Abstract
The effectiveness of plasmid DNA (pDNA) vaccines can be improved by the co-delivery of plasmid-encoded molecular adjuvants. We evaluated pDNAs encoding GM-CSF, Flt-3L, IL-12 alone, or in combination, for their relative ability to serve as adjuvants to augment humoral and cell-mediated immune responses elicited by prototype pDNA vaccines. In Balb/c mice we found that co-administration of plasmid-based murine GM-CSF (pmGM-CSF), murine Flt-3L (pmFlt-3L) or murine IL-12 (pmIL-12) could markedly enhance the cell-mediated immune response elicited by an HIV-1 env pDNA vaccine. Plasmid mGM-CSF also augmented the immune response elicited by DNA vaccines expressing HIV-1 Gag and Nef-Tat-Vif. In addition, the use of pmGM-CSF as a vaccine adjuvant appeared to markedly increase antigen-specific proliferative responses and improved the quality of the resulting T cell response by increasing the percentage of poly-functional memory CD8+ T cells. Co-delivery of pmFlt-3L with pmGM-CSF did not result in a further increase in adjuvant activity. However, the co-administration of pmGM-CSF with pmIL-12 did significantly enhanced Env-specific proliferative responses and vaccine efficacy in the murine vaccinia virus challenge model relative to mice immunized with the env pDNA vaccine adjuvanted with either pmGM-CSF or pmIL-12 alone. These data support the testing of pmGM-CSF and pmIL-12, used alone or in combination, as plasmid DNA vaccine adjuvants in future macaque challenge studies.
Keywords: plasmid DNA, cytokine, chemokine, molecular adjuvant, HIV
INTRODUCTION
Vaccination with plasmid DNA (pDNA) encoding a foreign antigen represents a simple and elegant means of inducing antigen-specific T cell and antibody responses in a number of animal model systems [1, 2]. DNA-based vaccines are non-infectious, are incapable of replication, do not express unwanted pathogenic proteins; and as a result have enhanced safety features compared to replication competent or attenuated viral vaccines. Unlike recombinant viral vector vaccines, which are often limited in their effectiveness due to pre-exiting or immunization induced anti-vector immunity, pDNA-based vaccines do not elicit anti-vector immunity and have the potential to be used for multiple booster immunizations.
DNA vaccines are known to be highly immunogenic and capable of eliciting protective immune responses in mice [3-5]. However, the immunogenicity of pDNA vaccines is generally much more limited in larger animals [2, 6, 7] and has proven disappointing in phase I clinical studies [8-10]. Therefore, multiple strategies have been developed and tested with the goal of improving pDNA vaccine potency. One area of focus has been on identification of the most effective pDNA vaccine delivery system [11-17], while others have sought to optimize expression of the encoded vaccine antigens [18, 19], or combine pDNA vaccines with other vaccine platforms in various prime-boost vaccination regimens [20-22]. In addition, pDNA elicited immune responses can be enhanced and modulated by the use of plasmid-based molecular adjuvants (reviewed in [23, 24]). Multiple groups have demonstrated the potential of coadministering pDNAs that express cytokine, β-chemokines or co-stimulatory molecules together with plasmids encoding target viral antigens. Among them, the cytokines interleukin-12 (IL-12, [25-27]), granulocyte-macrophage colony stimulating factor (GM-CSF, [25, 28-31]), and hematopoietic factor fms-like tyrosine kinase 3 ligand (Flt-3L, [32, 33]) are of particular interest.
It has been demonstrated that plasmid-based IL-12 or GM-CSF can act as effective pDNA vaccine adjuvants [25-31] and augment the magnitude of vaccine-elicited immune response. However, it has been recently reported that the quality of the antigen-specific T cell response rather than the magnitude of the response may be more important in the control of virus replication and disease progression. Indeed, using polychromatic flow cytometry to simultaneously measure five CD8+ T-cell functions (degranulation, expression of IFN-γ, MIP-1β, TNF-α and IL-2) in chronically HIV-infected individual, Betts et al. have reported that HIV-infected long-term nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells [34]. Further evidence for the importance of a multi-functional T cell response comes from vaccinia virus immunized individuals who maintain a high proportion of multi-functional vaccinia virus-specific T cells [35]. Taken together, these data suggest that the induction of vaccine-specific multi-functional CD8+ T cells may be an important component of an efficacious vaccine.
In the current study, we sought to evaluate the ability of plasmid-based adjuvants pmGM-CSF, pmFlt-3L and pmIL-12, used alone or in combination, to augment pDNA-elicited immune responses. Importantly, our analysis consisted of measuring adjuvant activity in terms of their effect on the magnitude and the quality of the antigen-specific immune response. Our results indicate that when the adjuvants were used individually, pmGM-CSF was most effective and enhanced the immune responses to HIV-1 antigen expressing pDNA by increasing the vaccine-elicited antigen-specific CD4+ and CD8+ T cell proliferation and improving the quality of multi-functional memory CD8+ T cell response. Importantly, co-administration of pmGM-CSF with pmIL-12 significantly enhanced HIV-1 env DNA vaccine elicited T cell proliferative responses and vaccine efficacy in the murine vaccinia virus challenge model relative to mice immunized with the env pDNA vaccine adjuvanted with pmGM-CSF or pmIL-12 alone.
MATERIAL AND METHODS
Plasmid DNAs
All HIV-1 genes used in this study including gag p55 (HXB2), nef (NL4-3), tat (NL4-3), vif (NL4-3) and env gp160 (primary isolate 6101 from David Montefiori, gene bank accession no. AAT36747) were RNA optimized. RNA optimization involved the introduction of multiple point mutations within the coding region inactivating endogenous inhibitory sequences, thus allowing for high level rev-independent protein expression as previously described [19, 36, 37]. Nef, tat and vif open reading frames (Nef amino acids 4-206; Tat amino acids 2-80; Vif amino acids 2-192) were fused inframe to generate a single Nef-Tat-Vif fusion protein (NTV) with protease cleavage sites (REKRAVVG) engineered between the Nef-Tat and Tat-Vif proteins to facilitate antigen processing. For safety reasons, the ability of Nef to alter T-cell signal transduction pathways and to down regulate cell surface expression of CD4 and MHC class I was inactivated by removal of the myristoylation signal [38]. In addition, the ability of Tat to transactivate nuclear gene expression was eliminated by the deletion of two cysteines (Cys30 and Cys34; [39]). Other amino acid sequence changes were also made in the Nef (A15T, G29R, V33A, N51T) and vif (N19R, R23S, R33G, D37G, N48H, K50R, H110Y, K155T, A167T) genes to introduce HIV-1 clade B consensus CTL epitopes [40].
Plasmid DNA-based expression vectors encoding HIV-1 derived genes, murine GM-CSF (pmGM-CSF) and murine fms-like tyrosine kinase 3-ligand (pmFlt-3L) were constructed using a human cytomegalovirus (HCMV) immediate early promoter and bovine growth hormone (BGH) polyadenylation (polyA) signal. In addition, a dual promoter expression plasmid encoding murine IL-12 p35 and p40 genes was also used. The mIL-12 p35 subunit was expressed under control of the HCMV immediate early promoter and SV40 polyA signal, while the mIL-12 p40 subunit was expressed under control of the simian cytomegalovirus (SCMV) and BGH polyA signal [41]. All expression vectors included a chimeric kanamycin resistance (kmr) gene, adenylyl 4’-nucleotidyl transferase type-1a [42, 43] and a ColE1 bacterial origin of replication required for selection and propagation of bacteria, respectively.
For each HIV plasmid DNA expression vector, in vitro expression of the encoded HIV-1 gene(s) was confirmed by Western blot after transient transfection of human rhabdomyosarcoma (RD) cells (ATCC; Manassas, VA) as previously described [41]. Production of murine IL-12 was confirmed after transient transfection of RD cells and screening cell supernatants for cytokine expression using an anti-murine IL-12 p70 capture ELISA (data not shown; Endogen, Woburn, MA). The in vitro expression of cDNAs for mGM-CSF and mFlt-3L were confirmed following transient transfection of 293 cells (ATCC; Manassas, VA) and subsequent cytokine- or protein-specific ELISA assays (data not shown).
Bioactivity of plasmid-expressed murine IL-12 was confirmed by assaying supernatants from transiently transfected RD cells for the capacity to induce IFN-γ secretion in murine splenocytes (data not shown). The biological activity of plasmid-expressed GM-CSF was confirmed by assaying supernatants from transiently transfected RD cells for their capacity to induce proliferation of FDCP1 cells [44].
Expression plasmids for inoculation of mice were produced by Puresyn, Inc. (Malvern, PA). Plasmids were propagated in Escherichia coli, isolated from cells by alkaline lysis, purified by column chromatography and formulated individually at a concentration of 2.5 mg/mL in isotonic citrate buffer (29.3 mM sodium citrate, 0.67 mM citric acid, 150 mM NaCl, 0.34 mM EDTA, pH = 6.4 – 6.7) containing 0.25% bupivacaine to allow for the formation of pDNA:bupivacaine complexes [45]. Final pDNA preparations were shown to consist of >90% supercoiled plasmid DNA and residual endotoxin was shown to be <30 EU/mg DNA (data not shown).
DNA Immunization of Mice
For these studies, 6-8 week old female Balb/c mice were used. Mice were purchased from Charles River Laboratories (Wilmington, MA) and maintained in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academic Press, Washington, DC, 1996). In addition, procedures for the use and care of the mice were approved by Wyeth Research’s Institutional Animal Care and Use Committee. Immediately prior to inoculation, the indicated pDNA expression vectors were mixed and administered by intramuscular (i.m.) injection into both quadriceps muscles (0.1 mL total injection volume, 0.05 mL per site) using an 18 gauge needle and 0.3 mL syringe.
IFN-γ ELISPOT Assay
Ten days after the final pDNA inoculation, mice were sacrificed and spleen cells were harvested. For the determination of vaccine-elicited IFN-γ ELISpot responses in individual mice, a mouse IFN-γ ELISpot kit (material number 551083, BD Biosciences, San Diego CA) was used. Ninety-six-well flat-bottom ELISpot plates (ImmunoSpot, Cellular Technology Limited, Cleveland OH) were coated overnight with a purified anti-mouse γ-interferon (mIFN-γ) monoclonal antibody (Material No. 51-2525KC, BD-Biosciences, San Diego CA) at a concentration of 10 mcg/mL, after which the plates were washed three times with sterile1x phosphate buffered saline (1x PBS) and then blocked for 2 hours with R10 complete culture medium (RPMI-1640 containing 10% FBS and 2 mM L-glutamine, 100 units/mL penicillin, 100 mcg/mL streptomycin sulfate, 1 mM sodium pyruvate, 1 mM HEPES, 100 mcM non-essential amino acids). Mouse spleens were homogenized by grinding the spleens between the frosted ends of two sterile microscope slides. The resulting homogenate was suspended in 10 mL of complete R05 culture medium (RPMI 1640 medium supplemented with 5% FBS, 2 mM L-glutamine, 100 units/mL penicillin, 100 mcg/mL streptomycin sulfate, 1 mM sodium pyruvate, 1 mM HEPES, 100 mcM non-essential amino acids) and splenocytes were subsequently isolated by Ficoll-Hypaque density gradient centrifugation and resuspended in complete R10 culture medium containing either 2 mcg/mL Con-A (Sigma), peptide pools (15mers overlapping by 11 amino acids; 2.5 mcM each final peptide concentration) spanning HIV-1HXB2 gag p55 (two pools containing approximately 61 peptides per pool), HIV-1NL43 nef (one pool containing 49 peptides), tat (one pool containing 23 peptides), vif (one pool containing 46 peptides), HIV-16101 env gp160 (four pools containing approximately 52 peptides per pool) or medium alone. Input cell numbers were 4 x 105 splenocytes per well (4 x 106 splenocytes/mL) and assayed in duplicate wells. Splenocytes were incubated for 22-24 hours at 37°C and then removed from the ELISpot plate by first washing three times with deionized water followed by incubation on ice for 10-20 minutes. Then plates were washed six times with 1x PBS containing 0.1% Tween-20. Thereafter, plates were treated with an anti-mouse IFN-γ biotinylated detection antibody (5.0 mcg/mL, Material No. 51-1818KZ, BD-Biosciences, San Diego CA) diluted with PBS/10%FBS and incubated overnight at 4°C. ELISpot plates were then washed 10 times with 1x PBS containing 0.1% Tween-20 and treated with 100 mcL per well of streptavidin-horseradish peroxidase conjugate (Catalog No. 51-9000209, BD-Biosciences, San Diego CA) diluted 1:100 with PBS/10%FBS and incubated for an additional 1 hour at room temperature. Unbound conjugate was removed by rinsing the plate six times with 1x PBS containing 0.1% Tween-20 and 3 times with 1x PBS. AEC Chromogen diluted 20 mcg/mL in AEC substrate solution (Catalog No. 551951, BD-Biosciences, San Diego CA) was then added (100 mcL/well) for 3-5 minutes before being rinsed away with water, after which the plates were air-dried, and the resulting spots counted using an Immunospot Reader (CTL Inc., Cleveland, OH). Peptide-specific IFN-γ ELISpot responses were considered positive if the response (minus media background) was ≥ 3 fold above the media response and ≥ 50 SFC/106 splenocytes.
Serum Gag specific IgG ELISA
Immulon 2HB plates were coated with 200 ng/mL (20ng/well) recombinant HIV-1IIIB gag p24 protein (Immunodiagnostics Inc.) in carbonate/bicarbonate buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) and incubated for a minimum of 13 hours at 4°C. Plates were washed 5 times with 1x PBS containing 0.1% Tween-20 (0.1% TPBS buffer) then blocked with 200 mcL of 3% BSA/0.1%TPBS for a minimum 2 hours at room temperature. Individual mouse serum samples were initially diluted at 1:150 and further diluted 1:3 across the plate in 1% BSA/0.1% TPBS buffer and incubated for a minimum of 13 hours at 4°C after which plates were washed 5 times with 0.1% TPBS buffer. Primary antibody: Biotin –SP-Affinipure Goat anti-mouse IgG (Jackson ImmunoResearch Laboratories Inc.) was added at a dilution of 1:20,000 in 1% BSA/0.1% TPBS buffer and incubated for a minimum of 2 hours at room temperature after which plates were washed 5 times with 0.1% TPBS buffer. Developing antibody: Streptavidin-Peroxidase conjugated anti-biotin antibody (500 units/mL, Roche Imunochemical, Indianapolis, IN) was added at a concentration of 1:10,000 in 1% BSA/0.1% TPBS buffer and incubated for 1 hours at room temperature. Plates were washed 5 times with 0.1% TPBS buffer. TMB substrate (3,3’, 5,5’-tetramethyl benzidine, Sigma) was added at 100 mcL/well and developed for 3 minutes after which the reaction was stopped by the addition of 1N sulfuric acid Stop solution (100 mcL/well). The plates were read at: wavelength 450 nm on plate reader. The serum IgG end point titer was determined to be the highest serum dilution in which the O.D.450 value was greater than the mean value from control naïve mice plus 3 standard deviations.
Serum Env specific IgG gp120 capture ELISA
Immulon 2HB plates were coated with 5 mcg/mL (500ng/well) of polyclonal sheep anti-HIV gp120 (Cliniqa) in PBS and incubated overnight at room temperature. Plates were then washed 5 times with PBS/0.1% Tween-20 buffer (0.1% TPBS) and blocked with 200 mcL of 3% BSA/0.1%TPBS for 1 hour at room temp. Then 100 mcL/well of HIV-16101 gp120 (200 ng/mL in 1%BSA/0.1%TPBS) was added and incubated for 1 hour at room temperature after which the plates were then washed 5 times with PBS/0.1% TPBS. Individual mouse serum samples were initially diluted 1:50 and further diluted 1:3 across the plate in 1% BSA/0.1% TPBS buffer (100 mcL/well) and incubated for a minimum of 13 hours at 4°C. The assay was continually processed as we described previously (see method of “Serum Gag specific IgG ELISA”). Briefly, plates were washed and incubated with biotin -SP-Affinipure Goat anti-mouse IgG (Jackson ImmunoResearch Laboratories Inc.) at a dilution of 1:20,000 in 1% BSA/0.1% TPBS for 2 hours at room temperature. Plates were then washed again, incubated with Streptavidin-Peroxidase conjugated anti-biotin antibody (500 units/mL, Roche Imunochemical, Indianapolis, IN) at a concentration of 1:10,000 in 1% BSA/0.1% TPBS buffer for 1 hours at room temperature, developed by adding TMB substrate (3,3’, 5,5’-tetramethyl benzidine, Sigma) and stopped by the addition of 1N sulfuric acid stop solution after 3 minutes. The plates were read at wavelength 450 nm on plate reader and the serum IgG end point titer was determined as the highest serum dilution in which the O.D.450 value was greater than the mean value from control naïve mice plus 3 standard deviations.
T cell proliferation assay
Mice spleens were harvested 10 days after final immunization and splenocytes were isolated and resuspended in R10 complete culture medium at 4 x 106 splenocytes/mL. In 24 well plates, 0.5 mL of cells from individual mice were add to appropriate wells with 0.5 mL of either RP-10 (as negative control), specific antigen HIV-16101 env gp120 (8 mcg/mL) or ConA (2 mcg/ml, as a positive control) and then cultured at 37°C for 6-7 days. For the last 24 hours, cells were pulsed with 10 mcM FITC-BrdU (BD Pharmingen) at 37°C, then harvested and transferred into a 96 U-bottom plate and surface stained with Anti-CD3-PerCP-Cy5.5, anti-CD4-PE and anti-CD8-APC (BD Pharmingen) for 15 mins on ice. Cell proliferation determination was carried out according to the BrdU Flow Kits instruction manual (BD Pharmingen). Briefly, cells were washed twice with FACS washing buffer (PBS/2% FBS), then incubated with 100 mcL cytofix/cytoperm for 15 minutes on ice. After washing with BD Perm/Wash buffer, cells were resuspended in 100 mcL Cytoperm plus buffer for 10 minutes on ice and washed twice with BD Perm/Wash buffer, incubated with 100 mcL BD cytofix/cytoperm again for 5 minutes at room temperature and washed twice with BD Perm/Wash buffer, resuspended in 100 mcL of diluted DNase (30 mcL stock DNase + 70 mcL PBS) and incubated at 37°C for 1 hour. After washed twice with BD Perm/Wash buffer, cells were stained with 50 mcL of anti-BrdU-FITC in BD Perm/Wash buffer for 20 minutes at room temperature, then washed twice with FACS washing buffer and resuspended in 300 mcL of FACS washing buffer. 1x105 events were acquired on a BD LSRII flow cytometer (BD Biosciences) and FACS data was analyzed using FACS Diva software (BD).
Intracellular cytokine staining of splenocytes
Ten days after the final immunization, the splenocytes were harvested and 3 millions splenocytes from individual mice were stimulated in RP10 with or without a single HIV-1HXB2 gag peptide pool (15mers overlapping by 11 amino acids; 122 total peptides) at an individual peptide concentration of 2.5 mcM with Golgiplug (Pharmingen) for 6 hrs at 37°C in 96 well U-bottom plates. Splenocytes were then washed twice in FACS washing buffer (PBS/2% FBS) and stained for 15 min in the dark at 4°C with surface marker monoclonal antibodies: anti-CD3-Pacific Blue, anti-CD8-APC, anti-CD44-PE-Cy5. Intracellular cytokine staining was carried out using the Cytofix/Cytoperm kit per the manufacturer’s instructions (BD Pharmingen). Briefly, cells were incubated with 100 mcL cytofix/cytoperm for 15 minutes on ice, washed with BD Perm/Wash buffer, stained with antibodies for intracellular cytokines: anti-IFN-γ-FITC, anti-TNF-α-PE-Cy7 and anti-IL-2-PE on ice for 30 minutes. After washing twice with FACS washing buffer, 5x105-106 events were acquired on a BD LSRII flow cytometer (BD Biosciences) and FACS data was analyzed using FACS Diva software (BD). All fluorochrome-labeled monoclonal antibodies were purchased from BD Pharmingen (BD, San Diego, CA).
Vaccinia virus titer in the ovaries of challenged mice
Ten days after the final DNA vaccination, mice (n=10/group) were challenged i.p. with 107 plaque forming units (pfu) of a recombinant vaccinia virus expressing HIV-16101 env gp120 (rVV-env). Five days after rVV-env challenge, mice were sacrificed; ovaries were removed, homogenized, sonicated, and assayed individually for infectious rVV-env by plating serial 10-fold dilutions on a monolayer of BSC-1 indicator cells. After 2 days of culture, the medium was removed; the BSC-1 cell monolayer was stained with 0.1% crystal violet (Sigma) for 5 minutes, and then dried. The number of plaques per well was counted visually.
Statistical Analysis
Comparisons of antigen-specific IFN-γ ELISpot responses and Log transformed serum IgG titer and rVV pfu in the different vaccination groups were performed using negative binomial regression. The negative binomial regression analyses were performed using the SAS Procedure GENMOD in SAS version 8.2 (Cary, NC). In all cases, p-values less than 0.05 indicated that the tests were statistically significant.
RESULTS
Ability of plasmid-based mGM-CSF and mFlt-3L to augment HIV env pDNA vaccine-elicited cell-mediated and serum antibody responses
To compare the relative adjuvant activity of plasmid-based murine GM-CSF (pmGM-CSF) and murine Flt-3L (PMFlt-3L), we first tested their ability to augment peak and memory antigen-specific IFN-γ ELISpot responses in mice following immunization with a prototype plasmid DNA vaccine expressing an RNA-optimized clade B primary isolate HIV-1 6101 env gp160 gene (env pDNA). Groups of Balb/c mice (n=8/group) were immunized by the i.m. route with a control empty vector, with env pDNA alone (100 mcg), or with env pDNA (100 mcg) combined with either 25 or 100 mcg of pmGM-CSF or pmFlt-3L. As a positive control, an additional group of mice were immunized with env pDNA (100 mcg) mixed with 25 mcg plasmid-based murine IL-12 (pmIL-12). Vaccine-elicited peak (ten days after a single immunization) and memory (five weeks after a prime-boost vaccination regimen) cell-mediated immune (CMI) responses were quantitated by IFN-γ ELISpot assay using overlapping HIV-16101 env gp160 peptides pools (Fig. 1A).
Fig. 1. Ability of various plasmid-based cytokines and chemokines to adjuvant the activity of a HIV-16101 env gp160 expressing plasmid DNA vaccine.

Balb/C mice (n=8/group) were immunized (i.m.) on day 0 and 21 with empty vector, with HIV-16101 env gp160 expressing pDNA (env pDNA, 100 mcg) alone or in combination with with 25 or 100 mcg of pmGM-CSF, pmFlt-3L, or 25 mcg of pmIL-12. (A) Peak (10 days post single immunization) and memory (five weeks post prime-boost) HIV-16101 env gp160 peptide-pool specific cell-mediated immune responses were quantitated in splenocytes by IFN-γ ELISpot assay (B) Serum anti-gp120 IgG titers six weeks post prime-boost were determined by gp120 capture ELISA. Data represent the mean values of each group with the standard error of the mean indicated. * indicates mean responses significantly (p < 0.05) different from those obtained with the env pDNA vaccine alone.
The results indicate that the co-administration of pmGM-CSF or pmFlt-3L significantly (p<0.05) enhanced peak env gp160-specific IFN-γ ELISpot responses. Compared to immunization with the env pDNA alone, co-delivery of pmGM-CSF or pmFlt-3L increased the peak env-specific IFN-γ ELISpot response 7.7- and 4.1-fold, respectively. Immunization with the env pDNA in combination with pmIL-12 also increased the peak env-specific IFN-γ ELISpot response 2.4-fold, though this difference did not achieve statistical significance.
The ability of these various plasmid-based cytokines and chemokines to augment env pDNA vaccine-elicited memory CMI responses were also evaluated in mice immunized twice on days 0 and 21 (Fig. 1A). Five weeks after the second immunization, splenocytes from individual mice were harvested and screened for the induction of env gp160-specific IFN-γ secretion by ELISpot assay. Mice immunized with the env pDNA vaccine in combination with 100 mcg of pmGM-CSF demonstrated an increased env gp160-specific memory IFN-γ ELISpot responses relative to mice immunized with the env pDNA vaccine alone, however, these responses did not achieve statistical significance. In contrast, the co-delivery of pmFlt-3L failed in augment memory responses.
Five weeks after the second immunization, mice were also screened for the induction of serum antibodies specific for HIV-16101 env gp120 by capture ELISA (Fig 1B). The results indicate that none of the plasmid-based cytokines or chemokines tested were able to significantly augment the ability of the env pDNA vaccine to elicit serum antibodies that could bind env gp120.
Plasmid-based mGM-CSF has a broad effective dose range.
We next sought to more precisely define the optimal dose of pmGM-CSF and pmFlt-3L to use as a pDNA vaccine adjuvant in mice. Also, we sought to determine if the co-administration of pmGM-CSF and pmFlt-3L would result in a further improvement in adjuvant activity. For these studies, groups of Balb/c mice (n=8/group) were immunized i.m. with a control empty vector (100 mcg), with the HIV-1 env pDNA vaccine alone (100 mcg), or with the env pDNA vaccine (100 mcg) combined with a dose range of pmGM-CSF or pmFlt-3L as indicated in Fig. 2. An additional group of mice were immunized with the env pDNA vaccine (100 mcg) in combination with 25 mcg each of pmGM-CSF and pFlt-3L. As a positive control, mice were immunized with the env pDNA vaccine (100 mcg) in combination with 25 mcg pmIL-12. Ten days after a single immunization, mouse splenocytes were harvested and screened for the induction of vaccine-elicited CMI responses by env gp160 peptide pool-specific IFN-γ ELISpot assay (Fig. 2). The results indicate that pmGM-CSF could act as a pDNA vaccine adjuvant over a broad dose range. Compared to mice immunized with the env pDNA vaccine alone, mice receiving the env pDNA vaccine in combination with 10, 25 or 100 mcg of pmGM-CSF all showed markedly enhanced env gp160 peptide pool-specific CMI responses (p<0.05). Similar results were seem with pmFlt-3L at the highest dose tested (100 mcg), however, the adjuvant activity of pmFlt-3L was highly variable at the lower 10 and 25 mcg doses (Fig. 2).
Fig. 2. Plasmid based mGM-CSF has a broad effective dosage range.

Balb/C mice (n=8/group) were immunized (i.m.) a single time with empty vector, with 100 mcg env pDNA alone or with env pDNA mixed with the indicated amount of pmGM-CSF or pmFlt-3L, or 25 mcg pmGM-CSF and pmFlt-3L in combination. Ten days post-immunization, splenocytes were isolated and env gp160-specific IFN-γ ELISpot responses were measured following stimulation with pooled env peptides (SFC, spot-forming cells). Data are reported as the mean value of each group with standard error of the mean indicated. * indicates mean responses significantly (p < 0.05) different from those obtained with the env pDNA vaccine alone.
Mice immunized with the env pDNA vaccine in combination with 25 mcg each of plasmid-based mGM-CSF and mFlt-3L also demonstrated significantly improved antigen-specific IFN-γ ELISpot responses (Fig. 2), however, these responses were not significantly improved relative to the group receiving the env pDNA vaccine in combination with 25 mcg plasmid-based mGM-CSF alone, suggesting that the addition of mFlt-3L pDNA contributed little to the observed adjuvant activity.
Adjuvant activity of pmGM-CSF and pmFlt-3L in combination with pmIL-12.
To further evaluate the ability of pmGM-CSF and pmFlt-3L to enhance pDNA vaccine-elicited immune responses, we examined their potential adjuvant activity when used in combination with pmIL-12. For this analysis, groups of Balb/c mice (n=8/group) were immunized with a control empty vector, with env pDNA alone (100 mcg), with env pDNA (100 mcg) combined with pmIL-12 (25 mcg) alone, or with env pDNA (100 mcg) and pmIL-12 (25 mcg) in combination with either pmGM-CSF, pmFlt-3L or a mixture of pmGM-CSF and pmFlt-3L as indicated in Fig. 3. Ten days after a single immunization, peak vaccine-elicited CMI responses were monitored by IFN-γ ELISpot assay using overlapping env gp160 peptides pools.
Fig. 3. In vivo adjuvant activity of various plasmid-based cytokines and chemokines in combination with plasmid mIL-12.

Balb/C mice (n=8/group) were immunized (i.m.) with empty vector, with env pDNA (100 mcg) alone or with env pDNA (100 mcg) mixed with 25 mcg pmIL-12 alone or pmIL-12 in combination with pmGM-CSF (25 mcg), pmFlt-3L (100mcg), or the combination of 25 mcg pmGM-CSF with pmFlt-3L (100 mcg). Ten days after a single immunization, env gp160-specific IFN-γ ELISpot responses were measured following stimulation with pooled env peptides (SFC, spot-forming cells). Data represent the mean values of each group with the standard error of the mean indicated. * indicates mean responses significantly (p < 0.05) different from those obtained with the env pDNA vaccine alone.
The results indicate that delivery of the env pDNA vaccine with pmIL-12 significantly (p>0.05) improves the induction of peak env gp160 peptide pool-specific IFN-γ ELISpot responses. However, the co-delivery of pmGM-CSF alone, pmFlt-3L alone, or pmGM-CSF and pmFlt-3L together with the env pDNA / pmIL-12 pDNA mix did not result in a further improvement in the env gp160-specific CMI response.
Plasmid-based mGM-CSF augments CMI and antibody responses to multiple HIV-1 antigens
To confirm that the adjuvant activity of pmGM-CSF and pmFlt-3L was not limited to plasmid-based HIV-1 env, we examined their adjuvant activity when co-delivered with prototype pDNA vaccines expressing either HIV-1HXB2 gag p55 or a fusion of the nef, tat and vif (ntv) accessory proteins derived from HIV-1NL43. For this analysis, groups of Balb/c mice (n=8/group) were immunized i.m. with a control empty vector, with the HIV-1 gag pDNA or ntv pDNA alone (100 mcg), or with the gag or ntv pDNA co-delivered with pmGM-CSF (25 mcg), pmFlt-3L (100 mcg) or pmIL-12 (25 mcg). Since 25 mcg of pmFlt-3L when used in combination with 25 mcg pmGM-CSF failed to further improve the in vivo adjuvant activity of pmGM-CSF (Fig. 2), we sought to test the adjuvant activity of 100 mcg of pmFlt-3L combined with 25 mcg of pmGM-CSF in combination with the HIV gag p55 pDNA vaccine.
Mice receiving the HIV-1 gag p55 pDNA vaccine were immunized on day 0 and boosted with the same vaccine at day 21. Mice receiving the HIV-1 ntv pDNA vaccine were immunized on day 0 and boosted with the same vaccine on days 21 and 42. Ten days after the final immunization, mice were euthanized and screened for the induction of gag p55 and ntv peptide pool-specific IFN-γ ELISpot responses (Fig. 4) and gag p24 binding serum antibodies (Fig. 5).
Fig. 4. In vivo adjuvant activity of plasmid-based mGM-CSF and mFlt-3L with HIV-1 gag and ntv expressing pDNA vaccines.

Balb/C mice (n=8/group) were (A) immunized i.m. with empty vector, with HIV-1 gag p55 pDNA (100 mcg) alone or with gag pDNA mixed with pmFlt-3L (100mcg), pmGM-CSF (25mcg), pmIL-12 (25 mcg), or pmGM-CSF (25 mcg) combined with pmFlt-3L (100 mcg) on day 0 and day 21; (B) immunized (i.m.) with empty vector, HIV-1 ntv pDNA (100 mcg) alone or with NTV pDNA mixed with pmFlt-3L (100 mcg), pmGM-CSF (25 mcg), pmIL-12 (25 mcg) on days 0, 21 and 42. Ten days after the final immunization, splenocytes were isolated and antigen specific IFN-γ ELISpot responses were measured following stimulation with pooled HIV gag p55 (A), or nef, tat, vif (B) peptides (SFC, spot-forming cells). Data are reported as the mean value of each group with standard error of the mean indicated. * indicates mean responses significantly (p < 0.05) different from those obtained with the gag or ntv pDNA vaccine alone.
Fig. 5. Plasmid based mGM-CSF augments HIV-1 pDNA vaccine elicited antigen specific antibody responses.
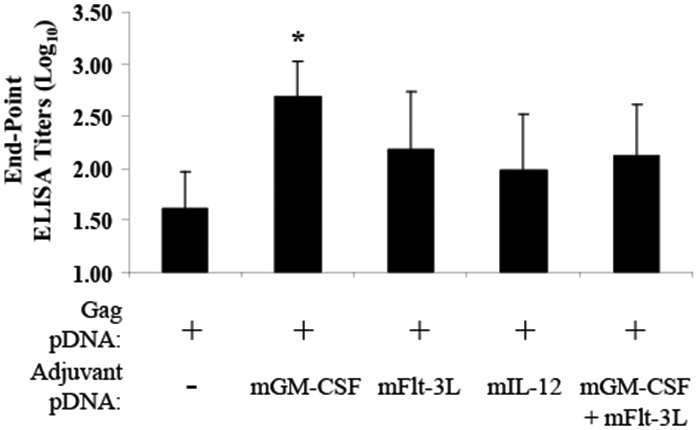
Balb/C mice (n=8/group) were immunized (i.m.) with empty vector, with 100mcg HIV-1 gag pDNA alone or with pag pDNA mixed with pmGM-CSF (25 mcg), pmFlt-3L (100 mcg), pmIL-12 (25 mcg) or pmGM-CSF (25 mcg) combined with pmFlt-3L (100 mcg). Mice were immunized on day 0 and day 21 and serum was harvested at 10 days post second immunization. Serum anti-gag p24 IgG titers were determined by ELISA. Data are reported as the mean log10 value of each group with the standard error of the mean indicated. * indicates mean responses significantly (p < 0.05) different from those obtained with the gag pDNA vaccine alone.
Compared to immunization with the gag p55 pDNA vaccine alone, co-delivery of pmGM-CSF significantly (p=0.024) improved the gag p55 peptide pool-specific IFN-γ ELISpot response (Fig. 4A). Co-delivery of pmIL-12 as an adjuvant also improved the gag p55 peptide pool-specific IFN-γ ELISpot response (Fig. 4A), though this difference did not achieve statistical significance (p=0.12). Similarly, co-delivery of pmGM-CSF with a pDNA vaccine expressing HIV ntv also significantly (p=0.015) improved the ntv peptide pool-specific IFN-γ ELISpot response (Fig. 4B). In contrast, co-delivery of pmFlt-3L failed to improve gag p55 or ntv peptide pool-specific CMI responses (Fig. 4).
Consistent with the data reported in Fig. 2, the combined use of pmGM-CSF and pmFlt-3L also significantly (p=0.030) improved gag p55 peptide pool-specific CMI responses relative to immunization with the gag p55 pDNA vaccine alone (Fig. 4A). However, these responses were not significantly improved relative to immunization with the gag p55 pDNA vaccine in combination with the pmGM-CSF pDNA alone, suggesting that pmFlt-3L contributed little to the improved gag p55 peptide pool-specific IFN-γ ELISpot responses.
The ability of pmGM-CSF and pmFlt-3L to augment HIV gag p24-specific serum antibody responses was monitored by ELISA. Compared to mice immunized with the gag p55 pDNA vaccine alone, mice receiving the gag p55 pDNA vaccine in combination with pmGM-CSF showed a significant (p=0.037) 1-log increase in serum gag p24 binding antibodies (Fig. 5). In contrast, immunization with the gag p55 pDNA vaccine in combination with pmIL-12, pmFlt-3L or pmGM-CSF + pmFlt-3L failed to significantly further improve the induction of gag p24-specific antibody responses.
Quantitative and qualitative characterization of antigen-specific CD8+ T cells by multiparameter flow cytometry
We next sought to determine to what extent pmGM-CSF or pmIL-12 could alter the functional quality of the pDNA vaccine-elicited antigen-specific CMI response in vivo. To this end, Balb/c mice were immunized with gag p55 pDNA alone (100 mcg), with 100 mcg gag p55 pDNA combined with 25 mcg pmIL-12 or pmGM-CSF (Fig. 6). Ten days after the final immunization, mouse splenocytes were harvested and HIV-1 gag p55 peptide pool-specific secretion of IFN-γ, IL-2 and TNF-α by total CD3+CD8+ and memory CD3+CD8+CD62dimCD44+ T cells was quantified by polychromatic flow cytometry.
Fig. 6. Quantitative and qualitative characterization of IL-2, TNFα and IFN-γ producing antigen-specific CD8+ T cells by multiparameter flow cytometry.
Balb/C mice (n=6/group) were immunized (i.m.) with 100mcg HIV-1 gag pDNA alone or with gag pDNA combined with 25 mcg pmGM-CSF or 25mcg pmIL-12 on day 0 and day 21. Ten days post second immunization splenocytes were harvested and the percentage of cytokine (IL-2, TNFα or IFN-γ) producing CD3+CD8+ T cells (A), or CD3+CD8+CD62dimCD44+ memory T cells (B) was quantitated by flow cytometry. Distribution of the cytokine response comprising seven functionally distinct populations producing IL-2, TNFα and IFN-γ, individually or in combination within CD3+CD8+ T cells (C) or CD3+CD8+CD62dimCD44+ memory T cells (D). Data are presented as the mean gag peptide-specific response (minus media background). Arrows show the percent T cells secreting all three cytokines.
The magnitude of the gag peptide pool-specific CMI response was estimated by summing the proportion of total CD3+CD8+ (Fig. 6A) and memory CD3+CD8+CD62dimCD44+ (Fig. 6B) T cells making either IFN-γ, IL-2 or TNF-α in response to peptide stimulation in vitro. The data indicate that the proportion of gag p55-specific total and memory T cells elicited by pDNA vaccine administration was not substantially increased via the co-delivery of pmIL-12 or pmGM-CSF.
The quality of the gag peptide pool-specific CMI response was characterized by determining the proportion of total CD3+CD8+ and memory CD3+CD8+CD62dimCD44+ T cells capable of secreting multiple cytokines in response to peptide stimulation in vitro (Fig. 6C-6D). Following immunization with the gag pDNA vaccine alone, 4.1% of total CD3+CD8+ and 4.8% memory CD3+CD8+CD62dimCD44+ antigen-specific T cells were found to be highly polyfunctional and secrete all three of the cytokines tested, i.e. IFN-γ, IL-2 or TNF-α. By comparison, the proportion of total CD3+CD8+ and memory CD3+CD8+CD62dimCD44+ antigen-specific T cells secreting all three cytokines increased 4.2- and 3.2-fold, respectively, when the gag pDNA vaccine was delivered in combination with pmGM-CSF. Collectively, these data suggest that co-delivery of pmGM-CSF as a vaccine adjuvant affects the quality of the antigen-specific total and memory CD8+ T cell response.
Plasmid-based mGM-CSF enhances antigen-specific T cell proliferation in vivo.
We then sought to determine to what extent pmGM-CSF could enhance pDNA vaccine antigen-specific T cell proliferation in vivo. To maximize the likely hood of differentiating amongst the various pDNA adjuvants, proliferative responses were measured ten days after a single suboptimal immunization. Balb/c mice were immunized with a control empty vector, with 100 mcg env gp160 pDNA alone, or combined with 25 mcg pmIL-12, 25 mcg pmGM-CSF, or 100 mcg env pDNA mixed with 25 pmcg mIL-12 in combination with 25 mcg of pmGM-CSF (Fig. 7). Ten days later mouse splenocytes were harvested and HIV-1 env gp120-specific CD4+ or CD8+ T cell proliferation was quantified using a flow-based BrdU incorporation assay (see Materials and Methods).
Fig. 7. Plasmid based mGM-CSF enhances antigen-specific T cell proliferation.

Balb/C mice (n=9/group) were immunized i.m. a single time with: empty vector, 100 mcg HIV env pDNA alone or env pDNA mixed with pmIL-12 (25 mcg), pmGM-CSF (25 mcg) or env pDNA mixed with the combination of 25mcg pmGM-CSF and 25mcg pmIL-12. On day 10 post-immunization, splenocytes were isolated and env-specific T cell proliferation was measured by FACS-based BrdU incorporation assay (see Materials and Methods) following stimulation with 4 mcg of purified gp120 protein for 6 days. Data represent the mean values of each group with the standard error of the mean indicated. * indicates mean value significantly (p < 0.05) different from those obtained with the env pDNA vaccine alone. **indicates mean value significantly (p<0.05) different from those obtained with the env pDNA mixed with plasmid-based mIL-12 or mGM-CSF.
Mice immunized a single time with the control empty pDNA vaccine or the env pDNA vaccine alone showed virtually no HIV-1 env gp120-specific CD4+ or CD8+ T cell proliferation (Fig 7). Immunization with the env pDNA vaccine in combination with pmIL-12 increased the env gp120-specific proliferative capacity of CD4+ and CD8+ T cells slightly. In contrast, the use of pmGM-CSF as a DNA vaccine adjuvant dramatically (p<0.05) increased the percentage of CD4+ and CD8+ T cells incorporating BrdU by 8.3- and 7.2-fold respectively, relative to mice immunized with the env pDNA vaccine alone. Interestingly, the co-delivery of pmGM-CSF with pmIL-12 as a vaccine adjuvant resulted in a further 3- fold increase in the proliferative capacity of gp120-specific CD4+ T cells relative to mice immunized with the env pDNA in combination with pmGM-CSF alone.
Taken together, these results suggest that pmGM-CSF when used as a pDNA vaccine adjuvant can enhance both antigen-specific CD4+ and CD8+ T cell proliferation, while the addition of pmIL-12 can lead to further enhancement of antigen-specific CD4+ T cell proliferation.
Plasmid based mGM-CSF and mIL-12 in combination augment the in vivo protective efficacy of an HIV-1 env pDNA vaccine
Finally, we sought to determine if the combined use of pmGM-CSF and pmIL-12 as pDNA vaccine adjuvants could increase the in vivo protective efficacy of a prototype HIV-1 env gp160 expressing pDNA vaccine. For this analysis, Balb/c mice (n=20/group) were immunized with a control empty vector pDNA (100 mcg), an HIV env gp160 pDNA (100 mcg) alone or combined with pmIL-12 (25 mcg), pmGM-CSF (25 mcg), or with pmIL-12 (25 mcg) and pmGM-CSF (25 mcg) in combination.
Ten days after a single pDNA immunization, half the mice were euthanized (n=10/group) and vaccine-elicited CMI responses were quantitated by IFN-γ ELISpot assay (Fig. 8A). At the same time, the remaining mice from each group (n=10) were challenged by the i.p. route with 107 pfu of a recombinant vaccinia virus expressing HIV-16101 env gp120 (rVV-env). Five days post rVV-env challenge, ovaries were harvested and rVV-env titers determined by plaque assay (Fig. 8B).
Fig. 8. Plamid based mGM-CSF and mIL-12 in combination augment the in vivo protective efficacy of a HIV-1 env pDNA vaccine.

Balb/C mice (n=20/group) were immunized (i.m.) with empty vector, HIV env pDNA (100mcg) alone or env pDNA in combination with pmIL-12 (25 mcg), pmGM-CSF (25 mcg), or the two cytokines in combination. Ten days after a single immunization: (A) splenocytes were isolated (n=10/group) and env-specific IFN-γ ELISpot responses were measured following stimulation with pooled env gp160 peptides (SFC, spot-forming cells). (B) mice (n=10/group) were challenged i.p. with 1x107 PFU rVV expressing HIV env gp120. Ovaries were harvested 5 days post challenge and rVV titers were determined by plaque assay. Data represent the mean values of each group with the standard error of the mean indicated. * indicates mean responses significantly (p < 0.05) different from those obtained following immunization with the env pDNA vaccine alone. (C) Correlation between the env-specific IFN-γ ELISpot response at time of challenge and the rVV titer seen in ovaries 5 days post-challenge.
The results indicate that compared to immunization with the env pDNA vaccine alone, co-immunization with pmGM-CSF increased env gp160 peptide pool-specific IFN-γ ELISpot responses 2.7 fold, a result which approached but did not achieve statistical significance (p=0.086, Fig. 8A). The increase in env-specific CMI responses seen in the mice receiving the pmGM-CSF adjuvant translated into a 0.9 log10 reduction in post challenge rVV-env titers relative to mice immunized with the env gp160 pDNA vaccine alone, a difference which also approached but did not achieve statistical significance (p=0.095, Fig. 8B). However, mice immunized with the env gp160 pDNA in combination with pmGM-CSF and pmIL-12 demonstrated a significant 4.1-fold increase (p=0.015) in env gp160 peptide pool-specific IFN-γ ELISpot responses and a 1.34 log10 reduction (p=0.012) in post challenge rVV-env titers relative to mice immunized with the env gp160 pDNA vaccine alone (Fig. 8A-8B). Looking at the data as a whole, there exists a clear correlation between the magnitude of the env-gp160 peptide pool-specific IFN-γ ELISpot response and the clearance of rVV-env post-challenge (Fig. 8C). These data suggest that the IFN-γ ELISpot response plays a key role in the clearance of rVV post-challenge and that the co-delivery of pmGM-CSF and pmIL-12 can serve as pDNA vaccine adjuvants and enhance the protective efficacy of vaccination in vivo.
Discussion
Plasmid DNA (pDNA) represents an attractive platform for the development of vaccines for the prevention of a wide range of infectious diseases and cancers. However, to date, candidate pDNA vaccines have not lived up to their promise in clinical studies [10] leading many to experiment with plasmid-based cytokines, chemokines and co-stimulatory molecules as pDNA vaccine adjuvants.
In this paper, we report that plasmid-based murine IL-12 (pmIL-12), murine GM-CSF (pmGM-CSF) or murine Flt-3L (pmFlt-3L) can be used as vaccine adjuvants and significantly enhance the ability of prototype pDNA vaccine to elicit antigen-specific cell-mediated immune responses in mice. Furthermore, we find that the use of pmGM-CSF as an adjuvant also markedly enhanced HIV antigen-specific T cell proliferation and increased the proportion of antigen-specific polyfunctional memory CD8+ T cells. In addition, though the combined use of pmGM-CSF with pmIL-12 did not lead to a further increase in the pDNA vaccine elicited IFN-γ ELIspot responses relative to immunization with pGM-CSF alone; the combined use of pmGM-CSF and pmIL-12 did dramatically enhance CD4+ T cells proliferation and pDNA vaccine protective efficacy in a murine rVV challenge model.
Flt3 ligand (Flt-3L) is a potent hematopoietic factor that induces profound expansion of progenitor cell pools in multiple tissues. In particular, Flt-3L is a growth factor for the in vivo and ex vivo generation of dendritic cells (DCs), which play a central role in the orchestration of the primary and memory phases of the immune response [46]. In mice, it has been reported that the co-delivery of pFlt-3L recruits DCs to the injection site and regional lymph nodes (LNs) and augments the immunogenicity of HIV pDNA vaccines [33]. In contrast, Sumida et al reported that the delivery of pFlt3L with an HIV env pDNA vaccine was inefficient at mobilizing DCs to the site of inoculation and exerted no detectable effects on vaccine immunogenicity [32]. In the present report, we find that pmFlt-3L demonstrated limited adjuvant activity in terms of its ability to augment peak env-specific T cell responses. However, co-delivery of pmFlt-3L had no measurable effect on env-specific memory T cell responses or env-specific antibody responses. In addition, we were unable to confirm the limited adjuvant activity of pmFlt-3L with other prototype pDNA vaccines expressing HIV gag or ntv.
IL-12 expressing pDNAs have been used by several groups to augment the humoral and cellular immune responses elicited by pDNA vaccines both in mice and monkey models [25-27]. Consistent with these previous reports, our results also show that the co-delivery of pmIL-12 alone can augment pDNA vaccine elicited antigen-specific cell-mediated immune responses. In addition, we found that the co-administration of an HIV env pDNA vaccine with a mixture of pmGM-CSF and pmIL-12 resulted in a significantly increased env-specific proliferative response relative to immunization with the env pDNA vaccine in combination with either pmGM-CSF or pmIL-12 alone. Following the combined use of pmGM-CSF and pmIL-12, vaccinated mice demonstrated an increase in env-specific IFN-γ ELISpot responses and showed a statistically significant increase in their ability to clear vaccinia virus following challenge relative to mice immunized with the env pDNA vaccine in combination with either pmGM-CSF or pmIL-12 alone.
In this study, the most pronounced adjuvant activity was seen when the prototype pDNA vaccines were delivered in combination with pmGM-CSF. GM-CSF has been reported to initiate the proliferation, differentiation, and activation of macrophages, neutrophils, and various professional antigen presenting cells [47, 48]. Delivered as a protein or a plasmid, GM-CSF has been shown to recruit and activate macrophages and dendritic cells at the site of inoculation [28, 49]. In mice, co-delivery of pmGM-CSF with a pDNA vaccine resulted in the recruitment of macrophages to the site of inoculation and specifically improved vaccine-elicited CD4+ T lymphocyte responses [28-30]. More recently, Qiu et al. have reported that co-administration of pmGM-CSF with an HIV gag pDNA vaccine in mice resulted in an improved antibody, CMI responses and protection from infection by a recombinant vaccinia virus expressing HIV-1 Gag [31]. Finally, Lai et al. have shown that co-delivery of pGM-CSF with the pDNA prime of a DNA/MVA vaccination regimen in rhesus macaques led to enhanced protection against the acute phase of a SHIV89.6P challenge [50]. Consistent with previous reports, our data confirm that pmGM-CSF, when co-delivered with an HIV pDNA vaccine, enhances vaccine-specific antibody and cell-mediated immune responses in mice. Our data also show that co-administration pmGM-CSF not only led to a dramatic augmentation of vaccine elicited CD4+ T cell proliferation [29], but also enhanced CD8+ T cell proliferation. In addition, our study furthers our understanding of the adjuvant activity of pmGM-CSF by demonstrating pmGM-CSF is able to act as molecular adjuvant for multiple pDNA vaccines expressing not only env and gag, but also nef, tat and vif.
CD8+ T lymphocytes with cytotoxic (CTL) potential are an important line of defense against many viral infections. Several studies suggest that during HIV infection these cells play a critical role in controlling viral replication [51, 52]. Recently, significant advances have been made with regard to the number of parameters that can be simultaneously detected by flow cytometry [53, 54]. These advances have dramatically increased our ability to characterize T-cell effector functions at the single-cell level [35] and contributed to the realization that there exists the potential for remarkable heterogeneity in the functional profile (quality) of the antigen-specific T cell response. With an increased ability to characterize T cell effector function, investigators are now reporting that the quality of the CD8+ T cell response, rather than simply the magnitude of the response, can be correlated with HIV disease progression [34]. For example, Betts et al. used polychromatic flow cytometry to characterize the quality of the HIV-specific CD8+ T cell responses by simultaneously measuring five CD8+ T-cell functions (surface CD107a mobilization for degranulation and the production of IFN-γ, MIP-1β, TNF-α and IL-2) in individuals with chronic HIV infection. It was found that non-progressors preferentially maintain HIV-specific CD8+ T cells that demonstrate all five functions on a cell-by-cell basis [34], suggesting that “multi-functional” antigen-specific T-cells are an important component of this protective immune response.
The importance of multi-functional T cell responses does not appear to be limited to HIV infection. In vaccinia virus immunized individuals, who often have lifelong protection against smallpox, highly polyfunctional CD8+ T cell responses were detected [35]. Together, these data suggest that the functional profile of vaccine-elicited CD8+ T cells might serve as a surrogate marker to predict vaccine efficacy. In the present study, compared to immunization with HIV gag pDNA alone, immunization with the HIV gag pDNA in combination with pmGM-CSF did not result in an increased number of antigen-specific CD8+ T cells as measured by intracellular secretion of IFN-γ, IL-2 or TNFα. However, our data demonstrate that mice receiving the HIV gag pDNA in combination with pmGM-CSF did demonstrate an increase in the proportion of antigen-specific CD8+ T cells with a polyfunctional phenotype (i.e. IFN-γ+, IL-2+, TNFα+). Taken together, our data suggests that pGM-CSF acts as a DNA vaccine adjuvant by not only enhancing the magnitude of T cells responses, but also serves to improve the quality of responses. Given the recent realization that not only the magnitude but also the quality of the antigen-specific T cell response appear to be important aspects of vaccine efficacy, these data appear to justify the continued pre-clinical and clinical evaluation of pGM-CSF as a DNA vaccine adjuvant.
Acknowledgements
We are grateful to P. Roth and E. Buzzell (National Cancer Institute-Frederick) for expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Gurunathan S, Stobie L, Prussin C, Sacks DL, Glaichenhaus N, Fowell DJ, et al. Requirements for the maintenance of Th1 immunity in vivo following DNA vaccination: a potential immunoregulatory role for CD8+ T cells. Journal of Immunology 2000;165(2):915–24. [DOI] [PubMed] [Google Scholar]
- [2].Donnelly JJ, Ulmer JB. DNA vaccines for viral diseases. Braz J Med Biol Res 1999;32(2):215–22. [DOI] [PubMed] [Google Scholar]
- [3].Fynan EF, Webster RG, Fuller DH, Haynes JR, Santoro JC, Robinson HL. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proceedings of the National Academy of Sciences of the United States of America 1993;90(24):11478–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Robinson HL, Hunt LA, Webster RG. Protection against a lethal influenza virus challenge by immunization with a haemagglutinin-expressing plasmid DNA. Vaccine 1993;11(9):957–60. [DOI] [PubMed] [Google Scholar]
- [5].Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 1993;259(5102):1745–9. [DOI] [PubMed] [Google Scholar]
- [6].Babiuk LA, van Drunen Littel-van den H, Babiuk SL. Immunization of animals: from DNA to the dinner plate. Vet Immunol Immunopathol 1999;72(1-2):189–202. [DOI] [PubMed] [Google Scholar]
- [7].Egan MA, Charini WA, Kuroda MJ, Schmitz JE, Racz P, Tenner-Racz K, et al. Simian immunodeficiency virus (SIV) gag DNA-vaccinated rhesus monkeys develop secondary cytotoxic T-lymphocyte responses and control viral replication after pathogenic SIV infection. Journal of Virology 2000;74(16):7485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].MacGregor RR, Boyer JD, Ugen KE, Lacy KE, Gluckman SJ, Bagarazzi ML, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis 1998;178(1):92–100. [DOI] [PubMed] [Google Scholar]
- [9].Wang R, Doolan DL, Le TP, Hedstrom RC, Coonan KM, Charoenvit Y, et al. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 1998;282(5388):p476–80. [DOI] [PubMed] [Google Scholar]
- [10].Lu S, Wang S, Grimes-Serrano JM. Current progress of DNA vaccine studies in humans. Expert Review of Vaccines 2008;7(2):175–91. [DOI] [PubMed] [Google Scholar]
- [11].Trimble C, Lin CT, Hung CF, Pai S, Juang J, He L, et al. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Vaccine 2003;21(25-26):4036–42. [DOI] [PubMed] [Google Scholar]
- [12].Herrmann JE, Chen SC, Jones DH, Tinsley-Bown A, Fynan EF, Greenberg HB, et al. Immune responses and protection obtained by oral immunization with rotavirus VP4 and VP7 DNA vaccines encapsulated in microparticles. Virology 1999;259(1):148–53. [DOI] [PubMed] [Google Scholar]
- [13].Chen SC, Jones DH, Fynan EF, Farrar GH, Clegg JC, Greenberg HB, et al. Protective immunity induced by oral immunization with a rotavirus DNA vaccine encapsulated in microparticles. Journal of Virology 1998;72(7):5757–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sharpe M, Lynch D, Topham S, Major D, Wood J, Loudon P. Protection of mice from H5N1 influenza challenge by prophylactic DNA vaccination using particle mediated epidermal delivery. Vaccine 2007;25(34):6392–8. [DOI] [PubMed] [Google Scholar]
- [15].Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen M, et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. Journal Of Immunology 2000;164(9):4635–40. [DOI] [PubMed] [Google Scholar]
- [16].Otten G, Schaefer M, Doe B, Liu H, Srivastava I, Megede Jz, et al. Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine 2004;22(19):2489–93. [DOI] [PubMed] [Google Scholar]
- [17].Luckay A, Sidhu MK, Kjeken R, Megati S, Chong S-Y, Roopchand V, et al. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J Virol 2007;81(10):5257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Andre S, Seed B, Eberle J, Schraut W, Bultmann A, Haas J. Increased Immune Response Elicited by DNA Vaccination with a Synthetic gp120 Sequence with Optimized Codon Usage. J Virol 1998;72(2):1497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schneider R, Campbell M, Nasioulas G, Felber B, Pavlakis G. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J Virol 1997;71(7):4892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Otten GR, Schaefer M, Doe B, Liu H, Srivastava I, Megede J, et al. Enhanced potency of plasmid DNA microparticle human immunodeficiency virus vaccines in rhesus macaques by using a priming-boosting regimen with recombinant proteins. Journal of Virology 2005;79(13):8189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Egan MA, Chong S-Y, Megati S, Montefiori DC, Rose NF, Sidhu M, et al. Priming with Plasmid DNAs Expressing IL-12 and SIV Gag Protein Enhances the Immunogenicity and Efficacy of an Experimental AIDS Vaccine Based on Recombinant Vesicular Stomatitis Virus. AIDS Research & Human Retroviruses 2005;7:629–643. [DOI] [PubMed] [Google Scholar]
- [22].Robinson HL. Prime boost vaccines power up in people. Nature Medicine 2003;9(6):642–3. [DOI] [PubMed] [Google Scholar]
- [23].Egan MA, Israel ZR. The use of cytokines and chemokines as genetic adjuvants for plasmid DNA vaccines. Clin Applied Immunol Rev 2002;2:255–87. [Google Scholar]
- [24].Barouch DH, Letvin NL, Seder RA. The role of cytokine DNAs as vaccine adjuvants for optimizing cellular immune responses. Immunological Reviews 2004;202:266–74. [DOI] [PubMed] [Google Scholar]
- [25].Moore AC, Kong W-p, Chakrabarti BK, Nabel GJ. Effects of antigen and genetic adjuvants on immune responses to human immunodeficiency virus DNA vaccines in mice. J Virol 2002;76(1):243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Boyer JD, Robinson TM, Kutzler MA, Parkinson R, Calarota SA, Sidhu MK, et al. SIV DNA vaccine co-administered with IL-12 expression plasmid enhances CD8 SIV cellular immune responses in cynomolgus macaques. Journal of Medical Primatology 2005;34(5-6):262–70. [DOI] [PubMed] [Google Scholar]
- [27].Schadeck EB, Sidhu M, Egan MA, Chong S-Y, Piacente P, Masood A, et al. A dose sparing effect by plasmid encoded IL-12 adjuvant on a SIVgag-plasmid DNA vaccine in rhesus macaques. Vaccine 2006;24(21):4677–87. [DOI] [PubMed] [Google Scholar]
- [28].Kusakabe K, Xin KQ, Katoh H, Sumino K, Hagiwara E, Kawamoto S, et al. The timing of GM-CSF expression plasmid administration influences the Th1/Th2 response induced by an HIV-1-specific DNA vaccine. Journal of Immunology 2000;164(6):3102–11. [DOI] [PubMed] [Google Scholar]
- [29].Barouch DH, Santra S, Tenner-Racz K, Racz P, Kuroda MJ, Schmitz JE, et al. Potent CD4+ T cell responses elicited by a bicistronic HIV-1 DNA vaccine expressing gp120 and GM-CSF. Journal of Immunology 2002;168(2):562–8. [DOI] [PubMed] [Google Scholar]
- [30].McKay PF, Barouch DH, Santra S, Sumida SM, Jackson SS, Gorgone DA, et al. Recruitment of different subsets of antigen-presenting cells selectively modulates DNA vaccine-elicited CD4+ and CD8+ T lymphocyte responses. European Journal of Immunology 2004;34(4):1011–20. [DOI] [PubMed] [Google Scholar]
- [31].Qiu J-T, Chang T-C, Lin C-T, Chen Y-M, Li FQ, Soong Y-K, et al. Novel codon-optimized GM-CSF gene as an adjuvant to enhance the immunity of a DNA vaccine against HIV-1 Gag. Vaccine 2007;25(2):253–63. [DOI] [PubMed] [Google Scholar]
- [32].Sumida SM, McKay PF, Truitt DM, Kishko MG, Arthur JC, Seaman MS, et al. Recruitment and expansion of dendritic cells in vivo potentiate the immunogenicity of plasmid DNA vaccines. Journal of Clinical Investigation 2004;114(9):1334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nayak BP, Sailaja G, Jabbar AM. Augmenting the immunogenicity of DNA vaccines: role of plasmid-encoded Flt-3 ligand, as a molecular adjuvant in genetic vaccination. Virology 2006;348(2):277–88. [DOI] [PubMed] [Google Scholar]
- [34].Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8(+) T cells. Blood 2006;107(12):4781–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8+ T cell responses. J Exp Med 2007;204(6):1405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schwartz S, Campbell M, Nasioulas G, Harrison J, Felber BK, Pavlakis GN. Mutational inactivation of an inhibitory sequence in human immunodeficiency virus type 1 results in Rev-independent gag expression. Journal of Virology 1992;66(12):7176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schwartz S, Felber BK, Pavlakis GN. Distinct RNA sequences in the gag region of human immunodeficiency virus type 1 decrease RNA stability and inhibit expression in the absence of Rev protein. Journal of Virology 1992;66(1):150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Aiken C, Konner J, Landau NR, Lenburg ME, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 1994;76(5):853–64. [DOI] [PubMed] [Google Scholar]
- [39].Sadaie MR, Rappaport J, Benter T, Josephs SF, Willis R, Wong-Staal F. Missense mutations in an infectious human immunodeficiency viral genome: functional mapping of tat and identification of the rev splice acceptor. PNAS 1988;85(23):9224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].HIV Immunology and HIV/SIV Vaccine Databases: Los Alamos National Laboratory, Theoretical Biology and Biophysics, Los Alamos, New Mexico. LA-UR 04-8162, 2003. [Google Scholar]
- [41].Egan MA, Megati S, Roopchand V, Garcia-Hand D, Luckay A, Chong S-Y, et al. Rational design of a plasmid DNA vaccine capable of eliciting cell-mediated immune responses to multiple HIV antigens in mice. Vaccine 2006;24(21):4510–23. [DOI] [PubMed] [Google Scholar]
- [42].Shaw KJ, Rather PN, Hare RS, Miller GH. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiological Reviews 1993;57(1):138–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sadaie Y, Burtis KC, Doi RH. Purification and characterization of a kanamycin nucleotidyltransferase from plasmid pUB110-carrying cells of Bacillus subtilis. Journal of Bacteriology 1980;141(3):1178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cooper CL, Brady G, Bilia F, Iscove NN, Quesenberry PJ. Expression of the Id family helix-loop-helix regulators during growth and development in the hematopoietic system. Blood 1997;89(9):3155–65. [PubMed] [Google Scholar]
- [45].Pachuk CJ, Ciccarelli RB, Samuel M, Bayer ME, Troutman RD, Zurawski DV, et al. Characterization of a new class of DNA delivery complexes formed by the local anesthetic bupivacaine. Biochimica et Biophysica Acta 2000;1468(1-2):20–30. [DOI] [PubMed] [Google Scholar]
- [46].Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. Journal of Experimental Medicine 1996;184(5):1953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jones TC. Future uses of granulocyte-macrophage colony-stimulating factor (GM-CSF). Stem Cells 1994;12 Suppl 1:229–39; discussion 39-40. [DOI] [PubMed] [Google Scholar]
- [48].Disis ML, Bernhard H, Shiota FM, Hand SL, Gralow JR, Huseby ES, et al. Granulocyte-macrophage colony-stimulating factor: an effective adjuvant for protein and peptide-based vaccines. Blood 1996;88(1):202–10. [PubMed] [Google Scholar]
- [49].Haddad D, Ramprakash J, Sedegah M, Charoenvit Y, Baumgartner R, Kumar S, et al. Plasmid vaccine expressing granulocyte-macrophage colony-stimulating factor attracts infiltrates including immature dendritic cells into injected muscles. Journal of Immunology 2000;165(7):3772–81. [DOI] [PubMed] [Google Scholar]
- [50].Lai L, Vodros D, Kozlowski PA, Montefiori DC, Wilson RL, Akerstrom VL, et al. GM-CSF DNA: An adjuvant for higher avidity IgG, rectal IgA, and increased protection against the acute phase of a SHIV-89.6P challenge by a DNA/MVA immunodeficiency virus vaccine. Virology 2007;369(1):153–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. Journal of Virology 1994;68(9):6103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. Journal of Virology 1994;68(7):4650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. Journal of Immunological Methods 2003;281(1-2):65–78. [DOI] [PubMed] [Google Scholar]
- [54].Perfetto SP, Chattopadhyay PK, Roederer M. Seventeen-colour flow cytometry: unravelling the immune system. Immunology 2004;4(8):648–55. [DOI] [PubMed] [Google Scholar]



