Abstract
Background
Experiencing anxiety and depression is very common in people living with dementia and mild cognitive impairment (MCI). There is uncertainty about the best treatment approach. Drug treatments may be ineffective and associated with adverse effects. Guidelines recommend psychological treatments. In this updated systematic review, we investigated the effectiveness of different psychological treatment approaches.
Objectives
Primary objective
To assess the clinical effectiveness of psychological interventions in reducing depression and anxiety in people with dementia or MCI.
Secondary objectives
To determine whether psychological interventions improve individuals' quality of life, cognition, activities of daily living (ADL), and reduce behavioural and psychological symptoms of dementia, and whether they improve caregiver quality of life or reduce caregiver burden.
Search methods
We searched ALOIS, the Cochrane Dementia and Cognitive Improvement Group's register, MEDLINE, Embase, four other databases, and three trials registers on 18 February 2021.
Selection criteria
We included randomised controlled trials (RCTs) that compared a psychological intervention for depression or anxiety with treatment as usual (TAU) or another control intervention in people with dementia or MCI.
Data collection and analysis
A minimum of two authors worked independently to select trials, extract data, and assess studies for risk of bias. We classified the included psychological interventions as cognitive behavioural therapies (cognitive behavioural therapy (CBT), behavioural activation (BA), problem‐solving therapy (PST)); 'third‐wave' therapies (such as mindfulness‐based cognitive therapy (MBCT)); supportive and counselling therapies; and interpersonal therapies. We compared each class of intervention with control. We expressed treatment effects as standardised mean differences or risk ratios. Where possible, we pooled data using a fixed‐effects model. We used GRADE methods to assess the certainty of the evidence behind each result.
Main results
We included 29 studies with 2599 participants. They were all published between 1997 and 2020. There were 15 trials of cognitive behavioural therapies (4 CBT, 8 BA, 3 PST), 11 trials of supportive and counselling therapies, three trials of MBCT, and one of interpersonal therapy. The comparison groups received either usual care, attention‐control education, or enhanced usual care incorporating an active control condition that was not a specific psychological treatment. There were 24 trials of people with a diagnosis of dementia, and five trials of people with MCI. Most studies were conducted in community settings. We considered none of the studies to be at low risk of bias in all domains.
Cognitive behavioural therapies (CBT, BA, PST)
Cognitive behavioural therapies are probably slightly better than treatment as usual or active control conditions for reducing depressive symptoms (standardised mean difference (SMD) ‐0.23, 95% CI ‐0.37 to ‐0.10; 13 trials, 893 participants; moderate‐certainty evidence). They may also increase rates of depression remission at the end of treatment (risk ratio (RR) 1.84, 95% CI 1.18 to 2.88; 2 studies, with one study contributing 2 independent comparisons, 146 participants; low‐certainty evidence). We were very uncertain about the effect of cognitive behavioural therapies on anxiety at the end of treatment (SMD ‐0.03, 95% CI ‐0.36 to 0.30; 3 trials, 143 participants; very low‐certainty evidence). Cognitive behavioural therapies probably improve patient quality of life (SMD 0.31, 95% CI 0.13 to 0.50; 7 trials, 459 participants; moderate‐certainty evidence) and activities of daily living at end of treatment compared to treatment as usual or active control (SMD ‐0.25, 95% CI ‐0.40 to ‐0.09; 7 trials, 680 participants; moderate‐certainty evidence).
Supportive and counselling interventions
Meta‐analysis showed that supportive and counselling interventions may have little or no effect on depressive symptoms in people with dementia compared to usual care at end of treatment (SMD ‐0.05, 95% CI ‐0.18 to 0.07; 9 trials, 994 participants; low‐certainty evidence). We were very uncertain about the effects of these treatments on anxiety, which was assessed only in one small pilot study.
Other interventions
There were very few data and very low‐certainty evidence on MBCT and interpersonal therapy, so we were unable to draw any conclusions about the effectiveness of these interventions.
Authors' conclusions
CBT‐based treatments added to usual care probably slightly reduce symptoms of depression for people with dementia and MCI and may increase rates of remission of depression. There may be important effect modifiers (degree of baseline depression, cognitive diagnosis, or content of the intervention). CBT‐based treatments probably also have a small positive effect on quality of life and activities of daily living. Supportive and counselling interventions may not improve symptoms of depression in people with dementia. Effects of both types of treatment on anxiety symptoms are very uncertain. We are also uncertain about the effects of other types of psychological treatments, and about persistence of effects over time. To inform clinical guidelines, future studies should assess detailed components of these interventions and their implementation in different patient populations and in different settings.
Keywords: Humans, Anxiety, Anxiety/therapy, Anxiety Disorders, Anxiety Disorders/therapy, Cognitive Dysfunction, Cognitive Dysfunction/therapy, Dementia, Dementia/complications, Dementia/therapy, Depression, Depression/therapy, Quality of Life
Plain language summary
Psychological treatments for depression and anxiety in dementia and mild cognitive impairment
Key points
‐ Psychological treatments based on cognitive behavioural therapy (which focuses on changing thoughts and behaviours) probably have small positive effects on depression, quality of life and daily activities in people with dementia or mild cognitive impairment (MCI).
‐ There is not enough evidence to know whether any psychological treatments are helpful for anxiety in people with dementia or MCI.
‐ More evidence is needed about different types of psychological treatments and which treatments may be best for which people.
What are dementia and mild cognitive impairment?
Dementia is a condition in which problems develop with cognition (memory and thinking skills). Someone with dementia is no longer able to manage all their daily activities independently. Mild cognitive impairment (MCI) is less severe and does not have a significant effect on daily activities. Some people with MCI will go on to develop dementia.
What do we mean by psychological treatments?
Psychological treatments, sometimes known as ‘talking therapies’, are based on psychological theories. They involve a therapist working together with an individual or a small group of people to develop skills and strategies to improve well‐being. These treatments can be adapted for people with cognitive impairment.
What did we want to find out?
Depression and anxiety are common in people with dementia and MCI, but the best way to treat them is unclear. Medicines often used to treat these problems may not be effective for people with dementia and may cause side effects, so many guidelines recommend trying psychological treatments first. We were interested in psychological therapies that aim to reduce symptoms of anxiety or depression or to improve the emotional well‐being of people with dementia or MCI. There are a variety of different types of psychological treatment. We wanted to find out how effective each treatment is for symptoms of depression and anxiety in people with dementia or MCI. We also wanted to find out about effects on quality of life, ability to manage daily activities and thinking skills, and to know if the treatments had any unwanted effects.
What did we do?
We searched for studies that compared a psychological treatment to usual care, or to usual care plus a treatment that was not a specific psychological treatment.
We divided the psychological treatments into several broad categories based on the theory behind them and the content of the treatment sessions, and we looked at each category separately. We summarised the results of the studies and rated our confidence in the evidence based on factors such as study methods and sizes.
What did we find?
We found 29 studies that included 2599 people with dementia or MCI. Most of the evidence we found was about treatments based on cognitive behavioural therapy (CBT, which aims to alter thoughts and behaviours) and treatments aimed at supporting well‐being, which we called counselling and supportive therapies. We also found a very small number of studies about mindfulness‐based cognitive therapy and interpersonal therapy. The majority of studies looked at the effect on depression, but very few studies had anxiety as an outcome.
The evidence we found suggests that:
‐ CBT‐based treatments probably improve symptoms of depression, quality of life and ability to manage daily activities at the end of the treatment period in people with dementia or MCI, although the effects were small. We could not be sure about any effect on anxiety. There was some evidence that the effect on depression might depend on how severe the symptoms of depression were before the start of treatment, whether people had a diagnosis of dementia or MCI and what type of treatment was used, but more research would be needed to be sure about this.
‐ Supportive and counselling treatments may have no effect on symptoms of depression at the end of treatment and there was not enough evidence for us to know if there was any effect on anxiety.
‐ We cannot be sure about the effect of mindfulness‐based therapies or interpersonal therapy because there were very few studies of these treatments.
There was limited information about unwanted effects associated with any of the treatments.
We also found 14 ongoing studies, so we can expect more evidence about our question to become available in the next few years.
What are the limitations of the evidence?
We could be moderately certain about the small positive effects of CBT‐based treatments on depression, quality of life and daily activities, but were less certain about other results. Most people in the review had dementia of mild to moderate severity so the results may not apply to people with MCI or more severe dementia. Very few studies included only people who had significant levels of depression before treatment, although these are the people most likely to be offered treatment in practice. There is not yet enough evidence to be able to say which people are most likely to benefit from which psychological treatments.
How up to date is the evidence?
This review is up to date to February 2021.
Summary of findings
Summary of findings 1. CBT treatments compared to treatment as usual for depression and anxiety for people with dementia and MCI.
| CBT treatments compared to treatment as usual for depression and anxiety for people with dementia and MCI | ||||
| Patient or population: depression and anxiety for people with dementia and MCI Setting: community or long‐term care Intervention: CBT treatments Comparison: treatment as usual (usual care, attention‐control education, diagnostic feedback, or services slightly above usual care comprising active control conditions) | ||||
| Outcomes | SMD (95% CI) meta‐analysis/ relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Depressive symptoms post‐treatment assessed with: GDS, HDRS, MADRS, PHQ‐9, and CSDD Follow‐up: range 8 weeks to 24 months | SMD 0.23 lower (0.37 lower to 0.10 lower) | 893 (13 RCTs) | ⊕⊕⊕⊝ Moderatea | Higher scores indicate higher symptoms of depression. |
| Depression remission post‐treatment assessed with: MADRS, and DSM‐III‐R Follow‐up: range 10 weeks to 12 weeks | RR 1.84 (1.18 to 2.88) | 146 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | A RR > 1 favours the intervention group. |
| Anxiety symptoms post‐treatment assessed with: GAI, RAID, and NPI‐A Follow‐up: range 3 months to 15 weeks | SMD 0.03 lower (0.36 lower to 0.30 higher) | 143 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,d | Higher scores indicate higher symptoms of anxiety. |
| Quality of life post‐treatment assessed with: DEMQOL, QoL‐AD, LSI‐A, and QoL‐AD NH Follow‐up: range 8 weeks to 15 weeks | SMD 0.31 higher (0.13 higher to 0.50 higher) | 459 (7 RCTs) | ⊕⊕⊕⊝ Moderatea | Higher scores indicate better quality of life. |
| Activities of daily living post‐treatment assessed with: ADL‐PI, SDS, WHODAS 2.0, B‐ADL, ADCS‐ADL, BADLS, and UPSA Follow‐up: range 12 weeks to 2 years | SMD 0.25 lower (0.40 lower to 0.09 lower) | 680 (7 RCTs) | ⊕⊕⊕⊝ Moderatea | Lower scores indicate better performance of ADL. |
| Cognition post‐treatment assessed with: MMSE Follow‐up: range 10 weeks to 2 years | SMD 0.13 higher (0.04 lower to 0.30 higher) | 535 (5 RCTs) | ⊕⊕⊝⊝ Lowa,d | Higher scores indicate better cognition. |
| Neuropsychiatric symptoms post‐treatment assessed with: NPI, RMBPC, and BEHAVE‐AD Follow‐up: range 10 weeks to 3 months | SMD 0.06 lower (0.26 lower to 0.14 higher) | 401 (5 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | Higher scores indicate more or more severe neuropsychiatric symptoms. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio; SMD: standardised mean difference | ||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||
aDowngraded once for serious limitations to study quality bDowngraded twice for very serious imprecision cDowngraded once for serious inconsistency dDowngraded once for serious imprecision
ADCS‐ADL: Alzheimer's Disease Cooperative Study – Activities of Daily Living Inventory; ADL‐PI: Activities of Daily‐Living Prevention Instrument; B‐ADL: Bayer Activities of Daily Living Scale; BADLS: Bristol Activities of Daily Living Scale; BEHAVE‐AD: Behavioral Pathology in Alzheimer's Disease; CBT: cognitive behavioural therapy; CSDD: Cornell Scale for Depression in Dementia; DEMQOL: Dementia Quality of Life Instrument; DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition, Revised; GAI: Geriatric Anxiety Inventory; GDS: Geriatric Depression Scale; HDRS: Hamilton Depression Rating Scale; LSI‐A: Life Satisfaction Index; MADRS: Montgomery‐Åsberg Depression Rating Scale; MMSE: Mini‐Mental State Examination; NPI: Neuropsychiatric Inventory; NPI‐A: Neuropsychiatric Inventory‐Anxiety; PHQ‐9: Patient Health Questionnaire‐9; QoL‐AD: Quality of Life‐Alzheimer’s Disease; QoL‐AD NH: Quality of Life in Alzheimer’s Disease in Nursing Homes; RAID: Rating Anxiety in Dementia; RMBPC: Revised Memory and Behavior Problems Checklist; SDS: Sheehan Disability Scale; UPSA: University of California San Diego Performance‐Based Skills Assessment; WHODAS 2.0: World Health Organization Disability Assessment Schedule 2.0
Summary of findings 2. Supportive and counselling interventions compared to treatment as usual for depression and anxiety for people with dementia.
| Supportive and counselling interventions compared to treatment as usual for depression and anxiety for people with dementia | ||||
| Patient or population: depression and anxiety for people with dementia Setting: community or long‐term care Intervention: supportive and counselling interventions Comparison: treatment as usual (usual care, attention‐control education, diagnostic feedback, or services slightly above usual care) | ||||
| Outcomes | SMD (95% CI) meta‐analysis | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Depressive symptoms post‐treatment assessed with: CSDD, BDI, GDS, and MADRS Follow‐up: range 9 weeks to 3 years | SMD 0.05 lower (0.18 lower to 0.07 higher) | 994 (9 RCTs) | ⊕⊕⊝⊝ Lowa,b | Higher scores indicate higher symptoms of depression. |
| Depression remission post‐treatment | Not measured | |||
| Anxiety symptoms post‐treatment assessed with: HADS Follow‐up: mean 3 months |
MD 0.80 lower (3.07 lower to 1.47 higher) | 24 (1 RCT) |
⊕⊝⊝⊝ Very lowa,c,d | Higher scores indicate higher symptoms of anxiety. |
| Quality of life post‐treatment assessed with: EQ‐5D, QoL‐AD, and 15D Follow‐up: range 9 weeks to 3 years | SMD 0.15 higher (0.02 higher to 0.28 higher) | 935 (8 RCTs) | ⊕⊕⊕⊝ Moderatea | Higher scores indicate better quality of life. |
| Activities of daily living post‐treatment assessed with: ADCS‐ADL and Barthel Index Follow‐up: range 6 months to 3 years | SMD 0.17 higher (0.01 lower to 0.34 higher) | 511 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,c | Lower scores indicate better performance of ADL. |
| Cognition post‐treatment assessed with: MMSE and CDT Follow‐up: range 10 weeks to 3 years | SMD 0.11 higher (0.03 lower to 0.26 higher) | 730 (6 RCTs) | ⊕⊕⊝⊝ Lowa,b | Higher scores indicate better cognition. |
| Neuropsychiatric symptoms post‐treatment assessed with: NPI and RMBPC Follow‐up: range 9 weeks to 3 years | SMD 0.11 higher (0.06 lower to 0.29 higher) | 538 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,c,d | Higher scores indicate more or more severe neuropsychiatric symptoms. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; OR: odds ratio; RR: risk ratio; SMD: standardised mean difference | ||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||
aDowngraded once for serious limitations to study quality bDowngraded once for serious imprecision cDowngraded twice for very serious imprecision dDowngraded once for serious inconsistency
ADCS‐ADL: Alzheimer's Disease Cooperative Study – Activities of Daily Living Inventory; BDI: Beck Depression Inventory; CDT: Clock Drawing Test; CSDD: Cornell Scale for Depression in Dementia; EQ‐5D: EuroQoL‐5 Dimension; 15D: 15D instrument; GDS: Geriatric Depression Scale; HADS: Hospital Anxiety and Depression Scale; MADRS: Montgomery‐Åsberg Depression Rating Scale; MMSE: Mini‐Mental State Examination; NPI: Neuropsychiatric Inventory; QoL‐AD: Quality of Life‐Alzheimer’s Disease; RMBPC: Revised Memory and Behavior Problems Checklist
Background
Description of the condition
People living with dementia are twice as likely as age‐matched controls to be diagnosed with major depressive disorder, which has a significant impact on quality of life (Enache 2011). The most recent comprehensive systematic review of the prevalence of major depressive disorder in all‐cause dementia has shown that it is common, affecting approximately 15.9% of the population (Asmer 2018). Prevalence is higher in vascular dementia (24.7%) than in dementia due to Alzheimer's disease (AD) (14.8%). Younger age, a family history of psychiatric disorder, and presence of sleep disturbances and aggression are all associated with higher rates of clinical depression in AD (Steck 2018). In mild cognitive impairment (MCI), depressive symptoms are also common, with overall pooled prevalence reported to be 32%, and greater burden reported in clinic‐based samples (Ismail 2017).
Although anxiety symptoms are common in people with dementia and MCI (Hwang 2004), there is a lack of consensus about how to define and conceptualise anxiety in the context of cognitive impairment. Starkstein 2007 has proposed a revision of diagnostic criteria for generalised anxiety disorder for people with dementia, consisting of excessive anxiety and worry (criteria A and B of the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV)) and any three of the symptoms of restlessness, irritability, muscle tension, fear, and respiratory symptoms. Anxiety is more common in individuals with dementia than those without. Prevalence rates for anxiety disorders are generally similar to those for depression, with overall estimates at around 14% for both dementia (Kuring 2018), and MCI populations (Chen 2018), and evidence of slightly higher estimates in clinic‐based samples.
Regarding the impact of the conditions, studies show that the severity of both anxiety and depression increases the severity of neurological impairment, which impacts on independence (Stogmann 2016), caregiver burden (Cheng 2017), and risk of entering long‐term care (Cepoiu‐Martin 2016). Symptoms of depression in MCI can be persistent and refractory to antidepressant medication (Devanand 2003), and generally predictive of higher rates of progression to AD dementia (Cooper 2015).
Description of the intervention
The main psychotherapeutic approaches in treating depression and anxiety in adults, according to the World Health Organization (WHO), are cognitive behavioural therapy (CBT), behavioural activation (BA), problem‐solving therapy (PST), interpersonal therapy (IPT), and integrative approaches such as counselling, and psychodynamic therapies (WHO 2019). 'Third‐wave CBT' approaches include interventions characterised by techniques that focus on the process, rather than the content of thoughts, helping people accept their thoughts in a non‐judgmental way (Hofmann 2010). Examples of 'third‐wave' approaches include interventions such as mindfulness‐based cognitive therapy (MBCT) (Teasdale 1995), and acceptance and commitment therapy (Lars‐Göran 2014). Other psychological interventions include humanistic approaches (Cain 2002), and systemic therapies (MacFarlane 2003). Offering psychological treatments to older people is generally worthwhile as it promotes improvements in depression and anxiety and increases general psychological well‐being (Hall 2016; Jonsson 2016; Kirkham 2016).
Pharmacological treatments for depression and anxiety are prescribed for people with dementia, despite evidence that their clinical effectiveness, at least for depression, may be limited (Dudas 2018; Orgeta 2017). Psychological interventions are currently recommended for the management of depression and anxiety for people with dementia and are part of current clinical guidelines in several countries, such as in the UK (NICE 2018). These interventions have been adapted for people who experience dementia and cognitive impairment, through the use of behavioural techniques, in order to compensate for losses in cognition and declining sensory function (Grant 1995).
A wide variety of psychological interventions has been studied within the context of improving affective function for people with dementia and cognitive impairment. CBT is an umbrella term encompassing a wide range of therapeutic approaches that combine cognitive components (aimed at thinking processes such as identifying and challenging unrealistic negative thoughts) and behavioural components (aimed at increasing rewarding activities). BA is a component of CBT but also considered a psychological intervention on its own, comparable to CBT in terms of clinical effectiveness for depression (Richards 2016). BA is focused on reducing depressive symptoms by supporting individuals to set goals and engage in pleasant activities on a daily basis, and to modify precipitants of distress (Teri 2003). Other psychotherapies include IPT, which focuses on the connection between depressive symptoms and interpersonal problems (Churchill 2018), cognitive analytic therapy, or other integrative therapies such as counselling. Interventions for people with dementia and cognitive impairment may also focus on building social support, aiming to foster social networks and to help people to cope with cognitive loss in order to reduce psychological distress.
Although there are a number of other types of interventions which incorporate some psychological elements and which target depression and anxiety in dementia, such as reminiscence (Woods 2018), and interventions focusing on environmental changes or exercise (Gitlin 2003; Rolland 2007), the present review focuses on psychological interventions; that is, interventions primarily based on psychological models as defined by WHO 2019.
How the intervention might work
Most psychological treatments based on the cognitive‐behavioural model emphasise the need to modify dysfunctional beliefs as well as incorporating components of behavioural therapy. Their aim is to challenge negative cognitions that maintain depressive symptomatology and anxiety symptoms (WHO 2019). An important component of CBT is monitoring and identifying thoughts and behaviours that contribute to depression or anxiety (Beck 1979). Treatment components of CBT for anxiety often include additional techniques such as teaching of relaxation skills (Stanley 2004). Modifications of CBT emphasise cognitive strategies in early‐stage dementia and behavioural strategies in later stages, reducing cognitive load and utilising concrete examples (Gellis 2009). PST aims to help people learn skills to actively, constructively, and effectively solve problems they face in their daily life (Nezu 2010). Treatment goals often include the development of a positive problem‐solving orientation, through the use of rational strategies, and reducing the tendency to avoid problems (D'Zurilla 2010). BA, which is an integral part of CBT, can also be provided as a brief psychotherapeutic approach on its own (Dimidjian 2006). Techniques incorporated into treatment include changing the way a person interacts with their environment, by increasing access to positive reinforcers of healthy behaviours, reducing behaviours that limit access to positive reinforcement, and addressing barriers to activation (Dimidjian 2006; Jacobson 2001).
Psychodynamic therapies use the therapeutic alliance between patient and therapist to support the development of self‐awareness, helping people to recognise and modify interpersonal patterns that contribute to psychological distress (Shedler 2010).
IPT focuses on stressful life events, interpersonal difficulties, and important life transitions that may contribute to the onset and maintenance of depressive symptoms, in order to help individuals connect with social support networks and improve the quality of their interpersonal relationships (Ravitz 2019).
Third‐wave CBT approaches conceptualise cognitions as psychological or 'private' events and use strategies such as mindful exercises and acceptance of unwanted thoughts to elicit change in the thinking process and reduce depression (Hofmann 2010).
Counselling interventions focus on enhancing subjective experiences during treatment by focusing on a range of issues such as needs, relationships, and attachments, using structured methods to encourage change (Churchill 2001). Social support interventions have been implemented in diverse chronic conditions, and aim to improve physical and psychosocial health by fostering emotional support and information exchange, empowering individuals to enhance their skills and daily functioning (Dennis 2003). Psychological therapies for people with dementia may involve both the individual and their caregiver(s) in the therapeutic approach (Teri 2003).
Why it is important to do this review
Recommendations stress that the treatment of anxiety and depressive symptoms is an essential part of the treatment of AD and other dementias (Alexopoulos 2005; Azermai 2012). Currently, pharmacological approaches are commonly used for anxiety and depression in dementia, despite research indicative of poor efficacy and side effects of antidepressants (Banerjee 2011; Dudas 2018). Evidence that more than 30% of people with dementia are being prescribed antidepressants indicates potentially inappropriate and unnecessary prescribing (Puranen 2017). Symptoms of anxiety and depression may also contribute to the overuse of antipsychotics, which are associated with substantial adverse effects, such as an increased risk of sedation, falls, and death (Van der Hooft 2008).
Although most current clinical practice guidelines recommend the use of non‐pharmacological interventions as the first line of treatment for both anxiety and depression in dementia (Hogan 2008; Salzman 2008), the evidence base supporting the efficacy of particular psychological interventions remains small. In the previous version of this review, published in 2014, we systematically synthesised evidence on a variety of psychological interventions that were generally based on well‐defined, theory‐driven psychological models that were primarily aimed at people with dementia.
Despite recent reviews being published on the effectiveness of psychological interventions for people with dementia, most focus on behavioural and psychological symptoms in general as opposed to depression and anxiety (Li 2021), and the majority provide narrative reviews of the available literature (Carrion 2018). Given the growth of research in the area and the importance of offering psychological treatments to people with dementia and MCI, this review aims to provide a comprehensive and up‐to‐date evaluation of current evidence, which will be useful for informing clinical guidelines and future research. In conducting this review, we followed the protocol that we published in the Cochrane Database of Systematic Reviews (Orgeta 2011).
Objectives
Primary objective
To assess the clinical effectiveness of psychological interventions in reducing depression and anxiety in people with dementia or MCI.
Secondary objectives
To determine whether psychological interventions improve individuals' quality of life, cognition, activities of daily living (ADL), and reduce behavioural and psychological symptoms of dementia, and whether they improve caregiver quality of life or reduce caregiver burden.
Methods
Criteria for considering studies for this review
Types of studies
We included studies in this review if they fulfilled these criteria:
they were randomised controlled trials (RCTs), including cluster‐randomised trials;
they included a control group (usual care) or a comparison group receiving no specific psychological intervention; and
they provided separate data on participants with dementia or MCI, or both, if the study was of a mixed population (also including older adults with normal cognition).
We identified ongoing studies but did not include these in the meta‐analysis.
Types of participants
The inclusion criteria for participants were:
people diagnosed with dementia of any type according to validated diagnostic criteria (Diagnostic and Statistical Manual (DSM) or International Classification of Diseases, or other validated criteria), and people with a diagnosis of MCI. Any definition of MCI was acceptable as long as the criteria used were published and included evidence of objective cognitive impairment but no dementia (e.g. Petersen 1999; Petersen 2003; Visser 2005)
any setting (e.g. home, community, long‐term care/nursing homes, hospital).
Types of interventions
For the purposes of this review, we defined a psychological intervention as an intervention that: (a) was designed to reduce anxiety and depression or improve adaptive functioning, or both; (b) was based on a psychological theory (for example, learning theory); and (c) involved a structured interaction between a facilitator and a participant which incorporated psychological methods (for example,behavioural, cognitive behavioural, family systems), in line with previous meta‐analytic studies and Cochrane Reviews in the general population (Churchill 2018; Uphoff 2020).We included interventions facilitated by psychologists, therapists in training,and other trained professionals. We grouped eligible interventions, where possible, in line with definitions provided by WHO 2019 into these five categories:
CBT therapies (which included CBT, PST, BA or behaviour management therapy);
third‐wave CBT therapies, including MBCT;
psychodynamic therapies (including brief psychotherapy and insight‐oriented psychotherapy);
interpersonal therapies; and
supportive and counselling therapies.
We excluded treatments identifiedas medication, exercise, reminiscence therapy, music therapy, art and drama therapy, yoga/meditation, befriending, or bibliotherapy. If an intervention could not be grouped into any of the preceding categories, we classified it as psychological, and included it in the review if there was some attempt to teach participants skills to reduce psychological distress such as depression and anxiety. We included both individual and group psychological interventions. The treatment could be of any intensity, duration, or frequency. Control conditions included no treatment (usual care) or a comparison group engaging in non‐specific psychosocial activity (for example, attention‐control, or active control conditions controlling for effects of staff attention or social contact). We did not consider comparisons with other therapeutic interventions in this review. We included studies that used combinations of different psychological treatments, or combinations of pharmacological and psychological interventions. In the case of combinations of pharmacological and psychological treatments, a comparison group of the pharmacological intervention alone or with the above control treatments was required.
Types of outcome measures
We included studies that measured any of the primary or secondary outcomes specified below. We pre‐specified clinically important outcomes which are relevant to people with dementia and MCI, carers, and healthcare providers.
Primary outcomes
Scale‐based measures of depressive symptoms (e.g. Cornell Scale for Depression in Dementia, Hamilton Depression Rating Scale (HDRS), Beck Depression Inventory, Geriatric Depression Scale (GDS)) and anxiety symptoms (e.g. Rating Anxiety in Dementia)
Depression remission (defined by a pre‐specified threshold on a depression scale or no longer meeting clinical criteria for depression). We accepted the authors' definition provided that the remission scores were based on changes in depression measures that were either clinician‐rated or other validated measures, and that these were similar to definitions used in the literature.
Secondary outcomes
Quality of life
Performance of activities of daily living (ADL)
Cognition
Neuropsychiatric symptoms
Caregivers' quality of life, burden, and depressive symptoms
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois) ‐ the Cochrane Dementia and Cognitive Improvement Group (CDCIG) Specialised Register. We searched all "Treatment MCI" and "Treatment Dementia" studies in combination with the following terms or phrases: Depression or Dysthymi* or "Adjustment Disorder/s" or "Mood Disorder/s" or "Affective Disorder/s" or "Affective Symptoms", Anxiety or Anxious or phobia/s or "Panic Disorder", psychotherapy, "cognitive therapy", "behaviour therapy", "cognitive behaviour therapy". ALOIS is maintained by the Information Specialists for CDCIG and contains dementia (prevention and treatment), mild cognitive impairment and cognitive improvement studies. The studies are identified from:
monthly searches of a number of major healthcare databases: MEDLINE, Embase, Cumulative Index to Nursing and Allied Health Literature (CINHAL), PsycINFO, and Latin American and Caribbean Health Science Information database (LILACS);
monthly searches of a number of trials registers: meta Register of Controlled Trials (mRCT); Umin Japan Trial Register; World Health Organization International Clinical Trials Registry Platform (ICTRP/WHO) portal (which covers ClinicalTrials.gov; ISRCTN; Chinese Clinical Trials Register; German Clinical Trials Register; Iranian Registry of Clinical Trials, and the Netherlands National Trials Register, plus others);
quarterly searches of the Central Register of Controlled Trials (CENTRAL) in the Cochrane Library; and
six‐monthly searches of a number of grey literature sources: ISI Web of knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses.
To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS website. We ran additional separate searches in many of the above sources to ensure that the most up‐to‐date results were retrieved. We describe the search strategies used in Appendix 1. The most recent search was carried out on 18 February 2021.
Searching other resources
We searched identified citations for additional trials and contacted the corresponding authors of identified trials for additional references and unpublished data. We scanned the reference lists of identified publications and all review papers that were related to psychological treatments for people with dementia and MCI.
Data collection and analysis
We used standard methodological procedures as recommended by Cochrane.
Selection of studies
Four review authors (VO, PL, RdPC, AM) worked independently to identify RCTs that met the inclusion criteria, and discussed any disagreements. We excluded those studies that clearly did not meet the inclusion criteria and obtained copies of the full texts of potentially relevant references. We documented reasons for exclusion of studies. Where necessary, we requested additional information from study authors.
Data extraction and management
Three review authors (VO, PL, AM) independently extracted data, using a standardised data extraction form which was piloted before use. We contacted the authors of the primary trials if there were doubts regarding missing data or methodological details of the trial. The extracted information included data on: participants, methods, interventions, outcomes, and results for all studies meeting the inclusion criteria; ongoing studies; and studies awaiting classification. These details included the following.
Participants: characteristics of the sample (age, diagnostic criteria, severity of cognitive impairment, and exclusion criteria).
Methods: data on methodologies used for randomisation, blinding, outcome reporting, and participant dropout.
Interventions: duration, intensity, type, and frequency of psychological and control interventions.
Outcomes: primary outcomes were ratings of depressive and anxiety symptoms, and depression remission. Secondary outcomes were measures of quality of life, ADL, cognition, and neuropsychiatric symptoms for people with dementia and MCI, and quality of life, depressive symptoms, and carer burden for caregivers.
Results: where data were available, we collected the number of participants for whom the outcome was measured in each group, means and standard deviations (SDs). We used change from baseline scores for all analyses reported (except for the reporting of findings of the study by Quinn 2016) and, if necessary, calculated the change scores. Calculations of the SD of change scores were based on an assumption that the correlation between measurements at baseline and those at subsequent time points is zero. This method overestimates the SD of the change from baseline, but is considered preferable in a meta‐analysis to take a conservative approach.
Assessment of risk of bias in included studies
We used Cochrane's tool for assessing risk of bias to evaluate the methodological quality of the included studies (Higgins 2011). The tool addresses six specific domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other sources of bias. Three review authors (VO, PL, AM) independently assessed each domain, resolving any differences through discussion with a fourth review author. In cases where no information was available to make a judgement, we explicitly stated this.
Measures of treatment effect
All outcomes except depression remission at post‐treatment were continuous, and a variety of different scales contributed data to each meta‐analysis. We therefore used the standardised mean difference (SMD) as the measure of treatment effect. For the dichotomous outcome of depression remission, we used risk ratio (RR). For reporting findings of the study by Quinn 2016, we used the mean difference as the measure of treatment effect.
Unit of analysis issues
We identified one trial that had multiple treatment groups: the Teri 1997 study, which contributed two independent comparisons of BA and PST. For this study, we combined the two control conditions and then divided the combined group in the meta‐analysis in line with the methods recommended by Cochrane (Higgins 2011).
Dealing with missing data
We reported the number of participants included in the final analysis as a proportion of all participants in the study. For each study, we noted what approach had been taken to missing data and considered how each method may have contributed to a risk of bias.
Assessment of heterogeneity
We used the I² statistic to assess heterogeneity amongst studies. We defined substantial heterogeneity as an I² of more than 70%.
Assessment of reporting biases
We assessed publication bias by producing funnel plots and inspecting them visually for all analyses combining six studies or more (Egger 1997). Due to the small number of studies combined in meta‐analyses, we did not conduct statistical tests for funnel plot asymmetry.
Data synthesis
We undertook separate comparisons of each of our intervention categories with control interventions at end of treatment, and ‐ where data were available ‐ also at post‐treatment follow‐up.
Where studies were sufficiently similar, we pooled data in meta‐analyses, using a fixed‐effect model to present overall estimated effects. This assumes that all studies are estimating the same (fixed) treatment effect. In practice, we were able to perform meta‐analyses for the following comparisons:
CBT interventions versus treatment as usual (TAU) immediately post‐intervention;
CBT interventions versus TAU at long‐term follow‐up;
supportive and counselling interventions versus TAU immediately post‐intervention.
We used Review Manager 5 to conduct all meta‐analyses (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We used subgroup analyses to evaluate the effect of these effect modifiers:
cognitive diagnosis: dementia versus MCI; and
depression at baseline: studies in which all participants met diagnostic criteria for a depressive disorder or exceeded a specified threshold on a depressive symptom scale at baseline versus studies with no depression‐related inclusion criteria.
Sensitivity analysis
We performed sensitivity analyses to examine the effect of type of control comparison (TAU or active control). Where possible, we performed sensitivity analyses by excluding from the analysis studies at high risk of bias.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to assess the certainty of the evidence for all outcomes, independently judged by two review authors (Guyatt 2011). Results of our analyses of CBT treatments versus TAU or active control are presented in Table 1, and of supportive and counselling interventions versus TAU in Table 2. Our summary of findings tables included these seven primary and secondary outcomes, measured at the end of the intervention period:
depressive symptoms;
depression remission;
anxiety symptoms;
quality of life;
activities of daily living;
cognition; and
neuropsychiatric symptoms.
Results
Description of studies
Results of the search
We identified a total of 4693 references through database searching. The search covered December 2011 to February 2021 and consisted of five searches (first search: 292 results; second search: 58 results, plus 3 studies identified by handsearching; third search: 715 results, plus 4 studies identified by handsearching; fourth search: 1511 results; fifth search: 2108 results, plus 2 studies identified by handsearching). The latest search was conducted on 18 February 2021.
We screened a total of 100 full‐text articles for eligibility: we excluded 53 with reasons (see Characteristics of excluded studies); 29 met the inclusion criteria (of which one contributed two independent comparisons, and six were included in the previous version of the review); 14 studies are ongoing (see Characteristics of ongoing studies); and four studies are awaiting classification whilst further information is obtained (see Characteristics of studies awaiting classification). See Figure 1 for a PRISMA flow diagram detailing the search process.
1.
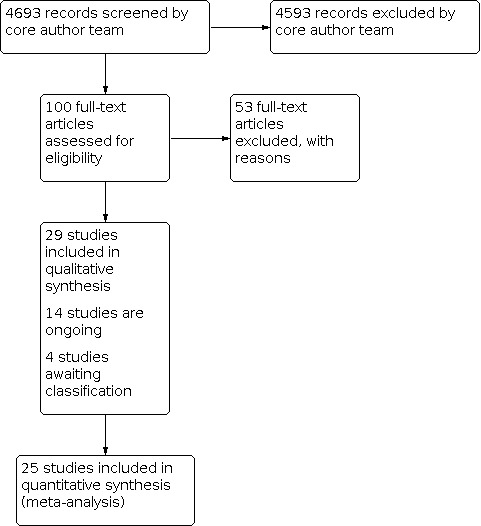
Study flow diagram
Included studies
See Characteristics of included studies.
Four studies evaluated the effectiveness of CBT versus TAU: Belleville 2018, Burgener 2008, Spector 2015, and Stanley 2013. Eight studies evaluated BA versus TAU: Kurz 2012, Lai 2020, Lichtenberg 2005, Lu 2016, Orgeta 2019, Rovner 2018 (BA versus an active control condition), Teri 1997 (pleasant‐event scheduling versus typical care control), and Travers 2017. Three studies evaluated PST versus TAU: Kiosses 2010 (PST versus an active control condition), Kiosses 2015 (PST versus an active control condition), and Teri 1997 (problem‐solving versus waiting‐list control). Eleven studies assessed the effectiveness of supportive and counselling interventions versus TAU: Bruvik 2013, Jha 2013, Koivisto 2016, Laakkonen 2016, Logsdon 2010, Marshall 2015, Nordheim 2019, Quinn 2016, Tappen 2009, Waldorff 2012, and Young 2014. Three studies evaluated MBCT versus TAU: Churcher Clarke 2017, Larouche 2019, and Wells 2013. One study evaluated IPT versus TAU: Burns 2005.
The majority of studies reported receiving national government funding (Belleville 2018; Bruvik 2013; Kiosses 2010; Kiosses 2015; Kurz 2012; Logsdon 2010; Lu 2016; Marshall 2015; Nordheim 2019; Quinn 2016; Rovner 2018; Spector 2015; Stanley 2013; Teri 1997; Waldorff 2012; Wells 2013). Two studies reported receiving university research funding (Burgener 2008; Young 2014). The studies by Burns 2005, Orgeta 2019, Travers 2017 and Tappen 2009 received funding from dementia research charities and trusts. One study reported receiving funding from both the pharmaceutical industry and government sectors (Koivisto 2016). Laakkonen 2016 and Larouche 2019 reported receiving funding from both the government and voluntary sectors. The studies by Churcher Clarke 2017, Jha 2013, Lai 2020, and Lichtenberg 2005 did not report their funding source.
Design
All 29 studies were RCTs that compared an eligible psychological intervention to a control intervention (TAU or waiting list or active control condition). Across all included studies, there were 2599 participants (n = 252 for CBT; n = 696 for BA; n = 137 for PST; n = 1381 for supportive and counselling interventions; n = 93 for MBCT; n = 40 for IPT).
CBT‐based therapies
Cognitive behavioural therapy (CBT)
Participants and setting
Belleville 2018 recruited people meeting criteria for single‐ or multiple‐domain amnestic MCI (Petersen 2004), from memory clinics in Canada. Burgener 2008 recruited people living with mild dementia in the USA but did not specify the setting. Spector 2015 recruited people with mild to moderate dementia and clinical anxiety living in the community in the UK. Stanley 2013 recruited people with mild to moderate dementia and clinical anxiety living in the community in the USA.
Intervention and control groups
Belleville 2018 evaluated group CBT versus a no‐contact control condition. Burgener 2008 tested the effectiveness of a multimodal intervention consisting of tai chi, CBT, and support groups versus a control condition which consisted of information about educational programs available. Both Spector 2015 and Stanley 2013 evaluated CBT‐based interventions adapted for people with anxiety and dementia versus TAU (medication or no treatment in Spector 2015; diagnostic feedback in Stanley 2013).
Outcomes
All the CBT trials assessed both depression and anxiety except Burgener 2008, which assessed only depression. We present the outcomes assessed across all CBT studies (Belleville 2018; Burgener 2008; Spector 2015; Stanley 2013), the details of participants recruited, and interventions evaluated, in Table 3. All four studies measured persistence of effects (assessed at 6 months, and 40 weeks), but for Burgener 2008 and Spector 2015, data were not available.
1. Table of cognitive behavioural therapies (CBT).
| Participants | Intervention and control conditions | Outcomes | |
| Belleville 2018 | Single‐or multiple‐domain amnestic MCI (Petersen 2004 criteria) n = 127; male (M) 57; female (F) 70 Mean age = 72.6 Mean MoCA = 24.4 Mean GDS‐15 = 3.3 |
Group CBT: cognitive restructuring of thoughts, beliefs, and attitudes helping participants modify thoughts causing unhelpful emotions, additional single booster session Eight 2‐hour group sessions over 8 weeks Control: no contact control condition |
1. Immediate and delayed episodic memory (Belleville 2006) 2. Depression (GDS‐15; Yesavage 1983) 3. Anxiety (GAI; Pachana 2007) 4. Well‐being (General Well‐Being Schedule; Dupuy 1977) 5. Memory strategies (Multifactorial Memory Questionnaire—Memory Strategies; Troyer 2002) 6. Subjective cognitive complaints (Questionnaire d’Auto‐Evaluation de la Mémoire; Van der Linden 1989) 7. Function (Activities of Daily‐Living Prevention Instrument; Galasko 2006) |
| Burgener 2008 | Mild dementia (CDR < 2.0) N = 43; M23; F20 Mean age = 76.9 Mean MMSE = 23.8 Mean GDS‐15 = 3.1 |
Multimodal intervention of tai chi, CBT, and support groups: strength and balance training, small group, and individual CBT which included challenging dysfunctional cognitions, developing positive coping skills, and enhancing personal control (Teri 1991); support groups focused on coping, problem‐solving, and developing relationships (Yale 1995) Tai chi was offered as a 1‐hour class 3 times a week over 20 weeks CBT was offered biweekly for 90 minutes for 20 weeks and social support groups bi‐weekly alternating with CBT over 20 weeks with each session lasting 90 minutes Control: attention‐control receiving information about educational programs available |
1. Cognition (MMSE; Folstein 1975) 2. Physical function (Single‐Leg Stance; Berg 1989, Berg Balance Scale; Rikki 1991, Cumulative Illness Rating Scale; Linn 1968) 3. Depression (GDS‐15; Yesavage 1983) 4. Self‐esteem (Rosenberg Self‐Esteem Scale; Rosenberg 1979) |
| Spector 2015 | Mild to moderate dementia (DSM‐IV; CDR range 0.5 to 2; clinical anxiety ≥ 11 RAID) N = 50; M20; F30 Mean age = 78.5 Mean MMSE = 20.9 Mean RAID = 19.7 Mean CSDD = 15.9 |
CBT: identifying and practicing strategies for feeling safe, challenging negative thoughts, and incorporating calming thoughts, and behavioural experiments A total of 10 sessions, each lasting 60 minutes over 15 weeks Control: treatment as usual which included either medication or no treatment |
1. Anxiety (RAID; Shankar 1999, HADS; Zigmond 1983) 2. Depression (CSDD; Alexopoulos 1988, HADS; Zigmond 1983) 3. Quality of life (QoL‐AD; Logsdon 1999) 4. Cognition (MMSE; Folstein 1975) 5. Neuropsychiatric symptoms (Neuropsychiatric Inventory; Cummings 1994) 6. Patient‐rated quality of the caregiving relationship (QCPR; Spruytte 2002) 7. Caregiver depression (HADS; Zigmond 1983) 8. Caregiver anxiety (HADS; Zigmond 1983) 9. Carer‐rated quality of the caregiving relationship (QCPR; Spruytte 2002) |
| Stanley 2013 | Mild to moderate dementia (confirmed by medical provider; clinical anxiety ≥ 1 NPI‐A) N = 32; M13; F19 Mean age = 78.6 Mean NPI‐A = 4.7 Mean GDS‐30 = 10.1 |
CBT: self‐monitoring for anxiety, deep breathing, and optional skills such as coping self‐statements, behavioural activation, and sleep management Up to 12 weekly home sessions, lasting 30 to 60 minutes over 6 months over the initial 3 months, and up to 8 brief telephone booster appointments Control: treatment as usual which incorporated diagnostic feedback but no additional contact |
1. Anxiety (NPI‐A; Cummings 1994, RAID; Shankar 1999, GAI; Pachana 2007) 2. Worry (Penn State Worry Questionnaire ‐ Abbreviated; Crittendon 2006) 3. Depression (GDS‐30; Yesavage 1983) 4. Quality of life (QoL‐AD; Logsdon 1999) 5. Caregiver distress related to anxiety symptoms (NPI‐A; Cummings 1994) 6. Caregiver depression (Patient Health Questionnaire‐9; Kroenke 2001) |
CBT: cognitive behavioural therapy; GAI: Geriatric Anxiety Inventory; CDR: Clinical Dementia Rating; CSDD: Cornell Scale for Depression in Dementia; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; GDS: Geriatric Depression Scale; HADS: Hospital Anxiety and Depression Scale; MCI: mild cognitive impairment; MMSE: Mini‐Mental State Examination; MoCA: Montreal Cognitive Assessment; NPI‐A: Neuropsychiatric Inventory‐Anxiety; RAID: Rating Anxiety in Dementia; QCPR: Quality of the Carer–Patient Relationship; QoL‐AD: Quality of Life‐Alzheimer’s Disease
Behavioural activation (BA)
Participants and setting
Kurz 2012 recruited people living with mild Alzheimer's disease (AD) in community settings in Germany, and Lai 2020 recruited people living with mild to moderate dementia in their own home in Hong Kong. The Lichtenberg 2005 study was conducted in special dementia care nursing home units in the USA. Both Lu 2016 and Rovner 2018 recruited people with a diagnosis of MCI living in the community in the USA, and Orgeta 2019 recruited people living with mild dementia in the community in the UK. In the Teri 1997 trial, participants lived in their own home (USA), and in the Travers 2017 study, participants were people with dementia living in nursing homes (Australia). Both the Teri 1997 and Travers 2017 studies recruited people with mild to moderate dementia and depression or depressive symptoms at baseline. In the Teri 1997 trial, all participants met Research Diagnostic Criteria (RDC) and Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition, Revised (DSM‐III‐R) criteria for major or minor depressive disorder and had a Hamilton Depression Rating Scale (HDRS) score of at least 10. In the study by Travers 2017, participants scored 4 or higher on the 12‐item Geriatric Depression Scale (GDS).
Intervention and control groups
Kurz 2012 evaluated a BA program versus a control intervention incorporating standard medical management for early dementia. Lai 2020 evaluated behavioural activity scheduling versus dementia care education over 10 weeks. Lichtenberg 2005 evaluated a behavioural treatment program adapted for people living with dementia in nursing homes versus usual care comprised of regular activities provided within the home. Lu 2016 evaluated an intervention primarily aimed at increasing meaningful activity through planning, versus a control intervention comprised of receiving an educational brochure and follow‐up calls every two weeks. Orgeta 2019 evaluated BA adapted for people living with mild dementia versus TAU, comprising treatments in line with the National Institute for Health and Care Excellence (NICE) guidelines. Rovner 2018 evaluated BA aimed at increasing cognitive, physical, and/or social activity versus a supportive control intervention focusing on conveying empathy and optimism. Teri 1997 evaluated pleasant‐event scheduling (of enjoyable activities) and modifying problem behaviours (behavioural and psychological symptoms) versus usual care, consisting of advice and information about support services available and a separate waiting list‐control group. Travers 2017 evaluated pleasant‐event scheduling of enjoyable activities versus a walking and talking intervention with a volunteer for 30 minutes each week.
Outcomes
All studies except Lai 2020 assessed depression. None of the trials assessed anxiety. We present the outcomes assessed across all BA studies (Kurz 2012; Lai 2020; Lichtenberg 2005; Lu 2016; Orgeta 2019; Rovner 2018; Teri 1997; Travers 2017), the details of participants recruited, and the interventions evaluated, in Table 4. Persistence of effects was assessed by Orgeta 2019 (six months) and Teri 1997 (six months), but study data were not available for the Teri 1997 study.
2. Table of behavioural activation (BA) therapies.
| Participants | Intervention and control conditions | Outcomes | |
| Kurz 2012 | Mild AD (ICD‐10; MMSE ≥ 21) N = 201; male (M) 113; female (F) 88 Mean age = 73.7 Mean MMSE = 25.1 Mean GDS‐30 = 8.9 |
BA: aimed at facilitating daily structuring, activity planning, resuming former activities based on individual goals, and mobilising support 12 weekly 1‐hour sessions over 12 weeks Control: site‐specific standard medical management for early dementia |
1. Function (Bayer Activities of Daily Living Scale; Hindmarch 1998, Aachen Functional Item Inventory; Böcker 2007) 2. Quality of life (DEMQOL; Smith 2005) 3. Depression (GDS‐30; Yesavage 1983) 4. Neuropsychiatric symptoms (NPI; Cummings 1994) 5. Caregiver depression (BDI; Beck 1961) 6. Caregiver burden (ZBI; Zarit 1980) 7. Memory (Wechsler Memory Scale Revised Logical Memory; Wechsler 1987) 8. Attention (Trail Making Test (TMT); Reitan 1958) 9. Verbal fluency (Regensburg Word Fluency Test; Aschenbrenner 2000) 10. Cognition (MMSE; Folstein 1975) |
| Lai 2020 | Mild and moderate dementia (ICD‐10; MoCA ≤ 21) N = 100; M52; F48 Mean age = 69.7 Mean MoCA = 18.1 |
BA: activity scheduling of pleasant activities in line with life values and goals, improving communication, monitoring of mood, and reassessment 10 weekly sessions provided over 10 weeks with weekly telephone follow‐ups Control: dementia care education over 10 weeks provided weekly |
1. Caregiver burden (ZBI; Zarit 1980) 2. Problem behaviours (Revised Memory and Behavior Problems Checklist; Teri 1992) 3. Caregiving time (Caregiver Activity Survey; Prince 2004) 4. Participant quality of life (Quality of Life‐Alzheimer’s Disease; Logsdon 1999) |
| Lichtenberg 2005 | Mild to moderate dementia N = 20; M2; F18 Mean age = 84.9 Mean MMSE = 14.2 Mean GDS‐15 = 4.0 |
BA: introduction to BA, pleasant‐event scheduling, mood monitoring, and relaxation strategies Three weekly sessions lasting 20 to 30 minutes each over 3 months Control: usual care comprised of regular activities taking place within the home |
1. Depression (GDS; Yesavage 1983, CSDD; Alexopoulos 1988) 2. Behavioural symptoms (Behavioral Pathology in Alzheimer's Disease; Reisberg 1987) |
| Lu 2016 | MCI (Albert 2011 et al. criteria) N = 40; M23; F17 Mean age = 73.8 38% scored ≥ 5 on PHQ‐9 |
BA: increasing meaningful activity through planning, addressing barriers to engagement, problem‐solving, and learning strategies for living with MCI Six biweekly 1‐hour sessions (2 in‐person and 4 telephone sessions) over 3 months Control: received educational brochure describing MCI, and opportunities to answer questions through biweekly follow‐up phone calls |
1. Congruence in function (Dementia Deficits Scale; Snow 2004) 2. Sense of confidence (Nowotny Confidence Subscale of the Nowotny Hope Scale; Nowotny 1989) 3. Meaningful activity (Canadian Occupational Performance Measure; Keihofner 2002) 4. Depression (PHQ‐9; Kroenke 2001) 5. Communication (Communication and Affective Expression Subscales of the Family Assessment Device; Miller 1985) 6. Function (Alzheimer's Disease Cooperative Study – Activities of Daily Living Inventory; Galasko 1997) 7. Life satisfaction (Life Satisfaction Index; Barrett 2006) 8. Carer depression (PHQ‐9; Kroenke 2001) 9. Caregiver outcomes (Bakas Caregiving Outcomes Scale; Bakas 2006) |
| Orgeta 2019 | Mild dementia (NINCDS‐ADRDA; scoring ≥ 18 on the MMSE) N = 63; M28; F35 Mean age = 80.4 Mean MMSE = 24.5 Mean CSDD = 7.2 |
BA: introduction to BA, pleasant‐event scheduling based on individuals' life values and preferences, through addressing barriers and practising relaxation strategies 8 weekly sessions lasting 90 minutes over 12 weeks Control: treatment as usual in line with the National Institute for Health and Care Excellence guidelines |
1. Function (Bristol Activities of Daily Living Scale; Bucks 1996) 2. Meaningful activity (Meaningful and Enjoyable Activities Scale; Tuijt 2020) 3. Depression (CSDD; Alexopoulos 1988) 4. Generic and dementia‐specific quality of life (EQ‐5D; EuroQoL 1990, DEMQOL; Smith 2005) 5. Neuropsychiatric symptoms (NPI; Cummings 1994) 6. Caregiver depression and anxiety (HADS; Zigmond 1983) 7. Caregiver quality of life (EQ‐5D; EuroQoL 1990, 12‐Item Short Form Health Survey; Ware 1996) |
| Rovner 2018 | Amnestic multiple‐ or single‐domain MCI (National Institute on Aging ‐ Alzheimer's Association) N = 221; M46; F175 Mean age = 75.8 Mean MMSE = 25.7 Mean GDS‐15 = 3.5 |
BA: increasing cognitive, physical, and/or social activity, in line with personal values, focusing on enhancement of treatment goals, and review of barriers Five in‐home 60‐minute sessions over 4 months and 6 in‐home 60‐minute follow‐up maintenance sessions over the next 20 months Control: supportive control intervention focusing on personal expression, conveying empathy, and optimism |
1. Cognition (Hopkins Verbal Learning Test–Revised; Benedict 1998, National Alzheimer’s Coordinating Center Uniform Data Set; Weintraub 2009, MMSE; Folstein 1975) 2. Function (University of California San Diego Performance‐Based Skills Assessment; Gomar 2011) 3. Activities (Florida Cognitive Activities Scale; Dotson 2008, US Health Interview Survey; Sturman 2005) 4. Depression (GDS‐15; Yesavage 1983) |
| Teri 1997 | Mild to moderate dementia (NINCDS‐ADRDA; diagnosis of major or minor depression; Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; HDRS ≥ 10) N = 33; M11; F22; 4‐arm RCT total N = 72 Mean age = 76.1 Mean MMSE = 16.3 Mean HDRS = 15.2 |
BA: pleasant‐event scheduling, mood monitoring, and modifying problem behaviours (behavioural and psychological symptoms) by addressing barriers and developing plans to maintain pleasant events for the future 9 weekly sessions lasting 60 minutes each over 9 weeks Control: treatment as usual incorporating typical advice and support services available in the community (and a separate waiting‐list control group) |
1. Depression (HDRS; Hamilton 1960, CSDD; Alexopoulos 1988, BDI; Beck 1961) 2. Cognition (MMSE; Folstein 1975, Dementia Rating Scale; Coblentz 1973) 3. Function (Record of Independent Living; Weintraub 1982; data not provided) 4. Caregiver depression (HDRS; Hamilton 1960) 5. Caregiver burden (ZBI; Zarit 1980; data not provided) |
| Travers 2017 | Mild to moderate dementia (SMMSE ≥ 10; GDS‐12R ≥ 4) N = 18; M2; F16 Mean age = 86.3 Mean SMMSE = 17.8 Mean GDS‐12R = 4.5 |
BA: pleasant‐event scheduling (scheduling of enjoyable activities), by developing individual plans, identifying barriers to engagement, and problem‐solving Sessions lasted 45 minutes over 8 weeks Control: receiving a walking‐and‐talking intervention (supervised social walking) for 30 minutes weekly |
1. Depression (GDS‐12R; Yesavage 1983) 2. Quality of life (Quality of Life in Alzheimer's Disease in Nursing Homes; Edelman 2005) |
BA: behavioural activation; BDI: Beck Depression Inventory; CSDD: Cornell Scale for Depression in Dementia; DEMQOL: Dementia Quality of Life Instrument; EQ‐5D: EuroQoL‐5 Dimension; GDS: Geriatric Depression Scale; HDRS: Hamilton Depression Rating Scale; ICD‐10: International Classification of Diseases, 10th Revision; MCI: mild cognitive impairment; MMSE: Mini‐Mental State Examination; MoCA: Montreal Cognitive Assessment; NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke ‐ Alzheimer's Disease and Related Disorders Association; NPI: Neuropsychiatric Inventory; PHQ‐9: Patient Health Questionnaire‐9; SMMSE: Standardized Mini‐Mental State Examination; ZBI: Zarit Burden Interview
Problem‐solving therapy (PST)
Participants and setting
Kiosses 2010 and Kiosses 2015 recruited older people with major depression, advanced cognitive impairment, and disability living in the community in the USA. Teri 1997 included people with mild to moderate dementia living in the community, also in the USA. In this study, all participants met RDC and DSM‐III‐R criteria for major or minor depressive disorder and had a HDRS score of at least 10. Kiosses 2010 recruited people with a diagnosis of unipolar major depressive disorder based on Structured Clinical Interview for DMS (SCID) and Structured Clinical Interview for DSM‐IV (SCID‐IV) criteria, and who had a score of 17 or higher on the HDRS. In the study by Kiosses 2015, all participants had non‐psychotic, unipolar major depressive disorder based on Structured Clinical Interview for DSM‐IV‐TR (SCID‐R) criteria, and scored 17 or higher on the Montgomery‐Åsberg Depression Rating Scale.
Intervention and control groups
Kiosses 2010 and Kiosses 2015 evaluated Problem Adaptation Therapy, aimed at reducing depression and disability versus a home‐delivered supportive control intervention focusing on empathic listening, encouragement, and use of reflection and emotion processing. Teri 1997 evaluated problem‐solving therapy versus usual care (consisting of advice and information about support services available and a separate waiting list‐control group).
Outcomes
Both the Kiosses 2010 and Kiosses 2015 studies measured depression at the end of 12‐week interventions, and Teri 1997 at the end of a 10‐week intervention. None of the PST studies measured anxiety as an outcome. We present the outcomes assessed across all PST studies (Kiosses 2010; Kiosses 2015; Teri 1997), the details of participants recruited, and the interventions evaluated, in Table 5. Teri 1997 measured persistence of effects at six months but data were not available.
3. Table of problem‐solving therapies (PST).
| Participants | Intervention and control conditions | Outcomes | |
| Kiosses 2010 | Major depression, cognitive impairment, and disability (major depression: SCID‐IV; ≥ 17 HDRS; cognitive impairment defined by < 2 standard deviations of mean of age‐matched controls on the Dementia Rating Scale (DRS; ≤ 30), or ≤ 18 on the Stroop Color and Word Test) N = 30; male (M) 9; female (F) 21 Mean age = 79.4 Mean MMSE = 26.6 Mean HDRS = 21.9 |
PST: aimed at reducing participants' depression and disability by facilitating problem‐solving, adaptive functioning, and engagement in pleasurable activities Weekly individual sessions over 12 weeks Control: home‐delivered supportive intervention consisting of empathic listening, reflection, emotional processing, and encouragement |
1. Depression (HDRS; Hamilton 1960) 2. Disability (Sheehan Disability Scale; Leon 1997) |
| Kiosses 2015 | Major depression, advanced cognitive impairment and disability (major depression: SCID‐R, mild cognitive deficits ≤ 7 on the DRS and ≥ 17 MADRS) N = 74; M23; F51 Mean age = 80.9 Mean DRS = 118.5 Mean MADRS = 21.2 |
PST: aimed at reducing the impact of negative emotions using a problem‐solving approach via specific tools Weekly individual home‐based sessions over 12 weeks Control: home‐delivered supportive intervention consisting of empathic listening, reflection, emotional processing, and encouragement |
1. Depression (MADRS; Montgomery 1979) 2. Disability (World Health Organization Disability Assessment Schedule 2.0; Ustun 2000) |
| Teri 1997 | Mild to moderate dementia (National Institute of Neurological and Communicative Disorders and Stroke ‐ Alzheimer's Disease and Related Disorders Association; diagnosis of major or minor depression; Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; HDRS ≥ 10) N = 39; M27; F12; 4‐arm RCT total N = 72 Mean age = 77.6 Mean MMSE = 16.8 Mean HDRS = 15.2 |
PST: problem‐solving situations of concern with the main focus being problem‐solving participant depression behaviours of specific concern to carers Weekly individual sessions of 60 minutes each over 9 weeks Control: treatment as usual incorporating typical advice, and support services available in the community (and a separate waiting‐list control group) |
1. Depression (HDRS; Hamilton 1960, Cornell Scale for Depression in Dementia; Alexopoulos 1988, Beck Depression Inventory; Beck 1961) 2. Cognition (MMSE; Folstein 1975, DRS; Coblentz 1973) 3. Function (Record of Independent Living; Weintraub 1982; data not provided) 4. Caregiver depression (HDRS; Hamilton 1960) 5. Caregiver burden (Zarit Burden Interview; Zarit 1980; data not provided) |
DRS: Dementia Rating Scale; HDRS: Hamilton Depression Rating Scale; MADRS: Montgomery–Åsberg Depression Rating Scale; MMSE: Mini‐Mental State Examination; PST: problem‐solving therapy; SCID: Structured Clinical Interview for DSM‐IV
Supportive and counselling therapies
Participants and setting
Bruvik 2013 recruited people living with mild dementia in the community in Norway. The studies by Jha 2013, Marshall 2015, and Quinn 2016 recruited people living with dementia in their own home in the UK. Both the Koivisto 2016 and the Laakkonen 2016 studies recruited people living with dementia in Finland. Logsdon 2010 recruited people living with mild to moderate dementia in the community in the USA. Nordheim 2019 recruited people living with mild to moderate dementia in community settings in Germany. Tappen 2009 recruited people living with dementia in a long‐term care facility in the USA, and Waldorff 2012 recruited community‐dwelling people living with mild AD in Denmark. Young 2014 recruited people living with mild dementia in China.
Intervention and control groups
Bruvik 2013 evaluated a counselling intervention versus a control condition comprised of receiving information about available services or support as required. Jha 2013 evaluated a well‐being recovery‐orientated counselling intervention versus a fixed package of care consisting of monthly visits for six months. Koivisto 2016 evaluated enhanced counselling support versus basic information about AD and access to regular services. Laakkonen 2016 evaluated a supportive therapy intervention based on psychosocial rehabilitation versus usual care which comprised regular health and social care services and advice about nutrition and exercise. Logsdon 2010 evaluated the effectiveness of structured support groups versus a waiting‐list control condition comprised of educational materials about dementia and support services available. Marshall 2015 evaluated a supportive therapy intervention known as Living Well with Dementia, incorporating psycho‐education about dementia, versus usual care. Nordheim 2019 evaluated couples‐based counselling versus standard care. Quinn 2016 evaluated a supportive group therapy intervention incorporating information about dementia versus TAU comprised of access to routine memory clinic services. Tappen 2009 assessed a counselling intervention adapted for people living with dementia in care homes versus usual care provided by staff at the long‐term care facility. Waldorff 2012 assessed the effectiveness of a counselling intervention versus information about local support available. Young 2014 evaluated a supportive therapy intervention incorporating provision of information and support about dementia versus standardised educational written materials about dementia.
Outcomes
All studies except Laakkonen 2016 measured depression, and only the study by Quinn 2016 measured anxiety as an outcome. We present the outcomes assessed across all supportive and counselling intervention studies (Bruvik 2013; Jha 2013; Koivisto 2016; Laakkonen 2016; Logsdon 2010; Marshall 2015; Nordheim 2019; Quinn 2016; Tappen 2009; Waldorff 2012; Young 2014), the details of participants recruited, and the interventions evaluated, in Table 6. Marshall 2015 assessed persistence of effects at 22 weeks, and Quinn 2016 assessed persistence of effects at six months post‐intervention (but data were not reported in a form that could be included in a meta‐analysis). The study by Waldorff 2012 examined persistence of effects of the intervention at three years.
4. Table of supportive and counselling therapies.
| Participants | Intervention and control conditions | Outcomes | |
| Bruvik 2013 | Mild dementia (ICD‐10) N = 230; male (M) 107; female (F) 123 Mean age = 78.4 Mean MMSE = 21.2 Mean CSDD = 8.0 |
CI: individual counselling sessions addressing unmet needs through problem‐solving, educational sessions about dementia, and social support meetings Five individual counselling sessions, one educational session, and 6 social support group meetings of 1 hour each over 12 months Control: received information about available services in local authority and were free to seek additional support |
1. Depression (CSDD; Alexopoulos 1988) 2. Carer depression (GDS; Yesavage 1983) |
| Jha 2013 | MCI or mild dementia (ICD‐10) N = 48; M16; F32 Mean age = 78.7 Mean MMSE = 22.0 Mean CSDD = 6.6 |
CI: well‐being and recovery orientated counselling intervention consisting of pre‐diagnostic counselling, a well‐being assessment, feedback primarily aimed at improving well‐being, post‐diagnostic counselling, and support Monthly visits for 6 months of 1 hour each Control: offered a fixed package of care of monthly visits for 6 months without being assessed for well‐being |
1. Well‐being (WHO 5‐item Well‐Being Index; Heun 1999) 2. Cognition (MMSE; Folstein 1975) 3. Depression (CSDD; Alexopoulos 1988) 4. Quality of life (EQ5D; EuroQoL 1990) 5. Caregiver burden (Zarit Burden Interview; Zarit 1980) |
| Koivisto 2016 | Mild AD (NINCDS‐ADRDA) N = 236; M115; F121 Mean age = 75.6 Mean MMSE = 21.5 Mean BDI = 10.7 |
CI: individual counselling, education, and both individual and support groups primarily aimed at enhancing knowledge, reducing social isolation, and support function Offered over 4 courses (total of 16 days) over 2 years Control: participated in annual follow‐up visits, and received regular healthcare services |
1. Institutionalisation 2. Dementia severity (CDR; Morris 1993) 3. Cognition (Consortium to Establish a Registry for Alzheimer's Disease neuropsychological battery; Chandler 2005, MMSE; Folstein 1975) 4. Function (ADCS‐ADL; Galasko 1997) 5. Neuropsychiatric symptoms (NPI; Cummings 1994) 6. Depression (BDI; Beck 1961) 7. Participant quality of life (QoL‐AD; Logsdon 1999) 8. Carer depression (BDI; Beck 1961) 9. Carer sense of coherence (Sense of Coherence Scale; Antonovsky 1987) 10. General physical and mental health for carers (GHQ; Goldberg 1985) 11. Carer quality of life (15D instrument; Sintonen 2001) |
| Laakkonen 2016 | Mild to moderate dementia (Finnish National Guidelines) N = 136; M85; F51 Mean age = 77.0 Mean MMSE = 20.8 |
ST: structured psychosocial rehabilitation program aimed at maintaining self‐efficacy, provision of social support, psycho‐education, use of resources, and problem‐solving skills in everyday life 4‐hour group sessions once a week over 8 weeks Control: usual care consisting of regular health and social services, and provision of advice on nutrition and exercise |
1. Quality of life (15D instrument; Sintonen 2001) 2. Cognition (CDR; Hughes 1982, Verbal Fluency; Morris 1989, Clock Drawing Test; Sunderland 1989) 3. Carer quality of life (RAND‐36 Item Health Survey; Hays 2001) 4. Carer sense of competence (SCQ; Vernooij‐Dassen 1996) 5. Carer mastery (Pearlin Mastery Scale; Pearlin 1978) |
| Logsdon 2010 | Mild to moderate dementia (diagnosed by physician) N = 142; M72; F70 Mean age = 74.9 Mean MMSE = 23.4 Mean GDS = 5.3 |
ST: structured support groups aimed at improving quality of life, sharing experiences, reducing feelings of isolation, and providing assistance with long‐term care planning Weekly 90‐minute sessions, taking place over 9 weeks Control: waiting‐list condition where people received written educational materials about dementia and AD, and services available |
1. Quality of life (QoL‐AD; Logsdon 1999, Medical Outcomes Study Short Form 36; Stewart 1988) 2. Depression (GDS; Yesavage 1983) 3. Family communication (Family Assessment Measure; Skinner 1983) 4. Perceived stress (Perceived Stress Scale; Cohen 1983) 5. Self‐efficacy (Self Efficacy Scale; Seeman 1996) 6. Caregiver reactions to problem behaviours (Revised Memory and Behavior Problems Checklist; Teri 1992) |
| Marshall 2015 | Mild dementia (NINCDS‐ADRDA) N = 58; M25; F33 Mean age = 75.6 Mean MMSE = 23.0 Mean CSDD = 6.2 |
ST: psycho‐education provided in a group setting aimed at improving quality of life, provision of information about dementia, coping with stress, and living as well as you can Weekly 75‐minute sessions over 10 weeks Control: a waiting‐list condition in which participants received usual care |
1. Quality of life (QoL‐AD; Logsdon 1999) 2. Depression (CSDD; Alexopoulos 1988) 3. Self‐esteem (RSES; Rosenberg 1965) 4. Cognition (MMSE; Folstein 1975) 5. Carer general health (GHQ; Goldberg 1985) |
| Nordheim 2019 | Mild to moderate dementia (National Institute on Aging ‐ Alzheimer's Association) N = 108; M66; F42 Mean age = 85.0 Mean MMSE = 22.8 Mean GDS‐15 = 5.5 |
CI: couples‐based counselling intervention incorporating information about dementia, couples communication training, coping and problem‐solving strategies, and relaxation techniques Offered twice every week, with sessions lasting 1 to 2 hours each for 10 to 12 weeks Control: received standard care incorporating 1 to 2 hours of consultation according to the standards of German memory clinics |
1. Quality of life (QoL‐AD; Logsdon 1999) 2. Carer quality of life (World Health Organization Quality of Life Brief scale; von Steinbüche 2006) 3. Cognition (MMSE; Folstein 1975) 4. Function (Barthel Index; Mahoney 1965, Instrumental Activities of Daily Living Scale; Lawton 1969, Overprotection Scale For Adults; Thompson 1993) 5. Carer sense of competence (SCQ; Vernooij‐Dassen 1996) 6. Dyadic coping (Dyadic Coping Inventory; Ledermann 2010) 7. Social support (Social Support Questionnaire; Fydrich 2009) 8. Participant depression (GDS‐15; Yesavage 1983) 9. Carer depression (GDS‐15; Yesavage 1983) |
| Quinn 2016 | Mild dementia (ICD‐10) N = 24; M18; F6 Mean age = 75.6 Mean MMSE = 23.6 Mean HADS = 4.6 |
ST: supportive group therapy intervention which incorporated information about dementia, managing memory difficulties, staying well, and accessing local resources Weekly 90‐minute sessions over 9 weeks Control: treatment as usual receiving routinely provided memory clinic services, such as a nurse‐led review, and access to psychiatry, psychology and social services |
1. Self‐efficacy (GSE; Schwarzer 1995) 2. Depression (HADS; Zigmond 1983) 3. Anxiety (HADS; Zigmond 1983) 4. Global distress (Clinical Outcomes in Routine Evaluation‐Outcome Measure; Evans 2002) 5. Quality of life (EQ5D; EuroQoL 1990) 6. Well‐being (ICEpop CAPability measure for Older people; Coast 2008) |
| Tappen 2009 | Moderate dementia (NINCDS‐ADRDA) N = 30; M3; F27 Mean age = 87.3 Mean MMSE = 11.4 Mean MADRS = 17.4 |
CI: individual counselling sessions primarily aimed at forming and maintaining supportive relationships, providing opportunities for expression, reducing isolation, and improving mood Three sessions per week, lasting 30 minutes each, for 16 weeks Control: usual care provided by staff at the long‐term care facility |
1. Mood (Dementia Mood Assessment Scale; Sunderland 1988, Alzheimer's Disease and Related Dementias Mood Scale; Tappen 2008) 2. Depression (MADRS; Montgomery 1979) |
| Waldorff 2012 | Mild AD (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; NINCDS‐ADRDA) N = 330; M151; F179 Mean age = 76.2 Mean MMSE = 24.0 Mean CSDD = 4.8 |
CI: individual counselling sessions, education, and telephone support, primarily aimed at preventing depression, loss of social networks, and retaining skills Up to 7 individual counselling sessions, 5 educational courses about dementia lasting 2 hours each, and telephone support (5 to 8 sessions) over 3 or 4 week intervals over 8 to 12 months Control: provided with information and guidance about local support services available |
1. Cognition (MMSE; Folstein 1975) 2. Depression (CSDD; Alexopoulos 1988) 3. Participant quality of life (EQ‐5D VAS; EuroQoL 1990, QoL‐AD; Logsdon 1999) 4. Neuropsychiatric symptoms (NPI; Cummings 1994) 5. Function (ADCS‐ADL; Galasko 1997) 6. Carer depression (GDS; Yesavage 1983) 7. Carer quality of life (EQ‐5D VAS; EuroQoL 1990) |
| Young 2014 | Mild dementia N = 39; M22; F17 Mean age = 80.3 Mean MMSE = 22.0 Mean GDS = 9.0 |
ST: incorporated provision of information and support about dementia, developing a positive lifestyle, adjusting to changes, and identifying sources of support Weekly 90‐minute sessions over 10 weeks Control: received standardised educational written material on dementia providing basic information |
1. Depression (GDS; Yesavage 1983) 2. Self‐esteem (RSES; Rosenberg 1965) 3. Self‐efficacy (GSE; Schwarzer 1995) 4. Coping with memory loss (Index for Managing Memory Loss; Keady 1995) 5. Participant quality of life (QoL‐AD; Logsdon 1999) |
ADCS‐ADL: Alzheimer's Disease Cooperative Study – Activities of Daily Living Inventory; BDI: Beck Depression Inventory; CDR: Clinical Dementia Rating; CI: counselling intervention; CSDD: Cornell Scale for Depression in Dementia; 15D: 15D instrument; EQ‐5D: EuroQoL‐5 Dimension; EQ‐5D VAS: EQ‐5D Visual Analogue Scale; GDS: Geriatric Depression Scale; GHQ: General Health Questionnaire; GSE: General Self‐Efficacy Scale; HADS: Hospital Anxiety and Depression Scale; ICD‐10: International Classification of Diseases, 10th Revision; MADRS: Montgomery–Åsberg Depression Rating Scale; MMSE: Mini‐Mental State Examination; NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke ‐ Alzheimer's Disease and Related Disorders Association; NPI: Neuropsychiatric Inventory; QoL‐AD: Quality of Life‐Alzheimer’s Disease; RSES: Rosenberg Self‐Esteem Scale; SCQ: Sense of Competence Questionnaire; ST: supportive therapy
Third‐wave mindfulness‐based interventions
Participants and setting
Churcher Clarke 2017 recruited people with mild to moderate dementia living in care homes in the UK. Both the Larouche 2019 and Wells 2013 studies recruited people with MCI living in the community in Canada and the USA, respectively.
Intervention and control groups
Churcher Clarke 2017 evaluated an adapted group‐based mindfulness intervention versus regular services provided within the home. Both Larouche 2019 and Wells 2013 evaluated mindfulness‐based group cognitive therapy versus usual care (comprised of psycho‐education about ageing and dementia and coping with cognitive decline in Larouche 2019; usual care in Wells 2013).
Outcomes
The studies by Churcher Clarke 2017 and Larouche 2019 assessed both depression and anxiety. Wells 2013 assessed only depression. We present the outcomes assessed across all third‐wave MBCT interventions (Churcher Clarke 2017; Larouche 2019; Wells 2013), the details of participants recruited, and the interventions evaluated, in Table 7. Larouche 2019 assessed persistence of effects at three months.
5. Table of third‐wave therapies.
| Participants | Intervention and control conditions | Outcomes | |
| Churcher Clarke 2017 | Mild to moderate dementia (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition) N = 31; male (M) 16; female (F) 15 Mean age = 80.6 Mean MMSE = 15.3 Mean CSDD = 7.3 |
TWI: adapted group‐based mindfulness intervention consisting of mindfulness meditation, mindful breathing, mindful movement, and practicing mindful listening, seeing, smelling, and touch 10 1‐hour group sessions, running twice a week for 5 weeks Control: regular services provided within the care home |
1. Depression (CSDD; Alexopoulos 1988) 2. Anxiety (Rating Anxiety in Dementia; Shankar 1999) 3. Quality of life (QoL‐AD; Logsdon 1999) 4. Cognition (MMSE; Folstein 1975) 5. Perceived stress (PSS; Cohen 1983) |
| Larouche 2019 | MCI (National Institute on Aging ‐ Alzheimer's Association) N = 48; for N = 45 M26; F19 Mean age = 70.9 Mean Montreal Cognitive Assessment = 24.4 Mean GDS‐30 = 7.9 |
TWI: mindfulness‐based group cognitive therapy intervention consisting of psycho‐education for mindfulness, handling obstacles and supporting meditation practice, and stress management Eight sessions of 2.5 hours each over 8 weeks Control: psycho‐education about aging and dementia, and information about coping with cognitive decline |
1. Depression (GDS; Yesavage 1983) 2. Anxiety (Geriatric Anxiety Inventory; Pachana 2007) 3. Quality of life (World Health Organisation Quality of Life Brief scale; von Steinbüche 2006, World Health Organisation Quality of Life for Older Persons; Leplège 2013) 4. Episodic memory (Episodic memory delayed and immediate recall; Moulin 2004) 5. Perceived stress (PSS; Cohen 1983) 6. Coping (Coping Orientation to Problems Experienced Inventory; Carver 1989) 7. Rumination (Ruminative Response Scale; Nolen‐Hoeksema 1991) 8. Mindfulness (Five Facet Mindfulness Questionnaire; Baer 2006) 9. Cortisol awakening response |
| Wells 2013 | MCI (diagnosed by neurologist; Grundman 2004 et al criteria) N =14 Mean age = 74.0 Mean MMSE = 27.0 Median CES‐D = 6.0 |
TWI: mindfulness‐based stress‐reduction intervention consisting of teaching of mindfulness, defined as non‐judgmental moment‐to‐moment awareness, through sitting and walking meditation, body scan, and mindful movement Eight 2‐hour weekly sessions over 8 weeks plus 1 mindfulness retreat day Control: usual care |
1. Cognition (Alzheimer's Disease Assessment Scale–Cognitive Subscale; Rosen 1984, Rey Auditory Verbal Learning Test; Rey 1964, Trail Making Test; Army Individual Test Battery 1944, Controlled Oral Word Association Test; Benton 1994, animal naming; Boston Naming Test; Kaplan 1978) 2. Resilience (Resilience Scale) 3. Hope (Herth Hope Index; Herth 1992) 4. Quality of life (QoL‐AD; Logsdon 1999) 5. Life orientation (Life Orientation Test; Scheier 1985) 6. Depression (CES‐D; Radloff 1977) 7. Mindfulness attention (Mindful Attention Awareness Scale; Brown 2003) |
GDS: Geriatric Depression Scale; CES‐D: Center for Epidemiologic Studies‐Depression; CSDD: Cornell Scale for Depression in Dementia; MMSE: Mini‐Mental State Examination; QoL‐AD: Quality of Life‐Alzheimer’s Disease; PSS: Perceived Stress Scale; TWI: third‐wave interventions
Interpersonal therapy (IPT)
Participants and setting
Burns 2005 recruited people with mild AD living in the community in the UK.
Intervention and control groups
The study evaluated a brief psychodynamic IPT intervention versus standard care which consisted of general advice about dementia, treatments and outpatient review.
Outcomes
Burns 2005 measured depression, but not anxiety, as an outcome. We present all other outcomes assessed by Burns 2005, the details of participants and of the intervention in Table 8. The study also assessed persistence of effects at three months.
6. Table of interpersonal therapies (IPT).
| Participants | Intervention and control groups | Outcomes | |
| Burns 2005 | Mild AD (National Institute of Neurological and Communicative Disorders and Stroke ‐ Alzheimer's Disease and Related Disorders Association) N = 40; male (M) 20; female (F) 20 Mean age = 75.8 Mean MMSE = 22.9 Mean CSDD = 5.5 |
IPT: aimed at identifying interpersonal conflicts or difficulties, causing or maintaining emotional distress, and encouraging practical changes Six sessions lasting 50 minutes each over 6 weeks Control: received standard care which consisted of general advice about diagnosis and treatment of dementia with outpatient review |
1. Depression (CSDD; Alexopoulos 1988) 2. Cognition (MMSE; Folstein 1975) 3. Function (Bristol Activities of Daily Living Scale; Bucks 1996) 4. Problem behaviours (Revised Memory and Behavior Problems Checklist; Teri 1992) 5. Global assessment (Clinical Global Impressions Scale; Guy 1976) 6. Carer depression (Beck Depression Inventory; Beck 1961) 7. Carer general health (General Health Questionnaire; Goldberg 1985) 8. Carer coping (Ways of Coping Checklist; Vitaliano 1985) |
CSDD: Cornell Scale for Depression in Dementia; IPT: interpersonal therapy; MMSE: Mini‐Mental State Examination
Excluded studies
We excluded a total of 53 studies after detailed examination, and provide the reasons for exclusion in the Characteristics of excluded studies table.
Risk of bias in included studies
Three review authors (VO, PL, AM) independently extracted information about risk of bias. Figure 2 presents a summary of judgments about each risk of bias item for each included study, and Figure 3 presents the assessments about each risk of bias item presented as percentages across all included studies (Characteristics of included studies). None of the studies were classified as having low risk of bias in all domains of risk assessment.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
For the domain of random sequence generation, we considered 11 studies to be at unclear risk of bias due to insufficient information on how the random assignment was performed (Burgener 2008; Kiosses 2010; Lichtenberg 2005; Logsdon 2010; Lu 2016; Marshall 2015; Stanley 2013; Tappen 2009; Teri 1997; Wells 2013; Young 2014). We judged the remaining studies to be at low risk of bias in this domain.
For the allocation concealment domain, we judged 14 studies to be at unclear risk of bias, due to insufficient information provided in the published reports (Burgener 2008; Churcher Clarke 2017; Kiosses 2010; Kiosses 2015; Kurz 2012; Larouche 2019; Lichtenberg 2005; Logsdon 2010; Stanley 2013; Tappen 2009; Teri 1997; Travers 2017; Wells 2013; Young 2014). We judged the remaining studies to be at low risk of bias in this domain.
Blinding
We rated all studies as having an unclear risk in the domain of performance bias, given that both personnel and participants were not blinded in most of the studies.
We assessed the majority of studies as low risk in the domain of detection bias, with most studies reporting that assessors were blind to treatment allocation. However, four studies did not provide information as to whether assessors were blind (Burgener 2008; Burns 2005; Logsdon 2010; Lu 2016), and we therefore rated these as having an unclear risk in this domain. We assessed one study, Travers 2017, to be at high risk of detection bias, as this study reported that it was not possible to blind all or some assessors to treatment allocation.
Incomplete outcome data
We rated most studies as low risk in the domain of attrition bias. However, we judged four studies to have an unclear risk of bias in this domain due to not providing sufficient information on how missing data were handled (Jha 2013; Lu 2016; Teri 1997; Tappen 2009).
Selective reporting
For four studies (Burns 2005; Kurz 2012; Quinn 2016; Teri 1997), there was evidence of selective reporting; we therefore judged these studies as having an unclear risk in this domain. All the remaining studies reported all pre‐specified outcomes, and we classified these as low risk of bias. We were able to access study protocols for the following studies: Belleville 2018, Kiosses 2015, Marshall 2015, Nordheim 2019, Orgeta 2019, Quinn 2016, Rovner 2018, Spector 2015, and Waldorff 2012. For the remaining studies, we relied on the list of outcomes provided in the methods section of the articles to assess this form of bias.
Other potential sources of bias
We identified no other apparent bias in each of the included studies.
Effects of interventions
We were able to conduct a number of meta‐analyses for the comparisons of CBT‐based interventions and supportive and counselling interventions with control conditions (which varied from usual care, to psycho‐education, and services above usual care including active control conditions for the CBT‐based treatments). We were able to extract data on the primary outcome of depressive symptoms for both CBT‐based interventions and supportive and counselling interventions versus TAU. Data on depression remission were only available for CBT‐based interventions. We also examined the effectiveness of CBT‐based treatments and supportive and counselling interventions on symptoms of anxiety versus TAU. Only one study of the supportive and counselling interventions assessed anxiety as an outcome and no studies assessed depression remission.
We were able to undertake meta‐analyses on the secondary outcomes of quality of life, ADL, cognition, neuropsychiatric symptoms, and carer depressive symptoms for both CBT‐based and supportive and counselling interventions versus control conditions. In addition, we examined the effectiveness of CBT‐based interventions versus usual care for carer burden.
We report narratively the results of the one study of IPT and the three studies of third‐wave mindfulness‐based interventions.
A negative effect size favours psychological interventions for the outcomes of depression, anxiety, ADL, neuropsychiatric symptoms, carer depression, and carer burden. A positive effect size favours psychological interventions for the outcomes of depression remission, quality of life, and cognition.
All outcomes were assessed at the end of the treatment periods. We were also able to examine the persistence of effects of CBT‐based treatments for five outcomes (depressive symptoms, anxiety symptoms, quality of life, ADL, and carer depressive symptoms).
Effects of CBT‐based treatments versus treatment as usual or active control
We present a summary of findings and assessment of the certainty of the evidence in the Table 1.
Primary outcomes
Depression
The meta‐analysis of effects of CBT‐based treatments (CBT, BA, PST) on depressive symptoms measured at the end of treatment included 893 participants and pooled data from 13 studies. We found that CBT‐based interventions were probably slightly better than TAU or active control (SMD ‐0.23, 95% CI ‐0.37 to ‐0.10; moderate‐certainty evidence; Analysis 1.1; Figure 4) in reducing depressive symptoms for people with dementia and MCI at post‐treatment, representing a small effect; with moderate heterogeneity between studies (I2 = 54%); and very low risk of publication bias when examining the funnel plot (Figure 5).
1.1. Analysis.

Comparison 1: Cognitive behavioural therapies versus treatment as usual, Outcome 1: Depressive symptoms post‐treatment
4.

Forest plot of comparison: 1 Cognitive behavioural therapies versus treatment as usual, outcome: 1.1 Depressive symptoms post‐treatment.
5.

Funnel plot of comparison: 1 Cognitive behavioural therapies versus treatment as usual, outcome: 1.1 Depressive symptoms post‐treatment.
We investigated whether the effect of CBT‐based treatments on depressive symptoms at the end of treatment varied with participants' level of depression at baseline (Analysis 1.1). One subgroup consisted of studies in which all participants had either a criterion‐based diagnosis of a depressive disorder or clinically significant depressive symptoms at baseline; in the other subgroup, there were no depression‐related participant inclusion criteria. There was a large effect favouring CBT treatments in studies which included people with a depression diagnosis or depressive symptoms at baseline (SMD ‐0.84, 95% CI ‐1.14 to ‐0.54; I² = 24%; 4 studies, 194 participants), but little or no effect of treatment in studies including people with no diagnosis of depression or depressive symptoms at baseline (SMD ‐0.08, 95% CI ‐0.23 to 0.07; I² = 0%; 9 studies, 699 participants). However, we also noted that the four studies which recruited participants who had either a criterion‐based diagnosis of a depressive disorder or clinically significant depressive symptoms at baseline included all three of the studies of problem‐solving therapy; these three studies accounted for 138 of the 194 participants in this subgroup.
In a second between‐study subgroup analysis, we investigated whether the effect of CBT‐based treatments on depressive symptoms at the end of treatment varied with cognitive diagnosis (dementia or MCI). Results are presented in Analysis 1.2; Figure 6. We found a significant difference between subgroups, suggesting that CBT interventions may be superior to TAU for people with dementia (SMD ‐0.40, 95% CI ‐0.57 to ‐0.23; I² = 44%; 10 studies, 554 participants), but not for people with MCI (SMD 0.03, 95% CI ‐0.18 to 0.24; I² = 0%; 3 studies, 339 participants).
1.2. Analysis.

Comparison 1: Cognitive behavioural therapies versus treatment as usual, Outcome 2: Depressive symptoms post‐treatment subgroup analysis ‐ type of cognitive diagnosis (dementia vs MCI)
6.

Forest plot of comparison: 1 Cognitive behavioural therapies versus treatment as usual, outcome: 1.2 Depressive symptoms post‐treatment subgroup analysis ‐ type of cognitive diagnosis (dementia vs MCI).
We found low‐certainty evidence that CBT treatments may be favoured in comparison to TAU or active control for depression remission at the end of treatment (risk ratio (RR) 1.84, 95% CI 1.18 to 2.88, I² = 0%; 2 studies, with one study contributing two independent comparisons, 146 participants; Analysis 1.3; Figure 7), representing a large effect.
1.3. Analysis.

Comparison 1: Cognitive behavioural therapies versus treatment as usual, Outcome 3: Depression remission post‐treatment
7.

Forest plot of comparison: 2 Cognitive behavioural therapies versus treatment as usual, outcome: 2.2 Depression remission post‐treatment.
CBT‐based interventions may not differ from control interventions in their long‐term effect on depressive symptoms (SMD ‐0.13, 95% CI ‐0.35 to 0.09; I² = 0%; 4 studies, 317 participants; low‐certainty evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1: Cognitive behavioural therapies versus treatment as usual, Outcome 4: Depressive symptoms long‐term
Anxiety
We found very low‐certainty evidence that CBT‐based treatments did not differ from control in reducing anxiety symptoms measured at end of treatment (SMD ‐0.03, 95% CI ‐0.36 to 0.30; I² = 36%; 3 studies, 143 participants; Analysis 1.5; Figure 8), and long‐term (SMD ‐0.06, 95% CI ‐0.47 to 0.35; I² = 40%; 2 studies, 95 participants; Analysis 1.6).
1.5. Analysis.

Comparison 1: Cognitive behavioural therapies versus treatment as usual, Outcome 5: Anxiety symptoms post‐treatment
8.

Forest plot of comparison: 2 Cognitive behavioural therapies versus treatment as usual, outcome: 2.4 Anxiety symptoms post‐treatment.
1.6. Analysis.

Comparison 1: Cognitive behavioural therapies versus treatment as usual, Outcome 6: Anxiety symptoms long‐term
Secondary outcomes
Quality of life
There was moderate‐certainty evidence that CBT‐based interventions probably improve patient carer‐rated quality of life compared to usual care at the end of treatment (SMD 0.31, 95% CI 0.13 to 0.50; I² = 29%; 7 studies, 459 participants; Analysis 1.7; Figure 9), representing a small effect. We found no evidence of publication bias (Figure 10). We found very low‐certainty evidence that CBT may also be associated with a small improvement in patient carer‐rated quality of life measured at long‐term follow‐up, but this result is imprecise and very uncertain (SMD 0.20, 95% CI ‐0.05 to 0.45; I² = 0%; 3 studies, 249 participants; Analysis 1.8).
1.7. Analysis.

Comparison 1: Cognitive behavioural therapies versus treatment as usual, Outcome 7: Quality of life post‐treatment
9.

Forest plot of comparison: 2 Cognitive behavioural therapies versus treatment as usual, outcome: 2.6 Quality of life post‐treatment.
10.

Funnel plot of comparison: 2 Cognitive behavioural therapies versus treatment as usual, outcome: 2.6 Quality of life post‐treatment.
1.8. Analysis.

Comparison 1: Cognitive behavioural therapies versus treatment as usual, Outcome 8: Quality of life long‐term
Activities of daily living (ADL)
There was moderate‐certainty evidence that CBT‐based interventions are probably better than TAU or active control at improving ADL for people with dementia and MCI at end of treatment (SMD ‐0.25, 95% CI ‐0.40 to ‐0.09; I² = 34%; 7 studies, 680 participants; Analysis 1.9; Figure 11), with no evidence of publication bias present (Figure 12). There was no evidence of a persistent effect measured at long‐term follow‐up (SMD ‐0.01, 95% CI ‐0.24 to 0.23; I² = 0%; 3 studies, 291 participants; very low‐certainty evidence; Analysis 1.10). There was no evidence of a significant difference between subgroups of people with dementia and those with MCI for activities of daily living at the end of treatment (Analysis 1.11; Figure 13).
1.9. Analysis.

Comparison 1: Cognitive behavioural therapies versus treatment as usual, Outcome 9: Activities of daily living post‐treatment
11.

Forest plot of comparison: 2 Cognitive behavioural therapies versus treatment as usual, outcome: 2.8 Activities of daily living post‐treatment.
12.

Funnel plot of comparison: 2 Cognitive behavioural therapies versus treatment as usual, outcome: 2.8 Activities of daily living post‐treatment.
1.10. Analysis.

Comparison 1: Cognitive behavioural therapies versus treatment as usual, Outcome 10: Activities of daily living long‐term
1.11. Analysis.

Comparison 1: Cognitive behavioural therapies versus treatment as usual, Outcome 11: Activities of daily living post‐treatment subgroup analysis ‐ type of cognitive diagnosis (dementia vs MCI)
13.

Forest plot of comparison: 1 Cognitive behavioural therapies versus treatment as usual, outcome: 1.11 Activities of daily living post‐treatment subgroup analysis ‐ type of cognitive diagnosis (dementia vs MCI).
Cognition
We found low‐certainty evidence that there may be little or no difference between CBT‐based interventions and usual care or active control for the cognitive function of people with dementia and MCI at the end of treatment (SMD 0.13, 95% CI ‐0.04 to 0.30; I² = 0%; 5 studies, 535 participants; Analysis 1.12; Figure 14).
1.12. Analysis.

Comparison 1: Cognitive behavioural therapies versus treatment as usual, Outcome 12: Cognition post‐treatment
14.

Forest plot of comparison: 2 Cognitive behavioural therapies versus treatment as usual, outcome: 2.10 Cognition post‐treatment.
Neuropsychiatric symptoms
There was very low‐certainty evidence that CBT‐based treatments may not differ from usual care for patient neuropsychiatric symptoms at the end of treatment (SMD ‐0.06, 95% CI ‐0.26 to 0.14; I² = 55%; 5 studies, 401 participants; Analysis 1.13; Figure 15).
1.13. Analysis.

Comparison 1: Cognitive behavioural therapies versus treatment as usual, Outcome 13: Neuropsychiatric symptoms post‐treatment
15.

Forest plot of comparison: 2 Cognitive behavioural therapies versus treatment as usual, outcome: 2.11 Neuropsychiatric symptoms post‐treatment.
Carer depression
We pooled six studies to assess the effectiveness of CBT‐based treatments compared to usual care on carers' depressive symptoms. We found very low‐certainty evidence that CBT‐based treatments may not differ from usual care in their effect at the end of treatment (SMD ‐0.09, 95% CI ‐0.29 to 0.10; I² = 53%; 6 studies, 413 participants; Analysis 1.14; Figure 16), with no evidence of publication bias (Figure 17). Similarly, we found very low‐certainty evidence of little or no difference between CBT‐based interventions and control conditions for carer depression measured at long‐term follow‐up (SMD 0.00, 95% CI ‐0.25 to 0.26; I² = 0%; 3 studies, 248 participants; Analysis 1.15).
1.14. Analysis.

Comparison 1: Cognitive behavioural therapies versus treatment as usual, Outcome 14: Carer depressive symptoms post‐treatment
16.

Forest plot of comparison: 2 Cognitive behavioural therapies versus treatment as usual, outcome: 2.12 Carer depressive symptoms post‐treatment.
17.

Funnel plot of comparison: 2 Cognitive behavioural therapies versus treatment as usual, outcome: 2.12 Carer depressive symptoms post‐treatment.
1.15. Analysis.

Comparison 1: Cognitive behavioural therapies versus treatment as usual, Outcome 15: Carer depressive symptoms long‐term
Carer burden
We found very low‐certainty evidence from two studies that CBT‐based treatments may have little or no effect on carer burden at the end of treatment (SMD 0.06, 95% CI ‐0.18 to 0.29; 2 studies, 289 participants; Analysis 1.16; Figure 18). There was substantial heterogeneity between these two studies (I² = 87%).
1.16. Analysis.

Comparison 1: Cognitive behavioural therapies versus treatment as usual, Outcome 16: Carer burden post‐treatment
18.

Forest plot of comparison: 2 Cognitive behavioural therapies versus treatment as usual, outcome: 2.14 Carer burden post‐treatment.
Effects of supportive and counselling interventions versus treatment as usual
We present a summary of findings and assessment of the certainty of the evidence in the Table 2.
Primary outcomes
Depression
Pooling data from nine studies showed that, at the end of the treatment period, supportive and counselling interventions may have little or no effect on depressive symptoms in people with dementia compared to TAU (SMD ‐0.05, 95% CI ‐0.18 to 0.07; I² = 4%; 994 participants; low‐certainty evidence; Analysis 2.1; Figure 19). We found no evidence of publication bias (Figure 20). Quinn 2016 conducted a very small pilot study (24 participants). They did not report data on depression outcomes at the end of treatment in a form that could be included in the meta‐analysis, but because of the size of the study, we do not consider this likely to have introduced important publication bias.
2.1. Analysis.

Comparison 2: Supportive and counselling interventions versus treatment as usual, Outcome 1: Depressive symptoms post‐treatment
19.

Forest plot of comparison: 2 Supportive and counselling interventions versus treatment as usual, outcome: 2.1 Depressive symptoms post‐treatment.
20.

Funnel plot of comparison: 2 Supportive and counselling interventions versus treatment as usual, outcome: 2.1 Depressive symptoms post‐treatment.
Anxiety
In this comparison, only Quinn 2016 reported anxiety as an outcome in a small pilot study of 24 participants (MD ‐0.80, 95% CI ‐3.07 to 1.47; very‐low certainty evidence; Analysis 2.2; Figure 21). This evidence is very uncertain and we cannot determine any effect of supportive and counselling interventions on anxiety symptoms in people with dementia.
2.2. Analysis.

Comparison 2: Supportive and counselling interventions versus treatment as usual, Outcome 2: Anxiety symptoms post‐treatment
21.

Forest plot of comparison: 2 Supportive and counselling interventions versus treatment as usual, outcome: 2.2 Anxiety symptoms post‐treatment.
Secondary outcomes
Quality of life
Moderate‐certainty evidence showed that supportive and counselling interventions were probably slightly favoured compared to TAU for patient quality of life measured at post‐treatment (SMD 0.15, 95% CI 0.02 to 0.28; I² = 36%; 8 studies, 935 participants; Analysis 2.3; Figure 22), representing a small effect; inspection of the funnel plot showed no evidence of publication bias (Figure 23). We were not able to include data from Quinn 2016 in the meta‐analysis, but in this small study (24 participants), people with dementia taking part in the supportive intervention also had higher quality of life at three months than those receiving usual care. Because of the small size of this study, we do not consider that its absence from the meta‐analysis is likely to have introduced bias.
2.3. Analysis.

Comparison 2: Supportive and counselling interventions versus treatment as usual, Outcome 3: Quality of life post‐treatment
22.

Forest plot of comparison: 2 Supportive and counselling interventions versus treatment as usual, outcome: 2.2 Quality of life post‐treatment.
23.

Funnel plot of comparison: 3 Supportive and counselling interventions versus treatment as usual, outcome: 3.2 Quality of life post‐treatment.
ADL
Usual care was slightly favoured compared to supportive and counselling interventions at the end of treatment for performance of ADL (SMD 0.17, 95% CI ‐0.01 to 0.34; I² = 9%; 3 studies, 511 participants; very low‐certainty evidence; Analysis 2.4; Figure 24), but this result was very uncertain so we are unable to draw any conclusion.
2.4. Analysis.

Comparison 2: Supportive and counselling interventions versus treatment as usual, Outcome 4: Activities of daily living post‐treatment
24.

Forest plot of comparison: 3 Supportive and counselling interventions versus treatment as usual, outcome: 3.3 Activities of daily living post‐treatment.
Cognition
We found low‐certainty evidence that supportive and counselling interventions may have little or no effect on cognition at the end of treatment (SMD 0.11, 95% CI ‐0.03 to 0.26; I² = 29%; 6 studies, 730 participants; Analysis 2.5; Figure 25); with no evidence of publication bias (Figure 26).
2.5. Analysis.

Comparison 2: Supportive and counselling interventions versus treatment as usual, Outcome 5: Cognition post‐treatment
25.

Forest plot of comparison: 2 Supportive and counselling interventions versus treatment as usual, outcome: 2.3 Cognition post‐treatment.
26.

Funnel plot of comparison: 3 Supportive and counselling interventions versus treatment as usual, outcome: 3.4 Cognition post‐treatment.
Neuropsychiatric symptoms
We found very low‐certainty evidence that supportive and counselling interventions may have little or no effect on patient neuropsychiatric symptoms at the end of treatment (SMD 0.11, 95% CI ‐0.06 to 0.29; I² = 10%; 3 studies, 538 participants; Analysis 2.6; Figure 27).
2.6. Analysis.

Comparison 2: Supportive and counselling interventions versus treatment as usual, Outcome 6: Neuropsychiatric symptoms post‐treatment
27.

Forest plot of comparison: 3 Supportive and counselling interventions versus treatment as usual, outcome: 3.5 Neuropsychiatric symptoms post‐treatment.
Carer depression
We found very low‐certainty evidence that supportive and counselling interventions may have little or no effect on carer depression at the end of the intervention period (SMD 0.02, 95% CI ‐0.13 to 0.17; I² = 40%; 4 studies, 704 participants; Analysis 2.7; Figure 28).
2.7. Analysis.

Comparison 2: Supportive and counselling interventions versus treatment as usual, Outcome 7: Carer depressive symptoms post‐treatment
28.

Forest plot of comparison: 2 Supportive and counselling interventions versus treatment as usual, outcome: 2.4 Carer depressive symptoms post‐treatment.
Persistence of effects of supportive and counselling interventions
Marshall 2015, Quinn 2016, and Waldorff 2012 were the only studies measuring persistence of effects long‐term, at 22 weeks, 6 months, and 3 years, respectively. Marshall 2015 reported no long‐term differences between a supportive intervention and a control intervention for all outcomes other than self‐esteem, which increased for the intervention group at 22 weeks. In the study by Quinn 2016 (data not in an extractable format suitable for meta‐analysis), depression was lower in the intervention group at six months and quality of life ratings higher; however, intervention participants had higher anxiety scores at long‐term follow‐up. Waldorff 2012 reported no differences between counselling and control groups on any of the outcomes assessed at three‐year follow‐up. We decided not to extract data from the Marshall 2015 and Waldorff 2012 studies due to the large variation in the long‐term follow‐up reported between these two studies (five months versus three years).
Third‐wave interventions
We decided not to pool any data from studies investigating third‐wave interventions as extractable data were available for only two of the studies (Churcher Clarke 2017; Larouche 2019), and these tested effectiveness of third‐wave interventions in different populations and settings: people living with dementia in nursing homes (Churcher Clarke 2017), versus people with MCI living in community settings (Larouche 2019).
Churcher Clarke 2017 reported a significant improvement in quality of life in the intervention group compared to control at end of treatment (10 weeks; P = 0.05), but no other significant differences were observed in the remaining outcomes tested, which included depression (P = 0.87), anxiety (P = 0.97), and cognition (P = 0.26).
In the study by Larouche 2019, there were no significant differences between intervention and control at end of treatment (eight weeks) or at three months on any of the outcomes tested, which were depression (P = 0.65), anxiety (P = 0.90), and quality of life (P = 0.98).
Wells 2013 reported that mindfulness‐based stress reduction was no different to treatment as usual in improving depressive symptoms (P = 0.35), or quality of life (P = 0.25) in people with MCI at end of treatment. The study authors reported that, in two cognitive measures, the control group performed better (P = 0.04; P = 0.01). We were not able to extract data for this study.
Interpersonal therapy
In the study by Burns 2005, there were no differences between IPT and TAU in any of the patient or carer outcomes at the end of treatment (six weeks) or at three‐month follow‐up.
Adverse events
Adverse events for CBT interventions
Orgeta 2019 and Spector 2015 reported no adverse events related to the intervention. In the Kiosses 2015 study, all adverse events were reported as unrelated to the study. Rovner 2018 reported details of serious adverse events over two years, of which none were related to the intervention. In the remaining trials, adverse events were not specified.
Adverse events for supportive and counselling interventions
Laakkonen 2016 and Marshall 2015 reported no adverse events. The remaining studies did not specify whether adverse events were present or not.
Adverse events for third‐wave interventions
Churcher Clarke 2017 and Wells 2013 reported no adverse events related to the study protocol. Larouche 2019 did not specify adverse events.
Adverse events for interpersonal therapy
Burns 2005 did not specify whether there were any adverse events.
Subgroup analyses
For the effects of CBT‐based treatments on depressive symptoms, we conducted subgroup analyses based on participants' cognitive diagnosis (MCI or dementia) and on the presence or absence of a criterion‐based depression diagnosis or clinically significant depressive symptoms at baseline. The results are presented above. We were unable to conduct corresponding subgroup analyses for the comparison of supportive and counselling therapies with TAU because there were no studies of these interventions in people with MCI, nor participants with depression at baseline.
Sensitivity analyses
Type of control comparison
CBT treatments
We explored the influence of type of control intervention to examine the impact of active control conditions on effect sizes. Three studies used a comparison control condition which could be classified as beyond usual care with potentially active elements (Kiosses 2010; Kiosses 2015; Rovner 2018). When we excluded these three trials from the meta‐analysis, there was little change in the SMD for depressive symptoms at the end of treatment (SMD ‐0.27, 95% CI ‐0.43 to ‐0.10; Analysis 3.1), very similar to the pooled SMD for all 13 trials (SMD ‐0.23, 95% CI ‐0.37 to ‐0.10). When these trials were excluded, the SMD for activities of daily living at the end of treatment was reduced and the result no longer reached statistical significance (SMD ‐0.10, 95% CI ‐0.31 to 0.12; Analysis 3.2). Excluding these trials had little effect on the results for cognition measured at the end of treatment (SMD 0.12, 95% CI ‐0.11 to 0.34; Analysis 3.3).
3.1. Analysis.

Comparison 3: Cognitive behavioural therapies versus treatment as usual ‐ type of control comparison (usual care versus active control), Outcome 1: Depressive symptoms post‐treatment
3.2. Analysis.

Comparison 3: Cognitive behavioural therapies versus treatment as usual ‐ type of control comparison (usual care versus active control), Outcome 2: Activities of daily living post‐treatment
3.3. Analysis.

Comparison 3: Cognitive behavioural therapies versus treatment as usual ‐ type of control comparison (usual care versus active control), Outcome 3: Cognition post‐treatment
Risk of bias
CBT treatments
We considered only one study to be at overall high risk of bias due to detection bias (Travers 2017). This study was included in two meta‐analyses of effects of CBT treatments ‐ on depressive symptoms post‐treatment and quality of life post‐treatment ‐ but excluding it had little effect on the results and did not alter the conclusions (Analysis 4.1; Analysis 4.2).
4.1. Analysis.

Comparison 4: Cognitive behavioural therapies versus treatment as usual ‐ excluding studies at high risk of bias, Outcome 1: Depressive symptoms post‐treatment
4.2. Analysis.

Comparison 4: Cognitive behavioural therapies versus treatment as usual ‐ excluding studies at high risk of bias, Outcome 2: Quality of life post‐treatment
Discussion
Summary of main results
The aim of the current review was to evaluate current evidence on the effects of psychological treatments for depression and anxiety for people with dementia and mild cognitive impairment (MCI). We found 29 studies meeting our inclusion criteria. Key findings of our review are that CBT treatments probably have a small positive effect for depressive symptoms for people with dementia and MCI immediately after treatment and probably provide a small benefit in terms of improving quality of life and activities of daily living at end of treatment. Beyond depressive symptoms, CBT interventions may benefit people with dementia in terms of depression remission at end of treatment; however, our certainty for this finding is low. We found no evidence that CBT treatments improve anxiety at end of treatment for people with dementia; however, these results were based on very low‐certainty evidence. Similarly, CBT treatments may not benefit people with dementia in terms of a reduction in neuropsychiatric symptoms at end of treatment, or improve outcomes for carers. However, our certainty in these findings remains low or very low, with findings overall imprecise and the numbers of studies low.
We also carried out analyses on effects of supportive and counselling interventions for people with dementia, and found that interventions such as counselling and social support and well‐being groups probably provide a small benefit in comparison to usual care in terms of improving quality of life at the end of treatment. We found no evidence that supportive and counselling interventions improve anxiety and depressive symptoms or cognition for people with dementia at end of treatment, but the results were generally imprecise and our confidence in these findings is low or very low. We found no evidence that supportive and counselling interventions confer benefit compared to treatment as usual for carers, such as reducing carer depression; however, the certainty of evidence is very low.
A limitation of the evidence is that most studies recruited people living with mild dementia, which means that results may not be applicable to people experiencing more advanced dementia. Additionally, there was variation between studies in terms of the nature, duration, and intensity of the psychological intervention being evaluated, so interpreting the data is not straightforward. However, despite these limitations, these results compare favourably with minimal or no benefits of pharmacological interventions in treating depression in dementia (Dudas 2018; Orgeta 2017), and highlight the need for continuing research in the area. The results may be particularly noteworthy as in some studies, the control group received additions to standard care, but we found that the effect of CBT‐based therapies on depressive symptoms was still present when we excluded studies in which the control intervention had potentially active elements (not amounting to a specific psychological treatment).
The psychological interventions we included all targeted symptoms of anxiety and depression through a structured psychological approach involving therapist and patient communication, which included directly teaching people with dementia and MCI skills to reduce anxiety and depression and improve general psychological well‐being. Nevertheless, the included trials evaluated a range of different psychological interventions, and some used a combination of treatments. The length and duration of interventions also varied in the studies, leading to differences in intensity and 'dosage' of the psychological treatments. Adherence to treatment appears to have been good in most studies, but adherence in randomised controlled trials may not translate into similar levels of adherence in everyday clinical settings. Despite these limitations, our review shows that psychological interventions may provide a small benefit to people with dementia and MCI by reducing depressive symptoms and improving quality of life and activities of daily living at end of treatment. Our findings also suggest that specific types of interventions, such as CBT, may potentially benefit people with dementia and MCI in several other outcomes, such as remission of symptoms of depression in the short term.
Overall completeness and applicability of evidence
The current review included 29 randomised controlled trials (RCTs), making it the largest systematic review on the topic. A total of 23 new studies have been published since the first version, which included only six studies. The large number of studies accumulating is not surprising given the recent focus in international policy on living well with dementia, and the clinical guideline recommendations in several countries on the use of psychological treatments for people with dementia (e.g. NICE 2018). The included studies were conducted in nine countries in total, with the majority of studies conducted in the USA (11 studies) and the UK (7 studies). The remainder were conducted in other European countries; one study was conducted in Australia; and two recent studies in China. The publication of a large number of studies from different countries contributing to this review is important as it strengthens our confidence in the reported findings. However, future research in other countries is highly recommended to maximise applicability of the evidence. Considering diversity, inclusion, and culture in future work, by involving diverse groups of participants in the development and evaluation of interventions, is important for improving equity of access to psychological interventions for people with dementia and MCI around the globe (WHO 2019).
Participants, outcome measures, and comparators
Our analyses pooled studies of both people with dementia and people with a diagnosis of MCI; however, most of the evidence was in people living with a diagnosis of dementia. We found a very small number of studies in people with MCI, and although we did conduct a subgroup analysis for the CBT‐based therapies, investigating the effect of cognitive diagnosis (dementia versus MCI), we consider the certainty of this result to be very low, which remains an important limitation of our review. Studies also differed in terms of whether participants had a diagnosis of depression or depressive symptoms at baseline. Although our subgroup analyses for CBT‐based therapies showed that the effect size varied with the presence or absence of a depression diagnosis or supra‐threshold depressive symptoms at baseline, this is low‐certainty evidence from a subgroup analysis. Furthermore, of the four studies recruiting people with depression at baseline, three specifically used PST. These were all of the studies using PST which were included in the review. Therefore, it is possible that the subgroup effect may be due to the type of CBT treatment as opposed to baseline depression. Nevertheless, our results raise the possibility that CBT approaches may reduce symptoms of depression mainly in people who are more depressed at baseline, making this an important variable to consider in future studies. We were also able to examine the effect of type of control comparison group on the results. Our sensitivity analyses showed that excluding studies in which the control comparison group was above usual care and had potentially 'active components' did not influence the effectiveness of psychological treatments for depressive symptoms measured at end of treatment. Although the effect was no longer significant for the outcome of activities of daily living measured at post‐treatment, this result should be interpreted with caution as very few studies remained in the analysis.
Overall, the review focused on a large number of outcomes for people with dementia and MCI, additionally analysed outcomes for carers, and followed our specified plan of considering depression and anxiety as primary outcomes. Our first primary outcome ‐ depressive symptoms at post‐treatment ‐ was evaluated by 13 studies with a total of 893 participants in CBT trials and by 9 studies with a total of 994 participants in supportive therapy and counselling trials. However, anxiety symptoms at post‐treatment were evaluated by only three studies which were generally small, with a total of 143 participants for CBT‐based therapies, and by one study with 24 participants for supportive and counselling therapies. Although there was no effect for CBT treatments, there was evidence of inconsistency and imprecision, limiting the certainty of the findings. We were able to test for several important outcomes, such as quality of life and activities of daily living. It is encouraging that CBT treatments were favoured compared to control conditions for several outcomes other than depressive symptoms, such as quality of life and activities of daily living. However, it is not clear whether these changes are clinically meaningful, and so findings should be interpreted with caution.
CBT versus supportive and counselling interventions
In view of the expanded evidence base for this updated review, we decided to conduct separate meta‐analyses for different types of psychological interventions, but we acknowledge that there is substantial overlap between some of the psychotherapeutic approaches. Also, although all interventions primarily targeted depression and anxiety and/or psychological stress, they were still to some extent heterogeneous within our groupings. We grouped together cognitive behavioural therapy, behavioural activation, and problem‐solving therapy interventions as cognitive behavioural therapies. We decided also to group together supportive and counselling interventions as these were similar in nature and aimed at supporting the well‐being of people with dementia. Although in most studies interventions were clearly defined, several studies evaluated a range of different psychological interventions. In some trials, such as in the Burgener 2008 study, a combination of treatments was evaluated (CBT and social support groups, which we categorised as primarily a CBT intervention). The settings of the studies varied (with some studies conducted in community settings and others taking place in long‐term care), and so did the intervention 'doses'. Heterogeneity across our analyses was low to moderate overall (except for the analyses investigating effects on carer burden), indicative of no major differences across studies that would preclude meta‐analyses.
Intervention adherence
Reference to intervention adherence was made in the majority of studies, indicating that psychological treatments are generally associated with high adherence across several settings. However, only a small number of studies made reference to assessing or monitoring fidelity, and only a minority of studies provided information about availability of intervention protocols. Although evidence generally of high adherence is encouraging, the lack of monitoring of fidelity parameters and access to treatment protocols is likely to make replicability of interventions difficult across settings. Nevertheless, adherence to treatment protocols can be a frequent barrier in interventions research in dementia, and our observations that, generally, studies did not report low levels of adherence is important for implementation of these interventions in real‐life settings for people with dementia and MCI.
Quality of the evidence
We used the GRADE approach to assess our confidence in the findings of the review by rating the risk of bias in each of the included studies, inconsistency and imprecision in the results, directness of the evidence, and publication bias for all outcomes. When comparing psychological treatments to TAU or active controls, we can be relatively confident in our finding of a small benefit of CBT treatments for depressive symptoms, quality of life and activities of daily living measured at the end of treatment, as certainty of evidence was moderate. Similarly, certainty of evidence was also moderate for the effects of supportive and counselling interventions on improving patient quality of life at the end of sessions. However, our confidence for a potential large benefit for depression remission at end of treatment for CBT interventions is low, as results were based on low‐certainty evidence.
Potential biases in the review process
We used a comprehensive and sensitive strategy to identify studies that could potentially meet the inclusion criteria for this review. We also contacted first authors of included studies, as well as authors of relevant references, to identify further studies. Four review authors (VO, PL, RdPC, and AM) independently selected studies, and three review authors (VO, PL, and AM) independently extracted data and carried out risk of bias assessments. We resolved any disagreements through discussion amongst the review author team, or by contacting the authors of the studies for further information. The present review presents and discusses all outcomes described in the protocol that were available for analysis, regardless of whether there was statistical significance.
Agreements and disagreements with other studies or reviews
The current review is distinctive in systematically synthesising the evidence base of psychological interventions to reduce anxiety or depression that are conducted primarily with people with dementia or MCI, rather than focusing on environmental changes or skills‐building interventions for family carers (Brodaty 2012). Previous reviews have concentrated on the effectiveness of other interventions of a psychosocial nature (including cognitive stimulation, cognitive rehabilitation, reminiscence, activities‐based interventions, promoting a healthy lifestyle, etc.), which are not aimed specifically at anxiety or depression. These reviews do suggest that non‐pharmacological interventions can be useful, and potentially cost‐effective, in terms of improving outcomes for people with dementia (Olazarán 2010).
Authors' conclusions
Implications for practice.
There is moderate‐certainty evidence that cognitive behavioural therapy (CBT) treatments, relative to treatment as usual (TAU) or active control, probably provide a small benefit for people with dementia and mild cognitive impairment (MCI) by slightly reducing depressive symptoms at the end of treatment and, with lower certainty, may also improve rates of remission of depression at the end of treatment. CBT treatments may also provide a small benefit for patient quality of life and activities of daily living compared to usual care or active control at the end of treatment. We found no evidence that supportive and counselling interventions improve symptoms of depression, but moderate‐certainty evidence of a small benefit in terms of improving quality of life at the end of treatment. For all types of psychological treatment, the evidence related to anxiety symptoms was of very low certainty and hence, inconclusive, so further research on the effectiveness of psychological interventions for reducing anxiety symptoms is necessary. Due to a lack of reporting, we were not able to determine whether these treatments have any adverse effects.
It is important to recognise that the results relate to psychological treatments which vary in content, and that most of the evidence is for people living with mild dementia in community settings.
Despite the limitations of the current evidence base, results indicative of potential benefit of these interventions are encouraging, especially in light of the lack of evidence of benefit and potential harms from pharmacological treatments.
Implications for research.
The evidence indicates that further small trials in cognitive behavioural therapy (CBT), behavioural activation (BA), or problem‐solving therapy (PST) for depression would not add greatly to the current evidence base, and that there should be a focus on large clinical trials that additionally investigate the cost‐effectiveness of these approaches in diverse settings. Assessment of treatment benefits in terms of reduced costs in health and social care are lacking. Future randomised controlled trials (RCTs) should adhere to the current highest standards of methodology and reporting, following the Consolidated Standards of Reporting Trials and should include long‐term follow‐up to determine whether any benefits persist. They will be most helpful if they focus on well‐defined psychological approaches, rather than on multimodal approaches which combine a variety of treatments. Future trials in the area should pay particular attention to baseline depression and anxiety, when designing and evaluating psychological interventions for people with dementia and MCI, and to the type of control intervention used as a comparison.
More research is needed into psychological treatments for people with MCI, acknowledging the challenge of how best to define MCI and that there is currently no standard definition universally accepted for use in clinical trials (Stephan 2013). Research is also needed to investigate the effect of the severity of dementia on treatment efficacy.
Initiatives aimed at harmonising and standardising interventions in the area would be important for pooling data in future meta‐analyses. There is a need to include a variety of key outcomes, particularly quality of life, and activities of daily living. There is also a need for novel treatments that have been developed alongside service users, understanding mechanisms of action and how these interventions can be sustained and maintained across settings to improve outcomes for people with dementia and MCI and those contributing to their care.
What's new
| Date | Event | Description |
|---|---|---|
| 18 February 2021 | New citation required and conclusions have changed | New search performed. New studies for inclusion and content revised. |
| 18 February 2021 | New search has been performed | New search performed. New studies included. |
History
Protocol first published: Issue 5, 2011 Review first published: Issue 1, 2014
Acknowledgements
We would like to thank the Cochrane Dementia and Cognitive Improvement Review Group; Sue Marcus, Managing Editor; and the external reviewers providing comments on our review.
We would like to thank peer reviewers, Tanya Davidson and Nicola Lautenschlager, and consumer reviewer, Catherine Hofstetter, for their comments and feedback.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| CENTRAL (The Cochrane Library) http://crso.cochrane.org/SearchSimple.php [Date of most recent search: 18 Jul 2019] |
#1 dement*:TI,AB,KY 8977 #2 alzheimer*:TI,AB,KY 8310 #3 ("lewy bod*" ):TI,AB,KY 301 #4 ("vascular cognit*" ):TI,AB,KY 143 #5 DLB:TI,AB,KY 135 #6 MESH DESCRIPTOR Dementia EXPLODE ALL TREES 4847 #7 MESH DESCRIPTOR Neurocognitive Disorders EXPLODE ALL TREES 8905 #8 ("organic brain disease" or "organic brain syndrome" ):TI,AB,KY 112 #9 ("major neurocognitive disorder"):TI,AB,KY 13 #10 (cerebro* ADJ2 deteriorat* ):TI,AB,KY 4 #11 (cerebro* ADJ2 insuffic* ):TI,AB,KY 167 #12 ("major neurocognitive disorder"):TI,AB,KY 13 #13 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 18320 #14 depress*:TI,AB,KY 59848 #15 dysthymi*:TI,AB,KY 735 #16 (adjustment disorder*):TI,AB,KY 372 #17 (mood disorder*):TI,AB,KY 2180 #18 (affective disorder*):TI,AB,KY 1310 #19 (affective symptom*):TI,AB,KY 674 #20 anxiety:TI,AB,KY 33227 #21 anxious:TI,AB,KY 2326 #22?phobi*:TI,AB,KY 2628 #23 (panic disorder):TI,AB,KY 1870 #24 BPSD:TI,AB,KY 124 #25 (behavioural and psychological symptoms of dementia):TI,AB,KY 49 #26 (neuropsychiatric symptom*):TI,AB,KY 436 #27 NPS:TI,AB,KY 348 #28 MESH DESCRIPTOR Behavioral Symptoms EXPLODE ALL TREES 17358 #29 MESH DESCRIPTOR Psychomotor Agitation 691 #30 MESH DESCRIPTOR Depression 9507 #31 MESH DESCRIPTOR Anxiety 6449 #32 MESH DESCRIPTOR Anxiety Disorders 3016 #33 #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 87661 #34 #13 AND #33 3422 #35 psychotherapy:TI,AB,KY 9215 #36 (cognitive therap*):TI,AB,KY 9801 #37 (behaviour therap*):TI,AB,KY 968 #38 counselling:TI,AB,KY 3043 #39 (cognitive analytic therapy):TI,AB,KY 15 #40 (interpersonal therap*):TI,AB,KY 105 #41 relaxation:TI,AB,KY 7987 #42 (non‐pharmacological intervention*):TI,AB,KY 417 #43 (non‐pharmacological treatment*):TI,AB,KY 318 #44 (psychodynamic therap*):TI,AB,KY 89 #45 (behavi* adj2 therap*):TI,AB,KY 15552 #46 (rational insight therap*):TI,AB,KY 0 #47 (problem‐solving therap*):TI,AB,KY 274 #48 CBT:TI,AB,KY 5106 #49 psychosocial:TI,AB,KY 9138 #50 psycho‐social:TI,AB,KY 292 #51 MESH DESCRIPTOR Psychotherapy EXPLODE ALL TREES 20475 #52 MESH DESCRIPTOR Psychotherapy, Multiple 10 #53 MESH DESCRIPTOR Psychotherapy, Group 1944 #54 MESH DESCRIPTOR Psychotherapy, Brief 914 #55 MESH DESCRIPTOR Psychotherapy, Rational‐Emotive 23 #56 #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 48646 #57 #34 AND #56 675 |
Oct 2018: 675 Jul 2019:‐ 153 |
| MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) [Date of most recent search: 18 Jul 2019] |
1 exp Dementia/ 2 Delirium/ 3 Wernicke Encephalopathy/ 4 Delirium, Dementia, Amnestic, Cognitive Disorders/ 5 dement*.mp. 6 alzheimer*.mp. 7 (lewy* adj2 bod*).mp. 8 deliri*.mp. 9 (chronic adj2 cerebrovascular).mp. 10 ("organic brain disease" or "organic brain syndrome").mp. 11 ("normal pressure hydrocephalus" and "shunt*").mp. 12 "benign senescent forgetfulness".mp. 13 (cerebr* adj2 deteriorat*).mp. 14 (cerebral* adj2 insufficient*).mp. 15 (pick* adj2 disease).mp. 16 (creutzfeldt or jcd or cjd).mp. 17 huntington*.mp. 18 binswanger*.mp. 19 korsako*.mp. 20 or/1‐19 21 "cognit* impair*".mp. 22 exp *Cognition Disorders/ 23 MCI.ti,ab. 24 ACMI.ti,ab. 25 ARCD.ti,ab. 26 SMC.ti,ab. 27 CIND.ti,ab. 28 BSF.ti,ab. 29 AAMI.ti,ab. 30 MD.ti,ab. 31 LCD.ti,ab. 32 QD.ti,ab. 33 AACD.ti,ab. 34 MNCD.ti,ab. 35 MCD.ti,ab. 36 ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 37 ((cognit* or memory or cerebr* or mental*) adj3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*)).ti,ab. 38 "preclinical AD".mp. 39 "pre‐clinical AD".mp. 40 ("preclinical alzheimer*" or "pre‐clinical alzheimer*").mp. 41 (aMCI or MCIa).ti,ab. 42 ("CDR 0.5" or "clinical dementia rating scale 0.5").ti,ab. 43 ("GDS 3" or "stage 3 GDS").ti,ab. 44 ("global deterioration scale" and "stage 3").mp. 45 "Benign senescent forgetfulness".ti,ab. 46 "mild neurocognit* disorder*".ti,ab. 47 (prodrom* adj2 dement*).ti,ab. 48 or/21‐47 49 20 or 48 50 depress*.ti,ab. 51 dysthymi*.ti,ab. 52 "adjustment disorder*".mp. 53 "mood disorder*".mp. 54 "affective disorder*".mp. 55 "affective symptom*".mp. 56 anxiety.mp. 57 anxious.mp. 58?phobi*.mp. 59 "panic disorder".mp. 60 BPSD.ti,ab. 61 "behavioural and psychological symptoms of dementia".mp. 62 ("neuropsychiatric symptom*" or NPS).mp. 63 exp Behavioral Symptoms/ or Psychomotor Agitation/ 64 Depression/ 65 Anxiety/ or Anxiety Disorders/ 66 or/50‐65 67 49 and 66 68 (psychotherapy or "cognitive therap*").mp. 69 "behaviour therap*".mp. 70 counselling.ti,ab. 71 "cognitive analytic therapy".mp. 72 "interpersonal therap*".mp. 73 relaxation.mp. 74 ("non‐pharmacological intervention*" or "non‐pharmacological treatment*").mp. 75 "psychodynamic therap*".mp. 76 (behavi* adj2 therap*).ti,ab. 77 "rational insight therap*".mp. 78 "problem‐solving therap*".mp. 79 CBT.ti,ab. 80 psychosocial.ti,ab. 81 psycho‐social.ti,ab. 82 exp Psychotherapy/ or Psychotherapy, Multiple/ or Psychotherapy, Group/ or Psychotherapy, Brief/ or Psychotherapy, Rational‐Emotive/ 83 or/68‐82 84 67 and 83 85 randomized controlled trial.pt. 86 controlled clinical trial.pt. 87 random*.ab. 88 trial.ab. 89 groups.ab. 90 or/85‐89 91 (animals not (humans and animals)).sh. 92 90 not 91 93 84 and 92 94 (2016* or 2017* or 2018* or 2013* or 2014* or 2015*).ed. 95 93 and 94 |
Oct 2018: 1029 Jul 2019: 342 |
| EMBASE (Ovid SP) 1974 to 17 October 2018 [Date of most recent search: 18 Jul 2019] |
1 exp dementia/ 2 Lewy body/ 3 delirium/ 4 Wernicke encephalopathy/ 5 cognitive defect/ 6 dement*.mp. 7 alzheimer*.mp. 8 (lewy* adj2 bod*).mp. 9 deliri*.mp. 10 (chronic adj2 cerebrovascular).mp. 11 ("organic brain disease" or "organic brain syndrome").mp. 12 "supranuclear palsy".mp. 13 ("normal pressure hydrocephalus" and "shunt*").mp. 14 "benign senescent forgetfulness".mp. 15 (cerebr* adj2 deteriorat*).mp. 16 (cerebral* adj2 insufficient*).mp. 17 (pick* adj2 disease).mp. 18 (creutzfeldt or jcd or cjd).mp. 19 huntington*.mp. 20 binswanger*.mp. 21 korsako*.mp. 22 CADASIL.mp. 23 or/1‐22 24 "cognit* impair*".mp. 25 exp cognitive defect/ 26 exp mild cognitive impairment/ 27 MCI.ti,ab. 28 ACMI.ti,ab. 29 ARCD.ti,ab. 30 SMC.ti,ab. 31 CIND.ti,ab. 32 BSF.ti,ab. 33 AAMI.ti,ab. 34 MD.ti,ab. 35 LCD.ti,ab. 36 QD.ti,ab. 37 AACD.ti,ab. 38 MNCD.ti,ab. 39 MCD.ti,ab. 40 ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 41 ((cognit* or memory or cerebr* or mental*) adj3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*)).ti,ab. 42 "preclinical AD".mp. 43 "pre‐clinical AD".mp. 44 ("preclinical alzheimer*" or "pre‐clinical alzheimer*").mp. 45 (aMCI or MCIa).ti,ab. 46 ("CDR 0.5" or "clinical dementia rating scale 0.5").ti,ab. 47 ("GDS 3" or "stage 3 GDS").ti,ab. 48 ("global deterioration scale" and "stage 3").mp. 49 "Benign senescent forgetfulness".ti,ab. 50 "mild neurocognit* disorder*".ti,ab. 51 (prodrom* adj2 dement*).ti,ab. 52 "age‐related symptom*".mp. 53 (episodic adj2 memory).mp. 54 ("pre‐clinical dementia" or "preclinical dementia").mp. 55 or/24‐54 56 23 or 55 57 depress*.ti,ab. 58 dysthymi*.ti,ab. 59 "adjustment disorder*".mp. 60 "mood disorder*".mp. 61 "affective disorder*".mp. 62 "affective symptom*".mp. 63 anxiety.mp. 64 anxious.mp. 65 "panic disorder".mp. 66 BPSD.ti,ab. 67 "behavioural and psychological symptoms of dementia".mp. 68 ("neuropsychiatric symptom*" or NPS).mp. 69 "Behavioral Symptom*".ti,ab. 70 agitat*.ti,ab. 71 depression/ 72 anxiety/ 73 anxiety disorder/ 74 or/57‐73 75 56 and 74 76 (psychotherapy or "cognitive therap*").mp. 77 "behaviour therap*".mp. 78 counselling.ti,ab. 79 "cognitive analytic therapy".mp. 80 "interpersonal therap*".mp. 81 relaxation.mp. 82 ("non‐pharmacological intervention*" or "non‐pharmacological treatment*").mp. 83 "psychodynamic therap*".mp. 84 (behavi* adj2 therap*).ti,ab. 85 "rational insight therap*".mp. 86 "problem‐solving therap*".mp. 87 CBT.ti,ab. 88 psychosocial.ti,ab. 89 psycho‐social.ti,ab. 90 psychotherapy/ 91 or/76‐90 92 75 and 91 93 randomized controlled trial/ 94 controlled clinical trial/ 95 trial.ti,ab. 96 (RCT or CCT).ti,ab. 97 randomly.ab. 98 "double‐blind*".ti,ab. 99 or/93‐98 100 92 and 99 101 (2013* or 2014* or 2015* or 2016* or 2017* or 2018*).em. 102 100 and 101 |
Oct 2018: 1615 Jul 2019: 334 |
| PsycINFO (Ovid SP) [Date of most recent search: 18 Jul 2019] |
1 exp Dementia/ 2 exp Delirium/ 3 exp Huntingtons Disease/ 4 exp Kluver Bucy Syndrome/ 5 exp Wernickes Syndrome/ 6 exp Cognitive Impairment/ 7 dement*.mp. 8 alzheimer*.mp. 9 (lewy* adj2 bod*).mp. 10 deliri*.mp. 11 (chronic adj2 cerebrovascular).mp. 12 ("organic brain disease" or "organic brain syndrome").mp. 13 "supranuclear palsy".mp. 14 ("normal pressure hydrocephalus" and "shunt*").mp. 15 "benign senescent forgetfulness".mp. 16 (cerebr* adj2 deteriorat*).mp. 17 (cerebral* adj2 insufficient*).mp. 18 (pick* adj2 disease).mp. 19 (creutzfeldt or jcd or cjd).mp. 20 huntington*.mp. 21 binswanger*.mp. 22 korsako*.mp. 23 ("parkinson* disease dementia" or PDD or "parkinson* dementia").mp. 24 or/1‐23 25 "cognit* impair*".mp. 26 exp Cognitive Impairment/ 27 MCI.ti,ab. 28 ACMI.ti,ab. 29 ARCD.ti,ab. 30 SMC.ti,ab. 31 CIND.ti,ab. 32 BSF.ti,ab. 33 AAMI.ti,ab. 34 MD.ti,ab. 35 LCD.ti,ab. 36 QD.ti,ab. 37 AACD.ti,ab. 38 MNCD.ti,ab. 39 MCD.ti,ab. 40 ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 41 ((cognit* or memory or cerebr* or mental*) adj3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*)).ti,ab. 42 "preclinical AD".mp. 43 "pre‐clinical AD".mp. 44 ("preclinical alzheimer*" or "pre‐clinical alzheimer*").mp. 45 (aMCI or MCIa).ti,ab. 46 ("CDR 0.5" or "clinical dementia rating scale 0.5").ti,ab. 47 ("GDS 3" or "stage 3 GDS").ti,ab. 48 ("global deterioration scale" and "stage 3").mp. 49 "Benign senescent forgetfulness".ti,ab. 50 "mild neurocognit* disorder*".ti,ab. 51 (prodrom* adj2 dement*).ti,ab. 52 "age‐related symptom*".mp. 53 (episodic adj2 memory).mp. 54 ("pre‐clinical dementia" or "preclinical dementia").mp. 55 or/25‐54 56 24 or 55 57 depress*.ti,ab. 58 dysthymi*.ti,ab. 59 "adjustment disorder*".mp. 60 "mood disorder*".mp. 61 "affective disorder*".mp. 62 "affective symptom*".mp. 63 anxiety.mp. 64 anxious.mp. 65 "panic disorder".mp. 66 BPSD.ti,ab. 67 "behavioural and psychological symptoms of dementia".mp. 68 ("neuropsychiatric symptom*" or NPS).mp. 69 Behavior Problems/ 70 "Depression (Emotion)"/ 71 Anxiety/ 72 or/57‐71 73 56 and 72 74 (psychotherapy or "cognitive therap*").mp. 75 "behaviour therap*".mp. 76 counselling.ti,ab. 77 "cognitive analytic therapy".mp. 78 "interpersonal therap*".mp. 79 relaxation.mp. 80 ("non‐pharmacological intervention*" or "non‐pharmacological treatment*").mp. 81 "psychodynamic therap*".mp. 82 (behavi* adj2 therap*).ti,ab. 83 "rational insight therap*".mp. 84 "problem‐solving therap*".mp. 85 CBT.ti,ab. 86 psychosocial.ti,ab. 87 psycho‐social.ti,ab. 88 Brief Psychotherapy/ or Geriatric Psychotherapy/ or Group Psychotherapy/ or Psychotherapy/ or Individual Psychotherapy/ 89 or/74‐88 90 73 and 89 91 exp Clinical Trials/ 92 trial.ti,ab. 93 (RCT or CCT).ti,ab. 94 randomly.ab. 95 "double‐blind*".ti,ab. 96 "single‐blind*".ti,ab. 97 randomi?ed.ti,ab. 98 or/91‐97 99 90 and 98 100 (2013* or 2014* or 2015* or 2016* or 2017* or 2018*).up. 101 99 and 100 |
Oct 2018: 470 Jul 2019: 134 |
| CINAHL (EBSCOhost) [Date of most recent search: 18 Jul 2019] |
S77 S75 AND S76 S76 EM 2013 OR EM 2014 OR EM 2015 OR EM 2016 OR EM 2017 OR EM 2018 S75 S61 AND S74 S74 S62 OR S63 OR S64 OR S65 OR S66 OR S67 OR S68 OR S69 OR S70 OR S71 OR S72 OR S73 S73 MH "Random Assignment" S72 MH "Single‐Blind Studies" or MH "Double‐Blind Studies" or MH "Triple‐Blind Studies" S71 MH "Crossover Design" S70 MH "Factorial Design" S69 MH "Placebos" S68 MH "Clinical Trials" S67 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" S66 TX crossover OR "cross‐over" S65 AB placebo* S64 TX random* S63 TX trial* S62 TX "latin square" S61 S42 AND S60 S60 S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 S59 (MH "Psychotherapy, Brief") S58 (MH "Psychotherapy, Group") S57 (MH "Psychotherapy+") S56 TX psycho‐social S55 TX psychosocial S54 TX CBT S53 TX problem‐solving therap* S52 TX rational insight therap* S51 TX behavi* N2 therap* S50 TX psychodynamic therap* S49 TX "non‐pharmacological intervention*" or "non‐pharmacological treatment*" S48 TX relaxation S47 TX interpersonal therap* S46 TX cognitive analytic therapy S45 TX counselling S44 TX behaviour therap* S43 TX psychotherapy or "cognitive therap*" S42 S21 AND S41 S41 S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 S40 (MH "Anxiety Disorders") S39 (MH "Anxiety") S38 (MH "Depression") S37 (MH "Psychomotor Agitation+") S36 (MH "Behavioral Symptoms+") S35 TX NPS S34 TX neuropsychiatric symptom* S33 TX behavioural and psychological symptoms of dementia S32 TX BPSD S31 TX panic disorder S30 TX?phobi* S29 TX anxious S28 TX anxiety S27 TX affective symptom* S26 TX affective disorder* S25 TX mood disorder*" S24 TX adjustment disorder* S23 TX dysthymi* S22 TX depress* S21 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 S20 TX "major neurocognitive disorder" S19 TX korsako* S18 TX binswanger* S17 TX huntington* S16 TX creutzfeldt or jcd or cjd S15 TX pick* N2 disease S14 TX cerebral* N2 insufficient* S13 TX cerebr* N2 deteriorat* S12 TX "benign senescent forgetfulness" S11 TX "normal pressure hydrocephalus" and "shunt*" S10 TX "organic brain disease" or "organic brain syndrome" S9 TX chronic N2 cerebrovascular S8 TX deliri* S7 TX lewy* N2 bod* S6 TX alzheimer* S5 TX dement* S4 (MH "Wernicke's Encephalopathy") S3 (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S2 (MH "Delirium") S1 (MH "Dementia+") |
Oct 2018: 617 Jul 2019: 438 |
| ISI Web of Science – all databases [includes: Web of Science (1945‐present); BIOSIS Previews (1926‐present); MEDLINE (1950‐present); Journal Citation Reports] [Date of most recent search: 18 Jul 2019] |
TOPIC:(dement* OR alzheimer* OR "vascular cognitive impairment" OR "lew* bod*" OR CADASIL OR "cognit* impair*" OR FTD OF FTLD OR "cerebrovascular insufficienc*" OR AD OR VCI) AND TOPIC:(depress* OR anxiety OR Agitation OR neuropsychiatric) AND TOPIC:(psychotherapy OR cognitive therapy OR counselling OR relaxation) AND TOPIC:(randomly OR randomised OR randomized OR "random allocat*" OR RCT OR CCT OR "double blind*" OR "single blind*" OR "double blind*" OR "single blind*" OR trial) | Oct 2018: 854 Jul 2019: 243 |
| LILACS (BIREME) [Date of most recent search: 18 Jul 2019] |
alzheimer OR alzheimers OR alzheimer’s OR dementia OR demenc$ [Words] and psychotherapy OR cognitive therapy OR counselling OR relaxation [Words] and randomly OR randomised OR randomized OR RCT OR "controlled trial" OR "double blind$" OR placebo [Words] | Oct 2018: 8 Jul 2019: 1 |
| ClinicalTrials.gov (www.clinicaltrials.gov) [Date of most recent search: 18 Jul 2019] |
depress* OR anxiety OR Agitation OR neuropsychiatric | dementia OR alzheimers OR cognition OR cognitive | psychotherapy OR cognitive therapy OR counselling OR relaxation | Start date on or after 01/01/2013 | Last update posted on or before 10/18/2018 | Oct 2018: 497 Jul 2019: 133 |
| ALOIS (via CRS web) [Date of most recent search: 18 Jul 2019] |
Depression or Dysthymi* or "Adjustment Disorder/s" or "Mood Disorder/s" or "Affective Disorder/s" or "Affective Symptoms", Anxiety or Anxious or phobia/s or "Panic Disorder", psychotherapy, "cognitive therapy", "behaviour therapy", "cognitive behaviour therapy" | Oct 2018: 17 Jul 2019:79 |
| ICTRP (http://apps.who.int/trialsearch) [Date of most recent search: 18 Jul 2019] |
depress* OR anxiety OR Agitation OR neuropsychiatric | dementia OR alzheimers OR cognition OR cognitive | psychotherapy OR cognitive therapy OR counselling OR relaxation | Start date on or after 01/01/2013 | Last update posted on or before 10/18/2018 | Oct 2018: 12 Jul 2019: 2 |
| Total before de‐duplication | Oct 2018: 5,794 Jul 2019: 1859 |
|
| Total after de‐duplication | Oct 2018: 4,319 Jul 2019:1510 |
|
| Total after first assessment based on title and abstract screening by CDCIG information specialist | Oct 2018: 717 | |
Data and analyses
Comparison 1. Cognitive behavioural therapies versus treatment as usual.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Depressive symptoms post‐treatment | 13 | 893 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.37, ‐0.10] |
| 1.1.1 Criterion‐based depression diagnosis at baseline | 4 | 194 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.84 [‐1.14, ‐0.54] |
| 1.1.2 No criterion‐based depression diagnosis at baseline | 9 | 699 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.23, 0.07] |
| 1.2 Depressive symptoms post‐treatment subgroup analysis ‐ type of cognitive diagnosis (dementia vs MCI) | 13 | 893 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.37, ‐0.10] |
| 1.2.1 Dementia | 10 | 554 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.57, ‐0.23] |
| 1.2.2 MCI | 3 | 339 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.18, 0.24] |
| 1.3 Depression remission post‐treatment | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.18, 2.88] |
| 1.4 Depressive symptoms long‐term | 4 | 317 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.35, 0.09] |
| 1.5 Anxiety symptoms post‐treatment | 3 | 143 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.36, 0.30] |
| 1.6 Anxiety symptoms long‐term | 2 | 95 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.47, 0.35] |
| 1.7 Quality of life post‐treatment | 7 | 459 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.31 [0.13, 0.50] |
| 1.8 Quality of life long‐term | 3 | 249 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.05, 0.45] |
| 1.9 Activities of daily living post‐treatment | 7 | 680 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.40, ‐0.09] |
| 1.10 Activities of daily living long‐term | 3 | 291 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.24, 0.23] |
| 1.11 Activities of daily living post‐treatment subgroup analysis ‐ type of cognitive diagnosis (dementia vs MCI) | 7 | 680 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.40, ‐0.09] |
| 1.11.1 Dementia | 4 | 346 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.52, ‐0.09] |
| 1.11.2 MCI | 3 | 334 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.40, 0.03] |
| 1.12 Cognition post‐treatment | 5 | 535 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.04, 0.30] |
| 1.13 Neuropsychiatric symptoms post‐treatment | 5 | 401 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.26, 0.14] |
| 1.14 Carer depressive symptoms post‐treatment | 6 | 413 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.29, 0.10] |
| 1.15 Carer depressive symptoms long‐term | 3 | 248 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.25, 0.26] |
| 1.16 Carer burden post‐treatment | 2 | 289 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.18, 0.29] |
Comparison 2. Supportive and counselling interventions versus treatment as usual.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Depressive symptoms post‐treatment | 9 | 994 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.18, 0.07] |
| 2.2 Anxiety symptoms post‐treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐3.07, 1.47] | |
| 2.3 Quality of life post‐treatment | 8 | 935 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [0.02, 0.28] |
| 2.4 Activities of daily living post‐treatment | 3 | 511 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.01, 0.34] |
| 2.5 Cognition post‐treatment | 6 | 730 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.03, 0.26] |
| 2.6 Neuropsychiatric symptoms post‐treatment | 3 | 538 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.06, 0.29] |
| 2.7 Carer depressive symptoms post‐treatment | 4 | 704 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.13, 0.17] |
Comparison 3. Cognitive behavioural therapies versus treatment as usual ‐ type of control comparison (usual care versus active control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Depressive symptoms post‐treatment | 13 | 893 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.37, ‐0.10] |
| 3.1.1 Treatment as usual | 10 | 568 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.43, ‐0.10] |
| 3.1.2 Active control condition | 3 | 325 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.39, 0.05] |
| 3.2 Activities of daily living post‐treatment | 7 | 680 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.40, ‐0.09] |
| 3.2.1 Treatment as usual | 4 | 355 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.31, 0.12] |
| 3.2.2 Active control condition | 3 | 325 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐0.64, ‐0.19] |
| 3.3 Cognition post‐treatment | 5 | 535 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.04, 0.30] |
| 3.3.1 Treatment as usual | 4 | 314 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.11, 0.34] |
| 3.3.2 Active control condition | 1 | 221 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.11, 0.42] |
Comparison 4. Cognitive behavioural therapies versus treatment as usual ‐ excluding studies at high risk of bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Depressive symptoms post‐treatment | 12 | 875 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.37, ‐0.10] |
| 4.2 Quality of life post‐treatment | 6 | 441 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.32 [0.13, 0.51] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Belleville 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial (RCT) of memory training versus group cognitive behavioural therapy (CBT) versus no contact control condition in people with mild cognitive impairment (MCI). | |
| Participants | Inclusion criteria: 1) people with single‐ or multiple‐domain MCI meeting Petersen 2004 criteria, 2) performing at least 1.5 standard deviations (SDs) below the average of age‐matched controls on standardised memory tests, 3) ≥ 24 on the Mini‐Mental State Examination (MMSE), 4) reporting a memory complaint, and 5) with normal vision and hearing. Exclusion criteria: 1) probable or possible Alzheimer's disease (AD) (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV); National Institute of Neurological and Communicative Disorders and Stroke ‐ Alzheimer's Disease and Related Disorders Association (NINCDS–ADRDA) criteria) or other forms of dementia, 2) significant cerebrovascular disorder/neurological disorder, 3) severe psychiatric illness or alcoholism, 4) general anaesthesia in the previous six months/head trauma, and 5) significant impairment in physical mobility. N = 127 (male (M) = 57, female (F) = 70) Mean age = 72.6 years Mean depression score at baseline = 3.3 on the Geriatric Depression Scale (GDS‐15) Mean cognition at baseline = 24.4 on the Montreal Cognitive Assessment (MoCA) |
|
| Interventions | Group CBT focusing on psycho‐education, exploring links between activities and ageing, cognitive restructuring of thoughts and beliefs, behavioural activation, and breathing techniques, delivered by qualified clinicians. 8 sessions of 2 hours each, plus a single booster session over 8 weeks. Control group: no contact condition (no additional contact other than assessments). |
|
| Outcomes | Primary outcomes Cognition 1) Immediate episodic memory ‐ word list and face name association tasks (z scores) 2) Delayed episodic memory ‐ as above Anxiety 3) Geriatric Anxiety Inventory (GAI) Depression 4) GDS‐15 Well‐being 5) General Well‐Being Schedule Secondary outcomes Memory strategies 6) Multifactorial Memory Questionnaire—Memory Strategies Subjective cognitive complaints 7) Questionnaire d’Auto‐Evaluation de la Mémoire Function 8) Activities of Daily Living–Prevention Instrument Outcomes assessed at 1 week, 3 months and 6 months. Outcomes at 3 (post‐treatment) and 6 (long‐term) months were included in the meta‐analysis. |
|
| Notes | Three‐arm RCT; cites government funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mentions use of a computerised random list. |
| Allocation concealment (selection bias) | Low risk | Mentions centralised randomisation by a statistician not involved in the project. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low levels of attrition; balanced between groups; specific reasons reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Bruvik 2013.
| Study characteristics | ||
| Methods | RCT of a multi‐component counselling intervention for depression for people with dementia versus treatment as usual. | |
| Participants | Inclusion criteria: 1) people with a diagnosis of dementia (International Classification of Diseases, 10th Revision (ICD‐10) criteria for research), 2) living at home, 3) scoring ≥ 15 on the MMSE, 4) able to give informed consent, and 4) an available carer scoring ≥ 15 on the Relative Stress Scale. N = 230 (M107, F123) Mean age = 78.4 years Mean depression at baseline = 8.0 on the Cornell Scale for Depression in Dementia (CSDD) Mean cognition at baseline = 21.2 (MMSE) |
|
| Interventions | Counselling intervention which incorporated individual counselling sessions addressing unmet needs through problem solving, community‐based educational sessions about dementia, and social support meetings focusing on coping with dementia, and pleasant events. 5 individual counselling sessions, one educational session, and 6 social support group meetings of 1 hour each over 12 months. Control group: received information about available services in local authority, and were free to seek additional support. |
|
| Outcomes | Primary outcomes Depression 1) CSDD Carer outcomes Depression 2) GDS Outcomes assessed at 12 months. Outcomes at 12 months (post‐treatment) were included in the meta‐analysis. |
|
| Notes | Cites government funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mentions use of a computer program. |
| Allocation concealment (selection bias) | Low risk | Mentions randomisation completed by a statistician. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided; probably both participants and personnel not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22 participants lost to follow‐up in intervention group, and 12 in control group; specific reasons reported; use of intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Burgener 2008.
| Study characteristics | ||
| Methods | RCT of multimodal CBT including tai chi exercises, and support group sessions for people with mild dementia versus attention control education. | |
| Participants | Inclusion criteria: 1) confirmed diagnosis of dementia (AD, Lewy body, vascular, frontal lobe, or mixed dementia), and 2) scoring < 2.0 on the Clinical Dementia Rating (CDR) scale. Diagnostic criteria for dementia were not specified and authors mentioned that participants were screened using the MMSE and the CDR. N = 43 (M23, F20) Mean age = 76.9 years Mean depression score at baseline = 3.1 (GDS‐15) Mean cognition at baseline = 23.8 (MMSE) |
|
| Interventions | Multimodal intervention combining tai chi (strength and balance training and relaxation), CBT (challenging dysfunctional cognitions and developing positive coping skills), and a support group (coping with dementia and positive problem solving). Tai chi 1‐hour classes offered weekly for 20 weeks, CBT of 90‐minute sessions offered bi‐weekly, support group of 90‐minute sessions offered bi‐weekly alternating with CBT. Control group: attention‐control receiving information about educational programs available. |
|
| Outcomes | Primary outcomes Cognition 1) MMSE Physical function 2) Single‐Leg Stance 3) Berg Balance Scale 4) Cumulative Illness Rating Scale Secondary outcomes Depression 5) GDS‐15 Self‐esteem 6) Rosenberg Self‐Esteem Scale (RSES) Outcomes measured at 20 weeks, and 40 (only for the control group) weeks. Outcomes at 20 weeks (post‐treatment) were included in the meta‐analysis. |
|
| Notes | Although the intervention was offered for 40 weeks, comparison data are provided only for 20 weeks; cites university research funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentions random assignment, no further details; insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided; probably both participants and personnel not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low levels of attrition; balanced between groups; specific reasons reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Burns 2005.
| Study characteristics | ||
| Methods | RCT of brief psychodynamic interpersonal therapy for people with mild dementia living in the community versus treatment as usual. | |
| Participants | Inclusion criteria: 1) diagnosis of dementia (NINCDS‐ADRDA), 2) CDR = 1 indicative of mild dementia, 3) ≥ 15 on the MMSE, 4) living in own home with carer in regular contact, and 5) ability to communicate verbally. N = 40 (M20, F20) Mean age = 75.8 years Mean depression at baseline = 5.5 (CSDD) Mean cognition at baseline = 22.9 (MMSE) |
|
| Interventions | Brief psychodynamic interpersonal therapy aimed at identifying interpersonal conflicts or difficulties causing or maintaining emotional distress. Components included finding and testing solutions to problems and encouraging practical changes. 6 individual sessions lasting 50 minutes each over 6 weeks. Control group: received standard care which consisted of general advice about diagnosis and treatment of dementia with outpatient review. |
|
| Outcomes | Primary outcomes Depression 1) CSDD Cognition 2) MMSE Function 3) Bristol Activities of Daily Living Scale (BADLS) Problem behaviours 4) Revised Memory and Behavior Problems Checklist (RMBPC) ‐ Carers reaction Global assessment 5) Clinical Global Impressions Scale Carer outcomes General Health 6) General Health Questionnaire (GHQ) Depression 7) Beck Depression Inventory (BDI) Coping 8) Ways of Coping Checklist Outcomes assessed at 8 weeks, and 3 months. |
|
| Notes | This study was not included in any meta‐analysis; cites voluntary sector funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mentions computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | Mentions randomisation completed by independent unit. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided; probably both participants and personnel not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided, insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition in the study; use of intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported except for carer depression. |
| Other bias | Low risk | No other apparent bias. |
Churcher Clarke 2017.
| Study characteristics | ||
| Methods | RCT of mindfulness‐based cognitive therapy (MBCT) for people with mild to moderate dementia in care homes versus treatment as usual. | |
| Participants | Inclusion criteria: 1) diagnosis of dementia (DSM‐IV criteria), 2) mild to moderate cognitive impairment with MMSE scores between 10 ‐ 26, 3) capacity to provide consent and some ability to communicate, 4) ability to see and hear well enough and maintain some concentration in a 45‐ to 60‐minute session, with minimal challenging behaviour, and 5) ability to speak English. Exclusion criteria: 1) major physical illness/disability, 2) learning disability, 3) practising meditation or yoga, and 4) history of brain lesions/head trauma. N = 31 (M16, F15) Mean age = 80.6 years Mean depression at baseline = 7.3 (CSDD) Mean cognition at baseline = 15.3 (MMSE) |
|
| Interventions | Adapted group‐based mindfulness intervention consisting of mindfulness meditation, mindful breathing, and movement, and practising mindful listening, seeing, smelling, and touch. Components included group discussions, orientation to the programme, and mindful warm‐up activities. 10 1‐hour group sessions, running twice a week for 5 weeks. Control group: received regular services provided within the care home. |
|
| Outcomes | Primary outcomes Depression 1) CSDD Anxiety 2) Rating Anxiety in Dementia (RAID) Quality of life 3) Quality of Life‐Alzheimer’s Disease (QoL‐AD) Cognition 4) MMSE Stress 5) Perceived Stress Scale (PSS) Outcomes assessed at 10 weeks (post‐treatment). |
|
| Notes | This study is not included in any meta‐analysis; no funding specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mentions use of a computer program. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided; probably both participants and personnel not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants lost in control group; specific reasons reported; use of intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Jha 2013.
| Study characteristics | ||
| Methods | RCT of a recovery‐orientated post‐diagnostic counselling intervention versus treatment as usual for people with MCI and mild dementia. | |
| Participants | Inclusion criteria: 1) people with memory problems, MCI, or suspected dementia (ICD‐10). N = 48 (M16, F32) Mean age = 78.7 years Mean depression at baseline = 6.6 (CSDD) Mean cognition at baseline = 22.0 (MMSE) |
|
| Interventions | A well‐being recovery‐orientated counselling intervention consisting of pre‐diagnostic counselling, therapeutic diagnostic consultation, and written feedback primarily aimed at improving well‐being. Components included well‐being assessment, psychiatric consultation, post‐diagnostic counselling and support. Monthly 1‐hour visits over 6 months. Control group: offered a fixed package of care of monthly visits for 6 months without being assessed for well‐being. |
|
| Outcomes | Primary outcomes Well‐being 1. WHO 5‐item Well‐Being Index Secondary outcomes Cognition 2. MMSE Depression 3. CSDD Quality of life 4. EuroQoL‐5 Dimension (EQ‐5D) Carer outcomes 5. Zarit Burden Interview (ZBI) Outcomes assessed at 6 months. Outcomes at 6 months (post‐treatment) were included in the meta‐analysis. |
|
| Notes | No funding specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mentions computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | Mentions random allocation sequence was concealed from the study team. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blind to treatment allocation; no information about personnel; probably not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Moderate to high levels of attrition; balanced between groups; specific reasons reported; insufficient detail of handling of missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Kiosses 2010.
| Study characteristics | ||
| Methods | RCT of problem adaptation therapy (PATH) vs. home‐delivered supportive therapy (ST) for older people with major depression, cognitive impairment, and disability. | |
| Participants | Inclusion criteria: 1) older people with a diagnosis of major depression (Structured Clinical Interview for DSM‐IV (SCID‐IV) criteria), 2) a score ≥ 17 on the HDRS, 3) cognitive impairment defined by a score lower than 2 SDs of the mean of age‐matched controls on the Dementia Rating Scale Initiation/Perseveration subscale (score ≤ 30), or ≤ 18 on the Stroop Color‐Word Test, without history/presence of psychiatric disorders, 4) disability defined by ≥ 1 on the Multilevel Assessment Instrument (MAI), 5) limited mobility, 6) available caregiver, and 7) not on psychotropic drugs/on stable dosage. Exclusion criteria: 1) Axis 1 disorder, 2) severe medical illness, 3) involved in psychotherapy, 4) scoring < 19 on the MMSE, 5) aphasia/dysarthria, and 6) inability to speak English. N = 30 (M9, F21) Mean age = 79.4 years Mean depression score at baseline = 21.9 (HDRS) Mean cognition at baseline = 26.6 (MMSE) |
|
| Interventions | Home‐delivered PATH aimed at reducing depression and disability, integrating environmental adaptation and caregiver participation, focusing on the patients' ecosystem. 12 weekly individual sessions. Control group: home‐delivered supportive active control comparison consisting of nonspecific support such as empathic listening, reflection, emotional processing, and encouragement. |
|
| Outcomes | Primary outcomes Depression 1) HDRS Disability 2) Sheehan Disability Scale Outcomes assessed at 6 and 12 weeks. Outcomes at 12 weeks (post‐treatment) were included in the meta‐analysis. |
|
| Notes | Cites government funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentions random assignment, no further details; insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided; probably both participants and personnel not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants lost to follow‐up in intervention group, and 3 in control group; specific reasons reported; use of intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Kiosses 2015.
| Study characteristics | ||
| Methods | RCT of PATH vs ST in reducing depression and disability in older people with major depression, cognitive impairment, and disability. | |
| Participants | Inclusion criteria: 1) older people with unipolar major depression DSM‐IV diagnosis (SCID‐R), 2) a score of ≥ 17 on the Montgomery–Åsberg Depression Rating Scale (MADRS), 3) at least mild cognitive deficits (≤ 7 on the Dementia Rating Scale (DRS)), 4) disability (defined by score ≥ 1 on the MAI), and 5) not taking antidepressants, cholinesterase inhibitors, or memantine, or on a stable dosage. Exclusion criteria: 1) other Axis I psychiatric disorder (except comorbid anxiety disorders), 2) acute/severe medical illness, 3) prescribed drugs known to cause depression, 4) involvement in psychotherapy, 5) advanced dementia (MMSE < 17), and 6) aphasia or inability to speak English. N = 74 (M23, F51) Mean age = 80.9 years Mean depression score at baseline = 21.2 (MADRS) Mean severity score at baseline = 118.5 (DRS) |
|
| Interventions | Individual home‐based problem adaptation therapy integrating a problem‐solving approach with compensatory strategies, environmental adaptations, and caregiver participation to improve participants’ emotion regulation. Individual weekly sessions over 12 weeks. Control group: home‐delivered supportive active control comparison consisting of nonspecific support such as empathic listening, reflection, emotional processing, and encouragement. |
|
| Outcomes | Primary outcomes Depression 1) MADRS Disability 2) World Health Organization Disability Assessment Schedule 2.0 Outcomes assessed at 4, 8, and 12 weeks. Outcomes at 12 weeks (post‐treatment) were included in the meta‐analysis. |
|
| Notes | Cites government funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mentions random assignment, and use of a Statistical Analysis Software randomisation function. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided; probably both participants and personnel not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 participants lost to follow‐up in intervention group, and 5 in control group; specific reasons reported; use of intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Koivisto 2016.
| Study characteristics | ||
| Methods | RCT of individual counselling, education, and support for people with mild dementia versus treatment as usual. | |
| Participants | Inclusion criteria: 1) very mild (CDR = 0.5) or mild (CDR = 1) AD diagnosed by specialists (NINCDS‐ADRDA), 2) able to speak and understand Finnish, 3) living in the community, 4) able to complete the Consortium to Establish a Registry for Alzheimer's Disease neuropsychological battery (CERAD‐NB), and 5) having a family carer. N = 236 (M115, F121) Mean age = 75.6 years Mean depression at baseline = 10.7 (BDI) Mean cognition at baseline = 21.5 (MMSE) |
|
| Interventions | Education, counselling, and social support sessions, including both group and individual sessions, aimed at enhancing knowledge, reducing social isolation and caregiver distress, supporting functional ability, and managing everyday life situations. 12 sessions in total over 2 years. Control group: participated in annual follow‐up visits, and received regular healthcare services. |
|
| Outcomes | Primary outcomes Institutionalisation 1. Rate of institutionalisation Secondary outcomes Disease severity 2. CDR Cognition 3. CERAD‐NB 4. MMSE Function 5. Alzheimer's Disease Cooperative Study – Activities of Daily Living Inventory (ADCS‐ADL) Depression 6. BDI Neuropsychiatric symptoms 7. Neuropsychiatric Inventory (NPI) Quality of life 8. QoL‐AD Carer outcomes Depression 9. BDI Sense of Coherence 10. Sense of Coherence Scale General health 11. GHQ Quality of life 12. 15D instrument (15D) Outcomes assessed at 36 months. Outcomes at 36 months (post‐treatment) were included in the meta‐analysis. |
|
| Notes | A 1‐item scale was used to measure quality of life in both people with dementia and carers; cites funding from pharmaceutical industry and government funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mentions computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | Mentions random allocation sequence was concealed from the study team. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided; probably both participants and personnel not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 30 participants lost to follow‐up in intervention group, and 76 in control group; specific reasons other than death not reported; use of intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Kurz 2012.
| Study characteristics | ||
| Methods | RCT of behavorial activation (BA) with memory rehabilitation elements versus treatment as usual for people with mild dementia. | |
| Participants | Inclusion criteria: 1) people with AD meeting criteria of mild AD (ICD‐10), 2) scoring ≥ 21 on the MMSE, and 3) looked after by a carer several times per week. Exclusion criteria: 1) acute psychiatric or physical disorder, 2) poor command of German, 3) undertaking psychotherapy or cognitive training, 4) regular visits to day care, and 5) alcohol or substance dependence. N = 201 (M113, F88) Mean age = 73.7 years Mean depression score at baseline = 8.9 (GDS‐30) Mean cognition at baseline = 25.1 (MMSE) |
|
| Interventions | BA aimed towards facilitating daily structuring, activity planning, and resuming former activities with a review of individual goals set by participants, and mobilising support based on neuro‐rehabilitation, and psychotherapy strategies. 12 weekly 1‐hour sessions over 12 weeks. Control group: site‐specific standard medical management for early dementia. |
|
| Outcomes | Primary outcomes Function 1) Bayer Activities of Daily Living Scale Secondary Outcomes Function 2) Aachen Functional Item Inventory Depression 3) GDS‐30 Quality of Life 4) Dementia Quality of Life Instrument (DEMQOL) Neuropsychiatric symptoms 5) NPI Cognition General cognition 6) MMSE Memory 7) Wechsler Memory Scale Revised Logical Memory 8) Trail Making Test (TMT) A Verbal fluency 9) Regensburg Word Fluency Test Caregiver outcomes Depression 10) BDI Burden 11) ZBI Outcomes assessed at 3 and 9 months. Outcomes at 3 (post‐treatment) and 9 (long‐term) months were included in the meta‐analysis; for the cognition meta‐analysis, 9‐month data were included (as data at 3 months were not available). |
|
| Notes | Cites government funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mentions use of a computerised random list. |
| Allocation concealment (selection bias) | Unclear risk | Mentions group allocation was concealed from outcome raters; insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided; probably both participants and personnel not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 participants lost to follow‐up in intervention group, and 4 in control group; specific reasons reported; use of intention‐to‐treat analysis and multiple imputations. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported except for cognition at 3 months. |
| Other bias | Low risk | No other apparent bias. |
Laakkonen 2016.
| Study characteristics | ||
| Methods | RCT of a social support intervention based on psychosocial rehabilitation versus usual care for people with dementia living in the community. | |
| Participants | Inclusion criteria: 1) people with a diagnosis of dementia (using Finnish National Guidelines), 2) living at home, 3) have a carer, and 4) are Finnish‐speaking. Exclusion criteria: 1) unable to walk, 2) unable to hear speech, and 3) having a terminal illness. N = 136 (M85, F51) Mean age = 77.0 years Mean cognition at baseline = 20.8 (MMSE) |
|
| Interventions | Social support based on psychosocial rehabilitation which was primarily aimed at maintaining self‐efficacy, and provision of information and social support. Principles included respecting participant autonomy, use of own resources, problem‐solving skills, and mastery of everyday life. 4‐hour group sessions once a week over 8 weeks. Control group: usual care consisting of regular health and social services, and provision of advice on nutrition and exercise. |
|
| Outcomes | Primary outcomes Health‐related quality of life 1) 15D Secondary outcomes Cognition 2) CDR 3) Verbal Fluency 4) Clock Drawing Test Carer outcomes Primary outcomes Health‐related quality of life 5) RAND 36‐Item Health Survey Secondary outcomes Sense of competence 6) Sense of Competence Questionnaire (SCQ) Mastery 7) Pearlin Mastery Scale (PM) Outcomes assessed at 3, 9, and 24 (costs only) months. Outcomes at 9 months (post‐treatment) were included in the meta‐analysis. |
|
| Notes | Cites funding from government and voluntary sector. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mentions use of a computerised random list. |
| Allocation concealment (selection bias) | Low risk | Mentions telephone‐based randomisation by a person not involved in the intervention unaware of peoples' identities. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Personnel not blind to treatment allocation; no information about participants; probably not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants lost to follow‐up in control group; specific reasons reported; use of intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported except the mental health subscale for carers. |
| Other bias | Low risk | No other apparent bias. |
Lai 2020.
| Study characteristics | ||
| Methods | RCT of activity scheduling and dementia education versus dementia care education for people with mild and moderate dementia and their spouses, living in the community. | |
| Participants | Inclusion criteria: 1) aged 65 to 80 years for both people with dementia and carers, 2) no chronic disease or other medical comorbidity for carers, 3) diagnosis of dementia (ICD‐10 criteria) by a psychiatrist, 4) scoring > 26 on MoCA for carers, and 5) a score ≤ 21 on MoCA for people with dementia. Exclusion criteria: 1) psychiatric disorder, or a known history of substance abuse for person with dementia or carer. N = 100 (M52, F48) Mean age = 69.7 years Mean cognition at baseline = 18.1 (MoCA) |
|
| Interventions | Dementia care education and activity scheduling focusing on pleasant activities in line with life values and goals, and improving communication. Additional components included enhancing motivation, weekly ratings of enjoyment, and achievement, monitoring of mood, and reassessment. 10 weekly sessions provided by occupational therapists over 10 weeks with weekly telephone follow‐ups. Control group: dementia care education over 10 weeks provided weekly. In the first two weeks, participants in both groups received health education (information on exercise and healthy eating, sleep management, counselling, acceptance therapy, and commitment therapy). |
|
| Outcomes | Primary outcomes Caregiver burden 1. ZBI Secondary outcomes Problem behaviours 2. RMBPC Caregiving time 3. Caregiver Activity Survey Quality of life for person with dementia 4. QoL‐AD Outcomes assessed at 12 weeks. Outcomes at 12 weeks (post‐treatment) were included in the meta‐analysis. |
|
| Notes | No funding specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mentions use of a computerised random list. |
| Allocation concealment (selection bias) | Low risk | Mentions allocation was concealed by using sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Personnel blind to treatment allocation; no information about participants; probably not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low levels of attrition; balanced between groups; specific reasons reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Larouche 2019.
| Study characteristics | ||
| Methods | RCT of MBCT for people with MCI versus psycho‐education. | |
| Participants | Inclusion criteria: 1) complaint about cognitive changes expressed by participant, relative, or clinician, 2) objective impairment in one or more cognitive domains, including at least episodic memory, with a performance under ‐1.5 SDs based on local norms, 3) preserved overall function, 4) absence of dementia, and 5) diagnosis of MCI based on National Institute on Aging ‐ Alzheimer's Association (NIA‐AA) criteria, validated in consensus meetings by two licensed neuro‐psychologists. Exclusion criteria: 1) history of neurological disease, traumatic brain injury, intracranial surgery, or stroke, 2) current psychiatric illness, or substance abuse in the last 12 months, 3) general anaesthesia or oncologic treatment in the past 6 months, 4) uncorrected vision/hearing impairments, 5) untreated or unstable metabolic condition, 6) recent treatment that may impact cognition, and 7) recent or sustained meditative experience. N = 48 (for N = 45 (M26, F19)) Mean age = 70.9 years Mean depression at baseline = 7.9 (GDS‐30) Mean cognition at baseline = 24.4 (MoCA) |
|
| Interventions | MBCT consisting of psycho‐education for mindfulness, handling obstacles, and supporting meditation practice, and stress management. Components included acceptance of one's situation, taking better care of oneself, and sustaining meditation practice. 8 sessions of two and a half hours each, over 8 weeks. Control group: received psycho‐education about ageing and dementia, and information about coping with cognitive decline. |
|
| Outcomes | Primary outcomes Depression 1) GDS Anxiety 2) GAI Perceived stress 3) PSS Quality of life 4) World Health Organization Quality of Life Brief scale (WHOQOL‐BREF) 5) World Health Organization Quality of Life for Older Persons Cognition 6) Episodic memory delayed and immediate recall Secondary outcomes Coping 7) Brief‐COPE (Coping Orientation to Problems Experienced Inventory) Mindfulness 8) Five Facet Mindfulness Questionnaire Rumination 9) Ruminative Response Scale Physiological stress 10) Cortisol awakening response Outcomes assessed at 8 weeks, and 3 months. |
|
| Notes | This study is not included in any meta‐analysis; cites funding from government and voluntary sector. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mentions random assignment via the use of Microsoft Excel random function and use of a freelance procedure. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant lost to follow‐up in intervention group, and 2 in control group; specific reasons reported; use of intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Lichtenberg 2005.
| Study characteristics | ||
| Methods | RCT of BA in specialist dementia care units in people with dementia versus treatment as usual. | |
| Participants | Inclusion criteria: 1) people with a diagnosis of dementia (criteria not specified). N = 20 (M2, F18) Mean age = 84.9 years Mean depression score at baseline = 4.0 (GDS‐15) Mean cognition at baseline = 14.2 (MMSE) |
|
| Interventions | BA intervention based on BA principles incorporating introduction to BA, pleasant‐event scheduling, mood monitoring, and relaxation strategies adapted for people living with dementia in nursing homes. 3 weekly sessions lasting 20 to 30 minutes each over 3 months. Control group: usual care comprised of regular activities taking place within the home. |
|
| Outcomes | Primary outcomes Behavioural symptoms 1) Behavioral Pathology in Alzheimer’s Disease Depression 2) GDS‐15 3) CSDD Outcomes assessed at 13 weeks. Outcomes at 13 weeks (post‐treatment) were included in the meta‐analysis. |
|
| Notes | No funding specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentions random assignment, no further details; insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided; probably both participants and personnel not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition in the study. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Logsdon 2010.
| Study characteristics | ||
| Methods | RCT of structured social support groups aimed at improving quality of life versus a waiting‐list control condition for people with dementia and their carers. | |
| Participants | Inclusion criteria: 1) people with a diagnosis of dementia confirmed by a primary care physician, 2) scoring ≥ 18 on the MMSE, 3) aware of their memory loss and able to communicate verbally, 4) able to participate independently in a group setting, 5) no history of severe mental illness, and 6) having an available carer. N = 142 (M72, F70) Mean age = 74.9 years Mean depression at baseline = 5.3 (GDS) Mean cognition at baseline = 23.4 (MMSE) |
|
| Interventions | Social support intervention known as the Early‐Stage Memory Loss programme comprised of structured support groups aimed at improving quality of life, sharing experiences, and concerns about dementia, reducing feelings of isolation, and providing assistance with long‐term care planning. 90‐minute sessions weekly over 9 weeks. Control group: waiting‐list condition where people received written educational materials about dementia and AD, and services available. |
|
| Outcomes | Primary outcomes Quality of life 1) QoL‐AD Health‐related quality of life 2) Medical Outcomes Study Short Form 36 Secondary outcomes Depression 3) GDS Communication 4) Family Assessment Measure Self‐efficacy 5) Self‐Efficacy Scale Perceived stress 6) PSS Problem behaviours 7) RMBPC ‐ Carer reaction Outcomes assessed at 9 weeks. Outcomes at 9 weeks (post‐treatment) were included in the meta‐analysis. |
|
| Notes | Cites government funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentions random assignment, no further details; insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided; probably both participants and personnel not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants lost to follow‐up in intervention group, and 2 in control group; specific reasons not reported; use of intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Lu 2016.
| Study characteristics | ||
| Methods | RCT of BA through enhancement of meaningful activity versus information support for people with MCI and their carers. | |
| Participants | Inclusion criteria: 1) a diagnosis of MCI (criteria by Albert 2011), 2) aged ≥ 60 years, 3) having a carer who scores ≥ 4 on the 6‐item MMSE, and 4) able to speak English. Exclusion criteria: 1) significant neurologic disease, 2) current major depression, and 3) carer having bipolar disorder or untreated schizophrenia. N = 40 (M23, F17) Mean age = 73.8 years 38% of the sample scored ≥ 5 on the Patient Health Questionnaire‐9 (PHQ‐9) indicative of depression. |
|
| Interventions | BA primarily aimed at increasing meaningful activity through planning, addressing barriers to engagement, problem solving, learning strategies for living with MCI, and establishing a manageable and realistic plan for meaningful activity. 6 biweekly 1‐hour sessions (2 in‐person and 4 telephone sessions) over 3 months. Control group: received educational brochure describing MCI, and had opportunities to answer questions through biweekly follow‐up phone calls. |
|
| Outcomes | Primary outcomes Function 1) Dementia Deficits Scale 2) Nowotny Hope Scale ‐ Confidence Subscale 3) ADCS‐ADL Meaningful activity 4) Canadian Occupational Performance Measure Depression 5) PHQ‐9 Communication 6) Communication and Affective Expression Subscales of the Family Assessment Device Quality of life 7) Life Satisfaction Index Carer outcomes Depression 8) PHQ‐9 Caregiver life changes 9) Bakas Caregiving Outcomes Scale Outcomes assessed at 3 months. Outcomes at 3 months (post‐treatment) were included in the meta‐analysis. |
|
| Notes | Cites government funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentions random assignment, no further details; insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Low risk | Mentions block‐randomisation approach with an independently‐generated allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Personnel blind to treatment allocation; no information about participants; probably not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details, insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 participants lost to follow‐up in intervention group, and 1 in control group; specific reasons reported; insufficient detail of handling of missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Marshall 2015.
| Study characteristics | ||
| Methods | RCT of Living Well with Dementia support groups aimed at improving quality of life for people with a recent diagnosis of dementia versus waiting‐list control. | |
| Participants | Inclusion criteria: 1) a recent diagnosis of dementia (NINCDS‐ADRDA; National Institute of Neurological Disorders and Stroke‐Association Internationale pour la Recherche et l’Enseignement en Neurosciences criteria), 2) participant acknowledging they have a memory problem, and 3) scoring ≥ 18 on the MMSE. Exclusion criteria: 1) pre‐morbid history of mental health problems, and 2) taking part in similar groups. N = 58 (M25, F33) Mean age = 75.6 years Mean depression at baseline = 6.2 (CSDD) Mean cognition at baseline = 23.0 (MMSE) |
|
| Interventions | Social support groups incorporating elements of psychotherapy and psycho‐education which comprised provision of information about dementia, coping with stress, use of memory aids, and living as well as you can. 75‐minute sessions weekly over 10 weeks. Control group: a waiting‐list condition in which participants received usual care. |
|
| Outcomes | Primary outcomes Quality of life 1) QoL‐AD Secondary outcomes Depression 2) CSDD Self‐esteem 3) RSES Cognition 4) MMSE Carer outcomes General health 5) GHQ Outcomes assessed at 10 and 22 weeks. Outcomes at 10 weeks (post‐treatment) were included in the meta‐analysis. |
|
| Notes | Cites government funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentions random assignment, no further details; insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Low risk | Mentions online secure system provided by a clinical trials unit. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blind to treatment allocation; no information about personnel; probably not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants lost to follow‐up in control group; specific reasons reported; use of intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Nordheim 2019.
| Study characteristics | ||
| Methods | RCT of a couples‐based counselling intervention for people with mild to moderate dementia and their partners versus standard care. | |
| Participants | Inclusion criteria: 1) community‐dwelling couples with one partner diagnosed with dementia (NIA‐AA criteria), and 2) having a diagnosis of mild to moderate dementia (≥ 15 on the MMSE). Exclusion criteria: 1) severe depression, 2) psychotic disorders, 3) addictive disorders for both partners, and 4) a dementia diagnosis in the spousal caregiver. N = 108 (M66, F42) Mean age = 85 years Mean depression score at baseline = 5.5 (GDS‐15) Mean cognition score at baseline = 22.8 (MMSE) |
|
| Interventions | Couples‐based counselling intervention incorporating information about dementia, couples communication training, coping and problem‐solving strategies, network and activity analysis, counselling for living space adaptations, and relaxation techniques provided by a psychotherapist and a social worker. Two weekly sessions of 1 to 2 hours each offered over 10 to 12 weeks. Control group: received standard care incorporating 1 to 2 hours of consultation according to the standards of German memory clinics. |
|
| Outcomes | Primary outcomes Quality of life 1) QoL‐AD Carer primary outcome Quality of life 2) WHOQOL‐BREF Other participant outcomes Activities of daily living 3) Barthel Index 4) Instrumental Activities of Daily Living Scale 5) Overprotection Scale For Adults Cognition 6) MMSE Depression 7) GDS‐15 Other carer outcomes Carer competence 8) SCQ Dyadic coping 9) Dyadic Coping Inventory Social support 10) Social Support Questionnaire Depression 11) GDS‐15 Outcomes assessed at 6 months. Outcomes at 6 months (post‐treatment) were included in the meta‐analysis. |
|
| Notes | Cites government funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mentions the use of a computer package to generate a random allocation sequence. |
| Allocation concealment (selection bias) | Low risk | Mentions central randomisation and that generating the random allocation sequence, enrolling participants, and assigning participants to interventions were strictly separated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blind to treatment allocation; no information about personnel; probably not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9 participants lost to follow‐up in intervention group, and 11 in control group; specific reasons reported; use of intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Orgeta 2019.
| Study characteristics | ||
| Methods | RCT of BA for people with mild dementia living in the community versus treatment as usual. | |
| Participants | Inclusion criteria: 1) people living with a diagnosis of dementia in the community (NINCDS‐ADRDA), 2) having a diagnosis of mild dementia (≥ 18 MMSE), and 3) an available carer to participate in the research and support the person in the intervention. Exclusion criteria: 1) at risk of self‐harm or a risk to others, 2) difficulties communicating in English, and 3) taking part in another intervention study. N = 63 (M28, F35) Mean age = 80.4 years Mean depression score at baseline = 7.2 (CSDD) Mean cognition at baseline = 24.5 (MMSE) |
|
| Interventions | BA adapted for people living with mild dementia, which incorporated introduction to BA, pleasant‐event scheduling based on life values and individual preferences, addressing barriers, and practising relaxation strategies. 8 weekly sessions lasting from 1 hour to 1 hour and 30 minutes over 12 weeks. Control group: treatment as usual comprising treatments in line with the National Institute for Health and Care Excellence guidelines. |
|
| Outcomes | Primary outcomes Function 1) BADLS Meaningful activity 2) Meaningful and Enjoyable Activities Scale Depression 3) CSDD Quality of life 4) DEMQOL Health‐related quality of life 5) EQ‐5D Neuropsychiatric symptoms 6) NPI Carer outcomes Depression 7) HADS Anxiety 8) HADS Health‐related quality of life 9) EQ‐5D 10) 12‐Item Short Form Health Survey Outcomes assessed at 3 and 6 months. Outcomes at 3 (post‐treatment) and 6 months (long‐term) were included in the meta‐analysis. |
|
| Notes | Cites voluntary sector funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mentions use of a computerised random list. |
| Allocation concealment (selection bias) | Low risk | Mentions centralised randomisation by a web‐based system and internet‐based sealed envelope codes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants lost to follow‐up in intervention group, and 4 in control group; specific reasons reported; use of intention‐to‐treat analysis and multiple imputations. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Quinn 2016.
| Study characteristics | ||
| Methods | RCT of a social support intervention versus treatment as usual for people with early‐stage dementia living in the community. | |
| Participants | Inclusion criteria: 1) people with a diagnosis of AD, vascular dementia, or mixed AD and vascular dementia (ICD‐10; World Health Organization, 1992 criteria), 2) being in the early‐stages of dementia with a score ≥ 20 on the MMSE, 3) able to provide informed consent, 4) on a stable dose of cholinesterase inhibitors/memantine, and 5) having a carer who is able to participate. Exclusion criteria: 1) history of significant neurological problems, psychiatric conditions, or cerebrovascular accidents, 2) significant anxiety or depression, 3) insufficient English, and 4) attending other group‐based psychosocial interventions. N = 24 (M18, F6) Mean age = 75.6 years Mean depression at baseline = 4.6 (HADS) Mean cognition at baseline = 23.6 (MMSE) |
|
| Interventions | Social support incorporating information about dementia, and practical ways of managing memory difficulties. Contents included staying well, planning for the future, and accessing local resources. 90‐minute weekly sessions over 8 weeks. Control group: treatment as usual receiving routinely‐provided memory clinic services, such as a nurse‐led review, and access to psychiatry, psychology, and social services. |
|
| Outcomes | Primary outcomes Self‐efficacy 1) General Self‐Efficacy Scale (GSE) Secondary outcomes Depression 2) HADS Anxiety 3) HADS General mental health 4) Clinical Outcomes in Routine Evaluation‐Outcome Measure Health‐related quality of life 5) EQ‐5D Well‐being 6) ICEpop CAPability measure for Older people Outcomes assessed at 3 and 6 months. Outcomes at 3 months (post‐treatment) for anxiety were included in the meta‐analysis. |
|
| Notes | We were not able to locate data for depression at 3 months, and data were not available from the published paper; cites government funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mentions use of a computer‐based algorithm. |
| Allocation concealment (selection bias) | Low risk | Mentions randomisation completed by an independent clinical trials unit. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided; probably both participants and personnel not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants lost to follow‐up in intervention group, and 1 in control group; specific reasons reported; use of intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported except for depression (3 months) and well‐being (3 and 6 months). |
| Other bias | Low risk | No other apparent bias. |
Rovner 2018.
| Study characteristics | ||
| Methods | RCT of BA aimed at increasing cognitive, physical, and social activity versus a supportive active control intervention in black older people with MCI. | |
| Participants | Inclusion criteria: 1) self‐identified black ethnicity, 2) ≥ 65 years, and 3) amnestic‐multiple or single‐domain MCI (NIA‐AA criteria). Exclusion criteria: 1) psychiatric diagnoses (e.g. major depression, dementia based on DSM‐IV), 2) on anti‐dementia medication, and 3) severe sensory deficits. N = 221 (M46, F175) Mean age = 75.8 years Mean depression score at baseline = 3.5 (GDS‐15) Mean cognition at baseline = 25.7 (MMSE) |
|
| Interventions | BA aimed at increasing cognitive, physical, and/or social activity, incorporating concepts from the health belief model to enhance cultural relevance, including respect for personal values, focusing on enhancement of treatment goals, and review of barriers. 5 in‐home 60‐minute treatment sessions over 4 months and 6 in‐home 60‐minute follow‐up maintenance sessions over the next 20 months. Control group: supportive control intervention which was comprised of a structured, nondirective intervention focusing on personal expression, conveying empathy, and optimism. |
|
| Outcomes | Primary outcomes Incidence of dementia 1) Hopkins Verbal Learning Test–Revised 2) National Alzheimer’s Coordinating Center Uniform Data Set Cognition 3) MMSE Secondary outcomes Function 4) University of California San Diego Performance‐Based Skills Assessment Function 5) Florida Cognitive Activities Scale 6) US Health Interview Survey Depression 7) GDS‐15 Outcomes measured at 6, 12, 18 and 24 months. Outcomes at 24 months (post‐treatment) were included in the meta‐analysis. |
|
| Notes | Cites government funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mentions use of a random numbers table. |
| Allocation concealment (selection bias) | Low risk | Mentions use of sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided; probably both participants and personnel not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 34 participants lost to follow‐up in intervention group, and 23 in control group; specific reasons not reported; use of intention‐to‐treat analysis and multiple imputations. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Spector 2015.
| Study characteristics | ||
| Methods | RCT of CBT for people with dementia and anxiety versus treatment as usual. | |
| Participants | Inclusion criteria: 1) participants with mild to moderate dementia (DSM‐IV criteria; CDR of 0.5, 1 or 2), 2) clinical anxiety determined by a score of ≥ 11 on RAID, 3) living in the community, 4) has a carer willing to participate in the intervention, 5) ability to understand and communicate in English, and 6) willing to engage in therapy involving discussion of thoughts and feelings. Exclusion criteria: 1) comorbid psychiatric disorder (e.g. psychosis), 2) challenging behaviour, 3) learning disability, and 4) diagnosis of severe physical illness. N = 50 (M20, F30) Mean age = 78.5 years Mean depression score at baseline = 15.9 (CSDD) Mean anxiety baseline score = 19.7 (RAID) Mean cognition at baseline = 20.9 (MMSE) |
|
| Interventions | Individual CBT which involved identifying and practicing strategies for feeling safe, challenging negative thoughts, and incorporating calming thoughts, behavioural experiments, and in between sessions, and telephone support encouraging ongoing work. 10 sessions, each lasting 60 minutes over 15 weeks. Control group: treatment as usual available to people with anxiety and dementia, which included either medication or no treatment. |
|
| Outcomes | Primary outcomes Anxiety 1) RAID 2) HADS‐A Secondary outcomes Depression 3) CSDD 4) HADS‐D Quality of life 5) QoL‐AD Cognition 6) MMSE Neuropsychiatric symptoms 7) NPI Quality of the care‐giving relationship 8) Quality of the Carer–Patient Relationship (QCPR) Carer outcomes Depression 9) HADS‐D Anxiety 10) HADS‐A Quality of the care‐giving relationship 11) QCPR Outcomes assessed at 15 weeks and 6 months. Outcomes at 15 weeks (post‐treatment) included in the meta‐analysis; 6‐month data not available. |
|
| Notes | Cites government funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mentions use of a computerised random list. |
| Allocation concealment (selection bias) | Low risk | Mentions centralised randomisation by a statistician not involved in the project. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants lost to follow‐up in intervention group, and 6 in control group; specific reasons reported; use of intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Stanley 2013.
| Study characteristics | ||
| Methods | RCT of CBT for anxiety for people with dementia versus treatment as usual. | |
| Participants | Inclusion criteria: 1) people with a diagnosis of dementia (confirmed by the participant’s medical provider), 2) with possible anxiety judged by scoring ≥ 4 on the Neuropsychiatric Inventory‐Anxiety (NPI‐A) subscale, 3) able to communicate in English, and 4) having a collateral/family carer able to take part. Exclusion criteria: 1) primary psychiatric diagnosis of major depression, 2) psychosis, 3) bipolar disorder, 4) suicidal intent, or 5) verbal or physical aggression. N = 32 (M13, F19) Mean age = 78.6 years Mean depression score at baseline = 10.1 (GDS‐30) Mean anxiety baseline score = 4.7 (NPI‐A) A total of 47% had a CDR of 0.5 or 1. |
|
| Interventions | CBT‐based intervention known as the Peaceful Mind Program, targeting anxiety in dementia, involving self‐monitoring for anxiety, deep breathing, and optional skills (i.e. coping self‐statements, behavioural activation, and sleep management). Up to 12 weekly in‐home sessions (lasting 30 to 60 minutes over 6 months) over the initial 3 months, and up to 8 brief telephone booster appointments during months 3 to 6. Control group: treatment as usual incorporating receiving diagnostic feedback but no additional contact other than assessments. |
|
| Outcomes | Primary outcomes Anxiety 1) NPI‐A 2) RAID 3) GAI Depression 4) GDS‐30 Worry 5) Penn State Worry Questionnaire–Abbreviated Quality of Life 5) QoL‐AD Carer outcomes Depression 6) PHQ‐9 Carer distress 7) NPI‐A ‐ Carer distress Outcomes assessed at 3 and 6 months. Outcomes at 3 (post‐treatment) and 6 months (long‐term) were included in the meta‐analysis. |
|
| Notes | Cites government funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors communicated that randomisation was completed in blocks; no further details, insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided; probably both participants and personnel not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants lost to follow‐up in intervention group, and 1 in control group; specific reasons reported; use of intention‐to‐treat analysis and multiple imputations. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Tappen 2009.
| Study characteristics | ||
| Methods | RCT of a counselling intervention versus treatment as usual for mood in people with moderate dementia living in nursing homes. | |
| Participants | Inclusion criteria: 1) diagnosis of probable AD (NINCDS‐ADRDA criteria), 2) MMSE ≤ 25, and 3) able to speak English. Exclusion criteria: 1) experiencing speech problems. N = 36 (M3, F33) Mean age = 87.3 years Mean depression at baseline = 17.4 (MADRS) Mean cognition at baseline = 11.4 (MMSE) |
|
| Interventions | Counselling intervention consisting of individual counselling sessions primarily aimed at forming and maintaining supportive relationships, providing opportunities for expression, reducing isolation, and improving mood. 3 sessions of 30 minutes each per week for 16 weeks. Control group: received usual care provided by staff of the long‐term care facility. |
|
| Outcomes | Primary outcomes Mood 1) Dementia Mood Assessment Scale 2) Alzheimer's Disease and Related Dementias Mood Scale Depression 3) MADRS Outcomes assessed at 16 weeks. Outcomes at 16 weeks (post‐treatment) were included in the meta‐analysis. |
|
| Notes | Cites voluntary sector funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentions random assignment, no further details; insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided; probably both participants and personnel not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Low levels of attrition; balanced between groups; specific reasons reported; insufficient detail of handling of missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Teri 1997.
| Study characteristics | ||
| Methods | RCT of BA versus problem‐solving therapy (PST) versus typical care versus waiting‐list control for people with dementia and depression. | |
| Participants | Inclusion criteria: 1) probable AD (NINCDS‐ADRDA criteria), 2) 6‐month history of cognitive problems, 3) living with carer in the community, 4) criteria for major or minor depressive disorder (DSM‐III‐R criteria), and 5) scoring ≥ 10 on the HDRS. N = 72 (M38, F34) Mean age = 76.4 years Mean depression score at baseline = 15.4 (HDRS) Mean cognition at baseline = 16.5 (MMSE) |
|
| Interventions | BA focusing on pleasant‐event scheduling, mood monitoring, modifying problem behaviours, addressing barriers, and developing plans to maintain events for the future. 9 weekly sessions lasting 60 minutes over 9 weeks. PST using a systematic approach to problem‐solve participant depression behaviours of specific concern to caregivers. 9 weekly sessions lasting 60 minutes over 9 weeks. Control group: treatment as usual incorporating typical advice and support services available in the community, and a separate waiting‐list control group. |
|
| Outcomes | Primary outcomes Depression 1) HDRS 2) CSDD 3) BDI Cognition 4) MMSE Severity of dementia 5) DRS Function 6) Record of Independent Living Carer outcomes Depression 7) HDRS Caregiver burden 8) ZBI Outcomes assessed at 10 weeks and 6 months. Outcomes at 10 weeks (post‐treatment) were included in the meta‐analysis; 6‐month data not available. |
|
| Notes | Note this study contributed data for both the BA and PST meta‐analysis; cites government funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentions random assignment, no further details; insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Personnel not blind to treatment allocation; no information about participants; probably not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Low levels of attrition; unclear whether they were balanced between groups; specific reasons not reported; insufficient detail about handling of missing data. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes at 3 months reported except for function; no outcomes reported at 6 months. |
| Other bias | Low risk | No other apparent bias. |
Travers 2017.
| Study characteristics | ||
| Methods | RCT of BA for improving mood and quality of life in nursing home residents with mild to moderate dementia versus a walking and talking intervention. | |
| Participants | Inclusion criteria: 1) had a score of ≥ 10 on the Standardized Mini‐Mental State Examination (SMMSE), 2) scored ≥ 4 on the GDS‐12R, 3) ability to communicate in English, and 4) living in the facility ≥ 3 months. Exclusion criteria: 1) receiving psychotherapy, and 2) having a terminal illness. N = 18 (M2, F16) Mean age = 86.3 years Mean depression score at baseline = 4.5 (GDS‐12R) Mean cognition at baseline = 17.8 (SMMSE) |
|
| Interventions | BA focusing on pleasant‐event scheduling that was feasible and practical, engaging in problem‐solving, and developing individually‐tailored plans to increase pleasant activities. 45‐minute sessions over 8 weeks. Control group: received a walking and talking intervention which involved spending 30 minutes walking and talking with a volunteer of the facility each week. |
|
| Outcomes | Primary outcomes Depression 1) GDS‐12R Quality of life 2) Quality of Life in Alzheimer’s Disease in Nursing Homes Outcomes assessed at 8 weeks. Outcomes at 8 weeks (post‐treatment) were included in the meta‐analysis. |
|
| Notes | Cites voluntary sector funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mentions random assignment, and use of the Statistical Package for the Social Sciences randomisation function. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided; probably both participants and personnel not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Raters were not blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One loss to follow‐up in control group due to death; analyses carried out with remaining participants. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Waldorff 2012.
| Study characteristics | ||
| Methods | RCT of a counselling intervention for depression versus treatment as usual for people with mild AD living in the community. | |
| Participants | Inclusion criteria: 1) being aged ≥ 50 years, 2) having a diagnosis of AD in the past 12 months (DSM‐IV; NINCDS‐ADRDA criteria), 3) scoring ≥ 20 on the MMSE, and 4) having a primary caregiver. Exclusion criteria: 1) having severe somatic or psychiatric comorbidity, 2) participating in other interventions, 3) and living in a nursing home, or having frontotemporal dementia. N = 330 (M151, F179) Mean age = 76.2 years Mean depression at baseline = 4.8 (CSDD) Mean cognition at baseline = 24.0 (MMSE) |
|
| Interventions | Counselling intervention consisting of individual counselling sessions, education, and telephone support, primarily aimed at preventing depression and loss of social networks. Up to 7 individual counselling sessions, 5 educational courses lasting 2 hours each, and 5 to 8 telephone support sessions over 3 to 4 week intervals over 8 to 12 months. Control group: provided with information and guidance about local support services available. |
|
| Outcomes | Primary outcomes Cognition 1) MMSE Depression 2) CSDD Health‐related quality of life 3) EQ‐5D Visual Analogue Scale (EQ‐5D VAS) Secondary outcomes Quality of life 4) QoL‐AD Neuropsychiatric symptoms 5) NPI Function 6) ADCS‐ADL Carer outcomes Depression 7) GDS Health‐related quality of life 8) EQ‐5D VAS Outcomes measured at 6 and 12 months, and 3 years. Outcomes at 12 months (post‐treatment) were included in the meta‐analysis. |
|
| Notes | Cites government funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mentions computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | Mentions random allocation sequence was concealed from the study team. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided; probably both participants and personnel not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 32 participants lost to follow‐up in intervention group, and 22 in control group; specific reasons other than death not reported; use of intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Wells 2013.
| Study characteristics | ||
| Methods | RCT of mindfulness‐based stress reduction (MBSR) for people with MCI versus treatment as usual. | |
| Participants | Inclusion criteria: 1) diagnosis of MCI as determined by a neurologist through history, physical examination, and neuropsychological testing (Wechsler Memory Scale Fourth Edition, MMSE, CDR; based on criteria by Grundman 2004), and 2) aged 55 to 90 years. Exclusion criteria: 1) actively practicing meditation or yoga, and 2) history of brain lesions or major head trauma. N = 14 (sex demographics not reported) Mean age = 74 years Median depression score at baseline = 6.0 on the Center for Epidemiologic Studies‐Depression (CES‐D) Mean cognition at baseline = 27.0 (MMSE) |
|
| Interventions | MBSR intervention consisting of teaching of mindfulness, defined as non‐judgmental moment‐to‐moment awareness, through sitting and walking meditation, body scan, and mindful movement. Eight 2‐hour weekly sessions over 8 weeks plus 1 mindfulness retreat day. Control group: received usual care. |
|
| Outcomes | Primary outcomes: Cognition 1) The Alzheimer's Disease Assessment Scale–Cognitive Subscale 2) Rey Auditory Verbal Learning Test 3) TMT A & B 4) Controlled Oral Word Association Test 5) Animal naming 6) Boston Naming Test Resilience 7) Resilience Scale Hope 8) Herth Hope Index Quality of life 9) QoL‐AD Life orientation 10) Life Orientation Test Depression 11) CES‐D Mindulness attention 12) Mindful Attention Awareness Scale Outcomes assessed at 8 weeks. |
|
| Notes | This study is not included in any meta‐analysis. We were not able to extract or locate data for this study. Data were not available from the published paper; cites government funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentions random assignment, no further details; insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided; probably both participants and personnel not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition in the study; use of intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Young 2014.
| Study characteristics | ||
| Methods | RCT of social support versus treatment as usual for people with mild dementia living in the community. | |
| Participants | Inclusion criteria: 1) people aged ≥ 60 years, 2) having a diagnosis of dementia, 3) with a score ≥ 18 on the MMSE, 4) aware of their memory loss, and 5) able to participate independently in a group. N = 39 (M22, F17) Mean age = 80.3 years Mean depression at baseline = 9.0 (GDS) Mean cognition at baseline = 22.0 (MMSE) |
|
| Interventions | Social support incorporating provision of information and support about dementia, developing a positive lifestyle, and adjusting to changes. Contents included identifying strengths, developing a plan for the future, and identifying sources of support. 90‐minute sessions over 10 weeks. Control group: received standardised educational written material on dementia providing basic information. |
|
| Outcomes | Primary outcomes Depression 1) GDS Secondary outcomes Self‐esteem 2) RSES Self‐Efficacy 3) GSE Coping with memory loss 4) Index for Managing Memory Loss Quality of life 5) QoL‐AD Outcomes assessed at 10 weeks. Outcomes at 10 weeks (post‐treatment) were included in the meta‐analysis. |
|
| Notes | A follow‐up paper reports data on quality of life (N = 64); cites university research funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentions random assignment, no further details; insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided; probably both participants and personnel not blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants lost to follow‐up in control group; specific reasons reported; use of intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other apparent bias. |
Abbreviations: AD: Alzheimer's disease; ADCS‐ADL: Alzheimer's Disease Cooperative Study – Activities of Daily Living Inventory; BA: behavioural activation; BADLS: Bristol Activities of Daily Living Scale; BDI: Beck Depression Inventory; Brief‐COPE: Coping Orientation to Problems Experienced Inventory; CBT: cognitive behavioural therapy; CDR: Clinical Dementia Rating; CERAD‐NB: Consortium to Establish a Registry for Alzheimer's Disease neuropsychological battery; CES‐D: Center for Epidemiologic Studies‐Depression; CSDD: Cornell Scale for Depression in Dementia; DEMQOL: Dementia Quality of Life Instrument; DRS: Dementia Rating Scale; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; EQ‐5D: EuroQoL‐5 Dimension; EQ‐5D VAS: EQ‐5D Visual Analogue Scale; F: female; 15D: 15D instrument; GAI: Geriatric Anxiety Inventory; GDS: Geriatric Depression Scale; GHQ: General Health Questionnaire; GSE: General Self‐Efficacy Scale; HADS: Hospital Anxiety and Depression Scale; HDRS: Hamilton Depression Rating Scale; ICD‐10: International Classification of Diseases, 10th Revision; M: male; MADRS: Montgomery–Åsberg Depression Rating Scale; MAI: Multilevel Assessment Instrument; MBCT: mindfulness‐based cognitive therapy; MBSR: mindfulness‐based stress reduction; MCI: mild cognitive impairment; MMSE: Mini‐Mental State Examination; MoCA: Montreal Cognitive Assessment; NIA‐AA: National Institute on Aging ‐ Alzheimer's Association; NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke ‐ Alzheimer's Disease and Related Disorders Association; NPI: Neuropsychiatric Inventory; NPI‐A: Neuropsychiatric Inventory‐Anxiety; PATH: problem‐adaptation therapy; PHQ‐9: Patient Health Questionnaire‐9; PM: Pearlin Mastery Scale; PSS: Perceived Stress Scale; PST: problem‐solving therapy; QCPR: Quality of the Carer–Patient Relationship; QoL‐AD: Quality of Life‐Alzheimer’s Disease; RAID: Rating Anxiety in Dementia; RCT: randomised controlled trial; RMBPC: Revised Memory and Behavior Problems Checklist; SCID: Structured Clinical Interview for DSM‐IV; SCQ: Sense of Competence Questionnaire; SDs: standard deviations; RSES: Rosenberg Self‐Esteem Scale; SMMSE: Standardized Mini‐Mental State Examination; ST: supportive therapy; TMT: Trail Making Test; WHOQOL‐BREF: World Health Organization Quality of Life Brief scale; ZBI: Zarit Burden Interview
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abraham 1992 | Ineligible population: in this randomised controlled trial (RCT) of group cognitive behavioural therapy (CBT) for nursing home residents with depression, participants with major cognitive impairment were excluded. |
| Almeida 2014 | Ineligible intervention: this RCT tests a computerised cognitive bias modification intervention for depression and quality of life in people with dementia and depression that trains participants' attentional resources to reduce repeated exposure and attention to negative stimuli. |
| Anderson 2018 | Ineligible population: in this RCT of CBT for depression and anxiety in older people living in long‐term care, participants with a Mini‐Mental State Examination (MMSE) score < 24 were excluded. |
| Auclair 2009 | Ineligible design and ineligible population: case vignette data from an RCT of couples' counselling of which a small number of participants had early‐stage Alzheimer's disease (AD). |
| Bailey 2017 | Ineligible intervention: RCT of a multi‐component intervention of question‐asking, reading, and reminiscence‐incorporating activities in people with dementia and depressive symptoms living in nursing homes. |
| Beck 2002 | Ineligible intervention: RCT of interventions aimed at increasing psychosocial activities (i.e. drawing, life review, massage): outcomes were direct observations of behaviour (video‐taped data). |
| Berwig 2020 | Ineligible trial design: a non‐randomised mixed‐method feasibility study of Marte Meo® counselling for people with behavioural variant frontotemporal dementia, and their primary carers. |
| Brodaty 2003 | Ineligible intervention: RCT of case management versus geriatric consultation versus standard protocol of care in people with dementia, depression, and/or psychosis living in nursing homes. |
| Chandler 2019 | Ineligible intervention: multi‐cluster RCT of memory‐compensation training, computerised cognitive training, yoga, support groups, and wellness education for people with mild cognitive impairment (MCI); support groups were unstructured, comprising reminiscence and discussing MCI‐related concerns. |
| Cheston 2003 | Ineligible design: pre‐post evaluation feasibility study of a 10‐week psychotherapy group aimed at addressing distress associated with forgetfulness in people with dementia. |
| Cheston 2009 | Ineligible design: pre‐post evaluation feasibility study of a 10‐week psychotherapy group aimed at sharing experiences of memory loss versus psycho‐education in people with mild dementia. |
| Collins 2018 | Ineligible design: pre‐post evaluation feasibility study of a 6‐week compassion‐focused group therapy for depression and anxiety, for people with mild to late‐stage dementia and their carers. |
| Craig 2018 | Ineligible design: pre‐post evaluation study testing the feasibility of compassion‐focused therapy for people with dementia and their carers. |
| Davison 2017 | Ineligible population: RCT of acceptance and commitment therapy for depression and anxiety for older people living in long‐term care, of which some had cognitive impairment (scoring between 19 and 23 on the MMSE). |
| Dozeman 2011 | Ineligible population: RCT of self‐help behavioural activation (BA) in older people living in nursing homes; excluded people with MMSE < 21; most people in this study did not have dementia or MCI. |
| Farrand 2016 | Ineligible design: single‐arm, non‐randomised feasibility study of BA self‐help for mood, well‐being, and quality of life in people with dementia supported by informal carers. |
| Finnema 2005 | Ineligible intervention: RCT of an emotion‐oriented care intervention for people with dementia, focusing on individuals' experiences, life history, and use of empathic skills by nursing staff. |
| Fischer‐Terworth 2011 | Ineligible trial design and ineligible intervention: controlled trial of a multi‐component intervention in people with mild to moderate dementia, incorporating music and psycho‐education of staff and family carers. |
| García‐Alberca 2017 | Ineligible trial design: pre‐post open trial evaluating the feasibility and acceptability of CBT for depression in people with AD living in long‐term care. |
| Helcer 2012 | Ineligible comparison: RCT of CBT plus memory training versus memory training alone in people with mild to moderate AD; no control group. |
| Hummel 2017 | Ineligible population: RCT of group CBT in older, medically‐ill participants with depression, of which only 20% had dementia; people with MMSE < 21 were excluded. |
| Hyer 2009 | Ineligible population: RCT of group CBT for older long‐term care residents with depression, of which only a small number had dementia; people with MMSE < 18 were excluded. |
| Ikemata 2017 | Ineligible intervention: RCT of progressive muscle relaxation versus a control intervention for neuropsychiatric symptoms, activities of daily living, and immune function in people with dementia living in nursing homes. |
| Joosten‐Weyn Banningh 2011 | Ineligible trial design: controlled trial of group psycho‐education and memory rehabilitation using CBT techniques for depression and well‐being in MCI versus a waiting‐list control group. |
| Kashimura 2020 | Ineligible design: a single‐arm, pre‐post study testing the feasibility and acceptability of a CBT program for depression and anxiety in people with dementia or MCI. |
| Klainin‐Yobas 2019 | Ineligible intervention: RCT of mindfulness versus health education for people with MCI; intervention did not have psychological elements, and was not aimed at stress reduction. |
| Kolanowski 2011 | Ineligible intervention: RCT of an activities‐based intervention based on the Needs‐Driven Dementia‐Compromised Behavior model, aimed at reducing agitation in people with dementia living in nursing homes. |
| Konnert 2009 | Ineligible population: RCT of group CBT in nursing home residents with depression, of which some experience cognitive impairment; people with MMSE ≤ 21 were excluded. |
| Kovach 2018 | Ineligible design: controlled cross‐over repeated‐measures study comparing mindfulness to cognitive therapeutic activity in frail older people and people with dementia; conditions randomised not participants. |
| Kulshreshtha 2019 | Ineligible design and ineligible comparison: pre‐post pilot study in African‐Americans with MCI, testing the feasibility and acceptability of CBT through web‐based or in‐person group sessions; no control group. |
| Lam 2010 | Ineligible intervention: RCT of an occupational therapy intervention for improving function and mood in people with mild and moderate dementia. |
| Lantz 1997 | Ineligible design and ineligible population: pre‐post evaluation study of a psychological intervention comprising modified meditation, relaxation, guided imagery, and body awareness for people living in nursing homes, of which some had dementia. |
| Leontjevas 2013 | Ineligible intervention and ineligible population: RCT of a multidisciplinary care management intervention for depression, incorporating pharmacological and psychosocial interventions, in older people living in nursing homes, of which some had dementia. |
| Luo 2020 | Ineligible population: cluster‐RCT of pleasant‐activity scheduling for residents living in a long‐term care facility; only people who did not experience cognitive impairment were recruited to the study. |
| Marciniak 2020 | Ineligible comparison: RCT of mindfulness‐based stress reduction practice on depression, cognition, and immunity in people with MCI versus cognitive training; no control group. |
| McSweeney 2012 | Ineligible intervention: cluster‐RCT of a multidisciplinary case management intervention for people with dementia and depression living in care homes. |
| Meeks 2015 | Ineligible population: RCT of BA for nursing home residents with depression, of which some experienced cognitive impairment (not further specified); people with MMSE < 14 were excluded. |
| NCT02083237 | Ineligible intervention: RCT of a psycho‐education and memory‐training intervention for people with MCI versus waiting‐list control; intervention does not meet criteria of a psychological treatment. |
| NCT04426838 | Ineligible comparison: RCT comparing face‐to‐face versus video‐conference‐based CBT for insomnia, incorporating stimulus control, sleep compression, relaxation, sleep hygiene, and cognitive restructuring for people with dementia and their carers; no control group. |
| Novelli 2018 | Ineligible intervention: RCT of the Tailored Activity Program ‐ Brazilian version on behavioural symptoms and quality of life in people with dementia versus usual care; intervention does not meet criteria of a psychological treatment. |
| O'Connor 2014 | Ineligible intervention: RCT of tailored activities based on individuals' abilities and interests for people with dementia who experience neuropsychiatric symptoms versus phone‐based education; intervention does not meet criteria of a psychological treatment. |
| Paller 2015 | Ineligible design: pre‐post evaluation study of mindfulness‐based stress reduction for quality of life and depression in people with a diagnosis of dementia or MCI, and older people with memory complaints. |
| Prakash 2019 | Ineligible intervention and ineligible population: RCT of progressive muscle relaxation therapy in older people, of which some had cognitive impairment; people with scores ≤ 17 on the MMSE were excluded. |
| Prick 2015 | Ineligible intervention: RCT of home‐based exercise, psycho‐education, support, and promotion of pleasant activities; intervention primarily aimed at carers; outcomes for people with dementia not assessed; intervention does not meet criteria of a psychological treatment. |
| Quintana‐Hernández 2016 | Ineligible intervention: RCT of a mindfulness‐based Alzheimer’s stimulation program versus cognitive stimulation therapy combined with progressive muscle relaxation versus usual care (control group); intervention did not have psychological elements and was not aimed at stress reduction. |
| Reinhardt 2014 | Ineligible population: RCT of problem‐solving therapy for depression versus social contact in older people living in long‐term care. Although people with mildly impaired cognitive status were not excluded, this sample was primarily people without cognitive impairment or dementia. |
| Rodriguez‐Sánchez 2018 | Ineligible intervention: RCT of a behaviour analysis intervention addressing specific behavioural problems for family carers of people with dementia attending day care centres versus a control group intervention. |
| Teri 2003 | Ineligible intervention: RCT of home‐based exercise combined with caregiver training aimed at increasing physical activity and decreasing problem behaviours for people with dementia. |
| Teri 2020 | Ineligible intervention: RCT of aerobic/endurance, strength, and balance/flexibility exercises, dementia education, and training carers to increase pleasant events; intervention does not meet criteria of a psychological treatment. |
| Van Haitsma 2015 | Ineligible intervention: RCT of individual activities for behavioural and psychological symptoms in people living with dementia in nursing homes; outcomes were observations of behaviour. |
| Vanova 2018 | Ineligible intervention: multi‐arm RCT of computer‐based cognitive rehabilitation versus computer‐based psychosocial stimulation (e‐health platform) versus combined cognitive rehabilitation and psychosocial stimulation versus control for cognition and mood for people with mild dementia and MCI; intervention does not meet criteria of a psychological treatment. |
| Whitlatch 2017 | Ineligible intervention: RCT of a psychosocial intervention aimed at developing a plan of future care, and increasing use of available services for people with dementia and their carers; intervention does not meet criteria of a psychological treatment. |
| Yesavage 1981 | Ineligible design and ineligible population: pre‐post study of supportive counselling versus cognitive training in people with symptoms of senile organic brain disease and moderate cognitive impairment. |
AD: Alzheimer's disease; BA: behavioural activation; CBT: cognitive behavioural therapy; MCI: mild cognitive impairment; MMSE: Mini‐Mental State Examination; RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Carreel 1990.
| Methods | Unknown whether this study was a randomised controlled trial (RCT) and whether it tested a psychological intervention |
| Participants | Older people with dementia and depression |
| Interventions | Group sessions with therapeutic aim described as conversation groups |
| Outcomes | Linguistic skills |
| Notes | Article in French |
ISRCTN42983681.
| Methods | RCT of psychodynamic interpersonal therapy principles in mild cognitive impairment (MCI) versus standard care |
| Participants | Unknown whether this sample has a diagnosis of MCI. Inclusion criteria: 1) diagnosis of MCI according to the criteria suggested by the International Psychogeriatric Association expert conference on MCI, 2) living with a spouse or partner, 3) ability to speak English, and 4) Mini‐Mental State Examination (MMSE) score > 26. Exclusion criteria: 1) participants/spouses living with serious physical condition. N = 40 |
| Interventions | Course of psychotherapy based on psychodynamic interpersonal therapy principles. Control group: receives standard care. |
| Outcomes | Primary outcome measure Clinical outcomes in routine evaluation Secondary outcome measures 1) Geriatric Depression Scale (GDS) 2) Beck Anxiety Inventory 3) Marital Intimacy Scale 4) MMSE 5) Quality of Life‐Alzheimer’s Disease (QoL‐AD) 6) Relationship Scales Questionnaire 7) Relationship Questionnaire – Attachment |
| Notes | This study appears as completed; data not published; unclear what criteria were used for MCI and whether there was a control group. |
NCT01550718.
| Methods | RCT of physical activity versus social activity versus no intervention for social and physical activity in early‐stage dementia |
| Participants | Inclusion criteria: 1) dementia diagnosis, 2) Clinical Dementia Rating scale score, 3) available care partner, 4) care recipient lives in the community or in a retirement home, and 5) both care partner and care recipient speak English. Exclusion criteria: 1) significant physical or psychiatric illness in either care partner or care recipient that would prevent participation, and 2) living out of the study area. N = 152 |
| Interventions | Social activity program (90‐minute weekly seminars of open discussion and socialising) versus a physical activity program (90‐minute weekly classes of physical exercises and discussion of health topics) versus no intervention. Control group: receives no intervention. |
| Outcomes | Primary outcomes Social activity 1) Pleasant Events Schedule‐AD Physical activity 2) Physical Activity Scale for the Elderly Quality of life 3) QoL‐AD Secondary outcomes Communication 4) Communication, Affective Expression, and Involvement subscales of the Family Assessment Measure Physical function 5) Physical Functioning Scale of the Medical Outcomes Study Short Form 36 Depression 6) GDS Outcomes measured at 1 and 4 months. |
| Notes | This study appears as ongoing; its status is unclear; unclear if the social support group has a psychological element. |
Wong 2016.
| Methods | Unclear design |
| Participants | Inclusion criteria: 1) clinical diagnosis of MCI, 2) able to give informed consent, and 3) at least 60 years of age. Exclusion criteria: 1) current/past experience with meditation/yoga, 2) prescribed cognitive intervention/electromagnetic stimulation, 3) acquired/traumatic brain injury, 4) psychoactive medication/intake of drugs that alter cognition, 5) drug/alcohol abuse, 6) on cholinesterase inhibitors, 7) having a psychiatric condition or neurological/cerebrovascular condition, 8) physical condition requiring treatment, 9) advanced cognitive decline, and 10) major physical or language impairment. |
| Interventions | Group‐based mindfulness training with both formal and informal practice over 8 weeks. Control group: receives standard care. |
| Outcomes | Cognition 1) Montreal Cognitive Assessment Psychological health 2) Depression Anxiety Stress Scales Mindfulness 3) Freiburg Mindfulness Inventory Function 4) Bayer Activities of Daily Living Scale Outcomes assessed at 8 weeks and 1 year. |
| Notes | Unclear whether this study is a randomised controlled trial. |
CBT: cognitive behavioural therapy; GDS: Geriatric Depression Scale; MCI: mild cognitive impairment; MMSE: Mini‐Mental State Examination; QoL‐AD: Quality of Life‐Alzheimer’s Disease; RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Aguirre 2017.
| Study name | Mindfulness‐based cognitive therapy (MBCT) programme for depression in people with early stages of dementia: study protocol for a randomised controlled feasibility study |
| Methods | Randomised controlled trial (RCT) |
| Participants | Inclusion criteria: 1) diagnosis of mild depression (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) criteria), and 2) mild to moderate dementia (Mini‐Mental State Examination (MMSE) scores ≥18). Exclusion criteria: 1) learning disability, 2) severe depression, or at high risk of self‐harm, 3) within 2 months of bereavement, 4) involved in other psychosocial intervention, and 5) experiencing psychosis. N = 32 |
| Interventions | Group MBCT sessions comprising meditation practice, discussion of mood and cognitive symptoms, and using both formal and informal mindfulness in distressing situations (i.e. mindful eating, body scan, mindful stretching, and sitting mindfulness meditation). Weekly 90‐minute sessions over 8 weeks plus homework exercises. Control group: treatment as usual incorporating General Practice (GP) and memory service support, antidepressant medication, and psychiatry review and appointments. |
| Outcomes | Primary outcomes Depression 1) Cornell Scale for Depression in Dementia (CSDD) 2) Patient Health Questionnaire‐9 (PHQ‐9) Secondary outcomes Quality of life 3) Quality of Life‐Alzheimer's Disease (QoL‐AD) Mindfulness 4) Cognitive and Affective Mindfulness Scale Anxiety 5) Generalized Anxiety Disorder 7‐item (GAD‐7) 6) Rating Anxiety in Dementia (RAID) Carer‐rated patient outcomes 7) CSDD carer‐rated 8) QoL‐AD carer‐rated 9) RAID carer‐rated Outcomes assessed at 3 and 6 months. |
| Starting date | 21 July 2016 |
| Contact information | elisa.aguirre@nelft.nhs.uk |
| Notes | www.isrctn.com/ISRCTN16382776 |
Ekman 2020.
| Study name | Evaluation of a novel psychological intervention tailored for patients with early cognitive impairment (PIPCI): study protocol of a randomized controlled trial |
| Methods | RCT |
| Participants | Inclusion criteria: 1) subjective cognitive decline or mild cognitive impairment (MCI) diagnosis (International Classification of Diseases, 10th Revision (ICD‐10) criteria; all MCI subtypes) aged < 70 years, 2) mild to moderate psychological symptoms that are indicated to be related to the participant’s cognitive impairment affecting participant's daily living and behaviour, 3) fluency in Swedish language, 4) access to a mobile telephone, and 5) signed informed consent. Exclusion criteria: 1) dementia diagnosis and/or occurrence of serious illness and/or injury or learning disability, 2) participation in other psychological treatment over the past 6 months, 3) severe psychiatric comorbidity, and/or severe psychiatric disorder, 4) antidepressant medication introduced or alterations in dosage < 6 months ago (i.e. unstable dose), 5) MMSE score < 26 and/or a Montreal Cognitive Assessment (MoCA) score < 24, 6) stroke or head trauma < 6 months ago, and 7) present substance abuse diagnosis. N = 120 |
| Interventions | Psychological intervention adjusted to people experiencing cognitive impairment, combining: cognitive behavioural therapy (CBT) and acceptance commitment therapy, including validation strategies, and psycho‐education. Intervention also aims to focus on increasing motivation for lifestyle changes, in line with personal goals alongside the teaching of behavioural activation (BA) and mindfulness. Weekly sessions of 55 minutes each over 10 weeks plus homework exercises. Control group: waiting‐list control group condition comprised of receiving regular health information that is provided after cognitive examination. |
| Outcomes | Primary outcomes Psychological health 1) Acceptance and Action Questionnaire‐II (AAQ‐II) Secondary outcomes Perceived stress 2) Perceived Stress Scale (PSS) Depression 3) Beck Depression Inventory Quality of life 4) Brunnsviken Brief Quality of Life Scale General health 5) Medical Outcomes Study Short Form 36 Executive function 6) Stroop Color and Word Test 7) Trail Making Test (TMT) A & B Memory 8) Brief Visuospatial Memory Test‐Revised Attention 9) Digit‐Span ‐ Wechsler Adult Intelligence Scale‐Fourth Edition (WAIS‐IV) 10) AX‐Continuous Performance Task Processing speed 11) Digit‐Symbol (WAIS‐IV) 12) Deary‐Liewald Reaction Time Task Biological health measures 13) Telomerase Costs‐effectiveness 14) Quality‐Adjusted Life Years Outcomes assessed at 11 weeks and 6 months. |
| Starting date | 01 February 2021 |
| Contact information | urban.ekman@ki.se |
| Notes | clinicaltrials.gov/ct2/show/NCT04356924 |
Forstmeier 2015.
| Study name | Cognitive behavioural treatment for mild Alzheimer’s patients and their caregivers (CBTAC): study protocol for a randomized controlled trial |
| Methods | RCT |
| Participants | Inclusion criteria:
1) 50 to 95 years, 2) diagnosis of probable or possible Alzheimer's disease (AD) (National Institute of Neurological and Communicative Disorders and Stroke ‐ Alzheimer's Disease and Related Disorders Association) criteria, 3) mild dementia severity (Clinical Dementia Rating (CDR) scores 0.5 or 1; MMSE ≥ 20), 4) with any non‐cognitive symptom that motivates acceptance of psychotherapeutic help, and 5) caregiver available to take part in sessions. Exclusion criteria: 1) concomitant alcohol/drug addiction, 2) malignant disease/severe organ failure, and 3) metabolic disorders, or other neurological conditions. N = 44 |
| Interventions | CBT multi‐component programme of goal setting, psycho‐education, engagement in pleasant activities, cognitive restructuring, caregiver training in behaviour management techniques, life review, and couples counselling. 25 weekly or bi‐weekly sessions over 9 months, with 5 single sessions with family caregiver. Control group: receives standard medical and psychosocial care which includes psycho‐education on dementia, medical treatment, social counselling by specialised staff, memory training, and self‐help group support for both participant and caregiver. |
| Outcomes | Primary outcomes Depression 1) Geriatric Depression Scale (GDS‐15) 2) CSDD 3) Depressive disorder (Structured Clinical Interview for DSM‐IV Axis I) Secondary outcomes Neuropsychiatric symptoms 4) Neuropsychiatric Inventory (NPI) 5) Apathy Evaluation Scale (AES) Quality of life 6) QoL‐AD Coping 7) Stress Coping Inventory (SCI) Carer outcomes Caregiver burden 8) Zarit Burden Interview (ZBI) Depression 9) Centre for Epidemiologic Studies ‐ Depression Anxiety 10) State‐Trait Anxiety Inventory Anger 11) State‐Trait Anger Expression Inventory Quality of life 12) Satisfaction with Life Scale 13) 12‐Item Short Form Health Survey (SF‐12) Coping 14) SCI Outcomes measured at 6 and 12 months. |
| Starting date | February 2011 |
| Contact information | s.forstmeier@psychologie.uzh.ch |
| Notes | clinicaltrials.gov/ct2/show/NCT01273272 |
Gildengers 2016.
| Study name | The design and implementation of an intervention development study: Retaining Cognition While Avoiding Late‐Life Depression (ReCALL) |
| Methods | RCT |
| Participants | Inclusion criteria: 1) 60 years or older, 2) PHQ‐9 > 1 with at least a score of 1 on question 1 or 2 (depressed mood or anhedonia), 3) adequate physical/sensory function and ability to engage in moderate‐intensity exercise, and 4) diagnosed with MCI by cognitive screening (Modified Mini‐Mental State Examination (3MS), TMT, Digit Symbol Substitution Test, and the Quick Mild Cognitive Impairment Screen), and by clinicians using the revised Petersen 2004 criteria. Exclusion criteria: 1) not meeting criteria for a major depressive episode/current anxiety disorder, except for specific phobia (DSM‐IV TR), 2) multiple sclerosis, 3) depression/substance disorder in the past year, 4) on antidepressants, and 5) lifetime history of bipolar disorder or schizophrenia. N = 94 |
| Interventions | Problem‐solving therapy aimed at teaching individuals to systematically approach and solve problems, exerting control, promoting pleasurable activities, and helping individuals avoid negatively‐charged situations. Offered up to 16 weeks; additional booster sessions at 3 and 9 months; no further information provided. Control group: enhanced usual care which includes mood and cognitive problems assessment, and referral for medical care. |
| Outcomes | Primary outcomes Depression 1) Onset of major depression (DSM‐IV TR) Anxiety 2) Onset of anxiety disorder (Primary Care Evaluation of Mental Disorders Questionnaire) Outcomes assessed at 16 weeks and every 3 months for 12 months. |
| Starting date | 26 June 2013 |
| Contact information | gildengersag@upmc.edu |
| Notes | clinicaltrials.gov/ct2/show/NCT01886586 |
ISRCTN11185706.
| Study name | Problem adaptation therapy for depression in dementia (PATHFINDER) |
| Methods | RCT |
| Participants | Inclusion criteria: 1) diagnosis of probable or mixed AD and vascular dementia (National Institute on Aging ‐ Alzheimer's Association (NIA‐AA) criteria), 2) mild to moderate dementia severity (Standardized Mini‐Mental State Examination (SMMSE) score > 10), 3) clinically significant depression (8+ on the CSDD), 4) aged > 50 years, 5) fluent in English, and 6) available family carer who can act as co‐therapist. Exclusion criteria: 1) rare dementia diagnosis, 2) initiation/change in dose of antidepressant/psychotropics in previous 4 weeks or plan to change treatment in the next 12 weeks, 3) engaging in psychological therapy, and 4) schizophrenia/bipolar disorder/other severe psychiatric disorder or suicidal ideation. N = 334 |
| Interventions | Problem‐adaptation therapy that aims at identifying problems hypothesised to maintain the person’s depression, identify solutions and pleasurable activities that the person enjoys, using environmental adaptations to help overcome physical and behavioural limitations. 10 face‐to‐face 1‐hour sessions delivered over 12 weeks. Control group: usual multidisciplinary care. |
| Outcomes | Primary outcomes Depression 1) CSDD Secondary outcomes Quality of life 2) Dementia Quality of Life Instrument (DEMQOL) and DEMQOL‐proxy 3) EuroQoL‐5 Dimension (EQ‐5D) Function 4) Bristol Activities of Daily Living Scale Cognition 5) SMMSE Anxiety 6) RAID Carer outcomes Burden 7) ZBI Quality of life 8) General Health Questionnaire‐12 Outcomes assessed at 3, 6, and 12 months. |
| Starting date | 01 September 2018 |
| Contact information | robert.howard@ucl.ac.uk |
| Notes | doi.org/10.1186/ISRCTN11185706 |
Janssen 2017.
| Study name | BA by mental health nurses for late‐life depression in primary care: a randomised controlled trial |
| Methods | Cluster RCT |
| Participants | Inclusion criteria: 1) older people ≥ 65 years, 2) with PHQ‐9 score > 9 recruited from primary care, and 3) who may have MCI or mild dementia. Exclusion criteria: 1) severe mental illness, 2) high suicide risk, drug and/or alcohol abuse, 3) prior psychotherapy, 4) change in medication, and 5) moderate to severe cognitive impairment. N = 200 |
| Interventions | BA encouraging engaging in rewarding activities and reducing avoidance behaviour, diminishing distress using functional analysis, activity registration, activity scheduling and relapse prevention. 8 weekly 30‐minute face‐to‐face sessions delivered by a trained mental health nurse. Control group: unrestricted access to GP treatment options including pharmacological treatment, psychological treatment by a mental heath nurse or a psychologist, or watchful waiting. |
| Outcomes | Primary outcomes Depression 1) Quick Inventory of Depressive Symptomatology 2) PHQ‐9 Depressive disorder and psychiatric comorbidity 3) Mini‐International Neuropsychiatric Interview Quality of life 4) EQ‐5D Outcomes assessed at 52 weeks. |
| Starting date | 25 August 2016 |
| Contact information | g.hendriks@propersona.nl |
| Notes | www.trialregister.nl/trial/5436 |
NCT01774448.
| Study name | The efficacy of mindfulness‐based cognitive therapy (MBCT) to improve depression symptoms and quality of life in individuals with dementia and their caregivers: a pilot study |
| Methods | RCT |
| Participants | Inclusion criteria: 1) early stages of dementia and aged between 50 and 85 years, 2) spousal caregiver (no signs of dementia/mild cognitive impairment), 3) evidence of at least mild depression (in at least one partner), and 4) able to speak, read, and write in English. Exclusion criteria: 1) neurological disorder (other than dementia for dementia group), and 2) a mental health diagnosis. N = 36 |
| Interventions | MBCT consisting of learning and practicing formal and informal mindfulness meditation, and participation in group discussion and inquiry. 1 session per week for 2 hours over an 8‐week intervention. Control group: waiting‐list control group where participants undergo a non‐intervention 8‐week period. |
| Outcomes | Primary outcomes Depression 1) GDS Secondary outcomes Apathy 2) AES Quality of life 3) QoL‐AD Anxiety 4) Depression Anxiety Stress Scales (DASS) Depression 5) DASS Self‐compassion 6) Self‐Compassion Scale Cognition 7) MoCA 8) 3MS Working memory 9) Digit Span Forward and Backward Tasks Cognitive flexibility 10) TMT Verbal fluency 11) Category fluency test Short‐term verbal memory 12) California Verbal Learning Test Attention 13) Attention Network Task Mindfulness 14) Five Facet Mindfulness Questionnaire Carer outcomes Coping 15) Brief‐COPE Burden 16) ZBI Outcomes assessed at 8 weeks. |
| Starting date | August 2013 |
| Contact information | lozen@uwaterloo.ca |
| Notes | clinicaltrials.gov/ct2/show/NCT01774448 |
NCT02013518.
| Study name | Cognitive behavioural therapy for persons with mild cognitive impairment or dementia in the early stages |
| Methods | RCT |
| Participants | Inclusion criteria: 1) a diagnosis of dementia (AD or mixed AD and vascular dementia; ICD‐10 criteria), 2) ≥ 20 on MMSE, 3) ≥ 5 on CSDD, and 4) a carer willing to participate in treatment, and has weekly contact. Exclusion criteria: 1) severe somatic/psychiatric diagnosis, 2) dementia with Lewy bodies or frontotemporal dementia, 3) poor command of Norwegian, and 4) doing psychotherapy. N = 200 |
| Interventions | CBT comprised of introduction to CBT principles, pleasant activities to improve mood and depressive symptoms, use of external memory aids to maintain independence/reduce demands on memory, active engagement in reminiscence to improve mood, and review of treatment goals. 1‐hour weekly sessions over 11 weeks. Control group: treatment as usual at the participating memory clinics. |
| Outcomes | Primary outcomes Depression 1) Montgomery‐Åsberg Depression Rating Scale 2) Hospital Anxiety and Depression Scale (HADS) 3) CSDD Self‐efficacy 4) General Self‐Efficacy Scale Quality of Life 5) QoL‐AD Cognition 6) MMSE 7) TMT 8) Consortium to Establish a Registry for Alzheimer’s Disease Word List Task Satisfaction with the intervention 9) Client Satisfaction Scale Neuropsychiatric Symptoms 10) NPI Use and costs of health care services 11) Resource Utilization in Dementia Carer outcomes Carer burden 12) Relative Stress Scale Outcomes assessed post‐intervention (no further information provided). |
| Starting date | September 2013 |
| Contact information | ingun.ulstein@aldringoghelse.no |
| Notes | clinicaltrials.gov/ct2/show/NCT02013518 |
NCT02777905.
| Study name | Group mindfulness‐based cognitive therapy for the treatment of late‐life depression and anxiety symptoms in primary care: a randomized controlled trial |
| Methods | RCT |
| Participants | Inclusion criteria: 1) at least 60 years of age and above, 2) depression or anxiety symptoms, or both, as indicated by scores of ≥ 10 on the PHQ‐9 or GAD‐7, and 3) normal cognition or MCI. Exclusion criteria: 1) mild, moderate, or severe dementia, 2) acute psychotic symptoms/severe personality problems/suicidal ideation, 3) change in psychotropic medication, 4) hearing impairment, and 5) unable to engage with intervention/ongoing active psychotherapy. N = 100 |
| Interventions | MBCT comprising group meditative practice, practicing mindfulness techniques, including both formal mindfulness meditation, and informal mindfulness practices, provision of meditation compact discs, and education about depression and anxiety. 2‐hour sessions per week once a week over 8 weeks. Control group: offered literature on mental health promotion and treatment as usual in the primary care health setting. |
| Outcomes | Primary outcomes Depression 1) PHQ‐9 Anxiety 2) GAD‐7 Physiological stress marker outcomes 3) cortisol 4) dehydroepiandrosterone 5) adrenaline 6) noradrenaline 7) aldosterone Inflammatory marker outcomes 8) C‐reactive protein 9) cytokines 10) epidermal growth factor 1 Outcomes assessed at 8 weeks. |
| Starting date | 19 May 2016 |
| Contact information | gabriela.torresplatas@mail.mcgill.ca |
| Notes | clinicaltrials.gov/ct2/show/NCT02777905 |
NCT03656159.
| Study name | Evaluation of a new cognitive behavioural therapy (CBT) to reduce psychological distress and improve quality of life of people with Alzheimer's disease and their caregivers |
| Methods | RCT |
| Participants | Inclusion criteria: 1) diagnosis of mild to moderate AD (score of 0.5 to 2 on the CDR), 2) have minimal self‐criticism to be able to participate in group therapy, 3) a score of ≥ 15 on the Kessler Psychological Distress Scale (either carer or participant must meet this criterion), 4) have a caregiver (≥ 8 hours weekly contact) who can participate, and 5) able to understand French. Exclusion criteria: 1) psychiatric disorder, 2) behavioural problems, and 3) living with disability or physical illness or a neurodegenerative disease. N = 30 |
| Interventions | CBT comprising behavioural activation, cognitive restructuring, stress/anger management strategies, knowledge about memory management, and sleep disorders. 8 weekly sessions of 2 hours each with a break of 15 minutes. Control group: non‐directive support group, providing time and space to discuss the impact of AD, help participants feel less alone and better understood and address implications of AD in daily life. |
| Outcomes | Primary outcomes Psychological distress 1) Kessler Psychological Distress Scale Anxiety 2) Geriatric Anxiety Inventory (GAI) Worry 3) Penn State Worry Questionnaire (PSWQ) Depression 4) GDS‐15 5) PHQ‐9 Sleep quality 6) Insomnia Severity Index Secondary outcomes Function 7) Disability Assessment for Dementia Quality of life 8) QoL‐AD Satisfaction with life 9) Satisfaction with Life Scale Pleasant activities 10) Entrevue Profil du Loisir Community integration 11) Community Integration Questionnaire Cognition 12) MoCA 13) Boston Naming Test 14) Verbal fluency test 15) Rey Auditory Verbal Learning Test 16) Famous faces naming test 17) Self‐Evaluation Questionnaire 18) Wechsler Memory Scale Third Edition 19) Rey‐Osterrieth Complex Figure Test 20) Frontal Assessment Battery 21) TMT 22) Victoria Stroop Test 23) Clock Drawing Test 24) Medication taken to manage anxiety, mood or insomnia Carer outcomes Quality of life 25) SF‐12 Outcomes assessed at 1 to 2 weeks, at 6 months, and 12 months |
| Starting date | 04 September 2018 |
| Contact information | sebastien.grenier@umontreal.ca |
| Notes | clinicaltrials.gov/ct2/show/NCT03656159 |
NCT03692988.
| Study name | Dignity therapy for patients with early dementia and their family (DTD) |
| Methods | RCT |
| Participants | Inclusion criteria: 1) able to sign informed consent, 2) diagnosis of very mild dementia, 3) ≥ 18 years, and 4) having a study partner (life partner, relative, close friend) available. Exclusion criteria: 1) unable to speak and read German, and 2) physical or cognitive incapacity to participate. N = 54 |
| Interventions | Dignity Therapy (DT) invites individuals with life‐limiting illnesses to reflect on matters of importance to them, and compiles them in a narrative document for the participant to share. No further information provided. Control group: waiting‐list control group. |
| Outcomes | Primary outcomes 1. Acceptance based on participation, refusal and dropout rate Satisfaction with DT 2. Dignity Therapy Evaluation Questionnaire Psychological distress 3. Distress Thermometer Depression 4. HADS‐D Anxiety 5. HADS‐A Outcomes assessed at 3 months. |
| Starting date | 02 October 2018 |
| Contact information | University of Zurich |
| Notes | clinicaltrials.gov/ct2/show/NCT03692988 |
Rodakowski 2019.
| Study name | Preventing disability in older adults with mild cognitive impairment: a Strategy Training intervention study |
| Methods | RCT |
| Participants | Inclusion criteria: 1) diagnosis of MCI (NIA‐AA criteria), 2) ≥ 60 years of age living in the community, 3) scoring ≥ 1 on the Patient Health Questionnaire‐2, and 4) self‐reported difficulty in at least 1 daily activity. Exclusion criteria: 1) central nervous system disorder (other than MCI), 2) major depression, 3) alcohol/substance use disorder, and 4) lifetime history of bipolar disorder/schizophrenia. N = 30 |
| Interventions | BA‐optimising engagement in meaningful and pleasurable activities through generating self‐selected goals, daily monitoring to demonstrate the value of participating in activities, increasing contact with others, and problem‐solving to remove barriers. No further information provided. Control group: enhanced‐usual care comprised of receiving feedback on thinking, memory, and mood status, and referral to services. |
| Outcomes | Primary outcomes Preclinical disability 1. Performance Assessment of Self‐Care Skills Secondary outcomes Cognition 2. Delis‐Kaplan Executive Function System 3. Repeatable Battery for the Assessment of Neuropsychological Status 4. 3MS Medical co‐morbidity 5. Cumulative Illness Rating Scale‐Geriatric 6. Current medication list Depression 7. PHQ‐9 Anxiety 8. GAD‐7 Insomnia 9. Pittsburgh Sleep Quality Index Physical Activity 10. Physical Activity Scale for the Elderly Social Support 11. Interpersonal Support Evaluation List‐12 Self‐appraisal of ability to take care of self 12. Appraisal of Self‐Care Agency Scale Coping 13. Ways of Coping Questionnaire Worry 14. PSWQ Outcomes assessed at 6 weeks, 3, 6, 9 and 12 months. |
| Starting date | November 2018 |
| Contact information | jur17@pitt.edu |
| Notes | www.ncbi.nlm.nih.gov/pmc/articles/PMC6512744/ |
Teti 2018.
| Study name | Cognitive behavioural therapy to enhance memory training efficacy in people with dementia: 1‐year follow‐up |
| Methods | RCT |
| Participants | Inclusion criteria: 1) people with a diagnosis of early to moderate AD. N = 44 |
| Interventions | CBT in conjunction with computer‐based memory training designed to target negative expectations and automatic distorted schemes of hopelessness and perceptions of poor self‐competency for the training exercises. Sessions delivered over 4 weeks. Control group: computer‐based memory training. |
| Outcomes | Primary outcomes 1) Cognitive function Secondary outcomes 2) Quality of life 3) Depression 4) Neuropsychiatric symptoms 5) Self‐efficacy |
| Starting date | Conference abstract |
| Contact information | annemarie.teti@hhchealth.org |
| Notes | First and last author have been contacted. |
Tran 2020.
| Study name | Occupational therapist‐led mindfulness‐based stress reduction for older adults living with subjective cognitive decline or mild cognitive impairment in primary care: a feasibility randomised control trial protocol |
| Methods | RCT |
| Participants | Inclusion criteria: 1) ≥ 60 years of age, 2) English fluency, 3) living independently, 4) reporting subjective decline or having a diagnosis of MCI (criteria not specified), and 5) patient in inter professional primary care clinic. Exclusion criteria: 1) history of prior participation in mindfulness‐based interventions or having 2 to 3 times per week or more of either mindfulness or yoga practice, 2) current history of significant medical, neurological or psychiatric condition, active psychosis, or bereavement, 3) alcoholism or other substance abuse, and 4) participating in other cognitive or memory training programmes. N = 40 |
| Interventions | Mindfulness‐based stress reduction led by occupational therapists offered via a tablet comprising lying down (body scan), sitting (attention on the breath), and mindful movement aimed at alleviating psychological symptoms. Group sessions of 3 hours delivered over 8 weeks. Control group: waiting‐list control receiving usual care. |
| Outcomes | Primary outcomes Satisfaction with performance 1) Canadian Occupational Performance Measure Secondary outcomes Depression 2) PHQ‐9 Anxiety 3) GAI Perceived Stress 4) PSS Mindfulness 5) Cognitive and Affective Mindfulness Scale‐Revised Quality of life 6) QoL‐AD Acceptance 7) AAQ‐II Outcomes assessed at 8, and 12 weeks. |
| Starting date | 03 October 2019 |
| Contact information | Todd.tran@wchospital.ca |
| Notes | clinicaltrials.gov/ct2/show/NCT03867474 |
AAQ‐II: Acceptance and Action Questionnaire‐II; AD: Alzheimer’s disease; AES: Apathy Evaluation Scale; BA: behavioural activation; CBT: cognitive behavioural therapy; CDR: Clinical Dementia Rating; CSDD: Cornell Scale for Depression in Dementia; DASS: Depression Anxiety Stress Scales; DEMQOL: Dementia Quality of Life Instrument; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; DT: Dignity Therapy; EQ‐5D: EuroQoL‐5 Dimension; GAD‐7: Generalized Anxiety Disorder 7‐item; GAI: Geriatric Anxiety Inventory; GDS: Geriatric Depression Scale; GP: General Practice; HADS: Hospital Anxiety and Depression Scale; ICD‐10: International Classification of Diseases, 10th Revision; MBCT: mindfulness‐based cognitive therapy; MCI: mild cognitive impairment; MMSE: Mini‐Mental State Examination; 3MS: Modified Mini‐Mental State Examination; MoCA: Montreal Cognitive Assessment; NIA‐AA: National Institute on Aging ‐ Alzheimer's Association; NPI: Neuropsychiatric Inventory; PHQ‐9: Patient Health Questionnaire‐9; PSS: Perceived Stress Scale; PSWQ: Penn State Worry Questionnaire; QoL‐AD: Quality of Life‐Alzheimer's Disease; RAID: Rating Anxiety in Dementia; RCT: randomised controlled trial; SCI: Stress Coping Inventory; SF‐12: 12‐Item Short Form Health Survey; SMMSE: Standardized Mini‐Mental State Examination; TMT: Trail Making Test; WAIS‐IV: Wechsler Adult Intelligence Scale‐Fourth Edition; ZBI: Zarit Burden Interview
Differences between protocol and review
We added the new outcomes of depression remission at the end of treatment and caregiver depressive symptoms at the end of treatment and post‐treatment follow‐up. We did not exclude studies on the grounds of inadequate reporting (missing information about study design and results).
For dichotomous outcomes, we used risk ratio (RR) to estimate treatment effects. Given the expanded evidence base, we compared each of our intervention categories separately to control interventions. Where possible, we performed separate comparisons at end of treatment and post‐treatment follow‐up.
The review protocol described subgroup analyses on the basis of intervention characteristics (intensity, duration, and frequency), and characteristics of the population (dementia severity, type, and setting). We did not carry out these subgroup analyses due to limited data available and due to the heterogeneity of interventions investigated.
The review protocol describes separate analyses for dementia and MCI. Due to the small number of studies identified in people with MCI, we pooled studies in dementia and MCI together and conducted a subgroup analysis to examine the effect of type of cognitive diagnosis.
We undertook an additional subgroup analysis examining the effect of depression diagnosis at baseline. This was not planned or specified in the protocol, but was conducted to address heterogeneity between studies. In addition, we explored the influence of type of control intervention to examine the impact of active control conditions on effect sizes, which was also not specified in the protocol.
Contributions of authors
VO ‐ correspondence; drafting review versions; search for trials; selection of RCTs; extraction of data; entry of data; data analysis; interpretation of statistical analyses; updating review.
PL ‐ drafting review versions; search for trials; selection of RCTs; extraction of data; entry of data; data analysis; interpretation of statistical analyses; updating review.
RdPC ‐ drafting review versions; search for trials; selection of RCTs; extraction of data; entry of data; data analysis; interpretation of statistical analyses; updating review.
AQ ‐ selection of RCTs of first review; extraction of data of first review; entry of data of first review; data analysis of first review; interpretation of statistical analyses of first review; comments on final draft of previous version of the review.
AS ‐ comments on final draft of previous version of the review.
MO ‐ comments on final draft of previous and current version of the review.
AM ‐ drafting review versions; search for trials; selection of RCTs; extraction of data; entry of data; data analysis; interpretation of statistical analyses; updating review.
Sources of support
Internal sources
-
New Source of support, UK
We would like to thank the Cochrane Dementia and Cognitive Improvement Group for its support.
External sources
-
NIHR, UK
This review update was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health
Declarations of interest
VO and PL were principal investigators in one of the included studies, and were not involved in data extraction for this study. AS and MO were principal investigators in one of the included studies, and were not involved in data extraction for this study. There are no other known conflicts of interest.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Belleville 2018 {published data only}
- Belleville S, Hudon C, Bier N, Brodeur C, Gilbert B, Grenier S, et al.MEMO+: Efficacy, durability and effect of cognitive training and psychosocial intervention in individuals with mild cognitive impairment. Journal of the American Geriatrics Society 2018;66(4):655-63. [DOI] [PubMed] [Google Scholar]
Bruvik 2013 {published data only}
- Bruvik FK, Allore HG, Ranhoff AH, Engedal K.The effect of psychosocial support intervention on depression in patients with dementia and their family caregivers: an assessor-blinded randomized controlled trial. Dementia and Geriatric Cognitive Disorders 2013;26:386-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burgener 2008 {published data only}
- Burgener SC, Yang Y, Gilbert R, Marsh-Yant S.The effects of a multimodal intervention on outcomes of persons with early-stage dementia. American Journal of Alzheimer’s Disease and Other Dementias 2008;23:382-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burns 2005 {published data only}
- Burns A, Guthrie E, Marino-Francis F, Busby C, Morris J, Russell E, et al.Brief psychotherapy in Alzheimer's disease: randomised controlled trial. British Journal of Psychiatry 2005;187:143-7. [DOI] [PubMed] [Google Scholar]
Churcher Clarke 2017 {published data only}
- Churcher Clarke A, Chan JM, Stott J, Royan L, Spector A.An adapted mindfulness intervention for people with dementia in care homes: feasibility pilot study. International Journal of Geriatric Psychiatry 2017;32(12):e123-31. [DOI] [PubMed] [Google Scholar]
Jha 2013 {published data only}
- Jha A, Jan F, Gale T, Newman C.Effectiveness of a recovery-orientated psychiatric intervention package on the wellbeing of people with early dementia: a preliminary randomised controlled trial.. International Journal of Geriatric Psychiatry 2013;28(6):589-96. [DOI] [PubMed] [Google Scholar]
Kiosses 2010 {published data only}
- Kiosses DN, Arean PA, Teri L, Alexopoulos GS.Home-delivered problem adaptation therapy (PATH) for depressed, cognitively impaired, disabled elders: a preliminary study. American Journal of Geriatric Psychiatry 2010;18:988-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kiosses 2015 {published data only}
- Kiosses DN, Ravdin LD, Gross JJ, Raue P, Kotbi N, Alexopoulos GS.Problem adaptation therapy for older adults with major depression and cognitive impairment: a randomized clinical trial. JAMA Psychiatry 2015;72(1):22-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koivisto 2016 {published data only}
- Koivisto AM, Hallikainen I, Välimäki T, Hongisto K, Hiltunen A, Karppi P, et al.Early psychosocial intervention does not delay institutionalization in persons with mild Alzheimer disease and has impact on neither disease progression nor caregivers' well-being: ALSOVA 3-year follow-up. International Journal of Geriatric Psychiatry 2016;31(3):273-83. [DOI] [PubMed] [Google Scholar]
Kurz 2012 {published data only}
- Kurz A, Thone-Otto A, Cramer B, Egert S, Frolich L, Gertz HJ, et al.Cordial: cognitive rehabilitation and cognitive-behavioral treatment for early dementia in Alzheimer disease. Alzheimer Disease and Associated Disorders 2012;26:246-53. [DOI] [PubMed] [Google Scholar]
Laakkonen 2016 {published data only}
- Laakkonen ML, Kautiainen H, Hölttä E, Savikko N, Tilvis RS, Strandberg TE, et al.Effects of self-management groups for people with dementia and their spouses - randomized controlled trial. Journal of the American Geriatrics Society 2016;64:752-60. [DOI] [PubMed] [Google Scholar]
Lai 2020 {published data only}
- Lai FH, Yan EW, Tsui WS, Yu KK.A randomized control trial of activity scheduling for caring for older adults with dementia and its impact on their spouse care-givers. Archives of Gerontology and Geriatrics 2020;90:104167. [DOI] [PubMed] [Google Scholar]
Larouche 2019 {published data only}
- Larouche E, Hudon C, Goulet S.Mindfulness mechanisms and psychological effects for aMCI patients: a comparison with psychoeducation. Complementary Therapies in Clinical Practice 2019;34:93-104. [DOI] [PubMed] [Google Scholar]
Lichtenberg 2005 {published data only}
- Lichtenberg PA, Kemp-Havican J, MacNeill SE, Schafer Johnson A.Pilot study of behavioral treatment in dementia care units. Gerontologist 2005;45:406–10. [DOI] [PubMed] [Google Scholar]
Logsdon 2010 {published data only}
- Logsdon RG, Pike KC, McCurry SM, Hunter P, Maher J, Snyder L, et al.Early-stage memory loss support groups: outcomes from a randomized controlled clinical trial. Journal of Gerontology: Psychological Sciences 2010;65B:691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2016 {published data only}
- Lu YY, Ellis J, Yang Z, Weaver MT, Bakas T, Austrom MG, et al.Satisfaction with a family-focused intervention for mild cognitive impairment dyads. Journal of Nursing Scholarship 2016;48(4):334-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2015 {published data only}
- Marshall A, Spreadbury J, Cheston R, Coleman P, Ballinger C, Mullee M, et al.A pilot randomised controlled trial to compare changes in quality of life for participants with early diagnosis dementia who attend a 'Living Well with Dementia' group compared to waiting-list control. Aging & Mental Health 2015;19(6):526-35. [DOI] [PubMed] [Google Scholar]
Nordheim 2019 {published data only}
- Nordheim J, Häusler A, Yasar S, Suhr R, Kuhlmey A, Rapp M, et al.Psychosocial intervention in couples coping with dementia led by a psychotherapist and a social worker: the DYADEM trial. Journal of Alzheimer's Disease 2019;68:745-55. [DOI] [PubMed] [Google Scholar]
Orgeta 2019 {published data only}
- Orgeta V, Tuijt R, Leung P, Verdaguer ES, Gould RL, et al.Behavioral activation for promoting well-being in mild dementia: feasibility and outcomes of a pilot randomized controlled trial. Journal of Alzheimer's Disease 2019;72:563-74. [DOI] [PubMed] [Google Scholar]
Quinn 2016 {published data only}
- Quinn C, Toms G, Jones C, Brand A, Edwards RT, Sanders F, et al.A pilot randomized controlled trial of a self-management group intervention for people with early-stage dementia (the SMART study). International Psychogeriatrics / IPA 2016;28(5):787-800. [DOI] [PubMed] [Google Scholar]
Rovner 2018 {published data only}
- Rovner BW, Casten RJ, Hegel MT, Leiby BE.Preventing cognitive decline in black individuals with mild cognitive impairment: a randomized clinical trial. JAMA Neurology 2018;75:1487-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spector 2015 {published data only}
- Spector A, Charlesworth G, King M, Lattimer M, Sadek S, Marston L, et al.Cognitive-behavioural therapy for anxiety in dementia: pilot randomised controlled trial. British Journal of Psychiatry 2015;206(6):509-16. [DOI] [PubMed] [Google Scholar]
Stanley 2013 {published data only}
- Stanley MA, Calleo J, Bush AL, Wilson N, Snow AL, Kraus-Schuman C, et al.The Peaceful Mind Program: a pilot test of a cognitive–behavioral therapy–based intervention for anxious patients with dementia. American Journal of Geriatric Psychiatry 2013;21(7):696-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tappen 2009 {published data only}
- Tappen RM, Williams CL.Therapeutic conversation to improve mood in nursing home residents with Alzheimer’s disease. Research in Gerontological Nursing 2009;2:267-75. [DOI] [PubMed] [Google Scholar]
Teri 1997 {published data only}
- Teri L, Logsdon RG, Uomoto J, McCurry SM.Behavioral treatment of depression in dementia patients: a controlled clinical trial. Journals of Gerontology Series B: Psychological Sciences & Social Sciences 1997;52(4):P159-66. [DOI] [PubMed] [Google Scholar]
Travers 2017 {published data only}
- Travers C.Increasing enjoyable activities to treat depression in nursing home residents with dementia: a pilot study. Dementia (London, England) 2017;16(2):204-18. [DOI] [PubMed] [Google Scholar]
Waldorff 2012 {published data only}
- Waldorff FB, Buss DV, Eckermann A, Rasmussen ML, Keiding N, Rishøj S, et al.Efficacy of psychosocial intervention in patients with mild Alzheimer’s disease: the multicentre, rater blinded, randomised Danish Alzheimer Intervention Study (DAISY). BMJ 2012;345:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 2013 {published data only}
- Wells RE, Kerr CE, Wolkin J, Dossett M, Davis RB, Walsh J, et al.Meditation for adults with mild cognitive impairment: a pilot randomized trial. Journal of the American Geriatrics Society 2013;61:642-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2014 {published data only}
- Young DK, Kwok TC, Ng PY.A single blind randomized control trial on support groups for Chinese persons with mild dementia. Clinical Interventions in Aging 2014;9:2105-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abraham 1992 {published data only}
- Abraham IL, Neundorfer MM, Currie LJ.Effects of group interventions on cognition and depression in nursing home residents. Nursing Research 1992;41:196-202. [PubMed] [Google Scholar]
Almeida 2014 {published data only}
- Almeida OP, MacLeod C, Flicker L, Ford A, Grafton B, Etherton-Beer C.RAndomised controlled trial to imProve depressIon and the quality of life of people with Dementia using cognitive bias modification: RAPID study protocol. BMJ Open 2014;23:e005623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2018 {published data only}
- Anderson K, Wickramariyaratne T, Blair A.A feasibility study of group‐based cognitive behaviour therapy for older adults in residential care. Clinical Psychologist 2018;22:[no pagination]. [DOI: 10.1111/cp.12109] [DOI] [Google Scholar]
Auclair 2009 {published data only}
- Auclair U, Epstein C, Mittelman M.Couples counseling in Alzheimer’s disease: additional clinical findings from a novel intervention study. Clinical Gerontologist 2009;32:130-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bailey 2017 {published data only}
- Bailey EM, Stevens AB, LaRocca MA, Scogin F.A randomized controlled trial of a therapeutic intervention for nursing home residents with dementia and depressive symptoms. Journal of Applied Gerontology 2017;36(7):895-908. [DOI] [PubMed] [Google Scholar]
Beck 2002 {published data only}
- Beck C, Vogelpohl T, Rasin J, Topps J, O’Sullivan P, Walls R, et al.Effects of behavioral interventions on disruptive behavior and affect in demented nursing home residents. Nursing Research 2002;51(4):219-28. [DOI] [PubMed] [Google Scholar]
Berwig 2020 {published data only}
- Berwig M, Dinand C, Becker U, Halek M.Application of Marte Meo® counselling with people with behavioural variant frontotemporal dementia and their primary carers (AMEO-FTD) – a non-randomized mixed-method feasibility study. Pilot and Feasibility Studies 2020;32:1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brodaty 2003 {published data only}
- Brodaty H, Draper BM, Millar J, Low L, Lie D, Sharah S, et al.Randomized controlled trial of different models of care for nursing home residents with dementia complicated by depression or psychosis. Journal of Clinical Psychiatry 2003;64:63-72. [DOI] [PubMed] [Google Scholar]
Chandler 2019 {published data only}
- Chandler MJ, Locke DE, Crook JE, Fields JA, Ball CT, Phatak VS, et al.Comparative effectiveness of behavioral interventions on quality of life for older adults with mild cognitive impairment: a randomized clinical trial. JAMA Network Open 2019;3(2):e193016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheston 2003 {published data only}
Cheston 2009 {published data only}
- Cheston R, Jones R.A small-scale study comparing the impact of psycho-education and exploratory psychotherapy groups on newcomers to a group for people with dementia. Aging & Mental Health 2009;13(3):420-5. [DOI] [PubMed] [Google Scholar]
Collins 2018 {published data only}
- Collins RN, Gilligan LJ, Poz R.The evaluation of a compassion-focused therapy group for couples experiencing a dementia diagnosis. Clinical Gerontologist 2018;41:474-86. [DOI] [PubMed] [Google Scholar]
Craig 2018 {published data only}
- Craig C, Hiskey S, Royan L, Poz R, Spector A.Compassion focused therapy for people with dementia: a feasibility study. International Journal of Geriatric Psychiatry 2018;33:1727-35. [DOI] [PubMed] [Google Scholar]
Davison 2017 {published data only}
- Davison TE, Eppingstall B, Runci S, O'Connor DW.A pilot trial of acceptance and commitment therapy for symptoms of depression and anxiety in older adults residing in long-term care facilities. Aging & Mental Health 2017;21(7):766-73. [DOI] [PubMed] [Google Scholar]
Dozeman 2011 {published data only}
- Dozeman E, Van Schaik DJ, Van Marwijk HW, Stek ML, Beekman AT, Van der Horst HE.Feasibility and effectiveness of activity-scheduling as a guided self-help intervention for the prevention of depression and anxiety in residents in homes for the elderly: a pragmatic randomized controlled trial. International Psychogeriatrics / IPA 2011;23(6):969-78. [DOI] [PubMed] [Google Scholar]
Farrand 2016 {published data only}
- Farrand P, Woodford J, Llewellyn D, Anderson M, Venkatasubramanian S, Ukoumunne OC, et al.Behavioural activation written self-help to improve mood, wellbeing and quality of life in people with dementia supported by informal carers (PROMOTE): a study protocol for a single-arm feasibility study. Pilot and Feasibility Studies 2016;2(42):[13 p.]. [DOI: 10.1186/s40814-016-0083-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Finnema 2005 {published data only}
- Finnema E, Droës R, Ettema T, Ooms M, Adèr H, Miel Ribbe M, et al.The effect of integrated emotion-oriented care versus usual care on elderly persons with dementia in the nursing home and on nursing assistants: a randomised clinical trial. International Journal of Geriatric Psychiatry 2005;20:330-43. [DOI] [PubMed] [Google Scholar]
Fischer‐Terworth 2011 {published data only}
- Fischer-Terworth C, Probst P.Evaluation of a TEACCH- and music therapy-based psychological intervention in mild to moderate dementia: a controlled trial. Geropsychology 2011;24:93-101. [Google Scholar]
García‐Alberca 2017 {published data only}
- García-Alberca JM.Cognitive-behavioral treatment for depressed patients with Alzheimer's disease. An open trial. Archives of Gerontology and Geriatrics 2017;71:1-8. [DOI] [PubMed] [Google Scholar]
Helcer 2012 {published data only}
- Helcer J, Santorelli G, Choi J.Cognitive behavioral therapy to combat hopelessness and low self efficacy in Alzheimer's disease. Alzheimer's & Dementia 2012;8(4 Suppl 1):P376. [Google Scholar]
Hummel 2017 {published data only}
- Hummel J, Weisbrod C, Boesch L, Himpler K, Hauer K, Hautzinger M, et al.AIDE-Acute Illness and Depression in Elderly Patients. Cognitive behavioral group psychotherapy in geriatric patients with comorbid depression: a randomized, controlled trial. Journal of the American Medical Directors Association 2017;18(4):341-9. [DOI] [PubMed] [Google Scholar]
Hyer 2009 {published data only}
- Hyer L, Yeager CA, Hilton N, Sacks A.Group, individual, and staff therapy: an efficient and effective cognitive behavioral therapy in long-term care. American Journal of Alzheimer’s Disease and Other Dementias 2008;23:528-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ikemata 2017 {published data only}
- Ikemata S, Momose Y.Effects of a progressive muscle relaxation intervention on dementia symptoms, activities of daily living, and immune function in group home residents with dementia in Japan. Japan Journal of Nursing Science 2017;14:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joosten‐Weyn Banningh 2011 {published data only}
- Joosten-Weyn Banningh LW, Prins JB, Vernooij-Dassen MJ, Wijnen HH, Olde Rikkert MG, Kessels RP.Group therapy for patients with mild cognitive impairment and their significant others: results of a waiting-list controlled trial. Gerontology 2011;57:444-54. [DOI] [PubMed] [Google Scholar]
Kashimura 2020 {unpublished data only}
- Kashimura M, Nomura T, Ishiwata A, Tateno A, Nogami A, Kitamura S, et al.Feasibility and acceptability of cognitive-behavioral therapy in older Japanese people with dementia: a single-arm intervention. Data on file.
Klainin‐Yobas 2019 {published data only}
- Klainin-Yobas P, Kowitlawakul Y, Lopez V, Tang CT, Hoek KE, Gan GL, et al.The effects of mindfulness and health education programs on the emotional state and cognitive function of elderly individuals with mild cognitive impairment: a randomized controlled trial. Journal of Clinical Neuroscience 2019;68:211-7. [DOI] [PubMed] [Google Scholar]
Kolanowski 2011 {published data only}
- Kolanowski A, Litaker M, Buettner L, Moeller J, Costa PT.A randomized clinical trial of theory-based activities for the behavioral symptoms of dementia in nursing home residents. American Journal of Geriatric Psychiatry 2011;59:1032-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Konnert 2009 {published data only}
- Konnert C, Dobson K, Stelmach L.The prevention of depression in nursing home residents: a randomised clinical trial of cognitive–behavioral therapy. Aging and Mental Health 2009;13:288-99. [DOI] [PubMed] [Google Scholar]
Kovach 2018 {published data only}
- Kovach CR, Evans CR, Sattell L, Rosenau K, Gopalakrishnan S.Feasibility and pilot testing of a mindfulness intervention for frail older adults and individuals with dementia. Research in Gerontological Nursing 2018;11(3):137-50. [DOI] [PubMed] [Google Scholar]
Kulshreshtha 2019 {published data only}
- Kulshreshtha A, Alonso A, Owen A, Goldstein FC, Hajjar I.Cognitive behavioral intervention focused on lifestyle improvement for African Americans with mild cognitive impairment: a pilot study. Alzheimer's & Dementia 2019;15(7S Part 9):P503. [Google Scholar]
Lam 2010 {published data only}
- Lam LC, Lui VW, Luk DN, Chau R, So C, Poon V, et al.Effectiveness of an individualized functional training program on affective disturbances and functional skills in mild and moderate dementia—a randomised control trial. International Journal of Geriatric Psychiatry 2010;25:133–41. [DOI] [PubMed] [Google Scholar]
Lantz 1997 {published data only}
- Lantz MS, Buchalter EN, McBee L.The Wellness Group: a novel intervention for coping with disruptive behavior among elderly nursing home residents. Gerontologist 1997;37(4):551-6. [DOI] [PubMed] [Google Scholar]
Leontjevas 2013 {published data only}
- Leontjevas R, Gerritsen DL, Smalbrugge M, Teerenstra S, Vernooij-Dassen MJ, Koopmans RT.A structural multidisciplinary approach to depression management in nursing-home residents: a multicentre, stepped-wedge cluster-randomised trial. Lancet 2013;381:2255-64. [DOI] [PubMed] [Google Scholar]
Luo 2020 {published data only}
- Luo H, Lou VW, Chen C, Chi I.The effectiveness of the positive mood and active life program on reducing depressive symptoms in long-term care facilities. Gerontologist 2020;60:193-204. [DOI] [PubMed] [Google Scholar]
Marciniak 2020 {published data only}
- Marciniak R, Šumec R, Vyhnálek M, Bendíčková K, Lázničková P, Forte G, et al.The effect of Mindfulness-Based Stress Reduction (MBSR) on depression, cognition, and immunity in mild cognitive impairment: a pilot feasibility study. Clinical Interventions in Aging 2020;15:1365–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
McSweeney 2012 {published data only}
- McSweeney K, Jeffreys A, Griffith J, Plakiotis C, Kharsas R, O’Connor DW.Specialist mental health consultation for depression in Australian aged care residents with dementia: a cluster randomized trial. International Journal of Geriatric Psychiatry 2012;27:1163-71. [DOI] [PubMed] [Google Scholar]
Meeks 2015 {published data only}
- Meeks S, Van Haitsma K, Schoenbachler B, Looney SW.BE-ACTIV for depression in nursing homes: primary outcomes of a randomized clinical trial. Journals of Gerontology Series B: Psychological Sciences & Social Sciences 2015;70(1):13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02083237 {unpublished data only}
- NCT02083237.Evaluation of an Intervention for Living With Mild Cognitive Impairment. clinicaltrials.gov/ct2/show/NCT02083237 (first received 11 March 2014).
NCT04426838 {published data only}
- NCT04426838.Cognitive behavioral therapy for insomnia for the dementia caregiving dyad. clinicaltrials.gov/ct2/show/NCT04426838 (first received 11 June 2020).
Novelli 2018 {published data only}
- Novelli MM, Machado SC, Lima GB, Cantatore L, Sena BP, Rodrigues RS, et al.Effects of the Tailored Activity Program in Brazil (TAP-BR) for persons with dementia: a randomized pilot trial. Alzheimer Disease & Associated Disorders 2018;32:339-45. [DOI] [PubMed] [Google Scholar]
O'Connor 2014 {published data only}
- O'Connor CM, Clemson L, Brodaty H, Jeon YH, Mioshi E, Gitlin LN.Use of the Tailored Activities Program to reduce neuropsychiatric behaviors in dementia: an Australian protocol for a randomized trial to evaluate its effectiveness. International Psychogeriatrics / IPA 2014;26(5):857-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paller 2015 {published data only}
- Paller KA, Creery JD, Florczak SM, Weintraub S, Mesulam MM, Reber PJ, et al.Benefits of mindfulness training for patients with progressive cognitive decline and their caregivers. American Journal of Alzheimer's Disease and Other Dementias 2015;30(3):257-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prakash 2019 {published data only}
- Prakash P, Nappinai S, Navaneetha, Malarvizhi, Pradeep T.The effectiveness of TIPER (Tailor Made Interventional Package to Enhance Relaxation) on depression, anxiety and stress among elders at old age homes, Puducherry. International Journal of Research in Pharmaceutical Sciences 2019;10(2):1124-6. [Google Scholar]
Prick 2015 {published data only}
- Prick AE, De Lange J, Twisk J, Pot AM.The effects of a multi-component dyadic intervention on the psychological distress of family caregivers providing care to people with dementia: a randomized controlled trial. International Psychogeriatrics / IPA 2015;27(12):2031-44. [DOI] [PubMed] [Google Scholar]
Quintana‐Hernández 2016 {published data only}
- Quintana-Hernández DJ, Miró-Barrachina MT, Ibáñez-Fernández IJ, Pino AS, Quintana-Montesdeoca MP, Rodríguez-de Vera B, et al.Mindfulness in the maintenance of cognitive capacities in Alzheimer's disease: a randomized clinical trial. Journal of Alzheimer's Disease 2016;50:217-32. [DOI] [PubMed] [Google Scholar]
Reinhardt 2014 {published data only}
- Reinhardt JP, Horowitz A, Cimarolli VR, Eimicke JP, Teresi JA.Addressing depression in a long-term care setting: a phase II pilot of problem-solving treatment. Clinical Therapeutics 2014;36(11):1531-7. [DOI] [PubMed] [Google Scholar]
Rodriguez‐Sánchez 2018 {published data only}
- Rodriguez-Sánchez E, Tamayo-Morales O, González-Sanchez J, Mora-Simón S, Losada-Baltar A, Unzueta-Arce J, et al.Behavioural intervention to reduce resistance in those attending adult day care centres: PROCENDIAS study protocol for a randomized clinical trial. Journal of Advanced Nursing 2018;74:1402-11. [DOI] [PubMed] [Google Scholar]
Teri 2003 {published data only}
- Terri L, Gibbons, LE, McCurry SM, Logsdon RG, Buchner DM, Barlow WE, et al.Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA 2003;290:2015-22. [DOI] [PubMed] [Google Scholar]
Teri 2020 {published data only}
- Teri L, Logsdon RG, McCurry SM, Pike KC, McGough EL.Translating an evidence-based multi-component intervention for older adults with dementia and caregivers. Gerontologist 2020;60:548-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Haitsma 2015 {published data only}
- Van Haitsma KS, Curyto K, Abbott KM, Towsley GL, Spector A, Kleban M.A randomized controlled trial for an individualized positive psychosocial intervention for the affective and behavioral symptoms of dementia in nursing home residents. Journals of Gerontology Series B: Psychological Sciences & Social Sciences 2015;70(1):35-45. [DOI] [PubMed] [Google Scholar]
Vanova 2018 {published data only}
- Vanova M, Irazoki E, García-Casal JA, Martínez-Abad F, Botella C, Shiells KR, et al.The effectiveness of ICT-based neurocognitive and psychosocial rehabilitation programmes in people with mild dementia and mild cognitive impairment using GRADIOR and ehcoBUTLER: study protocol for a randomised controlled trial. Trials 2018;19(100):[15 p.]. [DOI: 10.1186/s13063-017-2371-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitlatch 2017 {published data only}
- Whitlatch CJ, Heid AR, Femia EE, Orsulic-Jeras S, Szabo S, Zarit SH.The Support, Health, Activities, Resources, and Education program for early stage dementia: results from a randomized controlled trial. Dementia (London, England) 2017 Nov 24 [Epub ahead of print]. [DOI: 10.1177/1471301217743033] [DOI] [PubMed]
Yesavage 1981 {published data only}
- Yesavage JA, Westphal J, Rush L.Senile dementia: combined pharmacologic and psychologic treatment. Journal of the American Geriatrics Society 1981;29:164-71. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Carreel 1990 {published data only}
- Carreel C.Value of conversation groups in institutions for the elderly. Revue de Laryngologie 1990;111:319-23. [PubMed] [Google Scholar]
ISRCTN42983681 {published data only}
- ISRCTN42983681.Psychotherapy in mild cognitive impairment. isrctn.com/ISRCTN42983681 (first received 20 December 2006).
NCT01550718 {published data only}
- NCT01550718.Two interventions for early stage dementia: a comparative efficacy trial. clinicaltrials.gov/ct2/show/NCT01550718 (first received 12 March 2012).
Wong 2016 {published data only}
- Wong WP, Hassed C, Chambers R, Coles J.The effects of mindfulness on persons with mild cognitive impairment: protocol for a mixed-methods longitudinal study. Frontiers in Aging Neuroscience 2016;8:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Aguirre 2017 {published data only}
- Aguirre E, Stott J, Charlesworth G, Noone D, Payne J, Patel M, et al.Mindfulness-Based Cognitive Therapy (MBCT) programme for depression in people with early stages of dementia: study protocol for a randomised controlled feasibility study. Pilot and Feasibility Studies 2017;3(28):[7 p.]. [DOI: 10.1186/s40814-017-0143-x ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ekman 2020 {published data only}
- Ekman U, Kemani MK, Wallert J, Wicksell RK, Holmström L, Ngandu T, et al.Evaluation of a novel psychological intervention tailored for patients with early cognitive Impairment (PIPCI): study protocol of a randomized controlled trial. Frontiers in Psychology 2020;11:600841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forstmeier 2015 {published data only}
- Forstmeier S, Maercker A, Savaskan E, Roth T.Cognitive Behavioural Treatment for Mild Alzheimer's Patients and Their Caregivers (CBTAC): study protocol for a randomised controlled trial. Trials 2015;16(1):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gildengers 2016 {published data only}
- Gildengers AG, Butters MA, Albert SM, Anderson SJ, Dew MA, Erickson K, et al.The design and implementation of an intervention development study: Retaining Cognition while Avoiding Late-Life Depression (ReCALL). American Journal of Geriatric Psychiatry 2016;24(6):444-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN11185706 {unpublished data only}
- ISRCTN11185706.Problem adaptation therapy for depression in dementia (PATHFINDER) [Problem adaptation therapy for individuals with mild to moderate dementia and depression: the PATHFINDER trial]. isrctn.com/ISRCTN11185706 (first received 31 May 2018).
Janssen 2017 {published data only}
- Janssen N, Huibers MJ, Lucassen P, Voshaar RO, Van Marwijk H, Bosmans J, et al.Behavioural activation by mental health nurses for late-life depression in primary care: a randomized controlled trial. BMC Psychiatry 2017;17(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01774448 {published data only}
- NCT01774448.Mindfulness research program: designed to enhance wellbeing in people living with dementia and their spouses (MBCT_AD) [The efficacy of mindfulness-based cognitive therapy (MBCT) to improve depression symptoms and quality of life in individuals with dementia and their caregivers: a pilot study]. clinicaltrials.gov/ct2/show/NCT01774448 (first received 24 January 2013).
NCT02013518 {published data only}
- NCT02013518.Cognitive behavioural therapy for persons with mild cognitive impairment or dementia in the early stages. clinicaltrials.gov/ct2/show/NCT02013518 (first received 17 December 2013).
NCT02777905 {published data only}
- NCT02777905.Mindfulness-based cognitive therapy for depression and anxiety [Group mindfulness-based cognitive therapy for the treatment of late-life depression and anxiety symptoms in primary care: a randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT02777905 (first received 19 May 2016).
NCT03656159 {published data only}
- NCT03656159.Evaluation of a new CBT for people with Alzheimer's disease and their caregivers [Evaluation of a new cognitive behavioural therapy (CBT) to reduce psychological distress and improve quality of life of people with Alzheimer's disease and their caregivers]. clinicaltrials.gov/ct2/show/NCT03656159 (first received 4 September 2018).
NCT03692988 {published data only}
- NCT03692988.Dignity therapy for patients with early dementia and their family (DTD) [Dignity therapy: a brief psychological and existential intervention for patients with early dementia and their family: a randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT03692988 (first received 2 October 2018).
Rodakowski 2019 {published data only}
- Rodakowski J, Golias KW, Reynolds CF, Butters MA, Lopez OL, et al.Preventing disability in older adults with mild cognitive impairment: a Strategy Training intervention study. Contemporary Clinical Trials Communications 2019;15:[7 p.]. [DOI: 10.1016/j.conctc.2019.100368] [DOI] [PMC free article] [PubMed] [Google Scholar]
Teti 2018 {unpublished data only}
- Teti AM, Fiszdon M, Taylor B, Twamley EW, Pearlson GD, Choi J.Cognitive behavioral therapy to enhance memory training efficacy in people with dementia: 1-year follow-up. Alzheimer's and Dementia 2018;14(7):P285. [Google Scholar]
Tran 2020 {published data only}
- Tran T, Donnelly C, Nalder EJ, Trothen T, Finlayson M.Occupational therapist-led mindfulness-based stress reduction for older adults living with subjective cognitive decline or mild cognitive impairment in primary care: a feasibility randomised control trial protocol. BMJ Open 2020;10:e035299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Albert 2011
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman F, Fox NC, et al.The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia 2011;7:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alexopoulos 1988
- Alexopoulos GS, Abrams RC, Young RC.Cornell Scale for Depression in Dementia. Biological Psychiatry 1988;23:271-84. [DOI] [PubMed] [Google Scholar]
Alexopoulos 2005
- Alexopoulos GS, Jeste DV, Chung H, Carpenter D, Ross R, Docherty JP.The expert consensus guideline series. Treatment of dementia and its behavioral disturbances. Introduction: methods, commentary, and summary. Postgraduate Medicine 2005;Spec No:6-22. [PubMed] [Google Scholar]
Antonovsky 1987
- Antonovsky A.Unravelling the mystery of health. How people manage stress and stay well. San Francisco (CA): Jossey-Bass, 1987. [Google Scholar]
Army Individual Test Battery 1944
- Army Individual Test Battery.Manual of Directions and Scoring. Washington (DC): War Department, Adjutant General’s Office, 1944. [Google Scholar]
Aschenbrenner 2000
- Aschenbrenner S, Tucha O, Lange KW.Der Regensburger Wortflüssigkeitstest [Regensburg Word Fluency Test]. German edition. Göttingen, Germany: Hogrefe Verlag, 2000. [Google Scholar]
Asmer 2018
- Selim Asmer M, Kirkham J, Newton H, Ismail Z, Elbayoumi H, Leung RH, et al.Meta-analysis of the prevalence of major depressive disorder among older adults with dementia. Journal of Clinical Psyciatry 2018;79:17r11772. [DOI] [PubMed] [Google Scholar]
Azermai 2012
- Azermai M, Petrovic M, Elseviers MM, Bourgeois J, Van Bortel LM, Vander Stichele RH.Systematic appraisal of dementia guidelines for the management of behavioural and psychological symptoms. Ageing Research Reviews 2012;11:78-86. [DOI] [PubMed] [Google Scholar]
Baer 2006
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L.Using self-report assessment methods to explore facets of mindfulness. Assessment 2006;13:27-45. [DOI] [PubMed] [Google Scholar]
Bakas 2006
- Bakas T, Champion V, Perkins SM, Farran CJ, Williams LS.Psychometric testing of the revised 15-item Bakas Caregiving Outcomes Scale. Nursing Research 2006;55:346–55. [DOI] [PubMed] [Google Scholar]
Banerjee 2011
- Banerjee S, Hellier J, Dewey M, Romeo R, Ballard C, Baldwin R, et al.Sertraline or mirtazapine for depression in dementia (HTA-SADD): a randomised, multicentre, double-blind, placebo-controlled trial. Lancet 2011;378:403-11. [DOI] [PubMed] [Google Scholar]
Barrett 2006
- Barrett AJ, Murk PJ.Life Satisfaction Index for the Third Age-Short Form (LSITA_SF): An Improved and Briefer Measure of Successful Aging. St. Louis (MO): University of Missouri - St. Louis, 2006. [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M.An inventory for measuring depression. Archives of General Psychiatry 1962;4:561-71. [DOI] [PubMed] [Google Scholar]
Beck 1979
- Beck AT, Rush AJ, Shaw BF, Emery G.Cognitive Therapy of Depression. New York (NY): Guilford Press, 1979. [Google Scholar]
Belleville 2006
- Belleville S, Gilbert B, Fontaine F, Gagnon L, Ménard E, Gauthier S.Improvement of episodic memory in persons with mild cognitive impairment and healthy older adults: evidence from a cognitive intervention program. Dementia and Geriatric Cognitive Disorders 2006;22:486–99. [DOI] [PubMed] [Google Scholar]
Benedict 1998
- Benedict RH, Brandt J.Manual: Hopkins Verbal Learning Test - Revised/Brief Visuo-spatial Memory Test - Revised. Lutz (FL): Psychological Assessment Resources, 1998. [Google Scholar]
Benton 1994
- Benton AL, Hamsher K, Rey GL, Sivan AB.Multilingual Aphasia Examination. 3rd edition. Iowa City (IA): AJA Associates, 1994. [Google Scholar]
Berg 1989
- Berg K, Wood-Dauphinee S, William JI, Gayton D.Measuring balance in the elderly: preliminary development of an instrument. Physiotherapy Canada. Physiotherapie Canada 1989;41:304-11. [Google Scholar]
Böcker 2007
- Böcker M, Eberle N, Gauggel S.Aachen Functioning Item Bank. University Hospital of RWTH Aachen, Germany: Institute for Medical Psychology and Medical Sociology, 2007. [Google Scholar]
Brodaty 2012
- Brodaty H, Arasaratnam C.Meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. American Journal of Psychiatry 2012;169:946-53. [DOI] [PubMed] [Google Scholar]
Brown 2003
- Brown KW, Ryan RM.The benefits of being present: mindfulness and its role in psychological well-being. Journal of Personality and Social Psychology 2003;84:822-48. [DOI] [PubMed] [Google Scholar]
Bucks 1996
- Bucks RS, Ashworth DL, Wilcock GK.Assessment of activities of daily living in dementia: development of the Bristol Activities of Daily Living Scale. Age and Ageing 1996;25:113-20. [DOI] [PubMed] [Google Scholar]
Cain 2002
- Cain DJ, Seeman J.Humanistic Psychotherapies: Handbook of Research and Practice. Washington (DC): American Psycholoigcal Association, 2002. [Google Scholar]
Carrion 2018
- Carrion C, Folkvord F, Anastasiadou D, Aymerich M.Cognitive therapy for dementia patients: a systematic review. Dementia and Geriatric Cognitive Disorders 2018;46:1-26. [DOI] [PubMed] [Google Scholar]
Carver 1989
- Carver CS.You want to measure coping but your protocol’s too long: consider the brief COPE. International Journal of Behavioral Medicine 1997;4:92–100. [DOI] [PubMed] [Google Scholar]
Cepoiu‐Martin 2016
- Cepoiu-Martin M, Tam-Tham H, Patten S, Maxwell CJ, Hogan DB.Predictors of long-term care placement in persons with dementia: a systematic review and meta-analysis. International Journal of Geriatric Psychiatry 2016;31:1151-71. [DOI] [PubMed] [Google Scholar]
Chandler 2005
- Chandler MJ, Lacritz LH, Hynan LS, Barnard HD, Allen G, Deschner M, et al.A total score for the CERAD neuropsychological battery. Neurology 2005;65:102–6. [DOI] [PubMed] [Google Scholar]
Chen 2018
- Chen C, Hu Z, Jiang Z, Zhou F.Prevalence of anxiety in patients with mild cognitive impairment: a systematic review and meta-analysis. Journal of Affective Disorders 2018;236:211-21. [DOI] [PubMed] [Google Scholar]
Cheng 2017
- Cheng S.Dementia caregiver burden: a research update and critical analysis. Current Psychiatry Reports 2017;19:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Churchill 2001
- Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, et al.A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression. Health and Technology Assessment 2001;5:1-173. [DOI] [PubMed] [Google Scholar]
Churchill 2018
- Churchill R, Davies P, Caldwell DM, Moore TH, Jones HF, Lewis G.Interpersonal, cognitive analytic and other integrative therapies versus treatment as usual for depression. Cochrane Database of Systematic Reviews 2018, Issue 10. Art. No: CD008703. [DOI: 10.1002/14651858.CD008703.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coast 2008
- Coast J, Flynn TN, Natarajan L, Sproston K, Lewis J, Louviere JL, et al.Valuing the ICECAP capability index for older people. Social Science & Medicine 2008;67:874–82. [DOI] [PubMed] [Google Scholar]
Coblentz 1973
- Coblentz JM, Mattis S, Zingesser LH, Kasoff SS, Wisniewski HM, Katzman R.Presenile dementia: clinical evaluation of cerebrospinal fluid dynamics. Archives of Neurology 1973;29:299-308. [DOI] [PubMed] [Google Scholar]
Cohen 1983
- Cohen S, Kamarck T, Mermelstein R.A global measure of perceived stress. Journal of Health and Social Behavior 1983;24:385–96. [PubMed] [Google Scholar]
Cooper 2015
- Cooper C, Sommerlad A, Lyketsos CG, Livingston G.Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. American Journal of Psychiatry 2015;172:323-34. [DOI] [PubMed] [Google Scholar]
Crittendon 2006
- Crittendon J, Hopko DR.Assessing worry in older and younger adults: psychometric properties of an Abbreviated Penn State Worry Questionnaire (PSWQ-A). Journal of Anxiety Disorders 2006;20:1036–54. [DOI] [PubMed] [Google Scholar]
Cummings 1994
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J.The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308-14. [DOI] [PubMed] [Google Scholar]
D'Zurilla 2010
- D'Zurilla TJ, Nezu AM.Problem-solving therapy. In: Dobson KS, editors(s). Handbook of Cognitive-Behavioral Therapies. 3rd edition. New York (NY): Guilford Press, 2010:197-225. [Google Scholar]
Dennis 2003
- Dennis CL.Peer support within a health care context: a concept analysis. International Journal of Nursing Studies 2003;40:321-32. [DOI] [PubMed] [Google Scholar]
Devanand 2003
- Devanand DP, Pelton GH, Marston K.Sertraline treatment of elderly patients with depression and cognitive impairment. International Journal of Geriatric Psychiatry 2003;18:123–30. [DOI] [PubMed] [Google Scholar]
Dimidjian 2006
- Dimidjian S, Hollon SD, Dobson KS, Schmaling K, Kohlenberg RJ, Addis ME, et al.Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. Journal of Consulting and Clinical Psychology 2006;74:658-70. [DOI] [PubMed] [Google Scholar]
Dotson 2008
- Dotson VM, Schinka JA, Brown LM, Mortimer JA, Borenstein AR.Characteristics of the Florida cognitive activities scale in older African Americans. Assessment 2008;15:72-7. [DOI] [PubMed] [Google Scholar]
Dudas 2018
- Dudas R, Malouf R, McCleery J, Dening T.Antidepressants for treating depression in dementia. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD003944. [DOI: 10.1002/14651858.CD003944.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dupuy 1977
- Dupuy HJ.The general well-being schedule. In: McDowell I, Newall C, editors(s). Measuring Health: A Guide to Rating Scales and Questionnaires. 2nd edition. New York (NY): Oxford University Press, 1977:206–13. [Google Scholar]
Edelman 2005
- Edelman P, Fulton BR, Kuhn D, Chang CH.A comparison of three methods of measuring dementia specific quality of life: perspectives of residents, staff and observers. Gerontologist 2005;45(1):27–36. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C.Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Enache 2011
- Enache D, Winblad B, Aarsland D.Depression in dementia: epidemiology, mechanisms, and treatment. Current Opinion in Psychiatry 2011;24:461-72. [DOI] [PubMed] [Google Scholar]
EuroQoL 1990
- EuroQoL.EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 1990;1:199-208. [DOI] [PubMed] [Google Scholar]
Evans 2002
- Evans C, Connell J, Barkham M, Margison F, McGrath G, Mellor-Clark J, et al.Towards a standardised brief outcome measure: psychometric properties and utility of the CORE–OM. British Journal of Psychiatry 2002;180:51–60. [DOI] [PubMed] [Google Scholar]
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR.‘‘Mini-Mental State’’: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189-98. [DOI] [PubMed] [Google Scholar]
Fydrich 2009
- Fydrich T, Sommer G, Tydecks S, Brähler E.Social Support Questionnaire (F-SozU): Standardization of short form (K-14). Zeitschrift für Medizinische Psychologie 2009;18:43-48. [Google Scholar]
Galasko 1997
- Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M.An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Disease and Associated Disorders 1997;11:33-9. [PubMed] [Google Scholar]
Galasko 2006
- Galasko D, Bennett DA, Sano M.ADCS Prevention Instrument Project: assessment of instrumental activities of daily living for community dwelling elderly individuals in dementia prevention clinical trials. Alzheimer Disease & Associated Disorders 2006;20:152–69. [DOI] [PubMed] [Google Scholar]
Gellis 2009
- Gellis ZD, McClive-Reed KP, Brown EL.Treatments for depression in older persons with dementia. Clinical Care and Aging 2009;17:29-36. [PMC free article] [PubMed] [Google Scholar]
Gitlin 2003
- Gitlin LN, Winter L, Corcoran M, Dennis MP, Schinfeld S, Hauck WW.Effects of the home environmental skill-building program on the caregiver-care recipient dyad: 6-month outcomes from the Philadelphia REACH Initiative. Gerontologist 2003;43:532–46. [DOI] [PubMed] [Google Scholar]
Goldberg 1985
- Goldberg DP, Williams P.A User’s Guide to the General Health Questionnaire. Windsor, UK: NFER-Nelson, 1985. [Google Scholar]
Gomar 2011
- Gomar JJ, Harvey PD, Bobes-Bascaran MT, Davies P, Goldberg TE.Development and cross-validation of the UPSA short form for the performance-based functional assessment of patients with mild cognitive impairment and Alzheimer disease. American Journal of Geriatric Psychiatry 2011;19:915-22. [DOI] [PubMed] [Google Scholar]
Grant 1995
- Grant RW, Casey DA.Adapting cognitive behavioural therapy for the frail elderly. International Psychogeriatrics 1995;7(4):561-71. [DOI] [PubMed] [Google Scholar]
Grundman 2004
- Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, et al.Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Archives of Neurology 2004;61:59-66. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W.Clinical global impressions. In: ECDEU Assessment Manual for Psychopharmacology. Rockville (MD): National Institute of Mental Health, 1976. [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al.GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI] [PubMed] [Google Scholar]
Hall 2016
- Hall J, Kellett S, Berrios R, Bains MK, Scott S.Efficacy of cognitive behavioral therapy for generalized anxiety disorder in older adults: systematic review, meta-analysis, and meta-regression. American Journal of Geriatric Psychiatry 2016;24:1063-73. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M.A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hays 2001
- Hays RD, Morales LS.The RAND-36 measure of health-related quality of life. Annals of Medicine 2001;33:350–7. [DOI] [PubMed] [Google Scholar]
Herth 1992
- Herth K.Abbreviated instrument to measure hope: development and psychometric evaluation. Journal of Advanced Nursing 1992;17:1251–9. [DOI] [PubMed] [Google Scholar]
Heun 1999
- Heun R, Burkart M, Maier W, Bech P.Internal and external validity of the WHO Well-Being Scale in the elderly general population. Acta Psychiatrica Scandinavica 1999;99:171-8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al.The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hindmarch 1998
- Hindmarch I, Lehfeld H, De Jongh P, Erzigkeit H.The Bayer Activities of Daily Living Scale (B-ADL). Dementia and Geriatric Cognitive Disorders 1998;9:20–6. [DOI] [PubMed] [Google Scholar]
Hofmann 2010
- Hofmann SG, Sawyer AT, Fang A.The empirical status of the "new wave" of cognitive behavioral therapy. Psychiatric Clinics of North America 2010;33:701‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hogan 2008
- Hogan DB, Bailey P, Black S, Carswell A, Chertkow H, Clarke B, et al.Diagnosis and treatment of dementia: nonpharmacologic and pharmacologic therapy for mild to moderate dementia. Canadian Medical Association Journal 2008;179(10):1019-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 1982
- Hughes C, Berg I, Danzinger W, Coben L, Martin R.A new clinical scale for the staging of dementia. British Journal of Psychiatry 1982;140:566-72. [DOI] [PubMed] [Google Scholar]
Hwang 2004
- Hwang TJ, Masterman DL, Ortiz F, Fairbanks LA, Cummings JL.Mild cognitive impairment is associated with characteristic neuropsychiatric symptoms. Alzheimer Disease and Associated Disorders 2004;18(1):17-21. [DOI] [PubMed] [Google Scholar]
Ismail 2017
- Ismail Z, Elbayoumi H, Fischer CE, Hogan DB, Millikin CP, Schweizer T, et al.Prevalence of depression in patients with mild cognitive impairment: a systematic review and meta-analysis. JAMA Psychiatry 2017;74:58-67. [DOI] [PubMed] [Google Scholar]
Jacobson 2001
- Jacobson NS, Martell CR, Dimidjian S.Behavioral activation treatment for depression: returning to contextual roots. Clinical Psychology: Science and Practice 2001;8:255-70. [Google Scholar]
Jonsson 2016
- Jonsson U, Bertilsson G, Allard P, Gyllensvärd H, Söderlund A, Tham A, et al.Psychological treatment of depression in people aged 65 years and over: a systematic review of efficacy, safety, and cost-effectiveness. PLoS One 2016;11:e0160859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaplan 1978
- Kaplan EF, Goodglass H, Weintraub S.The Boston Naming Test. Philadelphia (PA): Lea & Febiger, 1978. [Google Scholar]
Keady 1995
- Keady J, Nolan M.IMMEL: assessing coping responses in the early stages of dementia. British Journal of Nursing 1995;4:309-14. [DOI] [PubMed] [Google Scholar]
Keihofner 2002
- Kielhofner G.The model of human occupation: theory and application. Baltimore (MD): Lippincott Williams & Wilkins, 2002. [Google Scholar]
Kirkham 2016
- Kirkham JG, Choi N, Seitz DP.Meta-analysis of problem solving therapy for the treatment of major depressive disorder in older adults. International Journal of Geriatric Psychiatry 2016;31:526-35. [DOI] [PubMed] [Google Scholar]
Kroenke 2001
- Kroenke K, Spitzer RL, Williams JB.The PHQ-9: Validity of a brief depression measure. Journal of General Internal Medicine 2001;16:606-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuring 2018
- Kuring JK, Mathias JL, Ward L.Prevalence of depression, anxiety and PTSD in people with dementia: a systematic review and meta-analysis. Neuropsycholy Review 2018;28:393-416. [DOI] [PubMed] [Google Scholar]
Lars‐Göran 2014
- Lars-Göran O.The efficacy of Acceptance and Commitment Therapy: an updated systematic review and meta-analysis. Behaviour Research Therapy 2014;61:105-21. [DOI] [PubMed] [Google Scholar]
Lawton 1969
- Lawton MP, Brody EM.Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179-86. [PubMed] [Google Scholar]
Ledermann 2010
- Ledermann T, Bodenmann G, Gagliardi S, Charvoz L, Verardi S, Rossier J, et al.Psychometricsof the dyadic coping inventory in three language groups. Swiss Journal of Psychology 2010;69:201-12. [Google Scholar]
Leon 1997
- Leon AC, Olfson M, Portera L, Farber L, Sheehan DV.Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. International Journal of Psychiatry in Medicine 1997;27:93–105. [DOI] [PubMed] [Google Scholar]
Leplège 2013
- Leplège A, Perret-Guillaume C, Ecosse E, Hervy M-P, Ankri J, Steinbüchel N.A new instrument to measure quality of life in older people: the French version of the WHOQOL-OLD. La Revue de Médecine Interne 2013;34:78-84. [DOI] [PubMed] [Google Scholar]
Li 2021
- Li W, Xu X, Wu F, Ni Y, Lan J.Comparative efficacy of non-pharmacological interventions on behavioural and psychological symptoms in elders with dementia: a network meta-analysis. Nursing Open 2021;8:2922-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linn 1968
- Linn BS, Linn MW, Gurel L.Cumulative illness rating scale. Journal of the American Geriatrics Society 1986;16:622-6. [DOI] [PubMed] [Google Scholar]
Logsdon 1999
- Logsdon RG, Gibbons LE, McCurry S, Teri L.Quality of life in Alzheimer’s disease: patient and caregiver report (QoL-AD). Journal of Mental Health and Aging 1999;5:21–32. [Google Scholar]
MacFarlane 2003
- MacFarlane MM.Systemic treatment of depression. Journal of Family Psychotherapy 2003;14:43-61. [Google Scholar]
Mahoney 1965
- Mahoney FI, Barthel DW.Functional evaluation: the Barthel index. Maryland State Medical Journal 1965;14:61-5. [PubMed] [Google Scholar]
Miller 1985
- Miller IW, Epstein NB, Bishop DS, Keitner GI.The McMaster Family Assessment Device: reliability and validity. Journal of Marital and Family Therapy 1985;11:345–56. [Google Scholar]
Montgomery 1979
- Montgomery SA, Åsberg M.A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382-9. [DOI] [PubMed] [Google Scholar]
Morris 1989
- Morris JC, Heyman A, Mohs RC, Hughes JP, Van Belle G, Fillenbaum G, et al.The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology 1989;39:1159-65. [DOI] [PubMed] [Google Scholar]
Morris 1993
- Morris JC.The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412-4. [DOI] [PubMed] [Google Scholar]
Moulin 2004
- Moulin CJ, James N, Freeman JE, Jones RW.Deficient acquisition and consolidation: intertrial free recall performance in Alzheimer's disease and mild cognitive impairment. Journal of Clinical and Experimental Neuropsychology 2004;26:1-10. [DOI] [PubMed] [Google Scholar]
Nezu 2010
- Nezu AM, Nezu CM, D'Zurilla TJ.Problem-solving therapy. In: Kazantzis N, Reinecke MA, Freeman A, editors(s). Cognitive and Behavioral Theories in Clinical Practice. New York (NY): Guilford Press, 2010:76-114. [Google Scholar]
NICE 2018
- National Institute for Health and Care Excellence (NICE).Dementia: assessment, management and support for people living with dementia and their carers; NICE guideline 97; June 2018. Available at www.nice.org.uk/guidance/ng97/resources/dementia-assessment-management-and-support-for-people-living-with-dementia-and-their-carers-pdf-1837760199109. [PubMed]
Nolen‐Hoeksema 1991
- Nolen-Hoeksema S, Morrow J.A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta earthquake. Journal of Personality and Social Psychology 1991;61:115–21. [DOI] [PubMed] [Google Scholar]
Nowotny 1989
- Nowotny ML.Assessment of hope in patients with cancer. Oncology Forum 1989;16:57–61. [PubMed] [Google Scholar]
Olazarán 2010
- Olazarán J, Reisberg B, Clare L, Cruz I, Peña-Casanova J, Ser T, et al.Nonpharmacological therapies in Alzheimer’s disease: a systematic review of efficacy. Dementia and Geriatric Cognitive Disorders 2010;30:161-78. [DOI] [PubMed] [Google Scholar]
Orgeta 2011
- Orgeta V, Spector AE, Orrell M.Psychological treatments for depression and anxiety in dementia and mild cognitive impairment. Cochrane Database of Systematic Reviews 2011, Issue 5. Art. No: CD009125. [DOI: 10.1002/14651858.CD009125] [DOI] [PMC free article] [PubMed] [Google Scholar]
Orgeta 2017
- Orgeta V, Tabet N, Nilforooshan R, Howard R.Efficacy of antidepressants for depression in Alzheimer's disease: systematic review and meta-analysis. Journal of Alzheimer's Disease 2017;58:725-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pachana 2007
- Pachana NA, Byrne GJ, Siddle H.Development and validation of the Geriatric Anxiety Inventory. International Psychogeriatrics 2007;19:103–14. [DOI] [PubMed] [Google Scholar]
Pearlin 1978
- Pearlin LI, Schooler C.The structure of coping. Journal of Health and Social Behavior 1978;19:2–21. [PubMed] [Google Scholar]
Petersen 1999
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E.Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology 1999;56:303-8. [DOI] [PubMed] [Google Scholar]
Petersen 2003
- Petersen RC.Conceptual Overview. In: Petersen RC, editors(s). Cognitive Impairment: Aging to Alzheimer's Disease. New York (NY): Oxford University Press, 2003:1-14. [Google Scholar]
Petersen 2004
- Petersen RC.Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
Prince 2004
- Prince M, 10/66 Dementia Research Group.Care arrangements for people with dementia in developing countries. International Journal of Geriatric Psychiatry 2004;19:170–7. [DOI] [PubMed] [Google Scholar]
Puranen 2017
- Puranen A, Taipale H, Koponen M, Tanskanen A, Tolppanen A, Tiihonen J, et al.Incidence of antidepressant use in community-dwelling persons with and without Alzheimer’s disease: 13-year follow-up. International Journal of Geriatric Psychiatry 2017;32:94-101. [DOI] [PubMed] [Google Scholar]
Radloff 1977
- Radloff LS.A CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement 1977;1:385–401. [Google Scholar]
Ravitz 2019
- Ravitz P, Watson P, Lawson A, Constantino MJ, Bernecker S, et al.Interpersonal psychotherapy: a scoping review and historical perspective (1974-2017). Harvard Review of Psychiatry 2019;27:165-80. [DOI] [PubMed] [Google Scholar]
Reisberg 1987
- Reisberg B, Borenstein MD, Salob SP, Ferris SH, Franssen E, Georgotas A.Behavioral symptoms in Alzheimer’s disease: phenomenology and treatment. Journal of Clinical Psychiatry 1987;48:9–15. [PubMed] [Google Scholar]
Reitan 1958
- Reitan RM.Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills 1958;8:271–276. [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5).Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rey 1964
- Rey A.L’Examen Clinique en Psychologie. Paris, France: Presses Universitaires de France, 1964. [Google Scholar]
Richards 2016
- Richards DA, Ekers D, McMillan D, Taylor RS, Sarah Byford S, Warren FC, et al.Cost and outcome of behavioural activation versus cognitive behavioural therapy for depression (COBRA): a randomised, controlled, non-inferiority trial. Lancet 2016;388:871-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rikki 1991
- Rikli R, Edwards D.Effects of a three year exercise program on motor function and cognitive processing speed in older women. Research Quarterly for Exercise and Sport 1991;62:61-7. [DOI] [PubMed] [Google Scholar]
Rolland 2007
- Rolland Y, Pillard F, Klapouszczak A, Reynish E, Thomas D, Andrieu S, et al.Exercise program for nursing home residents with Alzheimer's disease: a 1-year randomized, controlled trial. Journal of the American Geriatrics Society 2007;55:158-65. [DOI] [PubMed] [Google Scholar]
Rosen 1984
- Rosen WG, Mohs RC, Davis KL.A new rating scale for Alzheimer's disease. American Journal of Psychiatry 1984;141:1356–64. [DOI] [PubMed] [Google Scholar]
Rosenberg 1965
- Rosenberg M.Rosenberg Self-Esteem Scale (RSE): Acceptance and Commitment Therapy. Measures Package 1965;61:52. [Google Scholar]
Rosenberg 1979
- Rosenberg M.Conceiving the Self. New York (NY): Basic Books, 1979. [Google Scholar]
Salzman 2008
- Salzman C, Jeste DV, Meyer RE, Cohen-Mansfield J, Cummings J, Grossberg GT, et al.Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. Journal of Clinical Psychiatry 2008;69:889-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scheier 1985
- Scheier MF, Carver CS.Optimism, coping, and health: assessment and implication of generalized outcome expectancies. Health Psychology 1985;4:219-47. [DOI] [PubMed] [Google Scholar]
Schwarzer 1995
- Schwarzer R, Jerusalem M.Generalized Self-efficacy Scale. Windsor (UK): NFER-NELSON, 1995. [Google Scholar]
Seeman 1996
- Seeman T, McAvay G, Merrill S, Albert M, Rodin J.Self-efficacy beliefs and change in cognitive performance: MacArthur studies on successful aging. Psychology & Aging 1996;11:538–51. [DOI] [PubMed] [Google Scholar]
Shankar 1999
- Shankar KK, Walker M, Frost D.The development of a valid and reliable scale for Rating Anxiety in Dementia (RAID). Aging and Mental Health 1999;3:39–49. [Google Scholar]
Shedler 2010
- Shedler J.The efficacy of psychodynamic psychotherapy. American Psychologist 2010;65:98-109. [DOI] [PubMed] [Google Scholar]
Sintonen 2001
- Sintonen H.The 15D instrument of health related quality of life: properties and applications. Annals of Medicine 2001;33:328–36. [DOI] [PubMed] [Google Scholar]
Skinner 1983
- Skinner HA, Steinhauer PD, Santa-Barbara J.The family assessment measure. Canadian Journal of Community Mental Health 1983;2:91–104. [Google Scholar]
Smith 2005
- Smith SC, Lamping DL, Banerjee S, Harwood RS, Foley B, Smith P, et al.Measurementof health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and evaluation of current methodology. Health and Technology Assessment 2009;9:1–93. [DOI] [PubMed] [Google Scholar]
Snow 2004
- Snow AL, Norris MP, Doody R, Molinari VA, Orengo CA, Kunik ME.Dementia Deficits Scale. Rating self-awareness of deficits. Alzheimer Disease & Associated Disorders 2004;18:22–31. [DOI] [PubMed] [Google Scholar]
Spruytte 2002
- Spruytte N, Van Audenhove C, Lammertyn F, Storms G.The quality of the caregiving relationship in informal care for older adults with dementia and chronic psychiatric patients. Psychology and Psychotherapy: Theory, Research and Practice 2002;75:295-311. [DOI] [PubMed] [Google Scholar]
Stanley 2004
- Stanley MA, Diefenbach GJ, Hopko DR.Cognitive behavioral treatment for older adults with generalized anxiety disorder. A therapist manual for primary care settings. Behaviour Modification 2004;28:73-117. [DOI] [PubMed] [Google Scholar]
Starkstein 2007
- Starkstein SE, Jorge R, Petracca G, Robinson RG.The construct of generalized anxiety disorder in Alzheimer disease. American Journal of Geriatric Psychiatry 2007;15:42-9. [DOI] [PubMed] [Google Scholar]
Steck 2018
- Steck N, Cooper C, Orgeta V.Investigation of possible risk factors for depression in Alzheimer's disease: a systematic review of the evidence. Journal of Affective Disorders 2018;236:149-56. [DOI] [PubMed] [Google Scholar]
Stephan 2013
- Stephan BC, Minett T, Pagett E, Siervo M, Brayne C, McKeith IG.Diagnosing mild cognitive impairment (MCI) in clinical trials: a systematic review. BMJ Open 2013;3:e001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 1988
- Stewart AL, Hays R, Ware JE Jr.The MOS short-form general health survey: reliability and validity in a patient population. Medical Care 1988;26:724–32. [DOI] [PubMed] [Google Scholar]
Stogmann 2016
- Stogmann E, Moser D, Klug S, Gleiss A, Auff E, et al.Activities of daily living and depressive symptoms in patients with subjective cognitive decline, mild cognitive impairment, and Alzheimer's disease. Journal of Alzheimer's Disease 2016;49:1043-50. [DOI] [PubMed] [Google Scholar]
Sturman 2005
- Sturman MT, Morris MC, Mendes de Leon CF, Bienias JL, Wilson RS, Evans DA.Physical activity, cognitive activity, and cognitive decline in a biracial community population. Archives of Neurology 2005;62:1750-4. [DOI] [PubMed] [Google Scholar]
Sunderland 1988
- Sunderland T, Alterman IS, Yount D, Hill JL, Tariot PN, Newhouse PA.A new scale for the assessment of depressed mood in demented patients. American Journal of Psychiatry 1988;145:955-9. [DOI] [PubMed] [Google Scholar]
Sunderland 1989
- Sunderland T, Hill JL, Mellow AM, Lawlor BA, Gundersheimer J, Newhouse PA, et al.Clock drawing in Alzheimer’s disease. A novel measure of dementia severity. Journal of the American Geriatrics Society 1989;37:725-9. [DOI] [PubMed] [Google Scholar]
Tappen 2008
- Tappen RM, Williams CL.Development and testing of the Alzheimer’s Disease and Related Dementias Mood Scale. Nursing Research 2008;57:426-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Teasdale 1995
- Teasdale JD, Segal Z, Williams JM.How does cognitive therapy prevent depressive relapse and why should attentional control (mindfulness) training help? Behaviour Research and Therapy 1995;33:25‐39. [DOI] [PubMed] [Google Scholar]
Teri 1991
- Teri L, Gallagher-Thompson D.Cognitive-behavioural interventions for treatment of depression in Alzheimer's patients. Gerontologist 1991;31(3):413-6. [DOI] [PubMed] [Google Scholar]
Teri 1992
- Teri L, Truax R, Logsdon R.Assessment of behavioural problems in dementia: the Revised Memory and Behavior Problems Checklist. Psychology and Aging 1992;4:622-31. [DOI] [PubMed] [Google Scholar]
Thompson 1993
- Thompson SC, Sobolew-Shubin A.Perceptions of over protection in ill adults. Journal of Applied Social Psychology 1993;23:85-97. [Google Scholar]
Troyer 2002
- Troyer AK, Rich JB.Psychometric properties of a new meta-memory questionnaire for older adults. Journals of Gerontology - Series B Psychological Sciences and Social Sciences 2002;57:19-27. [DOI] [PubMed] [Google Scholar]
Tuijt 2020
- Tuijt R, Leung P, Profyri E, Orgeta V.Development and preliminary validation of the Meaningful and Enjoyable Activities Scale (MEAS) in mild dementia. International Journal of Geriatric Psychiatry 2020;35:944-52. [DOI] [PubMed] [Google Scholar]
Uphoff 2020
- Uphoff E, Ekers D, Robertson L, Dawson S, Sanger E, South E.Behavioural activation therapy for depression in adults. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013305. [DOI: 10.1002/14651858.CD013305.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ustun 2000
- Ustun B.WHODAS-II Disability Assessment Schedule. Washington (DC): NIMH Mental Health Research Conference, 2000. [Google Scholar]
Van der Hooft 2008
- Van der Hooft CS, Schoofs MW, Ziere G, Hofman A, Pols HA, Sturkenboom MC, et al.Inappropriate benzodiazepine use in older adults and the risk of fracture. British Journal of Clinical Pharmacology 2008;66:276-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Linden 1989
- Van der Linden M, Wijns C, Von Frenkell R.Un Questionnaire d’Auto-Evaluation de la Memoire (QAM). Brussels, Belgium: Editest, 1989. [Google Scholar]
Vernooij‐Dassen 1996
- Vernooij-Dassen MJ, Felling AJ, Persoon JM.Predictors of sense of competence in primary caregivers of demented persons. Social Science & Medicine 1996;43:41-9. [DOI] [PubMed] [Google Scholar]
Visser 2005
- Visser PJ, Scheltens P, Verhey FR.Do MCI criteria in drug trials accurately identify subjects with predementia Alzheimer's disease? Journal of Neurology, Neurosurgery, and Psychiatry 2005;76:1348-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vitaliano 1985
- Vitaliano PP, Russo J, Carr JE.The Ways of Coping Checklist: revision and psychometric properties. Multivariate Behavioral Research 1985;20:3-26. [DOI] [PubMed] [Google Scholar]
von Steinbüche 2006
- Steinbüchel N, Lischetzke T, Gurny M, Eid M.Assessing quality of life in older people: psychometric properties of the WHOQOL-BREF. European Journal of Ageing 2006;3:116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1996
- Ware J, Kosinski M, Keller SD.A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care 1996;34:220-33. [DOI] [PubMed] [Google Scholar]
Wechsler 1987
- Wechsler D.WMS-R: Wechsler Memory Scale - Revised (Manual). San Antonio (TX): Psychological Corporation, 1987. [Google Scholar]
Weintraub 1982
- Weintraub S, Barataz R, Mesulam M.Daily living activities in the assessment of dementia. In: Corkin S, Davis K, Cravden J, Usdin E, Wurtman J, editors(s). Alzheimer's disease: a report of progress in research. New York (NY): Raven Press, 1982:189-92. [Google Scholar]
Weintraub 2009
- Weintraub S, Salmon D, Mercaldo N.The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Disease & Associated Disorders 2009;23:91-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2019
- World Health Organization (WHO).mhGAP Intervention Guide, Version 2.0; 2016. www.who.int/publications/i/item/9789241549790 (accessed prior to 28 February 2022).
Woods 2018
- Woods RT, O'Philbin L, Farrell EM, Spector AE, Orrell M.Reminiscence therapy for dementia. Cochrane Database of Systematic Reviews 2018, Issue 3. Art. No: CD001120. [DOI: 10.1002/14651858.CD001120.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yale 1995
- Yale R.Developing Support Groups for Individuals With Early-Stage Alzheimer’s Disease: Planning, Implementation, and Evaluation. Baltimore (MD): Health Professions Press, 1995. [Google Scholar]
Yesavage 1983
- Yesavage JA, Brink TL, Rose TL.Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatry Research 1983;17:37-49. [DOI] [PubMed] [Google Scholar]
Zarit 1980
- Zarit SH, Reever KE, Bach-Petersen J.Relatives of the impaired elderly: correlates of feeling of burden. Gerontologist 1980;20:649–60. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP.The Hospital Anxiety and Depression Scale. Acta Psychologica Scandinavica 1983;67:361-70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Orgeta 2014
- Orgeta V, Qazi A, Spector AE, Orrell M.Psychological treatments for depression and anxiety in dementia and mild cognitive impairment. Cochrane Database of Systematic Reviews 2014, Issue 1. Art. No: CD009125. [DOI: 10.1002/14651858.CD009125] [DOI] [PMC free article] [PubMed] [Google Scholar]


