Abstract
In the past 2 decades, multidrug-resistant Streptococcus pneumoniae has been encountered with increasing frequency around the world. This has led to the need for newer agents in the treatment of S. pneumoniae infections. Oritavancin (LY333328) is a new glycopeptide antibiotic with activity against gram-positive organisms; however, there is limited information on the pharmacodynamics of oritavancin with respect to the treatment of S. pneumoniae. We utilized an in vitro pharmacodynamic model to compare the activity of oritavancin to that of vancomycin against two penicillin-, macrolide-, and ciprofloxacin-resistant S. pneumoniae isolates (R919 and R921) over a 48-h period. Both oritavancin and vancomycin achieved 99.9% (3-log) kill, with oritavancin achieving the limit of detection (102 CFU/ml) within 1 h and vancomycin achieving this limit at 24 h for both isolates. Detection of resistance was not observed for oritavancin or vancomycin during the 48-h experiments. The key pharmacodynamic parameter for oritavancin has not been well defined. In our experiment, the ratios of the area under the curve from 0 to 24 h to the MIC of oritavancin, oritavancin plus albumin, and vancomycin for both isolates were greater than 944.5, and the ratios of the maximum concentration of drug in serum to the MIC ranged from 73.7 to 7188.5. T>MIC was 100% for oritavancin and vancomycin for both isolates. Oritavancin is a unique and potent antimicrobial that warrants further investigation against multidrug-resistant S. pneumoniae.
Over the last 20 years, penicillin-resistant Streptococcus pneumoniae has been encountered with increasing frequency. Similarly, resistance is increasing to other antimicrobials such as the macrolides, cephalosporins, and more recently the fluoroquinolones (4, 7). The glycopeptide vancomycin still remains a viable option for resistant S. pneumoniae; however, vancomycin tolerance has recently been reported (11). New agents with activity against resistant S. pneumoniae are needed. Oritavancin (LY333328) is an investigational, semisynthetic glycopeptide with good activity against most sensitive and resistant gram-positive pathogens. Recent information suggests that its mechanism of action may be different from that of vancomycin, which would explain its activity against vancomycin-resistant organisms such as enterococci (1).
MATERIALS AND METHODS
Bacterial strains.
The study organisms consisted of two strains of S. pneumoniae, R919 and R921, that were derived from a clinical isolate, R79, by serial passage on agar plates containing various concentrations of ciprofloxacin (The Anti-Infective Research Lab), Detroit Receiving Hospital. The MIC of penicillin for both isolates is 2 μg/ml, that for erythromycin is 12 μg/ml, and the MICs of ciprofloxacin for R919 and R921 are 4 and 8 μg/ml, respectively.
Antimicrobial agents and medium.
Vancomycin (lot no. 2MU91M) was commercially purchased from Eli Lilly and Company (Indianapolis, Ind.). Oritavancin (LY333328; lot no. S107316) was supplied by Eli Lilly and Company. Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) supplemented with calcium (25 mg/ml) and magnesium (12.5 mg/ml) (SMHB) and 5% lysed horse blood (LHB) (Rockland, Inc., Gilbertsville, Pa.) was used for susceptibility testing. Todd-Hewitt Broth (THB) (Difco Laboratories) supplemented with 0.5% yeast extract (Difco Laboratories) was used in the in vitro pharmacodynamic models. To simulate the protein binding of oritavancin in vivo, human albumin (Baxter Healthcare Corporation, Glendale, Calif.) was added to all oritavancin in vitro pharmacodynamic models at the concentration of 4 g/dl.
Susceptibility testing.
MICs and minimum bactericidal concentrations (MBCs) of both oritavancin and vancomycin were determined by broth microdilution in SMHB plus 5% LHB, according to National Committee for Clinical Laboratory Standards guidelines for vancomycin (10). Samples (5 μl) from clear wells were plated on tryptic soy agar (TSA) plates with 5% sheep blood (SB) to determine MBCs. All cultures were incubated in candle jars with approximately 3% CO2 at a temperature of 37°C for 24 h. Oritavancin was additionally tested with the addition of albumin (4 g/dl).
In vitro pharmacodynamic model.
The in vitro pharmacodynamic model consists of a 250-ml one-compartment glass chamber with ports for the addition and removal of the THB with 0.5% yeast extract with or without albumin, injection of antibiotics, and removal of samples. Prior to each experiment, colonies from an overnight growth of bacteria on TSA plates with 5% SB were added to THB with 0.5% yeast extract to obtain a concentration of 106 CFU/ml. Fresh stock solutions of oritavancin and vancomycin were prepared daily and were stored at 2 to 8°C between dose administration times. Experimental regimens simulated antibiotic concentrations achieved in human plasma. Vancomycin was administered at a dose of 1 g every 12 h (four doses given) to achieve a peak concentration in serum (Cmax) of 30 μg/ml and a trough concentration of 7.5 μg/ml. To achieve targeted concentrations of oritavancin in plasma during the first 48 h of dosing in humans, oritvancin was administered at a loading dose of 5 mg/kg of body weight at 0 h, followed by 4 mg/kg at 24 h, to achieve a peak concentration of 100 μg/ml and 24-h trough concentration of 15 μg/ml. Each antibiotic was administered as a bolus into the models over 30 s using a hypodermic syringe. Fresh medium (SMHB) was continuously supplied and removed from the model along with the drug via a peristaltic pump (Masterflex; Cole-Parmer Instrument Company, Chicago, Ill.) set to simulate the half-lives (t1/2s) of vancomycin (6.5 h) and oritavancin (t1/2 at α phase [t1/2α] = 2 h); the pump ran in this manner for 8 h after dosing and then was changed to simulate a t1/2 of 12.3 h for the remaining 16 h of the 24 h dosing period (Eli Lilly and Company, unpublished data). Each model apparatus was placed in a water bath and maintained at 37°C for the entire 48 h study period. The pharmacodynamic model experiments were performed in duplicate, simultaneously, in order to ensure reproducibility.
Pharmacokinetic analysis.
Samples (0.5 ml) from each of the duplicate pharmacodynamic models were obtained at 0, 2, 4, 8, 24, 28, 32, and 48 h for determination of antibiotic concentrations. All samples were stored at −70°C until analysis. Vancomycin concentrations were determined using a fluorescence polarization immunoassay (Abbott Diagnostics TDx). This method has a sensitivity of 2.0 μg/ml for vancomycin. All vancomycin assays had a coefficient of determination of ≥0.92 and a between-day coefficient of variation ranging from 1.6 to 3.3%. Concentrations of oritavancin were determined by using a microbioassay with Micrococcus luteus ATCC 9341 as the indicator organism. Wells (diameter, 4 mm) were made in AMA #11 agar plates with 150- to 200-μl samples placed in each well. Plates were inoculated with an even lawn of indicator organism and incubated for 18 to 24 h at 37°C. All plates achieved a within-day and between-day coefficient of determination of ≥0.95. Oritivancin standard antibiotic concentrations were prepared with albumin (4 g/dl) and consisted of concentrations of 100, 25, and 5 μg/ml. The between-day coefficient of variation for oritavancin ranged from 2.2 to 6.1%. All standards and model samples were assayed in triplicate. The t1/2s of vancomycin and oritavancin were calculated from the slopes of the plots of drug concentration versus time. The area under the curve (AUC) obtained from the concentration-versus-time plot was calculated by the linear trapezoidal rule (PK Analyst programs; Micromath, Salt Lake City, Utah).
Pharmacodynamic analysis.
Samples (0.5 ml) were removed from each of the duplicate pharmacodynamic models at 0, 0.5, 1, 2, 4, 6, 8, 24, 28, 32, and 48 h. Each sample was then serially diluted in cold 0.9% sodium chloride, and bacterial counts were determined by placing 20-μl spots from the appropriately diluted samples in triplicate on TSA with 5% SB and incubated at 37°C for 24 h. We have previously determined that these methods have a limit of detection of 2 log10 CFU/ml (2). The total reduction in log10 CFU per milliliter over 48 h was determined by plotting time-kill curves based on the number of remaining organisms over the 48-h time period. The time to achieve a 99.9% bacterial reduction was determined by linear regression. The AUC/MIC and Cmax/MIC ratios were calculated for each drug. Resistance plates were done for vancomycin and oritavancin in each model by plating 24- and 48-h samples (100 μl) onto TSA plus 5% SB with four and eight times the MIC of each antibiotic and incubated at 37°C for 48 h.
The potential for antibiotic carryover was evaluated by serial dilution (range, 1:10 to 1:10,000) for all samples prior to plating. Antibiotic carryover was also evaluated by the incorporation of cholestyramine (lot no. M7K005A; Bristol-Myers Squibb Co., Princeton, N.J.), d-Ala–d-Ala (lot no. 77H1207; Sigma Chemicals, St. Louis, Mo.), polyaneholesulfonic acid (lot no. 104H05531; Sigma Chemicals), and polymeric absorbent beads (Amberlite; Rohm and Haas, Philadelphia, Pa.) into each serial dilution before plating.
Statistical analysis.
Changes in log10 CFU/milliliter at 8, 24, and 48 h were compared by analysis of variance (ANOVA), with Tukey's post-hoc test for multiple comparisons. P values of <0.05 were considered significant.
RESULTS
Susceptibility testing.
MIC and MBC results for R919 and R921 are summarized in Table 1. There was no difference between the MICs of oritavancin, oritavancin plus albumin, and vancomycin for both isolates. Oritavancin alone demonstrated the greatest activity, with an MIC of ≤0.015 for R919 and R921, followed by oritavancin plus albumin and then vancomycin.
TABLE 1.
MICs and MBCs for S. pneumoniae isolates R919 and R921a
| Antibiotic | MIC (MBC) for isolate as determined by microdilution
|
|
|---|---|---|
| R919 | R921 | |
| Oritavancin | ≤0.015 (≤0.015) | ≤0.015 (0.03) |
| Oritavancin + albumin | 0.03 (0.06) | 0.03 (0.125) |
| Vancomycin | 0.5 (1.0) | 0.5 (1.0) |
MICs and MBCs are given in micrograms per milliliter.
Pharmacodynamic and pharmacokinetic studies and resistance.
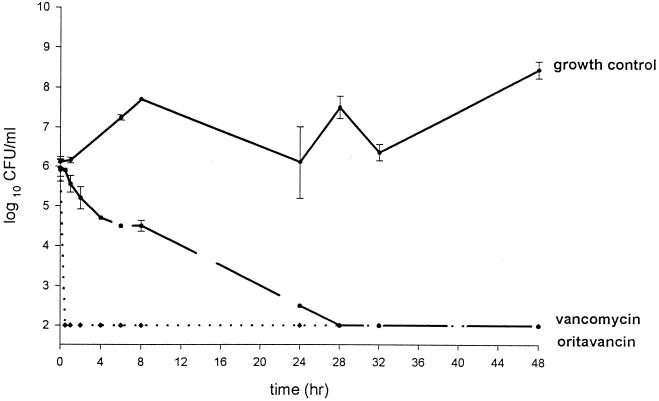
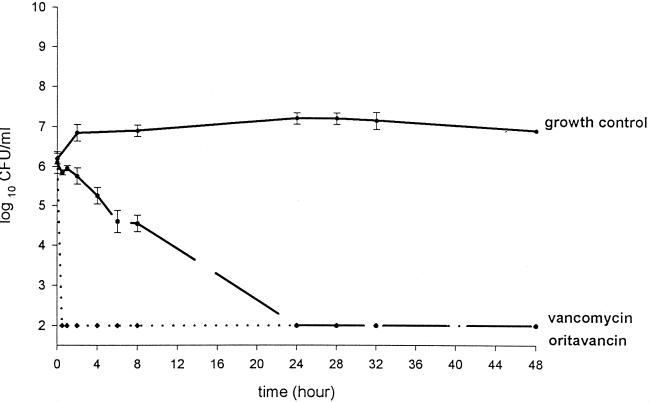
The activities of oritavancin and vancomycin are shown in Fig. 1 and 2. Oritavancin appeared to have more rapid bactericidal activity (P < 0.001) than vancomycin within the first 8 h, with the limit of detection in bacteria (102 CFU/ml) being reached in ≤1 h and remaining over the 48 h period for both R919 and R921. Vancomycin also lowered the colony counts of R919 and R921 to the level of the detection limits, and this action was sustained for both isolates at ≤28 h. There was no observed or statistical difference in CFU per milliliter between oritavancin and vancomycin at 24 and 48 h. Mean AUC from 0 to 24 h (AUC0–24)/MIC and Cmax/MIC ratios can be seen in Table 2. AUC0–24/MIC and Cmax/MIC ratios ranged from 944.5 to 36,505.8 and 72.7 to 7,188.5, respectively. Oritavancin alone showed the highest AUC0–24/MIC and Cmax/MIC ratios, followed by oritavancin plus albumin and then vancomycin. The mean Cmax, t1/2 and AUC0–24 can be seen in Table 3. No resistance to oritavancin or vancomycin was seen with either isolate.
FIG. 1.
Activities of oritavancin (LY333328) and vancomycin against isolate R919. Growth control is represented by the solid line, vancomycin activity is represented by a dashed line, and oritavancin activity is represented by dotted line. Error bars, standard deviations.
FIG. 2.
Activities of oritavancin (LY333328) and vancomycin against isolate R921. Growth control is represented by a solid line, vancomycin activity is represented by a dashed line, and oritavancin activity is represented by a dotted line. Error bars, standard deviations.
TABLE 2.
Pharmacokinetic parameters for vancomycin and oritavancina
| Antibiotic | Cmax (μg/ml) | t1/2 (h) | AUC0–24 |
|---|---|---|---|
| Oritavancin | 107.8 ± 10 | 2.9 ± 0.9b 11.6 ± 4.5c | 547.6 ± 89 |
| Vancomycin | 36.9 ± 1.5 | 5.9 ± 0.5 | 472.3 ± 41 |
Results reported as means ± standard deviations.
At 0 to 8 h.
At 8 to 24 h.
TABLE 3.
AUC0–24/MIC and Cmax/MIC ratios for both vancomycin and oritavancin
| Antibiotic | Ratio (mean ± SD) for R919 and R921
|
|
|---|---|---|
| AUC0–24/MIC | Cmax/MIC | |
| Oritavancina | 36,505.8 ± 5902.4 | 7,188.5 ± 675.5 |
| Oritavancin + albumin | 18,252.9 ± 2951.2 | 3,594.3 ± 337.7 |
| Vancomycin | 944.5 ± 81 | 73.7 ± 3.1 |
Both ratios are based on a MIC of 0.015 μg/ml.
Only serial saline dilution (1:10 or greater) of samples containing oritavancin was effective in carryover avoidance. Incorporation of cholyestyramine, d-Ala–d-Ala, or polyanetholesulfonic acid was less effective in diminishing antibiotic carryover then was serial dilution alone.
DISCUSSION
The incidence of multidrug-resistant S. pneumoniae continues to rise, with resistance to penicillin as high as 30% in some areas of the United States. Macrolide, cephalosporin, and trimethoprim-sulfamethoxazole resistance is also increasing. There is a need for newer agents active against resistant S. pneumoniae. Although the fluoroquinolones still remain viable options to treat resistant S. pneumoniae infections, the threat of resistance due to their increased use is evident. Reports from Canada and Hong Kong demonstrate an increase in S. pneumoniae fluoroquinolone resistance (4, 7).
S. pneumoniae continues to remain susceptible to vancomycin (MIC, 0.125 to 0.5 μg/ml); however, vancomycin-tolerant strains have been reported (5, 10). The new glycopeptide oritavancin may potentially be an alternative for treatment of multidrug-resistant S. pneumoniae. Previous studies have found MICs of oritavancin for S. pneumoniae to be around ≤0.01 to 0.25μg/ml (5). Our study showed that the two multidrug-resistant S. pneumoniae isolates were very susceptible to oritavancin (MICs, ≤0.015 to 0.03 μg/ml) and displayed significant killing in the 48 h models, with the limit of detection (102 CFU/ml) being attained in ≤1 h.
Unfortunately, in our study, antibiotic carryover could not be ruled out for some of our sampling points. This would suggest that kill curve data from the models at early time points where the oritavancin concentrations were highest might have overestimated oritavancin's activity. However, on the basis of the very low MICs obtained for isolates R919 and R921 and kill curve data from the pharmacodynamic models in which antibiotic carryover could be accounted for, oritavancin was capable of killing these organisms to the limits of detection early in the experiment, as is depicted by the data. Our data also suggested that there were no detectable effects of protein on oritavancin's bactericidal activity. Although minimal changes in MIC were seen by the addition of protein to the media, concentrations simulated in the model representing human pharmacokinetics appeared to be high enough that the effect of protein was not observed or was minimized. This is consistent with data from previous reports for oritavancin against Staphylococcus aureus and enterococci (2, 6, 8, 9, 12).
In conclusion, compared to vancomycin, oritavancin demonstrated more rapid and complete killing of two multidrug-resistant S. pneumoniae isolates. In light of increasing S. pneumoniae resistance, further investigation with oritavancin as a potential treatment option is warranted.
ACKNOWLEDGMENTS
We acknowledge Eli Lilly and Company for partial support of this project and Abbott Diagnostics for the fluorescence polarization immunoassay used in determining the vancomycin concentrations.
REFERENCES
- 1.Arthur M F, Depardieu F, Reynolds P, Courvalin P. Moderate resistance to glycopeptide LY333328 mediated by genes of the vanA and vanB clusters in enterococci. Antimicrob Agents Chemother. 1999;43:1875–1880. doi: 10.1128/aac.43.8.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baltch A L, Smith R P, Ritz W J, Bopp L H. Comparison of inhibitory and bactericidal activities and postantibiotic effects of LY333328 and ampicillin used singly and in combination against vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother. 1998;42:2564–2568. doi: 10.1128/aac.42.10.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cappelletty D M, Rybak M J. Bactericidal activities of cefprozil, penicillin, cefaclor, cefixime, and loracarbef against penicillin-susceptible and resistant Streptococcus pneumoniae in an in vitro pharmacodynamic infection model. Antimicrob Agents Chemother. 1996;40:1148–1152. doi: 10.1128/aac.40.5.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen D K, McGeer A, De Azavedo J C, Low D E. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N Engl J Med. 1999;341:233–239. doi: 10.1056/NEJM199907223410403. [DOI] [PubMed] [Google Scholar]
- 5.Garrote F G, Cercenado E, Alcala L, Bouza E. In vitro activity of the new glycopeptide LY333328 against multiply resistant gram-positive clinical isolates. Antimicrob Agents Chemother. 1998;42:2452–2455. doi: 10.1128/aac.42.9.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hershberger E, Aeshlimann J R, Moldovan T, Rybak M J. Evaluation of bactericidal activities of LY333328, vancomycin, teicoplanin, ampicillin-sulbactam, trovafloxacin, and RP59500 alone or in combination with rifampin or gentamicin against different strains of vancomycin-intermediate Staphylococcus aureus by time-kill curve methods. Antimicrob Agents Chemother. 1999;43:717–721. doi: 10.1128/aac.43.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho P, Que T, Tsang D N, Ng T, Chow K, Seto W. Emergence of fluoroquinolone resistance among multiply resistant strains of Streptococcus pneumoniea in Hong Kong. Antimicrob Agents Chemother. 1999;43:1310–1313. doi: 10.1128/aac.43.5.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mercier R C, Houlihan H H, Rybak M J. Pharmacodynamic evaluation of a new glycopeptide, LY333328, and in vitro activity against Staphylococcus aureus and Enterococcus faecium. Antimicrob Agents Chemother. 1997;41:1307–1312. doi: 10.1128/aac.41.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mercier R C, Penzak S R, Rybak M J. In vitro activities of an investigational quinolone, glycylcycline, glycopeptide, streptogramin, and oxazolidinone tested alone and in combinations against vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother. 1997;41:2573–2575. doi: 10.1128/aac.41.11.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility test for bacteria to grow aerobically. 3rd ed. Approved standard M7–A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 11.Novak R, Henriques B, Charpentier E, Normark S, Tuomanen E. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature. 1999;399:590–593. doi: 10.1038/21202. [DOI] [PubMed] [Google Scholar]
- 12.Zhanel G G, Kirkpatrick I D C, Hoban D J, Kabani A M, Karlowsky J A. Influence of human serum on pharmacodynamic properties of an investigational glycopeptide, LY333328, and comparator agents against Staphylococcus aureus. Antimicrob Agents Chemother. 1998;42:2427–2430. doi: 10.1128/aac.42.9.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]