Abstract
Background: In Arizona Helicobacter pylori prevalence of infection among Navajo adults is about 62% and gastric cancer incidence rate is 3–4 times higher than that of the non-Hispanic White population. Aim: The aim of this study was to estimate the prevalence of specific H. pylori virulence factors (cagA and vacA) among Navajo patients undergoing and their association with gastric disease. Methods: Virulence genes, cagA and vacA, in H. pylori were investigated in gastric biopsies from 96 Navajo patients over age 18 who were undergoing esophagogastroduodenoscopy. Biopsies from the antrum and fundus were used for molecular characterization to determine cagA type and number of EPIYA motifs and presence of alleles in the signal (s) and medium (m) regions of the vacA gene. Results: H. pylori infection was found in 22.9% of the biopsy samples. The cagA gene amplified in 57.6% of samples and showed a predominant “Western cagA” type, with the EPIYA-ABC motif (45.4%), most prevalent. The vacA allele s1bm1 was the most prevalent (54.5%). Conclusions: H. pylori genotypes were predominantly cagA Western-type and ABC EPIYA motifs. The vacA s1bm1 genotype was the most prevalent and seemed to be associated with gastritis. American Indian/Alaska Native populations are at higher risk for gastric cancer. It is important to identify genotypes of H. pylori and virulence factors involved in the high prevalence of H. pylori and associated disease among the Navajo population.
Keywords: Helicobacter pylori, gastric cancer, ulcers, gastritis, Navajos, Native Americans, American Indians, virulence factors
1. Introduction
Helicobacter pylori (H. pylori) is a fastidious microaerophilic Gram-negative rod. Infection with H. pylori is among the most common bacterial infections with nearly half of the global population estimated to be infected [1,2]. Colonization by the bacterium primarily occurs during childhood; an estimated 50% of children in developing countries and 10% of children in the United States have been colonized before the age of 10 [3,4]. Prevalence of infections with H. pylori is highly variable owing to age, race/ethnicity, gender, and socioeconomic factors, with most suggesting the higher prevalence in older age groups results from living conditions during their childhood [5,6].
Transmission of H. pylori has been reported to occur via gastro-oral, oral-oral, and fecal-oral routes [7,8]. Colonization of gastric mucosa is asymptomatic in most individuals and can persist for several decades [9,10]. Infections can cause mild to severe mucosal inflammation and are a risk factor in the etiology of several severe gastrointestinal outcomes including, peptic ulcer disease, chronic gastritis, and stomach or gastric cancer [1]. While a minority of chronically infected individuals develop gastric cancer [11], H. pylori infection has been attributed to nearly 89% of all non-cardia gastric cancers which represents over three-quarters of all gastric cancer worldwide [12]. Development of malignancy is a result of a complex interaction between environmental factors, host genetics, and virulence of the infecting H. pylori strain resulting in enhance or reduced inflammatory responses [13,14].
Colonization of gastric mucosa is accomplished by the action of virulence factors, (outer membrane proteins and adhesins) which enable adherence to the gastric epithelial cells [15,16]. Damage to the epithelium comes primarily from two well-studied virulence factors, the cytotoxin-associate gene A (cagA) and the vacuolating cytotoxin A gene (vacA) [17,18]. The cagA protein is one of the products encoded by the cag pathogenicity island (cag-PAI) which is responsible for the translocation of cagA to the cytoplasm of gastric epithelial cells in cagA-positive strains of H. pylori [19,20]. The severity and prevalence of gastric diseases are associated with cagA-positive H. pylori strains [21,22,23]. The prevalence of cagA-positive strains varies between ethnic groups and regions; in Asia cagA-positive strains reach >90% compared to 50–60% in Western countries [24,25,26]. The pro-inflammatory and carcinogenic activities of cagA are associated with the number and type of phosphorylation sites denominated EPIYA (Glu-Pro-Ile-Tyr-Ala) regions [18,27,28]. According to the amino acid sequences that flank this region, EPIYAs are classified as EPIYA-A, -B, -C, or -D. Western H. pylori strains preferentially express cagA containing EPIYA-A, and -B with one or more -C segments; while strains from Asia contain EPIYA-A, -B, and D motifs [27,29]. Gastric pathology is associated with EPIYA domains containing more than one EPIYA-C phosphorylation site [30,31].
The vacA protein is encoded by the vacA gene, present in all strains of H. pylori and variability in the 5′ signal (s) and middle (m) regions results in alleles that when expressed together increase the risk for gastric pathologies [17,32]. The combination of vacA alleles s1m1 results in strains producing high levels of this virulence factor; the s1m2 strains produce moderate levels, while the s2/m2 strains produce minimal concentrations or do not produce it at all [33,34]. The s1m1 and s1m2 genotypes generate vacA isoforms that cause direct damage to the gastric epithelium and stimulate an acute inflammatory process, which may lead to chronic gastritis or gastric ulcer [35,36]. The prevalence of genotypes of H. pylori that express virulent vacA isoforms varies with the geographic area, and infection with H. pylori vacA s1m1 type correlates with increased risk of gastric disease [26,37].
Despite the elevated burden of gastric cancer in certain communities and populations and the established link between H. pylori infection and no-cardia gastric cancer, data on H. pylori prevalence in Indigenous communities in the United States are sparse [26,38,39]. The Navajo Nation is a sovereign Native American nation with the largest territory in the United States. Prevalence of H. pylori in Navajo Nation has been reported at 68–70% [40] which is close to values found in Alaska Natives, 75% [38,41,42]. Perhaps what is more striking about the high H. pylori infection prevalence is that gastric cancer incidence is 3–4 times higher in Navajo Nation than among the non-Hispanic white population in Arizona [43]. These high rates of chronic infection likely contribute to disproportionately high rates of gastric cancer reported in Navajos and Alaska Natives [44,45,46,47].
Despite the high prevalence of H. pylori in these populations, there are few reports on the association between infection and gastroduodenal diseases, and still fewer on the types and distribution of vacA and cagA genotypes and clinical outcomes in patients with gastritis, peptic ulcers, or gastric cancer [26,48,49]. The aim of this study is to determine the genotypes of H. pylori present in Navajo adults in Northern Arizona and evaluate their association with clinical outcomes. Identifying the distribution of genotypes of H. pylori in Navajos will facilitate therapeutic and prevention strategies to improve clinical outcomes.
2. Materials and Methods
2.1. Ethical Considerations
We obtained resolutions of support and approval prior to community recruitment from the three participating Navajo chapter communities (chapters) and the two governing agency councils that incorporated the chapters. These resolutions were required before receiving the Navajo Nation Human Research Review Board for protocol approval. The Northern Arizona University Institutional Review Board also approved the final protocol and consent forms.
2.2. Patients and Sample Collection
Participants were recruited when visiting the gastroenterology clinic at Winslow Indian Health Care Clinic (WIHCC) as part of their already scheduled pre-procedure visit for esophagogastroduodenoscopy (EGD). The study was explained to the participants by the clinic nurse and consent forms signed prior to the EGD. Consent was received to obtain additional biopsy samples and to review medical records for histopathology findings, prior testing for H. pylori, and demographic characteristics. All patients signed an Informed Consent Form. The only exclusion criteria were that participants must be at least 18 years of age. Clinic staff completed medical record abstraction and removed all personal identifying information using a standardized data form. All data and samples were deidentified prior to transmission to the university laboratories for analysis and data entry.
During endoscopy, four biopsies were collected, two from the antrum and two from the fundus. One set of each was submitted for histopathological examination to be performed by an established laboratory contracted by WIHCC. The other set was placed in 400 µL RNAlater for transport to the NAU laboratory and DNA isolation.
2.3. H. pylori Strains and DNA Isolation
The strains H. pylori 26695 (ATCC 700392) and H. pylori 60190 (ATCC 49503), were used as reference isolates. Both strains have the genotype vacA s1/m1 and are cagA+. Biopsies were collected from the antrum and the fundus regions in separate tubes containing RNAlater (Sigma. Chemical Co., St Louis, MO, USA). DNA was isolated using the FastDNA Spin Kit (MP Biomedicals, LLC, Solon, OH, USA) and the FastPrep 24 instruments (MP Biomedicals LLC) as described by the manufacturer. DNA concentration was measured in a Nanodrop 1 (Thermo Fisher Scientific, Waltham, MA, USA) and stored at −20 °C until used.
2.4. Molecular Identification
The DNA samples were used to first identity the presence of H. pylori by PCR amplification of the H. pylori 16S rRNA gene. Positive and negative controls were included in each reaction. In some cases, the ~519 bp-amplified product was separated by electrophoresis in 1% agarose gels followed by SYBR Green staining and analysis under an ultraviolet (UV) light. Genotyping of positive strains for the identification of cagA and vacA genes was carried out as previously described [33,50] using the primers shown in Table 1.
Table 1.
Primers used for amplification and sequencing of 16S rRNA, cagA, and vacA genes.
| Primer | Use | Sequence | Target | Reference |
|---|---|---|---|---|
| 16S1/16S2 | Amplification | 5′ GCTAAGAGATCAGCCTATGTCC 3′ 5′ TGGCAATCAGCGTCAGGTAATG 3′ |
16S rRNA | [51] |
| VA1-F/VA1-R | Amplification and sequencing | 5′ ATGGAAATACAACAAACACAC 3′ 5′ CTGCTTGAATGCGCCAAAC 3′ |
vacA s | [33,50] |
| mF1/mR1 | Amplification and sequencing | 5′ ACCGCTCATBAAGATYAAYARCGCTC 3′ 5′ GCTAGGCGCTCTTTGAATTGC 3′ |
vacA m | [52] |
| glmM-F/glmM-R | Amplification | 5′ TAACCGAAGACATGCGCTG 3′ 5′ CATGAAAGATTTCTTCAATCAATCGCT 3′ |
glmM | [52] |
|
cagA-F1/ cagA-R1 |
Amplification | 5′ GATAACAGGCAAGCTTTTTGAGG 3′ 5′ CTGCAAAAGAATGTTTGGCAG 3′ |
5′ cagA | [53] |
| CAGTF/ CAGTR |
Amplification | 5′ ACCCTAGTCGGTAATGGGTTA 3 5′ GTAATTGTCTAGTTTCGC 3′ |
3′ cagA | [53] |
|
cagA-2/ cagA-4 |
Sequencing | 5′ GGAACCCTAGTCGGTAATG 3′ 5′ ATCTTTGAGCTTGTCTATCG 3′ |
3′ cagA | [54,55] |
2.5. CagA Gene Amplification
All H. pylori 16S rRNA gene-positive samples were used in PCR to detect the cagA and vacA genes using primers described in Table 1. Amplification to detect presence of the cagA was achieved by targeting the constant region using primers F1 and B1 [50,56]. A 349-bp product indicated presence of cagA. To determine the variability in the EPIYA domains, the 3′ variable region was amplified using primers cag2 and cag4 resulting in products of 550-850-bp [49,50,51,52,53,54,55,56]. The PCR reaction mix consisted of 1.7 mM MgCl2, 0.2 mM dNTPs, 1 U of Platinum® Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA), and 300 ng of total DNA in a total volume of 25 μL. The PCR conditions used for amplification were: 1 cycle at 94 °C for 5 min; 35 cycles at 94 °C for 40 s, 56 °C for 30 s, and 72 °C for 50 s; and a final extension cycle at 72 °C for 7 min. The PCR products were subjected to electrophoresis on a 1.5% agarose gel and analyzed under an ultraviolet (UV) light. Samples were considered cagA-positive when at least 1 of the 2 bands was observed.
2.6. vacA Gene Amplification
PCR analysis was performed on H. pylori DNA samples to genotype the vacA s and m regions and to detect the presence of the cagA gene using previously described primers (Table 1) [33,50]. Real-time PCR primers were tested for specificity against DNA collected from Helicobacter, Burkholderia, and Campylobacter. Genotyping of vacA was assessed by PCR with oligonucleotides specific for each region (Table 1).
The reaction mixture contained 1.5 mM MgCl2; 0.2 mM dNTPs; 5 pmol of oligonucleotides VAGF and VAGR, or 2.5 pmol of VAIF and VAIR, 1.5 U of Taq DNA polymerase and 200 ng of DNA, in a total volume of 25 μL. Amplification conditions were: 1 cycle at 94 °C for 5 min; 35 cycles at 94 °C for 1 min, 57o C for 1 min, 72 °C for 1 min; and a final extension cycle at 72 °C for 10 min. The PCR products were subjected to 1.5% agarose gel electrophoresis, stained with SYBR Green and visualized with ultraviolet light using a transluminator. In each PCR, DNA from strain 60190 (vacAs1m1/cagA+) was used as a positive control, and as a negative control, DNA was replaced with sterile deionized water. All reactions were performed in a gradient iCycler (BioRad, Hercules, CA, USA). The reference strains 26695 (GenBank AE000511), 60190 (GenBank U05676.1) and Tx30a (ATCC 51932) were used in the phylogenetic analysis of nucleotide and amino acid sequences, these strains are available in the NCBI (https://www.ncbi.nlm.nih.gov/ accessed on 15 June 2021). Amplified PCR products were gel-purified and subjected to sequencing with both forward and reverse primers using BigDye technology on an AB13700XL DNA sequencer (GeneWis, South Plainfield, NJ, USA).
2.7. Statistical Analysis
For all statistical comparisons, persons infected with mixed allelic subtypes (i.e., s1a/s1b) were categorized as s1b. Also, persons infected with both cagA-positive and cagA-negative H. pylori were categorized as infected with a cagA-positive strain. For the correlation of H. pylori genotypes with clinical data, we restricted the evaluation to three combinations of vacAs and vacAm regions with adequate sample sizes for evaluation: s1m1, s1m2, and s2m2. We compared clinical variables between these three genotypes with the likelihood ratio x2 test and analysis of variance (ANOVA) for categorical and continuous variables, respectively. All p values were two sided, and a p value of < 0.05 was considered statistically significant.
3. Results
Ninety-six (96) gastric biopsies sets were collected at WIHCC during EGD procedures during a 11-month timeframe (01/2018–05/2019; Table 2).
Table 2.
Comparing demographic and clinical characteristics by positive or negative biopsies.
| Total | Positive | Negative | p Value | |
|---|---|---|---|---|
| Total | 96 | 22 | 74 | |
| Age (mean) | 55.6 | 54.0 | 56.1 | 0.57 * |
| Gender | 0.08 # | |||
| Male | 25 | 10 | 15 | |
| Female | 71 | 12 | 59 | |
| Reason for Endoscopy | ||||
| Epigastric Pain | 17 | 5 | 12 | 0.153 § |
| Abdominal Pain | 13 | 4 | 9 | |
| GERD | 10 | 1 | 9 | |
| Bloating | 10 | 2 | 8 | |
| Right Upper Quadrant Pain | 6 | 2 | 4 | |
| Nausea | 5 | 1 | 4 | |
| Left Upper Quadrant Pain | 4 | 1 | 3 | |
| Gastritis | 3 | 1 | 2 | |
| GI Bleed | 2 | 2 | 0 | |
| Constipation | 2 | 0 | 2 | |
| Gastric Finding | ||||
| Normal | 14 | 2 | 12 | 0.21 § |
| Gastritis | 60 | 13 | 47 | |
| Gastric Atrophy & Fundal Gastritis | 4 | 3 | 1 | |
| Erosive Gastropathy | 4 | 3 | 1 | |
| Gastritis & Intestinal Metaplasia w/o Dysplasia | 7 | 2 | 5 |
* t test; # X2 square; § Fisher’s exact test.
There were no differences observed in mean age between those with positive biopsy samples for H. pylori (54 ± 12.4) or negative (56.1 ± 12.5). Women were less likely to have a positive biopsy (n = 12/71) compared with men (n = 10/25; p = 0.22). The frequency of H. pylori isolation was 22.9%. Most patients came from Leupp (26.4%), followed by Winslow (18.8%), with no difference by biopsy outcome (Χ2 = 9.9, p = 0.83).
3.1. Demographic Information in Positive Patients
Although 22 patients had at least one positive sample (22.9% of the 96 participants), positive samples collected from the antrum and fundus also were counted separately yielding 26 positive samples (13.5% of all biopsy samples analyzed). More positive samples were from the fundus (53.9%, n = 14) compared with the antrum (46.2%, n = 12). Both the antrum and fundus biopsies were positive in only 4 patients (18.2% participants (Table 2). Normal gastric findings were uncommon among those with positive biopsy samples (2 of 22 patients; 4.5%) compared with 12 of the 74 negative patients (16.2%). Gastritis was the most common gastric finding, in 62.5% of all participants, 59.0% of those with a positive biopsy and 63.5% of those with a negative biopsy (p = 0.8). Regardless of biopsy outcome, epigastric pain was the most common reason listed for undergoing the EGD (17.7%), followed by abdominal pain (13.5%) (Table 2).
3.2. PCR Amplification of cagA Gene Variable 3′ Region and EPIYA Motif Bioinformatic Analysis
Detection for 3′ variable region of the cagA gene in the 26 DNA samples from H. pylori positive strains resulted in 20 positive samples that were confirmed by agarose gel electrophoresis, indicating cagA+ infection in those patients (57.6%; Table 3). Isolates that did not generate amplification of cagA 3′ DNA end, were considered cagA negative and excluded from further molecular analysis. All cagA sequences from these isolates aligned well with the cagA 3′ ends of reference strains 26695 and 60190. The most common EPIYA pattern was the ABC (11/20 = 55%), ABCC (3/20 = 15%), with one AB pattern (5%) (Table 3).
Table 3.
Gastric findings and scores in Navajo patients and their association with H. pylori vacA and cagA genotypes.
| cagA | vacA Genotype | ||||||
|---|---|---|---|---|---|---|---|
| Gastric Disease | EPIYA | s1a,s1b | s1a | s1b | s2 | m1 | m2 |
| Normal | |||||||
| 0 | 0 | 0 | 1 | 0 | 2 | ||
| Gastritis | |||||||
| AB (1) | 0 | 0 | 1 | 1 | 1 | 1 | |
| ABC (7) | 1 | 1 | 9 | 1 | 8 | 2 | |
| Gastritis & ulcer | |||||||
| Erosive gastropathy | |||||||
| ABC (2) | 1 | 1 | 1 | 0 | 2 | 0 | |
| Gastric atrophy & Fundal gastritis | |||||||
| ABC (2) | 0 | 1 | 0 | 1 | 0 | ||
| ABCC (1) | 0 | 1 | 0 | 0 | 1 | 0 | |
| Gastric & Intestinal Metaplasia | |||||||
| ABCC (1) | 0 | 0 | 1 | 0 | 1 | 0 | |
| Gastritis & Intestinal metaplasia without dysplasia | |||||||
| ABCC (1) | 0 | 0 | 1 | 0 | 1 | 0 | |
| TOTAL | (15/20) | 2 | 3 | 14 | 3 | 15 | 5 |
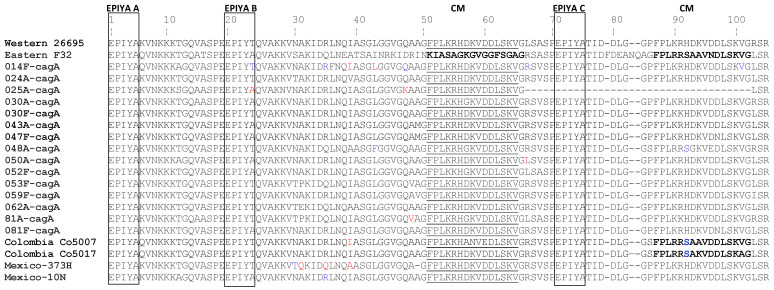
Based on CLUSTALW and Mega 7.0 sequence alignment, all sequenced cagA variable region showed the following patterns, EPIYA(K/Q)VNKKK(A/T)GQ that corresponds to EPIYA-A; the pattern E(P/S)IY(A/T)(Q/K)VAKKV(N/T)(A/Q)KI, to EPIYA-B; and the pattern EPIYATIDDL(G/R) to EPIYA-C (Figure 1).
Figure 1.
Amino acid sequence of the CagA 3’ terminal region from strains containing three EPIYA motifs. The reference strain F32 was isolated from a Japanese patient and it is representative of an Eastern sequence (ESS; AAF17597.1). The reference strain 26695 (NP207343.1) is representative of a Western CagA-specific sequence (WSS). The strains Co5007 (EU251000.1), Co5017 (EU250993.1) and 373H (JN390445.1) and 10N (JN390446.1) were isolated from indigenous groups in Colombia (Co) and Mexico, respectively. Boxes illustrate the regions containing the EPIYA domains and underline shows the cagA multimerization motif (CM).
Within this samples, no isolates corresponded to the EPIYA-D Eastern type: EPIYATIDFDEANQAG. The relationship between the number of EPIYA-C motifs or cagA types and gastric disease was not as clear because of the low number of isolates with more than one EPIYA C domain. Table 3 shows that as the number of EPIYA C motifs increased, the frequency of gastric disease decreased. H. pylori strains with only one EPIYA-C motif (ABC pattern) in the cagA proteins were more frequently associated with atrophic gastritis. Strains with two EPIYA-C motifs were more common among participants with intestinal metaplasia and erosive gastropathy. There was an almost equal distribution in the EPIYA B motif, with the threonine (53.3%) substituted for alanine (46.5%) as EPIYT instead of EPIYA (Figure 1). Phylogenetic analysis of cagA positive biopsies resulted in four distinctive groups. Group A contained eight of positive isolates which also contained the reference strain 26695, the Colombian isolate co5017 and the Mexican 373H isolate. Group B had five isolates which also contained the Mexican isolate 10N. In group C, two isolates were found which grouped closely to a Colombian co5007 and Asian isolate, F32 (Figure S1).
3.3. Presence of vacA in Native American Isolates
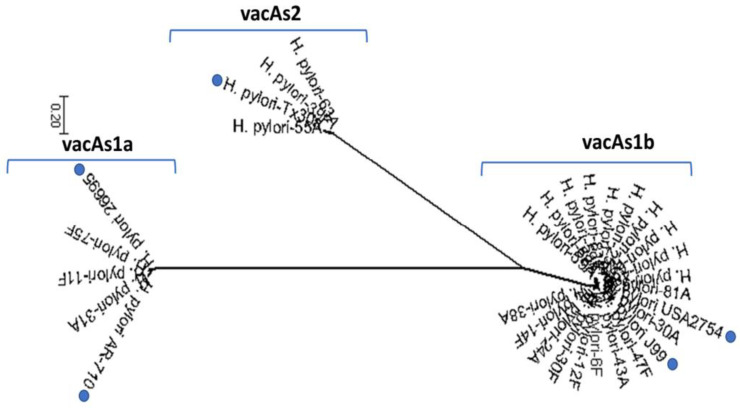
All analyzed isolates expressed the vacA gene. Two biopsies provided a mixed infection with s1a,s1b. The combination of s1b (70%) and m1 (75%) alleles was the most frequent; while allele s1a was found in three samples with m2 found in five samples (Table 3). Most of the isolates identified located with reference strains USA2754 and J99 indicating expression of the vacA s1b. One vacA s2 isolate placed with reference strainTx30a; while three others were s1a and located with reference strains 26695 and AR-710 (Figure 2). When analyzing the vacAm allele, two isolates with a vacAm1 allele (6F and 38A) were found closely associated with the m2 group (Figure S2). One vacAm2 isolate (31A) grouped closely to Colombian (NA1692) and Alaska (18 and 7), typical Amerindian isolates. Fewer vacAm2 genotypes were found in H. pylori in this study. The cagA+ vacA s1bm1 genotype was the most frequent isolate identified and patients with gastritis Table 3).
Figure 2.
Phylogenetic tree of Navajo vacAs alleles in Helicobacter pylori sequences. The vacAs1a alleles are shown on the left with two typical vacAs1a strains (26695-NC_000915.1:938415-942287 and AR-710-AY185128.1). Sequences shown on the right display vacAs1b alleles with reference strains (USA2754-AB057223.1, 60190-U05676.1 and J99-NC 000921.1). The vacAs2 allele is shown on the top with a representative strain (Tx30a-U29401.1) in this group.
4. Discussion
H. pylori is recognized as a major causative agent of chronic gastritis and peptic ulcer disease and has been identified as a major risk factor for development of gastric cancer. Native Americans in the southwestern region of the United States are at increased risk for development of gastric cancer. While studies looking at the association of H. pylori and gastric pathologies have taken place in Indigenous populations in Alaska and Canada [46,48,57,58,59], this is the first attempt to characterize the H. pylori genotypes and their association with gastric diseases among Navajo adults in northern Arizona.
H. pylori infection in Native American and Indigenous communities in Alaska is usually higher than their non-native counterparts and is comparable to that of developing countries, which may help explain the high rate of gastric cancer [40,41,42,43,44]. In a random sample of households of three communities on the Navajo Nation, we reported 56.4% prevalence and 72% of households had at least one person with a positive Urea Breath Test [5]. A retrospective study among Navajos living in New Mexico, reported seroprevalence for H. pylori of 74.4%; furthermore, 69.23% of patients with intestinal metaplasia were also positive for H. pylori [40]. Among Alaska Natives, 65% prevalence was reported for H. pylori on histology, and prevalence increased in patients with gastritis (66%) and chronic gastritis (87%) [44]. Prevalence using serologies is higher in some studies, with infection prevalence among Alaska Natives near 75% [44,46].
Gastric cancer is a multifaceted disease, with a wide range of associated risk factors, such as diet, obesity and chronic health conditions, and access to care and health care utilization [59,60,61]. However, because of the high prevalence of H. pylori and the disproportionately high incidence of gastric cancer, it is likely that H. pylori plays a central role in the gastric cancer observed among Native Americans and Alaska Natives [53]. Alaska Natives experience 4 times the mortality due to gastric cancer when compared to non-Hispanic White [62]. It is unknown whether genotypic differences in the circulating pathogen strain might also explain the high morbidity and mortality.
In our study, we collected gastric biopsies from 96 Navajo patients undergoing a scheduled EGD procedure. When biopsies were analyzed for presence of H. pylori, 22 out of 96 patients were positive (22.9%). This value is much lower than the near 60% observed using the urea breath test in these communities, most likely because it includes all biopsies, including those which may have been taken to confirm post-treatment H. pylori eradication. It is likely that many of the processed biopsies originated from patients that had or were currently undergoing antibiotic therapy. Because we were interested in the virulence factors, we did not subset the data. The 22.9% prevalence in EGD samples should not be used as an estimate of H. pylori prevalence in this study population.
The clinical relevance and geographical distribution of the virulent genotypes of H. pylori is still a matter of debate. Here, we report on the prevalence and relationship of virulence genes of H. pylori (vacA and cagA) with clinical status in patients from the Navajo Nation. The presence of cagA has been commonly associated with an increased risk for the development of gastric cancer [22,63]. In East Asia more than 90% of strains possess cagA which may help explain the high prevalence of gastric cancer in Asian countries [26,64]. We observed a 77% prevalence of cagA positive H. pylori in our isolates which is close to the 74% reported prevalence in Mexican patients with different types of chronic gastritis [50] and the 83.8% in Colombian patients with diverse gastric pathologies [37,65].
The type and number of EPIYA motifs in the 3′ region of cagA are predictors of virulence because of enhanced cagA phosphorylation [28,32,65]. Despite the limited number of patients with gastric atrophy and intestinal metaplasia in this series of patients, our data trend toward increasing number of EPIYA-C motifs being associated more severe disease (Table 3). Indeed, studies using H. pylori strains isolated from Japanese participants have shown that strains expressing cagA with higher numbers of EPIYA-C motifs were more prevalent in patients with atrophic gastritis and gastric cancer [33,66]. Another study in South Africa found that H. pylori strains expressing cagA proteins with four or more variable-region EPIYA-C motifs originated from patients with gastric cancer [51]. Further, Colombian studies showed not only these strains were associated with more severe lesions, but they were also more frequently found [30,37,65].
While we were unable to identify isolates with more than two EPIYA-C domains in this study population; 34.3% to 54.5% were reported in Colombian [22,60], 30% in Mexican [67], and 11.5% in Peruvian isolates [68]. Our study, as well as in a Mexican study investigating virulence factors in different Indigenous groups in Mexico, found that the most common vacA EPIYA domain was ABC [69]. None of the H. pylori strains in our study contained an EPIYA-D motif.
We found a predominance of cagA+ vacAs1m1 genotypes (76.9%) in the H. pylori isolates. Although all H. pylori strains express the vacA gene, its expression and structure may vary resulting in polymorphisms inducing different levels of cytotoxicity and gastric pathologies [70,71]. These polymorphisms are in the 5′ end, in the signal (s) and medial (m) regions for which strains expressing the combination of alleles s1m1 are capable of more cytotoxic activity in vitro than those expressing the vacAs2m2 alleles [33]. Our results are consistent with research in Latin America [49] and indigenous communities [26]. In a high-risk region from northeastern Brazil, a predominance of cagA+ vacAs1m1 isolates was reported, which were associated with more severe gastric pathologies [31]. In Mexico, the vacA s1m1 genotype and cagA EPIYA-ABC motif were present in patients with chronic gastritis [50]. The same pattern was observed in Peruvian Amerindians [71] and in Indigenous communities in Mexico [68].
The limited association between disease and virulent genotypes of H. pylori has provided conflicting results. In Alaska Natives 56% of isolates expressed vacAs1 and 39% s2 subtype where the genotype cagA+, vacAs1m1 was associated with a more severe pathology [45,46,47,48]. Furthermore, the vacA s1m1 genotype was the most prevalent. Our data are consistent with Miernyk et al. [48], although the infection prevalence among biopsies was low (22.9%), the prevalence of cagA positive H. pylori was 77%, vacA s1m1 was 80.7%. Further, western cagA positive strains with EPIYA ABC motifs were always associated with gastritis and a high percentage of vacA s1bm1 genotypes.
Although this is the first report on the expression of virulence genes in H. pylori isolates from the Navajo Nation, this study was limited by sample size. We tested biopsy samples from 96 participants with only 26 samples testing positive for H pylori. When comparing cagA and vacA to the participant’s histopathology findings, we did not have a large enough sample to definitively assess the association. Also, other microbial virulence factors (homB, oipA, babA, dupA) must also be considered [13,16]. We are continuing to recruit participants in this population and these issues will be addressed.
While our project focused on a relatively limited geographic area, this is an area where H. pylori prevalence and gastric cancer rates are disproportionally high, and implications of virulence factors is yet unknown. Gastric cancer is a complex disease with multiple etiologies each with a differing suite of risk factors [60]. Environmental factors have been shown to play an important role and will need to be considered [5,61]. While it is decreasing in most of the United States, a recent study showed that rates are almost universally higher among American Indians/Alaska Natives compared with geographically proximal White populations [58]. Improved understanding of the etiology of gastric cancer in the Navajo Nation will allow for incorporation of identified risk factors to improve prognosis.
Acknowledgments
We thank the participating tribal governmental entities for their support and review of this work, the board of directors at WIHCC who generously allowed us to secure gastric biopsies from their patients, participating patients, and the nursing and surgical staff who assisted with samples collection and patient consenting. We also thank the undergraduate students who helped with processing of these samples. We also thank Winslow Indian Health Care Center, specifically Alfreda Butler who collected patient’s information and provided consent forms.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/diseases10020019/s1. Figure S1. Phylogenetic tree of cagA in Navajo isolates compared with isolates from Mexico, 373H (JN390445.1) and 10N (JN390446.1); Colombia, Co5007 (EU251000.1) and Co5017 (EU250993.1); a Western (NP207343.1); and an Eastern H. pylori strain isolates (AAF17597.1). Figure S2. Phylogenetic tree of Navajo vacAm alleles in Helicobacter pylori sequences. vacAm1 sequences are on top with two typical m1 reference strains (USA2754-AB057223.1, 60190-U05676.1, and J99-NC 000921.1). The vacAm2 sequences are below and contain the reference strain Tx30a (U29401.1), as well as Colombian (Co1692-AB057311.1, Co1764-A057313.1, PS162A1-GU06444.1), Alaskan (Alaska7-AB057294.1, Alaska 18-AB057305.1), and Peruvian Amazon (Shi49-GU064499.1, Shi12-GU064505.1, Shi216-GU064505.1) isolates.
Author Contributions
Conceptualization, F.P.M., H.E.B., R.B.H.; methodology, F.P.M., H.E.B., S.K., M.M.; validation, F.P.M., H.E.B., R.B.H.; formal analysis, F.P.M., H.E.B., R.B.H., P.R.S.; investigation, F.P.M., G.J.; writing—original draft, F.P.M.; writing—review and editing, F.P.M., H.E.B., R.B.H., P.R.S.; funding acquisition, F.P.M., R.B.H., P.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the awards for the Partnership of Native American Cancer Prevention U54CA143924 (UACC) and U54CA143925 (NAU).
Institutional Review Board Statement
NAU ceded review of this protocol to the UA. This project was approved by the UA Institutional Review Board #1610912650), and the Navajo Nation Human Research Review Board (NNR-16-263T).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
These sequence data have been submitted to GenBank database under accession number MT875170, MT878479-MT878479. GenBank: www.ncbi.nlm.nih.gov (accessed on 15 June 2021).
Conflicts of Interest
The authors report no potential conflicts of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Makola D., Peura D.A., Crowe S.E. Helicobacter pylori infection and related gastrointestinal diseases. J. Clin. Gastroenterol. 2007;41:548–558. doi: 10.1097/MCG.0b013e318030e3c3. [DOI] [PubMed] [Google Scholar]
- 2.De Martel C., Forman D., Plummer D. Gastric Cancer. Epidemiology and Risk Factors. Gastroenterol. Clin. N. Am. 2013;42:219–240. doi: 10.1016/j.gtc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Perez G.I., Rothenbacher D., Brenner H. Epidemiology of Helicobacter pylori Infection. Helicobacter. 2004;9:1–6. doi: 10.1111/j.1083-4389.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 4.Camargo M.C., Yepez M.C., Cerón C., Guerrero N., Bravo L.E., Correa P., Fontham E.T. Age at acquisition of Helicobacter pylori infection: Comparison of two areas with contrasting risk of gastric cancer. Helicobacter. 2004;9:262–270. doi: 10.1111/j.1083-4389.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- 5.Harris R.B., Brown H.E., Begay R., Sanderson P., Chief C., Oren E., Monroy F.P. Helicobacter pylori prevalence and risk factors in three rural Navajo communities of Northern Arizona: A cross-sectional study. Int. J. Environ. Res. Public Health. 2022;19:797. doi: 10.3390/ijerph19020797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everhart J.E. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol. Clin. N. Am. 2000;29:559–578. doi: 10.1016/S0889-8553(05)70130-8. [DOI] [PubMed] [Google Scholar]
- 7.Blaser M.J. Helicobacter pylori eradication and its implications for the future. Aliment. Pharmacol. Ther. 1997;11:103–107. doi: 10.1046/j.1365-2036.11.s1.7.x. [DOI] [PubMed] [Google Scholar]
- 8.Bui D., Brown H., Harris R., Oren E. Serologic evidence for fecal-oral transmission of Helicobacter pylori. Am. J. Trop. Med. Hyg. 2016;94:82–88. doi: 10.4269/ajtmh.15-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Portal-Celhay C., Perez-Perez G.I. Immune responses to Helicobacter pylori colonization: Mechanisms and clinical outcomes. Clin. Sci. 2006;110:305–314. doi: 10.1042/CS20050232. [DOI] [PubMed] [Google Scholar]
- 10.Ernst P.B., Gold B.D. Helicobacter pylori in childhood: New insights into the immunopathogenesis of gastric disease and implications for managing infection in children. J. Pediatr. Gastroenterol. Nutr. 1999;28:462–473. doi: 10.1097/00005176-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Peek R.M., Blaser M.J. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 12.Boyle P., Levin B. World Cancer Report 2008. International Agency for Research on Cancer (IARC); Lyon, France: 2008. [Google Scholar]
- 13.Figura N., Tabaqchali S. Bacterial pathogenic factors. Curr. Opin. Gastroenterol. 1996;12:11–15. doi: 10.1097/00001574-199601001-00003. [DOI] [Google Scholar]
- 14.Con S.A., Takeuchi H., Con-Chin G.R., Con-Chin V.G., Yasuda N., Con-Wong R. Role of bacterial and genetic factors in gastric cancer in Costa Rica. World J. Gastroenterol. 2009;15:211–228. doi: 10.3748/wjg.15.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kusters J.G., Van Vliet A.H.M., Kuipers E.J. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006;19:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kao C.Y., Sheu B.S., Wu J.J. Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomed. J. 2016;39:14–23. doi: 10.1016/j.bj.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Argent R.H., Thomas R.J., Letley D.P., Rittig M.G., Hardie K.R., Atherton J.C. Functional association between the Helicobacter pylori virulence factors vacA and cagA. J. Med. Microbiol. 2008;57:145–150. doi: 10.1099/jmm.0.47465-0. [DOI] [PubMed] [Google Scholar]
- 18.Jones K.R., Joo Y.M., Jang S., Yoo Y.-J., Lee H.S., Chung I.-S., Olsen C.H., Whitmire J.M., Merrell D.S., Cha J.-H. Polymorphism in the cagA EPIYA motif impacts development of gastric cancer. J. Clin. Microbiol. 2009;47:959–968. doi: 10.1128/JCM.02330-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Covacci A., Censini S., Bugnoli M., Petracca R., Burroni D., Macchia G., Massone A., Papini E., Xiang Z., Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc. Natl. Acad. Sci. USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Backert S., Selbach M. Role of type IV secretion in Helicobacter pylori pathogenesis. Cell. Microbiol. 2008;10:1573–1581. doi: 10.1111/j.1462-5822.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- 21.Blaser M.J., Perez G.P., Kleanthous H., Cover T., Peek R.M., Chyou P.H., Stemmermann G.N., Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 22.Acosta N., Quiroga A., Delgado P., Bravo M.M., Jaramillo C. Helicobacter pylori cagA protein polymorphisms and their lack of association with pathogenesis. World. J. Gastroenterol. 2010;16:3936–3943. doi: 10.3748/wjg.v16.i31.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gwack J., Shin A., Kim C.-S., Ko K.-P., Kim Y., Jun J.K., Bae J., Park S.K., Hong Y.-C., Kang D., et al. CagA-producing Helicobacter pylori and increased risk of gastric cancer: A nested case-control study in Korea. Br. J. Cancer. 2006;95:639–641. doi: 10.1038/sj.bjc.6603309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torres J., Lopez L., Lazcano E., Camorlinga M., Flores L., Muñoz O. Trends in Helicobacter pylori infection and gastric cancer in Mexico. Cancer Epidemiol. Biomark. Prev. 2005;14:1874–1877. doi: 10.1158/1055-9965.EPI-05-0113. [DOI] [PubMed] [Google Scholar]
- 25.Murata-Kamiya N. Pathophysiological functions of the CagA oncoprotein during infection by Helicobacter pylori. Microbes Infect. 2011;13:799–807. doi: 10.1016/j.micinf.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Miernyk K., Morris J., Bruden D., McMahon B., Hurlburt D., Sacco F., Parkinson A., Hennessy T., Bruce M. Characterization of Helicobacter pylori cagA and vacA genotypes among Alaskans and their correlation with clinical disease. J. Clin. Microbiol. 2011;49:3114–3121. doi: 10.1128/JCM.00469-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higashi H., Tsutsumi R., Fujita A., Yamazaki S., Asaka M., Azuma T., Hatakeyama M. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc. Natl. Acad. Sci. USA. 2002;99:14428–14433. doi: 10.1073/pnas.222375399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizzato C., Torres J., Plummer M., Munoz N., Franceschi S., Camorlinga-Ponce M., Fuentes-Panana E.M., Canzian F., Kato I. Variations in Helicobacter pylori cytotoxin-associated genes and their influence in progression to gastric cancer: Implications for prevention. PLoS ONE. 2012;7:e29605. doi: 10.1371/journal.pone.0029605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panayotopoulou E.G., Sgouras D.N., Papadakos K., Kalliaropoulos A., Papatheodoridis G., Mentis A.F., Archimandritis A.J. Strategy to characterize the number and type of repeating EPIYA phosphorylation motifs in the carboxyl terminus of cagA protein in Helicobacter pylori clinical isolates. J. Clin. Microbiol. 2007;45:488–495. doi: 10.1128/JCM.01616-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quiroga A.J., Huertas A., Combita A.L., Bravo M.M. Variation in the number of EPIYA-C repeats in cagA protein from Colombian Helicobacter pylori strains and its ability middle to induce hummingbird phenotype in gastric epithelial cells. Biomedica. 2010;30:251–258. doi: 10.7705/biomedica.v30i2.188. [DOI] [PubMed] [Google Scholar]
- 31.Batista S.A., Rocha G.A., Rocha A.M., Saraiva I.E., Cabral M.M., Oliveira R.C., Queiroz D.M. Higher number of Helicobacter pylori cagA EPIYA C phosphorylation sites increases the risk of gastric cancer, but not duodenal ulcer. BMC Microbiol. 2011;11:61. doi: 10.1186/1471-2180-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basso D., Zambon C., Letley D.P., Stranges A., Marchet A., Rhead J.L., Schiavon S., Guariso G., Ceroti M., Nitti D., et al. Clinical Relevance of Helicobacter pylori cagA and vacA Gene Polymorphisms. Gastroenterology. 2008;135:91–99. doi: 10.1053/j.gastro.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 33.Atherton J.C., Cao P., Peek R.M., Tummuru M.K.R., Blaser M.J., Cover T.L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 34.Gangwer K.A., Shaffer C.L., Suerbaum S., Lacy D.B., Cover T.L., Bordenstein S.R. Molecular evolution of the Helicobacter pylori vacuolating toxin gene vacA. J. Bacteriol. 2010;192:6126–6135. doi: 10.1128/JB.01081-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martins L.C., De Corvelo Oliveira T.C., Demachki S., De Araújo M.T.F., Assumpcão M.B., Vilar S.C.A.J., Freitas F.B., Barbosa H.P.M., Fecury A.A., Amaral R.K.C.D., et al. Clinical and pathological importance of vacA allele heterogeneity and cagA status in peptic ulcer disease in patients from North Brazil. Mem. Inst. Oswaldo Cruz. 2005;100:875–881. doi: 10.1590/S0074-02762005000800009. [DOI] [PubMed] [Google Scholar]
- 36.Chang Y.H., Wang L., Lee M.S., Cheng C.W., Wu C.Y., Shiau M.Y. Genotypic characterization of Helicobacter pylori cagA and vacA from biopsy specimens of patients with gastroduodenal diseases. Mt. Sinai J. Med. 2006;73:622–626. [PubMed] [Google Scholar]
- 37.Arévalo-Galvis A., Trespalacios-Rangel A.A., Otero W., Mercado-Reyes M.M., Poutou-Piñales R.A. Prevalence of cagA, vacA, and iceA genes in H. pylori strains isolated from Colombian patients with functional dispepsia. Pol. J. Microbiol. 2012;61:33–40. doi: 10.33073/pjm-2012-004. [DOI] [PubMed] [Google Scholar]
- 38.Parkinson A.J., Bruce M.G., Zulz T. International circumpolar international surveillance, an Arctic network for surveillance of infectious diseases. Emerg. Infect. Dis. 2008;14:18–24. doi: 10.3201/eid1401.070717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nolen L.D., Bruden D., Miernyk K., McMahon B.J., Sacco F., Varner W., Mezzetti T., Hurlburt D., Tiesinga J., Bruce M.G. H. pylori-associated pathologic findings among Alaska native patients. Int. J. Circumpolar Health. 2018;77:1510715. doi: 10.1080/22423982.2018.1510715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stancioiu F., Ahmed S.H. Helicobacter pylori: Findings in a Native American Population. IHS Prim. Care Provid. 2005;30:59–63. [Google Scholar]
- 41.Kelly J.J., Lanier A.P., Schade T., Brantley J., Starkey B.M. Cancer disparities among Alaska native people, 1970–2011. Prev. Chronic Dis. 2014;11:E221. doi: 10.5888/pcd11.130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tveit A.H., Bruce M.G., Bruden D.L., Morris J., Reasonover A., Hurlburt D.A., Hennessy T.W., McMahon B. Alaska sentinel surveillance study of Helicobacter pylori isolates from Alaska native persons from 2000 to 2008. J. Clin. Microbiol. 2011;49:3638–3643. doi: 10.1128/JCM.01067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Navajo Epidemiology Report Cancer Among the Navajo, 2005–2013. [(accessed on 15 June 2021)]; Available online: https://www.nec.navajo-nsn.gov/Epi-Reports.
- 44.Sacco F., Bruce M.G., McMahon B.J., Bruden D. A prospective evaluation of 200 upper endoscopies performed in Alaska Native persons. Int. J. Circumpolar Health. 2007;66:144–152. doi: 10.3402/ijch.v66i2.18245. [DOI] [PubMed] [Google Scholar]
- 45.Parkinson A.J., Gold B.D., Bulkow L., Wainwright R.B., Swaminathan B., Khanna B., Petersen K.M., Fitzgerald M.A. High prevalence of Helicobacter pylori in the Alaska Native population and association with low serum ferritin levels in young adults. Clin. Diagn. Lab. Immunol. 2000;7:885–888. doi: 10.1128/CDLI.7.6.885-888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keck J.W., Miernyk K.M., Bulkow L.R., Kelly J.J., McMahon B.J., Sacco F., Hennessy T.W., Bruce M.G. Helicobacter pylori infection and markers of gastric cancer risk in Alaska Native persons: A retrospective case-control study. Can. J. Gastroenterol. Hepatol. 2014;28:305–310. doi: 10.1155/2014/892084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McMahon B.J., Bruce M.G., Koch A., Goodman K.J., Tsukanov V., Mulvad G., Borresen M.L., Sacco F., Barrett D.C., Westby S., et al. The diagnosis and treatment of Helicobacter pylori infection in Arctic regions with a high prevalence of infection: Expert Commentary. Epidemiol. Infect. 2016;144:225–233. doi: 10.1017/S0950268815001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miernyk K.M., Bruden D., Rudolph K.M., Hurlburt D.A., Sacco F., McMahon B.J., Bruce M.G. Presence of cagPAI genes and characterization of vacA s, i and m regions in Helicobacter pylori isolated from Alaskans and their association with clinical pathologies. J. Med. Microbiol. 2020;69:218–227. doi: 10.1099/jmm.0.001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atrisco-Morales J., Martínez-Santos V.I., Román-Román A., Alarcón-Millán J., De Sampedro-Reyes J., Carmen I.C.-D., Martínez-Carrillo D.N., Fernández-Tilapa G. vacA s1m1 genotype and cagA EPIYA-ABC pattern are predominant among Helicobacter pylori strains isolated from Mexican patients with chronic gastritis. J. Med. Microbiol. 2018;67:314–324. doi: 10.1099/jmm.0.000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Román-Román A., Martínez-Carrillo D.N., Atrisco-Morales J., Azúcar-Heziquio J.C., Cuevas-Caballero A.S., Castañón-Sánchez C.A., Reyes-Ríos R., Betancourt-Linares R., Reyes-Navarrete S., Carmen I.C.-D., et al. Helicobacter pylori vacA s1m1 genotype but not cagA or babA2 increase the risk of ulcer and gastric cancer in patients from Southern Mexico. Gut Pathog. 2017;9:1–12. doi: 10.1186/s13099-017-0167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Engstrand L., Nguyen A.M., Graham D.Y., el Zaatari F.A. Reverse transcription and polymerase chain reaction amplification of rRNA for detection of Helicobacter species. J. Clin. Microbiol. 1992;30:2295–2301. doi: 10.1128/jcm.30.9.2295-2301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toracchio S., Caruso R.A., Perconti S., Rigoli L., Betri E., Neri M., Verginelli F., Mariani-Costantini R. Evolutionarily-Related Helicobacter pylori Genotypes and Gastric Intraepithelial Neoplasia in a High-Risk Area of Northern Italy. Microorganisms. 2020;8:324. doi: 10.3390/microorganisms8030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamaoka Y., Kodama T., Kashima K., Graham D.Y., Sepulveda A.R. Variants of the 3′ region of the cagA gene in Helicobacter pylori isolates from patients with different H. pylori-associated diseases. J. Clin. Microbiol. 1998;36:2258–2263. doi: 10.1128/JCM.36.8.2258-2263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rudi J., Kolb C., Maiwald M., Kuck D., Sieg A., Galle P.R., Stremmel W. Diversity of Helicobacter pylori vacA and cagA genes and relationship to vacA and cagA protein expression, cytotoxin production, and associated diseases. J. Clin. Microbiol. 1998;36:944–948. doi: 10.1128/JCM.36.4.944-948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Argent R.H., Zhang Y., Atherton J.C. Simple method for determination of the number of Helicobacter pylori cagA variable-region EPIYA tyrosine phosphorylation motifs by PCR. J. Clin. Microbiol. 2005;43:791–795. doi: 10.1128/JCM.43.2.791-795.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Melkonian S.C., Pete D., Jim M.A., Haverkamp D., Wiggins C.L., Bruce M.G., White M.C. Gastric Cancer Among American Indian and Alaska Native Populations in the United States, 2005–2016. Am. J. Gastroenterol. 2020;115:1989–1997. doi: 10.14309/ajg.0000000000000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiggins C.L., Perdue D.G., Henderson J.A., Bruce M.G., Lanier A.P., Kelley J.J., Seals B.F., Espey D.K. Gastric cancer among American Indians and Alaska natives in the USA, 1999–2004. Cancer. 2008;113:1225–1233. doi: 10.1002/cncr.23732. [DOI] [PubMed] [Google Scholar]
- 58.Goodman K.J., Jacobson K., Veldhuyzen van Zanten S. Helicobacter pylori infection in Canadian and related Arctic Aboriginal populations. Can. J. Gastroenterol. 2008;22:289–295. doi: 10.1155/2008/258610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang P., Zhou Y., Chen B., Wan H.-W., Jia G.-Q., Bai H.-L., Wu X.-T. Overweight, obesity and gastric cancer risk: Results from a meta-analysis of cohort studies. Eur. J. Cancer. 2009;45:2867–2873. doi: 10.1016/j.ejca.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 60.Yusefi A.R., Bagheri Lankarani K., Bastani P., Radinmanesh M., Kavosi Z. Risk Factors for Gastric Cancer: A Systematic Review. Asian Pac. J. Cancer Prev. 2018;19:591–603. doi: 10.22034/APJCP.2018.19.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carmack A.M., Schade T.L., Sallison I., Provost E.M., Kelly J.J. Cancer in Alaska Native People: 1969–2013 the 45-Year Report. Alaska Native Tumor Registry, Alaska Native Epidemiology Center, Alaska Native Tribal Health Consortium; Anchorage, AK, USA: 2015. [Google Scholar]
- 62.Parsonnet J., Friedman G.D., Orentreich N., Vogelman H. Risk for gastric cancer in people with cagA positive or cagA negative Helicobacter pylori infection. Gut. 1997;40:297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sahara S., Sugimoto M., Vilaichone R.-K., Mahachai V., Miyajima H., Furuta T., Yamaoka Y. Role of Helicobacter pylori cagA EPIYA motif and vacA genotypes for the development of gastrointestinal diseases in Southeast Asian countries: A meta-analysis. BMC. Infect. Dis. 2012;12:223–230. doi: 10.1186/1471-2334-12-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sicinschi L.A., Correa P., Peek R.M., Camargo M., Piazuelo M., Romero-Gallo J., Hobbs S., Krishna U., Delgado A., Mera R., et al. CagA C-terminal variations in Helicobacter pylori strains from Colombian patients with gastric precancerous lesions. Clin. Microbiol. Infect. 2010;16:369–378. doi: 10.1111/j.1469-0691.2009.02811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beltrán-Anaya F.O., Poblete T.M., Román-Román A., Reyes S., De Sampedro J., Peralta-Zaragoza O., Rodríguez M., Del Moral-Hernández O., Illades-Aguiar B., Fernández-Tilapa G. The EPIYA-ABCC motif pattern in cagA of Helicobacter pylori is associated with peptic ulcer and gastric cancer in Mexican population. BMC Gastroenterol. 2014;14:223–230. doi: 10.1186/s12876-014-0223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reyes-Leon A., Atherton J.C., Argent R.H., Puente J.L., Torres J. Heterogeneity in the activity of Mexican Helicobacter pylori strains in gastric epithelial cells and its association with diversity in the cagA gene. Infect. Immun. 2007;75:3445–3454. doi: 10.1128/IAI.01951-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Devi S.M., Ahmed I., Khan A.A., Rahman S.A., Alvi A., Sechi L.A., Ahmed N. Genomes of Helicobacter pylori from native Peruvians suggest admixture of ancestral and modern lineages and reveal a western type cag-pathogenicity island. BMC Genom. 2006;7:191. doi: 10.1186/1471-2164-7-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Camorlinga-Ponce M., Perez-Perez G., González-Valencia G., Mendoza I., Peñaloza-Espinosa R., Ramos I., Kersulyte D., Reyes-Leon A., Romo C., Granados J., et al. Helicobacter pylori genotyping from american indigenous groups shows novel amerindian vacA and cagA alleles and Asian, African and European admixture. PLoS ONE. 2011;6:e27212. doi: 10.1371/journal.pone.0027212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sugimoto M., Yamaoka Y. The association of vacA genotype and Helicobacter pylori-related disease in Latin American and African populations. Clin. Microbiol. Infect. 2009;15:835–842. doi: 10.1111/j.1469-0691.2009.02769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McClain M.S., Beckett A.C., Cover T.L. Helicobacter pylori vacuolating toxin and gastric cancer. Toxins. 2017;9:316. doi: 10.3390/toxins9100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kersulyte D., Kalia A., Gilman R.H., Mendez M., Herrera P., Cabrera L., Velapatino B., Balqui J., De La Vega F.P.P., Ulloa C.A.R., et al. Helicobacter pylori from Peruvian Amerindians: Traces of human migrations in strains from remote Amazon, and genome sequence of an Amerind strain. PLoS ONE. 2010;5:e15076. doi: 10.1371/journal.pone.0015076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
These sequence data have been submitted to GenBank database under accession number MT875170, MT878479-MT878479. GenBank: www.ncbi.nlm.nih.gov (accessed on 15 June 2021).