Abstract
Objective
To estimate effectiveness of tickborne encephalitis (TBE) vaccination by time interval (<5, 5–10 and 10+years) postvaccination.
Design
A retrospective, matched case–control study
Participants
Cases—all adult (age 18–79) TBE cases in Switzerland reported via the national mandatory disease reporting surveillance system from 2006 to 2020 (final n=1868). Controls—community controls from a database of randomly selected adults (age 18–79) participating in a 2018 cross-sectional study of TBE vaccination in Switzerland (final n=4625).
Primary outcome measures
For cases and controls, the number of TBE vaccine doses received and the time since last vaccination were determined. Individuals were classified as being ‘unvaccinated’ (0 doses), ‘incomplete’ (1–2 doses) or ‘complete’ (3+ doses). Individuals with ‘complete’ vaccination were further classified by time since the last dose was received (<5 years, 5–10 years or 10+ years). A conditional logistic regression model was used to calculate vaccine effectiveness (VE: 100 × [1−OR]) for each vaccination status category.
Results
VE for incomplete vaccination was 76.8% (95% CI 69.0% to 82.6%). For complete vaccination, overall VE was 95.0% (95% CI 93.5% to 96.1%). When the most recent dose was received <5 years prior VE was 91.6% (95% CI 88.4% to 94.0%), 95.2% (95% CI 92.4% to 97.0%) when the most recent dose was received 5–10 years prior, and 98.5% (95% CI 96.8% to 99.2%) when the most recent dose was received 10+ years prior.
Conclusions
That VE does not decrease among completely vaccinated individuals over 10+ years since last vaccination supports the longevity of the protective response following complete TBE vaccination. Our findings support the effectiveness of 10-year TBE booster intervals currently used in Switzerland.
Keywords: TBE, Prevention, Protection, Duration, Breakthrough Infection
Strengths and limitations of this study.
Switzerland has a nearly complete national mandatory disease reporting surveillance system, and case data should be representative.
The case–control design allows direct comparison of vaccination status among cases and control individuals matched by sex, age and area of residence.
Despite a large number of tickborne encephalitis cases (n=1868), only a small number were vaccinated (n=151), preventing a more detailed analysis of factors which could affect vaccine effectiveness (VE) within groups.
Data on other factors which might impact the response to vaccination (chronic medical conditions, immunosuppression, age of first vaccination, whether individuals were vaccinated according to the recommended vaccination schedule) were not available.
As cases and controls were matched by age, VE values between age groups cannot be directly compared.
Background
Tickborne encephalitis (TBE) is a serious viral infection of the central nervous system which can result in permanent neurological injury and death. TBE is caused by the TBE virus (TBEV), which is transmitted by ticks of the Ixodidae family. Currently, TBEV is endemic throughout much of Europe.1 2 In Switzerland, mandatory reporting of TBE cases was initiated in 1988. Since then, both the incidence and geographical range of disease have continued to increase. In 2020, the country experienced its most severe disease season with an overall incidence of 5.16 cases/100 000 individuals; exceeding the WHO’s (WHO) definition of a ‘highly endemic’ area.1 2
Vaccination is highly protective against TBE; producing virus-neutralising antibodies which are associated with disease prevention, but are not universally considered the ‘correlate of protection’. Two vaccines are currently licensed by the European Medicines Agency (EMA): Encepur and FSME-Immun. Both are recommended for individuals living, working or travelling within endemic areas.2 Both are administered as a primary series of three doses given at day 0, 1–3 months and 5–12 months with booster doses every 36–60 months (3–5 years) thereafter, depending on age and vaccine formulation.3 4 In 2006, the Swiss Federal Office of Public Health (FOPH) amended its official recommendation for TBE vaccination, extending the Swissmedic and EMA-approved booster interval to 120 months (10 years).5 6
Previous studies have demonstrated sustained levels of virus-specific and neutralising antibodies up to and exceeding 10 years after TBE vaccination.7–9 Whether this translates to sustained effectiveness, however, is not clear. Additionally, irregular TBE vaccination has been associated with reduced vaccine effectiveness (VE),10 11 indicating that deviations from the established vaccination schedule can influence lasting immunity. Whether the prolonged TBE booster intervals in Switzerland impact VE is of great public health interest as reducing unnecessary vaccinations can improve cost-effectiveness and vaccine compliance. Here, we conducted a retrospective case–control study to evaluate TBE VE in Switzerland at <5, 5–10 and 10+ years postvaccination.
Methods
Study design
We used a retrospective, matched case–control study design, comparing all TBE cases among adults 18–79 in Switzerland reported via the national mandatory disease reporting surveillance system in the 15-year period between 2006 and 2020, to community controls selected from a 2018 nationwide study of TBE vaccination coverage.12 13
Selection of cases
TBE is a mandatory notifiable disease in Switzerland, with all confirmed TBE cases (based on serology and clinical picture14) reported by laboratories to the national disease surveillance system.14 15 Age, sex, canton of residence and information on vaccination status, including number of doses received and date of last vaccination, are reported to the Swiss FOPH by the submitting physician. From the Swiss FOPH, we obtained data for all TBE cases among residents of Switzerland aged 18–79 reported from 2006 to 2020. Among the 2450 eligible cases, 6 were excluded as sex was unknown, 550 were excluded as vaccination status was unknown and another 26 were excluded due to missing information on the number of vaccine doses received (final n=1868, figure 1).
Figure 1.
Study flow diagram. FOPH, Federal Office of Public Health; TBE, tickborne encephalitis.
Selection of controls
Community controls were selected from among a database of individuals participating in a 2018 cross-sectional study of TBE vaccination in Switzerland.12 13 In brief, adults with a Swiss mailing address in each of three age groups (18–39, 40–59, 60–79) were selected from each of the seven Swiss geographical regions (European NUTS-2 level) by disproportional stratified random sampling. Selected individuals (n=26 880; 1280 from each age group and region) were requested by mail to submit a copy of their vaccination record. A total of 4626 individuals submitted vaccination records. From these, date(s) of TBE immunisation were recorded into a database. All participants in this database were eligible for inclusion into this study. One case was excluded due to missing information on vaccine dose number (final n=4625, figure 1).
Matching
Cases and controls were matched on sex, age (within 5-year intervals) and canton of residence (half-cantons were combined). All possible matches for each case were considered. Matching was performed using nearest neighbour matching with the matchit function in R V.4.0.3,16 (R Foundation for Statistical Computing, Vienna, Austria).
Analysis
For cases and controls, the total number of TBE vaccine doses received and the time since the most recent vaccination were determined. Individuals were classified as being ‘unvaccinated’ (0 doses), ‘incomplete’ (1–2 doses) or ‘complete’ (3+ doses). Among those with ‘complete’ vaccination, individuals were further classified by the time since the last dose was received (<5 years, 5–10 years or 10+ years). Based on these criteria, a conditional logistic regression model was used to calculate Vaccine Effectiveness (VE: 100× [1−OR]) for each of the defined vaccination status categories. Statistical analyses were performed using Stata V.17.0 (StataCorp LLC, College Station, Texas, USA) and GraphPad Prism V.8.0 (GraphPad Software, San Diego, California, USA); p values <0.05 were considered statistically significant.
For the cross-sectional TBE vaccination coverage database used to select controls, potential participants were sent a letter explaining the study’s purpose and stating that, by submitting vaccination records, they were voluntarily consenting to participation.12 13 All data were anonymised and treated confidentially throughout the analysis.
Results
In total, 1828 cases and 3667 controls were matched for our study (figure 1, table 1). Among cases, 8.3% (n=151) had received at least one TBE vaccine dose, compared with 45.2% (n=1656) of controls (p<0.0001, χ2 test). Of vaccinated cases, 49.7% were ‘incomplete’ (1–2 doses) and 50.3% were ‘complete’ (3+ doses). Of vaccinated controls, 16.8% were ‘incomplete’ and 83.2 were ‘complete’ (p<0.0001, χ2 test).
Table 1.
Demographic breakdown of cases and controls and vaccine effectiveness by vaccination status
| Controls (n=3667) |
Cases (n=1828) |
% Vaccine Effectiveness (1-OR)*100 |
95% CI lower | 95% CI upper | P value | |
| Male | 1925 (52.5%) | 1159 (63.4%) | – | – | – | – |
| Female | 1742 (47.5%) | 669 (36.6%) | – | – | – | – |
| 18–39 | 1060 (28.9%) | 463 (25.3%) | – | – | – | – |
| 40–59 | 1257 (34.3%) | 800 (43.8%) | – | – | – | – |
| 60–79 | 1350 (36.8%) | 565 (30.9%) | – | – | – | – |
| Unvaccinated | 2011 (54.8%) | 1677 (91.7%) | Ref. | – | – | – |
| 1–2 doses (incomplete) | 279 (7.6%) | 75 (4.1%) | 76.8 | 69.0 | 82.6 | <0.001 |
| 3+ doses (complete) <5 years |
522 (14.2%) | 48 (2.6%) | 91.6 | 88.4 | 94.0 | <0.001 |
| 3+ doses complete 5–10 years |
397 (10.8%) | 20 (1.1%) | 95.2 | 92.4 | 97.0 | <0.001 |
| 3+ doses complete 10+ years |
458 (12.5%) | 8 (0.4%) | 98.5 | 96.8 | 99.2 | <0.001 |
| 3+ doses (complete) Any time |
1377 (37.6%) | 76 (4.2%) | 95.0 | 93.5 | 96.1 | <0.001 |
VE for incomplete vaccination was 76.8% (table 1). For complete vaccination, overall VE was 95.0%. When the most recent dose was received <5 years prior VE was 91.6%, 95.2% when the most recent dose was received 5–10 years prior, and 98.5% when the most recent dose was received 10+ years prior. Compared with <5 years prior, VE 10+ years prior was significantly higher (p<0.0001 conditional logistic regression). These values were also comparable between those aged 18–39, 40–59 and 60–79 (figure 2).
Figure 2.
Vaccine effectiveness by age. For each age group (18–39, 40–59 and 60–79), individuals were categorised as unvaccinated, incompletely vaccinated (1–2 doses), completely vaccinated (3+ doses) <5 years prior, 5–10 years prior or 10+ years prior and VE was calculated using the formula (VE=100×[1−OR]), with unvaccinated as the reference.
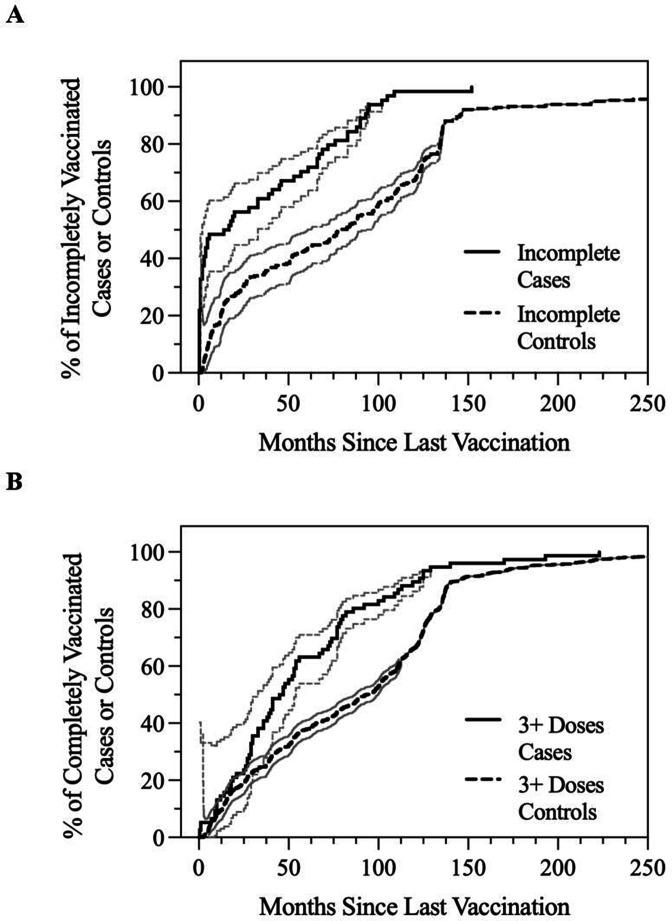
Among incompletely vaccinated TBE cases, the median time since last vaccination was 1.3 years (15.5 months), compared with 6.7 years (79.8 months) for incompletely vaccinated controls (p<0.0001 Mantel-Cox Log-rank test) (figure 3A). 37.5% of incompletely vaccinated cases occurred within 2 months of last vaccination. For completely vaccinated TBE cases, median time since last vaccination was 3.8 years (46.0 months) compared with 7.8 years (93.3 months) for controls (p<0.0001 Mantel-Cox Log-rank test, figure 3B). Comparing timing of last vaccination to the recommended booster vaccination scheme, 63.2% of cases had been last vaccinated within the preceding 5 years, 26.3% within the preceding 5–10 years and 10.5% had been last vaccinated more than 10 years prior. For controls, 38.0%, 28.8% and 33.3% of individuals had received a last vaccination <5, 5–10, or 10+ years prior, respectively (p<0.0001, χ2 test).
Figure 3.
Time since last vaccination among vaccinated cases and controls. (A) Among incompletely vaccinated cases (n=75) or controls (n=279), the percentage of individuals (with 95% CIs) that received their last vaccine dose by indicated times. (B) Among completely vaccinated cases (n=76) or controls (n=1377), the percentage of individuals (with 95% CIs) that received their last vaccine dose by indicated times.
Discussion
Here we used a retrospective, matched case–control study design to investigate TBE VE in Switzerland considering both incomplete and complete vaccination, and, among completely vaccinated individuals, different time intervals since last vaccination. Of the 8.3% of cases that had received at least one vaccine dose, 50.3% were completely vaccinated (3+ doses). Based on this definition, we estimate a failure rate of 4.2%, which is in line with TBE vaccine failure rates estimated in other studies,10 17–20 including two previous studies also using Swiss mandatory reporting data from the national database which we draw from here.21 22
Importantly, nearly half of vaccinated cases were among recipients of only 1–2 doses. Here, we calculated VE for incomplete vaccination at 76.8%, which was substantially and significantly less than that for complete vaccination (95.0%). Furthermore, we found that nearly 40% of the cases among incompletely vaccinated individuals occurred within 2 months of their last dose, suggesting they were exposed before they had developed a protective immune response to vaccination. As TBE is a strongly seasonal disease (peaking from April to October), and because primary vaccination for TBE according to the ‘conventional’ schedule takes a minimum of 5–9 months, vaccination should ideally begin in the fall or winter of the year prior to possible exposure. Alternatively, a rapid immunisation scheme (0, 7 and 21 days plus a fourth dose after 12–18 months for Encepur or 0 and 14 days plus a third dose after 5–12 months for FSME-Immun3 4), could potentially provide an option to limit a period of reduced protection. Previous studies have not found substantial differences in serological responses to TBE vaccination based on use of the ‘rapid’ or ‘conventional’ schedules.8 23 24 While our dataset did not include complete timing of TBE vaccination history, which could allow us to investigate VE by vaccination schedule, such an analysis could be informative.
Among completely vaccinated individuals, overall TBE VE was 95.0%. Interestingly, we did not observe a decrease in VE with increasing time since the last vaccine dose was received, consistent with the findings of a recent study evaluating TBE cases in vaccinated individuals using a different methodology.21 If anything, VE was lower in the first 5 years following last vaccination compared with last vaccination 10+ years prior. We further observed the median time to vaccine failure among completely vaccinated cases was 3.8 years (46.0 months). While not completely clear, this observation could potentially be explained by a fraction of individuals which remained insufficiently protected by vaccination. The median time to vaccine failure did not, however, differ between cases having received three doses or those having received more than four doses (not shown), suggesting no impact of adding an additional dose. It should also be noted that we did not observe appreciable differences in overall VE or VE over time between age groups (in agreement with findings summarised in a recent systematic review of TBE vaccine booster intervals25).
An important limitation of our study is the relatively small number of vaccinated cases (n=151). Furthermore, we do not have data on other factors which might impact the response to vaccination such that we could control for them in our analysis. Whether individuals have a chronic medical condition or immunosuppression,26–29 the age at which the person was first vaccinated,17 20 30 31 whether they were regularly vaccinated in their primary series,10 11 and possibly whether they received a ‘conventional’ or ‘rapid’ schedule, could potentially have an impact on our assessment of VE. An additional limitation is that, while we found TBE VE appeared similar between age groups, as we matched cases and controls by age we were unable to directly compare VE values. While some studies have demonstrated reduced serological responses to TBE vaccination and increased vaccine breakthrough in older individuals,17 20 30 31 other work has shown vaccination breakthrough to be comparable between older and younger age groups, and that VE remains high even among those aged 60+.32 33 Clarifying how TBE VE is impacted by age remains an important area warranting further study.
Taken together, and despite our study limitations, these results do not indicate a consistent decrease in TBE VE over time among fully vaccinated individuals, as might be predicted by antibody decay kinetics. These findings also highlight that antibody responses may not necessarily always be a suitable surrogate for VE estimates, which have been shown to decline with time in several publications.7 9 34 35
Conclusions
Despite that a substantial portion of the Swiss population has been vaccinated for TBE—42% coverage for one dose and 34% for three doses12—disease incidence continues to increase, indicating that current vaccination coverage is insufficient. To better address this, additional information related to vaccination uptake, schedule compliance, and effectiveness is needed. Our findings highlight the increased effectiveness of complete (3+ dose) vs incomplete (1–2 dose) TBE vaccination. That 40% of breakthroughs among incompletely vaccinated individuals occur within 1–2 months of vaccination suggests that individuals are being exposed before they have had time to develop a protective immune response to vaccination. That VE does not appreciably change or decrease among completely vaccinated individuals over 10+ years since last vaccination supports the longevity of the protective response following complete TBE vaccination; also among both younger and older age groups. In total, our findings support the effectiveness of 10-year TBE booster intervals currently used in Switzerland.
Supplementary Material
Footnotes
Contributors: KDZ and PL conceived the study. KDZ, SRH and PL designed the study, KDZ and PL acquired study funding. AJS and ESA provided case data. KDZ and SRH organised the database and analysed the data, KDZ wrote the first draft of the manuscript. KDZ, SRH, AJS, ESA, JSF and PL critically reviewed the manuscript and approved the submitted version. KDZ and PL are guarantor.
Funding: This work was funded, in part, by a competitive grant from the Swiss Federal Office of Public (grant number 19002540) awarded to KDZ and PL. KDZ was additionally supported by awards from the EMDO Stiftung (award number 1053) and the University of Zurich Forschungskredit for post-doctoral researchers (award number FK-20-059).
Disclaimer: The funders had no role in the study design, data analysis, preparation of the manuscript, or decision to publish.
Competing interests: The authors declare no competing interests.
Patient and public involvement: There was no patient and/or public involvement in the design, conduct, or dissemination plans of this research study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
To ensure participant privacy, the datasets analysed for this study are not publicly available. They are, however, available on request, without undue reservation, by contacting the study authors (phung.lang@uzh.ch).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The procedure and method of consent for the cross-sectional was approved by the Ethics Committee of the Canton of Zurich (approval number 2017-02-027).
References
- 1.Swiss Federal Office of Public Health. Zahlen zu Infektionskrankheiten - Zeckenenzephalitis FSME [Numbers for Infectious Illnesses - Tick-borne Encephalitis - TBE]., 2021. Available: https://www.bag.admin.ch/bag/de/home/zahlen-und-statistiken/zahlen-zu-
- 2.World Health Organization . Vaccines against tick-borne encephalitis: who position paper-recommendations. Vaccine 2011;29:8769–70. 10.1016/j.vaccine.2011.07.024 [DOI] [PubMed] [Google Scholar]
- 3.SEN . Encepur N Fachinfo [Technical Information]: Compendium.ch, 2020. Available: https://compendium.ch/product/105785-encepur-n-inj-susp/mpro
- 4.Swissmedic. FSME-Immun® CC Fachinfo [Technical Information]: Compendium.ch., 2020. Available: https://compendium.ch/product/1364944-fsme-immun-cc-inj-susp-sep-nadel/mpro
- 5.Swiss Federal Offic of Public Health. Empfehlungen zur Impfung gegen Zeckenenzephalitis [Recommendation for Vaccination against Tick-borne Encephalitis]. 2006. Available: https://www.bag.admin.ch/dam/bag/de/dokumente/nat-gesundheitspolitik/klimawandel/hitzewelle/hintergrundinfos/info-zecken/impfempfehlungen.pdf.download.pdf/impfempfehlungen.pdf
- 6.Swiss Federal Offic of Public Health. Schweizerischer Impfplan [Swiss Immunization Schedule]., 2020.https://www.bag.admin.ch/bag/de/home/gesund-leben/gesundheitsfoerderung-und-praevention/impfungen-prophylaxe/schweizerischer-impfplan.htmlhttps://www.bag.admin.ch/bag/de/home/gesund-leben/gesundheitsfoerderung-und-praevention/impfungen-prophylaxe/schweizerischer-impfplan.html
- 7.Paulke-Korinek M, Kundi M, Laaber B, et al. Factors associated with seroimmunity against tick borne encephalitis virus 10 years after booster vaccination. Vaccine 2013;31:1293–7. 10.1016/j.vaccine.2012.12.075 [DOI] [PubMed] [Google Scholar]
- 8.Beran J, Xie F, Zent O. Five year follow-up after a first booster vaccination against tick-borne encephalitis following different primary vaccination schedules demonstrates long-term antibody persistence and safety. Vaccine 2014;32:4275–80. 10.1016/j.vaccine.2014.06.028 [DOI] [PubMed] [Google Scholar]
- 9.Konior R, Brzostek J, Poellabauer EM, et al. Seropersistence of TBE virus antibodies 10 years after first booster vaccination and response to a second booster vaccination with FSME-IMMUN 0.5mL in adults. Vaccine 2017;35:3607–13. 10.1016/j.vaccine.2017.03.059 [DOI] [PubMed] [Google Scholar]
- 10.Heinz FX, Holzmann H, Essl A, et al. Field effectiveness of vaccination against tick-borne encephalitis. Vaccine 2007;25:7559–67. 10.1016/j.vaccine.2007.08.024 [DOI] [PubMed] [Google Scholar]
- 11.Heinz FX, Stiasny K, Holzmann H, et al. Vaccination and tick-borne encephalitis, central Europe. Emerg Infect Dis 2013;19:69–76. 10.3201/eid1901.120458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baroutsou V, Zens KD, Sinniger P, et al. Analysis of tick-borne encephalitis vaccination coverage and compliance in adults in Switzerland, 2018. Vaccine 2020;38:7825–33. 10.1016/j.vaccine.2020.10.022 [DOI] [PubMed] [Google Scholar]
- 13.Zens KD, Baroutsou V, Sinniger P, et al. A cross-sectional study evaluating tick-borne encephalitis vaccine uptake and timeliness among adults in Switzerland. PLoS One 2021;16:e0247216. 10.1371/journal.pone.0247216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobler PDG, Erber W, Bröker M. The TBE book. 2nd Edition. Global Health Press Pte Limited;, 2019. [Google Scholar]
- 15.Swiss Federal Office of Public Health Infektionskrankheiten melden - Zeckenenzephalitis FSME [Infectious Disease Reporting - Tick-borne Encephalitis - TBE]., 2021. Available: https://www.bag.admin.ch/bag/de/home/krankheiten/infektionskrankheiten-bekaempfen/meldesysteme-infektionskrankheiten/meldepflichtige-ik/meldeformulare.html#-1611150545
- 16.Ho DE, Imai K, King G. MatchIt : Nonparametric Preprocessing for Parametric Causal Inference. J Stat Softw 2011;42:1–28. 10.18637/jss.v042.i08 [DOI] [Google Scholar]
- 17.Hansson K, Rosdahl A, Insulander M. Tick-Borne encephalitis (TBE) vaccine failures: a ten-year retrospective study supporting the rationale for adding an extra priming dose in individuals from the age of 50 years. Clin Infect Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zavadska D, Odzelevica Z, Karelis G, et al. Tick-Borne encephalitis: a 43-year summary of epidemiological and clinical data from Latvia (1973 to 2016). PLoS One 2018;13:e0204844. 10.1371/journal.pone.0204844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindqvist R, Rosendal E, Weber E, et al. The envelope protein of tick-borne encephalitis virus influences neuron entry, pathogenicity, and vaccine protection. J Neuroinflammation 2020;17:284. 10.1186/s12974-020-01943-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lotrič-Furlan S, Bogovič P, Avšič-Županc T, et al. Tick-Borne encephalitis in patients vaccinated against this disease. J Intern Med 2017;282:142–55. 10.1111/joim.12625 [DOI] [PubMed] [Google Scholar]
- 21.Schmidt AJ, Altpeter E, Graf S. Tick-Borne encephalitis (TBE) in Switzerland: does the prolongation of vaccine booster intervals result in an increased risk of breakthroughs? J Travel Med 2021. [DOI] [PubMed] [Google Scholar]
- 22.Schuler M, Zimmermann H, Altpeter E, et al. Epidemiology of tick-borne encephalitis in Switzerland, 2005 to 2011. Euro Surveill 2014;19. 10.2807/1560-7917.ES2014.19.13.20756 [DOI] [PubMed] [Google Scholar]
- 23.Galgani I, Bunge EM, Hendriks L, et al. Systematic literature review comparing rapid 3-dose administration of the GSK tick-borne encephalitis vaccine with other primary immunization schedules. Expert Rev Vaccines 2017;16:919–32. 10.1080/14760584.2017.1358620 [DOI] [PubMed] [Google Scholar]
- 24.Rampa JE, Askling HH, Lang P, et al. Immunogenicity and safety of the tick-borne encephalitis vaccination (2009-2019): a systematic review. Travel Med Infect Dis 2020;37:101876. 10.1016/j.tmaid.2020.101876 [DOI] [PubMed] [Google Scholar]
- 25.Steffen R, Erber W, Schmitt HJ. Can the booster interval for the tick-borne encephalitis (TBE) vaccine 'FSME-IMMUN' be prolonged? - A systematic review. Ticks Tick Borne Dis 2021;12:101779. 10.1016/j.ttbdis.2021.101779 [DOI] [PubMed] [Google Scholar]
- 26.Dengler TJ, Zimmermann R, Meyer J, et al. Vaccination against tick-borne encephalitis under therapeutic immunosuppression. reduced efficacy in heart transplant recipients. Vaccine 1999;17:867–74. 10.1016/S0264-410X(98)00272-2 [DOI] [PubMed] [Google Scholar]
- 27.Hertzell KB, Pauksens K, Rombo L, et al. Tick-Borne encephalitis (TBE) vaccine to medically immunosuppressed patients with rheumatoid arthritis: a prospective, open-label, multi-centre study. Vaccine 2016;34:650–5. 10.1016/j.vaccine.2015.12.029 [DOI] [PubMed] [Google Scholar]
- 28.Panasiuk B, Prokopowicz D, Panasiuk A. Immunological response in HIV-positive patients vaccinated against tick-borne encephalitis. Infection 2003;31:45–6. 10.1007/s15010-002-2020-6 [DOI] [PubMed] [Google Scholar]
- 29.Jilich D, Maly M, Kosina P, et al. Immunogenicity and safety of rapid scheme vaccination against tick-borne encephalitis in HIV-1 infected persons. Epidemiol Infect. 2021;:e41.;149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stiasny K, Holzmann H, Heinz FX. Characteristics of antibody responses in tick-borne encephalitis vaccination breakthroughs. Vaccine 2009;27:7021–6. 10.1016/j.vaccine.2009.09.069 [DOI] [PubMed] [Google Scholar]
- 31.Andersson CR, Vene S, Insulander M, et al. Vaccine failures after active immunisation against tick-borne encephalitis. Vaccine 2010;28:2827–31. 10.1016/j.vaccine.2010.02.001 [DOI] [PubMed] [Google Scholar]
- 32.Erber W, Khan F, Zavadska D, et al. Effectiveness of TBE vaccination in southern Germany and Latvia. Vaccine 2022;40:819–25. 10.1016/j.vaccine.2021.12.028 [DOI] [PubMed] [Google Scholar]
- 33.Schmitt H-J, Dobler G, Zavadska D, et al. TBE vaccination breakthrough Cases-Does age matter? Vaccines 2021;9:932. 10.3390/vaccines9080932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rendi-Wagner P, Kundi M, Zent O, et al. Immunogenicity and safety of a booster vaccination against tick-borne encephalitis more than 3 years following the last immunisation. Vaccine 2004;23:427–34. 10.1016/j.vaccine.2004.07.002 [DOI] [PubMed] [Google Scholar]
- 35.Aerssens A, Cochez C, Niedrig M, et al. Analysis of delayed TBE-vaccine booster after primary vaccination. J Travel Med 2016;23:tav020.. 10.1093/jtm/tav020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To ensure participant privacy, the datasets analysed for this study are not publicly available. They are, however, available on request, without undue reservation, by contacting the study authors (phung.lang@uzh.ch).