Abstract
Afferent-loop syndrome (ALS) is known as a rare complication of partial or total gastrectomy and also occurs after pancreatoduodenectomy. The symptoms of ALS vary with the location of the mechanical obstruction, and the choice of therapeutic method should reflect the patient's condition and disease state. Herein, we report the use of endoscopic ultrasound (EUS)-guided afferent loop drainage with a plastic stent and its reintervention for malignant ALS. An 80-year-old man was admitted to our hospital with abdominal pain. Thirty-two months before, the patient underwent left hepatectomy with choledochojejunostomy and Roux-en-Y reconstruction for hilar biliary adenocarcinoma. An abdominal CT scan showed a dilated afferent loop and a low-density lesion in the peritoneum that suggested recurrence of hilar biliary adenocarcinoma and malignant ALS due to mechanical obstruction of the afferent loop caused by peritoneal dissemination. The recurrence site did not include the choledochojejunostomy anastomosis and was far distal to it. We employed a convex EUS scope and directly punctured the afferent loop from the stomach. We inserted one double pig-tail stent, and the ALS immediately improved. Five months later, ALS recurred, and we exchanged a plastic stent through the fistula. After reintervention, ALS did not recur before the patient's death due to cancer progression.
Keywords: Afferent-loop syndrome, Interventional endoscopic ultrasound, Plastic stent, Gastrojejunostomy
Introduction
Afferent-loop syndrome (ALS) is known as a rare complication of partial or total gastrectomy. The incidence rate of ALS after gastrectomy has been reported as 0.2–20% [1]. ALS also occurs after pancreatoduodenectomy (PD). In previous reports, the incidence rate of ALS after PD has been lower than after gastrectomy. Aoki et al. [2] reported the incidence rate of ALS as 0.3–1.0% after Billroth-II and 0.2% after Roux-en-Y. However, the incidence of ALS has been increasing in recent times. Pannala et al. [3] reported an incidence of 13% after PD for pancreatic adenocarcinoma. Furthermore, Ligresti et al. [4] reported the rate of malignant obstruction of the afferent loop as 33%. The mechanism of ALS is obstruction of the afferent loop, which induces stasis of intestinal and bile juice. The obstruction can be caused by tumor recurrence, radiation enteritis, or adhesion [5]. Of those, tumor recurrence is a major cause of ALS after PD for pancreatic adenocarcinoma. Due to the accumulation of biliary, pancreatic, or intestinal secretion in the afferent loop, symptoms of ALS vary with the location of the obstruction. The typical symptoms of ALS are vomiting, fever, abdominal pain, and jaundice. The most common clinical presentations are obstructive jaundice or acute cholangitis (50%) and abdominal pain (29%) [3]. The aim of therapy is to release the obstruction of the afferent loop or to drain the accumulated secretions. Therefore, the patient's condition or disease state should guide the choice of therapy. As surgical therapy is invasive, 25% of malignant ALS patients are not able to tolerate it [6], so less-invasive percutaneous or endoscopic techniques are often employed.
In ALS patients with obstructive jaundice or acute cholangitis, biliary drainage is mandatory and is performed using a percutaneous or endoscopic ultrasound (EUS) approach. Although percutaneous transhepatic biliary drainage has been applied in acute cholangitis or obstructive jaundice, permanent external catheterization is sometimes inevitable, especially for malignant ALS patients. In some cases, successful metal stent placement in the stenosis lesion through the percutaneous transhepatic biliary drainage route has been reported [7]. However, such stent placement is not successful in every case. While there are a few reports of direct percutaneous enteral stent placement [8, 9], EUS-guided biliary drainage (EUS-BD) is another choice for ALS patients, using EUS-guided hepaticogastrostomy or EUS-guided hepaticojejunostomy. Bie et al. [10] reported that EUS-BD seems to outperform percutaneous drainage. Since EUS-BD does not need scope insertion through the long distance of the jejunum, EUS-BD is often suitable, especially for patients with poor systematic status, although EUS-BD can sometimes cause severe complications. Therefore, EUS-BD should be performed only by interventional EUS experts. Another therapeutic option for ALS is release of the mechanical obstruction of the afferent loop. Surgical resection of the obstructive lesion, bypass, and enteral stent placement are effective therapies for this purpose. Although the surgical approach is usually necessary to perform resection or bypass, they are invasive for patients. On the other hand, enteral stent placement is performed using an endoscopic or percutaneous approach, which are less invasive than surgery. However, enteral stent placement is not always performed, due to the difficulty of reaching the obstruction. Ikeuchi et al. [11] reported the first case of ALS treated with a lumen-apposing metal stent (LAMS) in 2015, and some recent papers have reported that EUS-guided gastrojejunostomy with a LAMS is effective. Furthermore, drainage of the afferent loop with a metal stent or plastic stent has been reported [12, 13]. Here, we report the use of a plastic stent in EUS-guided afferent loop drainage and its reintervention for malignant ALS.
Case Report/Case Presentation
An 80-year-old man was admitted to our hospital with abdominal pain. Thirty-two months before, the patient underwent left hepatectomy with choledochojejunostomy and Roux-en-Y reconstruction for hilar biliary adenocarcinoma. S-1 adjuvant chemotherapy was performed after surgical therapy. The result of blood examination showed that the white blood cell count and C-reactive protein were elevated and that liver function and pancreatic enzymes were normal. Carbohydrate antigen 19-9 was remarkably elevated, at 15,610 U/mL. An abdominal computed tomography (CT) scan showed a dilated afferent loop and a low-density lesion in the peritoneum that suggested recurrence of the hilar biliary adenocarcinoma and malignant ALS due to mechanical obstruction of the afferent loop caused by peritoneal dissemination (Fig. 1). The recurrence site did not include the choledochojejunostomy anastomosis and was on the far distal side of it.
Fig. 1.
CT on admission (a: axial, b: coronal) showed a dilated afferent loop and a low-density lesion in the peritoneum causing mechanical obstruction.
We chose to use EUS-guided drainage of the afferent loop with a plastic stent. We employed a convex EUS scope, GF-UCT260 (Olympus, Tokyo, Japan), and visualized the dilated afferent loop. From the stomach, we punctured the afferent loop with a 19-gauge EchoTip Ultra (COOK, Winston Salem, NC, USA), dilated the fistula with an electrocautery catheter, a Cysto-Gastro-Set (Endo-Flex, Rohrdorf, Germany), and inserted a 0.025-inch guidewire into the afferent loop. Finally, we inserted a double pig-tail plastic stent (7Fr.-7cm double pig-tail stent, Zimmon type; COOK, Winston Salem, NC, USA) (Fig. 2a, b). The dilated afferent loop immediately began shrinking on CT after the procedure (Fig. 2c). Moderate peritonitis due to secretion leak occurred but was ameliorated using antibiotics. Other acute complications did not occur. A few days after EUS-guided drainage, the abdominal pain improved, and oral intake became possible. Seven days after the intervention, the patient was discharged from our hospital.
Fig. 2.
a, b EUS-guided plastic stent placement was successful. After the procedure, the CT scan (c) showed improvement of the dilation of the afferent loop.
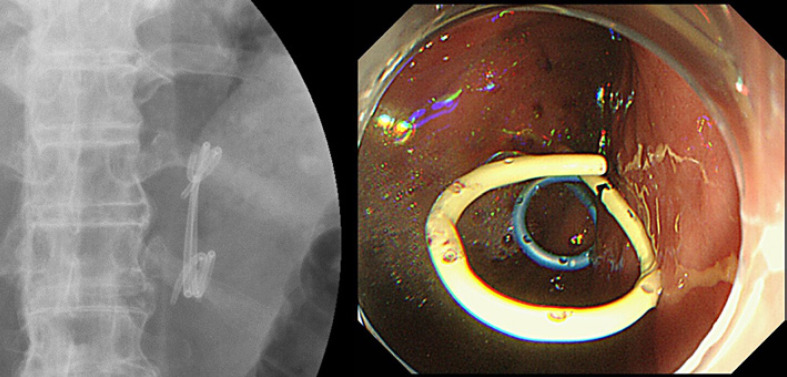
Five months after EUS-guided drainage, the patient complained of abdominal pain and was admitted to our hospital again. A CT scan disclosed recurrence of ALS. Although the plastic stent remained between the stomach and afferent loop, we diagnosed stent occlusion. We removed the occluded stent and inserted two plastic stents according to the step-up approach for walled-off necrosis of the pancreas. We employed a gastric scope (GIF-Q260J; Olympus, Tokyo, Japan). Before removal of the previous stent, we inserted a 0.025- and a 0.035-inch guidewire using an uneven double-lumen catheter. In this case, the afferent loop was slightly far from the stomach wall; therefore, we did not add balloon dilatation to avoid fistula injury. Since the fistula lumen was still narrow to deploy two new plastic stents, we deployed a 6Fr.-4cm Zimmon over the 0.025-inch guidewire first, and then, we removed the previous stent. Finally, over the 0.035-inch guidewire, we deployed the second double pig-tail stent, a 7Fr.-4cm Zimmon, into the remained gap in the fistula (Fig. 3). Recurrence of stent occlusion did not occur until the patient died of cancer progression 2 months after treatment.
Fig. 3.
Reintervention for recurrence of ALS was successful. Two plastic stents were inserted from the stomach through the fistula.
Discussion/Conclusion
Here, we demonstrated that a case of malignant ALS could be managed by EUS-guided drainage with a plastic stent, and that ALS recurrence could be relieved by a plastic stent exchange. Previous reports and studies have shown that EUS-guided therapy is effective, especially for palliative therapy for malignant ALS. In this case, the location of malignant recurrence appeared between the blind end of the afferent loop and the side of the choledochojejunostomy. As a result, acute cholangitis or obstructive jaundice did not occur in this case. Therefore, percutaneous or EUS-BD would not have been effective, and decompression of the afferent blind loop was necessary. Although percutaneous drainage of the afferent loop could be considered, a permanent external drainage tube might have been necessary and would have degraded the patient's quality of life. Thus, we regarded internal drainage as a suitable therapy for this case. There are no dedicated stents for ALS. In previous reports, the use of plastic stents, metal stents, and LAMSs has been reported. In our hospital, we could not use LAMSs immediately. Since the afferent loop did not attach to the wall of the stomach, there was a considerable distance between them. Therefore, we thought that metal stent insertion might be difficult and that the migration might occur. During the procedure, the insertion of the double pig-tail stent to the afferent loop was not difficult. Although a moderately acute complication, peritonitis, was observed, the symptoms of ALS and peritonitis immediately improved. The plastic stents were easy to use and effective in draining the afferent loop, although attention should be paid to preventing complications.
In this case, recurrence of ALS due to stent occlusion occurred 5 months later. However, the patient's symptoms were improved through the exchange of plastic stents. A double pig-tail stent is often used for biliary drainage, and stent occlusion usually occurs some months later. Despite the deployment of a plastic stent between the stomach and afferent loop, the stent patency was considerable. Similar to biliary drainage practice, the occluded stent was exchanged in this case. We could insert a guidewire beside the previous stent. Because sufficient time had passed after making a fistula, we thought that the risk of fistula injury was low. In fact, the dilation of the fistula with a balloon dilator was performed safely. Ogura et al. [14] have reported that a fistula made by EUS-guided hepaticojejunostomy with a covered metal stent was stable 7 days later, and transluminal treatment through the fistula was safe. In this case, we did not use a metal stent. However, 5 months was probably long enough to perform reintervention through the fistula safely. Matsumoto et al. [12] placed two plastic stents in the first session and did not report stent occlusion. Benallal et al. [13] reported the efficacy of EUS-guided gastroenterostomy using a metal stent and a plastic stent. In that study, stent occlusion did not occur for 3 months before the patient's death due to disease progression. The period of stent patency may be associated with the number of stents, or a metal stent may be favored over a plastic stent in terms of stent patency. Furthermore, other reports have shown that LAMSs for ALS are feasible and effective. LAMSs are often used in drainage for pancreatic fluid collection and walled-off necrosis, and it has been reported that long-term placement of LAMSs might cause pseudoaneurysm and hemorrhage. Unlike pancreatic fluid collection and walled-off necrosis, LAMSs for ALS often need to remain for some months and may have the potential to cause hemorrhage. Betés et al. [15] reported that hemorrhage after placement of LAMS for echoendoscopic anastomoses occurred in only one of thirty cases (3.3%). The efficacy and complications of LAMSs use for ALS remain to be investigated for a number of cases.
Although the first choice for ALS therapy is not certain, EUS-guided drainage of the afferent loop is feasible and safe. There are various stents to use for EUS-guided drainage of the afferent loop, and the outcome data for any particular stent are inadequate. Endoscopists, especially endoscopic retrograde cholangiopancreatography operators, are used to plastic stents and will choose them over metal stents or LAMSs on the basis of cost. We recommend plastic stents as the first choice of therapeutic methods for ALS under EUS-guided drainage of the afferent loop.
In conclusion, EUS-guided drainage of the afferent loop with plastic stents was effective and safe for ALS. Recurrence of ALS could be ameliorated with reintervention through the fistula. However, EUS-guided drainage of the afferent loop is not the best therapy for all ALS patients. We should choose therapeutic methods, including surgical therapy, in consideration of the cause of ALS, the location of the mechanical obstruction, and the patient's anatomy after previous surgeries, general condition, and prognosis.
Statement of Ethics
This study was reviewed and approved by the institutional review broad of the Kawasaki Medical School (approval no. 5438-00). Written informed consent was obtained from the next of kin of the patient for publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
Tomohiro Tanikawa, the corresponding author, Noriyo Urata, Katsunori Ishii, Ryo Katsumata, Ken Nishino, Mitsuhiko Suehiro, Miwa Kawanaka, Ken Haruma, and Hirofumi Kawamoto contributed to manuscript writing and literature review. All the authors read and approved the final manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1.Mitty WF, Jr, Grossi C, Nealon TF., Jr Chronic afferent loop syndrome. Ann Surg. 1970 Dec;172((6)):996–1001. doi: 10.1097/00000658-197012000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki M, Saka M, Morita S, Fukagawa T, Katai H. Afferent loop obstruction after distal gastrectomy with Roux-en-Y reconstruction. World J Surg. 2010 Oct;34((10)):2389–92. doi: 10.1007/s00268-010-0602-5. [DOI] [PubMed] [Google Scholar]
- 3.Pannala R, Brandabur JJ, Gan SI, Gluck M, Irani S, Patterson DJ, et al. Afferent limb syndrome and delayed GI problems after pancreaticoduodenectomy for pancreatic cancer: single-center, 14-year experience. Gastrointest Endosc. 2011 Aug;74((2)):295–302. doi: 10.1016/j.gie.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 4.Ligresti D, Amata M, Messina M, Traina M, Tarantino I. Single-step EUS-guided jejunojejunostomy with a lumen-apposing metal stent as treatment for malignant afferent limb syndrome. VideoGIE. 2020 Apr;5((4)):154–6. doi: 10.1016/j.vgie.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Termsinsuk P, Chantarojanasiri T, Pausawasdi N. Diagnosis and treatment of the afferent loop syndrome. Clin J Gastroenterol. 2020 Oct;13((5)):660–8. doi: 10.1007/s12328-020-01170-z. [DOI] [PubMed] [Google Scholar]
- 6.Moriura S, Takayama Y, Nagata J, Akutagawa A, Hirano A, Ishiguro S, et al. Percutaneous bowel drainage for jaundice due to afferent loop obstruction following pancreatoduodenectomy: report of a case. Surg Today. 1999;29((10)):1098–101. doi: 10.1007/s005950050652. [DOI] [PubMed] [Google Scholar]
- 7.Jinno N, Naitoh I, Nagura Y, Fujioka K, Mizuno Y, Momose J, et al. Percutaneous transhepatic self-expanding metallic stent placement for the treatment of malignant afferent loop obstruction. Intern Med. 2018 Feb 1;57((3)):333–7. doi: 10.2169/internalmedicine.9382-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chevallier P, Novellas S, Motamedi JP, Gugenheim J, Brunner P, Bruneton J-N. Percutaneous jejunostomy and stent placement for treatment of malignant Roux-en-Y obstruction: a case report. Clin Imaging. 2006;30((4)):283–6. doi: 10.1016/j.clinimag.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Laasch HU. Obstructive jaundice after bilioenteric anastomosis: transhepatic and direct percutaneous enteral stent insertion for afferent loop occlusion. Gut Liver. 2010 Sep;4((Suppl l)):S89–95. doi: 10.5009/gnl.2010.4.S1.S89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bie C, Bronswijk M, Vanella G, Pérez-Cuadrado-Robles E, van Malenstein H, Laleman W, et al. EUS-guided hepaticogastrostomy for patients with afferent loop syndrome: a comparison with EUS-guided gastroenterostomy or percutaneous drainage. Surg Endosc. 2021 Apr 28; doi: 10.1007/s00464-021-08520-z. [DOI] [PubMed] [Google Scholar]
- 11.Ikeuchi N, Itoi T, Tsuchiya T, Nagakawa Y, Tsuchida A. One-step EUS-guided gastrojejunostomy with use of lumen-apposing metal stent for afferent loop syndrome treatment. Gastrointest Endosc. 2015 Jul;82((1)):166. doi: 10.1016/j.gie.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto K, Kato H, Tomoda T, Sakakihara I, Yamamoto N, Noma Y, et al. A case of acute afferent loop syndrome treated by transgastric drainage with EUS. Gastrointest Endosc. 2013 Jan;77((1)):132–3. doi: 10.1016/j.gie.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Benallal DC, Hoibian S, Caillol F, Bories E, Presenti C, Ratone JP, et al. EUS-guided gastroenterostomy for afferent loop syndrome treatment stent. Endosc Ultrasound. 2018 Nov-Dec;7((6)):418–9. doi: 10.4103/eus.eus_41_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogura T, Nishioka N, Yamada M, Yamada T, Ueno S, Matsuno J, et al. Novel transluminal treatment protocol for hepaticojejunostomy stricture using covered self-expandable metal stent. Surg Endosc. 2021 Jan;35((1)):209–15. doi: 10.1007/s00464-020-07381-2. [DOI] [PubMed] [Google Scholar]
- 15.Betés M, Pérez-Longo P, Peralta S, Bojorquez A, Angós R, Chopitea A, et al. Feasibility and patency of echoendoscopic anastomoses with lumen apposing metal stents depending on the gastrointestinal segment involved. Sci Rep. 2021 Feb 17;11((1)):3992. doi: 10.1038/s41598-021-83618-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.