Abstract
Objective
A major factor in the growing world-wide epidemic of obesity and type 2 diabetes is the increased risk of transmission of metabolic disease from obese mothers to both first (F1) and second (F2) generation offspring. Fortunately, recent pre-clinical studies demonstrate that exercise before and during pregnancy improves F1 metabolic health, providing a potential means to disrupt this cycle of disease. Whether the beneficial effects of maternal exercise can also be transmitted to the F2 generation has not been investigated.
Methods
C57BL/6 female mice were fed a chow or high-fat diet (HFD) and housed in individual cages with or without running wheels for 2 wks before breeding and during gestation. Male F1 offspring were sedentary and chow-fed, and at 8-weeks of age were bred with age-matched females from untreated parents. This resulted in 4 F2 groups based on grandmaternal treatment: chow sedentary; chow trained; HFD sedentary; HFD trained. F2 were sedentary and chow-fed and studied up to 52-weeks of age.
Results
We find that grandmaternal exercise improves glucose tolerance and decreases fat mass in adult F2 males and females, in the absence of any treatment intervention of the F1 after birth. Grandmaternal exercise also improves F2 liver metabolic function, including favorable effects on gene and miRNA expression, triglyceride concentrations and hepatocyte glucose production.
Conclusion
Grandmaternal exercise has beneficial effects on the metabolic health of grandoffspring, demonstrating an important means by which exercise during pregnancy could help reduce the worldwide incidence of obesity and type 2 diabetes.
Keywords: Exercise, Glucose metabolism, Intergenerational effects, F2
Highlights
-
•
Grandmaternal exercise has profound effects on the metabolic health of grandoffspring as they age.
-
•
Grandmaternal exercise reverses the detrimental effects of grandmaternal high-fat diet on grandoffspring insulin sensitivity.
-
•
Grandmaternal exercise results in improved liver metabolic function in grandoffspring.
-
•
There is sex-specific regulation of glucose tolerance and insulin sensitivity in mice as they age.
1. Introduction
Type 2 diabetes (T2D) is a multi-factorial disease that arises from a combination of environmental and genetic factors. Environmental exposures can start early in life, and it is now well-recognized that poor maternal lifestyle can alter the intrauterine environment resulting in increased risk of disease transmission to offspring [1]. Studies focusing on metabolic disease have shown that under-nutrition in both humans [[2], [3], [4]] and rodent models [5,6] can increase the risk of first-generation (F1) offspring developing glucose intolerance and T2D later in life. Over-nutrition associated with poor diets during pregnancy in humans [7] and rodent models [[8], [9], [10], [11]] also increases the risk for development of obesity and T2D in F1 offspring. Thus, exposure of pregnant mothers (F0) to diet and nutritional stressors can generate a detrimental cycle of metabolic dysfunction, propagating the risk of metabolic disease to the next generation.
Maternal environment during pregnancy can affect not only F1, but also F2 through intergenerational effects [12]. When the pregnant F0 is exposed to an environmental stress the F1 germ cell precursors are simultaneously exposed to the stressor. These exposed F1 germs cells become the adult gametes that create the F2, thus affecting the grandoffspring [12]. Of the few human studies investigating environmental exposures in grandoffspring, under-nutrition during pregnancy was shown to increase adiposity [13] and obesity [14] in F2 adults, making them more prone to develop metabolic diseases. Grandparents’ health has been associated with F2 child and adolescent BMI and growth rate, showing that a healthy lifestyle of grandparents could reduce the risk of obesity in the grandchildren [15]. Since investigating the effects of maternal environmental exposures in second-generation middle-aged humans would take at least 6 decades, rodent models have been used for this purpose. Studies in mice show that under-nutrition in F0 mothers programmed the F2 generation to develop glucose intolerance and obesity [16], and exposing F0 mothers to over-nutrition during pregnancy resulted in the F2 generation developing a diabetic phenotype, even when the F1 generation was fed a standard chow-diet [17]. Similar results were found in rats, where grandmaternal high-fat diet during pregnancy resulted in F2 with glucose intolerance and increased body weight due to beta-cell dysfunction [18].
It is well known that exercise improves the health of people with T2D [19] and that exercise during pregnancy can protect the mother against the development of excessive weight gain, gestational diabetes and hypertension [20,21]. In addition to exercise improving the mother's health, importantly, studies of F0 maternal exercise revealed enhanced cardiac autonomic health, neuro-motor development, and reduced adiposity in F1 children aged 5 days to 6 months postpartum [[22], [23], [24], [25], [26]]. While maternal exercise has been shown to have these beneficial effects in young children, given the length of time needed for study, it is not surprising that the effects of maternal exercise on offspring metabolic health in humans has not been determined in adulthood, the stage of life where diabetes typically develops. Studies using rodent models have demonstrated that maternal exercise has marked effects on metabolic health in adult F1 offspring including prevention of glucose intolerance [8,9,11,[27], [28], [29]], reduction in insulin concentrations [[8], [9], [10]], decrease in body fat [8,9,27,30], and improved liver function [9]. While these studies clearly show that maternal exercise is a beneficial environmental exposure that improves the metabolic health of the F1 offspring, the effects of maternal exercise on the second generation are not known. Here, we determined that there are striking effects of grandmaternal exercise on the metabolic health of F2 as they age, suggesting that maternal exercise is an effective means to help prevent obesity and diabetes in multiple generations.
2. Research design and methods
2.1. Study approval
All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH) and were approved by the Joslin Diabetes Center Institutional Animal Care and Use Committee.
2.2. Mice and exercise paradigm
Beginning at 6-weeks of age, C57BL/6N virgin female mice (Charles River Laboratories) were singularly housed at 23 °C on a 12/12 h light/dark cycle. Mice were fed a chow (21% kcal from fat) or high-fat diet (60% fat) (9F5020 and D12492 HFD; Research Diets Inc.) and were randomly assigned to cages with (Exercise) or without (Sedentary) running wheels (Tecniplast) [8,9]. Females that ran less than 3 km/day pre-breeding were excluded from study. Two weeks after diet and exercise were started, one virgin male (sedentary and chow-fed) and two virgin females (one sedentary, one trained) were housed together for 3 days in cages without running wheels. After breeding, females were returned to their cages to continue their assigned exercise and diet treatments until pups were born. A schematic of the study is shown in Figure 1A.
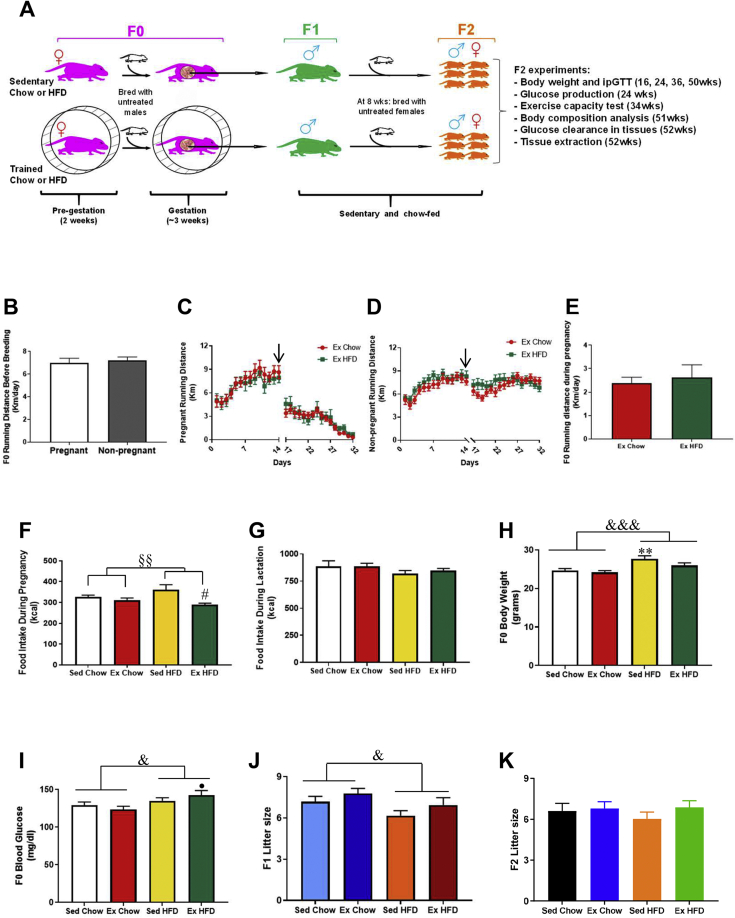
Figure 1.
Effects of grandmaternal diet and exercise on F0 phenotype and litter size. Schematic of the study (A). Running distance before breeding (2 weeks) in pregnant and non-pregnant females (B). Pre- and post-running distance from female mice that became pregnant (C) and from female mice that did not get pregnant (D). Black arrow: breeding start day (3 days without running). Running distance during pregnancy in Ex Chow and Ex HFD groups (E). Food intake during pregnancy (F). Food intake during lactation (G). Final body weight (H) and glycaemia (I) 3 wks after the end of exercise. F1 litter size (J) and F2 litter size (K). Two-way ANOVA: exercise main effect (§), diet main effect (&). Exercise main effect on food intake during pregnancy (P < 0.01). Diet main effect on body weight (P < 0.01), glycemia (P < 0.05) and F1 litter size (P < 0.05). Tukey's post-hoc test: ∗∗P < 0.01 vs. Sed Chow. #P < 0.05 vs. Sed HFD. •P < 0.05 vs. Ex Chow. Data are expressed as the mean ± SEM (n = 18–76/group).
The running wheels were locked at pup birth and two days after parturition litters were culled to 4–6 mice. After weaning, male F1 offspring were sedentary and chow-fed, and at 8-weeks of age the mouse with the weight closest to the average body weight of each litter was chosen to breed with age-matched virgin females that were sedentary, chow-fed and from untreated parents. F2 pups were weighed and culled to 4–6 mice per litter. If litters had less than 4 pups, then pups from other litters with more than 6 were added and fostered until weaning (day 21). These cross-fostered pups were euthanized at weaning. All F2 pups were left with their birthmothers until weaning, and then housed in groups (3–5 mice per cage) with their same sex siblings. F2 were housed in static cages (sedentary) from birth onwards and fed chow diet. Both male and female F2 were studied up to 52-weeks of age. Thus, four groups of F2 were generated based on their grandmaternal treatment: sedentary chow-fed (Sed Chow), exercise chow-fed (Ex Chow), sedentary high-fat fed (Sed HFD), and exercise high-fat fed (Ex HFD). Over the course of this study, 8 cohorts of F2 were generated, and more than 350 F2 mice were studied. Each cohort required 65 weeks of experiments, from F0 pre-breeding treatment to F2 at 52-weeks of age. Each litter corresponds to ∼3 mice per sex, with each experiment utilizing 3–16 litters. For measurements of glucose tolerance, body weight, fasting glucose, and food intake all mice from each litter were studied. For DEXA, exercise capacity, glucose clearance, and liver analysis, one male and one female mouse from each litter was selected for analysis based on having a body weight closest to the mean body weight for the entire litter.
2.3. Physiological parameters
For intraperitoneal glucose tolerance tests (ipGTT), F2 mice were fasted overnight (9pm-9am) and ipGTT performed as described [31]. Blood glucose response to the ipGTT was calculated as the area under the curve minus baseline for each mouse according to the trapezoidal method [32,33]. Plasma insulin concentrations were measured by ELISA (Crystal Chem, 90080) and HOMA-IR calculated using fasted plasma glucose values multiplied by the fasted serum insulin, divided by 22.5. For glucose clearance measurements, 52-week old mice were fasted overnight and tissue glucose uptake measured [34], an accumulation of 2-[3H]-deoxy-glucose-6-P in tissues determined, and glucose clearance rates calculated [35,36]. Offspring body weights were measured at 16, 24, 36 and 50-weeks of age. Food intake was measured weekly from weaning to 24-weeks of age and reported as kcal/day. At 51-weeks of age body composition measurements were made including lean mass, fat mass and bone mineral density by DEXA (Lunar PIXImus2). Exercise capacity was measured at 34-weeks of age [37].
2.4. Liver analyses
At 20-weeks of age, mice were anesthetized (pentobarbital; 100 mg/kg) and hepatocytes were perfused and collected for glucose production experiments [38]. Using separate mice from those where hepatocytes were isolated, at 52-weeks of age, mice were anesthetized, and livers were dissected. Gene expression was analyzed by qPCR (Table 1) [38]. For miRNAs analyzes, 100 ng of hepatic total RNA were used for RT reaction (miRCURY LNA RT; 339340; Qiagen). cDNA samples were analyzed by qPCR (miRCURY LNA miRNA PCR Assay; Qiagen; Table 2). qPCR was performed using ABI7900 RTPCR (Life Technologies) and fold change was calculated using the ΔΔCT method. Triglyceride and glycogen were assayed using ∼25 mg liver [39,40].
Table 1.
Primer sequences used to analyze liver F2 mRNA expressions.
| Target gene | Forward | Reverse |
|---|---|---|
| G6pc | AGGTCGTGGCTGGAGTCTTGTC | GTAGCAGGTAGAATCCAAGCGC |
| Fbp1 | TGCTGAAGTCGTCCTACGCTAC | TTCCGATGGACACAAGGCAGTC |
| Pgc1a | CCCTGCCATTGTTAAGACC | TGCTGCTGTTCCTGTTTTC |
| Pck1 | GGCGATGACATTGCCTGGATGA | TGTCTTCACTGAGGTGCCAGGA |
| Pklr | CGAAAAGCCAGTGATGTGGTGG | GATGCCATCGCTCACTTCTAGG |
| Pcx | GGATGACCTCACAGCCAAGCAT | GCAATCGAAGGCTGCGTACAGT |
| Pfkl | CCATCAGCAACAATGTGCCTGG | TGAGGCTGACTGCTTGATGCGA |
| Pdha1 | GTGAGAACAACCGCTATGGCATG | CGCAAACTTTGTTGCCTCTCGG |
| Pdk4 | ATCTAACATCGCCAGAATTAAACC | GGAACGTACACAATGTGGATTG |
| Cs | GACTACATCTGGACACACTCAATTCA | CGAGGGTCAGTCTTCCTCAGTAC |
| Idh3a | GCAGGACTGATTGGAGGTCTTG | GCCATGTCCTTGCCTGAATGT |
| Mdh2 | TCACTCCTGCTGAAGAACAGCC | CCTTTGAGGCAATCTGGCAACTG |
| Ogdh | GGTGTCGTCAATCAGCCTGAGT | ATCCAGCCAGTGCTTGATGTGC |
| Cd36 | ATGGGCTGTGATCGGAACTG | AGCCAGGACTGCACCAATAAC |
| Fatp4 | GACTTCTCCAGCCGTTTCCACA | CAAAGGACAGGATGCGGCTATTG |
| Acox1 | GGGAGTGCTACGGGTTACATG | CCGATATCCCCAACAGTGATG |
| Cpt1a | CAGCGAGTAGCGCATAGTCA | TGAGTGGCGTCCTCTTTGG |
| Lcad | TTTCCGGGAGAGTGTAAGGA | ACTTCTCCAGCTTTCTCCCA |
| Rpl13a | CTGCTCTCAAGGTTGTTCGGCT | CCTTCCGTTTCTCCTCCAGAGT |
Table 2.
Primers used to analyze liver F2 miRNA expressions.
| Target gene | GeneGlobe ID | Cat# |
|---|---|---|
| hsa-miR-23a-3p | YP00204772 | 339306 |
| hsa-miR-33-5p | YP00205690 | 339306 |
| hsa-miR-339-5p | YP00206007 | 339306 |
| hsa-miR-532-3p | YP00204003 | 339306 |
| Control primer set U6 snRNA (hsa, mmu) | YP00203907 | 339306 |
2.5. Statistical analysis
Data are means ± SEM. Unpaired t-test were used to compare sedentary vs trained F0. For F2 analysis, two-way analysis of variance (ANOVA) was performed followed by Tukey's post hoc test. A P value < 0.05 was statistically significant. Since the grandmother was treated, the “n” was determined by litter, not by the number of mice.
3. Results
3.1. Grandmaternal diet and exercise do not affect F2 litter size
Approximately 40% of mice were impregnated, and pre-breeding wheel running activity was not different between pregnant and non-pregnant females (Figure 1B). Pregnant dams decreased their running distance by ∼50% upon return to wheel cages post-breeding and continued to gradually decrease until delivery (Figure 1C), whereas there was no decrease in running distance post-breeding in females that did not get pregnant (Figure 1D). Although pregnancy decreased running distance the pregnant females still performed considerable running activity during gestation (2.49 ± 0.27 km/day), and there was no difference in running distance during pregnancy between chow and high-fat fed dams (Figure 1E).
Caloric intake in F0 during pregnancy was not different between chow-fed sedentary and trained females and was higher in high-fat fed sedentary compared to high-fat fed trained mice (Figure 1F), whereas there was no difference in caloric intake during the lactation period (Figure 1G). High-fat feeding of dams increased both dam body weight and glycemia, when measured after weaning, and therefore ∼3 weeks after training ceased (Figure 1H,I). Maternal high-fat diet decreased (Figure 1J), while maternal exercise tended to increase F1 litter size (P = 0.09). In contrast, there was no effect of grandmaternal diet or exercise on F2 litter size (Figure 1K).
3.2. Grandmaternal exercise decreases body weight and fat mass in F2 as they age
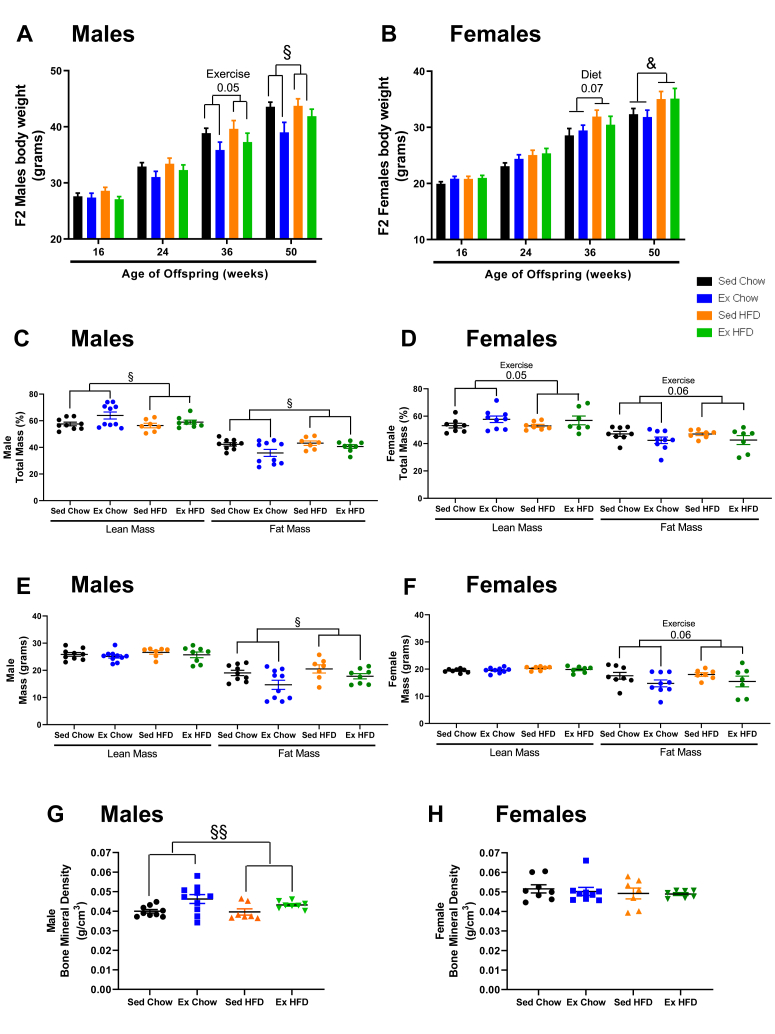
To investigate the effects of grandmaternal diet and exercise on the F2 generation, 8-week-old F1 male offspring from the four F0 treatment groups were bred with 8-week-old females from untreated mothers. There was no treatment of the F1 used for breeding or F2. Grandmaternal exercise did not affect F2 male offspring body weights at 16 or 24-weeks of age, but by 36 and 50-weeks there was a main effect of grandmaternal exercise to decrease F2 male offspring body weight independent of grandmaternal diet (Figure 2A). In contrast to the F2 males, there was no effect of grandmaternal exercise on female F2 body weight at any age. At 50-weeks, F2 females from high-fat fed grandmothers had increased body weights compared to F2 from chow-fed dams (Figure 2B). Food consumption in male and female F2 was not affected by grandmaternal diet or exercise (Suppl. Fig. S1A,B). Male and female F2 from exercise trained dams had a lower percent fat mass and higher percent lean mass at 51-weeks (Figure 2C,D). By analyzing the fat and lean mass per gram body weight, we found that these differences in percent fat and lean mass were due to a decrease only in fat mass (Figure 2E,F). Bone mineral density was increased in the F2 males from the grandmaternal exercise groups, but not in F2 females (Figure 2G,H). Exercise capacity in the F2 was not different among groups (Suppl. Fig. S1C,D). These results demonstrate that grandmaternal exercise, even in the absence of treatment of F1, has numerous beneficial effects on parameters of fat and bone health of the F2.
Figure 2.
Effects of grandmaternal exercise on F2 body weights and composition. Body weight of male (A) and female (B) F2 during the first year of life. Fat mass and lean mass in F2 male (C, E) and female (D, F) offspring and bone mineral density in F2 male (G) and female (H) offspring measured by DEXA (Lunar PIXImus2 mouse densitometer) at 51 wks of age from sedentary and trained grandmothers (F0) fed a chow (21% fat) or high-fat diet (60% fat). Two-way ANOVA: exercise main effect (§), diet main effect (&). Exercise tendency effect at 36wks (males P = 0.05), diet tendency effect at 36wks (females P = 0.07). Exercise effect at 50wks (males P = 0.02), diet effect at 50wks (females P = 0.03). Exercise effect on male % lean mass and % fat mass (C, P = 0.03); exercise tendency on female % lean mass and % fat mass (D, P = 0.05, P = 0.06). Exercise main effect on fat mass in grams in male (E, P = 0.01) and female (F, P = 0.06). Exercise main effect on male BMD (G, P = 0.005). Data are means ± SEM. N = 7–16 litter/group.
3.3. Grandmaternal exercise improves glucose tolerance and insulin sensitivity in F2
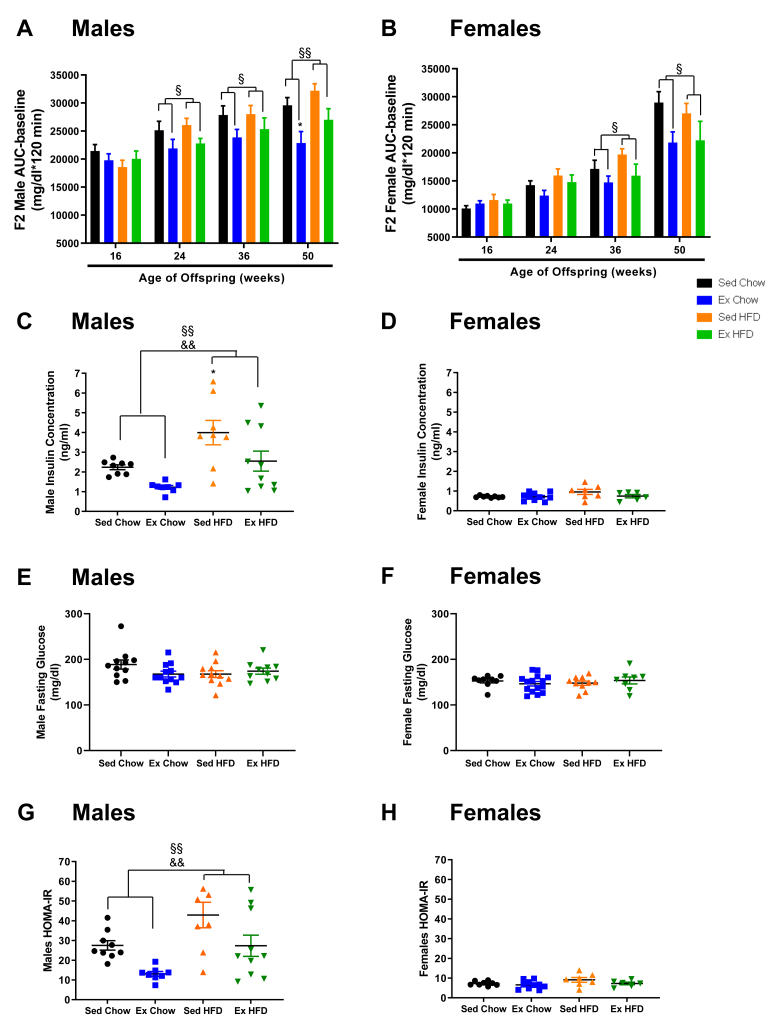
To investigate the effects of grandmaternal diet and exercise on systemic metabolism in the F2 generation, we performed glucose tolerance tests and calculated HOMA-IR (Figure 3A,B,G,H). For the F2 males, as they aged, there was a worsening of glucose tolerance in offspring from sedentary grandmothers. However, beginning at 24-weeks of age and through 36 and 50-weeks, if the grandmothers had trained, the F2 had marked improvements in glucose tolerance (Figure 3A). F2 females were similar to males, with female F2 from exercise trained grandmothers maintaining their glucose tolerance as they aged, whereas F2 from sedentary grandmothers had a worsening of glucose tolerance (Figure 3B). Grandmaternal high-fat diet did not have a main effect on glucose tolerance in F2 of either sex.
Figure 3.
Grandmaternal exercise training improves glucose tolerance and insulin sensitivity in F2. GTT (2g glucose/Kg bw, i.p.) of F2 male (A) and female (B) offspring. Fasting insulin concentrations (C, D), fasting glucose concentrations (E, F) and HOMA-IR (G, H) of F2 male and female offspring at 50 wks of age from sedentary and trained grandmothers (F0) fed a chow (21% fat) or high-fat diet (60% fat). Two-way ANOVA: exercise main effect (§), diet main effect (&). Exercise effect on glucose tolerance at 24wks (males P = 0.01), at 36wks (males P = 0.04, females P = 0.03) and at 50wks (males P = 0.001, females P = 0.01). Insulin in males: exercise effect (P = 0.007) and diet effect (P = 0.001). HOMA-IR in males: exercise effect (P = 0.002), diet effect (P = 0.002). Tukey's post-hoc test: ∗P < 0.05 vs. Sed C how. Data are mean ± SEM. N = 7–16 litter/group.
There were striking effects of grandmaternal exercise and diet on fasting insulin concentrations in F2 males. Insulin concentrations measured in F2 at 50-weeks were lower in F2 males of trained grandmothers, while grandmaternal high-fat diet resulted in higher F2 insulin concentrations (Figure 3C). Fasting glucose concentrations were not altered by grandmaternal diet or exercise in F2 males (Figure 3E). HOMA-IR demonstrated that grandmaternal exercise improved insulin sensitivity, while grandmaternal high-fat diet caused insulin resistance in F2 males (Figure 3G). Interestingly, insulin concentrations were markedly lower in F2 females compared to males, independent of grandmaternal treatment (Figure 3D). There was no effect of grandmaternal diet or exercise on insulin concentrations and fasting glucose in F2 females, although there was a tendency for higher insulin levels and HOMA-IR in F2 females from high-fat diet fed grandmothers (Figure 3D,F,H). These results demonstrate that grandmaternal exercise has marked effects to improve glucose homeostasis and insulin sensitivity in F2.
The improvement in glucose homeostasis in adult F2 from trained grandmothers could be related to the lower body weights that occur at some time points, but since the improvements in glucose tolerance preceded the lower F2 body weights, additional mechanisms must be involved. We next determined if the mechanism for the improved glucose tolerance in F2 from trained grandmothers was due to increases in glucose clearance in F2 muscle and adipose tissue. Interestingly, there was no effect of grandmaternal exercise on glucose clearance in F2 males or females (Suppl. Fig. S2). F2 male offspring from high-fat fed grandmothers had lower glucose clearance in tibialis anterior muscle, soleus muscle, gastrocnemius muscle, and perigonadal adipose tissue (Suppl. Fig. S2A), whereas there was no effect of grandmaternal diet on glucose clearance in any tissues from F2 females (Suppl. Fig. S2B). Lower rates of glucose clearance only in F2 males is consistent with the HOMA-IR data which show that high-fat feeding of grandmothers resulted in decreased insulin sensitivity only in F2 males.
3.4. Grandmaternal exercise affects F2 liver
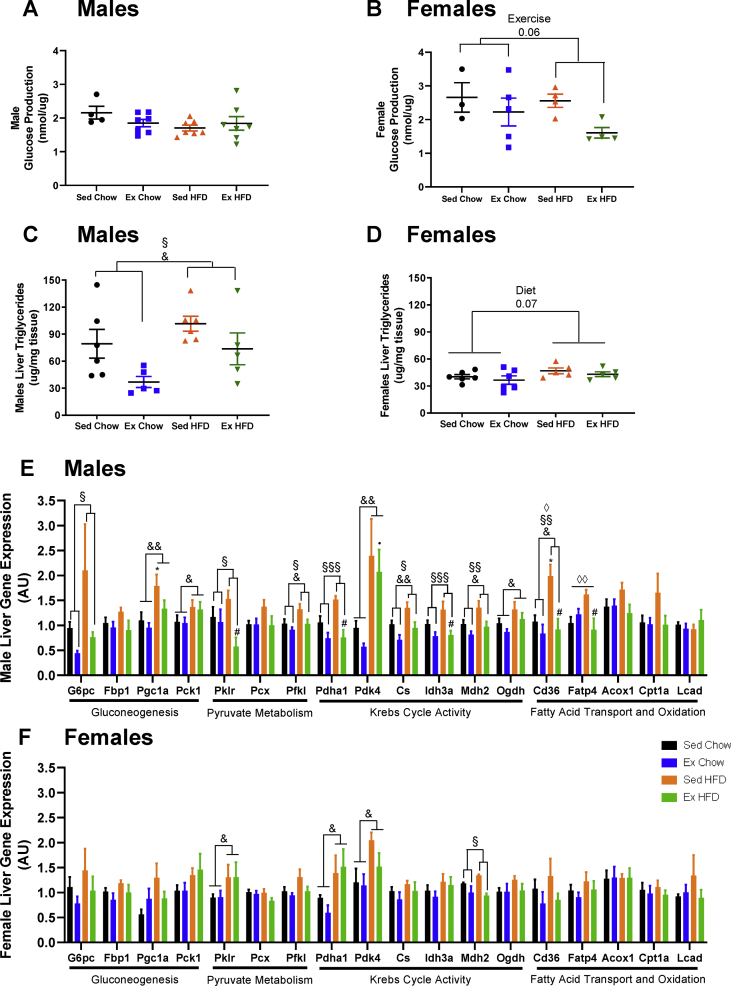
Given the lack of effect of grandmaternal exercise on F2 glucose clearance in muscle and adipose tissues, we next focused on liver, another tissue important in the regulation of glucose homeostasis. Basal rates of hepatic glucose production (HGP) were measured in isolated hepatocytes from F2 male and female offspring at 20-weeks, the oldest age that we find hepatocytes remain viable for HGP measurements. HGP was not different in F2 males from trained grandmothers (Figure 4A) whereas there was a tendency (P = 0.06) for decreased HGP in F2 females from trained grandmothers (Figure 4B). Liver triglyceride concentrations in F2 males at 52-weeks were higher if grandmothers were fed a high-fat diet, while grandmaternal exercise under both chow and high-fat diet conditions resulted in lower liver triglyceride concentrations (Figure 4C). F2 females had strikingly lower liver triglyceride concentrations compared to F2 males (Figure 4C,D). Grandmaternal exercise did not lower liver triglyceride concentrations in the F2 females, perhaps due to the already low concentrations, and high-fat diet fed grandmothers had a tendency for an increase in triglyceride concentrations (Figure 4D). Liver glycogen concentrations were not affected by grandmaternal diet or exercise in male or female F2 (Suppl. Fig. S3A,B). Thus, the effects of grandmaternal exercise on F2 glucose homeostasis includes changes in liver phenotype of F2.
Figure 4.
Effects of grandmaternal exercise on F2 liver phenotype. Basal glucose production in hepatocytes from F2 male (A) and female (B) offspring at 20 wks of age. Triglyceride content in liver from F2 male (C) and female (D) offspring at 52 wks of age. Liver gene expression from F2 male (E) and female (F) offspring at 52 wks of age from sedentary and trained grandmothers (F0) fed with a chow (21% fat) or high-fat diet (60% fat). Two-way ANOVA: exercise main effect (§), diet main effect (&), interaction (◊). Exercise main effect on female hepatic glucose production (B, P = 0.06). Exercise main effect (males TG P = 0.01). Diet main effect (males TG P = 0.03; females TG P = 0.07). Data are mean ± SEM. N = 5–6 litter/group. Tukey's post-hoc test: ∗P < 0.05 vs. Sed Chow, #P < 0.05 vs. Sed HFD, •P < 0.05 vs. Ex Chow. Data are mean ± SEM. N = 3–10 litter/group.
Because grandmaternal exercise affected F2 HGP and liver triglycerides we next measured the expression of numerous liver metabolic genes in F2. We have previously demonstrated that alternations in key liver metabolic genes in F1 offspring are an important mechanism by which maternal exercise significantly improves glucose homeostasis in F1 [9,38]. To determine if a similar adaptation to liver gene expression occurs in F2, we measured the expression of numerous genes involved in gluconeogenesis, pyruvate metabolism, Krebs cycle activity, and fatty acid transport and oxidation in livers from male (Figure 4E) and female (Figure 4F) F2 at 52-weeks. Grandmaternal diet resulted in an upregulation of numerous metabolic genes in F2 males, and grandmaternal exercise reversed some of these changes. Grandmaternal exercise or diet did not affect the expression of the housekeeping gene, Rpl13a. G6pc, a key regulator of gluconeogenesis, was significantly decreased in F2 males (Figure 4E) from exercise trained grandmothers and tended to decrease in F2 females (P = 0.1) (Figure 4F). Micro-RNAs (miRNAs) known to repress the expression of G6pc in liver [[41], [42], [43]] were not different in F2 males (Suppl. Fig. S3C) and only had a tendency for change in F2 females (Suppl. Fig. S3D), suggesting other mechanisms are potentially involved in controlling G6pc expression in F2. The fatty acid transporter gene, Cd36, was also decreased with grandmaternal exercise in F2 males (Figure 4E) and tended to decrease (P = 0.09) in F2 females (Figure 4F). Interestingly, Cd36 mRNA expression is positively correlated with hepatic triglyceride concentrations [44], consistent with our observation of decreased triglyceride liver concentrations in F2 from trained grandmothers and similar to what we have previously found in adult F1 females [9].
4. Discussion
While there has been growing evidence in recent years that maternal exercise has important effects to improve the metabolic health of first-generation offspring, remarkably, our current data demonstrate that maternal exercise has similarly robust effects to improve the metabolic health of second generation adult male and female offspring. We make the novel discovery that grandmaternal exercise training results in maintenance of glucose tolerance in F2 as they age, prevents the F2 from gaining excessive fat mass during adult life, and reduces weight gain with aging in F2 males. Moreover, the findings of decreased insulin concentrations and HOMA-IR in adult F2 male offspring demonstrate improved insulin sensitivity, suggesting that grandmaternal exercise may improve beta-cell function in F2. Another intriguing finding is that grandmaternal exercise resulted in increased bone density in F2 males. Although to our knowledge bone density has not been widely studied in the context of maternal exercise, interestingly, maternal exercise was reported to increase several genes in bone related to osteogenesis in F1 offspring [45]. Other tissues may also be involved, for example it will be interesting to study brown adipose tissues, since it has recently been implicated in maternal exercise effects on F1 [46]. Thus, grandmaternal exercise has effects on multiple F2 tissues, and all likely contribute to the health of the second-generation offspring.
The underlying mechanisms by which grandmaternal exercise improves F2 metabolic health is not known, but several factors are undoubtedly involved, including both direct and indirect exposures of the F2 [47,48]. A direct exposure occurs when the exercise performed by the pregnant female affects the F2 germ cells within the F1, while an indirect exposure of F2 occurs due to improvements in F1 metabolic health caused by F0 exercise. We studied F1 males and therefore the direct and/or indirect effects were transmitted through the F1 sperm. Future epigenetic studies of germ cells in utero and mature sperm of F1 male breeders will facilitate determining mechanisms of transmission, since previous studies of F1 sperm after exposure to adverse maternal environments including under-nutrition, over-nutrition and exercise have shown changes in DNA methylation [49] and small noncoding-RNAs [50,51].
In addition to the striking effects of grandmaternal exercise on F2, our study demonstrates that male and female offspring are metabolically distinct during the first year of life. Glucose tolerance, serum insulin, HOMA IR, and liver triglyceride content were all more favorable in F2 females, regardless of the grandmaternal treatment. This metabolically healthier phenotype of the F2 female mice was probably the reason that the beneficial effects of grandmaternal exercise was generally less pronounced compared to male F2. Previous work from our group also showed this healthier phenotype in the female offspring compared with males [9,38]. Another finding of note is that while grandmaternal high-fat diet worsened F2 insulin concentrations, HOMA-IR, and muscle and fat glucose clearance, we found no worsening of glucose tolerance in F2. This is interesting because it implicates duration of the high-fat feeding prior to conception as a determinant of F2 glucose tolerance, since previous studies using 8–9 weeks of pre-conception high-fat feeding showed impaired F2 glucose tolerance [17,18]. Thus, while grandmothers have tolerance to shorter periods of high fat feeding during pregnancy, the beneficial effects of exercise can occur in a shorter period of time.
One limitation of our study is that it was restricted to F2 generated from F1 males. F1 females may also transmit effects of grandmaternal exercise to the F2 generation, and this will be important to investigate in future studies. Another important area of investigation will be to understand if the beneficial effects of grandmaternal exercise in mice will translate to humans. We hypothesize they will, since human studies demonstrating the deleterious effects of grandmaternal under-nutrition on grandchildren in adulthood suggest that environmental exposures can be passed on to the F2 generation [13,14].
5. Conclusion
We conclude that grandmaternal exercise, in the absence of treatment of the F1 generation, has remarkable beneficial effects on the metabolic health of the F2 generation, demonstrating that maternal exercise is an advantageous maternal exposure that is transmitted through multiple generations. These findings suggest that maternal exercise could be an important tool to combat the detrimental cycle of metabolic dysfunction caused by obesity and type 2 diabetes in women of child-bearing age.
Funding
This work was supported by NIH awards R01 DK101043 (LJG), P30 DK036836 (Joslin Diabetes Center), and by American Diabetes Association grant #1-17-PMF-009 (ABA-W). JK was supported by research fellowships from Sunstar Foundation, JSPS Overseas Research Fellowships, Kanae Foundation for the promotion of medical science, and Meiji Yasuda Life Foundation of Health and Welfare.
Author contributions
A.B.A-W performed experiments analyzed the data and wrote and edited the manuscript. J. K. provided oversight for the epigenetics experiments and helped with animal experiments. K. R. helped with the epigenetics experiments. P. N. and N. M. helped with all animal experiments. M.F.H. performed experiments and helped analyzed the data. L.J.G. designed experiments, analyzed the data, and wrote and edited the manuscript. L.J.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All the authors read and approved the final manuscript.
Acknowledgments
The authors thank Afsah Dean, Allen Clermont, Meghan Halpin and Park Kyoungmin of the Joslin Diabetes Animal Physiology Core for support with animal studies. We thank Sarah Lessard, Brent Albertson, Noah Prince, Leslie Rowland, Tara MacDonald, Donato Rivas, Roberto Nava, Roeland Jan-Willem Middelbeek, Susana Llopis and Maria Vamvini for many helpful discussion and scientific input.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2022.101490.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Estampador A.C., Franks P.W. Genetic and epigenetic catalysts in early-life programming of adult cardiometabolic disorders. Diabetes, Metabolic Syndrome and Obesity. 2014;7:575–586. doi: 10.2147/DMSO.S51433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lumey L.H., Khalangot M.D., Vaiserman A.M. Association between type 2 diabetes and prenatal exposure to the Ukraine famine of 1932-33: a retrospective cohort study. Lancet Diabetes & Endocrinology. 2015;3(10):787–794. doi: 10.1016/S2213-8587(15)00279-X. [DOI] [PubMed] [Google Scholar]
- 3.Ravelli A.C., van der Meulen J.H., Michels R.P., Osmond C., Barker D.J., Hales C.N., et al. Glucose tolerance in adults after prenatal exposure to famine. The Lancet. 1998;351(9097):173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 4.Roseboom T., de Rooij S., Painter R. The Dutch famine and its long-term consequences for adult health. Early Human Development. 2006;82(8):485–491. doi: 10.1016/j.earlhumdev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Isganaitis E., Jimenez-Chillaron J., Woo M., Chow A., DeCoste J., Vokes M., et al. Accelerated postnatal growth increases lipogenic gene expression and adipocyte size in low-birth weight mice. Diabetes. 2009;58(5):1192–1200. doi: 10.2337/db08-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jimenez-Chillaron J.C., Hernandez-Valencia M., Reamer C., Fisher S., Joszi A., Hirshman M., et al. Beta-cell secretory dysfunction in the pathogenesis of low birth weight-associated diabetes: a murine model. Diabetes. 2005;54(3):702–711. doi: 10.2337/diabetes.54.3.702. [DOI] [PubMed] [Google Scholar]
- 7.Lahti-Pulkkinen M., Bhattacharya S., Wild S.H., Lindsay R.S., Raikkonen K., Norman J.E., et al. Consequences of being overweight or obese during pregnancy on diabetes in the offspring: a record linkage study in Aberdeen, Scotland. Diabetologia. 2019;62(8):1412–1419. doi: 10.1007/s00125-019-4891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanford K.I., Lee M.Y., Getchell K.M., So K., Hirshman M.F., Goodyear L.J. Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring. Diabetes. 2015;64(2):427–433. doi: 10.2337/db13-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanford K.I., Takahashi H., So K., Alves-Wagner A.B., Prince N.B., Lehnig A.C., et al. Maternal exercise improves glucose tolerance in female offspring. Diabetes. 2017;66(8):2124–2136. doi: 10.2337/db17-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raipuria M., Bahari H., Morris M.J. Effects of maternal diet and exercise during pregnancy on glucose metabolism in skeletal muscle and fat of weanling rats. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0120980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laker R.C., Lillard T.S., Okutsu M., Zhang M., Hoehn K.L., Connelly J.J., et al. Exercise prevents maternal high-fat diet-induced hypermethylation of the Pgc-1alpha gene and age-dependent metabolic dysfunction in the offspring. Diabetes. 2014;63(5):1605–1611. doi: 10.2337/db13-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heard E., Martienssen R.A. Transgenerational epigenetic inheritance: myths and mechanisms. Cell. 2014;157(1):95–109. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Painter R.C., Osmond C., Gluckman P., Hanson M., Phillips D.I., Roseboom T.J. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG. 2008;115(10):1243–1249. doi: 10.1111/j.1471-0528.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 14.Veenendaal M.V., Painter R.C., de Rooij S.R., Bossuyt P.M., van der Post J.A., Gluckman P.D., et al. Transgenerational effects of prenatal exposure to the 1944-45 Dutch famine. BJOG. 2013;120(5):548–553. doi: 10.1111/1471-0528.12136. [DOI] [PubMed] [Google Scholar]
- 15.Ding M., Strohmaier S., Schernhammer E., Yuan C., Sun Q., Michels K.B., et al. Grand-maternal lifestyle during pregnancy and body mass index in adolescence and young adulthood: an intergenerational cohort study. Scientific Reports. 2020;10(1):14432. doi: 10.1038/s41598-020-71461-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jimenez-Chillaron J.C., Isganaitis E., Charalambous M., Gesta S., Pentinat-Pelegrin T., Faucette R.R., et al. Intergenerational transmission of glucose intolerance and obesity by in utero undernutrition in mice. Diabetes. 2009;58(2):460–468. doi: 10.2337/db08-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gniuli D., Calcagno A., Caristo M.E., Mancuso A., Macchi V., Mingrone G., et al. Effects of high-fat diet exposure during fetal life on type 2 diabetes development in the progeny. The Journal of Lipid Research. 2008;49(9):1936–1945. doi: 10.1194/jlr.M800033-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y.H., Ye T.T., Liu C.X., Wang L., Chen Y.W., Dong Y. Maternal high-fat diet impairs glucose metabolism, beta-cell function and proliferation in the second generation of offspring rats. Nutrition and Metabolism. 2017;14:67. doi: 10.1186/s12986-017-0222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savikj M., Zierath J.R. Train like an athlete: applying exercise interventions to manage type 2 diabetes. Diabetologia. 2020;63(8):1491–1499. doi: 10.1007/s00125-020-05166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davenport M.H., Ruchat S.M., Poitras V.J., Jaramillo Garcia A., Gray C.E., Barrowman N., et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: a systematic review and meta-analysis. British Journal of Sports Medicine. 2018;52(21):1367–1375. doi: 10.1136/bjsports-2018-099355. [DOI] [PubMed] [Google Scholar]
- 21.Ming W.K., Ding W., Zhang C.J.P., Zhong L., Long Y., Li Z., et al. The effect of exercise during pregnancy on gestational diabetes mellitus in normal-weight women: a systematic review and meta-analysis. BMC Pregnancy and Childbirth. 2018;18(1):440. doi: 10.1186/s12884-018-2068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiebe H.W., Boule N.G., Chari R., Davenport M.H. The effect of supervised prenatal exercise on fetal growth: a meta-analysis. Obstetrics & Gynecology. 2015;125(5):1185–1194. doi: 10.1097/AOG.0000000000000801. [DOI] [PubMed] [Google Scholar]
- 23.May L.E., Scholtz S.A., Suminski R., Gustafson K.M. Aerobic exercise during pregnancy influences infant heart rate variability at one month of age. Early Human Development. 2014;90(1):33–38. doi: 10.1016/j.earlhumdev.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Clapp J.F., 3rd, Lopez B., Harcar-Sevcik R. Neonatal behavioral profile of the offspring of women who continued to exercise regularly throughout pregnancy. American Journal of Obstetrics and Gynecology. 1999;180(1 Pt 1):91–94. doi: 10.1016/s0002-9378(99)70155-9. [DOI] [PubMed] [Google Scholar]
- 25.McMillan A.G., May L.E., Gaines G.G., Isler C., Kuehn D. Effects of aerobic exercise during pregnancy on 1-month infant neuromotor skills. Medicine & Science in Sports & Exercise. 2019;51(8):1671–1676. doi: 10.1249/MSS.0000000000001958. [DOI] [PubMed] [Google Scholar]
- 26.Patel N., Godfrey K.M., Pasupathy D., Levin J., Flynn A.C., Hayes L., et al. Infant adiposity following a randomised controlled trial of a behavioural intervention in obese pregnancy. International Journal of Obesity. 2017;41(7):1018–1026. doi: 10.1038/ijo.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter L.G., Lewis K.N., Wilkerson D.C., Tobia C.M., Ngo Tenlep S.Y., Shridas P., et al. Perinatal exercise improves glucose homeostasis in adult offspring. American Journal of Physiology. Endocrinology and Metabolism. 2012;303(8):E1061–E1068. doi: 10.1152/ajpendo.00213.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vega C.C., Reyes-Castro L.A., Bautista C.J., Larrea F., Nathanielsz P.W., Zambrano E. Exercise in obese female rats has beneficial effects on maternal and male and female offspring metabolism. International Journal of Obesity. 2015;39(4):712–719. doi: 10.1038/ijo.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez-Twinn D.S., Gascoin G., Musial B., Carr S., Duque-Guimaraes D., Blackmore H.L., et al. Exercise rescues obese mothers' insulin sensitivity, placental hypoxia and male offspring insulin sensitivity. Scientific Reports. 2017;7:44650. doi: 10.1038/srep44650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero P., Guariglia D.A., Da Rocha F.F., Picoli C.C., Gilio G.R., Fabricio G.S., et al. Aerobic exercise training performed by parents reduces mice offspring adiposity. Journal of Sports Science. 2017:1–8. doi: 10.1080/02640414.2017.1405474. [DOI] [PubMed] [Google Scholar]
- 31.Zheng J., Alves-Wagner A.B., Stanford K.I., Prince N.B., So K., Mul J.D., et al. Maternal and paternal exercise regulate offspring metabolic health and beta cell phenotype. BMJ Open Diabetes Res Care. 2020;8(1) doi: 10.1136/bmjdrc-2019-000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allison D.B., Paultre F., Maggio C., Mezzitis N., Pi-Sunyer F.X. The use of areas under curves in diabetes research. Diabetes Care. 1995;18(2):245–250. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- 33.Matthews J.N., Altman D.G., Campbell M.J., Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300(6719):230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.An D., Lessard S.J., Toyoda T., Lee M.Y., Koh H.J., Qi L., et al. Overexpression of TRB3 in muscle alters muscle fiber type and improves exercise capacity in mice. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2014;306(12):R925–R933. doi: 10.1152/ajpregu.00027.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferre P., Leturque A., Burnol A.F., Penicaud L., Girard J. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochemical Journal. 1985;228(1):103–110. doi: 10.1042/bj2280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kramer H.F., Witczak C.A., Taylor E.B., Fujii N., Hirshman M.F., Goodyear L.J. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. Journal of Biological Chemistry. 2006;281(42):31478–31485. doi: 10.1074/jbc.M605461200. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald T.L., Pattamaprapanont P., Pathak P., Fernandez N., Freitas E.C., Hafida S., et al. Hyperglycaemia is associated with impaired muscle signalling and aerobic adaptation to exercise. Nature Metabolism. 2020;2(9):902–917. doi: 10.1038/s42255-020-0240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kusuyama J., Alves-Wagner A.B., Conlin R.H., Makarewicz N.S., Albertson B.G., Prince N.B., et al. Placental superoxide dismutase 3 mediates benefits of maternal exercise on offspring health. Cell Metabolism. 2021;33(5):939–956. doi: 10.1016/j.cmet.2021.03.004. e938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lessard S.J., Rivas D.A., Alves-Wagner A.B., Hirshman M.F., Gallagher I.J., Constantin-Teodosiu D., et al. Resistance to aerobic exercise training causes metabolic dysfunction and reveals novel exercise-regulated signaling networks. Diabetes. 2013;62(8):2717–2727. doi: 10.2337/db13-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toyoda T., An D., Witczak C.A., Koh H.J., Hirshman M.F., Fujii N., et al. Myo1c regulates glucose uptake in mouse skeletal muscle. Journal of Biological Chemistry. 2011;286(6):4133–4140. doi: 10.1074/jbc.M110.174938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia Y., Cong R., Li R., Yang X., Sun Q., Parvizi N., et al. Maternal low-protein diet induces gender-dependent changes in epigenetic regulation of the glucose-6-phosphatase gene in newborn piglet liver. Journal of Nutrition. 2012;142(9):1659–1665. doi: 10.3945/jn.112.160341. [DOI] [PubMed] [Google Scholar]
- 42.Wang B., Hsu S.H., Frankel W., Ghoshal K., Jacob S.T. Stat3-mediated activation of microRNA-23a suppresses gluconeogenesis in hepatocellular carcinoma by down-regulating glucose-6-phosphatase and peroxisome proliferator-activated receptor gamma, coactivator 1 alpha. Hepatology. 2012;56(1):186–197. doi: 10.1002/hep.25632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramírez C.M., Goedeke L., Rotllan N., Yoon J.H., Cirera-Salinas D., Mattison J.A., et al. MicroRNA 33 regulates glucose metabolism. Molecular and Cellular Biology. 2013;33(15):2891–2902. doi: 10.1128/MCB.00016-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alves-Bezerra M., Cohen D.E. Triglyceride metabolism in the liver. Comprehensive Physiology. 2017;8(1):1–8. doi: 10.1002/cphy.c170012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaeini A., Baghaban Eslaminejad M., Choobineh S., Mousavi N., Satarifard S., Shafieineek L. Effects of exercise prior or during pregnancy in high fat diet fed mice alter bone gene expression of female offspring: an experimental study. Journal of Reproductive BioMedicine. 2017;15(2):93–100. [PMC free article] [PubMed] [Google Scholar]
- 46.Son J.S., Zhao L., Chen Y., Chen K., Chae S.A., de Avila J.M., et al. Maternal exercise via exerkine apelin enhances brown adipogenesis and prevents metabolic dysfunction in offspring mice. Science Advances. 2020;6(16):eaaz0359. doi: 10.1126/sciadv.aaz0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kusuyama J., Alves-Wagner A.B., Makarewicz N.S., Goodyear L.J. Effects of maternal and paternal exercise on offspring metabolism. Nature Metabolism. 2020;2(9):858–872. doi: 10.1038/s42255-020-00274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sales V.M., Ferguson-Smith A.C., Patti M.E. Epigenetic mechanisms of transmission of metabolic disease across generations. Cell Metabolism. 2017;25(3):559–571. doi: 10.1016/j.cmet.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radford E.J., Ito M., Shi H., Corish J.A., Yamazawa K., Isganaitis E., et al. In utero effects. In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science. 2014;345(6198):1255903. doi: 10.1126/science.1255903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarker G., Sun W., Rosenkranz D., Pelczar P., Opitz L., Efthymiou V., et al. Maternal overnutrition programs hedonic and metabolic phenotypes across generations through sperm tsRNAs. Proceedings of the National Academy of Sciences of the U S A. 2019;116(21):10547–10556. doi: 10.1073/pnas.1820810116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stanford K.I., Rasmussen M., Baer L.A., Lehnig A.C., Rowland L.A., White J.D., et al. Paternal exercise improves glucose metabolism in adult offspring. Diabetes. 2018;67(12):2530–2540. doi: 10.2337/db18-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.