Abstract
During SARS-CoV-2 infection, the innate immune response can be inhibited or delayed, and the subsequent persistent viral replication can induce emergency signals that may culminate in a cytokine storm contributing to the severe evolution of COVID-19. Cytokines are key regulators of the immune response and virus clearance, and, as such, are linked to the—possibly altered—response to the SARS-CoV-2. They act via a family of more than 40 transmembrane receptors that are coupled to one or several of the 4 Janus kinases (JAKs) coded by the human genome, namely JAK1, JAK2, JAK3, and TYK2. Once activated, JAKs act on pathways for either survival, proliferation, differentiation, immune regulation or, in the case of type I interferons, antiviral and antiproliferative effects. Studies of graft-versus-host and systemic rheumatic diseases indicated that JAK inhibitors (JAKi) exert immunosuppressive effects that are non-redundant with those of corticotherapy. Therefore, they hold the potential to cut-off pathological reactions in COVID-19. Significant clinical experience already exists with several JAKi in COVID-19, such as baricitinib, ruxolitinib, tofacitinib, and nezulcitinib, which were suggested by a meta-analysis (Patoulias et al.) to exert a benefit in terms of risk reduction concerning major outcomes when added to standard of care in patients with COVID-19. Yet, only baricitinib is recommended in first line for severe COVID-19 treatment by the WHO, as it is the only JAKi that has proven efficient to reduce mortality in individual randomized clinical trials (RCT), especially the Adaptive COVID-19 Treatment Trial (ACTT-2) and COV-BARRIER phase 3 trials. As for secondary effects of JAKi treatment, the main caution with baricitinib consists in the induced immunosuppression as long-term side effects should not be an issue in patients treated for COVID-19.
We discuss whether a class effect of JAKi may be emerging in COVID-19 treatment, although at the moment the convincing data are for baricitinib only. Given the key role of JAK1 in both type I IFN action and signaling by cytokines involved in pathogenic effects, establishing the precise timing of treatment will be very important in future trials, along with the control of viral replication by associating antiviral molecules.
Keywords: COVID-19; cytokines; hematologic neoplasms; therapies, investigational; autoimmunity
Introduction
Cytokine receptors and the Janus kinase-Signal Transducers and Activators of Transcription pathway
Cytokines are alpha-helical proteins of 160–170 aminoacids that are secreted and act on target cells as a function of expression and exposure on their surface of specific receptors. They are fundamentally required for blood formation and regulation of the immune response. In blood formation, on commitment to differentiation of hematopoietic stem cells (HSCs), lineage specific cytokines regulate the survival, proliferation and differentiation of progenitors and the final blood levels.1
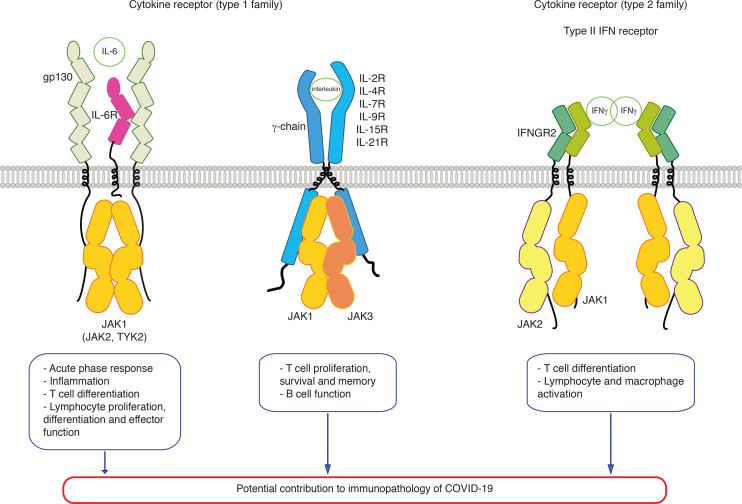
Cytokines act via specific cytokine receptors. The human genome codes for over 40 cytokine receptors. They all signal via Janus kinases (JAKs), initially called Just Another Kinases, that are appended non-covalently to their cytosolic tails. Four JAKs are coded by the human genome, namely JAK1, JAK2, JAK3 and TYK2. Activated receptors induce via JAKs the activation of Signal Transducers and Activators of Transcription (STATs). There are 7 STATs coded by the human genome. Several receptors use the same JAKs and sharing of JAKs allows specific signals by the different receptors and cytokines, but different outputs.1 The cytokine receptor superfamily is divided in type 1 and type 2 families (figure 1) and this distinction is derived from different sequence features, such as conserved WSXWS motifs in the extracellular domains and boxes 1 and 2 motifs in the cytosolic domains for type 1 (figure 2A).1 The type 1 family consists of homodimeric receptors (for Epo, Tpo, GCSF, Growth Hormone, Prolactin), heterodimeric receptors (for IL-3, IL-5, GM-CSF), using JAK2, hetero-oligomeric receptors, using JAK1 and to some extent JAK2 and TYK2 (represented by the IL6 family receptors),2 and finally the gamma chain using receptors (namely those for IL-2, IL-7, IL-9, IL-15, IL-21) (figure 1) which are composed of a specific chain bound to JAK1 and a common chain shared by all these receptors (the common gamma-chain or IL-2R subunit gamma), bound to JAK3. The type 2 family consists of receptors for type I interferon (bound to JAK1 and TYK2) (figure 3),3 for type II interferon or IFN-gamma (bound to JAK1 and JAK2)(figure 3)4 and receptors for IL-10, IL-20, IL-22 to IL-24 and others, using JAK1 or JAK2 and TYK2. The fundamental mechanism of activation of both type I and type II cytokine receptors is represented by cytokine-induced dimerization/oligomerization of receptor subunits, which brings the JAKs appended non-covalently to cytosolic domains into such relative proximity and conformation that they can activate the appanded JAKs and trigger the signaling cascade.5–8 This is starting with tyrosine phosphorylation of receptor tails, which then become attraction sites for SH2 containing signaling molecules and adaptors. The major substrate of JAKs is represented by the family of STATs.9 The choice of one of several STAT molecules is made as a function of which JAK and which sequence sites are tyrosine phosphorylated in receptors. In addition, adaptors linking cytokine receptors with ras-MAP-kinase and PI-3’-kinase-Akt are also attracted to receptors on JAK activation.1
Figure 1.
JAK-dependent cytokine receptors signaling involved in response to SARS-CoV-2 infection and potentially in COVID-19 immunopathology. EC, extracellular; IC, intracellular; IFN, interferon; JAK, Janus kinase.
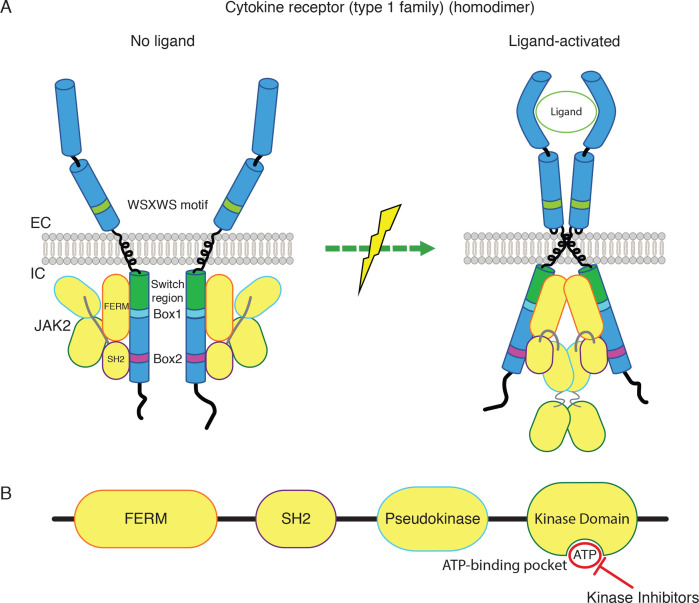
Figure 2.
Homodimeric cytokine receptor signaling via JAK2. (A) Homodimeric cytokine receptors and conformational changes on activation by ligands. JAK2 binds to boxes 1 and 2 motifs of the cytosolic domains of receptors, via the FERM and SH2 domains, respectively. The region separating the end of the transmembrane domain and the Box 1 is denoted ‘switch region’ and is required for ligand-activation of receptors and JAK2. (B) JAK2 domain structure and the activating mutations in myeloproliferative neoplasms (V617F and insertions and deletions mutating K539) shown as red stars. FERM, SH2, pseudokinase and kinase domains are shown from the NH2- to the COOH-terminus. kinase inhibitors currently in use act on the ATP-binding pocket of the kinase domain and inhibit both mutated and wild type forms of JAK2. EC, extracellular; IC, intracellular; IFN, interferon; JAK, Janus kinase.
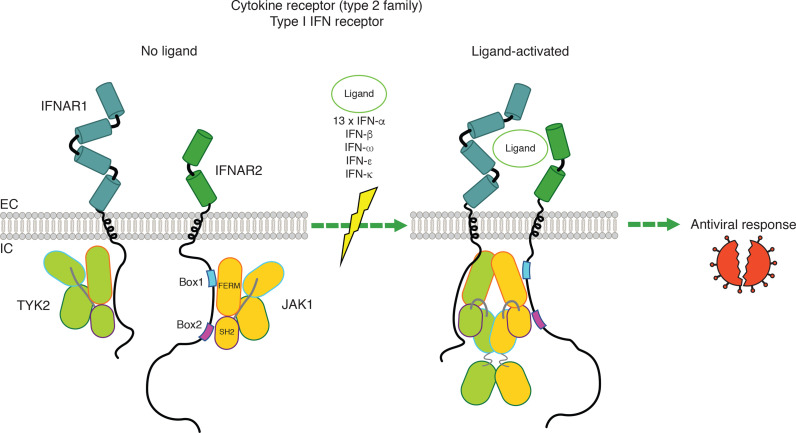
Figure 3.
Signaling by heterodimeric type I interferon (IFN) receptor and changes of conformation on activation of the IFN receptor by its ligands. JAK1 binds to boxes 1 and 2 motifs of the cytosolic domain of ifnar2 respectively via the FERM and SH2 domains. Tyk2 binds to the cytosolic domain of IFNAR1. EC, extracellular; IC, intracellular; IFN, interferon; IL, intereukin; JAK, Janus kinase.
It is important to stress two major features of cytokine receptor function: (1) in the absence of cytokines these receptors are completely inactive, unlike other receptor types which maintain a certain level of basal activity; (2) activation of JAKs is absolutely required for all signaling downstream of these receptors. In addition, JAKs are key to stabilize and chaperone cytokine receptors to the cell surface.10–13 JAKs bind to cytokine receptors’ cytosolic domains, namely the FERM domain binds to the Box 1 and the region between boxes 1 and 2, while the SH2 domain binds to Box 214–16 (figures 2A and 3). Box 1 is a proline-rich short sequence that is conserved in most type 1 cytokine receptors (such as EpoR or TpoR), while Box 2 is a sequence composed of hydrophobic and negatively charged residues.17 Interestingly, the region of the cytosolic tails located between the transmembrane domain and Box 1 is crucial for activation of JAKs, not for their binding; this is why this region of 10–14 aminoacids rich in positively charged residues18 has been denoted as « switch » region (figure 2A).5 10 These principles are correct for all type 1 cytokine receptors and are also globally correct for type 2 cytokine receptor, except that for the latter the boxes 1 and 2 are less well defined.1
SARS-CoV-2 and the immune system
Coronaviruses are enveloped spherical viruses with a diameter of 130–160 nm, with single stranded RNA unsegmented and positive polarity. They bind to the ACE2 receptor on target cells for entry and infection through envelope structures containing the S protein. SARS-CoV-2 belongs to the Coronavirinae subfamily Betacoronavirus genus and Sarbecovirus subgenus. SARS-CoV belongs to the same subgenus Sarbecovirus, while the Middle Eastern respiratory syndrome (MERS) virus belongs to another subgenus, Merbecovirus.19 SARS-CoV-2 entry, decapsidation, replication and virus release follow the path depicted in SARS-CoV and has been the subject of an extensive literature.
An impressive body of work has been accomplished in a very short time describing the roles of individual SARS-CoV-2 proteins in infection, replication and countering the immune system.20 With extensive sequencing and epidemiology studies, the different variants of the virus are being studied in detail with respect to infectivity, cytopathic effect21 and sensitivity to vaccine.22 23
Persistence of virus in the body,24 especially in the gut,25 given the enterocyte replication of the virus26 or only of transcripts from short integrated viral RNAs27 may contribute to memory response and may impact disease and other immune stimulations. Furthermore, long-term replication in the context of immunosuppression may promote selection for mutants of SARS-CoV-2.28–30
Immunopathology in COVID-19 and previously described SARS CoV and MERS infections
Experience with severe SARS-CoV-1 and MERS pointed to a major feature of these infections, which is to induce a delayed cytokine storm following an initially insufficient induction and action of type I interferons (IFN).31 32 The same has been clearly observed with SARS-CoV-2 which induces a biphasic disease consisting first of a flu-like phase, followed by a pulmonary and systemic disease, which pathological cytokine action and inflammation may lead to acute respiratory distress syndrome (ARDS).
In a standard viral infection, the viral genome and proteins induce, via Toll-like receptors, activation of the transcription factors required for rapid and sustained synthesis of type I IFNs alpha (produced by leucocytes, 17 subtypes) and beta (fibroblasts), epsilon (produced by reproductive tract epithelium), kappa (produced by epidermal keratinocytes) and omega (produced by leucocytes and is divergent from IFNs alpha and beta). There are 13 forms of IFN-alpha and 1 IFN-omega, which is closely related phylogenetically. IFN-epsilon and kappa are less homologous.33 34 All human 17 individual type I IFNs bind to the same receptor,35 36 namely the type I IFN receptor (heterodimer constituted of one IFNAR2 binding subunit, linked to JAK1, and one IFNAR1 signaling subunit, linked to TYK2) (figure 3),3 which induce, via a specific complex composed of STAT1, STAT2 and IRF9, genes that code for proteins that mediate an antiviral state in the neighboring cells.37
These IFNs are secreted by the initially infected cells and act on neighboring cells, inducing an antiviral state. As a consequence, the neighboring cells are protected and the antigen presenting cells can induce the adaptive immune response, composed of B cells that under the help of T cells will generate the plasmocytes that secrete neutralizing antibodies and also the formation of CD8+ cytotoxic T cells that will recognize and kill infected cells. Patients that exhibit anti-type I IFN antibodies or genetic defects in IFN induction mechanisms evolve towards a severe disease.38 39 Remarkably, autoantibodies against type I IFNs with neutralizing capacity are present in approximately 4% of uninfected individuals over 70 years old and are responsible for 20% of COVID-19 deaths.40 Furthermore, X-linked recessive TLR7 deficiency has been reported in ~1% of men under 60 years old with life-threatening COVID-19.41 Indeed, human TLR7 and plasmacytoid dendritic cells expressing TLR7 are essential for protective innate type I IFN immunity against SARS-CoV-2 in the respiratory tract. Finally, in a large study investigating genetic mechanisms of critical illness in COVID-19 by a genome-wide association study in 2244 critically ill patients with COVID-19 from 208 UK intensive care units, low expression of IFNAR2 or high expression of TYK2, are associated with life-threatening disease.42 These data clearly indicate that signaling by type I IFN and possibly other JAK-STAT pathway components are critical for COVID-19.
Type II IFN (IFN-gamma) (figure 1) is secreted by T and NK cells and plays a major role in T cell immunity.43 Type III IFN (IFN-lambda) is produced by epithelial cells at the local level and may function as an entry barrier for several viruses. IFN-lambda utilizes a distinct receptor system (IL-28R or IFN-lambda receptor alpha and IL-10 receptor beta, coupled also to JAK1 and TYK2, respectively).44 It is possible that defects in induction and action of IFN-lambda are also involved in COVID-19.
The presence of a very large genome with many non-structural proteins allows coronaviruses to target many of the proteins of the innate immunity machinery and especially the mechanisms of type I IFN induction and then signaling in target cells.31 45 This initial insufficient response allows rapid amplification of virus RNA which then reaches a threshold where it may induce the cytokine storm that usually appears 6–8 days after infection. The precise molecular link between high viral loads and aberrant cytokine/chemokine induction leading to the cytokine storm remains to be determined. Appearance of the cytokine storm is correlated with a decrease in lymphocytes, eosinophils, increase in D-dimers and importantly with an emergency myelopoiesis that generates insufficiently differentiated or skewed monocytic and granulocytic cells.
The SARS-CoV-2 infection impacts both lymphopoiesis and myelopoiesis. COVID-19 patients exhibit a decrease in the number of plasmacytoid and myeloid dendritic cells, different monocyte distribution subsets and activation patterns, as well as neutrophil phenotypic alterations.46 During COVID-19, defects in myelopoiesis occur with profound alterations of the myeloid compartment.46–48 In mild forms of COVID-19, inflammatory monocytes (HLA-DRhiCD11chiCD14+) with an IFN-stimulated gene signature are elevated.48 In severe forms, HLA-DRLow monocytes are present along with neutrophil precursors and dysfunctional mature neutrophils.48 Using single cell RNA sequencing of purified cell populations from COVID-19 patients it was demonstrated that while HLA-DRLow classical monocytes accumulate during severe disease, non-classical CD14LowCD16High monocytes disappear.47 In severe cases, classical monocytes release high levels of S100A8/S100A9, also called calprotectin.47 In addition, immunosuppressive CD10LowCD101-CXCR4+/- neutrophils are produced, that migrate to the lung. High levels of S100A8/S100A9 and altered frequency of non-classical monocytes are becoming markers of severe disease.47 However, the relationship between classic and non-classic monocytes is more complex.49
These changes in the myeloid and lymphoid compartments are a pivotal reason for further pathological mechanisms mediated by these cells at the level of lung pneumocytes and endothelial cells. It is important to emphasize that all step-by-step mechanistic details of these chains of events are still worked out, but what is certain is that while corticosteroid therapy is very useful, it is not sufficient to interrupt the immunopathology of severe of COVID-19.
During a controlled viral infection, early innate mechanisms executed by monocytes, NK cells and cytokines act to induce late adaptive immunity.50 Viral load peaks during the descending phase of the early innate response and coincides with the peak of development of adaptive response, then both viral load and intensity of adaptive response decrease, with virus becoming undetectable, while the adaptive response develops the long-term memory protection. In an uncontrolled infection there is persistent or delayed innate response with cytotoxicity, lymphopenia and immunosuppression. Mediators of toxicity during the delayed pathological immune response are IL-6, IL-1 and several other cytokines. Attempts to control these events using IL-6 or IL-1 blockers and corticosteroid therapy have led the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA) and WHO to indicate IL-6R blocker tocilizumab (and sarilumab)51–55 and IL-1 blocker anakinra (EMA only)56 57 in certain severe cases of COVID-19. These strategies would need to be compared with the use of JAK inhibitors (JAKi), but at present direct comparisons have not been performed.
Rationale for inhibiting JAKs in COVID-19
There are two major reasons why JAKs became prime targets of the pharmaceutical industry for inhibition. The first is that, in 2005, a unique somatic acquired JAK2 mutation (JAK2 V617F) was discovered to be responsible for 70% of a large group of myeloid cancers called myeloproliferative neoplasms (MPNs).58–61 This was a surprizing finding. Within MPNs, 95% of Polycythemia Vera (PV) and >60% of Essential Thrombocythemia (ET) and Primary Myelofibrosis (MF) were linked to this acquired mutations. 3% of PV are harboring a different set of JAK2 mutations in exon 12, where insertions or deletions lead to mutation of K539 in the linker between SH2 and pseudokinase domains, also inducing JAK2 persistent activation.62 The rest of ET and MF patients that do not harbor JAK2 V617F exhibit mutations that induce unusual modes of activation of TpoR that lead to persistent JAK2 activation.63 One is represented by mutations in TpoR (MPL) itself,64–67 the other is frameshifting mutations in calreticulin (CALR)68 69 that endow mutated CALR proteins the ability to bind and activate the same TpoR in the absence of ligand.70–73 Thus, for this very large group of diseases with increasing prevalence (0.4–0.7/1000, 5 times higher than chronic myeloid leukemia induced by the BCR-Abelson kinase (ABL) fusion protein) the cause is persistent activation of JAK2 in clonal HSCs, which gives a major advantage to progenitors of red blood cells, platelets and granulocytes, leading to clonal expansion of these lineages in the absence of cytokines.
In the same time with the identification of JAK2 V617F it was shown that homologous mutations activate constitutively JAK1 (V658F) and TYK2 (V678F)66 and these and other activating mutations were identified in T-acute lymphoblastoid leukemia (ALL), in B-ALL and several other conditions.74 75 Overall, these findings provided an impetus to search for potent inhibitors of JAKs.
The second reason why JAKs became prime targets for the pharmaceutical industry is represented by excessive activation of cytokines and their receptors in autoimmune diseases. The prime targets were members of the IL-2, IL-4, IL-7, IL-9, IL-15, IL-21 and especially the two JAK proteins associated with subunits of this group, namely JAK1 and JAK3.76 Inhibitors were thus seeked that could inhibit JAK1 and JAK3, or only JAK3. Such inhibitors were discovered and it was found that only inhibiting JAK3 exerts a much weaker effect than inhibiting JAK1 and JAK3, implying that JAK1 is the dominant JAK,77 and that JAK3 needs to be present in the complex, as a scaffold, but its catalytic activity is not as crucial as that of JAK1.77 78 Indeed, absence of JAK3 has a blocking effect while inhibiting the kinase domain only exerts a minor effect.77 79
More recently, increased interest in JAKi strategies arose for the need of new treatments for pathological inflammatory conditions like hemophagocytic lymphohistiocytosis (HLH) where overactive T cells and macrophages secrete numerous proinflammatory cytokines, including IFNγ, IL-1β, IL-2, IL-6, IL-10, IL-18 and TNF-α, involving the JAK-STAT pathway.80 Samewise in COVID-19, the JAK-STAT pathway is implicated in complement hyperactivation in SARS-CoV-2-infected respiratory epithelial cells81 and in the activation of CD4+ and CD8+ positive T cells, NK cells and monocytes which cooperate with elevated levels of IL-6, IL-9, IL-13, GM-CSF, IFNγ, IL-1β, IL-8, and IL-17. Indeed, very high levels of IL-2, IL-7, IL-10, TNF-α and G-CSF were described in COVID-19 patients requiring intensive care.82
Furthermore, JAK-STAT activation promotes senescence of SARS-CoV-2 infected cells, which amplifies inflammation.83
Therefore, JAKi, and especially JAK1/JAK2 inhibitors, were suggested as a potential therapy against systemic inflammation in COVID-1984 because treatment with a JAK1/JAK2 inhibitor to prevent lung injury in severe COVID-19 would be able to reduce cytokine action more effectively than blocking one cytokine at a time (like IL-6.85
Inhibitors
The major strategy used for obtaining kinase inhibitors is to obtain compounds that prevent ATP entrance in the ATP binding pocket. ATP is obligatory as a source of phosphate for catalysis. The small molecule inhibitor may bind and compete with ATP, or may bind to close—by sites or distant sites that allosterically prevent ATP binding to the ATP pocket. The kinase inhibitors may bind to active kinases and inhibit them (type I) or may bind to the inactive kinase, and block the transition to active kinase (type II).86 The very well-known kinase inhibitor imatinib inhibits the Abelson kinase (ABL), PDGF receptor and KIT receptor tyrosine kinases. Imatinib binds to kinases when they are inactive (type II), thus preventing activation. All current JAKi used in the clinics so far are type I inhibitors, and they only recognize and bind kinase domain of JAKs in their active conformation. This is on one hand a limitation, as prebinding activation is a prerequisite for binding, hence pathological signaling occurs at a certain point, but on the other hand the toxic effects due to blocking JAK2 for red blood cell and platelet formation will be less severe.
The industry identified several small molecule ATP competitors with nM affinity to each of the JAKs, or to several JAKs. Two molecules are mainly under focus of this review, baricitinib and ruxolitinib. Baricitinib exhibits an IC50 under 10 nM JAK1 and JAK2 (4.0–5.9 nM for JAK1 and 6.6–8.8 nM for JAK2), 787 nM for JAK3 and 61 nM TYK2, all determined at 1 mM ATP.87 These values are different as a function of the amount of ATP used and may also depend on the technique to assay inhibition of kinase activity.87–89 Ruxolitinib exhibits also IC50s of less than 10 nM for JAK1 and JAK2, specifically IC50 3.3–6.4 nM for JAK1, 2.8–8.8 nM for JAK2, 428–487 nM for JAK3 and approximately 10-fold selectivity for TYK2 (IC50 19–30 nM).87–89
They are both type I inhibitors with rather low half-life, 4 hours for ruxolitinib and 12.5 hours for baricitinib. In addition, we will discuss tofacitinib (JAK1 and JAK3 inhibitor), upadacitinib (JAK1 inhibitor) and nezulcitinib (pan JAKi, inhaled). We will not discuss the strictly JAK1 specific filgotinib, as its clinical development is most recent. Also, a JAK2 inhibitor, fedratinib which was only recently approved in the clinics after a pause in development, will not be discussed. However, it appears that although all their differences a JAKi class effect is emerging, with potential relevance in COVID-19.
Structural bases for inhibitor action. The first crystal structure of ruxolitinib was solved in complex with the kinase domain of c-Src,90 while the structure of the JAK2 kinase domain was solved in complex with a pan JAKi.91 The X-ray crystal structure of baricitinib was initially solved in complex with a member of the Numb-associated kinases, namely BMP2-inducible kinase.92 Only in 2021, in a very recent study which is being published in Blood the structures of ruxolitinib and of baricitinib were reported with the JAK2 kinase domain.93 Of great interest, these structures allowed the first examination of ruxolitinib and baricitinib engaged with their main target (figure 2B). It also afforded design of ruxolitinib and baricitinib modified molecules where a linker was introduced to a solvent exposed carbon (C2 of the pyrimidine ring) of each inhibitor to which pomalidomide or thalidomide were linked. Proteolysis-targeting compounds were created and showed that they hold the potential to degrade JAK2 in target cells.93
JAKi has known a broadening use in the clinics over the past decade and new molecules keep being designed.94 Ruxolitinib, one of the oldest JAKi, is the agent most used in hematology patients whereas other JAKi such as baricitinib and tofacitinib are used more commonly in systemic rheumatic diseases (tofacitinib for patients with rheumatoid arthritis, psoriatic arthritis or ulcerative colitis and baricitinib for patients with rheumatoid arthritis).
There is extensive literature on the effects of JAKi in MPNs (in MF and complicated PV). In these conditions, the treatment is effective for alleviating symptoms, improving quality of life, overall survival, decreasing spleen size, but is not curative, as allele burdens do not significantly decrease. Most importantly, resistance to treatment exists, that is not linked to further mutations in the driver JAK2 gene. This is very different from the situation with imatinib and BCR/ABL1 driven chronic myeloid leukemia where mutations in ABL kinase domain, the main target, are explaining resistance.95 These results have been interpreted to suggest that the targets of JAK2 inhibitors such as ruxolitinib are non-mutated JAK1 and JAK2 in inflammatory cells, suggesting that JAKi may act as effective anti-inflammatory agents in MPNs,96 which is indeed the goal of their use in systemic rheumatic diseases or in newer indications such as or HLH80 or graft-versus-host disease (GVHd).97–102
That ruxolitinib is able to be effective in cases of GVHd resistant to corticotherapy clearly indicates its immunosuppressive effects are not identical to those of corticotherapy.97 98 The mechanisms assessed in mouse models of GVHd involve JAKi-mediated suppression of pro-inflammatory cytokines and of the proliferation of effector T cells, with ruxolitinib impairing differentiation of CD4+ T cells into IFNγ- and IL17A-producing cells.103
Experience with JAKi in COVID-19
Significant clinical experience has been accumulated on the use of JAKi for treating COVID-19 and associated inflammatory status (table 1).
Table 1.
Clinical experience with JAK2 inhibitors in COVID-19
| Study ID no | Brief title of the study | Promoter/Sponsor | Status (first posted) | Patient’s condition/inclusion criteria | No of patients | Study type | Study phase | Centers | Intervention/drugs | Primary outcome measures | Reference |
| June 2020 (published) | COVID-19-induced (mild to severe) pneumonia | 4 | Cases report | Pilot study | Monocentric | Baricitinib |
|
Stebbing et al117 | |||
| NCT04438629 | Evaluation of Immune Response in COVID-19 Patients (IMMUNOVID) | Azienda Ospedaliera Universitaria Integrata Verona (sponsor) and Pederzoli Hospital of Peschiera, Italy | March–June 2020 (published), recruiting | COVID-19-induced pneumonia | 88 (20 on baricitinib) | Observational, longitudinal, prospective | Bicentric | Baricitinib, 4 mg two times per day 2 days, 4 mg/day 7 days |
|
Bronte et al118 | |
| NCT04358614 | Baricitinib Therapy in COVID-19: A Pilot Study on Safety and Clinical Impact | Hospital of Prato, Italy | completed (published October 2020) | COVID-19 moderate pneumonia | 12 patients in the pilot study 191 (113 on baricitinib) in the second phase |
Observational, longitudinal, retrospective | Phase 2 Phase 3 |
Multicentric (seven in Italy) | Baricitinib 4 mg/d and antiviral therapy (lopinavir/ritonavir) for 2 weeks vs SOC (hydroxychloroquine and lopinavir/ritonavir) for 2 weeks |
Mortality rate | Cantini et al120 |
| NCT04401579 | Adaptive COVID-19 Treatment Trial 2 | US National Institute of Allergy and Infectious Diseases | Completed (published March 2021) | COVID-19 hospitalized patients | 1033 (515 on baricitinib) | Interventional, randomized, double-blind, placebo-controlled | Phase 3 | Multicentric (67 international centers) | Remdesivir plus baricitinib 4 mg/d, up to 2 weeks vs Remdesivir plus placebo |
Time to recovery | Kalil et al121 |
| NCT04421027 | A Study of Baricitinib (LY3009104) in Participants With COVID-19 (COV-BARRIER) | Eli Lilly and Company | Completed (published August 2021) | O2-dependent COVID-19 patients with risk indicators of aggravation | 1585 | Interventional, randomized, double-blind, placebo-controlled | Phase 3 | Multicentric | Baricitinib 4 mg/d and SOC for 2 weeks vs Placebo and SOC for 2 weeks |
|
Marconi et al124 |
| NCT04469114 | Tofacitinib in Hospitalized Patients With COVID-19 Pneumonia (STOP-COVID) | Hospital Israelita Albert Einstein, Brazil (sponsor) | Completed (published) | COVID-19 hospitalized patients with pneumonia | 289 | Interventional, randomized, double-blind, parallel-design, placebo-controlled | Phase 3 | Multicentric (15 in Brazil) | Tofacitinib, 10 mg Twice daily up to 14 days vs Placebo |
Death or respiratory failure until Day 28 | Guimaraes et al131 |
| Hammersmith Hospital, London, UK | June 2020 (published) | HSCT for CML Diabetes Male |
1 | Case report | Monocentric | Tocilizumab 8 mg/kg (two doses, no effect) Ruxolitinib 5 mg Twice daily 3 days, 10 mg Twice daily 8 days, 5 mg Twice daily 10days |
Case report (severe disease) | Innes et al142 | |||
| Aachen University Hospital, Aachen, Germany | May 2020 (published) | MF high-risk for COVID-19 (arterial hypertension, obesity, hyperuricemia, chronic kidney disease) Male |
1 | Case report | Monocentric | Ruxolitinib, 10 mg BD (patient on drug before COVID-19) | Case report (mild disease) | Koschmieder et al143 | |||
| No 47 (Italian Agency for Drug (AIFA) and Istituto Spallanzani) No 17 104 (RUXO-COVID (institutional ethic committee in Florence) |
RUXO-COVID | Azienda Ospedaliera-Universitaria Careggi, Florence, Italy | April-May 2020 (inclusions) August 2020 (published) |
Severe COVID-19 (no mechanical ventilation at diagnosis) | 34 | Observational, prospective | Monocentric | Ruxolitinib (compassionate use), 5 mg Twice daily 1–2 days, 10 mg Twice daily 1–2 days, |
|
Vannucchi et al144 | |
| Schwarzwald–Baar–Klinikum Villingen-Schwenningen, Germany | March-April 2020 (inclusions), June 2020 (published) | Severe COVID-19 | 105 consecutive patients, 14 patients on ruxolitinib |
Retrospective, observational (multidisciplinary board decision on specific medical treatment) | pilote case series | Monocentric | Ruxolitinib, 7.5 mg Twice daily 1–7 days to 15 mg Twice daily | CIS | La Rosee et al145 | ||
| NCT04338958 | Ruxolitinib in COVID-19 Patients With Defined Hyperinflammation (RuxCoFlam) | University of Jena, Germany (Sponsor) | Completed (published) | COVID-19 patients with hyperinflammation (COVID-19 Inflammation Score (CIS) ≥10/16) | 193 | Interventional, single arm (open), non-randomized | Phase 2 | Multicentric | Ruxolitinib, 10 mg Twice daily to 20 mg Twice daily | Improvement in CIS | La Rosee et al145 |
| NCT04337359 (expanded access for ruxolitinib) | Ruxolitinib Managed Access Program for Patients Diagnosed With Severe/Very Severe COVID-19 Illness | Policlinico S.Marco Gruppo San Donato University and Research Hospital, Zingonia, Bergamo, Italy | March–April 2020 (inclusions), November 2020 (published) | Severe COVID-19 (no mechanical ventilation at diagnosis) | 75 (32 on ruxolitinib, 43 as control) | Interventional, non-randomized | Monocentric | Ruxolitinib, 5 mg Twice daily 7 days, 5 mg/d 3 days + Methyl-prednisolone 1 mg/kg/day IV 3 days, 0.5 mg/kg/day 5 days, then oral prednisone tapered over 2 weeks |
Evolution of the COVID-19 disease | D'Alessio et al146 | |
| February 2020 (included), July 2020 (published) | Severe COVID-19 | 41 (20 on ruxolitinib, 21 as control) | Interventional, prospective, single-blind, randomized and controlled | Phase 2 | Multicentric (three in China) | Ruxolitinib, 5 mg Twice daily and standard-of-care (SOC) vs Placebo (C vitamin, 100 mg Twice daily) and SOC |
Time to clinical improvement | Cao et al147 | |||
| trial register no. 81 April 2020 (Italian COVID-19 Ethical Committee) NCT04361903 |
Ruxolitinib for the Treatment of ARDS in Patients With COVID-19 Infection (COVID-19: Ruxolitinib for the Treatment of cytokinE Storm resPiratory dIstREss Syndrome. RESPIRE Study) |
August 2020 (published) | COVID-19-related ARDS | 18 | Observational, retrospective | Multicentric (three in Italy) | Ruxolitinib, 20 mg Twice daily 2 days and de-escalation to 5 mg Twice daily | Degree of respiratory impairement | Capochiani et al148 | ||
| MPN-COVID | European Leukemia Network | MPNs | 175 | Observational, retrospective | Multicentric, in 38 European centers (France, Germany, Italy, Poland, Spain and the UK) |
|
Barbui et al151 | ||||
| Royal Berkshire Hospital NHS Foundation Trust, Reading, UK | COVID-19 with secondary haemophagocytic lymphohistiocytosis (sHLH) Patients with medical history of lymphoma |
2 | Cases report | Monocentric | Ruxolitinib + Tocilizumab |
Case report (severe diseases) | Portsmore et al153 | ||||
| NCT04362137 | Phase 3 Randomized, Double-blind, Placebo-controlled Multi-center Study to Assess the Efficacy and Safety of Ruxolitinib in Patients With COVID-19 Associated Cytokine Storm (RUXCOVID) | Novartis Pharmaceuticals | Completed | COVID-19-related cytokine storm | 432 | Interventional, randomized, double-blind, placebo-controlled | Phase 3 | Multicentric | Ruxolitinib, 5 mg Twice daily vs Placebo |
|
|
| NCT04377620 | Assessment of Efficacy and Safety of Ruxolitinib in Participants With COVID-19-Associated ARDS Who Require Mechanical Ventilation (RUXCOVID-DEVENT) | Incyte Corporation | Terminated | COVID-19-associated ARDS AND intubation and mechanical ventilation |
211 | Interventional, randomized, double-blind, placebo-controlled | Phase 3 | Multicentric | Ruxolitinib, 5 mg or 15 mg, Twice daily and SOC vs Placebo and SOC |
Death rate | |
| NCT04402866 | TD-0903 for ALI Associated With COVID-19 | Theravance Biopharma (sponsor) | completed (published) | Oxygen-requiring patients with COVID-19-associated:
|
235 | Randomized, double-blind, parallel-group trial, placebo-controlled | Phase 2 | Multicentric (the UK, Moldova and Ukrain) | Nezulcitinib (ascending-dose cohorts from 1 to 10 mg/day) for up to 7 days vs Placebo |
No of respiratory failure-free days (Baseline through Day 28) | Singh et al157 |
ALI, acute lung injury; ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; JAK, Janus kinases; MF, myelofibrosis.
JAKi in use in systemic rheumatic diseases
The effect of the COVID-19 pandemic on people with inflammatory or autoimmune rheumatic diseases remains unclear and, as immune-compromised patients, people with systemic rheumatic diseases are at increased risk of infection, including by SARS-CoV-2.104 This is due to their underlying immune conditions and to immune-modulating therapies such as biologics. Whether background immunosuppressive medications put individuals with rheumatic disease at an increased or decreased risk for severe SARS-CoV-2 infection is unknown, and evidence is lacking to guide treatment decisions.105 This population may, however, represent an interesting group to study as some disease-modifying drugs commonly used to treat rheumatic diseases, such as hydroxychloroquine, or biologics targeting interleukin IL-6 (as tocilizumab)51 52 106 107 or sarilumab,108 IL-1 (as anakinra)56 or JAKi are being or have been assessed in patients with severe COVID-19.
It should be added that in times of broad vaccination against COVID-19 in Western countries, one study demonstrated that the overall response rate to COVID-19 vaccine in patients suffering from systemic rheumatic diseases treated with JAKi remained high,109 in line with rates reported with other immunosuppressants.110
In this review, we will develop on the use of JAKi for the treatment of severe form of COVID-19.
Baricitinib
Baricitinib, a JAK1/JAK2 inhibitor, was suggested as soon as February 2020 as a potential treatment for COVID-19 acute respiratory disease111 using BenevolentAI’s knowledge graph. BenevolentAI is a large repository of structured medical information that include different and numerous connections extracted by machine learning. Adaptation/customization to COVID-19 was applied to this resource and approved drugs that may inhibit the viral infection were searched. Baricitinib was identified as a potential molecule that could inhibit infection of lung cells by SARS-CoV-2. One of the key cell subtypes expressing the ACE2 receptor are lung AT2 alveolar cells. Viruses enter by endocytosis and many small molecule kinase inhibitors were shown to prevent entry into cells of different virus types.112 113 One regulator of endocytosis is AP2-associated protein kinase 1 (AAK1), which belongs to the group of Numb-Associated Kinases. A prediction was formulated that inhibition of AAK1 could prevent SARS-CoV-2 entry and then also intracellular virion assembly. One of the molecules predicted to inhibit AAK1 was baricitinib which also binds to another endocytosis regulator, cyclin G-associated kinase. The prediction was that baricitinib would inhibit both virus entry (via AAK1 targeting) and inflammation by targeting JAK1 and JAK2 downstream many cytokine receptors during cytokine storm.111
This work was extended and the affinity and selectivity of the identified drugs were examined in order to point to anti-inflammatory and antiviral drugs. Baricitinib, fedratinib and ruxolitinib were highlighted as having similar JAK-STAT inhibitory potency, baricitinib stood out due to its AAK1 inhibition capacity. In a EMEA trial with baricitinib for autoimmune diseases in 4214 patients, a small increase in upper respiratory infection has been seen, but the incidence of severe infections was similar to placebo.114 That baricitinib holds the potential to inhibit SARS-CoV-2 infection was also reviewed in reference 115.
Experimental evidence showed that indeed baricitinib exerts an antiviral effect separated from its anti-inflammatory effects.116 117 In a case series of patients with bilateral COVID-19 pneumonia, baricitinib treatment was associated with clinical and radiologic recovery, a rapid decline in SARS‐CoV‐2 viral load, inflammatory markers and IL6 levels. Collectively, these data supported further evaluation of the anti‐cytokine and anti‐viral activity of baricitinib and suggested its assessment in randomized trials in hospitalized COVID-19 patients.117
Indeed, baricitinib reduced the immune dysregulation in severe COVID-19 patients. A group of 20 patients was treated in an observational, longitudinal trial (IMMUNOVID) with baricitinib at 4 mg two times per day for 2 days, followed by 4 mg per day for another 7 days (NCT04438629).118 Treated patients exhibited markedly reduced levels of IL6, IL1β and TNFα, recovery of circulating T and B cells and increased antibody production against the Spike protein. This was associated with a reduction in the need for oxygen therapy. This study suggested that baricitinib can prevent progression to severe disease.
Baricitinib reduced lung inflammation in a rhesus macaque model of SARS-CoV-2 infection.119 While viral shedding was not reduced by baricitinib and T cell responses were similar, animals treated with baricitinib showed reduced inflammation, decreased lung infiltration and exhibited a reduction in lung pathological changes. Lung macrophages production of cytokines and chemokines responsible for inflammation and neutrophil recruitment was reduced by baricitinib.119
A beneficial effect of baricitinib was reported in COVID-19 moderate pneumonia in a retrospective multicenter study (NCT04358614).120 In this study baricitinib reduced COVID-19 mortality rate, intensive care unit admissions of COVID-19 pneumonia and SARS-CoV-2 viral burden in nasopharyngeal swabs and when used during 14 days, did not induce adverse effects.
Results from the trial of the ACTT-2 trial assessing baricitinib plus remdesivir for hospitalized adults with COVID-19 (NCT04401579)121 give support to the approach of inhibiting JAK1/JAK2 in severe COVID-19 disease. This adaptive, double-blind, randomized and placebo-controlled phase 3 trial on 1033 patients (515 receiving both drugs and 518 receiving just remdesivir) evaluated baricitinib plus remdesivir in hospitalized adults with COVID-19. Remdesivir was administered for ≤10 days, and either baricitinib or placebo (control) for ≤14 days. The primary outcome was time to recovery and a secondary outcome was clinical status at day 15. The combination of baricitinib plus remdesivir was superior to remdesivir alone in both counts with a 1-day shortening of recovery time in the baricitinib arm (median 7 days, 95% CI (6 to 8 days) vs 8 days in the control group, 95% CI (7 to 9 days)) and 30% higher odds of improvement in clinical status at day 15 (OR, 1.3; 95% CI (1.0 to 1.6)). Patients receiving high-flow oxygen or noninvasive ventilation at enrollment had a time to recovery of 10 days with combination treatment and 18 days with control (rate ratio for recovery, 1.51; 95% CI (1.10 to 2.08)). The 28-day mortality rate was 5.1% in the combination group and 7.8% in the control group (HR for death, 0.65; 95% CI (0.39 to 1.09)). Thus, baricitinib plus remdesivir was superior to remdesivir alone in reducing recovery time and accelerating improvement in clinical status among patients with COVID-19, notably among those receiving high-flow oxygen or non-invasive ventilation. Following the result of this trial, the FDA issued on November 19, 2020 an emergency use authorization (EUA) for baricitinib in combination with remdesivir, for the treatment of suspected or laboratory confirmed COVID-19 in hospitalized adults and pediatric patients 2 years of age or older requiring supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).122
The role of addition of glucocorticoids was not evaluated in the ACTT-2 trial, but dexamethasone stayed permitted in standard indications as ARDS. As a matter of fact, the adapative, randomized, blinded controlled phase 3 ACTT-4 trial, which primary objective was to evaluate the clinical efficacy of baricitinib in combination with remdesivir vs dexamethasone and remdesivir as assessed by the mechanical ventilation-free survival by day 29, was closed to enrolement in April 2021, prior to full enrolment, after intermediate efficacy analysis found that neither treatment regimen was significantly better than the other.123
Then, results were announced from the phase 3 COV-BARRIER (NCT04421027) trial of baricitinib in hospitalized COVID-19 patients.124 The international, double-blinded, placebo-controlled study randomized 1525 patients to baricitinib or standard of care alone. Although the study did not meet its primary endpoint for improvement in progression to non-invasive ventilation or invasive mechanical ventilation, or death, there was a statistically significant improvement in mortality for patients treated with baricitinib in addition to standard of care, including corticosteroids and remdesivir.124 Importantly, the COV-BARRIER study showed that the survival benefits provided by baricitinib were independent of the presence or absence of concomitant use of steroids (mostly dexamethasone). Following these results, the FDA revised the EUA for baricitinib on July 28, 2021, to an EUA authorizing the drug alone for the treatment of COVID-19 in hospitalized adults and pediatric patients 2 years of age or older requiring supplemental oxygen, non-invasive or invasive mechanical ventilation, or ECMO. It has to be mentioned that baricitinib was approved expressely mentioning the fact that it was not ‘FDA-approved as a treatment for COVID-19’.125
In conclusion, in the USA126 and Japan127 (in consideration by the EMA) baricitinib in combination with low-dose dexamethasone is currently recommended for the treatment of COVID-19 patients requiring high-flow oxygen or non-invasive ventilation or low-flow oxygen but with significantly elevated inflammatory markers. The same conclusions were reached by the WHO from the eighth version of its ‘A living WHO guideline on drugs for COVID-19’ from January 2022, which strongly recommended baricitinib for patients with severe or critical COVID-19 in combination with corticosteroids.128
In all those indications, baricitinb was indicated as an alternative treatment to the IL-6 inhibitor tocilizumab. To date in an extremely quickly evolving field, use of baricitinib is not recommended in patients requiring mechanical ventilation or ECMO, as it was judged that more data were needed in this population. Baricitinib seemed nevertheless a reasonable alternative to tocilizumab if the latter is not available.
As for patients with systemic rheumatic diseases, the indication to discontinue treatment in patients with known exposure to SARS-CoV-2 seems prevalent but is still debated and should be discussed with specialist physicians keeping in mind the risk of disease flare.129
Tofacitinib
Tofacitinib is a JAK1 (IC50 15 nM) and JAK3 (IC50 45–55 nM) inhibitor known to be effective against cytokines signaling via JAK1 and JAK3.77 87 It also inhibits JAK2 with less potency (IC50 71–77 nM), while the IC50 for TYK2 is 472–489 nM.87 130 The efficacy and safety of tofacitinib in patients hospitalized with COVID-19 pneumonia has been recently reported in the STOP-COVID trial, a randomized, double-blind, interventional phase 3 trial (NCT04469114) of 289 patients randomized 1:1 between placebo and tofacitinib 10 mg two times per day; most patients received also glucocorticoids.131 Tofacitinib led in hospitalized patients with COVID-19 pneumonia to a lower risk of death or respiratory failure through day 28 when compared with placebo. Specifically, death from any cause through day 28 occurred in 2.8% of the patients in the tofacitinib group vs 5.5% in the placebo group (HR, 0.49; 95% CI 0.15 to 1.63), with a cumulative incidence of death or respiratory failure through day 28 of 18.1% vs 29.0% in the tofacitinib vs placebo group (risk ratio, 0.63; 95% CI 0.41 to 0.97; p=0.04).131 These results let the US National Institute of Health (NIH) to recommend tofacitinib as an alternative to baricitinib when unavailable.126 Of note, the WHO recommended against the use of tofacitinib in January 2022, considering lack of evidence from small trials and possible increase in serious side effects with tofacitinib.128 132
Upadacitinib
Upadacitinib was reported to be a JAK1 specific inhibitor.133 It exhibits sub nM IC50 for JAK1 (0.76 nM), 2 nM for JAK2 and >100 nM for JAK3 and TYK2.130 Its in vitro pharmacology was determined in primary peripheral mononuclear cells from healthy subjects in comparison with baricitinib and tofacitinib.134 In primary cells upadacitinib also inhibited JAK2 downstream IL3 type cytokines, not only JAK1 downstream inflammatory cytokines like IL6.134 Given this profile of inhibition, this inhibitor may be useful in counteracting the broad signaling via JAK1 and JAK2 in cytokine storm. Of interest an in-silico docking study found significant binding of kinase inhibitors like upadacitinib to the RNA dependent RNA polymerase kinase-like folded NiRAN domain.135
JAKi most in use in hematology patients
Ruxolitinib
Ruxolitinib is a JAK1 and JAK2 inhibitor used for around 10 years in the clinics for the treatment of MPNs MF and PV.
In general, hematological cancer patients exhibit enhanced mortality on SARS-CoV-2 infection when compared with healthy individuals.136 137 A cohort study in Wuhan on hospitalized hematological cancer patients showed an incidence of 10% that developed COVID-19 infection. While the incidence was relatively similar to that in healthcare providers (7%), the mortality rate of hematological patients (no MPN in the study) was increased to 62% vs 0% of healthcare providers,138 estimated 41 times higher than hematological patients without SARS-CoV-2 infection.139 Interestingly, a lower rate of SARS-CoV-2 infection was recorded both in China and elsewhere for BCR/ABL1 positive chronic myeloid leukemia.137 140 However, no significant attenuation of T cell response was detected in MPN patients compared with healthy subjects.141
Ruxolitinib was tested in several clinical settings, from one patient studies to phase 3 clinical trials (table 1, ClinicalTrials.gov). However, although a positive impression first came from case reports or small, uncontrolled or non-randomized cases series142–148 that ruxolitinib might be helpful to fight COVID-19, these results were not confirmed in larger randomized trials. Press releases have been issued after the results of two phase 3, randomized, placebo-controlled clinical trials with the use of ruxolitinib in hospitalized non-ventilated patients (RUXCOVID, NCT04362137) and in ventilated patients (RUXCOVID-DEVENT, NCT04377620). Neither study met its primary endpoint. In the RUXCOVID study, 432 patients were enrolled. The results showed no improvement in the proportion of patients who experienced death, respiratory failure requiring mechanical ventilation or admission to the intensive care unit, by day 29 for patients receiving ruxolitinib 5 mg bidaily compared with those receiving standard of care alone (12.0% vs 11.8%; p=0.769). There was also no clinically relevant benefit in mortality rate by day 29 or in time to recovery.
The phase 3 RUXCOVID-DEVENT study evaluated ruxolitinib 5 mg bidaily or 15 mg bidaily as a treatment for patients with COVID-19 associated ARDS on mechanical ventilation. There was no statistically significant improvement in mortality through day 29 compared with placebo. However, when US study participants were analyzed separately there was significance, as it also could be detected for the overall population when data from both treatment arms were pooled. The safety findings in both RUXCOVID and in RUXCOVID-DEVENT were consistent with those expected for ruxolitinib and for patients with COVID-19 infection.
The development of ruxolitinib in the COVID-19 setting has not proceeded after these results149 and ruxolitinib has not be retained by the WHO128 or other drug agencies as a drug to be used in the setting of COVID-19.
Many reasons could explain the fact that ruxolitinib, which has the same spectrum of JAKs inhibition as baricitinib, did not improve patients with COVID-19. One would be that BenevolentAI did not predict ruxolitinib as having a possible impact on reducing SARS-CoV-2 pathogenicity. Furthermore, it is not clear if patients in the RUXCOVID and RUXCOVID-DEVENT trials were constantly on antiviral therapy. Nevertheless, coadministration of remdesivir with baricitinib in the COV-BARRIER trial concerned less than 20% of the patients and the placebo and test arms were appropriately balanced.124 Hence, it is unlikely that it influenced the results of the study. Doses of ruxolitinib in the RUXCOVID trial were also lower than in most publications, but higher doses did not improve outcome in the RUXCOVID-DEVENT trial. As for the best time of intervention, no phase 3 study evaluated early treatment with ruxolitinib but both RUXCOVID and RUXCOVID-DEVENT studied different time of evolution of the disease and neither met its endpoint. Finally, best efficacy of baricitinib or tofacitinib is achieved in combination with corticosteroids, and such combination was not studied in a dedicated trial in the case of ruxolitinib. Once again however, publications mainly focus on trials with positive ending. Details of failed ones are much more difficult to get access to and the decision behind the arrest in the development of ruxolitinib can only be interfered from press releases by Novartis/Incyte.
The COVID-19 pandemia had a major impact on how MPN patients are managed.150 Studies on the evolution of MPN patients with respect to COVID-19 infection led by default to examination of effects of ruxolitinib because many patients were under treatment and sudden discontinuation of ruxolitinib is generally avoided in MPNs due to potential rebound effects on myeloid proliferation.
In a retrospective study with 175 MPN patients, it was reported that MPN patients’ mortality was higher than in the general population and was 48% in MF, while ET, PV and prefibrotic MF appeared more similar to the general population.151 That ruxolitinib treatment was significantly more frequent in patients who died in comparison with survivors (p=0.006) may be explained because the severity of the MPN was higher and necessitated treatment. Following multivariable analysis, ruxolitinib treatment alone did apparently not impact on mortality in this study. In an independent manner, it was established that discontinuing ruxolitinib in MPN patients with COVID-19 is associated with increased mortality.151 Therefore, in MPN patients on treatment with ruxolitinb who would develop COVID-19, its discontinuation should always be considered with care and discussed in concertation with the referent hematologistand guided by the presence of objective clinical reasons for discontinuation like worsening clinical condition, inability to take peroral medication or contraindications for ruxolitnib treatment (thrombocytopenia, anemia, bleeding, and bacterial sepsis), all of whom might be negative prognostic factors per se.152
A combination of anti-IL-6 therapy and ruxolitinib was proposed153 based on the resemblance of inflammation in severe COVID-19 with the inflammatory picture detected in the pathological condition HLH.154 This laboratory profile predicted severe evolution.155 Such patients were thought to possibly benefit from combining IL-6 inhibitor tocilizumab and ruxolitinib. In latest guidelines on COVID-19; however, no combination of tocilizumab and any JAKi is recommended.
Nezulcitinib
Nezulcitinib is a panJAKi with IC50s of 10.3 nM, 10.6 nM, 10.2 NM and 9.2 nM for JAK1, JAK2, JAK3 and TYK2, respectively156 that can be administered by inhalation. A phase 1 study in healthy subjects of inhaled nezulcitinib demonstrated safety and minimal plasma exposure when given by inhalation. This was also supported by no effects on NK activity. Single and multiple doses of inhaled nezulcitinib at 1, 3 and 10 mg had no safety issues.156 A phase 2 clinical trial with inhaled nezulcitinib in severe COVID-19 is underway and initially reported trends for improved oxygenation and clinical status, shortened hospitalization, and fewer deaths vs placebo (NCT04402866).157 However, in the long-run the top-line data news reported that inhaled nezulcitinib failed to meet the primary end-point in hospitalized patients with COVID-19 with acute lung injury and impaired oxygenation.158
Meta-analyses and comparative assessment of JAKi
Several meta-analysis studies were reported on safety and efficacy of use of JAKi, especially baricitinib and ruxolitinib in COVID-19.159–161
One examined effect of JAKi in patients with COVID-19 published between Jan 1, 2020 and March 6, 2021. Six cohort studies and five clinical trials were assessed with 2367 patients.159 Precise timing of start of JAKi treatment was not consistently reported. JAKi decreased use of invasive mechanical ventilation, but only had marginal effects on rates of intensive care unit admissions and ARDS. The precise effect of timing of JAKi treatment which could be critical could not be assessed and correlated with effects.159 The quality assessment of the published studies was performed with the Newcastle-Ottawa and Jadad scales.159
In another study160 several electronic databases (PubMed, EuropePMC, the Cochrane Central Register of Controlled Trials) were searched up to December 11, 2020 for published studies bearing keywords “COVID-19′′ along with (“JAK inhibitor” or “Ruxolitinib” or “Tofacitinib” or “Fedratinib” or “Baricitinib”) and (“Severe” or “Mortality”). Five studies with 1190 patients were included. Use of JAKi was significantly associated with a reduced risk of mortality and clinical improvement in COVID-19 hospitalized patients.
The same overall conclusions were reported by a meta-analysis,161 which examined two databases for RCTs in hospitalized patients, where patients were assigned to JAKi or standard of care. Pooling data from 4 such trials with 1338 subjects and treatment with either baricitinib, ruxolitinib, tofacitinib and nezulcitinib. Treatment with a JAKi led to a significant reduction in the risk of COVID-19 death by 43%.161
Although these meta-analyses provide a positive image on the efficacy of JAKi in COVID-19, there are several weaknesses. There is redundancy in the studies interrogated. Head-to-head comparisons of different inhibitors in similar settings was not performed. In addition, lack of systematic information on the timing of start of treatment and the variability of simultaneous glucocorticoid treatment further complicate interpretation.
Potential adverse effects
There are known adverse effects of the JAKi, which have been described in phase 3 clinical trials with ruxolitinib in MF and PV and then observed for other JAK2 inhibitors.162–164 Given that JAK2 is essential for formation of red blood cells and platelets, anemia and thrombocytopenia can be induced by the use of JAK2 inhibitors.162–164 In addition, given the involvement of JAKs in the immune response especially via IFNγ, reactivation of latent herpes simplex, zona-zoster, hepatitis B infections and tuberculosis are well known potential adverse effects. Several patients treated for MPNs with ruxolitinib developed aggressive B cell lymphomas.165 Given that the timeframe of administration of JAKi is rather short (less than 30 days) in COVID-19 the expectation is that the effects reported after long-term use will not be detected.
Several JAKi are being investigated for potential thrombotic risk.166 This is apparently the case for tofacitinib and upacitinib (JAK1 inhibitor).166 This issue is of great relevance given the thrombotic risk of COVID-19. Also, baricitinib should be examined for potential thrombotic risk.167 In September 2021, FDA issued an updated warning about increased risk of serious heart-related events, cancer, bloot clots and death for JAKi that treat certain chronic inflammatory conditions.168 This warning is based on a review of a large randomized safety clinical trial involving tofacitinib, but the warning has been extended to other JAKi treating such chronic inflammatory condition, such as baricitinib and upacitinib. This warning may not be relevant for treatment with JAKi in COVID-19 due to the short treatment duration, while the safety trial concerned chronic longer exposure to JAKi.
A couple of potential adverse effects of JAKi including cardiotoxicity and hepatotoxicity, as well as toxicity of combinations including JAKi have been reviewed in.169 The coadministration, of either baricitinib or ruxolitinib, along with IL-6 inhibitors (eg, tocilizumab, siltuximab) could produce additive immunosuppression with possible severe bacterial or fungal infections. Ruxolitinib inhibits colchicine metabolism and this is relevant in patients with renal or hepatic impairment. Favipiravir may enhance baricitinib exposure. In contrast, no adverse effects have been reported for simultaneous use of JAKi and remdesivir or dexamethasone.
Conclusions
Although the pandemic situation seems to wear-off in western countries and vaccines against SARS-CoV-2 have led to limiting hospitalisations, predicting the future evolution of SARS-CoV-2 infection in the human population is difficult. Many questions remain concerning new outbreaks, evolution of the virus or how long protection following natural infection or vaccination will last. Therefore, the potential for drugs to treat people suffering from COVID-19 remains of interest.
The major targets for inhibition in COVID-19 are the entry and replication of SARS-CoV-2 and the immunopathological phenomena triggered by the delayed cytokine storm inducing lung pathology and ARDS. Since cytokines act via JAKs, JAK1 and JAK2 inhibitors may emerge as potential useful therapeutic tools in the COVID-19 pathology. A JAK1 and JAK2 inhibitor, baricitinib also inhibits the Numb Associated Kinase AAK1, which is involved in endocytosis of the virus. Hence baricitinib would act both via inhibiting the immunopathology and inflammation due to JAK1/JAK2 inhibition, but also via direct inhibition of virus entry and assembly due to AAK1 inhibition. Positive results from the double-blind, randomized, placebo-controlled ACCT-2 and COV-BARRIER trials led to the FDA recommendation of baricitinib in certain settings of COVID-19 and its recommendation in U.S. NIH practice guideline for treatment of COVID-19. The same conclusison were reached by the expert committee of the WHO that recommends baricitinib in severe and criticial COVID-19 since the eighth version of its ‘living guideline on drugs for COVID-19’ from January 2022. Second, the JAK2 and JAK3 inhibitor tofacitinib also got recommended by the U.S. NIH in case of baricitinib unavailability. However other alternative exists, such as IL-6 receptor blockers, which should be used in priority according to latest WHO guidelines.
Ruxolitinib is the oldest JAKi on the market and the only one used in the hematological setting. It is often used in the treatment of MF and certain PV patients, and it was shown that on SARS-CoV-2 infection the inhibitor is generally not stopped and should be continued to control the MPN. It has been suggested that ruxolitinib may reduce immunopathological reactions as it does for GVHd. Although small non-controlled studies suggested some benefit for ruxolitinib in the treatment of COVID-19 and meta-analyses suggested a positive effect, the two randomized phase 3 studies failed to meet their primary endpoints, which led to suspension of ruxoltinib development program in the setting of COVID-19. The different outcomes between these RCTs and smaller studies point to the complexity of conducting clinical studies in patients with COVID-19. The outcomes may have been affected by differences in standards of care at different sites and in different regions at different times during the course of the pandemic. These standards of care include varying proportions of patients receiving corticosteroids, which has been shown to affect the outcomes for hospitalized patients. The studies also enrolled patients at different stages of COVID-19 and patients with more advanced disease may respond better to JAKi. Differences in outcomes between ruxolitinib and baricitinib in different studies may also reflect the doses used.
The results on JAKi clinical use analyzed in several meta-analyses and in the studies detailed above suggest but do not prove a role for JAKi in COVID-19, as the only robust data are for an inhibitor that also exerts other effects than JAKi. Better designed trials with precisely controlled time of introduction of JAKi will be critical in the future. A favorable action of JAKi would be predicted by the mechanistic understanding of the COVID-19 immunopathology involving cytokines that require JAK1 for signaling. However, robust clinical confirmation is still needed. In principle, JAKi can be envisaged at times of development of cytokine storm but should not be used too early in COVID-19, as type I IFN signaling requires JAK1. Careful timing is required since pathological secretion of IL-6/IL-1 may occur in the same time-frame with the requirement for type I IFN action to clear the virus, and therefore JAKi may need to be associated with antiviral molecules.
As more molecules keep being approved in emergency for treatment of COVID-19, RCTs are important to compare their definitive effect and determine the best indication(s) for each molecule. Also, RCTs with combination therapies would be all the more important for patient who might be already on treatment with JAKi—which it might be dangerous to stop abruptly in an emergency situation.
More data will emerge on secondary effects of JAKi in the setting of COVID-19. For example, both JAKi and COVID-19 were linked to potential thrombotic risk of their own but no evidence to date exist for an increased thrombotic risk while using JAKi for treating COVID-19. Moreover, adverse effects known from long-term treatment with JAKi, as immunosuppression, are less likely to be relevant in COVID-19, where treatment is of short duration, but should be kept in mind.
Footnotes
Correction notice: This article has been corrected since it was first published online. The author Stefan Constantinescu has been updated to Stefan N Constantinescu.
Contributors: SC, PG, PL and GL planned, wrote and reviewed review.
Funding: Funding to SC is acknowledged from Ludwig Institute for Cancer Research, Fondation contre le cancer, Salus Sanguinis and Fondation 'Les avions de Sébastien', projects Action de recherché concertée (ARC) 16/21-073 and WelBio F 44/8/5 - MCF/UIG -10 955. GL is the recipient of a PhD Fellowship from Fondation 'Les avions de Sébastien'. GL was supported by a PhD fellowship from the Fondation 'Les avions de Sébastien'.
Competing interests: SC is cofounder of MyeloPro Diagnostics and Research, Vienna and of AlsaTech, Boston, MA, companies that aim to treat myeloproliferative neoplasms (MyeloPro) and neurodegenerative diseases (AlsaTech). PL is an employee of Incyte which developed ruxolitinib and owns stock of Incyte.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Watowich SS, Wu H, Socolovsky M, et al. Cytokine receptor signal transduction and the control of hematopoietic cell development. Annu Rev Cell Dev Biol 1996;12:91–128. 10.1146/annurev.cellbio.12.1.91 [DOI] [PubMed] [Google Scholar]
- 2.Heinrich PC, Behrmann I, Haan S, et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 2003;374:1–20. 10.1042/BJ20030407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uzé G, Schreiber G, Piehler J, et al. The receptor of the type I interferon family. Curr Top Microbiol Immunol 2007;316:71–95. 10.1007/978-3-540-71329-6_5 [DOI] [PubMed] [Google Scholar]
- 4.Stark GR, Kerr IM, Williams BR, et al. How cells respond to interferons. Annu Rev Biochem 1998;67:227–64. 10.1146/annurev.biochem.67.1.227 [DOI] [PubMed] [Google Scholar]
- 5.Constantinescu SN, Huang LJ, Nam H, et al. The erythropoietin receptor cytosolic juxtamembrane domain contains an essential, precisely oriented, hydrophobic motif. Mol Cell 2001;7:377–85. 10.1016/s1097-2765(01)00185-x [DOI] [PubMed] [Google Scholar]
- 6.Seubert N, Royer Y, Staerk J, et al. Active and inactive orientations of the transmembrane and cytosolic domains of the erythropoietin receptor dimer. Mol Cell 2003;12:1239–50. 10.1016/s1097-2765(03)00389-7 [DOI] [PubMed] [Google Scholar]
- 7.Moraga I, Wernig G, Wilmes S, et al. Tuning cytokine receptor signaling by re-orienting dimer geometry with surrogate ligands. Cell 2015;160:1196–208. 10.1016/j.cell.2015.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilmes S, Hafer M, Vuorio J, et al. Mechanism of homodimeric cytokine receptor activation and dysregulation by oncogenic mutations. Science 2020;367:643–52. 10.1126/science.aaw3242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briscoe J, Guschin D, Rogers NC, et al. JAKs, STATs and signal transduction in response to the interferons and other cytokines. Philos Trans R Soc Lond B Biol Sci 1996;351:167–71. 10.1098/rstb.1996.0013 [DOI] [PubMed] [Google Scholar]
- 10.Huang LJ, Constantinescu SN, Lodish HF. The N-terminal domain of Janus kinase 2 is required for Golgi processing and cell surface expression of erythropoietin receptor. Mol Cell 2001;8:1327–38. 10.1016/s1097-2765(01)00401-4 [DOI] [PubMed] [Google Scholar]
- 11.Ragimbeau J, Dondi E, Alcover A, et al. The tyrosine kinase Tyk2 controls IFNAR1 cell surface expression. Embo J 2003;22:537–47. 10.1093/emboj/cdg038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Royer Y, Staerk J, Costuleanu M, et al. Janus kinases affect thrombopoietin receptor cell surface localization and stability. J Biol Chem 2005;280:27251–61. 10.1074/jbc.M501376200 [DOI] [PubMed] [Google Scholar]
- 13.Radtke S, Jörissen A, de Leur HS-V, et al. Three dileucine-like motifs within the interbox1/2 region of the human oncostatin M receptor prevent efficient surface expression in the absence of an associated Janus kinase. J Biol Chem 2006;281:4024–34. 10.1074/jbc.M511779200 [DOI] [PubMed] [Google Scholar]
- 14.Lupardus PJ, Ultsch M, Wallweber H, et al. Structure of the pseudokinase-kinase domains from protein kinase Tyk2 reveals a mechanism for Janus kinase (JAK) autoinhibition. Proc Natl Acad Sci U S A 2014;111:8025–30. 10.1073/pnas.1401180111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallweber HJA, Tam C, Franke Y, et al. Structural basis of recognition of interferon-α receptor by tyrosine kinase 2. Nat Struct Mol Biol 2014;21:443–8. 10.1038/nsmb.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrao RD, Wallweber HJ, Lupardus PJ. Receptor-Mediated dimerization of JAK2 FERM domains is required for Jak2 activation. Elife 2018;7:e38089. 10.7554/eLife.38089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ihle JN, Gilliland DG. Jak2: normal function and role in hematopoietic disorders. Curr Opin Genet Dev 2007;17:8–14. 10.1016/j.gde.2006.12.009 [DOI] [PubMed] [Google Scholar]
- 18.Murakami M, Narazaki M, Hibi M, et al. Critical cytoplasmic region of the interleukin 6 signal transducer gp130 is conserved in the cytokine receptor family. Proc Natl Acad Sci U S A 1991;88:11349–53. 10.1073/pnas.88.24.11349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol 2015;1282:1–23. 10.1007/978-1-4939-2438-7_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartenian E, Nandakumar D, Lari A, et al. The molecular virology of coronaviruses. J Biol Chem 2020;295:12910–34. 10.1074/jbc.REV120.013930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajah MM, Hubert M, Bishop E, et al. SARS-CoV-2 alpha, beta, and delta variants display enhanced spike-mediated syncytia formation. Embo J 2021;40:e108944. 10.15252/embj.2021108944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Liu J, Xia H, et al. BNT162b2-Elicited neutralization against new SARS-CoV-2 spike variants. N Engl J Med 2021;385:472–4. 10.1056/NEJMc2106083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu K, Werner AP, Koch M, et al. Serum neutralizing activity elicited by mRNA-1273 vaccine. N Engl J Med 2021;384:1468–70. 10.1056/NEJMc2102179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Donnell JS, Chappell KJ. Chronic SARS-CoV-2, a cause of post-acute COVID-19 sequelae (Long-COVID)? Front Microbiol 2021;12:724654. 10.3389/fmicb.2021.724654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021;591:639–44. 10.1038/s41586-021-03207-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamers MM, Beumer J, van der Vaart J, et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020;369:50–4. 10.1126/science.abc1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Richards A, Barrasa MI, et al. Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc Natl Acad Sci U S A 2021;118:e2105968118. 10.1073/pnas.2105968118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med 2020;383:2291–3. 10.1056/NEJMc2031364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kemp SA, Collier DA, Datir RP, et al. SARS-CoV-2 evolution during treatment of chronic infection. Nature 2021;592:277–82. 10.1038/s41586-021-03291-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corey L, Beyrer C, Cohen MS, et al. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med Overseas Ed 2021;385:562–6. 10.1056/NEJMsb2104756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Channappanavar R, Fehr AR, Zheng J, et al. Ifn-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J Clin Invest 2019;129:3625–39. 10.1172/JCI126363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017;39:529–39. 10.1007/s00281-017-0629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LaFleur DW, Nardelli B, Tsareva T, et al. Interferon-kappa, a novel type I interferon expressed in human keratinocytes. J Biol Chem 2001;276:39765–71. 10.1074/jbc.M102502200 [DOI] [PubMed] [Google Scholar]
- 34.Marks ZRC, Campbell N, deWeerd NA, et al. Properties and functions of the novel type I interferon epsilon. Semin Immunol 2019;43:101328. 10.1016/j.smim.2019.101328 [DOI] [PubMed] [Google Scholar]
- 35.Lazear HM, Schoggins JW, Diamond MS. Shared and distinct functions of type I and type III interferons. Immunity 2019;50:907–23. 10.1016/j.immuni.2019.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manry J, Laval G, Patin E, et al. Evolutionary genetic dissection of human interferons. J Exp Med 2011;208:2747–59. 10.1084/jem.20111680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li MMH, MacDonald MR, Rice CM. To translate, or not to translate: viral and host mRNA regulation by interferon-stimulated genes. Trends Cell Biol 2015;25:320–9. 10.1016/j.tcb.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020;370:eabd4585. 10.1126/science.abd4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020;370:eabd4570. 10.1126/science.abd4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asano T, Boisson B, Onodi F, et al. X-linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci Immunol 2021;6:eabl4348. 10.1126/sciimmunol.abl4348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bastard P, Gervais A, Le Voyer T, et al. Autoantibodies neutralizing type I IFNs are present in ~4% of uninfected individuals over 70 years old and account for ~20% of COVID-19 deaths. Sci Immunol 2021;6:eabl4340. 10.1126/sciimmunol.abl4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pairo-Castineira E, Clohisey S, Klaric L, et al. Genetic mechanisms of critical illness in COVID-19. Nature 2021;591:92–8. 10.1038/s41586-020-03065-y [DOI] [PubMed] [Google Scholar]
- 43.Roberts RM, Liu L, Alexenko A. New and atypical families of type I interferons in mammals: comparative functions, structures, and evolutionary relationships. Prog Nucleic Acid Res Mol Biol 1997;56:287–325. 10.1016/s0079-6603(08)61008-9 [DOI] [PubMed] [Google Scholar]
- 44.Kotenko SV, Gallagher G, Baurin VV, et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol 2003;4:69–77. 10.1038/ni875 [DOI] [PubMed] [Google Scholar]
- 45.Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID-19: current state of the science. Immunity 2020;52:910–41. 10.1016/j.immuni.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peruzzi B, Bencini S, Capone M, et al. Quantitative and qualitative alterations of circulating myeloid cells and plasmacytoid dC in SARS-CoV-2 infection. Immunology 2020;161:345–53. 10.1111/imm.13254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silvin A, Chapuis N, Dunsmore G, et al. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell 2020;182:1401–18. 10.1016/j.cell.2020.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schulte-Schrepping J, Reusch N, Paclik D, et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell 2020;182:1419–40. 10.1016/j.cell.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rébillard R-M, Charabati M, Grasmuck C, et al. Identification of SARS-CoV-2-specific immune alterations in acutely ill patients. J Clin Invest 2021;131:e145853. 10.1172/JCI145853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vardhana SA, Wolchok JD. The many faces of the anti-COVID immune response. J Exp Med 2020;217:e20200678. 10.1084/jem.20200678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.RECOVERY Collaborative Group . Tocilizumab in patients admitted to hospital with COVID-19 (recovery): a randomised, controlled, open-label, platform trial. Lancet 2021;397:1637–45. 10.1016/S0140-6736(21)00676-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Investigators R-C, Gordon AC, Mouncey PR, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med Overseas Ed 2021;384:1491–502. 10.1056/NEJMoa2100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.WHO . Who recommends life-saving interleukin-6 receptor blockers for COVID-19 and urges producers to join efforts to rapidly increase access, 2021. [PMC free article] [PubMed] [Google Scholar]
- 54.FDA . Coronavirus (COVID-19) update: FDA Authorizes drug for treatment of COVID-19. U.S. FDA, 2021. [Google Scholar]
- 55.EMA . Ema recommends approval for use of RoActemra in adults with severe COVID-19, 2021. [Google Scholar]
- 56.Kyriazopoulou E, Poulakou G, Milionis H, et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med 2021;27:1752–60. 10.1038/s41591-021-01499-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.EMA . Ema recommends approval for use of Kineret in adults with COVID-19, 2021. [Google Scholar]
- 58.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054–61. 10.1016/S0140-6736(05)71142-9 [DOI] [PubMed] [Google Scholar]
- 59.James C, Ugo V, Le Couédic J-P, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005;434:1144–8. 10.1038/nature03546 [DOI] [PubMed] [Google Scholar]
- 60.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005;7:387–97. 10.1016/j.ccr.2005.03.023 [DOI] [PubMed] [Google Scholar]
- 61.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 2005;352:1779–90. 10.1056/NEJMoa051113 [DOI] [PubMed] [Google Scholar]
- 62.Scott LM, Tong W, Levine RL, et al. Jak2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 2007;356:459–68. 10.1056/NEJMoa065202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood 2017;129:667–79. 10.1182/blood-2016-10-695940 [DOI] [PubMed] [Google Scholar]
- 64.Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006;3:e270. 10.1371/journal.pmed.0030270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 2006;108:3472–6. 10.1182/blood-2006-04-018879 [DOI] [PubMed] [Google Scholar]
- 66.Staerk J, Lacout C, Sato T, et al. An amphipathic motif at the transmembrane-cytoplasmic junction prevents autonomous activation of the thrombopoietin receptor. Blood 2006;107:1864–71. 10.1182/blood-2005-06-2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pietra D, Brisci A, Rumi E, et al. Deep sequencing reveals double mutations in cis of Mpl exon 10 in myeloproliferative neoplasms. Haematologica 2011;96:607–11. 10.3324/haematol.2010.034793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013;369:2379–90. 10.1056/NEJMoa1311347 [DOI] [PubMed] [Google Scholar]
- 69.Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013;369:2391–405. 10.1056/NEJMoa1312542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chachoua I, Pecquet C, El-Khoury M, et al. Thrombopoietin receptor activation by myeloproliferative neoplasm associated calreticulin mutants. Blood 2016;127:1325–35. 10.1182/blood-2015-11-681932 [DOI] [PubMed] [Google Scholar]
- 71.Araki M, Yang Y, Masubuchi N, et al. Activation of the thrombopoietin receptor by mutant calreticulin in CALR-mutant myeloproliferative neoplasms. Blood 2016;127:1307–16. 10.1182/blood-2015-09-671172 [DOI] [PubMed] [Google Scholar]
- 72.Elf S, Abdelfattah NS, Chen E, et al. Mutant calreticulin requires both its mutant C-terminus and the thrombopoietin receptor for oncogenic transformation. Cancer Discov 2016;6:368–81. 10.1158/2159-8290.CD-15-1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pecquet C, Chachoua I, Roy A, et al. Calreticulin mutants as oncogenic rogue chaperones for TpoR and traffic-defective pathogenic TpoR mutants. Blood 2019;133:2669–81. 10.1182/blood-2018-09-874578 [DOI] [PubMed] [Google Scholar]
- 74.Flex E, Petrangeli V, Stella L, et al. Somatically acquired JAK1 mutations in adult acute lymphoblastic leukemia. J Exp Med 2008;205:751–8. 10.1084/jem.20072182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raivola J, Haikarainen T, Abraham BG, et al. Janus kinases in leukemia. Cancers 2021;13:800. 10.3390/cancers13040800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Demoulin JB, Renauld JC. Interleukin 9 and its receptor: an overview of structure and function. Int Rev Immunol 1998;16:345–64. 10.3109/08830189809043001 [DOI] [PubMed] [Google Scholar]
- 77.Haan C, Rolvering C, Raulf F, et al. Jak1 has a dominant role over Jak3 in signal transduction through γc-containing cytokine receptors. Chem Biol 2011;18:314–23. 10.1016/j.chembiol.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 78.Losdyck E, Hornakova T, Springuel L, et al. Distinct Acute Lymphoblastic Leukemia (ALL)-associated Janus Kinase 3 (JAK3) Mutants Exhibit Different Cytokine-Receptor Requirements and JAK Inhibitor Specificities. J Biol Chem 2015;290:29022–34. 10.1074/jbc.M115.670224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thomis DC, Gurniak CB, Tivol E, et al. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science 1995;270:794–7. 10.1126/science.270.5237.794 [DOI] [PubMed] [Google Scholar]
- 80.Keenan C, Nichols KE, Albeituni S. Use of the JAK inhibitor ruxolitinib in the treatment of hemophagocytic lymphohistiocytosis. Front Immunol 2021;12:614704. 10.3389/fimmu.2021.614704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yan B, Freiwald T, Chauss D, et al. SARS-CoV-2 drives JAK1/2-dependent local complement hyperactivation. Sci Immunol 2021;6:eabg0833. 10.1126/sciimmunol.abg0833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee S, Yu Y, Trimpert J, et al. Virus-Induced senescence is a driver and therapeutic target in COVID-19. Nature 2021;599:283–9. 10.1038/s41586-021-03995-1 [DOI] [PubMed] [Google Scholar]
- 84.Heidel F, Hochhaus A. Holding CoVID in check through JAK? the MPN-approved compound ruxolitinib as a potential strategy to treat SARS-CoV-2 induced systemic hyperinflammation. Leukemia 2020;34:1723–5. 10.1038/s41375-020-0898-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meletiadis J, Tsiodras S, Tsirigotis P. Interleukin-6 blocking vs. JAK-STAT inhibition for prevention of lung injury in patients with COVID-19. Infect Dis Ther 2020;9:707–13. 10.1007/s40121-020-00326-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leroy E, Constantinescu SN. Rethinking JAK2 inhibition: towards novel strategies of more specific and versatile Janus kinase inhibition. Leukemia 2017;31:1023–38. 10.1038/leu.2017.43 [DOI] [PubMed] [Google Scholar]
- 87.Clark JD, Flanagan ME, Telliez J-B. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem 2014;57:5023–38. 10.1021/jm401490p [DOI] [PubMed] [Google Scholar]
- 88.Quintás-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 2010;115:3109–17. 10.1182/blood-2009-04-214957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fridman JS, Scherle PA, Collins R, et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol 2010;184:5298–307. 10.4049/jimmunol.0902819 [DOI] [PubMed] [Google Scholar]
- 90.Duan Y, Chen L, Chen Y, et al. C-Src binds to the cancer drug ruxolitinib with an active conformation. PLoS One 2014;9:e106225. 10.1371/journal.pone.0106225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lucet IS, Fantino E, Styles M, et al. The structural basis of Janus kinase 2 inhibition by a potent and specific pan-Janus kinase inhibitor. Blood 2006;107:176–83. 10.1182/blood-2005-06-2413 [DOI] [PubMed] [Google Scholar]
- 92.Sorrell FJ, Szklarz M, Abdul Azeez KR, et al. Family-Wide structural analysis of human Numb-Associated protein kinases. Structure 2016;24:401–11. 10.1016/j.str.2015.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang Y, Min J, Jarusiewicz JA, et al. Degradation of Janus kinases in CRLF2-rearranged acute lymphoblastic leukemia. Blood 2021;138:2313-2326. 10.1182/blood.2020006846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McLornan DP, Pope JE, Gotlib J, et al. Current and future status of JAK inhibitors. Lancet 2021;398:803–16. 10.1016/S0140-6736(21)00438-4 [DOI] [PubMed] [Google Scholar]
- 95.Cortes J, Goldman JM, Hughes T. Current issues in chronic myeloid leukemia: monitoring, resistance, and functional cure. J Natl Compr Canc Netw 2012;10 Suppl 3:S1–13. 10.6004/jnccn.2012.0184 [DOI] [PubMed] [Google Scholar]
- 96.Tefferi A. Primary myelofibrosis: 2021 update on diagnosis, risk-stratification and management. Am J Hematol 2021;96:145–62. 10.1002/ajh.26050 [DOI] [PubMed] [Google Scholar]
- 97.Zeiser R, von Bubnoff N, Butler J, et al. Ruxolitinib for Glucocorticoid-Refractory acute graft-versus-host disease. N Engl J Med 2020;382:1800–10. 10.1056/NEJMoa1917635 [DOI] [PubMed] [Google Scholar]
- 98.Zeiser R, Burchert A, Lengerke C, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia 2015;29:2062–8. 10.1038/leu.2015.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jagasia M, Perales M-A, Schroeder MA, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood 2020;135:1739–49. 10.1182/blood.2020004823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Risitano AM, Peffault de Latour R. Ruxolitinib for steroid-resistant acute GVHD. Blood 2020;135:1721–2. 10.1182/blood.2020005364 [DOI] [PubMed] [Google Scholar]
- 101.Zeiser R, Polverelli N, Ram R, et al. Ruxolitinib for Glucocorticoid-Refractory chronic graft-versus-host disease. N Engl J Med Overseas Ed 2021;385:228–38. 10.1056/NEJMoa2033122 [DOI] [PubMed] [Google Scholar]
- 102.FDA . Fda approves ruxolitinib for chronic graft-versus-host disease. U.S. FDA, 2021. [Google Scholar]
- 103.Spoerl S, Mathew NR, Bscheider M, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood 2014;123:3832–42. 10.1182/blood-2013-12-543736 [DOI] [PubMed] [Google Scholar]
- 104.Liu Y, Sawalha AH, Lu Q. COVID-19 and autoimmune diseases. Curr Opin Rheumatol 2021;33:155–62. 10.1097/BOR.0000000000000776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hausmann JS, Kennedy K, Simard JF, et al. Immediate effect of the COVID-19 pandemic on patient health, health-care use, and behaviours: results from an international survey of people with rheumatic diseases. Lancet Rheumatol 2021;3:e707–14. 10.1016/S2665-9913(21)00175-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med 2021;384:20-30. 10.1056/NEJMoa2030340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crichton ML, Goeminne PC, Tuand K, et al. The impact of therapeutics on mortality in hospitalised patients with COVID-19: systematic review and meta-analyses Informing the European respiratory Society living guideline. Eur Respir Rev 2021;30:210171. 10.1183/16000617.0171-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.CORIMUNO-19 Collaborative group . Sarilumab in adults hospitalised with moderate-to-severe COVID-19 pneumonia (CORIMUNO-SARI-1): an open-label randomised controlled trial. Lancet Rheumatol 2022;4:e24–32. 10.1016/S2665-9913(21)00315-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seror R, Camus M, Salmon J-H, et al. Do JAK inhibitors affect immune response to COVID-19 vaccination? data from the MAJIK-SFR registry. Lancet Rheumatol 2022;4:e8–11. 10.1016/S2665-9913(21)00314-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ramirez GA, Asperti C, Cucca V, et al. Challenges to vaccination against SARS-CoV-2 in patients with immune-mediated diseases. Vaccines 2021;9:1147. 10.3390/vaccines9101147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020;395:e30–1. 10.1016/S0140-6736(20)30304-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Constantinescu SN, Cernescu CD, Popescu LM. Effects of protein kinase C inhibitors on viral entry and infectivity. FEBS Lett 1991;292:31–3. 10.1016/0014-5793(91)80826-o [DOI] [PubMed] [Google Scholar]
- 113.Pu S-Y, Xiao F, Schor S, et al. Feasibility and biological rationale of repurposing sunitinib and erlotinib for dengue treatment. Antiviral Res 2018;155:67–75. 10.1016/j.antiviral.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stebbing J, Phelan A, Griffin I, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis 2020;20:400–2. 10.1016/S1473-3099(20)30132-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang X, Zhang Y, Qiao W, et al. Baricitinib, a drug with potential effect to prevent SARS-COV-2 from entering target cells and control cytokine storm induced by COVID-19. Int Immunopharmacol 2020;86:106749. 10.1016/j.intimp.2020.106749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stebbing J, Sánchez Nievas G, Falcone M, et al. Jak inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv 2021;7:eabe4724. 10.1126/sciadv.abe4724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stebbing J, Krishnan V, de Bono S, et al. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol Med 2020;12:e12697. 10.15252/emmm.202012697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bronte V, Ugel S, Tinazzi E, et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Invest 2020;130:6409–16. 10.1172/JCI141772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hoang TN, Pino M, Boddapati AK, et al. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell 2021;184:460–75. 10.1016/j.cell.2020.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cantini F, Niccoli L, Nannini C, et al. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J Infect 2020;81:647–79. 10.1016/j.jinf.2020.06.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N Engl J Med 2021;384:795–807. 10.1056/NEJMoa2031994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.FDA . Coronavirus (COVID-19) update: FDA Authorizes drug combination for treatment of COVID-19. U.S. FDA, 2020. [Google Scholar]
- 123.NIH . Nih closes enrollment in trial comparing COVID-19 treatment regimens. U.S. NIoH, 2021. [Google Scholar]
- 124.Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med 2021;9:1407–18. 10.1016/S2213-2600(21)00331-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.FDA . Coronavirus (COVID-19) update: July 30, 2021. U.S. FDA, 2021. [Google Scholar]
- 126.NIH . COVID-19 treatment guidelines. National Institutes of Health U.S, 2021. [PubMed] [Google Scholar]
- 127.Yamakawa K, Yamamoto R, Terayama T, et al. Japanese rapid/living recommendations on drug management for COVID-19: updated guidelines (September 2021). Acute Med Surg 2021;8:e706. 10.1002/ams2.706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Agarwal A, Rochwerg B, Lamontagne F, et al. A living who guideline on drugs for covid-19. BMJ 2020;370:m3379. 10.1136/bmj.m3379 [DOI] [PubMed] [Google Scholar]
- 129.Mikuls TR, Gravallese EM, Saag KG. COVID-19: care of adult patients with systemic rheumatic disease. Waltham, MA: UpToDate, 2021. [Google Scholar]
- 130.Reddig A, Voss L, Guttek K, et al. Impact of different JAK inhibitors and methotrexate on lymphocyte proliferation and DNA damage. J Clin Med 2021;10:1431. 10.3390/jcm10071431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guimarães PO, Quirk D, Furtado RH, et al. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med 2021;385:406–15. 10.1056/NEJMoa2101643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kmietowicz Z. Covid-19: who recommends baricitinib and sotrovimab to treat patients. BMJ 2022;376:o97. 10.1136/bmj.o97 [DOI] [PubMed] [Google Scholar]
- 133.Genovese MC, Smolen JS, Weinblatt ME, et al. Efficacy and safety of ABT-494, a selective JAK-1 inhibitor, in a phase IIb study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol 2016;68:2857–66. 10.1002/art.39808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McInnes IB, Byers NL, Higgs RE, et al. Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res Ther 2019;21:183. 10.1186/s13075-019-1964-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sonkar C, Doharey PK, Rathore AS, et al. Repurposing of gastric cancer drugs against COVID-19. Comput Biol Med 2021;137:104826. 10.1016/j.compbiomed.2021.104826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood 2020;136:2881–92. 10.1182/blood.2020008824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Breccia M, Piciocchi A, De Stefano V, et al. COVID-19 in Philadelphia-negative myeloproliferative disorders: a GIMEMA survey. Leukemia 2020;34:2813–4. 10.1038/s41375-020-01032-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.He W, Chen L, Chen L, et al. COVID-19 in persons with haematological cancers. Leukemia 2020;34:1637–45. 10.1038/s41375-020-0836-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol 2020;7:e737–45. 10.1016/S2352-3026(20)30251-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li W, Wang D, Guo J, et al. COVID-19 in persons with chronic myeloid leukaemia. Leukemia 2020;34:1799–804. 10.1038/s41375-020-0853-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Harrington P, Harrison CN, Dillon R, et al. Evidence of robust memory T‐cell responses in patients with chronic myeloproliferative neoplasms following infection with severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). Br J Haematol 2021;193:692–6. 10.1111/bjh.17402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Innes AJ, Cook LB, Marks S, et al. Ruxolitinib for tocilizumab‐refractory severe COVID‐19 infection. Br J Haematol 2020;190:e198–200. 10.1111/bjh.16979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Koschmieder S, Jost E, Cornelissen C, et al. Favorable COVID-19 course despite significant comorbidities in a ruxolitinib-treated patient with primary myelofibrosis. Eur J Haematol 2020;105:655–8. 10.1111/ejh.13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vannucchi AM, Sordi B, Morettini A, et al. Compassionate use of JAK1/2 inhibitor ruxolitinib for severe COVID-19: a prospective observational study. Leukemia 2021;35:1121–33. 10.1038/s41375-020-01018-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.La Rosée F, Bremer HC, Gehrke I, et al. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation. Leukemia 2020;34:1805–15. 10.1038/s41375-020-0891-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.D'Alessio A, Del Poggio P, Bracchi F, et al. Low-Dose ruxolitinib plus steroid in severe SARS-CoV-2 pneumonia. Leukemia 2021;35:635–8. 10.1038/s41375-020-01087-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cao Y, Wei J, Zou L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol 2020;146:137–46. 10.1016/j.jaci.2020.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Capochiani E, Frediani B, Iervasi G, et al. Ruxolitinib rapidly reduces acute respiratory distress syndrome in COVID-19 disease. Analysis of data collection from respire protocol. Front Med 2020;7:466. 10.3389/fmed.2020.00466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Novartis . Novartis provides update on RUXCOVID study of ruxolitinib for hospitalized patients with COVID-19, 2020. [Google Scholar]
- 150.Palandri F, Piciocchi A, De Stefano V, et al. How the coronavirus pandemic has affected the clinical management of Philadelphia-negative chronic myeloproliferative neoplasms in Italy—a GIMEMA MPN WP survey. Leukemia 2020;34:2805–8. 10.1038/s41375-020-0953-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Barbui T, Vannucchi AM, Alvarez-Larran A, et al. High mortality rate in COVID-19 patients with myeloproliferative neoplasms after abrupt withdrawal of ruxolitinib. Leukemia 2021;35:485–93. 10.1038/s41375-020-01107-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lucijanic M, Kusec R. Ruxolitinib withdrawal due to the COVID-19. Leukemia 2021;35:1218–1. 10.1038/s41375-021-01214-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Portsmore S, Tran Nguyen TN, Beacham E, et al. Combined IL‐6 and JAK/STAT inhibition therapy in COVID‐19‐related sHLH, potential game changer. Br J Haematol 2020;190:525–8. 10.1111/bjh.16966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Prilutskiy A, Kritselis M, Shevtsov A, et al. SARS-CoV-2 infection-associated hemophagocytic lymphohistiocytosis. Am J Clin Pathol 2020;154:466–74. 10.1093/ajcp/aqaa124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846–8. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pfeifer ND, Lo A, Bourdet DL, et al. Phase I study in healthy participants to evaluate safety, tolerability, and pharmacokinetics of inhaled nezulcitinib, a potential treatment for COVID-19. Clin Transl Sci 2021;14:2556–65. 10.1111/cts.13123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Singh D, Bogus M, Moskalenko V, et al. A phase 2 multiple ascending dose study of the inhaled pan-JAK inhibitor nezulcitinib (TD-0903) in severe COVID-19. Eur Respir J 2021;58:2100673. 10.1183/13993003.00673-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Theravance B, Theravance Biopharma I. Announces Top-Line results from phase 2 study of Nezulcitinib in patients hospitalized with acute lung injury due to Covid-19, 2021. [Google Scholar]
- 159.Chen C-X, Wang J-J, Li H, et al. JAK-inhibitors for coronavirus disease-2019 (COVID-19): a meta-analysis. Leukemia 2021;35:2616–20. 10.1038/s41375-021-01266-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wijaya I, Andhika R, Huang I, et al. The use of Janus kinase inhibitors in hospitalized patients with COVID-19: systematic review and meta-analysis. Clin Epidemiol Glob Health 2021;11:100755. 10.1016/j.cegh.2021.100755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Patoulias D, Doumas M, Papadopoulos C, et al. Janus kinase inhibitors and major COVID-19 outcomes: time to forget the two faces of Janus! a meta-analysis of randomized controlled trials. Clin Rheumatol 2021;40:4671–4. 10.1007/s10067-021-05884-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Harrison C, Kiladjian J-J, Al-Ali HK, et al. Jak inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med Overseas Ed 2012;366:787–98. 10.1056/NEJMoa1110556 [DOI] [PubMed] [Google Scholar]
- 163.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med Overseas Ed 2012;366:799–807. 10.1056/NEJMoa1110557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med 2015;372:426–35. 10.1056/NEJMoa1409002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Porpaczy E, Tripolt S, Hoelbl-Kovacic A, et al. Aggressive B-cell lymphomas in patients with myelofibrosis receiving JAK1/2 inhibitor therapy. Blood 2018;132:694–706. 10.1182/blood-2017-10-810739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mehta P, Ciurtin C, Scully M, et al. Jak inhibitors in COVID-19: the need for vigilance regarding increased inherent thrombotic risk. Eur Respir J 2020;56:2001919. 10.1183/13993003.01919-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jorgensen SCJ, Tse CLY, Burry L, et al. Baricitinib: a review of pharmacology, safety, and emerging clinical experience in COVID‐19. Pharmacotherapy 2020;40:843–56. 10.1002/phar.2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.FDA . Janus Kinase (JAK) inhibitors: Drug Safety Communication - FDA Requires Warnings about Increased Risk of Serious Heart-related Events, Cancer, Blood Clots, and Death. U.S. FDA, 2021. [Google Scholar]
- 169.Gatti M, Turrini E, Raschi E, et al. Janus Kinase Inhibitors and Coronavirus Disease (COVID)-19: Rationale, Clinical Evidence and Safety Issues. Pharmaceuticals 2021;14:738. 10.3390/ph14080738 [DOI] [PMC free article] [PubMed] [Google Scholar]