Abstract
Background:
Endoscopic papillectomy is a minimally invasive treatment for benign tumors of the ampulla of Vater or early ampullary carcinoma. However, reported recurrence rates are significant and risk factors for recurrence are unclear.
Objective:
The aims of this study were to evaluate the efficacy and safety of endoscopic papillectomy and to identify risk factors for recurrence and adverse events.
Methods:
All patients who underwent endoscopic papillectomy at five tertiary referral centers between January 2008 and December 2018 were included. Recurrence was defined as the detection of residue on one of the follow-up endoscopies. Treatment success was defined as the absence of tumor residue on the last follow-up endoscopy.
Results:
A total of 227 patients were included. The resections were en bloc in 64.8% of cases. The mean lesion size was 20 mm (range: 3–80) with lateral extension in 23.3% of cases. R0 resection was achieved in 45.3% of cases. The recurrence rate was 30.6%, and 60.7% of recurrences were successfully treated with additional endoscopic treatment. Finally, treatment success was achieved in 82.8% of patients with a median follow-up time of 22.3 months. R1 resection, intraductal invasion, and tumor size > 2 cm were associated with local recurrence. Adverse events occurred in 36.6% of patients and included pancreatitis (17.6%), post-procedural hemorrhage (11.0%), perforation (5.2%), and biliary stenosis (2.6%). The mortality rate was 0.9%.
Conclusion:
Endoscopic papillectomy is an effective and relatively well-tolerated treatment for localized ampullary tumors. In this series, R1 resection, intraductal invasion, and lesion size > 2 cm were associated with local recurrence.
Keywords: ampullary tumor, endoscopic papillectomy, endoscopic resection, post-ERCP pancreatitis
Introduction
Ampullary adenomas are rare entities, with a prevalence of 0.1% according to autopsy studies. 1 Symptoms are usually non-specific or related to pancreato-biliary obstruction.2,3 The diagnosis may also be made incidentally in asymptomatic patients undergoing upper gastrointestinal endoscopy or during a screening procedure in patients with a genetic predisposition such as familial adenomatous polyposis (FAP). Complete resection is required for ampullary lesions, whether malignant or not, due to the potential for malignant transformation, according to the adenoma-adenocarcinoma sequence. 4 Historically, the treatment was based on surgery and was associated with high morbidity and mortality.3,5–7 Two types of surgery can be performed, pancreaticoduodenectomy and surgical papillectomy, depending on the stage of the lesion or history of previous papillectomy. 8 Nowadays, endoscopic papillectomy (EP) is considered the most interesting alternative to surgery for the curative treatment of ampullary adenoma, based on the previously published promising results, which reported a success rate of over 70%.9,10 Recently, EP has also been indicated for early ampullary carcinoma, as it could be curative for Tis tumors considering that there is no lympho-vascular invasion or lymph node metastasis at this stage.11–13 Adequate staging is therefore recommended, favorably by endoscopic ultrasound (EUS) and abdominal magnetic resonance cholangiopancreatography for staging of ampullary tumors. In addition, endoscopic indications for EP are increasing, in particular, in patients with short intraductal involvement or large lesions. Indeed, thermal ablation, radiofrequency, or other advanced endoscopic treatments allow curative resections in these cases. 14
However, adverse events are more frequent after EP than after other endoscopic procedures, 2 and recurrence remains a main concern. Large-scale studies are needed to determine the risk factors for adverse events and whether they can be prevented. Furthermore, the recurrence rate is 11.8% with variable risk factors. 2
Thus, the primary aim of this present study was to determine the treatment success rate after EP for ampullary tumors. Secondary aims were to identify risk factors for recurrence and adverse events.
Methods
Patients
All consecutive patients who underwent EP at five tertiary referral centers between January 2008 and December 2018 were included in this multicenter, retrospective, observational study. According to French law, it is not necessary to seek the opinion of an ethics committee for a retrospective study. The research was carried out in accordance with the Helsinki Declaration. Informed consent of the procedure modalities and risks was collected in each patient. Patient data were collected retrospectively using electronic patient records. Baseline patient data included age, sex, American Society of Anesthesiologic (ASA) score, circumstance of detection (incidental, symptoms, follow-up of a genetic predisposition), and ongoing anticoagulant or antiplatelet therapy.
Ampullary lesions and EP
The results of initial biopsies and preoperative evaluation by EUS were collected. Morphological characteristics of lesions were size, presence of lateral extension, or intraductal invasion. EP could be performed with or without submucosal injection at the discretion of the endoscopist. The type of polypectomy snare and current were not documented. The resection was qualified as en bloc or piecemeal. Administration of rectal indomethacin and prophylactic pancreatic or biliary stenting were also reported. The final histological diagnosis and the quality of resection (lateral and deep margins) graded as R0 or R1 were collected from the pathology reports. The different steps of the procedure are described in Figure 1.
Figure 1.
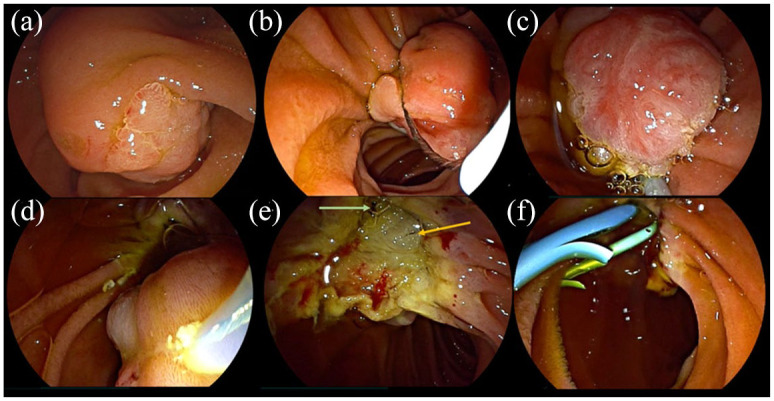
Example of an endoscopic papillectomy procedure for ampullary adenoma with high-grade dysplasia: (a) Inspection of the lesion. (b) Positioning of the snare at the oral side of the ampulla. (c) Capture of the lesion and resection. (d) Retrieval of the resected specimen. (e) Inspection of the scar: biliary orifice (left arrow) and pancreatic orifice (right arrow). (f) Placement of a pancreatic stent and a biliary stent.
Definitions and outcomes
Patient follow-up was based on endoscopic examinations, at a frequency set by the referring endoscopist according to the appearance of the EP scar and the results of biopsies. Patients without at least one follow-up endoscopy were considered lost to follow-up. Recurrence was defined as detection of macroscopic residue or histological evidence of adenoma/adenocarcinoma on biopsy during follow-up (true recurrence or insufficient initial treatment). Further endoscopic procedures (mucosectomy, radiofrequency, argon plasma coagulation) were collected. Treatment success was defined as complete excision of the lesion, regardless of the number of sessions required and detection of a recurrence in the follow-up period, if this was amenable to endoscopic treatment again (endoscopically managed lesions). It included definitive resection, that is, lesions that were definitively managed by a single endoscopic session and did not require any adjunctive therapeutic approach, and remission after further endoscopic procedures for local relapse identified on subsequent endoscopies.
Adverse events
Adverse events were graded according to the American Society for Gastrointestinal Endoscopy (ASGE) lexicon, 15 and their treatment if any were recorded. Hemorrhage was defined as a decrease in hemoglobin level of at least 2 g/dl or the need for transfusion. Treatment could be medical, endoscopic (sclerosis, clips, coagulation forceps, hemostatic powder or gel), or radiological (embolization). It was also investigated whether post–endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis resulted in increased length of hospitalization. Mortality was also recorded.
Statistical analysis
Quantitative data were described by mean and standard deviation or median and interquartile range (IQR); categorical data were described by frequency and percentage. Risk factors for recurrence were analyzed using Cox proportional-hazards models. The proportional-hazards assumption was tested using Schoenfeld residuals. Variables with p value < 0.20 in the univariable analysis were included in a multiple regression model. Backward selection in the multivariable analysis was then performed so that variables with a p value < 0.05 were kept in the final model. Hazard ratios (HRs) were estimated with their 95% confidence interval (CI). Risk factors for adenocarcinoma and adverse events were analyzed by logistic regression, with the same variable selection strategy. Analyses were performed using Stata version 16.0 software (StataCorp, Texas, USA).
The reporting of this study conforms to the STROBE statement.
Results
We included 227 patients (mean age: 61.0 ± 14.8 years; 52.9% male). Patient characteristics are presented in Table 1.
Table 1.
Demographic, clinical, procedural, and lesion characteristics in the 227 patients who underwent endoscopic papillectomy.
| Characteristics | |
|---|---|
| Age (years), mean ± SD | 61 ± 14.8 |
| Sex, n (%) | |
| Male | 120 (52.9) |
| Female | 107 (47.1) |
| ASA score, n (%) | |
| 1 | 82 (37.4) |
| 2 | 103 (47.0) |
| 3 | 34 (15.6) |
| 4 | 0 (0.0) |
| Context, n (%) | |
| Lynch syndrome | 4 (1.8) |
| Familial adenomatous polyposis | 48 (21.1) |
| Sporadic | 175 (77.1) |
| Circumstances of detection, n(%) | |
| Incidental | 134 (60.1) |
| Acute pancreatitis | 12 (5.4) |
| Cholestasis/cholangitis | 25 (11.2) |
| Follow-up for genetic predisposition | 52 (23.3) |
| Anticoagulant/antiplatelet therapy, n (%) | |
| Yes | 45 (19.8) |
| No | 182 (80.2) |
| Withdrawal before resection, n (%) | 41 (91.1) |
| Tumor size (mm), mean (range) | 20 (3–80) |
| Lateral extension, n (%) | 53 (23.3) |
| Intraductal invasion, n (%) | 35 (15.4) |
| Submucosal injection before resection | 112 (54.6) |
| Missing data | 12 |
| Type of resection | |
| En bloc | 147 (64.8) |
| Piecemeal | 80 (35.2) |
| Rectal indomethacin administration | 31 (58.5) |
| Missing data | 174 |
| Stenting | |
| Pancreatic | 161 (70.9) |
| Biliary | 53 (23.3) |
| Final histological diagnosis, n (%) | |
| Normal histology | 5 (2.2) |
| Inflammatory lesion | 7 (3.1) |
| Low-grade dysplasia | 108 (47.6) |
| High-grade dysplasia | 75 (33.0) |
| Adenocarcinoma | 24 (10.5) |
| Neuroendocrine tumor | 2 (0.9) |
| Others (hamartoma, paraganglioma) | 4 (1.8) |
| Unknown | 2 (0.9) |
| R0 resection | |
| Yes | 102 (45.3) |
| No | 123 (54.7) |
| Missing data | 2 |
ASA, American Society of Anesthesiology; SD, standard deviation.
Characteristics of the lesions and procedural data
Lesion characteristics and procedural data are shown in Table 1. At a previous examination, 165 lesions had been biopsied (72.7%). Preoperative EUS was performed in 74.0% of patients, all of whom had usT1N0 lesions. The mean tumor size was 20 mm (range: 3–80). Pre-therapeutic evaluation revealed lateral extension in 23.3% of cases and intraductal invasion in 15.4% of cases. Submucosal injection was performed in 54.6% of cases. The resection was mainly en bloc (64.8%). A pancreatic stent and a biliary stent were placed in 70.9% and 23.3% of cases, respectively.
Regarding the histological diagnosis of the resected specimen, 47.6% of the lesions were ampullary adenomas with at most low-grade dysplasia, 33.0% high-grade dysplasia, and 10.5% invasive adenocarcinoma. There were also 3.1% hyperplastic or inflammatory lesions and 2.7% various tumors (three gangliocytic paragangliomas, two neuroendocrine tumors, and one hamartoma). The papilla was considered normal in histology analysis in 2.2% of cases. For two patients, no pathology report was available. The concordance rate of histological diagnosis between standard biopsies and resected specimen was 60.0%. Discrepancies were mainly due to underdiagnosis (71%). For adenocarcinoma, in particular, underdiagnosis by biopsy occurred in 72% of cases. Resection was described as R0 in 45.3% of pathology reports.
Recurrence and endoscopic success
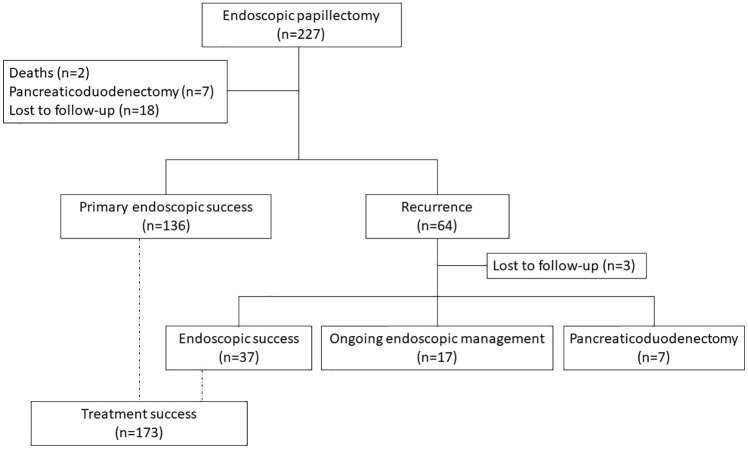
The median follow-up was 22.3 months (IQR: 8.7–46.4). Eighteen patients were lost to follow-up. The flow chart is presented in Figure 2.
Figure 2.
Flow chart describing the study flow from endoscopic procedure to the end of follow-up.
Recurrence occurred in 64 patients (30.6%) with a median time of recurrence of 6.8 months (IQR: 2.5–22.2). Most recurrences corresponded to R1 resection (73.4%). In multivariable analysis, factors associated with recurrence were lesion size > 2 cm (HR: 1.80; 95% CI: 1.07–3.04; p = 0.027), intraductal invasion (HR: 2.41; 95% CI: 1.35–4.31; p = 0.003), and R1 resection (HR: 2.04; 95% CI: 1.11–3.74; p = 0.022) (Table 2). In 136 patients, definitive resection was achieved (65.1%). All patients who had a recurrence, except for three patients lost to follow-up, underwent further endoscopic procedures. These further endoscopic procedures resulted in remission in 37 patients (60.7%) with a median of 1.27 successive treatments (IQR: 1–2). Twenty-four patients could not be successfully treated with endoscopic treatment alone at the end of the study. Of these, 7 underwent additional pancreaticoduodenectomy, while the remaining 17 patients were still undergoing endoscopic treatment. Thus, treatment success was achieved in 173 patients (82.8%).
Table 2.
Univariable and multivariable analysis of factors associated with recurrence after endoscopic papillectomy.
| Variable | Univariable | Multivariable (n = 186) | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| FAP/Lynch syndrome | 0.65 (0.37–1.14) | 0.13 | ||
| Tumor size (reference: <1 cm) | ||||
| 1–2 cm | 2.16 (0.90–5.20) | 0.087 | ||
| >2 cm | 3.65 (1.56–8.50) | 0.003 | 1.80 (1.07–3.04) | 0.027 |
| Lateral extension | 1.82 (1.09–3.03) | 0.021 | ||
| Intraductal invasion | 2.69 (1.53–4.72) | 0.001 | 2.41 (1.35–4.31) | 0.003 |
| Piecemeal resection | 1.99 (1.22–3.26) | 0.006 | ||
| Histology (reference: normal) | ||||
| Low-grade dysplasia | 1.75 (0.24–12.94) | 0.59 | ||
| High-grade dysplasia | 2.79 (0.38–20.73) | 0.32 | ||
| Adenocarcinoma | 3.07 (0.38–24.86) | 0.29 | ||
| R1 resection | 2.50 (1.14–4.43) | 0.002 | 2.04 (1.11–3.74) | 0.022 |
CI, confidence interval; FAP, familial adenomatous polyposis; HR, hazard ratio.
Of the 24 patients with adenocarcinomas, 8 had a definitive resection; 7 underwent additional cephalic pancreaticoduodenectomy (CPD), among them 2 died after surgery; and 1 patient was lost to follow-up. Eight patients underwent additional endoscopic treatment: 2 patients had a remission; 4 could not have complete treatment, among them 1 underwent CPD; and 2 were lost to follow-up. As a result, treatment success occurred in 10 early ampullary adenocarcinomas. In multivariable analysis, factors associated with adenocarcinoma were cholestasis or cholangitis (HR: 19.7; 95% CI: 7.03–55.3; p < 0.001) and tumor size > 2 cm (HR: 2.92; 95% CI: 1.13–7.52; p = 0.027) (Table 3).
Table 3.
Univariable and multivariable analysis of factors associated with a diagnosis of adenocarcinoma after endoscopic papillectomy.
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Circumstances of lesion detection (reference: incidental) | ||||
| Follow-up for genetic predisposition | 1.02 (0.31–3.42) | 0.973 | ||
| Post-ERCP pancreatitis | 4.00 (0.38–42.08) | 0.248 | ||
| Cholestasis/cholangitis | 16.80 (5.96–47.37) | <0.001 | ||
| FAP/Lynch syndrome | 0.49 (0.16–1.48) | 0.206 | 19.7 (7.03–55.3) | <0.001 |
| Tumor size | ||||
| 1–2 cm | 4.99 (0.63–39.8) | 0.13 | ||
| >2 cm | 8.95 (1.14–70.6) | 0.037 | 2.92 (1.13–7.52) | 0.027 |
CI, confidence interval; ERCP, endoscopic retrograde cholangiopancreatography; FAP, Familial adenomatous polyposis; OR, odds ratio.
Recurrence-free survival was not significantly different between centers (p = 0.77).
Adverse events of EP
The rate of adverse events was 36.6% (n = 83) (Table 4). The main adverse event was post-ERCP pancreatitis (17.6%), which increased the length of hospital stay in half the cases (53%). Prophylactic pancreatic stenting significantly reduced the risk of pancreatitis (HR: 0.41; 95% CI: 0.20–0.66; p < 0.014). The second most common adverse event was hemorrhage (11.0%), which required endoscopic management in 36% of cases and embolization in only one case (4%). Lesion size > 2 cm was the only factor associated with hemorrhage (HR: 3.31; 95% CI: 1.41–7.79; p < 0.006). Other adverse events were perforation (5.2%), for which no risk factor could be identified, and biliary stenosis (2.6%). No cases of cholangitis were reported. The mortality rate was 0.9%; all deaths were caused by severe post-ERCP pancreatitis with multi-organ failure.
Table 4.
Adverse events of endoscopic papillectomy in the 227 patients who underwent endoscopic papillectomy.
| Adverse events | n (%) |
|---|---|
| Post-ERCP pancreatitis | 40 (17.6) |
| Increased length of stay | 18 (53.0) |
| Missing data | 6 |
| Hemorrhage | 25 (11.0) |
| Endoscopic management | 9 (36.0) |
| Artery embolization | 1 (4.0) |
| Perforation | 12 (5.2) |
| Biliary stenosis | 6 (2.6) |
| Total | 83 (36.6) |
ERCP, endoscopic retrograde cholangiopancreatography.
The rate of patients with at least one adverse event was not significantly different between patients with sporadic lesions (31.4%) and patients with a genetic predisposition (32.7%; p = 0.86).
Discussion
EP is a challenging treatment of relatively rare entities, ampullary adenomas, and early ampullary adenocarcinomas, that is generally performed by experts in tertiary centers. Thus, our study of 227 patients is the largest series of EP performed to date to our knowledge. In our study, the recurrence rate was 30.6%, with treatment success in 82.8% of patients, achieved after a single definitive endoscopic session in 65.1% of patients. Adverse events occurred in 36.6% of patients and severe post-ERCP pancreatitis in about 8%.
Pre-therapeutic evaluation before EP is essential; however, the accuracy of biopsies is low. In our study, the concordance rate of biopsy specimens with post-papillectomy specimen was only 60.0%, which is similar to previous studies.16,17 Careful examination and multiple biopsies targeting suspicious areas (erosions or bleeding areas, architectural disorganization) of ampullary lesions at initial endoscopy are necessary to increase histologic yield and reduce false-negative rates. A second reading by an experienced pathologist may be recommended. The recent European Society for Gastrointestinal Endoscopy (ESGE) guidelines recommend not performing EP when adenoma is not proven, 8 as normal or inflammatory tissue was found in the post-EP histological analysis in 8–13% of cases in other retrospective series.18,19 Consistently, 5.3% of final histological diagnoses were consistent with normal or inflammatory lesions in our series.
The optimal selection criteria for curative endoscopic treatment of ampullary tumors have been clarified by the recent ESGE guidelines, which recommends pancreaticoduodenectomy for T1 tumors and considers transduodenal ampullectomy or EP for Tis tumors. 8 Several recent studies have shown that endoscopic treatment can be curative in well-differentiated adenocarcinomas without invasion of the duodenal submucosa or lympho-vascular infiltration and with an intraductal invasion of less than 1 cm.11–13 In our study, 10 of the 24 adenocarcinomas were successfully treated by EP; however, the stage was not known due to the retrospective nature of the data. Interestingly, the malignant nature of the lesion did not emerge as a risk factor for recurrence. In contrast, cholestasis or cholangitis at diagnosis and lesion size > 2 cm were associated with malignancy, suggesting that the presence of these factors requires accurate pre-therapeutic evaluation, en bloc resection, and close follow-up. In our study, pre-therapeutic EUS was performed in 74.0% of patients. The use of intraductal ultrasound (IDUS) could be more accurate in visualizing the mucosal layers compared with conventional EUS. Indeed, for T1 lesions, the accuracies of IDUS and EUS are 86% and 62%, respectively.17,20 However, the accessibility of IDUS still limits its use.
With regard to the resection technique, we found a high rate of submucosal injection (54.6%) compared with the rate of lesions with lateral duodenal extension (23.3%), which are conventionally the only ones requiring submucosal injection. These discordant results are probably related to a center effect. Indeed, in one center, 91.5% (n = 75/82) of EPs were performed with submucosal injection regardless of lateral extension. Some studies have suggested that submucosal injection was associated with more frequent residual tumor and shorter recurrence-free survival, and did not reduce post-procedural adverse event.21,22 The simple snare technique is now recommended, except for laterally spreading ampullary tumors. 8 Notably, an association between recurrence rate and submucosal injection was not identified in our study. A relatively high rate of biliary stenting was also observed (23.3%); this procedure is usually performed to reduce the risk of cholangitis and biliary stenosis, but these events are rare and the evidence in favor of biliary stenting is weak. 2
With a treatment success rate of 82.8%, this series confirms that EP is an efficient curative treatment for benign lesions and early adenocarcinomas of the papilla of Vater. Previous studies have reported a median success rate of 72.5%, with a recurrence rate of 11.8%. 2 Differences between studies result in part from the heterogeneity of inclusion criteria and the definition of success. Some studies define success as complete R0 resection without recurrence during follow-up,2,23 whereas in this real-life series, success was defined as the absence of residual tumor at the end of follow-up, regardless of any recurrence during follow-up, as it can be efficiently treated by endoscopic treatment.24,25 In fact, in our study, 60.7% of recurrences were successfully treated with additional endoscopic treatment. This rate might be underestimated because some patients at the end of study were still undergoing endoscopic treatment for recurrence and might be cured after several endoscopic sessions.
The recurrence rate in our study was 30.6%, which is higher than previously reported. 2 This could be explained by the high rate of R1 resections (54.7%) and piecemeal resections (35.2%). Indeed, the lesions in our study were significantly larger than in previous studies (20 mm versus 15.7 mm), 2 making en bloc resection and consequently R0 resection more difficult. However, this recurrence rate is probably overestimated as the diagnosis of recurrence was sometimes based on the detection of a macroscopic residue at the follow-up endoscopy, without systematic biopsies. Thus, a simple inflammatory scar tissue could be overly considered as an adenomatous residue. Endoscopic follow-up is essential because recurrence can occur up to 65 months after resection. 26 In our study, the median time to recurrence was 6.8 months with a median follow-up of 22.3 months. In some patients, recurrences occurred after normal follow-up endoscopies (n = 9) and up to 54 months after EP. However, the risk factors for recurrence are not well defined. Ridtitid et al. 26 have shown that jaundice at diagnosis, intraductal invasion, piecemeal resection, and adenocarcinoma were associated with recurrence. In some studies, lesion size was also a risk factor.27,28
In our study, the factors associated with recurrence in multivariate analysis were R1 resection (HR: 2.04), intraductal invasion (HR: 2.41), and lesion size > 2 cm (HR: 1.80). Indeed, large lesions are more often resected in a piecemeal fashion leading to a higher risk of incomplete resection. In addition, EP is frequently incomplete in case of intraductal invasion with residual endobiliary dysplasia. However, endobiliary radiofrequency has been reported as an effective additional therapy for the treatment of residual endobiliary adenomas, with success rates of 70%. 29 Intraductal invasion should not be a contraindication to EP but should prompt therapeutic discussions. If endoscopic resection is chosen, close follow-up and additional radiofrequency treatment may be suggested, especially for inoperable patients. Thus, close follow-up should be performed with a first endoscopy within the first 3 months, at 6 and 12 months after EP. 8 Thereafter, annual follow-up should be extended for at least 5 years, especially in case of initial R1 resection, lesion > 2 cm, or intraductal invasion.
Although EP remains less morbid than surgery, it is a technique with a higher rate of adverse events than other endoscopic procedures. In the literature, the adverse events rate is 24.9%, while mortality is rare, less than 0.3%.2,16,17,26 In our study, the adverse events rate was 36.6%. Although this rate is high, most adverse events were moderate and accessible to medical or endoscopic treatment. The two main adverse events were post-ERCP pancreatitis (17.6%) and hemorrhage (11.0%). Previous studies have shown that prophylactic pancreatic stenting and rectal indomethacin administration significantly decreased the risk of post-ERCP pancreatitis in high-risk patients.2,30–32 In our study, only pancreatic stenting was significantly associated with a reduced risk of post-ERCP pancreatitis (HR: 0.41). The retrospective nature of the study prevented us from investigating why only 70.9% of patients underwent pancreatic stenting. Similarly, the use of rectal indomethacin did not appear to be a protective factor for post-ERCP pancreatitis, probably due to underreporting. Current guidelines recommend pancreatic stenting and rectal indomethacin administration to prevent the risk of post-ERCP pancreatitis.8,33 For post-procedural hemorrhage, the only risk factor identified was lesion size > 2 cm (HR: 3.31). Lateral extension did not significantly increase the risk of bleeding. A possible explanation could be that submucosal injection was often associated with adrenaline, which facilitates local hemostasis after resection. The perforation rate and mortality were 5.2% and 0.9%, respectively, too low to determine significant risk factors. Death occurred only in patients with severe post-ERCP pancreatitis, emphasizing the need for pancreatitis prophylaxis.
This study is important for several reasons. First, as one of the largest series published, it provides new data on this procedure. As highlighted by a recent expert consensus based on a Delphi process,34,35 data are scarce on this relatively rare procedure, with randomized prospective studies difficult to build, making consensus difficult to achieve. In this context, retrospective data are useful as they help evidence-based decisions, and, in the future, enable to perform meta-analyses. In addition, it provides new analyses on risk factors of recurrence and adverse effects enabled by its statistical power. The main limitation of this real-life study is its retrospective nature, resulting in a heterogeneous endoscopic follow-up and reporting. While some patients were followed closely and over a long period of time (several years), others were followed only for a few months. However, the median follow-up time (22.3 months) remained longer than the median time to recurrence (6.8 months). In addition, despite a high number of patients, sub-group analyses may have been underpowered to demonstrate significant differences.
In conclusion, this large cohort adds to the evidence that EP is an effective treatment for ampullary adenomas and early ampullary carcinoma. Recurrence was more common in case of R1 resection, intraductal invasion, and size larger than 20 mm, but subsequent endoscopic treatment was often feasible. However, endoscopists should be trained in the prevention and management of adverse events because EP remains a relatively risky procedure.
Footnotes
Author contributions: Hannah Gondran: Conceptualization; Data curation; Formal analysis; Investigation; Writing – original draft.
Nicolas Musquer: Conceptualization; Formal analysis; Methodology; Writing – original draft.
Enrique Perez-Cuadrado-Robles: Formal analysis; Supervision.
Pierre Henri Deprez: Data curation; Writing – review & editing.
François Buisson: Data curation; Writing – review & editing.
Arthur Berger: Data curation; Writing – review & editing.
Elodie Cesbron-Métivier: Data curation; Writing – review & editing.
Timothee Wallenhorst: Data curation; Writing – review & editing.
Nicolas David: Data curation; Writing – review & editing.
Franck Cholet: Data curation; Writing – review & editing.
Bastien Perrot: Formal analysis; Methodology; Software; Writing – original draft.
Lucille Quénéhervé: Formal analysis; Writing – review & editing.
Emmanuel Coron: Conceptualization; Supervision; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Arthur Berger  https://orcid.org/0000-0003-0387-4734
https://orcid.org/0000-0003-0387-4734
Lucille Quénéhervé  https://orcid.org/0000-0001-5443-2579
https://orcid.org/0000-0001-5443-2579
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Hannah Gondran, Institut des Maladies de l’Appareil Digestif (IMAD), CHU Nantes, Nantes, France.
Nicolas Musquer, Institut des Maladies de l’Appareil Digestif (IMAD), CHU Nantes, Nantes, France.
Enrique Perez-Cuadrado-Robles, Service de gastroentérologie, Hôpital européen Georges Pompidou, Assistance publique des hôpitaux de Paris, Paris, France; Service d’hépato-gastro-entérologie, Cliniques universitaires Saint-Luc, Brussels, Belgium.
Pierre Henri Deprez, Service d’hépato-gastro-entérologie, Cliniques universitaires Saint-Luc, Brussels, Belgium.
François Buisson, Service d’hépato-gastro-entérologie, CHU Angers, Angers, France.
Arthur Berger, Service d’hépato-gastro-entérologie, CHU Angers, Angers, France.
Elodie Cesbron-Métivier, Service d’hépato-gastro-entérologie, CHU Angers, Angers, France.
Timothee Wallenhorst, Service des Maladies de l’Appareil Digestif, CHU Pontchaillou, Université de Rennes 1, Rennes, France.
Nicolas David, Service d’hépatogastroen térologie, La Cavale Blanche, CHRU Brest, Brest, France.
Franck Cholet, Service d’hépatogastroen térologie, La Cavale Blanche, CHRU Brest, Brest, France.
Bastien Perrot, Biostatistics and Methodology Unit, Department of Clinical Research and Innovation, Centre Hospitalier Universitaire de Nantes, Nantes, France.
Lucille Quénéhervé, Service d’hépatogastroen térologie, La Cavale Blanche, CHRU Brest, Brest, France.
Emmanuel Coron, Department of Gastroenterology and Hepatology, University Hospital of Geneva (HUG), rue Gabrielle Perret-Gentil 4, 1211, Genève 1205, Switzerland.
References
- 1. Kimura W, Ohtsubo K. Incidence, sites of origin, and immunohistochemical and histochemical characteristics of atypical epithelium and minute carcinoma of the papilla of Vater. Cancer 1988; 61: 1394–1402. [DOI] [PubMed] [Google Scholar]
- 2. Spadaccini M, Fugazza A, Frazzoni L, et al. Endoscopic papillectomy for neoplastic ampullary lesions: a systematic review with pooled analysis. United European Gastroenterol J 2020; 8: 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martin JA, Haber GB. Ampullary adenoma: clinical manifestations, diagnosis, and treatment. Gastrointest Endosc Clin N Am 2003; 13: 649–669. [DOI] [PubMed] [Google Scholar]
- 4. Tran TC, Vitale GC. Ampullary tumors: endoscopic versus operative management. Surg Innov 2004; 11: 255–263. [DOI] [PubMed] [Google Scholar]
- 5. Bourgouin S, Ewald J, Mancini J, et al. Predictive factors of severe complications for ampullary, bile duct and duodenal cancers following pancreaticoduodenectomy: multivariate analysis of a 10-year multicentre retrospective series. Surgeon 2017; 15: 251–258. [DOI] [PubMed] [Google Scholar]
- 6. Lai ECH, Lau SHY, Lau WY. Measures to prevent pancreatic fistula after pancreatoduodenectomy: a comprehensive review. Arch Surg 2009; 144: 1074–1080. [DOI] [PubMed] [Google Scholar]
- 7. Farnell MB, Sakorafas GH, Sarr MG, et al. Villous tumors of the duodenum: reappraisal of local vs. extended resection. J Gastrointest Surg 2000; 4: 13–21; discussion 22–23. [DOI] [PubMed] [Google Scholar]
- 8. Vanbiervliet G, Strijker M, Arvanitakis M, et al. Endoscopic management of ampullary tumors: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2021; 53: 429–448. [DOI] [PubMed] [Google Scholar]
- 9. Binmoeller KF, Boaventura S, Ramsperger K, et al. Endoscopic snare excision of benign adenomas of the papilla of Vater. Gastrointest Endosc 1993; 39: 127–131. [DOI] [PubMed] [Google Scholar]
- 10. Heise C, Abou Ali E, Hasenclever D, et al. Systematic review with meta-analysis: endoscopic and surgical resection for ampullary lesions. J Clin Med 2020; 9: E3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamamoto K, Itoi T, Sofuni A, et al. Expanding the indication of endoscopic papillectomy for T1a ampullary carcinoma. Dig Endosc 2019; 31: 188–196. [DOI] [PubMed] [Google Scholar]
- 12. Woo SM, Ryu JK, Lee SH, et al. Feasibility of endoscopic papillectomy in early stage ampulla of Vater cancer. J Gastroenterol Hepatol 2009; 24: 120–124. [DOI] [PubMed] [Google Scholar]
- 13. Yoon SM, Kim M-H, Kim MJ, et al. Focal early stage cancer in ampullary adenoma: surgery or endoscopic papillectomy? Gastrointest Endosc 2007; 66: 701–707. [DOI] [PubMed] [Google Scholar]
- 14. Pérez-Cuadrado-Robles E, Piessevaux H, Moreels TG, et al. Combined excision and ablation of ampullary tumors with biliary or pancreatic intraductal extension is effective even in malignant neoplasms. United European Gastroenterol J 2019; 7: 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 2010; 71: 446–454. [DOI] [PubMed] [Google Scholar]
- 16. Kang SH, Kim KH, Kim TN, et al. Therapeutic outcomes of endoscopic papillectomy for ampullary neoplasms: retrospective analysis of a multicenter study. BMC Gastroenterol 2017; 17: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Napoleon B, Gincul R, Ponchon T, et al. Endoscopic papillectomy for early ampullary tumors: long-term results from a large multicenter prospective study. Endoscopy 2014; 46: 127–134. [DOI] [PubMed] [Google Scholar]
- 18. Laleman W, Verreth A, Topal B, et al. Endoscopic resection of ampullary lesions: a single-center 8-year retrospective cohort study of 91 patients with long-term follow-up. Surg Endosc 2013; 27: 3865–3876. [DOI] [PubMed] [Google Scholar]
- 19. Alali A, Espino A, Moris M, et al. Endoscopic resection of ampullary tumours: long-term outcomes and adverse events. J Can Assoc Gastroenterol 2020; 3: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Itoh A, Goto H, Naitoh Y, et al. Intraductal ultrasonography in diagnosing tumor extension of cancer of the papilla of Vater. Gastrointest Endosc 1997; 45: 251–260. [DOI] [PubMed] [Google Scholar]
- 21. Hyun JJ, Lee TH, Park J-S, et al. A prospective multicenter study of submucosal injection to improve endoscopic snare papillectomy for ampullary adenoma. Gastrointest Endosc 2017; 85: 746–755. [DOI] [PubMed] [Google Scholar]
- 22. Chung KH, Lee SH, Choi JH, et al. Effect of submucosal injection in endoscopic papillectomy of ampullary tumor: propensity-score matching analysis. United European Gastroenterol J 2018; 6: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sauvanet A, Chapuis O, Hammel P, et al. Are endoscopic procedures able to predict the benignity of ampullary tumors? Am J Surg 1997; 174: 355–358. [DOI] [PubMed] [Google Scholar]
- 24. Sakai A, Tsujimae M, Masuda A, et al. Clinical outcomes of ampullary neoplasms in resected margin positive or uncertain cases after endoscopic papillectomy. World J Gastroenterol 2019; 25: 1387–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee R, Huelsen A, Gupta S, et al. Endoscopic ampullectomy for non-invasive ampullary lesions: a single-center 10-year retrospective cohort study. Surg Endosc 2020; 35: 684–692. [DOI] [PubMed] [Google Scholar]
- 26. Ridtitid W, Tan D, Schmidt SE, et al. Endoscopic papillectomy: risk factors for incomplete resection and recurrence during long-term follow-up. Gastrointest Endosc 2014; 79: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ito K, Fujita N, Noda Y, et al. Impact of technical modification of endoscopic papillectomy for ampullary neoplasm on the occurrence of complications. Dig Endosc 2012; 24: 30–35. [DOI] [PubMed] [Google Scholar]
- 28. Irani S, Arai A, Ayub K, et al. Papillectomy for ampullary neoplasm: results of a single referral center over a 10-year period. Gastrointest Endosc 2009; 70: 923–932. [DOI] [PubMed] [Google Scholar]
- 29. Camus M, Napoléon B, Vienne A, et al. Efficacy and safety of endobiliary radiofrequency ablation for the eradication of residual neoplasia after endoscopic papillectomy: a multicenter prospective study. Gastrointest Endosc 2018; 88: 511–518. [DOI] [PubMed] [Google Scholar]
- 30. Napoléon B, Alvarez-Sanchez MV, Leclercq P, et al. Systematic pancreatic stenting after endoscopic snare papillectomy may reduce the risk of postinterventional pancreatitis. Surg Endosc 2013; 27: 3377–3387. [DOI] [PubMed] [Google Scholar]
- 31. Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc 2005; 62: 367–370. [DOI] [PubMed] [Google Scholar]
- 32. Elmunzer BJ, Scheiman JM, Lehman GA, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med 2012; 366: 1414–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. ASGE Standards of Practice Committee, Chathadi KV, Khashab MA, et al. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc 2015; 82: 773–781. [DOI] [PubMed] [Google Scholar]
- 34. Fritzsche JA, Fockens P, Barthet M, et al. Expert consensus on endoscopic papillectomy using a Delphi process. Gastrointest Endosc 2021; 94: 760–773.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barakat MT, Adler DG. Endoscopic ampullectomy: can expert input shape endoscopic practice? Gastrointest Endosc 2021; 94: 774–775. [DOI] [PubMed] [Google Scholar]