Abstract
Introduction
Prognosis of patients with COVID-19 depends on the severity of the pulmonary affection. The most severe cases may progress to acute respiratory distress syndrome (ARDS), which is associated with a risk of long-term repercussions on respiratory function and neuromuscular outcomes. The functional repercussions of severe forms of COVID-19 may have a major impact on quality of life, and impair the ability to return to work or exercise. Social inequalities in healthcare may influence prognosis, with socially vulnerable individuals more likely to develop severe forms of disease. We describe here the protocol for a prospective, multicentre study that aims to investigate the influence of social vulnerability on functional recovery in patients who were hospitalised in intensive care for ARDS caused by COVID-19. This study will also include an embedded qualitative study that aims to describe facilitators and barriers to compliance with rehabilitation, describe patients’ health practices and identify social representations of health, disease and care.
Methods and analysis
The "Functional Recovery From Acute Respiratory Distress Syndrome (ARDS) Due to COVID-19: Influence of Socio-Economic Status" (RECOVIDS) study is a mixed-methods, observational, multicentre cohort study performed during the routine follow-up of post-intensive care unit (ICU) functional recovery after ARDS. All patients admitted to a participating ICU for PCR-proven SARS-CoV-2 infection and who underwent chest CT scan at the initial phase AND who received respiratory support (mechanical or not) or high-flow nasal oxygen, AND had ARDS diagnosed by the Berlin criteria will be eligible. The primary outcome is the presence of lung sequelae at 6 months after ICU discharge, defined either by alterations on pulmonary function tests, oxygen desaturation during a standardised 6 min walk test or fibrosis-like pulmonary findings on chest CT. Patients will be considered to be socially disadvantaged if they have an "Evaluation de la Précarité et des Inégalités de santé dans les Centres d’Examen de Santé" (EPICES) score ≥30.17 at inclusion.
Ethics and dissemination
The study protocol and the informed consent form were approved by an independent ethics committee (Comité de Protection des Personnes Sud Méditerranée II) on 10 July 2020 (2020-A02014-35). All patients will provide informed consent before participation. Findings will be published in peer-reviewed journals and presented at national and international congresses.
Trial registration number
Keywords: COVID-19, intensive & critical care, respiratory infections
Strengths and Limitations.
RECOVIDS is an observational study involving a large number of centres in France, and comprising a population of patients with acute respiratory distress syndrome (ARDS) due to COVID-19.
Patients will undergo extensive pulmonary function testing and imaging at 6 and 12 months after ARDS due to COVID-19 to search for persisting lung sequelae.
A potential limitation of this study is that the participating centres may be unable to perform all the planned examinations and follow-up within the specified timeframe, due to the ongoing pandemic, which has profoundly disorganised the delivery of healthcare.
Slow recruitment may affect inclusion capacity, or completion of the full protocol by the patients included.
Introduction
Background and rationale
Since January 2020, the world has been embroiled in a pandemic caused by an emerging new coronavirus, named SARS-CoV-2, and the resulting disease, namely COVID-19. Around 5% of symptomatic patients will require admission to intensive care unit (ICU), representing severe cases of COVID-19, and of these, 50%–70% will need mechanical ventilation.1–4 Mortality in these patients varies between countries and over time, and ranges from 25% to 40% or even 70% according to different studies.1 5
The initial and long-term prognosis of patients suffering from COVID-19 depends on the severity of the pulmonary affection, and in the most severe cases, patients may progress to acute respiratory distress syndrome (ARDS). ARDS is associated with a risk of potential long-term repercussions on respiratory function, but also on neuromuscular outcomes, due to the long duration of the ICU stay.6 The functional repercussions (both physical and psychological) of severe forms of COVID-19 may have a major impact on quality of life (QoL), and impair the ability to return to work or exercise. In addition to comorbidities (eg, age >70 years, cardiovascular disease, diabetes, obesity, chronic respiratory or renal failure or cancer) known to be associated with a higher risk of developing severe forms, social inequalities in healthcare may influence the medium and long-term prognosis, as was shown to be the case during the H1N1 pandemic in 2009.7 Indeed, racial, ethnic and financial disparities have been shown to exist, and the socially disadvantaged are widely affected during the current pandemic, as demonstrated among the population of New York8 and in a large homeless shelter population in Boston.9 In France, similar findings were observed in Seine-Saint-Denis, which is the French department with the highest rate of poverty. In this department, there was a sudden and massive influx of patients with COVID-19 into hospitals in March 2020,10 and the virus spread extremely rapidly due to the very high population density, with overcrowding in many places, and higher rates of multigenerational households. A recent literature review11 highlighted that socioeconomic data are often overlooked, but are crucial to identify the most vulnerable groups.
Objectives
We aim to conduct a prospective, multicentre study to investigate the association between social vulnerability and functional (physical and psychological) recovery in patients who were hospitalised in the ICU for ARDS caused by SARS-CoV-2. We hypothesise that patients with a disadvantaged socioeconomic position will have poorer functional recovery at 6 months after discharge from the ICU than those with a more affluent socioeconomic position.
Primary objective
To evaluate respiratory functional recovery at 6 months after discharge from the ICU after ARDS due to SARS-CoV-2 in patients according to socioeconomic position. Patients will be considered to be socially disadvantaged or not (hereafter termed deprived or non-deprived patients) if they have an "Evaluation de la Précarité et des Inégalités de santé dans les Centres d’Examen de Santé" (EPICES) score ≥30.17 or <30.17 at inclusion, respectively.12 13
Secondary objectives
To describe and compare the modalities of rehabilitation at 6 and 12 months after ICU discharge between those who are socially disadvantaged and those who are not. Rehabilitation refers here to the individualised treatment performed in real conditions with the purpose of improving patients’ functional capacities (respiratory, physical) and QoL. It can vary from specifically dedicated programmes in specialised centre to home physiotherapy.
To investigate the influence of socioeconomic position on respiratory function and the condition of the pulmonary parenchyma at 6 months, and the changes at 12 months in patients found to have impaired respiratory function at 6 months.
To describe the frequency of loss of taste and smell, and the course of this affection at 6 months.
To investigate the influence of socioeconomic position on muscle function, cognitive function, the frequency of symptoms of post-traumatic stress disorder, anxiety and QoL at 6 and 12 months after ICU discharge.
To investigate the influence of socioeconomic position on nutritional status at 6 months.
To investigate the influence of socioeconomic position on mortality at 12 months.
Objective of the qualitative study
This study will also include an embedded qualitative study that aims to identify, describe and categorise the facilitators and barriers to compliance with the rehabilitation programme, patients’ health practices, their social representations of health, disease and care, and their available and mobilisable resources enabling access to healthcare and services (in particular, whether they are influenced by socioeconomic position or not).
Study design
The RECOVIDS is a mixed-methods, observational, multicentre cohort study. It will be performed in the course of the routine care and routine evaluation of post-ICU functional recovery as implemented in France for all patients who suffer from ARDS.
Methods
Study setting
The RECOVIDS study will be performed in 38 centres spread across all of mainland France. The ICUs of participating centres will recruit patients to the study, while the units that perform the routine follow-up of patients with post-ARDS will be in charge of the follow-up consultations for the purposes of this study. These may be post-ICU recovery units, respiratory medicine departments or rehabilitation departments.
The current protocol is reported in compliance with the Standard Protocol Items: Recommendations for Interventional Trials statement.
Eligibility criteria
Inclusion criteria
All patients admitted to an ICU in any of the participating centres for SARS-CoV-2 infection proven by PCR (regardless of the type of sample used) are eligible. To be included, patients must have undergone chest CT scan at the initial phase of management (ie, immediately prior to or during the ICU stay); have received respiratory support (mechanical or not) or high-flow nasal oxygen; and have ARDS diagnosed according to the Berlin 2012 definition.14 For patients who received high-flow nasal oxygen, a flow of at least 50 L/min with FiO2 >50% and a PaO2/FiO2 ratio ≤200 are required for inclusion. All patients must provide oral consent after having been informed about the study.
Exclusion criteria
Patients who meet any one or more of the following criteria will not be included: limited autonomy prior to hospital admission with a walking perimeter <50 m or WHO performance status 3 or 4; a history of chronic respiratory insufficiency defined by the use of long-term oxygen therapy (LTOT) or non-invasive ventilation (NIV) in the home (except for patients with sleep apnoea and/or obesity hypoventilation syndrome (OHS)); a history of central or peripheral neurological disorders limiting motor autonomy and impairing the ability to perform the walk test or pulmonary function tests (PFTs); refusal to participate; age <18 years; patients not affiliated or not benefiting from national health insurance, in accordance with French legislation; patients under guardianship, curatorship or protected adults; inability to comprehend and consent to the study. Patients will also be excluded if they fail to attend the first evaluation at 6 months after ICU discharge (death or refusal) or if they do not have the required tests to enable evaluation of the primary endpoint.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Additional non-inclusion criteria for the qualitative study
Impaired comprehension or expression precluding a semistructured interview; patients unable to express themselves adequately in French; patients who were discharged less than 1 month previously.
Outcomes
Primary outcome
The primary outcome is the presence of lung sequelae at 6 months after ICU discharge, defined either by alterations on PFTs, oxygen desaturation during a standardised 6 min walk test or fibrosis-like pulmonary findings on chest CT. The details of each criterion are presented in table 1.
Table 1.
Definition of lung sequelae
| Criteria | Test | Definition |
| Alteration of PFT | PFT | DLCO <80% of the theoretical value FVC <80% of the theoretical value |
| Oxygen desaturation on 6MWT | 6MWT in room air (or with the usual oxygen flow if impossible) | Delta SpO2 (SpO2 prewalk-SpO2 postwalk) ≥4% and SpO2 postwalk <90% |
| Fibrosis-like pulmonary findings | Thin-slice non-enhanced chest CT | At least one of the following:
|
DLCO, diffusion capacity of the lung for carbon monoxide; FVC, forced vital capacity; 6MWT, 6 min walk test; PFT, pulmonary function test; SpO2, peripheral capillary oxygen saturation.
Chest CT interpretation will be centralised and performed independently by two experienced chest radiologists using a standardised form.
Secondary outcomes
The secondary outcomes and their definitions are given in table 2.
Table 2.
Secondary outcomes
| Outcome | Description/score | Timepoint |
| Rehabilitation | Type, frequency, modalities (ambulatory/hospital), number of sessions performed/number of scheduled sessions after ICU discharge | 6, 12 months |
| Respiratory status | Dyspnoea (mMRC)26 | 6, 12 months |
| Oxygen saturation by pulse oximetry (SpO2) at rest and in room air | 6, 12 months | |
| 6MWT performed according to the ERS/ATS guidelines27 | 6 months 12 months for patients with desaturation during exercise, or walked distance <90% of the theoretical value on 6MWT at 6 months |
|
| Functional respiratory status: PFT including spirometry (FEV1, FVC, FEV1/FVC), plethysmography (TLC, RV, FRC, VC, RV/TLC) and diffusion capacity of the lung (DLCO, DLCO/VA) according to ERS/ATS guidelines28 29 | 6 months 12 months for patients with at least one of the following: FVC <80% of the theoretical value, FEV1 <80% of the theoretical value, DLCO <80% at 6 months |
|
| Lung parenchyma | Thin-slice non-enhanced chest CT | 6 months 12 months for patients with pulmonary involvement (fibrosis-like lesions, reticular and/or residual ground glass opacities, mosaic pattern) on chest CT at 6 months |
| Olfaction and taste | Questionnaire and quantification of alteration using visual analogue scale | Inclusion, 6 months |
| Neuromuscular status | Muscular autonomy (ADL30, 6MWT) | 6, 12 months |
| Cognitive status | MoCA31 | 6, 12 months |
| Psychological status | Post-traumatic stress disorder (PTSD) (IES-R)32
Anxiety and/or depression (HADS)33 |
6, 12 months |
| Quality of life | SF-3634
VSRQ35 |
6, 12 months |
| Vital status | Medical files or contact with patient or his/her relatives | 12 months |
| Nutritional state | Weight, food intake | Inclusion and 6 months |
| Qualitative study: barriers and facilitators to rehabilitation, as perceived by the patients |
Semistructured interviews after return to home | Between 8 and 10 months |
ADL, activities of daily living; DLCO, diffusion capacity of the lung for carbon monoxide; ERS/ATS, European Respiratory Society/American Thoracic Society; FEV1, forced expiratory volume in 1 s; FRC, functional residual capacity; FVC, forced vital capacity; HADS, Hospital Anxiety and Depression Scale; ICU, intensive care unit; IES-R, Impact of Event Scale Revised; mMRC, modified Medical Research Council; MoCA, Montreal Cognitive Assessment; 6MWT, 6 min walk test; PFT, pulmonary function test; RV, residual volume; SF-36, Short Form Health Survey modified 36; SpO2, peripheral capillary oxygen saturation; TLC, total lung capacity; VA, alveolar volume; VC, vital capacity; VSRQ, Visual Simplified Respiratory Questionnaire.
The flow chart and study design are presented in figure 1.
Figure 1.
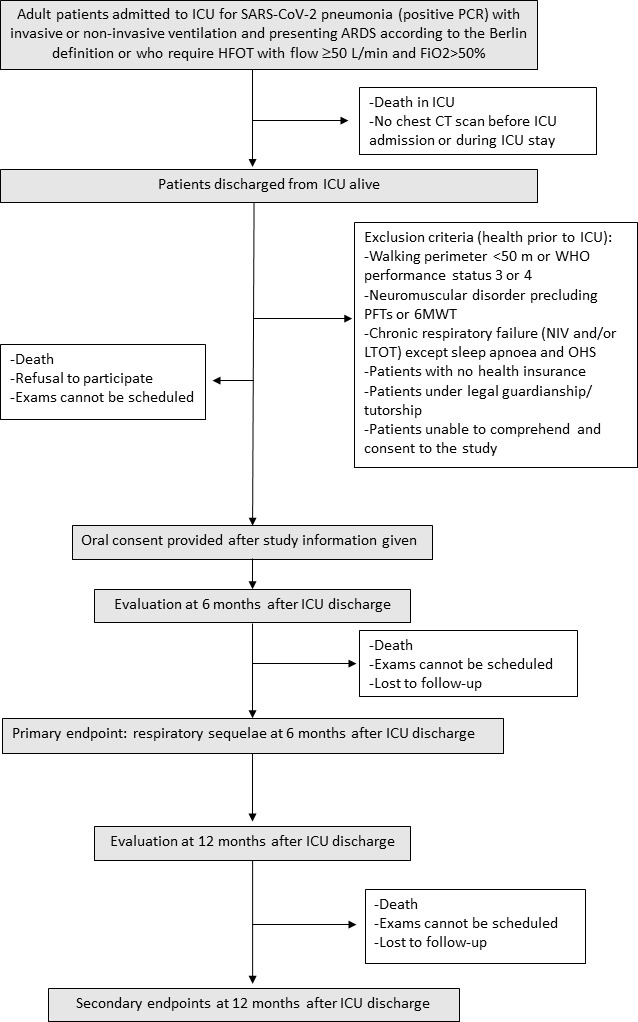
Flow chart of the study. HFOT, high-flow oxygen therapy; 6MWT, 6 min walk test; ARDS, acute respiratory distress syndrome; ICU, intensive care unit; PFT, pulmonary function test; OHS, obesity hypoventilation syndrome; LTOT, long-term oxygen therapy; NIV, non-invasive ventilation.
Variables of interest
The main variable of interest is social deprivation, assessed at the individual level using the EPICES score (Evaluation of Deprivation and Inequalities in Health Examination Centres). The EPICES score measures social and material deprivation using 11 items relating to social conditions, leisure activities and family/social support.12 13 Patients with an EPICES score ≥30.17 are considered as socially deprived.13 Deprivation will also be assessed at the level of the IRIS (îlots regroupés pour l'information statistique, i.e. aggregated units for statistical information) using the French European Deprivation Index.15
We also collect data on demographic characteristics (age, gender), living conditions (own home, retirement home), level of education (defined by the highest diploma obtained), occupational position according to seven categories of the French National Statistics Office, comorbidities (Charlson Comorbidity Index) and life habits (smoking status, level of alcohol intake, self-reported physical activity before COVID-19 at baseline and variables relating to healthcare utilisation; ie, chronic disease, home help, time required to get to the nearest doctor).
Data related to ICU stay will include the Simplified Acute Physiological Score (SAPS II)16 and Sequential Organ Failure Assessment (SOFA) score17 at ICU admission, as well as life support therapy (mechanical ventilation, vasopressors and/or inotropic agents, renal replacement therapy, high-flow nasal cannula), ARDS (severity and management) and length of ICU stay.
Study conduct
Patients will be screened at ICU discharge, during hospitalisation in post-ICU units, or contacted by phone if they have been discharged to home. Patients who meet study inclusion criteria will be informed about the study orally. They will notably be informed that they can withdraw from the study at any time without having to give a reason, and without it affecting their care in any way. The first scheduled follow-up visit will be performed at 6±1 months, and the second at 12±1 months after ICU discharge. The timing of data collection is summarised in table 3.
Table 3.
Timing of data collection during the study
| Screening | Inclusion | M6 (M5–M7) | M8–M10 | M12 (M11–M13) | |
| Eligibility assessment | X | ||||
| Information and consent | X | ||||
| Inclusion and non-inclusion criteria | X | ||||
| Clinical examination | |||||
| SpO2, mMRC | X | X* | |||
| Paraclinical examinations | |||||
| 6MWT | X | X* | |||
| PFT: spirometry, plethysmography and DLCO | X | X† | |||
| Chest CT | X | X‡ | |||
| Olfaction and taste questionnaire and visual analogue scale | X | X | |||
| Neuromuscular assessment | X | X | |||
| ADL Katz | X | X | |||
| Cognitive state: MoCA | X | X | |||
| Self-reported questionnaires: HADS, IES-R, SF-36, VSRQ | X | X | |||
| Nutritional status: weight, food intake | X | X | |||
| Data collection | |||||
| Baseline characteristics | X | ||||
| Sociodemographic characteristics | X | ||||
| Comorbidities | X | ||||
| Life habits | X | ||||
| Healthcare utilisation | X | ||||
| Data related to ICU stay | X | ||||
| SOFA, SAPS II | X | ||||
| Life support therapy | X | ||||
| Management | X | ||||
| ARDS | X | ||||
| Rehabilitation after ICU discharge | X | X | |||
| Vital status | X | X | |||
| Semistructured interview | X |
*For patients with desaturation at effort, or walked distance <90% of the theoretical value on 6MWT at 6 months
†For patients with at least one of the following: FVC <80% of the theoretical value, FEV1 <80% of the theoretical value, DLCO <80% at 6 months
‡For patients with pulmonary involvement (fibrosis-like lesions, reticular and/or residual ground glass opacities, mosaic pattern) on chest CT at 6 months
ADL, activities of daily living; ARDS, acute respiratory distress syndrome; DLCO, diffusion capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HADS, Hospital Anxiety and Depression Scale; ICU, intensive care unit; IES-R, Impact of Event Scale Revised; mMRC, modified Medical Research Council; MoCA, Montreal Cognitive Assessment; 6MWT, 6 min walk test; PFT, pulmonary function test; SAPS II, Simplified Acute Physiological Score; SF-36, Short Form Health Survey modified 36; SOFA, Sequential Organ Failure Assessment; SpO2, peripheral capillary oxygen saturation; VSRQ, Visual Simplified Respiratory Questionnaire.
For the qualitative study, semistructured interviews (of approximately 60 min duration) will be performed over the telephone by a sociologist between 8 and 10 months after discharge in a sample of approximately 30 patients who had been home for at least 1 month.
If PFT or CT tests were performed between ICU discharge and the first 6-month visit, they can be used as the values for the 6-month follow-up only if the results were normal (table 2).
If the chest CT scan at 6 months shows mosaic attenuation of the pulmonary parenchyma, then CT will be repeated at 12 months with forced expiration acquisition.
Sample size and recruitment
Given the lack of specific COVID-19 data that can be used as a basis for formal sample size calculations, the sample size is based on the estimated number of admissions in the participating centres. Given the number of patients admitted to ICU during the first wave in spring 2020, we expect to recruit 500 patients across all 38 participating centres.
This sample size will make it possible to detect an OR ranging from 0.342 to 0.601 at a two-sided significance level of 5%, power of 80%, with a social deprivation rate of 50% (based on the IVOIRE cohort study18) and assuming a functional recovery rate at 6 months that varies from 10% to 50% in patients surviving ARDS.19
Regarding the qualitative study, a multicentre sample of 30 patients (15 deprived and 15 non-deprived) will be randomly selected from among the patients who accept to participate in the study. This sample size should be sufficient to achieve data saturation.20
The first patient was included in September 2020.
Data management
Data will be collected using an electronic case report form (CleanWEB, Telemedicine Technologies, Boulogne-Billancourt, France) by an investigator, with the help of a clinical research technician. Data will be handled according to French legislation governing the management and privacy of personal nominative data. Data will be rendered anonymous using a code consisting of the first letter of the surname and first name of the patient, the number of the participating centre and the order of subject inclusion in the centre. All participant information will be stored on a secure server and will be collected, shared and maintained in strict respect of confidentiality.
Radiological data will be rendered anonymous then transferred to the coordinating centre for central interpretation using a secure digital transfer network (Nexus, NEHS Digital, Malakoff, France). At reception, the data will be downloaded to a secure server dedicated to the study data. If the participating centre has no access to digital transfer technologies, the images may be sent on anonymised CD-ROMs.
Each patient’s study identifier and their postal address will be used to identify the socioeconomic characteristics and the availability of healthcare services in the patient’s area of residence. To this end, each physical address will be geolocated and attributed to a specific territory, with the associated European Deprivation Index.
Statistical analysis
Descriptive analysis of patient groups
Patient characteristics will be described in each group (deprived/non-deprived) and will be presented as number and percentage for categorical variables, or as mean±SD (or median and (Q1; Q3) depending on data distribution) for continuous variables. Data will be compared between deprived and non-deprived groups using the χ2 or Fisher’s exact test for categorical variables, and the Student t-test or non-parametric test for continuous variables, as appropriate.
We will compare the characteristics of patients who fail to attend the 6-month visit for the evaluation of the primary endpoint with those in whom the primary endpoint was successfully evaluated.
Main analysis
The impact of deprivation on the recovery rate (ie, absence of lung sequelae) at 6 months will be analysed using logistic regression. Analyses will be adjusted for age, gender, SAPS II at ICU admission, Charlson Comorbidity Index score, respiratory support (high-flow oxygen therapy (HFOT) vs mechanical ventilation) and its duration, ARDS severity (PaO2/FiO2), length of ICU stay, tracheotomy ventilatory weaning, rehabilitation (none, usual care, rehabilitation) and duration of rehabilitation.
Secondary analyses
All secondary outcomes will be described in each group (deprived/non-deprived) and then analysed using a similar strategy to that used in the main analysis. Modelling will be adapted according to nature of secondary outcome and model hypotheses will be respected (logistic, generalised linear regression, mixed model, Wilcoxon test, log-rank test, …).
Analysis of qualitative data
All interviews will be recorded and transcribed in full. The interviews will be performed by a qualified sociologist. To reduce bias and ensure triangulation of analyses, the discourse of the study interviews will first be analysed by the sociologist, then the coding will be reviewed in an interdisciplinary meeting bringing together investigators and researchers in public health. The interviews and analyses will be performed according to the methods described by Paillé and Mucchielli.21 The interview guide will be developed conjointly by the sociologist and the project team (interdisciplinary team comprising physicians and public health researchers) and may be refined according to the findings of the first few interviews.
Interviews will be analysed using an inductive approach.22
Ethics and dissemination
The study protocol and the informed consent form were approved by an independent ethics committee (Comité de Protection des Personnes Sud Méditerranée II) on 10 July 2020 (2020-A02014-35).
Before enrolling patients into the study, researchers will obtain the patient’s informed consent according to the procedures described above.
Findings will be published in peer-reviewed journals and presented at national and international congresses to present the results to healthcare professionals involved in the management of patients with COVID-19.
Data availability statement
The study team is available to collaborate with other research teams on reasonable request to access study data. Expressions of interest to access study data, made out to the corresponding author, will be considered and then group-level or individual-level deidentified data may be shared as appropriate.
Discussion
The current pandemic caused by SARS-CoV-2 has led to a massive influx of patients with severe pneumonia and ARDS in ICUs worldwide. A large proportion of these patients will not survive their infection.5 Among the survivors, there is a possibility that they may not recover fully in terms of functional outcomes, dependency (physical and/or mental) or that they may be left with other long-term repercussions that will rapidly become a major public health problem, given the extent of the pandemic.
This large multicentre study aims to use a wide range of tests and complementary examinations to assess the overall health of patients with COVID-19 at 6 months and 1 year after discharge from the ICU. We investigate the health outcomes seen through the prism of socioeconomic position, and notably the level of social deprivation, using a mixed-methods approach. Social deprivation is associated with cumulative disadvantages, including unstable living conditions and financial or other difficulties that together mediate differences in health outcomes and life expectancy.23 In the present study, social deprivation is defined according to the EPICES score, which has previously been used by our group and others.18 23–25 We hypothesise that the relationship between socioeconomic position and functional recovery could be mediated by social isolation or more difficult access to care opportunities. The results of this study will help orient public policy regarding personalised medical and social follow-up, with a view to avoiding medium-term to long-term complications, which in turn may deteriorate and impair QoL or ultimately lead to death, thereby amplifying the social inequalities in healthcare even further in France.
Supplementary Material
Acknowledgments
The authors thank Fiona Ecarnot, PhD (EA3920, University of Franche-Comté, Besançon, France) for editorial assistance.
Footnotes
Twitter: @laurafederici, @Quenot
Contributors: Conception and design: P-LD, IF, EK, NM-B, J-PR, CB, J-PQ. Acquisition, analysis and interpretation of data: P-LD, MD, NM-B, AR, CC, JM, DS, GP, AA, CDa, BS, PK, LF, ER, MB, LL, TV, SN, BLC, GB, MM, MN, MR, WO, HG, CSG, JB, AD, XM, GB, EA-M, PA, ND, GLB, A-FM, SH, VB, NS, M-AH, SDB, G-DC, JD, NT, CDe, SG, J-PR, ML, MG, J-PQ. Critical revision of the protocol manuscript for important intellectual content: all authors. Final approval of the version to be published: all authors. All authors agree to be accountable for all aspects of the work and to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1. COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators . Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med 2021;47:60–73. 10.1007/s00134-020-06294-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–42. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 3. Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475–81. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Phua J, Weng L, Ling L, et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med 2020;8:506–17. 10.1016/S2213-2600(20)30161-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020;395:1763–70. 10.1016/S0140-6736(20)31189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lowcock EC, Rosella LC, Foisy J, et al. The social determinants of health and pandemic H1N1 2009 influenza severity. Am J Public Health 2012;102:e51–8. 10.2105/AJPH.2012.300814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wadhera RK, Wadhera P, Gaba P, et al. Variation in COVID-19 hospitalizations and deaths across New York City boroughs. JAMA 2020;323:2192–5. 10.1001/jama.2020.7197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baggett TP, Keyes H, Sporn N, et al. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA 2020;323:2191–2. 10.1001/jama.2020.6887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lapostolle F, Goix L, Vianu I, et al. COVID-19 epidemic in the Seine-Saint-Denis department of greater Paris: one month and three waves for a tsunami. Eur J Emerg Med 2020;27:274–8. 10.1097/MEJ.0000000000000723 [DOI] [PubMed] [Google Scholar]
- 11. Khalatbari-Soltani S, Cumming RC, Delpierre C, et al. Importance of collecting data on socioeconomic determinants from the early stage of the COVID-19 outbreak onwards. J Epidemiol Community Health 2020;74:jech-2020-214297–623. 10.1136/jech-2020-214297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bihan H, Ramentol M, Fysekidis M, et al. Screening for deprivation using the EPICES score: a tool for detecting patients at high risk of diabetic complications and poor quality of life. Diabetes Metab 2012;38:82–5. 10.1016/j.diabet.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 13. Labbe E, Blanquet M, Gerbaud L, et al. A new reliable index to measure individual deprivation: the EPICES score. Eur J Public Health 2015;25:604–9. 10.1093/eurpub/cku231 [DOI] [PubMed] [Google Scholar]
- 14., Ranieri VM, Rubenfeld GD, et al. , ARDS Definition Task Force . Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307:2526–33. 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 15. Pornet C, Delpierre C, Dejardin O, et al. Construction of an adaptable European transnational ecological deprivation index: the French version. J Epidemiol Community Health 2012;66:982–9. 10.1136/jech-2011-200311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPs II) based on a European/North American multicenter study. JAMA 1993;270:2957–63. 10.1001/jama.1993.03510240069035 [DOI] [PubMed] [Google Scholar]
- 17. Vincent JL, Moreno R, Takala J, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. on behalf of the Working group on sepsis-related problems of the European Society of intensive care medicine. Intensive Care Med 1996;22:707–10. 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 18. Quenot J-P, Helms J, Labro G, et al. Influence of deprivation on initial severity and prognosis of patients admitted to the ICU: the prospective, multicentre, observational IVOIRE cohort study. Ann Intensive Care 2020;10:20. 10.1186/s13613-020-0637-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herridge MS, Cheung AM, Tansey CM, et al. One-Year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 2003;348:683–93. 10.1056/NEJMoa022450 [DOI] [PubMed] [Google Scholar]
- 20. Hennink MM, Kaiser BN, Marconi VC. Code saturation versus meaning saturation: how many interviews are enough? Qual Health Res 2017;27:591–608. 10.1177/1049732316665344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paillé P, Mucchielli A. L'analyse qualitative en sciences humaines et sociales. In: Colin A, ed. Qualitative analysis in human and social sciences. Paris, 2012. [Google Scholar]
- 22. Charmaz K. Constructing Grounded theory. 2nd ed. London: Sage Publications, 2014. [Google Scholar]
- 23. Béjot Y, Bourredjem A, Mimeau E, et al. Social deprivation and 1-year survival after stroke: a prospective cohort study. Eur J Neurol 2021;28:800–8. 10.1111/ene.14614 [DOI] [PubMed] [Google Scholar]
- 24. Guilloteau A, Binquet C, Bourredjem A, et al. Social deprivation among socio-economic contrasted French areas: using item response theory analysis to assess differential item functioning of the EPICES questionnaire in stroke patients. PLoS One 2020;15:e0230661. 10.1371/journal.pone.0230661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Letellier N, Carrière I, Cadot E, et al. Individual and neighbourhood socioeconomic inequalities in cognitive impairment: cross-sectional findings from the French CONSTANCES cohort. BMJ Open 2020;10:e033751. 10.1136/bmjopen-2019-033751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Williams N. The MRC breathlessness scale. Occup Med 2017;67:496–7. 10.1093/occmed/kqx086 [DOI] [PubMed] [Google Scholar]
- 27. Holland AE, Spruit MA, Troosters T, et al. An official European respiratory Society/American thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014;44:1428–46. 10.1183/09031936.00150314 [DOI] [PubMed] [Google Scholar]
- 28. Graham BL, Brusasco V, Burgos F. ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J 2017;2017:49. [DOI] [PubMed] [Google Scholar]
- 29. Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American thoracic Society and European respiratory Society technical statement. Am J Respir Crit Care Med 2019;200:e70–88. 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–86. 10.1093/geront/9.3_Part_1.179 [DOI] [PubMed] [Google Scholar]
- 31. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 32. Bienvenu OJ, Williams JB, Yang A, et al. Posttraumatic stress disorder in survivors of acute lung injury: evaluating the impact of event Scale-Revised. Chest 2013;144:24–31. 10.1378/chest.12-0908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 34. Ware JE, Sherbourne CD. The mos 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 35. Perez T, Arnould B, Grosbois J-M, et al. Validity, reliability, and responsiveness of a new short visual simplified respiratory questionnaire (VSRQ) for health-related quality of life assessment in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2009;4:9–18. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study team is available to collaborate with other research teams on reasonable request to access study data. Expressions of interest to access study data, made out to the corresponding author, will be considered and then group-level or individual-level deidentified data may be shared as appropriate.


