Abstract
Objectives:
The aim of this study is to present the clinical, oncological, and functional results of locally aggressive benign bone tumors treated with extended intralesional curettage without the use of adjuvant in a tertiary orthopedic oncology center.
Method:
A total of 172 patients treated with surgical curettage and high-speed burrs for the diagnosis of aneurysmal bone cyst, giant cell tumor, osteoblastoma, chondroblastoma, and chondromyxoid fibroma were included in the study. Demographic, radiological, and clinical data of the patients were analyzed.
Results:
One-hundred seventy two patients (101 (59%) female and 71 (41%) male) with a mean age of 23 years (6–84). The mean follow-up period was 48 months (18–108). In the study, a total of 8 (4.6%) patients had postoperative complications, 17 (9.9%) patients had recurrence in the postoperative period. Diameter greater than 5 cm was found to be a risk factor for recurrence (p < 0.004). The probability of developing complications was found to be significantly higher in patients with recurrence (p < 0.001). There was no significant relationship between recurrence and age, tumor type, and tumor stage.
Conclusion:
Successful treatment results can be obtained with extended surgical curettage, high-speed burr, and cauterization without the use of chemical adjuvants in locally aggressive bone tumors.
Keywords: Treatment, local aggressive, bone tumor, curettage, recurrence
Introduction
Although locally aggressive bone tumors are generally benign in nature, with a very low incidence of metastasis, they exhibit a progressive clinical pattern. These tumors constitute approximately 10% of all bone tumors 1 and are divided into five subgroups: aneurysmal bone cyst (ABC), giant cell tumor (GCT), chondroblastoma, osteoblastoma, and chondromyxoid fibroma (CMF). These tumors display differing malignancy potential; therefore, treatments with low rates of recurrence are preferred.
The general approach to the treatment of locally aggressive benign bone tumors is extended intralesional curettage. Some authors suggest the application of various chemical agents during or after surgery to reduce recurrence rates. 2 Liquid nitrogen, alcohol, phenol, hydrogen peroxide, electrocauterization, argon, plasma coagulation, and iodine have been used as local chemical adjuvants in the eradication of these tumors. However, these agents have side effects (e.g. wound healing problems, nerve paralysis, tendon rupture, bleeding, infection, pathological fracture, and/or skin and muscle necrosis); some agents may have limited availability. Considering the similarities in terms of prognosis and cost-effectiveness, methods such as surgery, burring, and cauterization should be considered.
Although there have been previous studies on chemical adjuvant applications in the literature, data on patients treated with extended curettage without adjuvant are limited. The aim of this study is to present the clinical, oncological, and functional results of locally aggressive benign bone tumors treated with extended intralesional curettage without the use of adjuvant in a tertiary orthopedic oncology center.
Method
This was a retrospective study of patients treated in our institute between January 2007 and December 2015. Patients in this study underwent surgical treatment for the diagnosis of locally aggressive benign bone tumor (i.e. ABC, GCT, osteoblastoma, chondroblastoma, or CMF). In total, 264 locally aggressive bone tumors were evaluated; 218 patients hospitalized during the study period underwent surgery. Twelve patients with secondary tumors, 21 patients without follow-up, and 13 patients who underwent reoperation were excluded; thus, 172 patients were included in the study. Medical records, plain radiographs, computerized tomography (CT), and magnetic resonance imaging (MRI) scans were all reviewed. All biopsy and surgical curettage materials were reviewed and confirmed by the Department of Pathology of our University Hospital. The study protocol was approved by the ethics committee (09.2016.497). Written informed consent was obtained from all subjects before the study.
The inclusion criteria were patients newly diagnosed with locally aggressive benign bone tumors who had undergone treatment with wide surgical curettage and high-speed burring. All patients who attended our clinic were evaluated, without any restrictions. Patients were excluded if they met the following criteria: lack of medical information or radiographs; unclear tumor location on radiographs; inadequate radiographs; application of plaque screws; lesions <5 mm; existing bone tumor diagnosis; recurrent lesions, reoperation, or secondary tumors; receipt of a chemical adjuvant after curettage; and poor postoperative follow-up.
The following patient data were recorded retrospectively from orthopedic oncology, surgery, and pathology records, according to tumor type and clinical features: demographic and clinical data (e.g. age, sex, admission symptoms, tumor distribution, localization, volume and stage, preoperative embolization, and filling material applied after tumor curettage), recurrence distribution data (e.g. tumor type, diameter, stage, and presence of pathological fracture), and postoperative complications. In addition, radiological imaging findings (MRI and X-ray images), radiological staging, histopathological findings, previously applied treatment modalities, and surgical procedures were also examined. The relationship between the tumor diameters and recurrence rates was made based on prior literature 3 that showed a relationship between lesion diameter at the first examination and pathological fracture. Tumor staging was performed in accordance with the Enneking Staging system: Stage 1, Latent; Stage 2, Active; Stage 3, Aggressive. 4 All patients underwent preoperative plain radiography (anteroposterior/lateral); lesion diameter and volume were calculated using these radiographs. The following formulas were used to calculate the approximate volume according to the shape of the lesion: Cylindrical, ABC × 0.785 (π × A/2 × B/2 × C); Spherical, ABC × 0.52 (4/3 × π × A/2 × B/2 × C/2). 3
All surgeries for study patients were performed by the same surgeon. Preoperatively all patients underwent a closed biopsy procedure using a percutaneous Jamshidi needle for a definitive diagnosis. Intralesional extended curettage was the main surgical procedure. The technique of curettage was based on using curettes and high-speed burrs. Initially, a large window was opened on the bony cortex for Stage I and Stage II lesions. This window was enlarged to visualize the whole cavity and the gross lesion was curetted. At the next step, the inner cortical wall of the cavity was burred down with a high-speed burr device and then cauterized. This procedure was repeated several times. For Stage III lesions that already had a destroyed cortical wall, same procedure was performed after removal of tumor from the cavity and surrounding soft tissue. Liberal saline wash was used to remove tumor debris. All corners of the lesion were repeatedly curetted. To prevent damage to the epiphyseal plate, lesions located close to the epiphysis received additional attention. In lesions that crossed the epiphyseal line, burring was not applied to the adjacent edge; for such lesions, only curettage was performed. At the final step, the cavity was filled with allograft in 74 patients, cement (PMMA: polymethyl methacrylate) in 38 patients, and autograft in 15 patients.
During the postoperative period, all patients underwent immobilization to reduce complications. Passive and active range of motion (ROM) exercises were started immediately after surgery for lesions around the knee joint. For all lower extremity lesions, gradual weight-bearing was started at the immediate postoperative period. For the following 2 weeks, they were allowed to walk with a single crutch and full weight-bearing was allowed 2 months after surgery. For all upper extremity lesions, patients were given a sling for 1 week for pain relief, and passive ROM exercises were started. Active exercises were started 2 weeks later. Lifting heavy objects was prohibited for at least 2 months for all Grade II and III upper extremity lesions. In patients with a proximal femur location, a walking spica cast or long leg splints extending under the hip were applied.
Patients were followed up at 3-month intervals for the first year after surgery and at 6-month intervals for the second year after surgery. At each visit, patients were evaluated by radiography and physical examination. Bone healing was defined as opacification and cortical thickening on radiographs. Imaging methods were used for standardization. Radiologic evaluations were performed by two authors participating in the study. Patients with ⩾90% healing were considered to exhibit full recovery, while those with <90% were considered to exhibit partial recovery. 5 Furthermore, 80%–90% obliteration was defined as “significant partial healing” if cortical thickening had been achieved. Patients with recurrence were assessed by imaging studies. Extended intralesional curettage and grafting were performed in 15 of 17 patients with recurrence; block resection was performed in the remaining 2 patients. Clinical improvement and patient function were assessed by means of the Musculoskeletal Tumor Society (MSTS) Score system. 6 Patients completed the MSTS questionnaire at the last postoperative outpatient examination.
Statistical analysis
SPSS Statistics software, version 21 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Recurrence rates and parameters affecting recurrence were evaluated according to the diagnoses. Data are expressed as mean values, standard deviations, and percentages. Kaplan–Meier survival analysis was used to compare recurrence-free survival values. The chi-square test and Fisher’s exact test were used to compare recurrence rates among patients stratified according to age, diameter, tumor localization, and applied filling material; they were also used to compare recurrence and complications. p < 0.05 was considered indicative of statistical significance.
Results
Of the remaining 172 patients, the diagnoses were as follows: 88 (51.2%) with ABC, 51 (29.7%) with giant cell tumor, 19 (11%) with osteoblastoma, 7 (4.1%) with chondroblastoma, and 7 (4.1%) with CMF.
The mean follow-up period was 48 months (range = 18–108 months). The mean age of patients was 23 years (range = 6–84 years); their demographic and clinical characteristics and tumor distributions are shown in Table 1. Microfracture was detected in 55 (35.2%) of the patients who presented with complaints of pain and swelling in the tumor area. Mean lesion volume was 125.06 cm3 in patients with pathological fractures. Regarding tumor distribution, ABCs were in the proximal humerus, while giant cell tumors were in the distal femur. Figure 1 shows a patient diagnosed with a proximal femur located at ABC; Figure 2 shows a patient with a proximal tibia located at GCT. The majority of tumors were located in the lower limb bones in 91 (52.9%) patients. The mean diameter of tumor lesions was 6 cm (range = 1–18 cm) and the mean volume was 96 cm3 (range = 1.96–207.1 cm3). Of the 172 patients, 50% (86 patients) exhibited Stage 2 tumors and 50% (86 patients) exhibited Stage 3 tumors. Spaces formed after curettage were filled with allograft in 74 (43%) patients, autograft in 15 (8.7%) patients, and cement in 38 (22%) patients. Embolization was performed in 12 (6.9%) patients before surgery to reduce bleeding. Locations of lesions in patients undergoing embolization were the pelvis in 7 (58.3%) patients, femur in 3 (25%) patients, and humerus in 2 (16.6%) patients.
Table 1.
Demographic, clinical, and tumor distribution of patients.
| Variable | Value, n (%) | |||
|---|---|---|---|---|
| Sex | Male | 71 (41%) | ||
| Female | 101 (59%) | |||
| Age (mean) | 23 (min: 6 to max: 84) | |||
| Presenting symptoms | Pain-swelling | Microfracture (+) | 156 (90.6%) | 55 (35.2%) |
| Microfracture (−) | 101 (64.7%) | |||
| Pathological fracture | 16 (9.3%) | |||
| Tumor distribution | Aneurysmal bone cyst | 88 (51.2%) | ||
| Giant cell tumor | 51 (29.7%) | |||
| Osteoblastoma | 19 (11%) | |||
| Chondroblastoma | 7 (4%) | |||
| Chondromyxoid fibroma | 7 (4%) | |||
| Tumor localization | Lower extremity | 91 (52.9%) | ||
| Upper extremity | 62 (36%) | |||
| Pelvis | 15 (8.7%) | |||
| Vertebral | 4 (2.3%) | |||
| Tumor diameter (cm) (mean) | 6 cm (min: 1 to max: 18 cm) | |||
| Tumor volume (mm3) (mean) | 96 cm3 (min: 1.96 to max: 207.1) | |||
| Tumor stage (Enneking) | Stage 1 | 0 | ||
| Stage 2 | 86 (50%) | |||
| Stage 3 | 86 (50%) | |||
| Graft | Allograft | 74 (43%) | ||
| Cement (PMMA) | 38 (22%) | |||
| Autograft | 15 (8.7%) | |||
| None | 45 (26.2%) | |||
| Application of preoperative embolization | Embolization (+) | 12 (6.9%) | ||
| Embolization (−) | 160 (93.1%) | |||
PMMA: polymethyl methacrylate; cm: centimeter; cm3: cubic centimeter.
Values are expressed as numbers and percent (%).
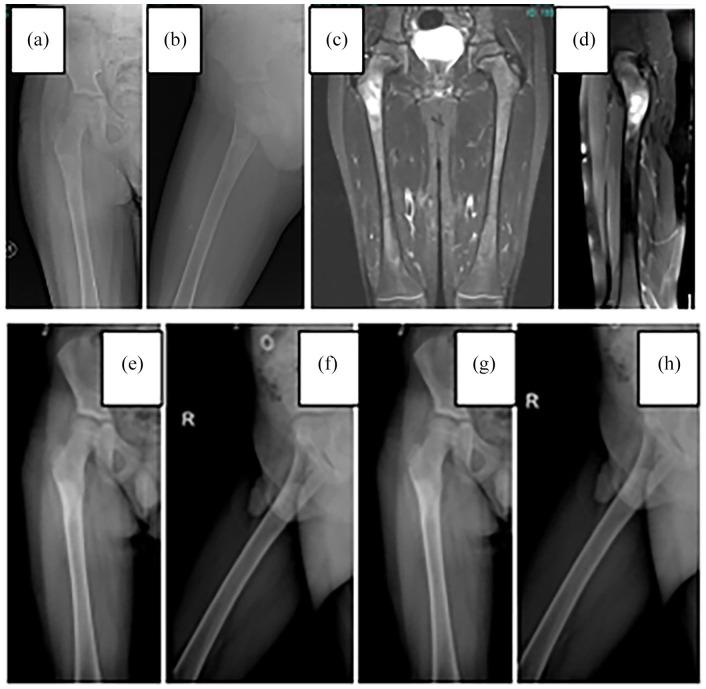
Figure 1.
Aneurysmal bone cyst in the proximal femur: (a) and (b) preoperative graph, (c) and (d) preoperative MRI sections, (e) and (f) postoperative control graph, and (g) and (h) sixth month control chart.
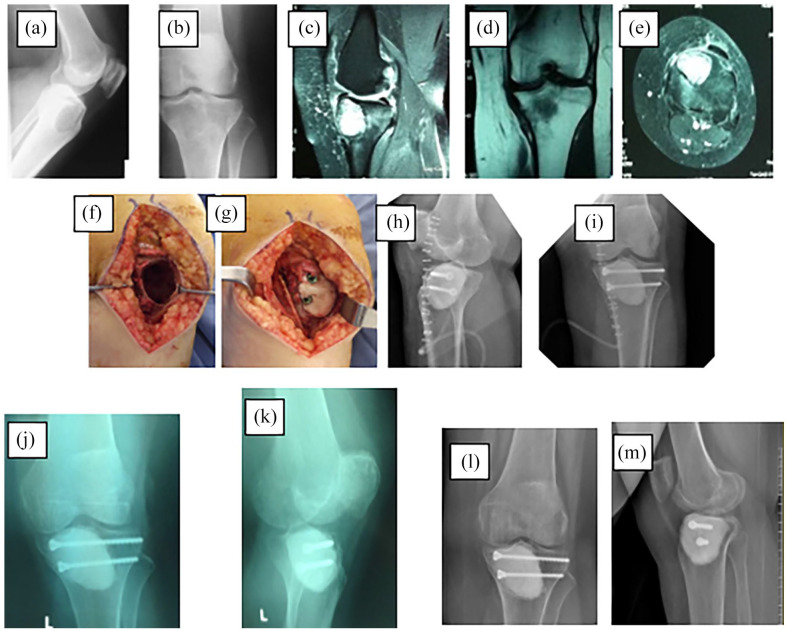
Figure 2.
Giant cell tumor in the proximal tibia: (a) and (b) preoperative graph, (c)–(e) preoperative MRI sections, (f) and (g) photos during surgery, (h) and (i) postoperative control graph, (j) and (k) third month control graph, and (l) and (m) third year control chart.
In this study, a total of 17 (9.9%) cases developed recurrence (Table 2). When we group according to tumor types: aneurysmal bone cyst 10 (5.8%), giant cell tumor 6 (3.5%), and recurrence with 1 (0.6%) osteoblastoma.
Table 2.
Recurrence distribution of patients according to tumor type.
| Diagnosis | Total | |||||||
|---|---|---|---|---|---|---|---|---|
| ABC | GCT | OSBL | CBL | CMF | ||||
| Recurrence | None | Number | 78 | 45 | 18 | 7 | 7 | 155 |
| % | 50.3% | 29.0% | 11.6% | 4.5% | 4.5% | 100% | ||
| Yes | Number | 10 | 6 | 1 | 0 | 0 | 17 | |
| % | 5.8% | 3.5% | 0.6% | 0% | 0% | 9.9% | ||
| Total | Number | 88 | 51 | 19 | 7 | 7 | 172 | |
| % | 51.2% | 29.7% | 11.0% | 4.1% | 4.1% | 100% | ||
ABC: aneurysmal bone cyst; GCT: giant cell tumor; OSBL: osteoblastoma; CBL: chondroblastoma; CMF: chondromyxoid fibroma.
Values are expressed as numbers and percent (%).
The recurrence rates were compared among patients according to their clinical and tumor characteristics (Table 3). Recurrence rates of patients with tumors below and above 5 cm in diameter were 1.2% (2 patients) and 8.7% (15 patients), respectively (p = 0.004). Recurrence rates of patients with Stage 2 and Stage 3 tumors were 1.7% (3 patients) and 8.1% (14 patients), respectively (p = 0.009). There were no statistically significant relationships between recurrence rates and other parameters.
Table 3.
Recurrence distribution data according to clinical and tumor characteristics of patients.
| Variable | Recurrence (−) (n = 155) | Recurrence (+) (n = 17) | p-value | |
|---|---|---|---|---|
| Age | 23.05 ± 14.09 | 28.4 ± 15.4 | 0.143 | |
| Tumor | Aneurysmal bone cyst | 78 (45.3%) | 10 (5.8%) | 0.661 |
| Giant cell tumor | 45 (26.2%) | 6 (3.5%) | ||
| Osteoblastoma | 18 (10.5%) | 1 (0.6%) | ||
| Chondroblastoma | 7 (4.1%) | 0 | ||
| Chondromyxoid fibroma | 7 (4.1%) | 0 | ||
| Tumor diameter | <5 cm | 77 (44.8%) | 2 (1.2%) | 0.004 |
| ⩾5 cm | 78 (45.3%) | 15 (8.7%) | ||
| Tumor stage | Stage 2 | 83 (48.3%) | 3 (1.7%) | 0.009 |
| Stage 3 | 72 (41.9%) | 14 (8.1%) | ||
| Graft | Allograft | 67 (39%) | 7 (4%) | 0.887 |
| Cement (PMMA) | 35 (20.2%) | 3 (1.8%) | ||
| Autograft | 13 (7.5%) | 2 (1.2%) | ||
| None | 42 (24.4%) | 3 (1.8%) | ||
| Pathological fracture | Pathological fracture (+) | 12 (7%) | 4 (2.3%) | 0.057 |
| Pathological fracture (−) | 143 (83.1%) | 13 (7.6%) | ||
cm: centimeter.
Values are expressed as mean value ± SD or numbers and percent (%).
p < 0.05 is considered statistically significant.
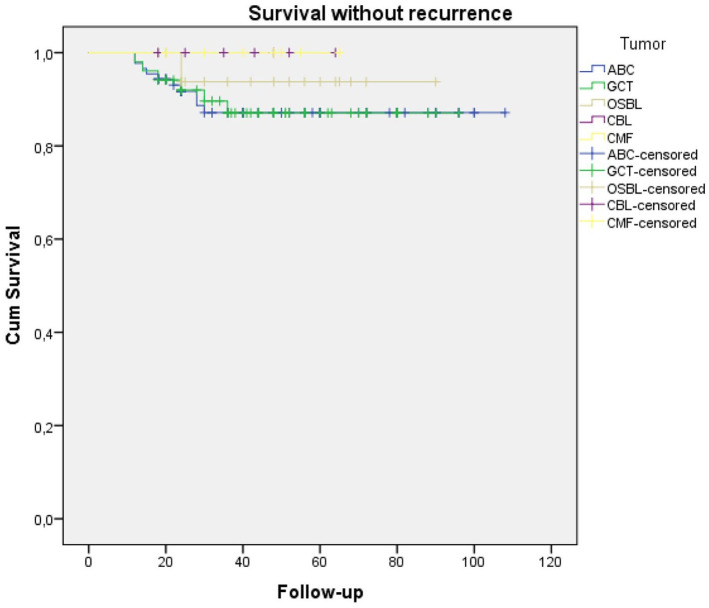
In total, 8 (4.6%) patients developed postoperative complications (Table 4). Surgical site infections developed in five patients, of which two were superficial and were treated with antibiotherapy + debridement. Three infections were deep and required two-stage revision surgery. In one patient with a diagnosis of CMF in the proximal tibia, a varus deformity developed after medial epiphysis injury, which required corrective osteotomy at 4 years postoperatively. An 8-year-old patient diagnosed with an ABC required reoperation when a pathological fracture was detected at 3 months postoperatively. A 14-year-old patient with an ABC in a proximal tibial lesion showed osteoarthritis at 15 months postoperatively. The mean MSTS at the last clinical follow-up was 95.5% (83.3%–98.6%). As a result of the recurrence-free survival analysis between the five diagnostic groups in the study, no statistically significant difference was found between the groups (p = 0,655). The Kaplan–Meier survival analysis curve is shown in Figure 3.
Table 4.
Data of patients with postoperative complications.
| Tumor type | Age (years) | Gender | Postoperative complication | |
|---|---|---|---|---|
| 1 | Aneurysmal bone cyst | 51 | Female | Surgical site infection |
| 2 | Aneurysmal bone cyst | 53 | Female | Surgical site infection |
| 3 | Aneurysmal bone cyst | 55 | Female | Surgical site infection |
| 4 | Aneurysmal bone cyst | 8 | Male | Pathological fracture |
| 5 | Aneurysmal bone cyst | 14 | Male | Osteoarthritis |
| 6 | Aneurysmal bone cyst | 46 | Female | Surgical site infection |
| 7 | Giant cell tumor | 23 | Male | Surgical site infection |
| 8 | Chondromyxoid fibroma | 8 | Female | Varus deformity |
Figure 3.
Kaplan–Meier survival analysis curve for the recurrence-free survival analysis between the five diagnostic groups in the study.
Discussion
In this study, the rates of recurrence after surgical treatment of locally aggressive bone tumors (i.e. with extended intralesional curettage, burring, and cauterization) were similar to the reported rates of recurrence achieved with the use of adjuvants. Regardless of the type of treatment, an advanced tumor stage at the time of diagnosis and a diameter of the primary tumor of 5 cm significantly increase the rates of recurrence.
The aim of tumor treatment is complete removal of tumor tissue, minimization of the possibility of recurrence, and maintenance of maximum patient function. Treatment and management of benign, locally aggressive bone tumors remain controversial among orthopedists. Extended intralesional surgical curettage remains a widely used method, but its success remains controversial. Investigators have generally divided these tumors into separate groups and different procedures have been performed, including block resection, curettage, burring, cauterization, and adjuvant treatments. There have been a few studies in which a common therapeutic approach is applied to all locally aggressive benign bone tumors.
Recurrence rates are the primary consideration in the success of surgical techniques used for tumor treatments. In recent studies, 2 the recurrence rates after surgical treatments in locally aggressive bone tumors have been approximately 50%. Gradually, block resections have begun to reduce these rates. As we explained in section “Method,” we perform extended surgical curettage. While only tumor tissue is removed in curettage, curettage is expanded until healthy tissues are seen in extended curettage. In en bloc resection, the tumor tissue is completely removed, leaving intact tissue around it. According to some non-randomized studies, 1 recurrence rates decreased by 25% to 50% with wide curettage. Despite reductions in recurrence rate, the morbidity and decline in quality of life caused by limb loss have encouraged surgeons to search for new options. Therefore, various chemical adjuvants have been used recently, including liquid nitrogen, phenol, radiotherapy, bisphosphonates, and argon beam coagulant. Recurrence rates have decreased by up to 10%; 1 however, adverse effects have been reported, such as tissue and cartilage toxicity. In comparative studies, the advantages and disadvantages of chemical adjuvants have not been clearly demonstrated. In this study, we showed that similar recurrence rates can be achieved without the use of chemical adjuvants. In addition, we avoided the side effects associated with chemical adjuvants. In this respect, we have demonstrated the superiority of our surgical treatment, compared to treatment with chemical adjuvants.
Described as a hyperplastic process reactive to intraosseous or subperiosteal bleeding, the etiology of ABC remains unclear; however, the lesion can grow rapidly and destroy bone, causing pain and pathological fracture. 5 Recurrence is particularly undesirable, because ABC may exhibit locally aggressive behavior; this type of tumor is typically treated surgically. The recurrence rate after surgical treatment reported in our previous study 5 in 64 patients diagnosed with ABC was (recurrence rate information missing) after curettage, burring, and cauterization without the use of chemical adjuvants. The majority of our 9.9% recurrence rate was the recurrence (5.3%) in 10 patients diagnosed with ABC. We attribute the degree of success obtained with 88 patients to the development of our surgical techniques and experience over time; these were the most important factors in reducing this rate. In a similar study with fewer patients, 7 the rate of recurrence obtained after surgical curettage, burring, cauterization, and grafting was 12%. Our achievement of a similar or better recurrence rate with a suitable surgical approach in the treatment of ABC, compared with studies that applied chemical adjuvants, supports the success of adjuvant-free surgical treatments in the main ABC group of locally aggressive bone tumors. We attribute our low rates to our clinical experience and the performance of all operations by a single surgeon experienced in this field.
Various surgical methods have been proposed for treatment of GCT, which is the second most common group of locally aggressive bone tumors, but there remains no established protocol. Denosumab is an effective treatment when these tumors cannot be surgically removed or when surgical resection is likely to lead to severe morbidity (e.g. loss of limbs or joints).8,9 In the literature, there have been studies of patients with GCT who were treated with different chemical adjuvants. In most of these studies, recurrence rates ranged from 10% to 20%. Shi et al. 10 reported a 17% recurrence rate in their series of patients with GCT who were treated with long-term radiotherapy. Recurrence rates after nitric oxide and argon beam coagulator were 15.9% (16 patients) and 10%, respectively, in studies of patients with GCT who were treated with surgical curettage and adjuvant therapy. The complication rate was reported as 11.7% (six patients with pathological fractures, three patients with skin problems, one patient with peroneal palsy, and two patients with osteoarthritis). 11 The level 3 study of Frank et al. 12 in patients with GCT showed that application of phenol as an adjuvant did not affect the risk of recurrence. In this study, although the number of patients diagnosed with GCT was low compared to the literature, our non-adjuvant recurrence rate was 3.5% (six patients). Our data indicate the superiority of the surgical technique we used in patients with GCT, compared to the use of chemical adjuvants. However, while the recurrence rates of CMF were 26.3% in the Mayo clinical series, 13 we attribute the lack of reduction in this study to the low number of patients. Machak and Snetkov, 14 who applied curettage and burr together in the treatment of GCT and named it the combined curettage technique, showed that this technique appears to provide the most potent and comprehensive impact on residual tumor cells located in risk zones.
Chemical adjuvants used during or after surgery can cause side effects such as adjacent tissue necrosis, wound healing problems, nerve paralysis, and tendon rupture after Argon Ray Coagulator treatment; 15 wound healing defects, skin necrosis, and peroneal nerve paralysis after application of nitric oxide; 11 and bleeding, infection, pathological fracture, neural damage, skin burns, and muscle burns after radiofrequency ablation. 16 Concerns about the efficacy and associated morbidity of these adjuvants have often been raised, and the indication for their use is still being debated. 17 In this study, we observed mainly surgical site infections. In terms of cost-effectiveness, complications due to chemical adjuvant treatment can worsen an already difficult clinical situation. We presume that chemicals with serious complications and no superiority in rates of recurrence are harmful, rather than beneficial. Thus, it may be appropriate to perform surgical treatment by means of extended intralesional curettage without the use of chemical adjuvants.
Among the strengths of this study were the treatment of a large number of locally aggressive bone tumors with a single treatment approach. Several studies have evaluated the results of tumor treatment among patients in separate groups; to the best of our knowledge, this study is the first to examine all patients with a single treatment approach.
This study had some limitations. First, it was retrospective in nature and did not include a control group for data comparison. Second, the data were patient-dependent, which may have led to selection bias when evaluating the MSTS. Third, identical treatment was applied to patients with different diagnoses. However, when patients were considered as a diagnostic group, the recurrence rates were comparable to or better than those published in the literature; thus, a single surgical technique can be successfully applied in treatment of five different tumors. Ideal power analysis could not be performed because there were no study samples in the literature that fully matched this study (including five different locally aggressive tumors). Finally, we used PMMA in 38 (22%) patients. Cement is considered a chemical adjuvant by some clinicians. An important consideration involves the occurrence of lesion necrosis due to the heat and tumor cell death. PMMA clearly exhibits a heat effect during application, but its ability to cause tumor cell necrosis is controversial. In an animal study conducted by Wilkins et al., 18 bone necrosis occurred at ⩾60°C; there was no necrosis at <48°C and the area where PMMA was applied did not reach temperatures >46°C. The results of an animal study by Malawer et al. 19 support the hypothesis that no bone necrosis occurs around a cavity filled with PMMA.
Conclusion
Successful treatment results can be achieved in locally aggressive bone tumors by means of extended surgical curettage, burring, and cauterization without the use of chemical adjuvants. Additional advantages include a reduction in operative costs and the elimination of chemical-related side effects. Further prospective randomized controlled trials and trials are needed to compare different treatment modalities and outcomes.
Acknowledgments
The authors thank the staff of Marmara University Orthopedics and Traumatology Department. Their help and support was important to the formation of this work.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The study protocol was approved by the Ethics Committee, Marmara University (09.2016.497).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from all subjects before the study.
ORCID iD: Ali Erkan Yenigül  https://orcid.org/0000-0002-2690-9488
https://orcid.org/0000-0002-2690-9488
References
- 1. Azar FM, Canale ST, Beaty JH. Campbell’s operative orthopaedics e-book. New York: Elsevier, 2016. [Google Scholar]
- 2. Malek F, Krueger P, Hatmi ZN, et al. Local control of long bone giant cell tumour using curettage, burring and bone grafting without adjuvant therapy. Int Orthop 2006; 30(6): 495–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kundu ZS, Gupta V, Sangwan SS, et al. Curettage of benign bone tumors and tumor like lesions: a retrospective analysis. Indian J Orthop 2013; 47(3): 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campanacci M, Capanna R, Picci P. Unicameral and aneurysmal bone cysts. Clin Orthop Relat Res 1986; 204: 25–36. [PubMed] [Google Scholar]
- 5. Erol B, Topkar MO, Caliskan E, et al. Surgical treatment of active or aggressive aneurysmal bone cysts in children. J Pediatr Orthop B 2015; 24(5): 461–468. [DOI] [PubMed] [Google Scholar]
- 6. Enneking WF, Dunham W, Gebhardt MC, et al. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res 1993; 286: 241–246. [PubMed] [Google Scholar]
- 7. Gibbs CP, Jr, Hefele MC, Peabody TD, et al. Aneurysmal bone cyst of the extremities. J Bone Joint Surg Am 1999; 81(12): 1671–1678. [DOI] [PubMed] [Google Scholar]
- 8. Yayan J. Denosumab for effective tumor size reduction in patients with giant cell tumors of the bone: a systematic review and meta-analysis. Cancer Control 2020; 27(3): 1073274820934822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Branstetter DG, Nelson SD, Manivel JC, et al. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res 2012; 18(16): 4415–4424. [DOI] [PubMed] [Google Scholar]
- 10. Shi W, Indelicato DJ, Reith J, et al. Radiotherapy in the management of giant cell tumor of bone. Am J Clin Oncol 2013; 36(5): 505–508. [DOI] [PubMed] [Google Scholar]
- 11. Malawer MM, Bickels J, Meller I, et al. Cryosurgery in the treatment of giant cell tumor. A long-term followup study. Clin Orthop Relat Res 1999; 359: 176–188. [DOI] [PubMed] [Google Scholar]
- 12. Klenke FM, Wenger DE, Inwards CY, et al. Giant cell tumor of bone: risk factors for recurrence. Clin Orthop Relat Res 2011; 469(2): 591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu CT, Inwards CY, O’Laughlin S, et al. Chondromyxoid fibroma of bone: a clinicopathologic review of 278 cases. Hum Pathol 1998; 29(5): 438–446. [DOI] [PubMed] [Google Scholar]
- 14. Machak GN, Snetkov AI. The impact of curettage technique on local control in giant cell tumour of bone. Int Orthop 2021; 45(3): 779–789. [DOI] [PubMed] [Google Scholar]
- 15. Lewis VO, Wei A, Mendoza T, et al. Argon beam coagulation as an adjuvant for local control of giant cell tumor. Clin Orthop Relat Res 2007; 454: 192–197. [DOI] [PubMed] [Google Scholar]
- 16. Santiago FR, Del Mar Castellano Garcia M, Montes JL, et al. Treatment of bone tumours by radiofrequency thermal ablation. Curr Rev Musculoskelet Med 2009; 2(1): 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bickels J, Campanacci DA. Local adjuvant substances following curettage of bone tumors. J Bone Joint Surg Am 2020; 102(2): 164–174. [DOI] [PubMed] [Google Scholar]
- 18. Wilkins RM, Okada Y, Sim FH, et al. Methyl methacrylate replacement of subchondral bone: a biochemical and morphologic analysis. In: Enneking WF. (ed.) Limb-Sparing Surgery in Musculoskeletal Oncology. New York: Churchill Livingstone, 1984, pp. 479–485. [Google Scholar]
- 19. Malawer MM, Marks MR, McChesney D, et al. The effect of cryosurgery and polymethylmethacrylate in dogs with experimental bone defects comparable to tumor defects. Clin Orthop Relat Res 1988; 226: 299–310. [PubMed] [Google Scholar]