon et al., 2011). The GSEA plots were created based on the GSEA output with in-house R scripts.
Acknowledgments
We thank Stern-Ginossar lab members, Oren Kobiler, and Igor Ulitsky for providing valuable feedback. We thank Eain A. Murphy for the TB40E-GFP virus strain. We thank the Weizmann flow cytometry unit for technical assistance. This study was supported by a European Research Council consolidator grant (CoG-2019-864012) and by the Israel Science Foundation (1526/18). N.S.-G. is a member of the European Molecular Biology Organization (EMBO) Young Investigator Program.
Author contributions
B.R., M.S., and N.S.-G. conceived and designed the project. B.R., M.S., R.L.S., and M.L. performed the experiments. B.R. and A.N. prepared figures and performed all bioinformatics and statistical analyses. B.R., M.S., and N.S.-G. wrote the manuscript. All authors had access to the study data and read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Published: April 12, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2022.110653.
Contributor Information
Michal Schwartz, Email: michalsc@weizmann.ac.il.
Noam Stern-Ginossar, Email: noam.stern-ginossar@weizmann.ac.il.
Supplemental information
(A) Temporal class of viral genes and their expression levels in RPKM in RNA-seq time course of HCMV-infected fibroblasts, related to Figure 2. (B) RPKM values for viral genes from RNA-seq time course of HCMV-infected monocytes, related to Figures 1 and 4. (C) 3′ end RNA sequencing reads of HCMV-infected CD14+ monocytes treated with diverse epigenetic drugs, screen-based hits, related to Figure 5. (D) RPKM values for all genes (viral and human) from TSA time course, related to Figures 6 and 7.
Data and code availability
-
•
All next-generation sequencing data files were deposited and made publicly available in Gene Expression Omnibus (GEO). Accession numbers are listed in the key resources table.
-
•
This paper does not report original code
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request, Noam Stern-Ginossar (noam.stern-ginossar@weizmann.ac.il)
References
- AuCoin D.P., Smith G.B., Meiering C.D., Mocarski E.S. Betaherpesvirus-conserved cytomegalovirus tegument protein ppUL32 (pp150) controls cytoplasmic events during virion maturation. J. Virol. 2006;80:8199–8210. doi: 10.1128/JVI.00457-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarkh Y., Dölken L., Nagel M., Gilden D., Cohrs R.J. Synthesis and decay of varicella zoster virus transcripts. J. Neurovirol. 2011;17:281–287. doi: 10.1007/s13365-011-0029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigley T.M., Reitsma J.M., Mirza S.P., Terhune S.S. Human cytomegalovirus pUL97 regulates the viral major immediate early promoter by phosphorylation-mediated disruption of histone deacetylase 1 binding. J. Virol. 2013;87:7393–7408. doi: 10.1128/JVI.02825-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst E.-M., Messerle M. Analysis of human cytomegalovirus oriLyt sequence requirements in the context of the viral genome. J. Virol. 2005;79:3615–3626. doi: 10.1128/JVI.79.6.3615-3626.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braspenning S.E., Sadaoka T., Breuer J., Verjans G.M.G.M., Ouwendijk W.J.D., Depledge D.P. Decoding the architecture of the varicella-zoster virus transcriptome. MBio. 2020;11 doi: 10.1128/mBio.01568-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne E.P., Shenk T. Human cytomegalovirus UL83-coded pp65 virion protein inhibits antiviral gene expression in infected cells. Proc. Natl. Acad. Sci. U S A. 2003;100:11439–11444. doi: 10.1073/pnas.1534570100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J., Angulo A., Amaratunga D., Guo H., Jiang Y., Wan J.S., Bittner A., Frueh K., Jackson M.R., Peterson P.A., et al. DNA microarrays of the complex human cytomegalovirus genome: profiling kinetic class with drug sensitivity of viral gene expression. J. Virol. 1999;73:5757–5766. doi: 10.1128/jvi.73.7.5757-5766.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S., Caviness K., Buehler J., Smithey M., Nikolich-Žugich J., Goodrum F. Transcriptome-wide characterization of human cytomegalovirus in natural infection and experimental latency. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E10586–E10595. doi: 10.1073/pnas.1710522114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington J.M., Khoury E.L., Mocarski E.S. Human cytomegalovirus ie2 negatively regulates alpha gene expression via a short target sequence near the transcription start site. J. Virol. 1991;65:887–896. doi: 10.1128/jvi.65.2.887-896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbs C.S., Matlaf L., Harkins L.E. Methods for the detection of cytomegalovirus in glioblastoma cells and tissues. Methods Mol. Biol. 2014;1119:165–196. doi: 10.1007/978-1-62703-788-4_11. [DOI] [PubMed] [Google Scholar]
- Collins-McMillen D., Rak M., Buehler J.C., Igarashi-Hayes S., Kamil J.P., Moorman N.J., Goodrum F. Alternative promoters drive human cytomegalovirus reactivation from latency. Proc. Natl. Acad. Sci. U. S. A. 2019;116:17492–17497. doi: 10.1073/pnas.1900783116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crough T., Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin. Microbiol. Rev. 2009;22:76–98. doi: 10.1128/CMR.00034-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaher R.J., Jacob R.J., Steiner M.R., Allen W.R., Hill J.M., Miller C.S. Histone deacetylase inhibitors induce reactivation of herpes simplex virus type 1 in a latency-associated transcript–independent manner in neuronal cells. J. Neurovirol. 2005;11:306–317. doi: 10.1080/13550280590952817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarchi J.M., Schmidt C.A., Kaplan A.S. Patterns of transcription of human cytomegalovirus in permissively infected cells. J. Virol. 1980;35:277–286. doi: 10.1128/jvi.35.2.277-286.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depledge D.P., Palser A.L., Watson S.J., Lai I.Y.-C., Gray E.R., Grant P., Kanda R.K., Leproust E., Kellam P., Breuer J. Correction: specific capture and whole-genome sequencing of viruses from clinical samples. PLoS One. 2012;7 doi: 10.1371/journal.pone.0027805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhard F., Baptista M.A.P., Krammer T., Hennig T., Lange M., Arampatzi P., Jürges C.S., Theis F.J., Saliba A.-E., Dölken L. scSLAM-seq reveals core features of transcription dynamics in single cells. Nature. 2019;571:419–423. doi: 10.1038/s41586-019-1369-y. [DOI] [PubMed] [Google Scholar]
- Forte E., Butun F.A., Marinaccio C., Schipma M.J., Piunti A., Schroeder M.W., Kandpal M., Shilatifard A., Abecassis M., Hummel M. Epigenetic reprogramming of host and viral genes by human cytomegalovirus infection in kasumi-3 myeloid progenitor cells at early times postinfection. J. Virol. 2021;95 doi: 10.1128/JVI.00183-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatherer D., Seirafian S., Cunningham C., Holton M., Dargan D.J., Baluchova K., Hector R.D., Galbraith J., Herzyk P., Wilkinson G.W.G., et al. High-resolution human cytomegalovirus transcriptome. Proc. Natl. Acad. Sci. U. S. A. 2011;108:19755–19760. doi: 10.1073/pnas.1115861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W. Structure and assembly of the virion. Intervirology. 1996;39:389–400. doi: 10.1159/000150509. [DOI] [PubMed] [Google Scholar]
- Griessl M., Renzaho A., Freitag K., Seckert C.K., Reddehase M.J., Lemmermann N.A.W. Stochastic episodes of latent cytomegalovirus transcription drive CD8 T-cell “memory inflation” and avoid immune evasion. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.668885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves I.J., Sinclair J.H. Knockdown of hDaxx in normally non-permissive undifferentiated cells does not permit human cytomegalovirus immediate-early gene expression. J. Gen. Virol. 2007;88:2935–2940. doi: 10.1099/vir.0.83019-0. [DOI] [PubMed] [Google Scholar]
- Groves I.J., Jackson S.E., Poole E.L., Nachshon A., Rozman B., Schwartz M., Prinjha R.K., Tough D.F., Sinclair J.H., Wills M.R. Bromodomain proteins regulate human cytomegalovirus latency and reactivation allowing epigenetic therapeutic intervention. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2023025118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., Gao S.-J. A novel role of SIRT1 in gammaherpesvirus latency and replication. Cell Cycle. 2014;13:3328–3330. doi: 10.4161/15384101.2014.968431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopcraft S.E., Pattenden S.G., James L.I., Frye S., Dittmer D.P., Damania B. Chromatin remodeling controls Kaposi’s sarcoma-associated herpesvirus reactivation from latency. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H.-L., Shiflett L.A., Kobayashi M., Chao M.V., Wilson A.C., Mohr I., Huang T.T. TOP2β-Dependent nuclear DNA damage shapes extracellular growth factor responses via dynamic AKT phosphorylation to control virus latency. Mol. Cell. 2019;74:466–480.e4. doi: 10.1016/j.molcel.2019.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren-Shaul H., Kenigsberg E., Jaitin D.A., David E., Paul F., Tanay A., Amit I. MARS-seq2.0: an experimental and analytical pipeline for indexed sorting combined with single-cell RNA sequencing. Nat. Protoc. 2019;14:1841–1862. doi: 10.1038/s41596-019-0164-4. [DOI] [PubMed] [Google Scholar]
- Knipe D.M., Howley P.M., Griffin D.E., Lamb R.A., Martin M.A., Roizman B., Straus S.E., Rubin D.H. Fourth Edition. Volumes I and II. Lippincott Williams And Wilkins, Philadelphia (2001); 2002. Fundamental Virology; pp. 1029–1030. (Fields Virology). [Google Scholar]
- Koyuncu E., Budayeva H.G., Miteva Y.V., Ricci D.P., Silhavy T.J., Shenk T., Cristea I.M. Sirtuins are evolutionarily conserved viral restriction factors. MBio. 2014;5 doi: 10.1128/mBio.02249-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna B.A., Lau B., Jackson S.E., Wills M.R., Sinclair J.H., Poole E. Transient activation of human cytomegalovirus lytic gene expression during latency allows cytotoxic T cell killing of latently infected cells. Sci. Rep. 2016;6:24674. doi: 10.1038/srep24674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., Trapnell C., Pop M., Salzberg S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon A., Subramanian A., Pinchback R., Thorvaldsdóttir H., Tamayo P., Mesirov J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–1740. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderman J.A., Kobayashi M., Rayannavar V., Fak J.J., Darnell R.B., Chao M.V., Wilson A.C., Mohr I. Immune escape via a transient gene expression program enables productive replication of a latent pathogen. Cell Rep. 2017;18:1312–1323. doi: 10.1016/j.celrep.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinowski L., Lidschreiber M., Windhager L., Rieder M., Bosse J.B., Rädle B., Bonfert T., Györy I., de Graaf M., Prazeres da Costa O., et al. Real-time transcriptional profiling of cellular and viral gene expression during lytic cytomegalovirus infection. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough S.H., Spector D.H. Transcription in human fibroblasts permissively infected by human cytomegalovirus strain AD169. Virology. 1983;125:31–46. doi: 10.1016/0042-6822(83)90061-2. [DOI] [PubMed] [Google Scholar]
- McWatters B.J.P., Stenberg R.M., Kerry J.A. Characterization of the human cytomegalovirus UL75 (glycoprotein H) late gene promoter. Virology. 2002;303:309–316. doi: 10.1006/viro.2002.1614. [DOI] [PubMed] [Google Scholar]
- Meier J.L., Stinski M.F. Regulation of human cytomegalovirus immediate-early gene expression. Intervirology. 1996;39:331–342. doi: 10.1159/000150504. [DOI] [PubMed] [Google Scholar]
- Murphy J.C., Fischle W., Verdin E., Sinclair J.H. Control of cytomegalovirus lytic gene expression by histone acetylation. EMBO J. 2002;21:1112–1120. doi: 10.1093/emboj/21.5.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitzsche A., Paulus C., Nevels M. Temporal dynamics of cytomegalovirus chromatin assembly in productively infected human cells. J. Virol. 2008;82:11167–11180. doi: 10.1128/JVI.01218-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor C.M., Murphy E.A. A myeloid progenitor cell line capable of supporting human cytomegalovirus latency and reactivation, resulting in infectious progeny. J. Virol. 2012;86:9854–9865. doi: 10.1128/JVI.01278-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.-J., Kim Y.-E., Pham H.T., Kim E.T., Chung Y.-H., Ahn J.-H. Functional interaction of the human cytomegalovirus IE2 protein with histone deacetylase 2 in infected human fibroblasts. J. Gen. Virol. 2007;88:3214–3223. doi: 10.1099/vir.0.83171-0. [DOI] [PubMed] [Google Scholar]
- Pei Y., Robertson E.S. The Crosstalk of epigenetics and metabolism in herpesvirus infection. Viruses. 2020;12:1377. doi: 10.3390/v12121377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Maidji E., Fisher S.J., McDonagh S., Tabata T. In: HCMV Persistence in the Population: Potential Transplacental Transmission. Arvin A., Campadelli-Fiume G., Mocarski E., Moore P.S., Roizman B., Whitley R., Yamanishi K., editors. Cambridge University Press; 2007. Human herpesviruses: biology, therapy, and immunoprophylaxis. Chapter 45. [PubMed] [Google Scholar]
- Phillips S.L., Bresnahan W.A. The human cytomegalovirus (HCMV) tegument protein UL94 is essential for secondary envelopment of HCMV virions. J. Virol. 2012;86:2523–2532. doi: 10.1128/JVI.06548-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radsak K., Fuhrmann R., Franke R.P., Schneider D., Kollert A., Brücher K.H., Drenckhahn D. Induction by sodium butyrate of cytomegalovirus replication in human endothelial cells. Arch. Virol. 1989;107:151–158. doi: 10.1007/BF01313887. [DOI] [PubMed] [Google Scholar]
- Reeves M.B. Chromatin-mediated regulation of cytomegalovirus gene expression. Virus Res. 2011;157:134–143. doi: 10.1016/j.virusres.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves M.B., Sinclair J.H. Analysis of latent viral gene expression in natural and experimental latency models of human cytomegalovirus and its correlation with histone modifications at a latent promoter. J. Gen. Virol. 2010;91:599–604. doi: 10.1099/vir.0.015602-0. [DOI] [PubMed] [Google Scholar]
- Reeves M., Murphy J., Greaves R., Fairley J., Brehm A., Sinclair J. Autorepression of the human cytomegalovirus major immediate-early promoter/enhancer at late times of infection is mediated by the recruitment of chromatin remodeling enzymes by IE86. J. Virol. 2006;80:9998–10009. doi: 10.1128/JVI.01297-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves M.B., MacAry P.A., Lehner P.J., Sissons J.G.P., Sinclair J.H. Latency, chromatin remodeling, and reactivation of human cytomegalovirus in the dendritic cells of healthy carriers. Proc. Natl. Acad. Sci. U. S. A. 2005;102:4140–4145. doi: 10.1073/pnas.0408994102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K., Zhang W., Chen X., Ma Y., Dai Y., Fan Y., Hou Y., Tan R.X., Li E. An epigenetic compound library screen identifies BET inhibitors that promote HSV-1 and -2 replication by bridging P-TEFb to viral gene promoters through BRD4. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffert R.T., Kalejta R.F. Human cytomegalovirus gene expression is silenced by Daxx-mediated intrinsic immune defense in model latent infections established in vitro. J. Virol. 2007;81:9109–9120. doi: 10.1128/JVI.00827-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M., Stern-Ginossar N. The transcriptome of latent human cytomegalovirus. J. Virol. 2019;93 doi: 10.1128/JVI.00047-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckert C.K., Griessl M., Büttner J.K., Scheller S., Simon C.O., Kropp K.A., Renzaho A., Kühnapfel B., Grzimek N.K.A., Reddehase M.J. Viral latency drives “memory inflation”: a unifying hypothesis linking two hallmarks of cytomegalovirus infection. Med. Microbiol. Immunol. 2012;201:551–566. doi: 10.1007/s00430-012-0273-y. [DOI] [PubMed] [Google Scholar]
- Shishkin A.A., Giannoukos G., Kucukural A., Ciulla D., Busby M., Surka C., Chen J., Bhattacharyya R.P., Rudy R.F., Patel M.M., et al. Simultaneous generation of many RNA-seq libraries in a single reaction. Nat. Methods. 2015;12:323–325. doi: 10.1038/nmeth.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnayder M., Nachshon A., Krishna B., Poole E., Boshkov A., Binyamin A., Maza I., Sinclair J., Schwartz M., Stern-Ginossar N. Defining the transcriptional landscape during cytomegalovirus latency with single-cell RNA sequencing. MBio. 2018;9 doi: 10.1128/mBio.00013-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnayder M., Nachshon A., Rozman B., Bernshtein B., Lavi M., Fein N., Poole E., Avdic S., Blyth E., Gottlieb D., et al. Single cell analysis reveals human cytomegalovirus drives latently infected cells towards an anergic-like monocyte state. Elife. 2020;9 doi: 10.7554/eLife.52168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair J. Chromatin structure regulates human cytomegalovirus gene expression during latency, reactivation and lytic infection. Biochim. Biophys. Acta. 2010;1799:286–295. doi: 10.1016/j.bbagrm.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Sinclair J., Sissons P. Latency and reactivation of human cytomegalovirus. J. Gen. Virol. 2006;87:1763–1779. doi: 10.1099/vir.0.81891-0. [DOI] [PubMed] [Google Scholar]
- Sinzger C., Hahn G., Digel M., Katona R., Sampaio K.L., Messerle M., Hengel H., Koszinowski U., Brune W., Adler B. Cloning and sequencing of a highly productive, endotheliotropic virus strain derived from human cytomegalovirus TB40. E. J. Gen. Virol. 2008;89:359–368. doi: 10.1099/vir.0.83286-0. [DOI] [PubMed] [Google Scholar]
- Slobedman B., Cao J.Z., Avdic S., Webster B., McAllery S., Cheung A.K., Tan J.C., Abendroth A. Human cytomegalovirus latent infection and associated viral gene expression. Future Microbiol. 2010;5:883–900. doi: 10.2217/fmb.10.58. [DOI] [PubMed] [Google Scholar]
- Staras S.A.S., Dollard S.C., Radford K.W., Flanders W.D., Pass R.F., Cannon M.J. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin. Infect. Dis. 2006;43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- Stenberg R.M. The human cytomegalovirus major immediate-early gene. Intervirology. 1996;39:343–349. doi: 10.1159/000150505. [DOI] [PubMed] [Google Scholar]
- Stern-Ginossar N., Weisburd B., Michalski A., Le V.T.K., Hein M.Y., Huang S.-X., Ma M., Shen B., Qian S.-B., Hengel H., et al. Decoding human cytomegalovirus. Science. 2012;338:1088–1093. doi: 10.1126/science.1227919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U. S. A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J., Sadanari H., Sato H., Fukuda S. Sodium butyrate-inducible replication of human cytomegalovirus in a human epithelial cell line. Virology. 1991;185:271–280. doi: 10.1016/0042-6822(91)90774-6. [DOI] [PubMed] [Google Scholar]
- Terhune S.S., Schröer J., Shenk T. RNAs are packaged into human cytomegalovirus virions in proportion to their intracellular concentration. J. Virol. 2004;78:10390–10398. doi: 10.1128/JVI.78.19.10390-10398.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum S.M., Streblow D.N., Monroe M.E., Smith P., Auberry K.J., Pasa-Tolic L., Wang D., Camp D.G., 2nd, Rodland K., Wiley S., et al. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J. Virol. 2004;78:10960–10966. doi: 10.1128/JVI.78.20.10960-10966.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenknecht N., Reuter N., Scherer M., Reichel A., Müller R., Stamminger T. Contribution of the major ND10 proteins PML, hDaxx and Sp100 to the regulation of human cytomegalovirus latency and lytic replication in the monocytic cell line THP-1. Viruses. 2015;7:2884–2907. doi: 10.3390/v7062751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M.W., Stinski M.F. Temporal patterns of human cytomegalovirus transcription: mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J. Virol. 1982;41:462–477. doi: 10.1128/jvi.41.2.462-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weekes M.P., Tomasec P., Huttlin E.L., Fielding C.A., Nusinow D., Stanton R.J., Wang E.C.Y., Aicheler R., Murrell I., Wilkinson G.W.G., et al. Quantitative temporal viromics: an approach to investigate host-pathogen interaction. Cell. 2014;157:1460–1472. doi: 10.1016/j.cell.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurochko A.D., Hwang E.S., Rasmussen L., Keay S., Pereira L., Huang E.S. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-kappaB during infection. J. Virol. 1997;71:5051–5059. doi: 10.1128/jvi.71.7.5051-5059.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Temporal class of viral genes and their expression levels in RPKM in RNA-seq time course of HCMV-infected fibroblasts, related to Figure 2. (B) RPKM values for viral genes from RNA-seq time course of HCMV-infected monocytes, related to Figures 1 and 4. (C) 3′ end RNA sequencing reads of HCMV-infected CD14+ monocytes treated with diverse epigenetic drugs, screen-based hits, related to Figure 5. (D) RPKM values for all genes (viral and human) from TSA time course, related to Figures 6 and 7.
Data Availability Statement
-
•
All next-generation sequencing data files were deposited and made publicly available in Gene Expression Omnibus (GEO). Accession numbers are listed in the key resources table.
-
•
This paper does not report original code
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request, Noam Stern-Ginossar (noam.stern-ginossar@weizmann.ac.il)