Abstract
Melanomas harboring an activating BRAFV600 mutation account for 50% of all advanced melanomas. The approval of BRAF-targeted therapy revolutionized treatment of these patients with achievement of impressive responses. However, development of resistance to these drugs is a significant problem, and as such, duration of response remains low, with median progression free survival of around 11–15 months. Immune checkpoint blockers exploit the immune system to eradicate cancer and can produce durable disease control that results in long-term, treatment-free survival in some patients. These drugs have shown very impressive survival in patients with BRAF-mutated melanoma. Thus, there is a need to continue to utilize emerging data to achieve long-term disease control for patients with advanced melanoma. Combining targeted therapy with immune therapy may be one possible way to achieve this goal. In this review, the mechanisms of action of these two pathways, including the mechanistic basis of this combination, are summarized, along with results of completed and ongoing trials in triple therapy.
Keywords: BRAF mutation, checkpoint inhibitor, combination immunotherapy, melanoma, targeted therapy
Introduction
Melanoma is a malignant tumor that arises from melanocytes and accounts for the most skin cancer related deaths, making it the deadliest cutaneous malignancy. 1 The incidence of melanoma has been continuously rising over the last decade. 2 About 50% of patients with cutaneous melanoma have tumors that are positive for BRAFV600 mutations. 3 During the last decade, the overall survival (OS) and progression free survival (PFS) of patients with metastatic melanoma have improved drastically with the approval of new effective signal transduction–targeted therapies (BRAF inhibitor (BRAFi) and MEK inhibitor (MEKi)) and immune checkpoint blockers (ICBs) (anti-CTLA-4, anti-PD-L1, and anti-PD-1 antibodies). BRAF targeting agents and immune checkpoint blockade have managed to achieve 5-year survival rates in advanced melanoma, of over 30%.4,5
BRAF mutation in melanoma
The mitogen-activated protein kinase (MAPK)/extracellular-signal-regulated kinase (ERK) pathway is the most commonly mutated pathway in melanoma, 6 leading to activation of MAPK/ERK signaling through gain of function mutation in BRAF. 7 Amino acid substitution at V600, which leads to a constitutive activation of BRAF, is seen in majority of cases. 8 BRAFi and MEKi impair the signal transduction pathways that regulate proliferation and survival of melanoma cells. These inhibitors act fast, often within days, and achieve high tumor response rates, with overall response rate (ORR) of ~75%, in patients harboring an activating mutation in the BRAF oncogene. 5 However, serious adverse events (AEs) have been seen in up to 71% patients on long-term follow-up. All grade AEs including pyrexia (69%), chills (60%), fatigue (58%), diarrhea (49%), anemia (22%), and rash (33%), requiring dose reductions, are seen in up to two-thirds of patients. 5 Cutaneous squamous cell carcinoma and cardiomyopathy are also seen. At the same time, despite impressive initial response rates, long-term follow-up studies show a PFS of only 11–15 months and 5-year survival rates of approximately 30%.5,9 Resistance to BRAFi, because of reactivation of MAPK pathway in 70–80%, is the leading cause of loss of response.
Immune checkpoint blockade in melanoma
Immune checkpoint blockade works by directing the attention of the immune system against the cancer to actively kill the tumor cells. ICBs block the programmed death 1/ programmed death ligand 1 (PD-1/PDL-1) axis or the CTLA-4 pathway. PD-1/PDL-1 blockers work by blocking the PD-1 signaling axis, allowing for restoration of activity of exhausted CD8 effector T-cells, thus reversing dysfunctional antitumor T-cell states. 10 These drugs induce durable antitumor responses in approximately 50% of patients. 11 CTLA-4 blockade results in unrestrained CD28-mediated positive co-stimulation through B7-1 and B7-2 ligands. 12 When compared to BRAF-targeted therapy, ICBs have lower tumor response rates. For example, in unresectable and metastatic melanoma, nivolumab alone or in combination with ipilimumab achieved response rates of 44% and 58%, respectively. 11 However, in patients with tumor responses, ICBs have delivered more durable responses. The best survival outcomes in melanoma have been shown with the combination of nivolumab and ipilimumab, with 6.5 years of median follow-up, median PFS was 11.5 months, median OS of 72.1 months, and a 6.5-year survival rate of 49%.13,14
BRAF-mutated melanoma and immunotherapy
Immune checkpoint blockade with anti-PD-1 alone or in combination with CTLA-4 blockers has demonstrated impressive tumor responses, specifically in BRAFV600-mutated melanoma. Patients with BRAFV600-mutated tumors who had received one line of therapy (including BRAF-directed therapy), when subsequently treated with pembrolizumab, demonstrated a PFS of 8.4 months and median OS of 32.7 months with a 5-year survival of 38.7%. 15 Subset analysis demonstrated that patients with BRAF-mutant tumors when treated with first-line anti-PD-1 therapy had a better ORR (47%) and OS (median OS not reached at 5-year follow-up), while patients who had received prior BRAFi/MEKi therapy had ORR of 32% and a median OS of 20.4 months. 15 The combination of nivolumab and ipilimumab has demonstrated a median PFS of 16.8 months, with a median OS of 60 months and 5-year survival of 60% in BRAF-mutated advanced melanoma. 11
Whether first-line BRAF/MEKi therapy or ICB would be preferred in patients with BRAF-mutant melanoma has also been an active area of research. Multiple mechanisms have been described leading to development of resistance to BRAFi/MEKi, and it appears that cross-resistance to ICBs may exist. 16 Retrospective analyses have shown that BRAF-mutated melanoma treated with anti-PD-1 therapy in the second line (after progression on BRAF/MEKi therapy) had a much lower response with ORR of 18% and a median OS of 8.4 months than seen in treatment-naïve patients with anti-PD-1. 17 The randomized phase 3 DREAMseq trial evaluating sequential therapy in advanced melanoma also clearly demonstrated a survival advantage in patients receiving ICB (ipilimumab + nivolumab) first followed by BRAFi/MEKi (dabrafenib and trametinib) at progression than patients who received BRAFi/MEKi first and ICB at progression. OS at 2 years was 72% in the first group versus 52% in the second group. 18 This remains an area of intense research; however, initial data strongly indicate that ICB first followed by BRAFi/MEKi leads to better survival in advanced BRAF-mutant melanoma. However, what is not yet known is what role there may be for a triplet combination of BRAF/MEKi with anti-PD-1 immunotherapy.
BRAFi- and MEKi-mediated immunomodulation leads to improved antitumor activity of immunotherapy
The constitutive upregulation of the MAPK signaling pathway by a BRAF mutation can lead to a protumorigenic microenvironment with an ineffective antitumor immune response, through a complex and myriad set of interconnected mechanisms.
BRAF-mutant tumors show a reduced expression of IFNγ, TNFα, and IL-2 as well as CD40L on CD4+ tumor-infiltrating lymphocytes (TILs), leading to the development of immunologically ‘cold’ tumors, enabling evasion of effective T-cell responses. 19 These tumors have also been shown to induce accumulation of regulatory T-cells (Tregs) and myeloid-derived suppressor cells (MDSCs) in BRAFV600E/PTEN-driven murine model of melanoma. 19 In vitro BRAFV600E melanoma cells have been shown to suppress maturation of dendritic cells and consequently the production of IL-12 and TNFα,20,21 as well as an increased expression of immunomodulatory cytokines like IL-6 and IL-10, which promote recruitment of immunosuppressive cells such as MDSCs and Tregs to the tumor microenvironment. 21 Downregulation of human leukocyte antigens (HLA) class 1 molecules on cell surface of melanoma cells, through internalization and intracellular sequestration, leading to attenuation of antigen presentation and CD8+ T-cell recognition has also been demonstrated. 22
Inhibition of the upregulated BRAF and MEK pathway attenuates and reverses the protumor microenvironment and enhances immune stimulatory signaling. BRAF inhibition leads to increased CD40L and IFNγ expression of intratumoral CD4+ TILs and reduces Tregs and MDSCs in murine models. 19 BRAFi reverse downregulation of HLA class I and upregulated CD70 molecules, leading to a reduction in immunosuppressive markers including IL-1 A, IL-6, IL-8, IL-10, and vascular endothelial growth factor (VEGF), thereby counteracting tumor-induced immune escape mechanisms.21,23 BRAFi ± MEKi treatment is associated with an increased CD8+ T-cell infiltration into the tumor microenvironment24 –26 and impaired T-cell receptor (TCR)–mediated apoptosis of tumor antigen–specific T-cells, producing improved antitumor T-cell responses.27,28 BRAFi ± MEKi drugs also increase expression of the melanoma differentiation antigens MART-1 (Melanoma Antigen Recognized by T-cells), gp100 (Glycoprotein 100), TYRP1 (tyrosinase related protein 1), and DCT (dopachrome tautomerase), leading to an enhanced recognition of the tumor by T-cells.24,29
Synergistic effects of combining MAPK inhibitors with immune checkpoint inhibitors (preclinical setting)
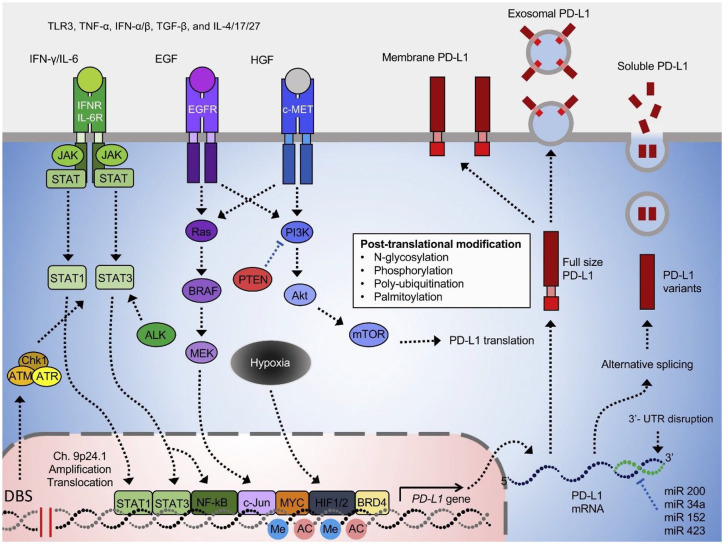
Mouse model studies have provided the initial background data for subsequent human clinical trials of MAPK inhibitors and ICBs. A combination of BRAFi with ICBs have demonstrated increased number of TILs, decreased tumor volume, enhanced response and significantly prolonged survival as compared with BRAFi alone in BRAFV600E immunocompetent mice. 30 Similarly, in mouse model of syngeneic BRAFV600E-driven melanoma, combination of BRAFi/MEKi with adoptive cell transfer showed complete tumor regression, increased T-cell infiltration into tumors, improved in vivo cytotoxicity, and global immune–related gene upregulation. 31 BRAFi therapy was also shown to increase the expression of PD-1 and TIM-3 on infiltrating T-cells, and PD-L1 and PD-L2 on tumor cells, suggesting enhanced response to tumor control with triple therapy. 30 With prolonged use of BRAFi/MEKi, melanoma cells tend to upregulate PDL-2 and tumor-infiltrating immune cells with loss of CD8+ T-cells and decrease in the ratio of CD8+ T-cells to Tregs,24,27,30,32 leading to decrease in tumor immunogenicity. Over time, BRAFi/MEKi therapy increases PDL-1 expression in PDL-1 negative tumors. 33 This can be utilized to determine the benefit of concurrent versus sequential therapy with BRAFi/MEKi and ICBs (Figure 1).
Figure 1.
The regulatory mechanism of PDL-1 expression shows possible synergistic mechanism between BRAFi/MEKi and ICB therapy. 34
BRAFi, BRAF inhibitor; MEKi, MEK inhibitor; ICB, immune checkpoint blocker.
Trials combining BRAFi/MEKi and immunotherapy
Phase 1 studies
One of the first phase 1 studies of this combination enrolled 15 patients with BRAFV600-mutated metastatic melanoma for treatment with dabrafenib, trametinib, and pembrolizumab (NCT02130466). 35 In all, 13 of the 15 patients were treated as first-line, while 2 had received adjuvant therapy prior. The combination had an ORR of 73% (similar to BRAFi/MEKi therapy alone), 5 and at a median follow-up of 27 months had a continued response in 40% of patients. However, this was associated with high toxicity profile, albeit similar to BRAFi/MEKi, 5 with 73% patients experiencing a grade 3/4 toxicity (most common being elevation of liver function tests and pyrexia). This study showed that this triple-combined therapy may benefit a subset of patients with BRAFV600-mutated metastatic melanoma by increasing the frequency of long-lasting antitumor responses. 35
Another phase 1b study (ClinicalTrials.gov, number NCT01656642) evaluated the safety and antitumor activity of combining atezolizumab (anti-PD-L1) with vemurafenib and cobimetinib in patients with BRAFV600-mutated metastatic melanoma in the first-line setting. The 28-day run-in phase with cobimetinib and vemurafenib alone showed an increase in proliferating CD4+ T-helper cells but not an increase in T-regulatory cells. The triplet regimen also had substantial toxicity, with grade 3/4 toxicity rate of 67%. The ORR of 71.8% was higher than expected, and about 39% patients had ongoing response at 29.9-month follow-up. 36
Phase 2/3 studies in first-line metastatic setting
Keynote-022 was a randomized phase 2 trial where treatment-naïve patients received dabrafenib and trametinib together with pembrolizumab (triplet; n = 60) or placebo (doublet; n = 60). Primary endpoint of median PFS was 16.9 months with triplet and 10.7 months with doublet therapy (hazard ratio (HR), 0.53; 95% confidence interval (CI), 0.34–0.83). Two-year PFS was 41% for triplet and 16.3% for doublet therapy, while median duration of response (DOR) was 25.1 months and 12.1 months, respectively. Median OS was not reached with triplet and was 26.3 months with doublet (HR, 0.64; 95% CI, 0.38–1.06). With triplet and doublet, respectively, OS at 24 months was 63.0% and 51.7%, respectively. Grade 3–5 treatment-related adverse events (TRAEs) occurred in 58% patients receiving triplet and 25% receiving doublet, while serious TRAEs were seen in 40% and 23%, respectively. One person died from pneumonitis in the triplet arm. AE requiring dose interruption were seen in 83% in triplet arm (most commonly fever and diarrhea) and 68% in doublet arm (most commonly fever and neutropenia).37,38
One of the first randomized phase 3 clinical trials (IMspire150) in patients with BRAFV600 mutant advanced melanoma randomized 514 patients to vemurafenib and cobimetinib with or without atezolizumab. Like the phase 1 study, this trial also had a 28-day run-in where patients in the triplet arm only received vemurafenib and cobimetinib. At a median follow-up of 18.9 months, the triple combination resulted in a significant improvement in the progression-free survival (15.1 months) compared with placebo-controlled double therapy with vemurafenib and cobimetinib (10.6 months; HR, 0.78; 95% CI, 0.63–0.97; p = .025). The triple therapy did not increase the objective response rate. TRAEs were seen in 79% and 73%, respectively. 39 The significant improvement in the primary endpoint of PFS in this trial led to the U.S. Food and Drug Administration (FDA) approval of this regimen in metastatic melanoma in July 2020.
The phase 3 COMBI-i trial consisted of first-line spartalizumab (PD-1 blocker), dabrafenib, and trametinib versus dabrafenib and trametinib with placebo in patients advanced BRAFV600–mutant melanoma (NCT02967692). 40 However, unlike IMspire150 trial, the randomized portion of COMBI-i did not show a significantly improved PFS over dabrafenib + trametinib (median PFS, 16.2 months versus 12.0 months, respectively; HR, 0.82; 95% CI, 0.65–1.03; p = 0.042 (one-sided)). PFS at 24 months were 44% versus 36% in the triplet and doublet arms, respectively. Median OS was not reached across treatment arms. The objective response rates were again similar at 69% in the spartalizumab, dabrafenib, and trametinib arm versus 64% in the doublet arm; median DOR was not reached versus 20.7 months, respectively. TRAEs grade ⩾ 3 occurred in 55% versus 33% of patients treated with the triplet versus the doublet. 40 Given the negative results of this trial, this regimen was not considered for FDA approval.
With regard to the conflicting PFS results of these phase 3 trials, the differences in the drugs involved, the statistical designs and patient characteristics in the studies might explain the differences. Furthermore, these trials were designed in era when BRAFi/MEKi therapy was thought to be a reasonable control arm to compare with a triplet regimen, but these trials do not answer the question of the regimens compared with anti-PD-1 or PD-1 + CTLA-4 immunotherapy.
A biomarker analysis in the COMBI-i study was conducted to determine any subsets of patients who derived more benefit from the triplet regimen versus dabrafenib and trametinib alone. Patients with higher lactate dehydrogenase (LDH) levels, more than three sites of metastasis, and bulkier disease derived more benefit from the triplet regimen. 41 This is in line with prior studies that have shown that BRAFi/MEKi are most effective in patients with normal LDH and less than three metastatic sites. 41 Interestingly, patients with melanoma with higher tumor mutational burden (TMB ⩾ 10 mut/Mb) also derived more benefit with the COMBI-i triplet regimen; in high TMB patients, median PFS was 23.9 versus 11.8 months when treated with the triplet versus doublet regimen compared with 12.8 versus 12 months for low TMB patients. Further biomarker analyses are ongoing to examine what patients may benefit more from triplet therapy.
Triple therapy in other settings
Central nervous system (CNS) metastatic disease
Brain metastases is a devastating complication of melanoma and is associated with poor outcomes and survival. Dabrafenib and trametinib have shown intracranial response (ICR) of 58% in untreated BRAF-mutant melanoma brain metastases in the COMBI-MB study, with median DOR of 6.5 months. 42 ICBs in this setting have shown more encouraging results with longer ICRs in patients with asymptomatic brain metastases. In the Checkmate 204 trial, the combination of ipilimumab and nivolumab has shown an ICR of 58%, 43 in patients with asymptomatic brain metastases, while median DOR and PFS were not yet reached after a median follow-up of 20.6 months. 44
However, the large triple therapy trials to date have excluded this patient population. One of the few triple therapy studies to include this patient population was a single arm phase II study (TRIDeNT; NCT02910700) in BRAF-mutated patients refractory to anti-PD1 therapy. Patients received nivolumab, dabrafenib, and trametinib. The ICR in a group of seven patients with asymptomatic or mildly symptomatic brain metastases was 57%, including CR rate of 28%, with a median PFS of 8 months. 45 Of note, this trial is also exploring the triplet regimen in the anti-PD-1 refractory melanoma setting; in the 17 anti-PD-1 refractory patients, ORR was 88%, with median PFS of 8.2 months.
Several larger triplet trials are ongoing in melanoma brain metastases. The first study in the specific population is a phase 2 study evaluating atezolizumab, cobimetinib, and vemurafenib in patients with BRAF-mutant melanoma with brain metastases; that trial includes patients with symptomatic brain metastases requiring upto 8 mg dexamethasone per day (NCT03625141). 46 Another large randomized phase 2 study, SWOG S2000, is comparing encorafenib, binimetinib, and nivolumab versus ipilimumab and nivolumab in BRAF-mutant melanoma with brain metastases (NCT04511013). This trial also includes and stratifies for patients with symptomatic brain metastases requiring up to 8 mg dexamethasone.
Neoadjuvant application
In several studies, a strong association of pathological response (pCR) with recurrence-free survival (RFS) and OS, has been observed, with neoadjuvant therapy in melanoma. 47 Pooled data suggest that pCR with neoadjuvant immunotherapy produced better RFS and OS than pCR with targeted therapy. 47 Thus, triple therapy in the neoadjuvant setting assumes importance with ongoing and planned trials of triplet therapy in this setting, such as the NeoTrio trial exploring dabrafenib, trametinib, and pembrolizumab neoadjuvantly in stage III resectable melanoma (NCT02858921). The Neo-VC trial (NCT 02303951) using vemurafenib, cobimetinib, and atezolizumab in the neoadjuvant setting in stage 3c/4 melanoma was, however, terminated because of poor accrual. The NEO-TIM study (NCT04722575) is another interesting study where BRAF-positive patients will receive neoadjuvant vemurafenib and cobimetinib followed by adjuvant atezolizumab (arm A) and neoadjuvant vemurafenib, cobimetinib, and atezolizumab followed by adjuvant atezolizumab (arm B).
Discussion
Patients with advanced BRAFV600-mutant melanoma treated with BRAFi and MEKi therapies in either first or subsequent line therapy have shown excellent initial tumor response rates, but these responses tend to not be durable. On the other hand, ICB therapies have lower tumor response rates, with longer lasting responses, if used first-line; tumor response and survival rates to ICB drop in patients with BRAF-mutant melanoma if given after progression on prior BRAFi/MEKi therapy. Initial data from studies exploring optimal sequencing suggest that upfront ICB may lead to improved survival compared with upfront BRAF/MEKi therapy. However, triplet therapy has not yet been compared with upfront ICB.
Triplet therapy with BRAFi/MEKi and immune checkpoint inhibitor therapy combined has the potential to significantly enhance duration of treatment response and survival in patients with advanced BRAF-mutant melanoma. The strong immune-modulating effects of BRAF and MEK inhibition in the form of enhanced antitumor immunity and increased expression of PDL-1 can synergistically enhance the effect of immune checkpoint inhibitors. At the same time, the concern for overlap in resistance pathways and development of cross-reactivity makes the rationale for triplet therapy, as compared with sequential therapy, more compelling.
However, as the PFS results from the IMspire150 study were positive and the COMBI-i study were negative, results from phase 3 trials to date have not yet definitely answered the question of which patients should be considered for triplet therapy. The trials to date have shown that there is a delayed PFS benefit that starts 6–8 months after initiation of triplet therapy, as compared with BRAFi/MEKi alone. Whether this will be clinically significant, especially given the increased toxicity rates and overlapping toxicity profile of these regimens, remains to be seen.
As such, there has not yet been widespread use of the FDA-approved vemurafenib and cobimetinib with atezolizumab triplet regimen; in addition to its toxicity rates, the complicated dosing regimen with a 28-day doublet run in period and dose adjustment of vemurafenib during triplet portion may also be a barrier to use for some patients. In addition, the role of triplet regimens in the front-line metastatic setting as compared to treating with upfront ICB such as nivolumab + ipilimumab is also not yet clear. Longer follow-up and more information about median DOR and PFS from the triplet trials may help to define where these regimens may fit into guidelines for management of melanoma.
Biomarker analyses are ongoing to identify subpopulations of patients who may derive increased benefit from triplet therapy, with initial analyses suggesting patients with increased TMB, higher LDH, three or more sites of metastasis and bulkier disease may have increased benefit. In clinical practice, patients with bulky and particularly symptomatic metastases (painful or bleeding tumors, for example) are more likely to be started on upfront BRAFi/MEKi therapy, with many clinicians subsequently adding in anti-PD-1 therapy, leading to a de facto triplet regimen for a time period, before subsequent stopping of BRAF/MEKi. This approach is also commonly utilized in patients who have received upfront BRAFi/MEKi with subsequent progression of their melanoma. These patients frequently have widespread metastatic progression, and abruptly stopping BRAF-targeted therapy before starting ICB often leads to a faster rate of tumor growth that may become uncontrolled before ICB is able to start working. As such, in order to try to avoid this, in clinical practice, BRAFi/MEKi may be continued for a period of time after starting ICB.
Ongoing trials targeting specific cohorts of patients such as those with melanoma brain metastases (including symptomatic disease), or anti-PD-1 refractory patients, or neoadjuvant use, are ongoing. Results from these studies may further help to define the role of triplet therapy. There are additional questions that may also require exploration such as whether there are differences in efficacy between the different triplet regimens, issues with treatment compliance, and financial considerations and cost.
Acknowledgments
Figure 1 is reprinted from Cha et al. 34 with permission from Elsevier.
Footnotes
Author contributions: Shahla Bari: Writing – original draft.
Jameel Muzaffar: Writing – original draft.
Zeynep Eroglu: Writing – original draft and writing – review and editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Disclosures for ZE: research funding from Novartis and Pfizer. Advisory boards are Novartis, Genentech, OncoSec, Eisai, Natera and Regeneron.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Contributor Information
Shahla Bari, Department of Cutaneous Oncology, Moffitt Cancer Center, Tampa, FL, USA.
Jameel Muzaffar, Department of Head and Neck Oncology, Moffitt Cancer Center, Tampa, FL, USA.
Zeynep Eroglu, Department of Cutaneous Oncology, Moffitt Cancer Center, 12902 USF Magnolia Drive, Tampa, FL 33612, USA.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Whiteman DC, Green AC, Olsen CM. The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol 2016; 136: 1161–1171. [DOI] [PubMed] [Google Scholar]
- 3. Kakadia S, Yarlagadda N, Awad R, et al. Mechanisms of resistance to BRAF and MEK inhibitors and clinical update of US Food and Drug Administration-approved targeted therapy in advanced melanoma. Onco Targets Ther 2018; 11: 7095–7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol 2019; 30: 582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Long GV, Eroglu Z, Infante J, et al. Long-term outcomes in patients with BRAF V600-mutant metastatic melanoma who received dabrafenib combined with trametinib. J Clin Oncol 2018; 36: 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am 2009; 23: 529–545, ix. [DOI] [PubMed] [Google Scholar]
- 7. Satyamoorthy K, Li G, Gerrero MR, et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res 2003; 63: 756–759. [PubMed] [Google Scholar]
- 8. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002; 417: 949–954. [DOI] [PubMed] [Google Scholar]
- 9. Robert C, Grob JJ, Stroyakovskiy D, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 2019; 381: 626–636. [DOI] [PubMed] [Google Scholar]
- 10. Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515: 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramagopal UA, Liu W, Garrett-Thomson SC, et al. Structural basis for cancer immunotherapy by the first-in-class checkpoint inhibitor ipilimumab. Proc Natl Acad Sci USA 2017; 114: E4223–E4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017; 377: 1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. CheckMate 067: 6.5-year outcomes in patients (pts) with advanced melanoma. J Clin Oncol 2021; 39(Suppl. 15): 9506–9506. [Google Scholar]
- 15. Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 2019; 20: 1239–1251. [DOI] [PubMed] [Google Scholar]
- 16. Hugo W, Shi H, Sun L, et al. Non-genomic and immune evolution of melanoma acquiring MAPKi resistance. Cell 2015; 162: 1271–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kreft S, Gesierich A, Eigentler T, et al. Efficacy of PD-1-based immunotherapy after radiologic progression on targeted therapy in stage IV melanoma. Eur J Cancer 2019; 116: 207–215. [DOI] [PubMed] [Google Scholar]
- 18. Atkins MB, Lee SJ, Chmielowski B, et al. DREAMseq: a phase III trial – ECOG-ACRIN EA6134. In: ASCO plenary series, abstract 356154, November 2021, https://ascopubs.org/doi/abs/10.1200/JCO.2021.39.36_suppl.356154
- 19. Ho PC, Meeth KM, Tsui YC, et al. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNγ. Cancer Res 2014; 74: 3205–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ott PA, Henry T, Baranda SJ, et al. Inhibition of both BRAF and MEK in BRAF(V600E) mutant melanoma restores compromised dendritic cell (DC) function while having differential direct effects on DC properties. Cancer Immunol Immunother 2013; 62: 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sumimoto H, Imabayashi F, Iwata T, et al. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med 2006; 203: 1651–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bradley SD, Chen Z, Melendez B, et al. BRAFV600E co-opts a conserved MHC class I internalization pathway to diminish antigen presentation and CD8+ T-cell recognition of melanoma. Cancer Immunol Res 2015; 3: 602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whipple CA, Boni A, Fisher JL, et al. The mitogen-activated protein kinase pathway plays a critical role in regulating immunological properties of BRAF mutant cutaneous melanoma cells. Melanoma Res 2016; 26: 223–235. [DOI] [PubMed] [Google Scholar]
- 24. Frederick DT, Piris A, Cogdill AP, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res 2013; 19: 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu C, Peng W, Xu C, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res 2013; 19: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wilmott JS, Long GV, Howle JR, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res 2012; 18: 1386–1394. [DOI] [PubMed] [Google Scholar]
- 27. Song C, Piva M, Sun L, et al. Recurrent tumor cell-intrinsic and -extrinsic alterations during MAPKi-induced melanoma regression and early adaptation. Cancer Discov 2017; 7: 1248–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dushyanthen S, Teo ZL, Caramia F, et al. Agonist immunotherapy restores T cell function following MEK inhibition improving efficacy in breast cancer. Nat Commun 2017; 8: 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boni A, Cogdill AP, Dang P, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res 2010; 70: 5213–5219. [DOI] [PubMed] [Google Scholar]
- 30. Cooper ZA, Juneja VR, Sage PT, et al. Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade. Cancer Immunol Res 2014; 2: 643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu-Lieskovan S, Mok S, Homet Moreno B, et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci Transl Med 2015; 7: 279–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cooper ZA, Reuben A, Spencer CN, et al. Distinct clinical patterns and immune infiltrates are observed at time of progression on targeted therapy versus immune checkpoint blockade for melanoma. Oncoimmunology 2016; 5: e1136044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kakavand H, Wilmott JS, Menzies AM, et al. PD-L1 expression and tumor-infiltrating lymphocytes define different subsets of MAPK inhibitor-treated melanoma patients. Clin Cancer Res 2015; 21: 3140–3148. [DOI] [PubMed] [Google Scholar]
- 34. Cha JH, Chan LC, Li CW, et al. Mechanisms controlling PD-L1 expression in cancer. Mol Cell 2019; 76: 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ribas A, Lawrence D, Atkinson V, et al. Combined BRAF and MEK inhibition with PD-1 blockade immunotherapy in BRAF-mutant melanoma [Erratum in: Nat Med 2019; 25: 1319]. Nat Med 2019; 25: 936–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sullivan RJ, Hamid O, Gonzalez R, et al. Atezolizumab plus cobimetinib and vemurafenib in BRAF-mutated melanoma patients. Nat Med 2019; 25: 929–935. [DOI] [PubMed] [Google Scholar]
- 37. Ascierto PA, Ferrucci PF, Fisher R, et al. Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat Med 2019; 25: 941–946. [DOI] [PubMed] [Google Scholar]
- 38. Ferrucci PF, Di Giacomo AM, Del Vecchio M, et al. KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer 2020; 8: e001806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gutzmer R, Stroyakovskiy D, Gogas H, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomized, double-blind, placebo-controlled, phase 3 trial [Erratum in: Lancet 2020; 396: 466]. Lancet 2020; 395: 1835–1844. [DOI] [PubMed] [Google Scholar]
- 40. Nathan P, Dummer R, Long GV, et al. Spartalizumab plus dabrafenib and trametinib (Sparta-DabTram) in patients (pts) with previously untreated BRAF V600–mutant unresectable or metastatic melanoma: results from the randomized part 3 of the phase III COMBI-i trial. Ann Oncol 2020; 31(Suppl. 4): S1142–S1215. [Google Scholar]
- 41. Dummer R, Lebbé C, Atkinson V, et al. Combined PD-1, BRAF and MEK inhibition in advanced BRAF-mutant melanoma: safety run-in and biomarker cohorts of COMBI-i. Nat Med 2020; 26: 1557–1563. [DOI] [PubMed] [Google Scholar]
- 42. Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicenter, multicohort, open-label, phase 2 trial. Lancet Oncol 2017; 18: 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicenter randomized phase 2 study. Lancet Oncol 2018; 19: 672–681. [DOI] [PubMed] [Google Scholar]
- 44. Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med 2018; 379: 722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Burton EM, Amaria RN, Glitza IC, et al. Safety and efficacy of TRIplet combination of nivolumab (N) with dabrafenib (D) and trametinib (T) [TRIDeNT] in patients (pts) with BRAF-mutated metastatic melanoma (MM): a single center phase II study. Ann Oncol 2019; 30(Suppl. 5): V534–V535. [Google Scholar]
- 46. Queirolo P, de la Cruz Merino L, Abajo Guijarro AM, et al. A phase II study evaluating atezolizumab (A), cobimetinib (C), and vemurafenib (V) in patients (pts) with BRAF-mutant melanoma and central nervous system (CNS) metastases (mets). J Clin Oncol 2020; 38(Suppl. 15): TPS10081. [Google Scholar]
- 47. Menzies AM, Amaria RN, Rozeman EA, et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat Med 2021; 27: 301–309. [DOI] [PubMed] [Google Scholar]