Abstract
Background and Objectives:
Here we aimed to characterize clinical outcomes in those receiving treatment at a Veterans Health Administration (VHA) methadone maintenance treatment program (MMT) during the COVID 19 pandemic in which SAMSHA regulations for MMTs were changed to provide a greater number of methadone allotments and decreased clinic-visit frequency.
Methods:
We report results of a single-site, pre-post cohort study of urine drug screen data 3 months before and after an increase in allotments of take-home medication from the methadone clinic. One hundred twenty-nine patients met inclusion criteria for this study. The study was reviewed by the NYHHS IRB committee and granted final approval by the Research and Development Committee.
Results:
The sample was predominately male, average age 66years and average years in most recent treatment is 4.1 years. No statistical significance was found between period 1 and period 2 in the positive test detection for nonprescribed opiates, methadone and illicit substances (P > .05), number of new medical illnesses or overdoses. We controlled for participant age, substance use disorder diagnosis, psychiatric disorder diagnosis, and number of years in treatment.
Discussion/Conclusions:
The results of the study illustrate the relative safety of the changes made at this particular MMT during the pandemic. Additionally, there was continued adherence to methadone treatment with minimal change in illicit substance use during period 1 and period 2.
Scientific Significance:
To these authors’ knowledge this paper is one of the first to examine clinical outcomes in those with opioid addiction prescribed methadone from MMTs during the COVID 19 pandemic.
Keywords: Methadone, opiate substitution treatment, addiction medicine
Introduction
The opioid crisis remains a major cause of morbidity and mortality in the United States, with the Substance Abuse and Mental Health Services Administration (SAMHSA) estimating the 1-year prevalence of opioid use disorder at 1.6 million, or 0.6% of the US population, in 2019. 1 Meta-analyses of cohort studies have suggested a standardized mortality of 12 to 20 associated with opioid use disorder (OUD) compared with the overall population.2,3 Among the longest-established treatments for OUD is medication assisted treatment (MAT) with methadone, a medication that is associated with a significant improvement in retention in OUD treatment compared to no medication. 4 Retention in treatment, in turn, is associated with a significant mortality benefit. 5 These findings emphasize the importance of maintaining continuity of care in methadone maintenance therapy programs (MMTs) to reduce the disease burden of the opioid epidemic.
In 2020, the onset of the coronavirus disease 2019 (COVID-19) pandemic prompted significant changes in the delivery of MMT. Prior to March 2020, SAMHSA required patients beginning MMT to present in person to clinic at least 6 days a week for supervised consumption of methadone and receive no more than 1 dose per week for unsupervised, offsite consumption. Larger allotments of “take-home” medication were restricted to patients with a longer tenure in treatment (eg, 3 days’ supply at 180 days in treatment, 2 weeks’ supply at 1 year, and a month’s supply at 2 years; 42 CFR § 8). When the COVID-19 pandemic was declared a state of emergency in the United States, SAMHSA indefinitely relaxed this restrictions, allowing MMTs to prescribe 14 to 28-days of take-home methadone to any patient in MMT regardless of duration of prior treatment. 6 The intent of this policy change was to alleviate the requirement for MMT patients to make frequent in-person clinic visits, thereby promoting social isolation and limiting the spread of a respiratory disease outbreak. 7
Until 2020 the pre-pandemic regulations restricting methadone take-homes had remained essentially unchanged since the introduction of MMT in the United States as a measure to prevent misuse of the medication, including diversion, excessive dose, or overdose. 8 However, the safety benefit of these policies, and of the practice of methadone administration under supervision, have been little studied. A 2017 systematic review illustrated that the quality of evidence comparing the safety and efficacy of supervised opioid substitution therapy to that of unsupervised therapy is overall low. 9 Few studies have directly compared take-home methadone contingent on urine drug screen results or other treatment adherence metrics to take-home independent of treatment adherence, and those studies that have done so examined relatively small cohorts.10-12
The aims of this paper are to characterize the issues of methadone prescription safety during the COVID 19 pandemic in which SAMSHA relaxed regulations for MMTs, thereby providing a greater number of methadone allotments and decreased clinic-visit frequency. We did so by looking at clinical data from a large, Veterans Health Administration (VHA) hospital-affiliated MMT. Specifically, we characterize changes in medication adherence, illicit substance use, rates of infection, or mortality during 2 time points—before SAMSHA’s relaxation of MMT regulation and during the pandemic related changes. To these authors’ knowledge this paper is one of the first to examine clinical outcomes in those with OUD prescribed methadone from MMTs during the COVID 19 pandemic.
Methods
Design
We report results of a single-site, pre-post cohort study of urine drug screen data 3 months before and after an increase in allotments of take-home medication from the methadone clinic at the Manhattan VA Medical Center, part of the VA NY Harbor Healthcare System (NYHHS). We examined medical records from the period 12/16/2019–3/15/2020 (“Period 1”) and the period 3/16/2020-6/15/2020 (“Period 2”) to obtain patient characteristics and outcomes data. The study was reviewed by the NYHHS IRB committee and granted final approval by the Research and Development Committee, with a waiver of HIPAA and informed consent.
Study setting and population
This study was conducted at the Methadone Maintenance Treatment program (MMT) of the Manhattan VA Medical Center. The MMT utilizes a multidisciplinary team composed of counselors, social workers, psychologists, nurses, and psychiatrists to provide clinic-based buprenorphine and methadone as well as office-based buprenorphine to New York City’s veteran population. The MMT’s methadone program falls under the jurisdiction of NY State’s Office of Addiction Services and Supports (OASAS) and complies with federal guidelines set for all methadone clinics. Each veteran signs an agreement at the time of enrollment detailing criteria for discharge, including diversion and behavior compromising the safety of peers and staff. The clinic additionally requires enrolled patients to meet a counselor once per week and to submit observed urine toxicology on clinic visit days.
On March 16, 2020, the OASAS relaxed its methadone restrictions to match SAMHSA’s emergency guidance. 13 Accordingly, the MMT adapted to the new policy by stopping new clinic enrollments and relaxed its take-home dose allowances to accommodate this interim policy. Following a discussion of clinic staff, the MMT assigned a level of risk to each patient and established the following protocol for take-home methadone prescriptions: 27 days of take-home medication for low-risk patients, 13 days for medium-risk patients, 6 days for high-risk patients, and 4 days for few select patients deemed at highest risk. Although there were no strict risk criteria, high-risk features included frequent missed appointments, medication diversion, overdoses, or urine specimens positive for benzodiazepines or alcohol. Patients who were stable on >6 days of take-home methadone pre-pandemic were generally considered low risk. Patients continued to meet with counselors once per week or each time they visited the clinic and to provide urine drug screen (UDS) specimens at each MMT visit in which methadone is dispensed. Clinicians reduced take-home allotments for patients exhibiting increases in medication diversion or substance use after the initial increase in take-home doses.
The patients in this study were identified through a review of VA electronic health records (EHR). Patients were eligible for inclusion if they were enrolled in the MMT at the beginning of the study period (12/16/2019) and could be followed to the end of the study. Eleven patients enrolled in the study during the beginning of Period 1 left the MMT prior to the end of Period 2 and were not included in analyses. They were administratively discharged (unable to meet the requirements for continued treatment) or moved to a different MMT.
Measures
Following the identification of subjects for inclusion, electronic charts were reviewed, and patient characteristics and outcomes data were extracted to REDCap (Research Electronic Data Capture), a secure, web-based software platform designed to support data capture for research studies.14,15
Baseline characteristics
Baseline characteristics isolated from Veterans Health Administration (VHA) electronic health records included demographics (age, gender, and race/ethnicity), substance disorder history (including non-opioid substance use disorders), psychiatric disorder history (non-substance use disorder such as PTSD, mood disorder, anxiety, disorder, sleep disorder, psychotic disorder, etc), infectious disease history, and years in MMT leading to Period 1, average methadone dosage. All the baseline characteristics were determined at Period 1. The diagnosis of infectious disease, substance use disorder and psychiatric disorder were made by the patient’s primary care, other medical or psychiatric providers, or clinicians working with the patients in the MMT.
Outcome measures
The outcomes of interest in this study were methadone adherence and nonprescribed substance use, as indicated by urine drug screen (UDS) data. The MMT drug panel is comprised of alcohol, amphetamines, barbiturates, benzodiazepines, cannabis, cocaine, opiates (heroin and fentanyl), oxycodone and methadone. To assess methadone adherence, the percent of UDS positive for methadone in each period were calculated for each subject. Two measures of nonprescription substance use were calculated from UDS data: percent of UDS positive for opiates and percent of UDS positive for any nonprescribed substance other than cannabis. Any drug includes amphetamines, barbiturates, benzodiazepines, cocaine, opiates (heroin and fentanyl), oxycodone, or alcohol. Cannabis was excluded from this analysis because abstinence from recreational cannabis use is not a primary goal of treatment or counseling at the MMT; as such, many clinic patients consistently use recreational cannabis while maintaining abstinence from other non-prescribed substances.
Additionally, we sought to characterize the incidence rate of any new medical complications of opioid use disorder during Period 2 of the study, such as substance overdose, and infectious disease complications (aspiration pneumonia, hepatitis, HIV, skin, and soft tissue infection).
Statistical analysis
The xtset command was used to set the balanced panel data. One-way tabulations were used to summarize sociodemographic and diagnostic variables of the study population. Summary statistics were given for all predictor variables. Two-way cross-tabulations were used with summarize outcome variables over time. Pearson Chi-squares were performed in order to assess differences across time periods.
Multiple logistic regression was performed using the xtlogit command to assess the relationship between predictor variables and each of 4 outcomes: opiate drug positivity, methadone positivity, and any drug positivity. For each outcome, 3 models were created. Model 1 included only the time period and did not control for any other predictor variables. Model 2 included the time period as well as the numbers of years in treatment. Model 3 includes all Model 2 covariates as well as participant age, substance use disorder diagnosis, psychiatric disorder diagnosis, and percent reduction in visit frequency. These covariates were chosen to be examined as they have been shown in previous studies to impact relapse rates, and may confound the outcomes of interest in this study.
Random-effects model estimators were used in order to accommodate time-invariant regressors. Hausman tests were also performed and supported the use of random effects estimators in methadone positivity. Likelihood-ratio tests on regression rho statistics confirmed the choice to use individual-specific effects models instead of the pooled OLS models on all 4 outcomes.
Two-sided design-based tests and an alpha level of 0.05 was used to evaluate statistical significance in all multiple logistic regressions. All data management and analyses were conducted using Stata/IC 15.
Results
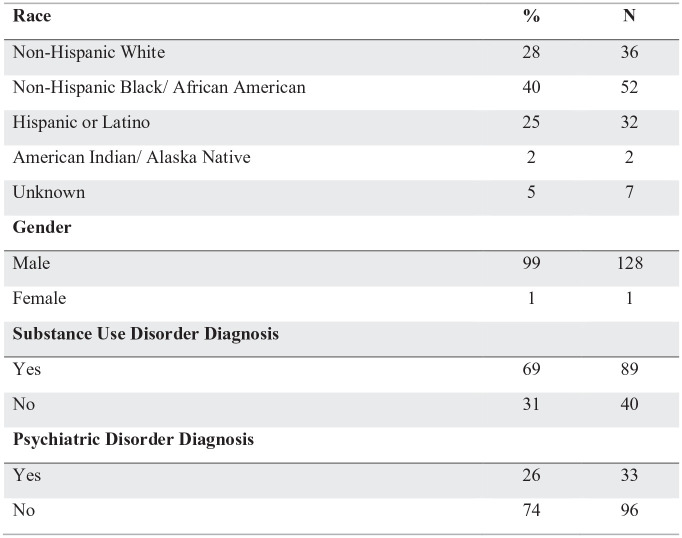
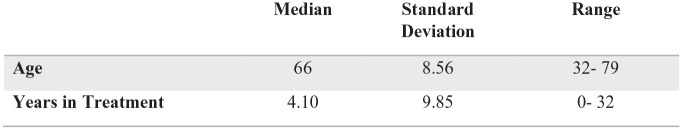
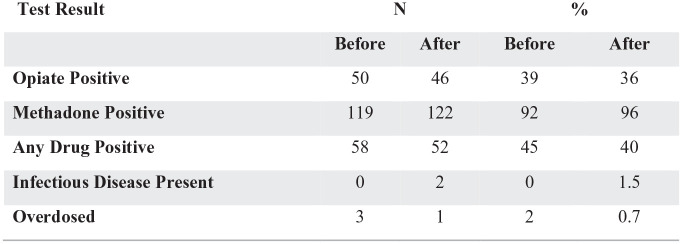
A total of 129 (128 male, 1 female) patients were enrolled in the MMT at Period 1 and met inclusion criteria for the study. Contributions of gender differences was not analyzed due to the predominately male sample. Demographic features of the study population are described in Figure 1. Within the sample, 69% (n = 89) met diagnostic criteria for another substance use. Additionally, 71% (n = 103) met criteria for a co-occurring substance use disorder and nonsubstance psychiatric diagnosis. Figure 2 illustrates the range of ages (32-79) of the sample, and years in treatment during the current program (4.10 years) when entering Period 1. Figure 3 shows a summary of the urine drug screens detecting substances during Period 1 versus Period 2. No statistical significance was found between Period 1 and Period 2 in the positive test detection for nonprescribed opiates, methadone and any illicit drug. During Period 1 and period 2 there was 3 overdoses and 1 overdose respectively.
Figure 1.
Demographics table (N = 129).
*P-level > .05.
Figure 2.
Summary statistics continued (N = 129).
Figure 3.
Summary statistics continued (N = 129).*
*Chi-square tests were conducted for each outcome variables and none were statistically significant with a P-level > .05.
Table 1 shows outcomes in opioid positive urine drug screens under 3 models. Without controlling for any other covariates, the changes made to clinic guidelines during the COVID-19 pandemic was not a significant predictor of methadone positivity. In model 2, changes made to clinic guidelines was not a statistically significant predictors of methadone positivity. In model 3, when controlling for age, comorbid substance use diagnosis, comorbid psychiatric disorder diagnosis, and percent-reduction in visit frequency, neither years in treatment, nor changes made to clinic guidelines during COVID-19 were significant predictors of methadone positivity. Number of years in treatment was a statistically significant predictor in model 2 and 3.
Table 1.
Opioid positive urine drug screens controlling for covariates.
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Opioid Positive UDS (Yes/No) | Opioid Positive UDS (Yes/No) | Opioid Positive UDS (Yes/No) | |
| Opioid Positive UDS (Yes/No) | |||
| Period 1 | 1.00 [1.00, 1.00] | 1.00 [1.00, 1.00] | 1.00 [1.00, 1.00] |
| Period 2 | 0.69 [0.29, 1.62] | 0.82 [0.34, 1.98] | 0.82 [0.34, 1.98] |
| Years in Treatment | 0.87** [0.78, 0.96] | 0.87** [0.78, 0.97] | |
| Age | 1.00 [0.90, 1.12] | ||
| Subs Use Diagnosis = 0 | |||
| Subs Use Diagnosis = 1 | 0.25 [0.03, 2.08] | ||
| Psych Diagnosis = 0 | |||
| Psych Diagnosis = 1 | 3.01 [0.32, 28.08] | ||
| % Reduction in visit frequency | 0.31 [0.01, 6.59] | ||
| / | |||
| lnsig2u | 18.15*** [7.48, 44.06] | 18.20*** [7.79, 42.51] | 17.26*** [7.32, 40.73] |
| Observations | 258 | 250 | 250 |
| R 2 | |||
| Adjusted R2 | |||
| N_pop | |||
| N_sub | |||
| F |
Exponentiated coefficients; ci in brackets.
P < .05. **P < .01. ***P < .001.
Model 1: Controlled only for changes made to clinic guidelines during period 1 and 2 Model 2: Controlled for years in treatment.
Model 3: Controlled for age, comorbid substance use diagnosis, comorbid psychiatric disorder diagnosis, percent-reduction in visit frequency, years in treatment, and changes made during period 1 and period 2.
UDS, urine drug screen Period 1: control time period.
Period 2, during COVID 19 changes.
Psychiatric diagnosis excludes substance use disorder diagnosis.
SUD, Substance use disorder (other than opioid use disorder diagnosis).
Table 2 shows outcomes in methadone positive urine drug screens under same 3 models. Without controlling for any other covariates, changes made to clinic guidelines during COVID-19 was not a significant predictor of opiate positivity. When controlling for participant years in treatment, changes made to clinic guidelines during COVID-19was not a significant predictor of methadone positivity, while number of years in treatment was a significant predictor. For every additional year of treatment, participants had 14% less odds of testing positive for opiate use. However, when controlling for age, comorbid substance use diagnosis, comorbid psychiatric disorder diagnosis, and percent-reduction in visit frequency, nor changes made to clinic guidelines during COVID-19 were significant predictors of opiate positivity.
Table 2.
Methadone positive urine drug screens controlling for covariates.
| Model 1 |
Model 2 |
Model 3 |
|
|---|---|---|---|
| Methadone Positive UDS (Yes/No) | Methadone Positive UDS (Yes/No) | Methadone Positive UDS (Yes/No) | |
| Methadone positive UDS (Yes/No) | |||
| Period 1 | 1.00 [1.00, 1.00] | 1.00 [1.00, 1.00] | 1.00 [1.00, 1.00] |
| Period 2 | 0.57 [0.17, 1.96] | 0.80 [0.22, 2.95] | 0.81 [0.22, 2.92] |
| Years in Treatment | 0.90 [0.79, 1.03] | 0.91 [0.81, 1.02] | |
| Age (years) | 1.05 [0.94, 1.16] | ||
| Other SUDs Diagnosis = 0 | |||
| Presence of Other SUDs Diagnosis = 1 | 1.23 [0.19, 8.09] | ||
| No other psychiatric diagnosis = 0 | |||
| Presence of one or more psychiatric diagnosis = 1 | 8.18 [0.51, 130.23] | ||
| % Reduction in visit frequency | 0.11 [0.00, 3.64] | ||
| / | |||
| lnsig2u | 5.46* [1.14, 26.15] | 6.13* [1.17, 32.00] | 4.31 [0.64, 28.94] |
| Observations | 258 | 250 | 250 |
| R 2 | |||
| Adjusted R2 | |||
| N_pop | |||
| N_sub | |||
| F |
Exponentiated coefficients; ci in brackets.
P < .05. ** P < .01. ***P < .001.
Model 1: Controlled only for changes made to clinic guidelines during period 1 and 2 Model 2: Controlled for years in treatment
Model 3: Controlled for age, comorbid substance use diagnosis, comorbid psychiatric disorder diagnosis, percent-reduction in visit frequency, years in treatment, and changes made during period 1 and period 2.
UDS: urine drug screen.
Period 1: control time period.
Period 2: during COVID 19 changes.
Psychiatric diagnosis excludes substance use disorder diagnosis.
SUD, Substance use disorder (other than opioid use disorder diagnosis) SUD: Substance use disorder (other than opioid use disorder diagnosis).
Table 3 shows outcomes in any drug positive (not including cannabis) urine drug screens under same 3 models. Without controlling for any other covariates, the changes made to clinic guidelines during COVID-19 was not a significant predictor of opiate positivity. When controlling for age, comorbid substance use diagnosis, comorbid psychiatric disorder diagnosis, and percent-reduction in visit frequency, changes made to clinic guidelines during COVID-19 were not significant predictors of opiate positivity. Number of years in treatment was a statistically significant predictor in model 2 and 3.
Table 3.
Any drug positive urine drug screens controlling for covariates.
| Model 1 |
Model 2 |
Model 3 |
|
|---|---|---|---|
| Any Drug Positive UDS (Yes/No) | Any Drug Positive UDS (Yes/No) | Any Drug Positive UDS (Yes/No) | |
| Any Drug Positive UDS (Yes/No) |
|||
| Period 1 | |||
| Period 2 | 0.57 [0.24, 1.36] | 0.61 [0.25, 1.48] | 0.61 [0.25, 1.48] |
| Years in Treatment | 0.86** [0.78, 0.95] | 0.86** [0.77, 0.95] | |
| Age (years) | 0.98 [0.87, 1.09] | ||
| Other SUDs Diagnosis = 0 | |||
| Presence of Other SUDs Diagnosis = 1 | 0.53 [0.06, 4.32] | ||
| No other psychiatric diagnosis = 0 | |||
| Presence of one or more psychiatric diagnosis = 1 | 3.96 [0.41, 38.62] | ||
| % Reduction in visit frequency | 1.20 [0.05, 27.59] | ||
| / | |||
| lnsig2u | 19.86*** [8.12, 48.60] | 17.79*** [7.49, 42.22] | 17.75*** [7.49, 42.07] |
| Observations | 258 | 250 | 250 |
| R 2 | |||
| Adjusted R2 | |||
| N_pop | |||
| N_sub | |||
| F |
Exponentiated coefficients; ci in brackets.
P < .05. **P < .01. ***P < .001.
Model 1: Controlled only for changes made to clinic guidelines during period 1 and 2 Model 2: Controlled for years in treatment.
Model 3: Controlled for age, comorbid substance use diagnosis, comorbid psychiatric disorder diagnosis, percent-reduction in visit frequency, years in treatment, and changes made during period 1 and period 2.
UDS, urine drug screen.
Period 1: control time period.
Period 2: during COVID 19 changes.
Psychiatric diagnosis excludes substance use disorder diagnosis.
SUD, Substance use disorder (other than opioid use disorder diagnosis) SUD: Substance use disorder (other than opioid use disorder diagnosis).
Discussion
Emerging evidence has demonstrated the importance of protecting individuals with OUD from COVID 19, with studies showing an increased risk of hospitalization or death in patients with SUDs in general and OUD in particular.16–18 Furthermore, experiences of patients and providers during past disasters illustrates the importance of studying best MMT practices during states of emergency. In one survey of MMTs following the 9/11 terrorist attacks in New York City, personnel from multiple clinics reported disruptions in regular operation posed by street closures, public transit changes, and the overall climate of fear in the city; due to these factors, these clinics reported decreased patient retention in the aftermath of the attacks. 19 In a survey of New Yorkers who use substances conducted following Hurricane Sandy, 35 of 149 (22.9%) individuals enrolled in MMT prior to the disaster reported requiring unprescribed opioid substances to avoid withdrawal in the wake of the storm due to an insufficient supply of take-home medication. 20 Alongside the present pandemic, these episodes illustrate the range of circumstances under which judicious increases in take-home methadone may be vital to maintaining continuity of care in MMT.
Limitations of our study include that some nuance was lost when dichotomizing the outcome variables. Since multiple tests were administered to each participant both before and after COVID-19 regulations were put in place, the subtle difference in drug screen outcomes were not fully appreciated. This study also had a limited sample size which may have precluded the possibility of picking up on the true relationship of changes made to clinic guidelines during COVID-19 and subsequent methadone administration rules and any of the outcome variables. This study also takes place at a Veterans Health Administration Hospital (VHA) which skews to a predominately older male patient population. The findings therefore cannot be generalized to all MMT programs. Additionally, we do not take a stratified look at the outcome differences between outcomes within the low-risk, moderate or high-risk clinic patients. However, given the size of the sample studied here and the overall aims of the study, it is unlikely that stratification by risk level would have yielded meaningful outcome differences.
The results of the study illustrate the relative safety of the changes made at this particular MMT during the pandemic as there were no statistically significant differences between overdose rates and new infectious diseases. Furthermore, there was no statistically significant changes in urine drug screen outcomes for non-prescribed opiates, methadone or any illicit drug (outside of cannabis), which in this study indicated adherence to methadone treatment and minimal change in illicit substance use during Period 1 and Period 2. Of note, the patient’s in care at the MMT program studied here, had an average retention of 4.1 years. The length of retention at the start of period 1 suggests overall stability in the sample, and may contribute to the lack of significant findings between these 2 time periods. The outcomes of this study cannot be more broadly defined as each MMT in the country during this period came up with their own scheduling changes for patients, which may have differed from the scheduling changes made at this particular MMT. Further research is required to determine whether reduced restrictions can be broadly applied to non-pandemic, non-disaster, times. As noted earlier, between the creation of MMT and the pandemic there has not been any controlled studies examining more relaxed prescribing practices of methadone for OUD. Our study does not answer the question of the safety of relaxed methadone prescribing outside of circumstances such as COVID-19, with possible applications during other disasters. However, our study does beg the question as to whether the pre-COVID SAMSHA requirements were generalized too broadly as methadone clinics throughout the country serve patients of various demographic backgrounds and diverse treatment needs.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All the authors have made substantive contributions to the article and assume full responsibility for its content; and all those who have made substantive contributions to the article have been named as authors.
ORCID iD: Pantea Farahmand  https://orcid.org/0000-0002-2105-7159
https://orcid.org/0000-0002-2105-7159
Data Access: Data, samples or models can be accessed by contacting the writers of this paper.
References
- 1. Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health [press release]. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration 2020. [Google Scholar]
- 2. Degenhardt L, Bucello C, Mathers B, et al. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction (Abingdon, England). 2011;106(1):32-51. [DOI] [PubMed] [Google Scholar]
- 3. Hser Y-I, Evans E, Grella C, Ling W, Anglin D. Long-term course of opioid addiction. Harvard Rev Psychiatry. 2015;23(2):76-89. [DOI] [PubMed] [Google Scholar]
- 4. Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3):CD002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ (Clinical research ed). 2017;357:j1550-j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Substance Abuse Mental Health Services Administration. FAQs: Provision of methadone and buprenorphine for the treatment of Opioid Use Disorder in the COVID-19 emergency. 2020. Accessed May 19, 2020. https://www.samhsa.gov/sites/default/files/faqs-for-oud-prescribing-and-dispensing.pdf
- 7. Wilder-Smith A, Freedman DO. Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J Travel Med. 2020;27(2):taaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Novick DM, Salsitz EA, Joseph H, Kreek MJ. Methadone medical maintenance: an early 21st-century perspective. J Addict Dis. 2015;34(2-3):226-237. [DOI] [PubMed] [Google Scholar]
- 9. Saulle R, Vecchi S, Gowing L. Supervised dosing with a long-acting opioid medication in the management of opioid dependence. Cochrane Database Syst Rev. 2017;4(4):CD011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stitzer ML, Iguchi MY, Felch LJ. Contingent take-home incentive: effects on drug use of methadone maintenance patients. J Consult Clin Psychol. 1992;60(6):927-934. [DOI] [PubMed] [Google Scholar]
- 11. Stitzer M, Bigelow G, Lawrence C, Cohen J, D’Lugoff B, Hawthorne J. Medication take-home as a reinforcer in a methadone maintenance program. Addict Behav. 1977;2(1):9-14. [DOI] [PubMed] [Google Scholar]
- 12. Gerra G, Saenz E, Busse A, et al. Supervised daily consumption, contingent take-home incentive and non-contingent take-home in methadone maintenance. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(2):483-489. [DOI] [PubMed] [Google Scholar]
- 13. COVID-19 OTP Guidance and Frequently Asked Questions [press release], 2020. [Google Scholar]
- 14. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang QQ, Kaelber DC, Xu R, Volkow ND. COVID-19 risk and outcomes in patients with substance use disorders: analyses from electronic health records in the United States. Mol psychiatry. 2020;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baillargeon J, Polychronopoulou E, Kuo YF, Raji MA. The impact of substance use disorder on COVID-19 outcomes. Psychiatr Serv. 2021;72(5):578-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allen B, El Shahawy O, Rogers ES, Hochman S, Khan MR, Krawczyk N. Association of substance use disorders and drug overdose with adverse COVID-19 outcomes in New York City: January–October 2020. J Public Health. 2020;43(3):462-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frank B, Dewart T, Schmeidler J, Demirjian A. The impact of 9/11 on New York City’s substance abuse treatment programs: a study of program administrators. J Addict Dis. 2006;25(1):5-14. [DOI] [PubMed] [Google Scholar]
- 20. Pouget ER, Sandoval M, Nikolopoulos GK, Friedman SR. Immediate impact of hurricane sandy on people who inject drugs in New York City. Subst Use Misuse. 2015;50(7):878-884. [DOI] [PMC free article] [PubMed] [Google Scholar]