Abstract
Cathelicidin-derived antimicrobial peptides are a component of the peptide-based host defense of neutrophils and epithelia, with a widespread distribution in mammals. We recently reported the cDNA sequences of three putative horse myeloid cathelicidins, named eCATH-1, -2, and -3. A Western analysis was performed to investigate their presence in neutrophils and processing to mature peptides. eCATH-2 and eCATH-3, but not eCATH-1, were found to be present in uncleaved forms in horse neutrophils. The corresponding mature peptides were detected in inflammatory sites, suggesting that processing of the propeptides takes place upon neutrophil activation. A functional characterization was then performed with synthetic eCATH peptides. Circular dichroism measurements indicated an amphipathic α-helical conformation of these peptides in an anisotropic environment, and in vitro assays revealed a potent activity and a broad spectrum of antimicrobial activity for eCATH-1 and a somewhat more restricted spectrum of activity for eCATH-2. Conversely, a strong dependence on salt concentration was observed when the activity of eCATH-3 was tested. This peptide efficiently killed bacteria and some fungal species, i.e., Cryptococcus neoformans and Rhodotorula rubra, in low-ionic-strength media, but the activity was inhibited in the presence of physiological salt medium. This behavior could be modified by modulating the amphipathicity of the molecule. In fact, the synthetic analogue LLK-eCATH-3, with a slightly modified sequence that increases the hydrophobic moment of the peptide, displayed a potent activity in physiological salt medium against the strains resistant to eCATH-3 under these conditions.
The antimicrobial peptides of innate host defense systems display a rich repertoire of diverse structures (6, 23). These peptides show a broad spectrum of antimicrobial activity in vitro and provide a valuable means of defense against invading pathogens. In mammals, both epithelium- and myeloid cell-derived antimicrobial peptides have been described (17, 18, 20). Defensins and cathelicidins are major components of this peptide-based defense system (5, 19, 36, 37). The cathelicidin peptides in particular are characterized by a marked structural variety (19, 37). Members of this family are stored in unprocessed forms (propeptides) in the secretory granules of neutrophils. These propeptides contain a conserved cathelin propiece that must be removed to liberate microbicidal peptides (19, 37), which may be released into phagosomes or outside the cell (27, 39). Neutrophils from different species may vary substantially in their cathelicidin contents, apparently with only 1 congener present in humans, while up to 10 congeners can be found in other species. In addition to myeloid cells (2, 11, 22), the human LL-37 peptide has also been found to be expressed in other blood cells (1) and in epithelia (3, 14, 15), and recent reports strongly suggest a protective role of this peptide against bacterial infections in vivo (4).
Prompted by the structural heterogeneity of the cathelicidin peptides, we recently examined horse myeloid cells for novel members of this family. There were no reports on cathelicidins from this species, and in addition, we were intrigued by the limited repertoire of antimicrobial peptides identified in the equine neutrophils. Earlier studies, in fact, reported the virtual absence of defensins and the presence in these cells of lysozyme (28) and of two Cys-rich peptides (eNAP-1 and -2) with no structural homology (9, 10). Our search led to the identification of three myeloid cell-derived cDNAs that putatively encode novel cathelicidins, denoted eCATHs (which stands for “equine cathelicidins”) (30). Only two of these, i.e., eCATH-2 and -3, however, were found to be expressed in significant amounts (30). A functional analysis of the novel eCATHs was then undertaken to compare their activities and infer their contributions to the microbicidal functions of these cells. In the present study we investigated their processing and analyzed their in vitro biological activities using synthetic peptides whose sequences corresponded to the deduced sequences to evaluate their antimicrobial and cytotoxic potentials. We also report on structure-activity relationship studies that identify the structural features of eCATH-3 that account for the low level of activity of this peptide.
MATERIALS AND METHODS
Western analysis of horse neutrophils and neutrophil granules.
Neutrophils were isolated from fresh blood of eCATH-1 gene-positive (30) healthy horses as described previously (35) and were denatured with 10% trichloroacetic acid or incubated with 0.1% Triton X-100 in phosphate-buffered saline for 5 to 120 min, as described previously (38). Neutrophil granules were isolated as reported elsewhere (38) and were solubilized with 0.1% Triton X-100 (TX-100) for 5 to 30 min in the presence or absence of 50 μM N-methoxy-succinyl-Ala-Ala-Pro-Val chloromethyl ketone (AAPV-CMK; Sigma). Protein was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting as described previously (38). Samples of tracheobronchial secretions from five horses affected by chronic obstructive pulmonary disease (COPD) and from one horse with acute bronchiolitis were a generous gift from Antonio Pellegrini (Institut für Veterinarphysiologie, University of Zurich). Protein separation from these secretions was achieved by SDS-PAGE with a Tris-tricine buffer system. For matrix-assisted laser desorption/ionization (MALDI) mass spectrometry, peptides were eluted from slices of the polyacrylamide gel by stirring overnight with formic acid-water-isopropanol (1:3:2 [vol/vol]). The rabbit antisera used in the Western analysis were raised by repeated injections of chemically synthesized eCATH-1, eCATH-2, and eCATH-3 peptides, and the immunoglobulin G fractions of the antisera were obtained as described previously (38). Antigen specificity and a lack of cross-reactivity were determined by Western blotting.
Peptide synthesis.
Peptides that corresponded to the deduced sequences of eCATH-1, -2, and -3 and the residue 5-40 and 7-40 eCATH-3 fragments were synthesized by the solid-phase method with a synthesizer (9050; Milligen). In addition, an analogue of eCATH-3 named LLK-eCATH-3 (see Fig. 1) with a slightly modified sequence was synthesized to improve the amphipathicity of the parent peptide. In particular, His13 and Lys20 were changed to Leu to improve the hydrophobic sector of the helix, and Ile30 was changed to Lys to maintain a similar mean residue hydrophobicity (−0.22 versus −0.25) (13). Peptides were synthesized on 9-fluorenylmethoxy carbonyl (Fmoc)–l-Ser tert-butyl (tBu)–polyethylene glycol (PEG)–polystyrene (PS) resin (eCATH-1, eCATH-3, LLK-eCATH-3, and eCATH-3 fragments 5-40 and 7-40) or Fmoc–l-Pro–PEG–PS resin (eCATH-2). Couplings were carried out with a five- to eightfold excess of an equimolar mixture of Fmoc-amino acid, HOBt, and TBTU in the presence of N-methylmorpholine. HOBt and TBTU were replaced with the more efficient coupling reagent HATU in the case of difficult couplings, as predicted by the Peptide Companion software (CoshiSoft, Tucson, Ariz.). Amino acid side chains were protected with trityl (His, Gln), 2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl (Arg), t-butoxycarbonyl (Lys, Trp), and t-butyl (Ser, Thr, Asp, Glu). Peptide deprotection and cleavage from the resin were carried out with a mixture of trifluoroacetic acid (TFA)-ethandithiol-water-triisopropylsilane (92.5:2.5:2.5:2.5 [vol/vol]) for 2 h at room temperature. After filtration of the resins, the peptides were precipitated in methylbutyl ether, and the precipitates were washed several times by centrifugation at 26,000 × g for 10 min and then lyophilized. Crude peptides were resuspended in 0.1% TFA and were purified by reversed-phase high-pressure liquid chromatography on a preparative (19 by 300 mm) C18 Delta-Pak column (Waters, Milford, Mass.) with appropriate 0 to 60% water-acetonitrile gradients in the presence of 0.1% TFA. The purities of the synthetic peptides were assessed by analytical reversed-phase high-pressure liquid chromatography, using a Symmetry C18 column (Waters), and molecular masses were determined by electrospray mass spectrometry (ES-MS) with an API I instrument (SCIEX; Perkin Elmer) as a quality control of the synthesis.
FIG. 1.
Amino acid sequences of the equine cathelicidin peptides eCATH-1, -2, and -3, as deduced from cDNA (30). The analogue LLK-eCATH-3 was synthesized to improve the amphipathicity of the parent eCATH-3 peptide (see Fig. 3). Dashes denote identical residues in the LLK-eCATH-3 and eCATH-3 sequences.
CD spectroscopy.
Circular dichroic (CD) spectra were recorded at 25°C on a Jasco J-600 spectropolarimeter with a cell path length of 2 mm. Peptides were dissolved in 5 mM sodium phosphate buffer (pH 7.0) at a concentration of 10 to 30 μM in the absence or presence of trifluoroethanol (TFE) up to 45% (vol/vol). The α-helical content (fα) was estimated by the equation ([θ] − [θ]rc)/([θ]α − [θ]rc), where [θ] is the mean residue ellipticity in units of degree · square centimeters · decimoles−1 at 222 nm, [θ]rc is the ellipticity for a random coil peptide, and [θ]α is the ellipticity for a 100% helical peptide given by −39,500(1 − 4/n), where n is the number of residues in the peptide (8).
Analytical assays.
The peptide concentration was determined by measuring the absorbance of phenylalanine at 257.5 nm (eCATH-1, eCATH-3, and LLK-eCATH-3) or the absorbance of tryptophan at 280 nm (eCATH-2) by using extinction coefficients of 195.1 and 5,630 M−1 cm−1 for Phe and Trp, respectively. Ion concentrations in Sabouraud medium (920 mg of Na+ per liter, 199 mg of K+ per liter, <530 mg of Cl− per liter, <8 mg of Ca2+ per liter, <8 mg of Mg2+ per liter) were determined with an automated analyzer for clinical chemistry (ILAB900; Instrumentation Laboratory).
Antimicrobial and cytotoxic activities and membrane permeabilization.
The MICs of the purified eCATHs were determined by the microdilution susceptibility test in 96-well microdilution plates as reported previously (31). The antibacterial activity was measured in Mueller-Hinton broth (Difco) with the following logarithmic-phase microorganisms (2.5 × 105 to 5.0 × 105 CFU ml−1): Escherichia coli ATCC 25922 and ML35, Salmonella enterica serovar Typhimurium ATCC 14028, Salmonella enterica serovar Enteritidis (clinical isolate), Pseudomonas aeruginosa ATCC 27853, Serratia marcescens ATCC 8100, Klebsiella pneumoniae ATCC 13883 and SK1 (horse isolate), Staphylococcus aureus ATCC 25923 and a methicillin-resistant S. aureus (MRSA) clinical isolate, Staphylococcus epidermidis ATCC 12228, Streptococcus equinus (horse isolate), and Bacillus megaterium Bm11. The MICs of eCATH-3 were also determined in 10 mM citrate (pH 5.0 and 6.0) or 10 mM phosphate (pH 6.0, 7.0, and 8.0) buffers containing 100 mM NaCl by using E. coli ATCC 25922 (106 CFU ml−1). In addition, the activity of eCATH-3 was also determined by incubating bacteria with various concentrations of the peptide for 1 h at 37°C in 10 mM sodium phosphate buffer (pH 7.4). The bacteria were then diluted and plated, and the numbers of CFU were counted after incubation at 37°C for 16 to 18 h. The antifungal activity of the synthetic peptides was evaluated with two American Type Culture Collection (ATCC) strains of Cryptococcus neoformans and clinical isolates of Candida spp., Pichia etchellsii, Rhodotorula rubra, and C. neoformans. Fungi were grown on Sabouraud dextrose agar at 30°C for 36 to 48 h. The MICs were determined by the microdilution susceptibility test in 96-well microdilution plates in Sabouraud dextrose liquid or in RPMI 1640 medium with 2.5 × 104 to 5.0 × 104 CFU ml−1, according to the guidelines of the National Committee for Clinical Laboratory Standards, as outlined for the the M-27 method, adapted to microdilution assays. The effects of the eCATH peptides on the permeabilities of the outer and inner membranes of E. coli ML-35 were evaluated by following the unmasking of β-lactamase and β-galactosidase activities as described previously (31). To investigate the kinetics of inactivation and membrane permeabilization, C. neoformans was grown on solid Sabouraud medium, collected, and diluted with Sabouraud dextrose liquid at the required density (final concentration), (6 × 104 to 8 × 104/ml) and incubated with or without peptide at 30°C for up to 560 min. The same assays were performed with RPMI 1640 medium in place of Sabouraud medium. Ten-microliter aliquots of each sample were diluted with sterile saline and plated on solid Sabouraud medium, and viable colonies were counted after incubation at 30°C for 48 h. One hundred-microliter aliquots of the same samples were withdrawn to evaluate membrane integrity. The aliquots were cooled to 4°C and incubated with propidium iodide at a final concentration of 10 μg/ml for 5 min. Uptake of propidium iodide was determined by cytofluorimetric analysis of the red fluorescent cells on a Facscan instrument (Becton Dickinson, Mansfield, Mass.) equipped with Cell Quest software and standardized with Calibrite beads (Becton Dickinson). An electronic gate was set around the fungal cells by using their forward scatter and side scatter properties. The cell population within the channels with fluorescence intensities of 1 to 10 was regarded as nonpermeabilized on the basis of a comparison with cells incubated without peptides. Cell debris was excluded from analysis by appropriately raising the forward scattering threshold. The hemolytic activity was measured by determining at 415 nm the hemoglobin release of 10% (vol/vol) suspensions of fresh human or horse erythrocytes incubated with peptides for 30 min at 37°C.
RESULTS
Processing of horse cathelicidins.
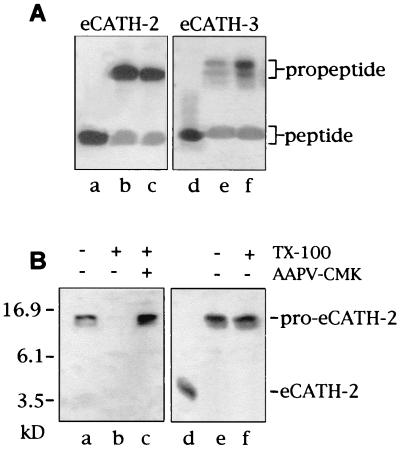
We recently identified three novel cathelicidins in horses as deduced from myeloid cDNA and detected two corresponding polypeptides named eCATH-2 and eCATH-3 in equine neutrophils (30). As with pig and cattle congeners (27, 38), the horse cathelicidins were found to be stored in unprocessed forms (pro-eCATHs) (30), with putative cleavage sites for elastase between the N-terminal cathelin domain and the C-terminal antimicrobial domain. We sought evidence that these propeptides undergo controlled proteolysis in vivo to generate antimicrobial peptides by analyzing two common neutrophil-dominated inflammatory disorders of horses, i.e., COPD and acute bronchiolitis. Six samples of tracheobronchial secretions of horses affected by these diseases were analyzed by Western blotting with antibodies to synthetic eCATH peptides (the sequences are reported in Fig. 1). All revealed the mature eCATH-2 and eCATH-3, in addition to the respective propeptides (representative samples are shown in Fig. 2A, lanes b, c, e, and f). These results thus provide evidence for in vivo processing of the proforms in inflammatory settings. The peptides were then eluted from polyacrylamide gels and analyzed by MALDI-TOF mass spectrometry. A molecular mass of 3,575.32 Da was determined for the purified eCATH-2, in agreement with the mass deduced from cDNA (Table 1). The eCATH-3 sample was a mixture of three different products with molecular masses of 4,679.81, 4,110.93, and 3,924.52 Da. These matched the masses deduced from cDNA for the mature eCATH-3 peptide and for eCATH-3 fragments lacking four and six N-terminal residues, denoted eCATH-3(5–40) and eCATH-3(7–40), respectively, in Table 1. These fragments likely were produced by partial proteolysis of the eCATH-3 peptide in the protease-rich inflammatory medium. The results of this analysis thus indicate that the processing sites for the maturation of eCATH-2 and -3 were correctly deduced from cDNA. In addition, the masses determined for the native peptides rule out the presence of posttranslational modifications of the sequences.
FIG. 2.
(A) Western analysis of bronchial secretions using antibodies to eCATH-2 (lanes a to c) and eCATH-3 (lanes d to f). Lane a, synthetic eCATH-2 peptide; lane d, synthetic eCATH-3 peptide; lanes b and e, tracheobronchial secretions from a horse affected by COPD; lanes c and f, tracheobronchial secretions from a horse affected by acute bronchiolitis. (B) Western analysis of total granule populations from horse neutrophils (lanes a to c) and whole horse neutrophils (lanes d to f) using antibodies to eCATH-2. Lane a, TCA-precipitated granules; lane b, neutrophil granules after incubation with TX-100 for 10 min; lane c, neutrophil granules after incubation with TX-100 for 10 min in the presence of the elastase inhibitor AAPV-CMK; lane d, synthetic eCATH-2; lane e, TCA-precipitated neutrophils; lane f, neutrophils after incubation with TX-100 for 120 min.
TABLE 1.
Mass spectrometric analysis of the synthetic eCATH peptides and of the native peptides eluted from polyacrylamide gels of tracheobronchial secretions of horses affected by COPD
| Peptide | Mass (Da)
|
||
|---|---|---|---|
| Synthetic peptidesa | Peptides eluted from polyacrylamide gelsb | Calculated | |
| eCATH-1 | 3,140.72c | 3,141.00 | |
| eCATH-2 | 3,574.86 | 3,575.32 | 3,575.17 |
| eCATH-3 | 4,679.96 | 4,679.81 | 4,679.56 |
| LLK-eCATH-3 | 4,655.89 | 4,655.58 | |
| eCATH-3(5–40) | 4,110.54 | 4,110.93 | 4,110.88 |
| eCATH-3(7–40) | 3,924.26 | 3,924.52 | 3,924.67 |
Masses determined by electrospray-mass spectrometry.
Masses determined by MALDI–time-of-flight mass spectrometry.
Standard deviation of mass determinations was between ±0.12 and ±0.61.
A Western analysis of horse neutrophils was then performed by using antibodies to synthetic eCATH peptides to ascertain that processing is mediated by neutrophil elastase. With this aim, neutrophils or total neutrophil granules were incubated with the nondenaturing detergent TX-100 for various lengths of time in the presence or absence of an elastase inhibitor. Protein was separated by SDS-PAGE and immunoblotted. This approach has already been used to show that the cow congeners are processed to mature peptides in neutrophils or neutrophil granules lysed under nondenaturing conditions (38). Immunoblots of TX-100-treated samples are shown in Fig. 2B. The propeptides rapidly disappeared after solubilization of total neutrophil granules with TX-100 (Fig. 2B, lane b, for eCATH-2), and the absence of intermediate and/or mature forms in these blots denoted uncontrolled proteolysis. The addition of the elastase inhibitor AAPV-CMK before granule solubilization was sufficient to prevent proteolysis (Fig. 2B, lane c, for proeCATH-2), suggesting that the propeptides are susceptible to elastase and are unaffected by the other granule enzymes. At variance with cattle (38), however, we could not set experimental conditions mimicking a milieu in which controlled proteolysis of the horse propeptides may take place. For instance, when horse neutrophils rather than total neutrophil granules were incubated for up to 120 min with TX-100, only unprocessed forms were detected (shown for pro-eCATH-2 in Fig. 2B, lane f), in keeping with the presence of highly effective cytosolic inhibitor(s) of the granule proteases (35). Conversely, cow propeptides of this family are rapidly processed to microbicidal peptides when bovine neutrophils are solubilized with TX-100 (38). These species-distinctive differences likely depend on the relative abundance of the elastase and/or cytosolic elastase inhibitor(s).
Structural features of eCATH peptides.
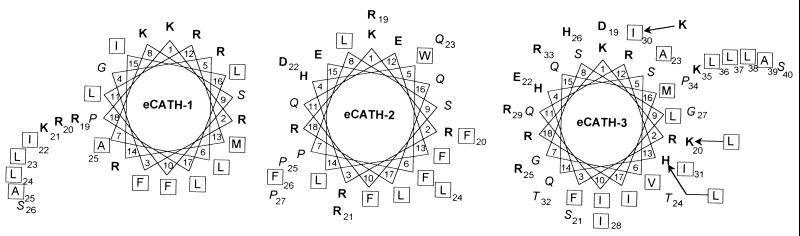
The mature eCATH peptides showed features common to a variety of antimicrobial peptides, including a net positive charge at neutral pH and a predicted α-helical conformation. No obvious similarity to other sequences was found after scanning the SwissProt database with the BLAST algorithm. Unlike eCATH-2 and -3, a polypeptide or peptide that corresponds to the low-abundance eCATH-1 trascript was not detected in horse myeloid cells (30). A putative eCATH-1 peptide would display the highest density of positive charges (9 basic residues out of 26) and the relatively high mean hydrophobic moment per residue (0.38) (13) typical of antimicrobial peptides. The biological properties of eCATH-1 were therefore investigated in parallel with those of eCATH-2 and -3. As predicted from secondary structure analysis (29), eCATH-1 may assume an α-helical conformation in a stretch encompassing residues 5 to 16, with a possible loop at Leu 17 and Pro 18, whereas eCATH-2 and eCATH-3 are predicted to assume α-helical conformations in the regions spanning residues 10 to 21 and 8 to 23, respectively. When fitted into a helical wheel, the three peptides showed moderate amphipathicities (Fig. 3). The mean hydrophobic moment per residue was low when it was calculated for the complete sequences and increased only if the predicted α-helical stretches were considered (mean hydrophobic moments per residue of 0.38 versus 0.46 for eCATH-1, 0.25 versus 0.81 for eCATH-2, and 0.35 versus 0.43 for eCATH-3).
FIG. 3.
Helical wheel representation of the eCATH peptides. Charged residues are in boldface, hydrophobic residues are boxed, and small, hydrophilic, neutral residues are in italics. Arrows in the eCATH-3 wheel indicate substitutions introduced to obtain the LLK-eCATH-3 analogue with improved amphipathicity.
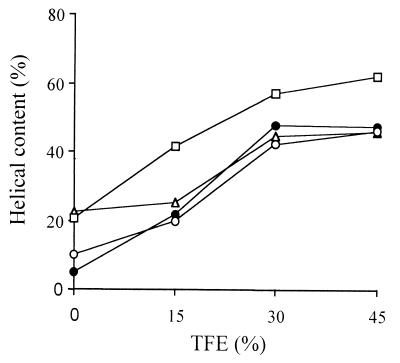
The structure and activities of the three eCATHs were examined by using synthetic peptides that corresponded to the deduced sequences (30). The peptides were synthesized by Fmoc chemistry, and their molecular masses were confirmed by ES-MS (Table 1). The secondary structure was investigated by CD spectroscopy in the presence of the helicogenic solvent TFE (Fig. 4). The estimated helical contents for eCATH-1, -2, and -3 with 45% TFE were 47.3, 46.0, and 46.3%, respectively, and increased only slightly with higher concentrations of TFE. These values are consistent with the predicted secondary structure. Unlike eCATH-1 and eCATH-3, a 22.7% α-helical content was already observed in aqueous buffer for eCATH-2, suggesting self-association of the peptide in solution. In keeping with this conclusion, eCATH-2 was shown to precipitate in Mueller-Hinton broth at concentrations above 114 μg/ml. This behavior was also observed with the peptides PMAP-37 (33) and LL-37 (21) and likely depends on the presence of negatively charged residues in all these peptides, which may favor ionic intermolecular interactions.
FIG. 4.
Helical content of the eCATH peptides in the absence or presence of TFE. The α-helical content was estimated from mean residue ellipticity at 222 nm. ●, eCATH-1; ▵, eCATH-2; ○, eCATH-3; □, LLK-eCATH-3.
Biological activities of eCATH peptides.
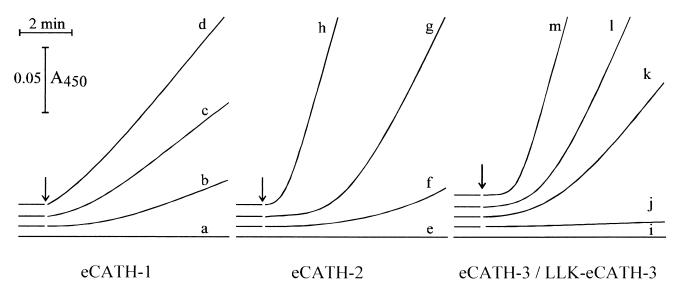
The synthetic peptides were tested in vitro against a panel of bacteria and fungi. The MICs for the bacteria tested (Table 2) revealed significant differences in their activities. eCATH-1 was the most effective when assayed in Mueller-Hinton broth, with MICs of 3 to 12 μg/ml for gram-negative organisms and MICs of 6 to 24 μg/ml for gram-positive organisms. Higher MICs (50 μg/ml) of this peptide were observed only for the MRSA strain. eCATH-2 displayed up to 16-fold higher MICs under the same experimental conditions, with the most significant differences being observed for Salmonella, Pseudomonas, and Streptococcus species (MICs, ≥114 μg/ml). Both peptides were also shown to cause a fast and complete permeabilization of the outer membrane of E. coli ML-35 at 1.2 to 3 μg/ml (data not shown), whereas the inner membrane permeabilization was more efficient with eCATH-2 than eCATH-1 (Fig. 5). Quite unexpectedly, eCATH-3 proved to be ineffective against all the bacterial strains under these conditions, with MICs of >150 μg/ml (Table 2) over a pH range of 5 to 7, and did not permeabilize the outer membrane (data not shown) or inner membrane (Fig. 5) of E. coli ML-35 at 100 μg/ml. The peptide, however, efficiently killed bacteria in low-ionic-strength medium (Fig. 6). Also important, the 5-40 and 7-40 N-terminally truncated eCATH-3 fragments detected in the tracheobronchial secretions displayed the same activity profile as the full-length peptide (data not shown), thus ruling out the possibility that N-terminal digestion of the peptide is required for improved activity.
TABLE 2.
Antibacterial activities of eCATH peptidesa
| Organism and strain | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| eCATH-1 | eCATH-2 | eCATH-3 | LLK-eCATH-3 | |
| Escherichia coli ATCC 25922 | 3 | 14 | >150 | 4.5 |
| Escherichia coli ML35 | 3 | 14 | >150 | 4.5 |
| Salmonella enterica serovar Typhimurium ATCC 14028 | 6 | 114 | >150 | 9 |
| Salmonella enterica serovar Enteritidisb | 6 | 114 | >150 | 9 |
| Pseudomonas aeruginosa ATCC 27853 | 12.5 | ≥114 | >150 | 18 |
| Serratia marcescens ATCC 8100 | 12.5 | 28.5 | >150 | 4.5 |
| Klebsiella pneumoniae ATCC 13883 | 3 | 28.5 | >150 | 9 |
| Klebsiella pneumoniae SK1c | 6 | 114 | >150 | 18 |
| Staphylococcus aureus ATCC 25923 | 25 | 57 | >150 | 18 |
| Staphylococcus aureusb (MRSA) | 50 | 114 | >150 | 18 |
| Staphylococcus epidermidis ATCC 12228 | 6 | 28.5 | >150 | 18 |
| Streptococcus equinusc | 25 | >114 | >150 | 9 |
| Bacillus megaterium Bm11 | 12.5 | 14 | >150 | 9 |
MICs were determined in Mueller-Hinton broth after incubation at 37°C for 16 to 18 h. Results are the means of three to five independent determinations with a divergence of not more than one MIC.
Horse isolate.
Clinical isolate.
FIG. 5.
Kinetics of the inner membrane permeabilization of E. coli ML-35 by the eCATH peptides. Permeabilization was determined by recording spectrophotometrically the hydrolysis of o-nitrophenyl-β-d-galactopyranoside, a normally impermeant substrate of the cytoplasmic β-galactosidase. Experiments were carried out in 10 mM sodium phosphate buffer (pH 7.4) with 100 mM NaCl. Traces a, e, and i, untreated bacteria; bacteria were treated with 0.6, 3, and 15 μg of eCATH 1 per ml (traces b, c, and d, respectively); 0.7, 3.5, and 7 μg of eCATH-2 per ml (traces f, g, and h, respectively); 90 μg of eCATH-3 per ml (trace j); and 2, 4.5, and 9 μg of LLK-eCATH-3 per ml (traces k, l, and m, respectively). The time of addition of peptides is indicated by arrows. The results are representative of two to three independent determinations.
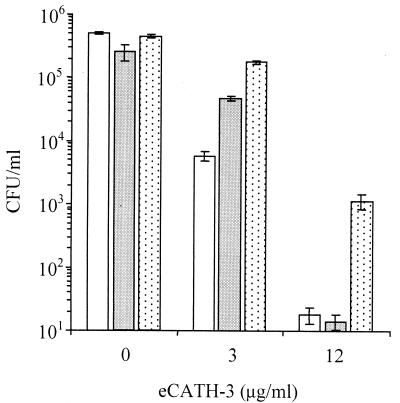
FIG. 6.
Antibacterial activity of eCATH-3 in low-salt medium. E. coli ATCC 25922 (open bars), S. enterica serovar Typhimurium ATCC 14028 (dark bars), and S. aureus ATCC 25923 (dotted bars). Bacterial cells (2 × 105 to 5 × 105 CFU/ml) were incubated with the indicated amounts of eCATH-3 in 10 mM sodium phosphate buffer (pH 7.4) for 1 h at 37°C. The cells were then serially diluted in sterile saline, plated in Mueller-Hinton agar, and incubated for 16 to 18 h to allow colony counts to be performed. The results are the means of three independent determinations. Error bars indicate standard deviations.
Marked differences in antifungal activity were also observed. Antifungal activity was determined in Sabouraud medium (pH 5) and RPMI 1640 medium (pH 7) (Table 3). eCATH-1 and eCATH-3, but not eCATH-2, were found to be active in Sabouraud medium against clinical isolates of C. neoformans. eCATH-1 exhibited a comparable anticryptococcal activity (Table 3) and a faster killing kinetics (Fig. 7) when assayed in RPMI 1640 medium. However, eCATH-3 was ineffective (MIC, >150 μg/ml) against C. neoformans in this medium, and conversely, eCATH-2 gained activity (MIC, 30 μg/ml) against C. neoformans in this medium. The peptides showed similar behaviors and only slightly higher MICs for R. rubra. By contrast, other fungi such as Candida spp. and P. etchellsii appeared to be equally resistant to all the peptides in Sabouraud and RPMI 1640 media (Table 3). The ability of eCATH-3 to neutralize C. neoformans and R. rubra in Sabouraud medium but not in RPMI 1640 medium may be explained by the different ionic strengths of the media. Compared with RPMI 1640 medium, Sabouraud medium is a low-salt medium, and a low ionic strength is also required for the antibacterial activity of this peptide (Table 2 and Fig. 6). Conversely, the differences in the pHs of the two media (pH 5 versus pH 7) do not seem to play a role, as eCATH-3 was found to be inactive in RPMI 1640 medium at both pH 7 and pH 5 (data not shown).
TABLE 3.
Antifungal activities of eCATH peptidesa
| Organism and strain | MIC (μg/ml)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| eCATH-1
|
eCATH-2
|
eCATH-3
|
LLK-eCATH-3
|
|||||
| Sab | RPMI | Sab | RPMI | Sab | RPMI | Sab | RPMI | |
| Candida albicans | >100 | >100 | >114 | >114 | >150 | >150 | >150 | >150 |
| Candida parapsilosis | >100 | >100 | >114 | >114 | >150 | >150 | >150 | >150 |
| Candida tropicalis | >100 | >100 | >114 | >114 | >150 | >150 | >150 | >150 |
| Pichia etchellsii | >100 | >100 | >114 | >114 | >150 | >150 | >150 | >150 |
| Cryptococcus neoformans | 6 | 6 | >114 | >114 | 9 | >150 | 150 | 75 |
| Cryptococcus neoformans | 12.5 | 6 | >114 | 21 | 9 | >150 | 150 | 37.5 |
| Cryptococcus neoformans ATCC 90113 | 12.5 | 6 | >114 | 28.5 | 18 | >150 | >150 | 18 |
| Cryptococcus neoformans ATCC 52817b | 12.5 | 6 | >114 | 28.5 | 18 | >150 | 150 | 18 |
| Rhodotorula rubra | 50 | 3 | >114 | 57 | 37.5 | >150 | 150 | 18 |
MICs determined in Sabouraud medium (Sab) or RPMI 1640 medium (RPMI) after incubation at 30°C for 48 h. All fungi except the ATCC strains are clinical isolates. Results are the means of two to four independent experiments with a divergence of not more than one MIC.
Acapsular mutant.
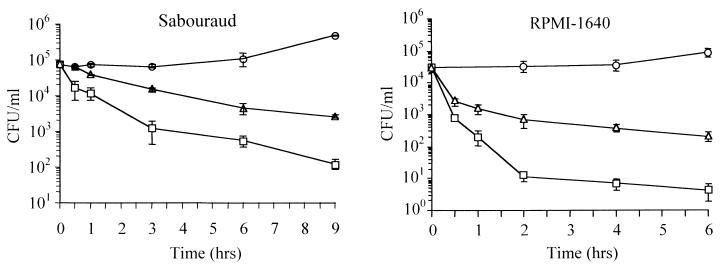
FIG. 7.
Kinetics of inactivation of C. neoformans by eCATH peptides. Fungi were incubated at 30°C for the indicated times in the absence or presence of 31 μg of eCATH-1 per ml and 46 μg of eCATH-3 or LLK-eCATH-3 per ml (10 μM peptides), serially diluted in buffered saline, and plated in solid Sabouraud medium to allow colony counts to be performed. The experiment on the left side was performed in Sabouraud medium in the absence (○) or presence of eCATH-3 (▵) or eCATH-1 (□). The experiment shown on the right side was performed in RPMI 1640 medium in the absence (○) or presence of LLK-eCATH-3 (▵) or eCATH-1 (□). Each point is the mean of three independent experiments. Error bars indicate standard deviations.
To get an insight into the anticryptococcal activities of the active peptides, the role of the glucuronoxylomannan-rich polysaccharide capsule of C. neoformans was evaluated by assaying the peptides against an acapsular strain of C. neoformans. The MIC were not significantly different from those obtained with the encapsulated strains both in Sabouraud medium and in RPMI 1640 medium (Table 3), and the killing kinetics were also comparable (data not shown), suggesting that the structure of the capsule is not a primary target or a barrier for these peptides.
Potential toxic effects to mammalian cells were also examined, in addition to the antimicrobial activity, by assaying the peptides in vitro against erythrocytes from human and horse peripheral blood. Human erythrocytes (10% [vol/vol] suspensions) were insensitive to eCATH-3 (0.6% lysis at 468 μg/ml) and were barely affected by eCATH-1, with 4.6% hemolysis with 314 μg of peptide per ml, whereas 21% hemolysis was observed with eCATH-2 at the same concentration. Similar results were obtained with horse erythrocytes (data not shown).
Structure-activity relationship studies of eCATH3 peptide.
As indicated by the antimicrobial assays, eCATH-3 was effective only in low-ionic-strength medium. This is unusual, as most cathelicidin peptides retain their activity at physiological salt concentrations. We focused on this peptide in an attempt to identify structural features that could explain this behavior. Several reports point to a strong correlation between amphipathicity and antimicrobial activity (12, 32), and it seemed reasonable to associate the low efficiency of eCATH-3 to the low hydrophobic moment of the peptide. To test whether an increased amphipathicity of the helix would improve the performance, a peptide (LLK-eCATH-3) with a slightly modified sequence was synthesized. His13 and Lys20 were changed to Leu to improve the hydrophobic sector of the helix, and Ile30 was changed to Lys to maintain a similar mean residue hydrophobicity (−0.22 versus −0.25) (13). The modified LLK-eCATH-3 showed increased mean hydrophobic moments per residue (from 0.43 to 0.62) in the predicted helical region from residues 8 to 23 and a higher helical content (62 versus 46% in 45% TFE) (Fig. 4). The substitutions caused a modest increase in the hemolytic effects (4.8% at 465 μg/ml) and a dramatic change in the antimicrobial activity. LLK-eCATH-3 proved active in Mueller-Hinton broth against most bacterial strains resistant to the parent peptide under these conditions. The MICs were in the range of 5 to 20 μg/ml (Table 2), and the kinetics of the outer membrane (data not shown) and the inner membrane (Fig. 5) permeabilization of E. coli ML-35 were comparable to those of eCATH-2. The peptide also gained activity against C. neoformans and R. rubra in RPMI 1640 medium and, similar to eCATH-2, proved much less efficient in Sabouraud medium (Fig. 7). Thus, the effects of LLK-eCATH-3 on bacteria indicate a correlation between the antibacterial activity and the amphipathicity of the molecule, whereas the effects on fungi appear to be more complex and may deserve further studies aimed at evaluation of the influence of the medium on the activity, as observed with other peptides (34).
DISCUSSION
The aim of the present study was to examine the biological properties of the equine cathelicidin peptides and evaluate their potential contribution to the peptide-based host defense of horse neutrophils. These cells lack defensins, and the defense peptides identified in addition to the eCATHs include lysozyme (28) and two unrelated peptides, eNAP-1 and eNAP-2, active in low-salt medium against clinical isolates of equine pathogens (9, 10). The eNAPs show high cysteine contents and are members of two different protein families, i.e., the granulins (9) and the four-disulfide core protein family (10). The novel eCATHs are α-helical and show substantial similarity with respect to helical content and amphipathic character. Alignments of the nucleotide sequences with cathelicidins from other species suggest that the gene duplications that gave origin to the horse members of this family postdate the separation of perissodactyls and artiodactyls.
Despite their structural similarity and relatively recent origin (30), we found significant differences in the in vitro activities of the three peptides. eCATH-1 and eCATH-3 are at opposite ends of the spectrum, with the former being a potent antibacterial agent with a broad spectrum of activity and the latter displaying antimicrobial activity only in low-salt medium. eCATH-2 shows intermediate potency and an intermediate spectrum of activity. These, however, may have been underestimated because of the tendency of this peptide to aggregate at physiological pH.
The strong antimicrobial activity and virtual absence of hemolytic effects of eCATH-1 suggest a good target selectivity of this peptide toward microbial cells, and on the basis of these features, the peptide appears to be best suited to the host defense. This notwithstanding, the eCATH-1 gene is present in only approximately 50% of the animals analyzed (30), and even when it is present, its levels of expression are low. In addition, the corresponding eCATH-1 protein has not been detected by eCATH-1-specific antibodies in the myeloid cells of these animals. The possibility cannot be ruled out that eCATH-1 is expressed at higher concentrations only under particular conditions and/or in cell types other than neutrophils. Various studies have in fact demonstrated expression of members of the cathelicidin family in other tissues in addition to myeloid cells (1–3, 14–16).
The modest in vitro activity of eCATH-3 raises the question as to the ability of this peptide to perform under in vivo conditions. eCATH-3 had MICs of 2 to 20 μg/ml for bacterial cells in low-ionic-strength medium and is ineffective when tested up to 114 μg/ml in physiological salt medium. An explanation for the low efficiency of the peptide has been provided by the use of the synthetic analogue LLK-eCATH-3, which established a correlation between the biological activity and the amphipathicity of the molecule. When considering the in vivo activity of eCATH-3, however, one should take into account not only the in vitro performance but also the abundance of the peptide at sites of release. Both Northern and Western analyses indicate that eCATH-3 is abundantly expressed in myeloid cells, and it is likely that the ion-dependent inhibition of the activity is overcome under conditions that determine local accumulation of large amounts of peptide, i.e., in phagosomes, where the factors released from neutrophil granules can reach remarkably high concentrations (24).
Our analysis demonstrates the presence of eCATH-3 in inflammatory secretions, indicating that the peptide can also be released in extracellular fluids. It remains to be determined if it reaches sufficiently high local concentrations to be effective by itself or possibly has a role in these regions by acting synergistically with other defense molecules. Synergism between defense peptides has been established in several studies aimed at evaluation of the complexity of the interactions of multiple peptides in vivo (25, 26, 40). One should, however, also consider the intriguing possibility that microbial killing is not the only or principal function of eCATH-3, as this peptide might have another still unidentified biological role(s). This hypothesis is in keeping with an increasing number of studies that reveal that the mammalian antimicrobial peptides are multifunctional host defense effector molecules (7, 19). Thus, while this study has provided a good in vitro characterization of the antimicrobial and cytotoxic activities of the eCATH peptides, further work should explore the possibility of a diversified use of these peptides by the horse host defense.
ACKNOWLEDGMENTS
This work was supported by the Italian Ministry for University and Research (MURST) P.R.I.N. Cofin. 2000, Istituto Superiore di Sanità National Research Project AIDS (grant 50B.41), CNR Target Project on Biotechnology, Commissariato di Governo della Regione FVG, and Regione Friuli Venezia Giulia.
We thank A. Pellegrini (University of Zurich) for horse tracheobronchial secretions, P. De Paoli (Centro di Riferimento Oncologico di Aviano, Pordenone) and F. De Bernardis (Istituto Superiore di Sanità, Rome, Italy) for the fungal strains, D. Garozzo (Istituto per la Chimica e la Tecnologia dei Materiali Polimerici, CNR Catania, Italy) for MALDI mass spectrometry, N. Bortolotti (University of Udine) for ion measurements, M. Benincasa (University of Trieste) and L. Tomasinsig (University of Udine) for assistance with preparation of the manuscript, and A. Tossi (University of Trieste) for critically reading the manuscript.
REFERENCES
- 1.Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, Kiessling R, Jornvall H, Wigzell H, Gudmundsson G H. The human antimicrobial and chemotactic peptides LL-37 and α-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. [PubMed] [Google Scholar]
- 2.Agerberth B, Gunne H, Odeberg J, Kogner P, Boman H G, Gudmundsson G H. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci USA. 1995;92:195–199. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bals R, Wang X, Zasloff M, Wilson J M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has a broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA. 1998;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bals R, Weiner D J, Meegalla R L, Wilson J M. Transfer of a cathelicidin peptide antibiotic gene restores bacterial killing in a cystic fibrosis xenograft model. J Clin Investig. 1999;103:1113–1117. doi: 10.1172/JCI6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bevins C L, Martin-Porter E, Ganz T. Defensins and innate host defence of the gastrointestinal tract. Gut. 1999;45:911–915. doi: 10.1136/gut.45.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boman H G. Gene-encoded peptide antibiotics and the concept of innate immunity: an update review. Scand J Immunol. 1998;48:15–25. doi: 10.1046/j.1365-3083.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 7.Chan Y R, Gallo R L. PR-39, a syndecan-inducing antimicrobial peptide, binds and affects p130(Cas) J Biol Chem. 1998;273:28978–28985. doi: 10.1074/jbc.273.44.28978. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y-H, Yang J T, Chau K H. Determination of the helix and β form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974;13:3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- 9.Couto M A, Harwig S S L, Cullor J S, Hughes J P, Lehrer R I. Identification of eNAP-1, an antimicrobial peptide from equine neutrophils. Infect Immun. 1992;60:3065–3071. doi: 10.1128/iai.60.8.3065-3071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couto M A, Harwig S S L, Cullor J S, Hughes J P, Lehrer R I. eNAP-2, a novel cysteine-rich bactericidal peptide from equine leukocytes. Infect Immun. 1992;60:5042–5047. doi: 10.1128/iai.60.12.5042-5047.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowland J B, Johnsen A H, Borregaard N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995;368:173–176. doi: 10.1016/0014-5793(95)00634-l. [DOI] [PubMed] [Google Scholar]
- 12.Dathe M, Wieprecht T, Nikolenko H, Handel L, Maloy W L, MacDonald D L, Beyermann M, Bienert M. Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Lett. 1997;403:208–212. doi: 10.1016/s0014-5793(97)00055-0. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg D. Three dimensional structure of membrane and surface proteins. Ann Rev Biochem. 1984;47:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- 14.Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, Gudmundsson G H. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 15.Frohm Nilsson M, Sandstedt B, Sorensen O, Weber G, Borregaard N, Stahle-Backdahl M. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun. 1999;67:2561–2566. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallo R L, Kim K, Bernfield M, Kozak C, Zanetti M, Merluzzi L, Gennaro R. Identification of CRAMP, a cathelin related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem. 1997;272:13088–13093. doi: 10.1074/jbc.272.20.13088. [DOI] [PubMed] [Google Scholar]
- 17.Ganz T, Lehrer R I. Antimicrobial peptides of leukocytes. Curr Opin Hematol. 1997;4:53–58. doi: 10.1097/00062752-199704010-00009. [DOI] [PubMed] [Google Scholar]
- 18.Ganz T, Weiss J. Antimicrobial peptides of phagocytes and epithelia. Semin Hematol. 1997;34:343–354. [PubMed] [Google Scholar]
- 19.Gennaro R, Zanetti M. Structural features and biological activities of the cathelicidin-derived antimicrobial peptides. Biopolymers. 2000;55:31–49. doi: 10.1002/1097-0282(2000)55:1<31::AID-BIP40>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Huttner K M, Bevins C L. Antimicrobial peptides as mediators of epithelial host defense. Pediatr Res. 1999;45:785–794. doi: 10.1203/00006450-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Johansson J, Gudmundsson G H, Rottenberg M E, Berndt K D, Agerberth B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J Biol Chem. 1998;273:3718–3724. doi: 10.1074/jbc.273.6.3718. [DOI] [PubMed] [Google Scholar]
- 22.Larrick J W, Hirata M, Balint R F, Lee J, Zhong J, Wright S C. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehrer R I, Ganz T. Antimicrobial peptides in mammalian and insect host defence. Curr Opin Immunol. 1999;11:23–27. doi: 10.1016/s0952-7915(99)80005-3. [DOI] [PubMed] [Google Scholar]
- 24.Lehrer R I, Lichtenstein A K, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 25.Levy O, Ooi C E, Weiss J, Lehrer R I, Elsbach P. Individual and synergistic effects of rabbit granulocyte proteins on Escherichia coli. J Clin Investig. 1994;94:672–682. doi: 10.1172/JCI117384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagaoka I, Hirota S, Yomogida S, Ohwada A, Hirata M. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm Res. 2000;49:73–79. doi: 10.1007/s000110050561. [DOI] [PubMed] [Google Scholar]
- 27.Panyutich A, Shi J, Boutz P L, Zhao C, Ganz T. Porcine polymorphonuclear leukocytes generate extracellular microbicidal activity by elastase-mediated activation of secreted proprotegrins. Infect Immun. 1997;65:978–985. doi: 10.1128/iai.65.3.978-985.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellegrini A, Waiblinger S, von Fellenberg R. Purification of equine neutrophil lysozyme and its antibacterial activity against gram-positive and gram-negative bacteria. Vet Res Commun. 1991;15:427–435. doi: 10.1007/BF00346538. [DOI] [PubMed] [Google Scholar]
- 29.Rost B, Sander C. Prediction of protein secondary structure at better than 70% accuracy. J Mol Biol. 1993;232:584–599. doi: 10.1006/jmbi.1993.1413. [DOI] [PubMed] [Google Scholar]
- 30.Scocchi M, Bontempo D, Boscolo S, Tomasinsig L, Giulotto E, Zanetti M. Novel cathelicidins in horse leukocytes. FEBS Lett. 1999;457:459–464. doi: 10.1016/s0014-5793(99)01097-2. [DOI] [PubMed] [Google Scholar]
- 31.Skerlavaj B, Romeo D, Gennaro R. Rapid membrane permeabilization and inhibition of vital functions of gram-negative bacteria by bactenecins. Infect Immun. 1990;58:3724–3730. doi: 10.1128/iai.58.11.3724-3730.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tossi A, Sandri L, Giangaspero A. Amphipathic, α-helical antimicrobial peptides. Biopolymers. 2000;55:4–30. doi: 10.1002/1097-0282(2000)55:1<4::AID-BIP30>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 33.Tossi A, Scocchi M, Zanetti M, Storici P, Gennaro R. PMAP-37, a novel pig myeloid antibacterial peptide. Eur J Biochem. 1995;228:941–946. doi: 10.1111/j.1432-1033.1995.tb20344.x. [DOI] [PubMed] [Google Scholar]
- 34.Turner J, Cho Y, Dinh N N, Waring A J, Lehrer R I. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother. 1998;42:2206–2214. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Fellenberg R, Kohler L, Grunig G, Pellegrini A. Comparison of neutrophil elastases and of neutrophil protease inhibitors in the horse and man. Am J Vet Res. 1985;46:2480–2484. [PubMed] [Google Scholar]
- 36.White S H, Wimley W C, Selsted M E. Structure, function, and membrane integration of defensins. Curr Opin Struct Biol. 1995;5:521–527. doi: 10.1016/0959-440x(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 37.Zanetti M, Gennaro R, Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
- 38.Zanetti M, Litteri L, Gennaro R, Horstmann H, Romeo D. Bactenecins, defense polypeptides of bovine neutrophils, are generated from precursor molecules stored in the large granules. J Cell Biol. 1990;111:1363–1371. doi: 10.1083/jcb.111.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zanetti M, Litteri L, Griffiths G, Gennaro R, Romeo D. Stimulus-induced maturation of probactenecins, precursors of neutrophil antimicrobial polypeptides. J Immunol. 1991;146:4295–4300. [PubMed] [Google Scholar]
- 40.Zarember K, Elsbach P, Shin-Kim K, Weiss J. p15s (15-kD antimicrobial proteins) are stored in the secondary granules of rabbit granulocytes: implications for antibacterial synergy with the bactericidal/permeability-increasing protein in inflammatory fluids. Blood. 1997;89:672–679. [PubMed] [Google Scholar]