Abstract
Objectives
Elevated low-density lipoprotein cholesterol (LDL-C) increases the risk of recurrent cardiovascular disease (CVD) events. We examined use of lipid-lowering therapy (LLT) following ischaemic stroke, and estimated benefits from guideline-based up-titration of LLT.
Methods
The Norwegian COgnitive Impairment After STroke (Nor-COAST) study, a multicentre prospective cohort study, collected data on LLT use, dose intensity and LDL-C levels for 462 home-dwelling patients with ischaemic stroke. We used the Secondary Manifestations of Arterial Disease-Reduction of Atherothrombosis for Continued Health (SMART-REACH) model to estimate the expected benefit of up-titrating LLT.
Results
At discharge, 92% received LLT (97% statin monotherapy). Patients with prestroke dementia and cardioembolic stroke aetiology were less likely to receive LLT. Older patients (coefficient −3 mg atorvastatin per 10 years, 95% CI −6 to −0.5) and women (coefficient −5.1 mg atorvastatin, 95% CI −9.2 to −0.9) received lower doses, while individuals with higher baseline LDL-C, ischaemic heart disease and large artery stroke aetiology received higher dose intensity. At 3 months, 45% reached LDL-C ≤1.8 mmol/L, and we estimated that 81% could potentially reach the target with statin and ezetimibe, resulting in median 5 (IQR 0–12) months of CVD-free life gain and median 2% 10-year absolute risk reduction (IQR 0–4) with large interindividual variation.
Conclusion
Potential for optimisation of conventional LLT use exists in patients with ischaemic stroke. Awareness of groups at risk of undertreatment and objective estimates of the individual patient’s benefit of intensification can help personalise treatment decisions and reduce residual cholesterol risk.
Trial registration number
Keywords: hyperlipidemias; stroke; pharmacology, clinical; medication adherence
Key questions.
What is already known about this subject?
Target achievement for low-density lipoprotein cholesterol (LDL-C) is suboptimal in patients with established cardiovascular disease.
Achieved LDL-C levels is largely dependent on dose intensity of lipid-lowering drugs prescribed, baseline LDL-C levels and patients’ adherence to therapy.
What does this study add?
We explore clinical factors influencing physician’s prescription patterns after ischaemic stroke, where age, sex, ischaemic stroke subtype, coexisting coronary artery disease and baseline LDL-C were related to dose intensity.
We estimated that 81% could potentially reach an LDL-C ≤1.8 mmol/L with statin plus ezetimibe leading to median 5 months cardiovascular disease-free life-gain.
Estimated benefit from intensification of therapy varies between individuals.
How might this impact on clinical practice?
Awareness of patient groups at risk of undertreatment, like women, and awareness of an individual patient’s vascular risk and the benefits of intensifying treatment might help avoid undertreatment and overtreatment.
The Secondary Manifestations of Arterial Disease-Reduction of Atherothrombosis for Continued Health(SMART-REACH) model can be used to objectively estimate expected benefit.
Introduction
The incidence of fatal and non-fatal cardiovascular disease (CVD) events has decreased over the last decades.1 2 In Norway, mortality from stroke has decreased by nearly 40% in the last 10 years, and mortality from myocardial infarction has decreased by approximately 50%.1 A large proportion of Norwegian stroke patients suffer minor strokes and are independent and home-dwelling after 3 months.3 Thus, a growing number of patients are living with established CVD and need optimal secondary prevention and follow-up to prevent recurrent events.
Patients with ischaemic stroke are at high risk of recurrent CVD events.4 In Norway, one of four strokes is recurrent, despite more than 90% of patients being prescribed guideline-recommended medications at discharge.3 Drugs lowering low-density lipoprotein cholesterol (LDL-C) are one of the strategies to reduce the risk of recurrence.5–8 Although the optimal LDL-C target remains unclear,6 recent studies indicate that lower treatment targets are more beneficial.8 There has been an increase in both statin use and dose over time,9 10 but gaps still exist between guideline-recommendations2 6 11 12 and current practice with suboptimal target achievement for LDL-C.6 9 13–15 This gap could be associated with both patient-related factors, such as poor drug adherence and perceived adverse effects,12 13 and physician-related factors like choice of drug type and dose intensity.12 13 Awareness of an individual patient’s CVD risk, perceived risk of adverse effects and the expected harm-benefit ratio may also influence how lipid-lowering therapy (LLT) is prescribed and used.2 6 12 15 16
Little is known about the current use of LLT among patients with recent ischaemic stroke, especially the dose intensity of LLT. Moreover, a considerable interindividual variation in risk of recurrent events, competing risks and remaining life expectancy exist,4 with corresponding variation in the net benefit from more intensive LLT.4 17 Objective estimates of an individual patient’s benefit might assist in making well-balanced decisions on whether to intensify treatment or not. Our study therefore aimed to address two sets of questions. First, how do current prescription patterns and achieved LDL-C reduction differ in subgroups of patients who had stroke? Next, what is the expected treatment benefit when theoretically up-titrating LLT according to guidelines?
Methods
Study population
Home-dwelling patients from the multicentre, prospective Nor-COAST (Norwegian COgnitive Impairment After STroke) cohort study were included (n=729), online supplemental figure S1. Patients admitted with ischaemic stroke at five Norwegian stroke units were consecutively included between May 2015 and March 2017.18 Patients were assessed with self-report questionnaires, clinical examinations and blood sampling after 3 and 18 months (online supplemental methods). We excluded patients who died within the first 3 months (n=29), nursing home patients (n=36) and patients lacking medication information (n=3). Patients between 45 and 80 years (n=462) were included as we used a risk prediction model derived and validated in this age range.4 17
openhrt-2022-001972supp001.pdf (383.6KB, pdf)
Use of LLT
LLT was identified using the following Anatomical Therapeutic Chemical classification system codes: C10AA, C10AX and C10B. We used the Defined Daily Doses (DDDs)19 to convert to atorvastatin equivalent doses by the following formula: (Dose of statin/DDD for that statin)×DDD for atorvastatin=atorvastatin equivalent dose. High-intensity statins (HIS) was defined as ≥40 mg atorvastatin, ≥20 mg rosuvastatin or 80 mg simvastatin per day.6 We measured medication adherence by the 4-item Morisky Medication Adherence Scale (MMAS-4), where a score of 4 points was defined as high adherence.20
LDL-C control and expected LDL-C with up-titration of LLT
LDL-C ≤1.8 mmol/L was defined as target attainment2 11 and 3-month levels were used as the basis for theoretical intensification. Guidelines recommend statins at maximally tolerated dose (step 1) and use of ezetimibe (step 2) in patients unable to achieve the target with statin monotherapy or statin intolerance.2 6 11 The potent and expensive proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors are mainly considered for patients still not reaching targets (step 3).6 11
We included patients receiving LLT at discharge in these analyses. We used mean % reduction in LDL-C derived from randomised clinical trials as previously presented and validated21 (online supplemental methods, online supplemental table S1). First, all patients with LDL-C >1.8 mmol/L not using HIS was up-titrated to HIS, assuming a 50.2% mean reduction in LDL-C (the effect of atorvastatin 80 mg).21 If the expected LDL-C then was >1.8 mmol/L, ezetimibe was added, assuming a mean 22.7% reduction in LDL-C.21 We also estimated the effect of adding ezetimibe without increased statin doses, assuming patients already used maximally tolerated statin.
Estimated benefit from up-titration of LLT
We estimated individual benefit in terms of gain in months free of recurrent stroke, myocardial infarction or cardiovascular mortality17 and 10-year absolute risk reduction (ARR), by using the externally validated SMART-REACH (Secondary Manifestations of Arterial Disease-Reduction of Atherothrombosis for Continued Health) model.17 The model is a competing risk-adjusted lifetime model previously validated in Nor-COAST,4 which uses the following predictors: sex, smoking, diabetes mellitus, systolic blood pressure, total cholesterol, creatinine, number of locations of CVD (coronary, cerebral and/or peripheral arterial disease), atrial fibrillation and heart failure (online supplemental methods and online supplemental table S2).
We first calculated the life expectancy without recurrent cardiovascular events based on 3-month levels of predictors in the model, defined as the median estimated survival without a recurrent event.17 We next estimated potential treatment benefit defined as the difference in CVD-free life expectancy with and without up-titration of LLT. CVD-free life expectancy with achieved LDL-C level after up-titration was calculated by incorporating an HR of 0.78 for major cardiovascular events per 1.0 mmol/L reduction in LDL-C5 in the SMART-REACH model. The individuals’ 10-year ARR was defined as the difference between current 10-year CVD risk and the 10-year risk with achieved LDL-C levels after up-titration.
Statistical analysis
Logistic and linear regression was used with LLT prescription (yes/no) and atorvastatin equivalent dose (mg/day) as dependent variables, respectively, to identify predictors of LLT use and intensity. Potential predictors were selected a priori based on previous studies9 10 22 and clinical reasoning, leading to inclusion of the following covariates, first one at a time, and next, adjusted for age and sex: age, sex, LDL-C, prestroke LLT, frailty by the 5-item Fried criteria13 as a continuous variable from 0 (robustness) to 5 (frail), the Global Deterioration Scale as continuous variable from 1 (normal) to 7 (severe dementia). A history of ischaemic heart disease was included as a categorical variable (yes/no). Stroke subtype was divided into five categories according to the Trial of Org 10 172 in Acute Stroke Treatment classification: large artery disease, cardioembolic stroke, small vessel disease, undetermined strokes and other aetiology (grouped with ‘undetermined’). We report coefficients or odds ratios (OR) with 95% confidence intervals (CI). Two‐sided p values <0.05 were regarded as statistically significant. However, due to multiple comparisons, p values between 0.01 and 0.05 should be interpreted with caution. We imputed missing data for LDL-C and covariates to predict CVD risk by means of single imputation using predictive mean matching (online supplemental table S2 shows extent of missing data). Data analysis was performed using Stata V.16 or R V.4.0.2.
Patient and public involvement
The Nor-COAST study included two user representatives from the national unions for patients with stroke and dementia. The user representatives have been actively participating in the planning and performance of the study. They have been invited to Nor-COAST meetings and separate meetings have been held two to three times per year. They become consecutively informed about the study progress and are invited to share their views on endpoints, ethics, and dissemination of the results to the users.
Results
Baseline characteristics and LLT at discharge
Mean age was 69.0 years (SD 8.1), 38% were female, 24% were smoking and 35% (n=161) were already using LLT at admission (table 1). At discharge, 92% (n=427) were prescribed LLT, of whom 422 received statins, either alone (n=414) or in combination with ezetimibe (n=8), whereas five patients were receiving ezetimibe alone. Mean statin dose was 41 mg (SD 21) atorvastatin equivalent dose and 64% (n=276) received HIS. Type and doses of LLT are shown in online supplemental table S3.
Table 1.
Clinical characteristics at index stay by lipid-lowering therapy use at discharge
| Prescribed lipid-lowering therapy (n=427) | Not prescribed lipid-lowering therapy (n=35) | Total population (n=462) |
|||
| Non-high intensity statin (n=146) | High-intensity statin* (n=276) | Any† (n=427) |
|||
| Demographics | |||||
| Age (years) | 70.4 (8.0) | 68.0 (8.0) | 68.8 (8.1) | 70.7 (8.2) | 69.0 (8.1) |
| Sex, female | 57 (39) | 105 (38) | 163 (38) | 14 (40) | 177 (38) |
| Education | 12.3 (3.8) | 12.6 (3.7) | 12.6 (3.7) | 11.5 (3.4) | 12.5 (3.7) |
| Home care services | 7 (5) | 5 (3) | 15 (4) | 5 (14) | 20 (4) |
| Cardiovascular characteristics | |||||
| Atrial fibrillation | 38 (26) | 46 (17) | 84 (20) | 16 (46) | 100 (22) |
| Diabetes mellitus | 32 (22) | 50 (18) | 84 (20) | 6 (17) | 90 (20) |
| History of hypertension | 84 (58) | 146 (53) | 233 (55) | 17 (49) | 250 (54) |
| Prestroke lipid-lowering therapy | 69 (47) | 89 (32) | 160 (37) | 1 (3) | 161 (35) |
| Previous cerebrovascular disease | 41 (28) | 52 (19) | 97 (23) | 10 (29) | 107 (23) |
| Ischaemic heart disease | 30 (21) | 46 (17) | 77 (18) | 2 (6) | 79 (17) |
| Peripheral artery disease | 15 (10) | 19 (7) | 34 (8) | 0 (0) | 34 (7) |
| Heart failure | 2 (1) | 6 (2) | 8 (2) | 3 (9) | 11 (2) |
| Glomerular filtration rate (mL/min/1.73 m²) | 79 (15) | 78 (16) | 79 (16) | 77 (21) | 79 (16) |
| Body mass index (kg/m2) | 26.2 (4.2) | 27.0 (4.3) | 26.7 (4.2) | 26.0 (3.7) | 26.7 (4.2) |
| Current smoker | 34 (23) | 101 (37) | 100 (24) | 9 (26) | 109 (24) |
| Physically active | 36 (25) | 77 (28) | 115 (27) | 8 (23) | 123 (27) |
| Lipid levels at index stay | |||||
| Total cholesterol (mmol/L) | 4.6 (1.2) | 5.1 (1.3) | 5.0 (1.3) | 4.7 (1.4) | 5.0 (1.3) |
| LDL cholesterol (mmol/L) | 2.8 (0.9) | 3.3 (1.1) | 3.1 (1.1) | 3.0 (1.3) | 3.1 (1.1) |
| HDL cholesterol (mmol/L) | 1.4 (0.6) | 1.4 (0.6) | 1.4 (0.6) | 1.3 (0.4) | 1.4 (0.5) |
| Stroke characteristics and other comorbidities | |||||
| NIHSS discharge | 1.4 (1.8) | 1.7 (2.4) | 1.6 (2.2) | 2.0 (3.9) | 1.7 (2.4) |
| Stroke subtype (n=447) | |||||
| Large artery disease | 10 (7) | 38 (14) | 48 (12) | 1 (3) | 49 (11) |
| Cardioembolic | 34 (24) | 54 (20) | 88 (21) | 15 (43) | 103 (23) |
| Small vessel disease | 35 (25) | 62 (24) | 99 (24) | 5 (14) | 104 (23) |
| Other cause | 5 (4) | 6 (2) | 11 (3) | 1 (3) | 12 (3) |
| Undetermined or multiple causes | 59 (41) | 104 (39) | 166 (40) | 13 (37) | 179 (40) |
| Charlson comorbidity index | 3.8 (1.7) | 4.3 (1.9) | 3.6 (1.8) | 4.1 (1.9) | 3.6 (1.8) |
| Frail | 14 (10) | 16 (6) | 30 (7) | 2 (6) | 32 (7) |
| Cognitive impairment | 3 (2) | 4 (2) | 7 (2) | 6 (17) | 13 (3) |
| Independent functional status at discharge‡ | 102 (70) | 196 (71) | 303 (71) | 21 (60) | 324 (70) |
| Other secondary preventive drugs at discharge | |||||
| Antithrombotic drugs | 144 (99) | 275 (100) | 424 (99) | 34 (97) | 458 (99) |
| Antihypertensive drugs | 113 (77) | 205 (74) | 321 (75) | 25 (71) | 346 (75) |
| Total number of medications | 5.3 (2.6) | 5.2 (2.4) | 5.2 (2.5) | 4.0 (3.0) | 5.1 (2.6) |
Values are n (%) or mean (SD) (n observations). Detailed definitions in online supplemental methods.
*Defined as ≥40 mg atorvastatin, ≥20 mg rosuvastatin or 80 mg simvastatin per day.
†5 patients received ezetimibe monotherapy.
‡Defined as ≤2 on Modified Rankin Scale.
HDL, high density lipoprotein; LDL, low density lipoprotein; NIHSS, National Institutes of Health Stroke Scale.
Unadjusted and adjusted associations between patient characteristics and prescription of LLT (yes/no) at discharge are shown in online supplemental table S4. Patient characteristics associated with dose intensity at discharge are shown in table 2.
Table 2.
Linear regression with statin dose intensity (mg)* as dependent variable, for participants prescribed statin monotherapy at discharge (n=414)
| Unadjusted analysis | Age- and sex-adjusted analysis | ||||
| n | Coefficient (95% CI) | P value | Coefficient (95% CI) | P value | |
| Age, years | 414 | −0.30 (−0.55 to −0.05) | 0.019 | −0.26 (−0.51 to −0.01) | 0.039 |
| Sex, female | 414 | −5.1 (−9.2 to −0.9) | 0.017 | −4.5 (−8.6 to −0.3) | 0.036 |
| LDL-C†, (mmol/L) | 414 | 2.7 (0.9 to 4.5) | 0.004 | 2.8 (0.9 to 4.6) | 0.003 |
| Prestroke use of LLT | 414 | −2.4 (−6.6 to 1.8) | 0.268 | −1.8 (−6.1 to 2.4) | 0.402 |
| Frailty‡ | 414 | 0.2 (−2.0 to 2.3) | 0.889 | 1.3 (−0.9 to 3.5) | 0.249 |
| Cognitive impairment§ | 408 | 0.2 (−3.0 to 3.4) | 0.918 | 0.8 (−2.4 to 4.0) | 0.626 |
| Ischaemic heart disease | 414 | 6.1 (0.8 to 11.4) | 0.024 | 6.7 (1.3 to 12.1) | 0.016 |
| Index stroke aetiology¶ | 399 | ||||
| Large artery disease | Reference category | Reference category | |||
| Cardioembolic stroke | −11.8 (−19.4 to −4.2) | 0.002 | −11.6 (−19.1 to −4.1) | 0.003 | |
| Small vessel disease | −11.3 (−18.8 to −3.8) | 0.003 | −11.3 (−18.8 to −3.9) | 0.003 | |
| Undetermined or multiple causes | −9.2 (−16.2 to −2.3) | 0.010 | −9.4 (−16.3 to −2.4) | 0.008 | |
*Atorvastatin equivalent dose.
†Measured at first day after admission.
‡Measured by modified Fried Frailty criteria with 0 as reference corresponding to robust, and 5 to frail.
§Prestroke, measured by Global Deterioration Scale with 1 as reference corresponding to normal cognitive function and 7 to severe dementia.
¶Classified according to the TOAST (Trial of Org 10 172 in Acute Stroke Treatment) classification.
LDL-C, low-density lipoprotein cholesterol.
Achieved LDL-C levels and LLT at follow-up
For patients prescribed LLT at discharge (n=427), mean LDL-C decreased from 3.1 (SD 1.1) to 2.1 (SD 0.7) mmol/L 3 months poststroke. For LLT naïve patients the corresponding decrease was from 3.5 (SD 1.0) to 2.0 (SD 0.7) mmol/L. In total, 45% (n=193) achieved the LDL-C target of ≤1.8 mmol/L and 33% of these had reached the target by receiving non-HIS, 62% by HIS, 1% by ezetimibe monotherapy, 2% by statin plus ezetimibe and 2% without LLT (discontinued). In total, 14 patients had discontinued statins between discharge and 3 months. In total, 58% (n=249) had LDL-C ≤2.0 mmol/L, 11% (n=45) ≤1.4 mmol/L and 2% (n=10) ≤1.0 mmol/L and 78% reported high medication adherence at 3 months.
Lipid profiles according to subgroups of patients are shown in online supplemental table S5, where women, younger patients and patients with no prestroke LLT had higher LDL-C at admission. LLT for patients not reaching the target by subgroups of patients is shown in online supplemental table S6. Target attainment in different subgroups of LLT regimens is shown in online supplemental figure S2. Table 3 shows characteristics in categories defined by quartiles of relative LDL-C reduction. Patients with the largest reduction were younger, had higher LDL-C at index stay, 82% were prescribed HIS and 86% reported optimal adherence. Among patients with the smallest LDL-C reduction, 78% had prestroke LLT.
Table 3.
Characteristics in categories defined by quartiles of % LDL-cholesterol reduction from index stay to the 3-month visit for patients prescribed LLT at discharge (n=427)
| ≤Q1 <8% reduction (n=107) |
Q1–Q2 9%–35% reduction (n=107) |
Q2–Q3 36%–51% reduction (n=107) |
Q3 >51% reduction (n=106) |
|
| Median % reduction (IQR) | −6 (−28 to 0) | 23 (16 to 29) | 44 (39 to 48) | 57 (54 to 61) |
| Age, mean (SD) | 70.3 (8.1) | 69.3 (7.8) | 68.9 (8.3) | 66.9 (7.9) |
| Sex, female | 28 (26) | 42 (39) | 44 (41) | 49 (46) |
| Body mass index (kg/m2), mean (SD) | 26.7 (4.1) | 26.6 (4.8) | 26.5 (4.1) | 27.0 (3.9) |
| Current smoker at admission | 26 (24) | 22 (21) | 23 (22) | 29 (27) |
| Hypertension | 81 (76) | 66 (62) | 44 (41) | 42 (40) |
| Prestroke use of LLT | 83 (78) | 51 (48) | 18 (17) | 8 (8) |
| Diabetes mellitus | 28 (26) | 20 (19) | 19 (18) | 17 (16) |
| History of ischaemic heart disease | 41 (28) | 19 (18) | 13 (12) | 4 (4) |
| Prior stroke | 45 (42) | 29 (27) | 11 (10) | 12 (11) |
| Charlson comorbidity index | 4.3 (1.8) | 3.8 (2.0) | 3.2 (1.4) | 3.1 (1.8) |
| Frail | 7 (7) | 9 (8) | 6 (6) | 8 (8) |
| Cognitive impairment | 4 (4) | 3 (3) | 0 (0) | 0 (0) |
| Stroke subtype (n=412) | ||||
| Large artery disease | 10 (9) | 14 (14) | 13 (12) | 11 (11) |
| Cardioembolic stroke | 33 (31) | 24 (24) | 18 (17) | 13 (13) |
| Small vessel disease | 19 (18) | 24 (24) | 27 (26) | 29 (29) |
| Other | 3 (3) | 3 (3) | 5 (5) | 0 (0) |
| Undetermined | 40 (38) | 36 (35) | 43 (41) | 47 (47) |
| LDL-C at index stay, mean (SD) | 2.1 (0.8) | 2.8 (0.8) | 3.5 (0.9) | 4.0 (0.9) |
| LDL-C at 3 months, mean (SD) | 2.4 (0.8) | 2.1 (0.6) | 2.0 (0.5) | 1.7 (0.4) |
| 10-year CVD risk (%),* median (IQR) | 50 (38–63) | 43 (33–54) | 40 (30–52) | 37 (29–49) |
| Discontinued statin between 0 and 3 months | 7 (7) | 6 (6) | 1 (1) | 0 (0) |
| Optimal medication adherence† (n=351) | 70/87 (81) | 67/87 (77) | 69/90 (77) | 75/87 (86) |
| Non-high intensity statin | 50 (47) | 37 (35) | 37 (35) | 19 (18) |
| High-intensity statin | 50 (47) | 64 (60) | 69 (64) | 87 (82) |
| At target at 3 months | 29 (27) | 41 (38) | 47 (44) | 76 (72) |
Values are n/N (%) if other not specified. Detailed definitions of variables in online supplemental methods.
*Estimated by the Secondary Manifestations of Arterial Disease-Reduction of Atherothrombosis for Continued Health (SMART-REACH) model.
†Corresponding to 4 points on Morisky Medication Adherence Scale 4.
CVD, cardiovascular disease; GFR, glomerular filtration rate; LDL-C, low-density lipoprotein cholesterol; LLT, lipid-lowering therapy.
In total, 73% of the 352 patients with available medication lists at 18 months reported high medication adherence and 11% (n=38) had discontinued statins (10% of men and 13% of women, p=0.337, 9% with HIS and 14% with non-HIS, p=0.229), of whom four had switched to ezetimibe monotherapy. Treatment patterns for those still persistent to statins are shown in online supplemental figure S3. Of patients with no LLT at discharge or 3 months (n=26), six patients started between 3 and 18 months.
Expected LDL-C levels when theoretically up-titrating LLT
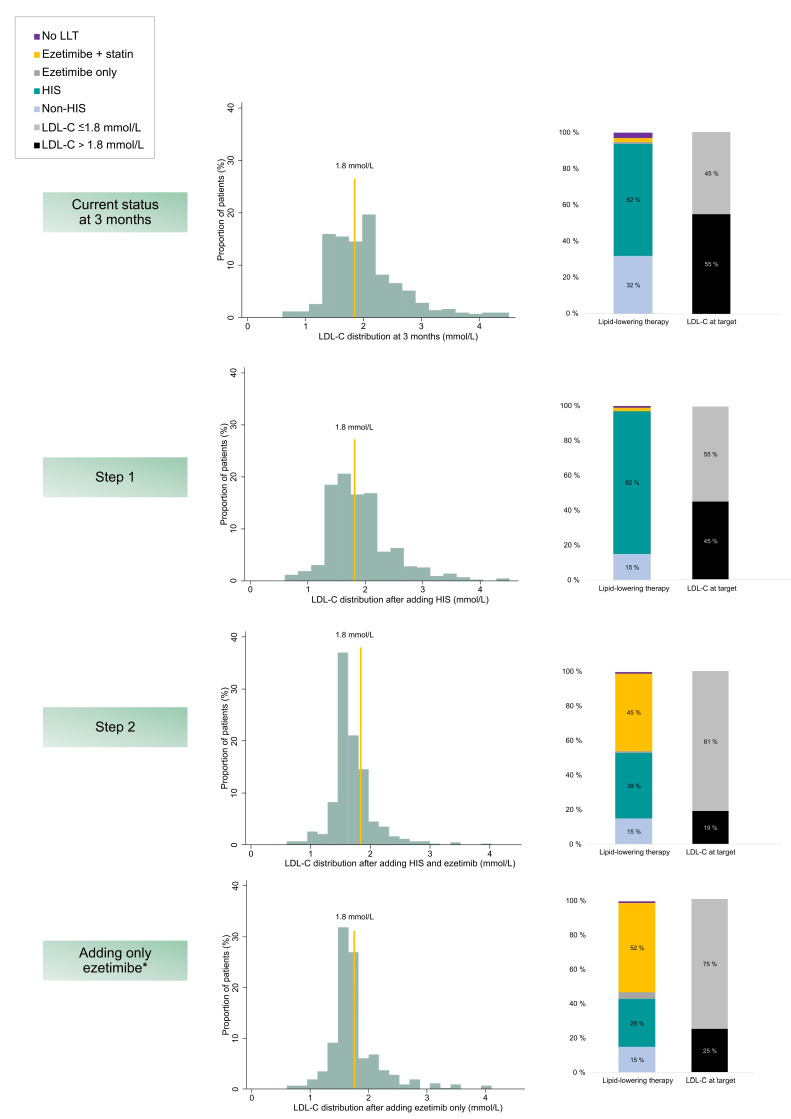
Figure 1 shows LDL-C distribution after theoretically up-titrating LLT according to guidelines, proportions achieving the guideline target for each step and proportions using different LLT. Of the n=234 patients not at target at 3 months, n=87 were receiving non-HIS and could undergo up-titration to HIS (step 1), online supplemental figure S4. Up-titration in these subjects would result in an additional n=43 achieving an LDL-C level ≤1.8 mmol/L (overall cohort with LDL-C ≤1.8 mmol/L; 55% (n=236) at this stage). Of the remaining 45% (n=191) not at the LDL-C target, six patients were already receiving concomitant ezetimibe. Ezetimibe could be added to the remaining 44% (n=185) receiving HIS who were not at the target (step 2). After this step, an additional 26% would have reached the target (total at target, 81% (n=347)). After intensification, mean LDL-C was 1.7 mmol/L (SD 0.4). Mean LDL-C for those not reaching the target after intensification (n=80) was 2.2 mmol/L (SD 0.4). Assuming all patients were already using maximally tolerated statin dose and only ezetimibe could be added, 75% (n=319) could potentially reach the target.
Figure 1.
Distribution of LDL-C, proportions at target ≤1.8 mmol/L and LLT in use at 3 months and after hypothetically up-titrating LLT according to guideline-recommendations. First (step 1) by adding/up-titrating to high intensity statin, and next (step 2) by adding ezetimibe. *Assuming already on maximally tolerated statin dose. Proportions are n of the total population (n=427). Patients with no LLT, are patients who have discontinued prescribed LLT between discharge and 3 months. LDL-C, low-density lipoprotein cholesterol; LLT, lipid-lowering therapy; HIS, high-intensity statin.
Expected benefit when theoretically up-titrating LLT
For all patients prescribed LLT (n=427), the median 10-year CVD risk was 42% (IQR 31%–54%) and lifetime risk was 70% (IQR 64%–76%). Median CVD-free life expectancy was 80.2 years (IQR 76.2–83.2). Overall median estimated lifetime benefit when up-titrating LLT was 5 months (IQR 0–12). Median CVD-free life gain was <6 months for 52% (n=220), 6–12 months for 27% (n=115) and >12 months for 22% (n=92). Estimated median 10-year ARR was 2% (IQR 0%–4%).
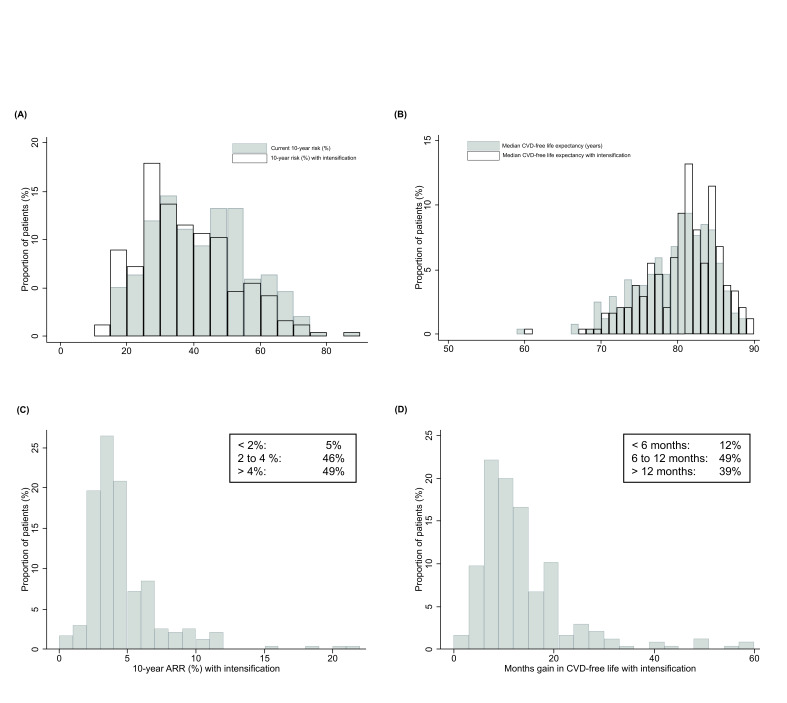
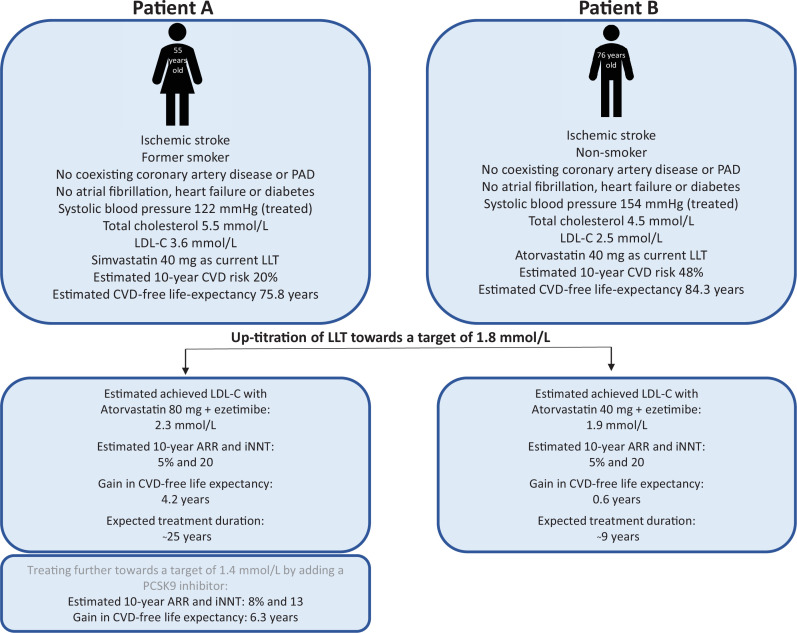
For patients with LDL-C above 1.8 mmol/L (n=234), the median estimated lifetime benefit by up-titrating was 11 months (IQR 7–17), with 39% having >12 months of estimated CVD-free life gain (figure 2). Characteristics for patients stratified by tertiles of months of gain in CVD-free life are shown in online supplemental table S7. Estimated 10-year ARR for these patients was median 4% (IQR 3%–5%), and the median 10-year risk level could be reduced from 40% (IQR 31%–52%) to 35% (IQR 27%–46%). Further up-titration to the LDL-C target 1.4 mmol/L would lead to median 17 months (IQR 11–25) of estimated lifetime benefit (online supplemental figure S5). Two illustrative patient examples are shown in figure 3.
Figure 2.
Estimated prognostic impact of intensification of lipid-lowering therapy according to the guideline-recommendations for patients with LDL-C above 1.8 mmol/L at 3 months (n=234). The top row shows (A) the distribution of the estimated 10-year CVD before and after intensification and (B) estimated median life-expectancy free from CVD events before and after intensification. The bottom row shows (C) distribution of estimated 10-year ARRs with intensification and (D) distribution in gain in months free from CVD events with intensification. ARR, absolute risk reduction; CVD, cardiovascular disease; LDL-C, low-density lipoprotein cholesterol.
Figure 3.
Two patient examples. The benefit of intensification of current lipid-lowering therapy estimated by the SMART-REACH model for a patient aged 55 years (A) versus 76 years (B) and expected treatment duration. ARR, absolute risk reduction; CVD, cardiovascular disease; iNNT, individual number-needed-to-treat (1 divided by ARR); LDL-C, low-density lipoprotein cholesterol; LLT, lipid-lowering therapy; PAD, peripheral artery disease; PCSK9, proprotein convertase subtilisin/kexin type 9.
Discussion
In this observational study of patients ≤80 years, we showed high LLT prescription rates, and although LDL-C levels in many cases were not far from target, less than half of patients reached the target. Age, sex, stroke aetiology and baseline LDL-C were related to LLT intensity prescribed. Younger patients, women and patients receiving HIS had larger % LDL-C reduction. We estimated that 81% could potentially reach the target with well-established low-cost drugs leading to median of 11 months CVD-free life-gain for patients with elevated LDL-C, but with large interindividual variation.
The prescription rates and mean statin doses were higher in Nor-COAST than in other studies.9 14 15 22–24 In total, 63% of those not reaching the target reported using HIS, illustrating that highest tolerated statin dose monotherapy may for many patients not yield target attainment.12 14 However, a previous study has noted that LDL-C levels down to mean 1.4 mmol/L is possible with optimal adherence and optimised dose of statin plus ezetimibe.25 The Nor-COAST study was conducted between 2015 and 2018 and most physicians were presumably treating towards a target of LDL-C <2.0 mmol/L.26 However, most patients with dose adjustments had their dose reduced in line with other studies,27 few used alternative LLT and 11% discontinued statins within 18 months.
As in other studies,9 10 22 27 female sex and advanced age were associated with lower dose intensity. Other studies have shown that females less often receive evidence-based CVD drugs, more often experience adverse effects and have lower awareness of their CVD risk.2 Current prescription patterns in the elderly might be explained by heterogeneity in underlying health status,2 6 16 as well as age and polypharmacy being risk factors for adverse effects and interactions.6 Although emerging evidence supports similar relative CVD risk reductions regardless of age,2 previous guidelines recommendations have been less concise. The ARR with intensified LLT can be substantial, while actual increase in life-expectancy in elderly might be limited due to risk of both CVD events and competing risks (figure 3).2 6 16
Cardioembolic stroke was associated with no LLT prescription, while large artery disease aetiology and coexisting coronary disease was associated with higher dose intensity. Evidence has historically been more robust for these patients,7 8 14 15 and previous studies have reported that coronary patients receive LLT and HIS more often than patients with peripheral and cerebrovascular disease.14 15 However, the large overlap between ischaemic stroke subtypes, different CVD entities and the high prevalence of atherosclerosis regardless of stroke aetiology illustrate the need for optimal lipid control in all subtypes.28 Furthermore, consistent relative treatment effects across multiple subgroups of patients have been demonstrated in landmark meta-analyses5 6 and observational studies show reduced risk of CVD events and mortality with statins also in cardioembolic stroke.29 Though, some of these patients might not have atherosclerosis and treating lipids less intensively might better harmonise with the individual patients’ expected benefit.
Concordance with guidelines might not be the ultimate marker of successful treatment for all.30 However, not achieving targets might well be influenced by lack of familiarity with guidelines, physicians’ and patients’ preferences and uncertainty of clinical benefit of LLT which might lead to misinterpretations about the benefit-harm tradeoffs.12 14–16 27 Statin intolerance and narrow reimbursement criteria for PCSK9-inhibitors might also be important reasons.15 Moreover, levels are often not far from targets; the physicians might then take a more pragmatic approach. Comparable to a large simulation study,21 81% was expected to reach LDL-C ≤1.8 mmol/L with safe, effective low-cost drugs. Though, the efficiency is likely to be lower in real-life settings (online supplemental table S8) and PCSK9 inhibitors would be required especially if aiming for more stringent targets.6 12 21 However, the estimated individual benefit of a more intensive approach varies, depending on baseline CVD risk, level of LDL-C, remaining life-expectancy and competing risks.2 6 17 Benefit on group level was largest in younger patients with relatively high LDL-C levels, however, younger age also means longer treatment duration and thereby higher costs to achieve those benefits (figure 3). Amount of benefit considered meaningful is also highly subjective and conditional on adverse effects, costs and patient preferences.31 Furthermore, only estimating further up-titration for patients with LDL-C above 1.8 mmol/L underestimated the actual potential benefit, since CVD risk is linearly related to LDL-C reduction5 6 (online supplemental figure S5).
Strengths and limitations
The strengths of this study include prospective consecutively inclusion and assessing LLT intensity three time-points,6 11 whereas previous studies are hampered by retrospective design9 23 with data collected a long period after an event9 23 27 or solely at discharge.10 22 Although proportions with frailty and dementia were low, including clinical information about these features and ischaemic stroke aetiology is a strength that previous studies lack or have based on registry data and diagnostic codes only.9 10 Using a lifetime risk prediction model adjusted for competing risk avoids overestimating treatment benefit in older individuals and underestimation of benefit in younger individuals.17 The Nor-COAST participants have characteristics comparable to patients in the Norwegian Stroke Registry32 and generalisation at least to Norwegian stroke patients and comparable populations is plausible.
Several limitations merit considerations. Information regarding drug-related adverse effects or patient preferences was not available. Information bias due to obtaining LLT use by interview and self-reported medication adherence might overestimate the actual LLT use. MMAS-4 is a general tool, not specific to LLT, and patients may consider their overall adherence as good although adherence to LLT is suboptimal. Other methods of measuring adherence to LLT like use of pharmacy registry data could have given other results. Self-reported use might also lead to a conservative estimate of the expected LDL-C levels achieved with intensified LLT. We did not account for interindividual variations in percentage LDL-C reduction achieved with the same drug dose.6 12 Whereas most variables only had limited missingness, there was considerable missing for LDL-C at 3 months (24%). Our cohort does not represent a randomized controlled trial setting from which the LDL-C reductions and hazard ratio were retrieved, and these results only give an indication of the impact of conventional LLT.2 30
In conclusion, almost all patients who had stroke received LLT, but below 50% reached the LDL-C target. We show potential for reducing residual cholesterol risk with safe, effective well-established low-cost LLT. Awareness of patient groups at risk of undertreatment, like women, and awareness of the individual patient’s benefit of intensifying treatment might help avoid undertreatment and overtreatment. The SMART-REACH model can be used to objectively estimate expected benefit. When benefits are known, these can be balanced against potential costs and perceived side-effects, to assist physicians and patients in well-informed treatment decisions.
openhrt-2022-001972supp002.pdf (700.1KB, pdf)
Acknowledgments
We gratefully acknowledge all participants, the Nor-COAST research group and the dedicated study staff at participating hospitals. MMAS Research Morisky Widget Software US Copyright Office Number TX 8-816-517 is protected by US Copyright laws. Permission for use is required. A license agreement was made between St. Olav University Hospital and MMAS Research LLC. A license is available from: MMAS Research LLC. Email: strubow@morisky.org.
Footnotes
Twitter: @marinorgy
Contributors: MNG, SHJH, JD, OS, IS, FLJV and HE contributed to conception and design. MNG drafted the manuscript. MNG, HE and IS contributed to data acquisition. MNG, SHJH and SL contributed to analysis, and MNG, SHJH, JD, OS, SL, IS, FLJV and HE contributed to interpretation and critically revising the manuscript. All authors gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy. MNG accepts as guarantor full responsibility for the finished work, had access to the data, and controlled the decision to publish.
Funding: The Nor-COAST study was funded by the Norwegian Health Association and Norwegian University of Science and Technology (NTNU). The work of MNG was funded by Dam Foundation and the Liaison Committee between the Central Norway Regional Health Authority and NTNU.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the Norwegian Regional Committee for Medical and Health Research Ethics North (REC number 2017/1462). The REC number for the Nor-COAST main study is 2015/171. Participants gave informed consent to participate in the study before taking part.
References
- 1.Public Health Report . Cardiovascular disease in Norway. Norwegian Institute of public health, 2020. Available: https://www.fhi.no/en/op/hin/health-disease/cardiovascular-disease-in-norway---/
- 2.Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–337. 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 3.Norwegian Stroke Registry . Annual report, 2018. Available: https://www.kvalitetsregistre.no/sites/default/files/1_arsrapport_2018_hjerneslag_0.pdf
- 4.Gynnild MN, Hageman SHJ, Dorresteijn JAN, et al. Risk stratification in patients with ischemic stroke and residual cardiovascular risk with current secondary prevention. Clin Epidemiol 2021;13:813–23. 10.2147/CLEP.S322779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–81. 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–88. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 7.Amarenco P, Bogousslavsky J, Callahan A, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549–59. 10.1056/NEJMoa061894 [DOI] [PubMed] [Google Scholar]
- 8.Amarenco P, Kim JS, Labreuche J, et al. A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med 2020;382:9. 10.1056/NEJMoa1910355 [DOI] [PubMed] [Google Scholar]
- 9.Yang Z, Edwards D, Massou E, et al. Statin use and high-dose statin use after ischemic stroke in the UK: a retrospective cohort study. Clin Epidemiol 2019;11:495–508. 10.2147/CLEP.S201983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjölander M, Eriksson M, Glader E-L. Social stratification in the dissemination of statins after stroke in Sweden. Eur J Clin Pharmacol 2013;69:1173–80. 10.1007/s00228-012-1454-8 [DOI] [PubMed] [Google Scholar]
- 11.Norwegian Guideline for Prevention of Cardiovascular Disease . The Norwegian Directorate of health, 2017. Available: https://www.helsedirektoratet.no/retningslinjer/forebygging-av-hjerte-og-karsykdom
- 12.Averna M, Banach M, Bruckert E, et al. Practical guidance for combination lipid-modifying therapy in high- and very-high-risk patients: a statement from a European atherosclerosis Society Task force. Atherosclerosis 2021;325:99–109. 10.1016/j.atherosclerosis.2021.03.039 [DOI] [PubMed] [Google Scholar]
- 13.Gynnild MN, Aakerøy R, Spigset O, et al. Vascular risk factor control and adherence to secondary preventive medication after ischaemic stroke. J Intern Med 2021;289:355-368. 10.1111/joim.13161 [DOI] [PubMed] [Google Scholar]
- 14.Ray KK, Molemans B, Schoonen WM, et al. EU-wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: the dA Vinci study. Eur J Prev Cardiol 2021;28:1279–89. 10.1093/eurjpc/zwaa047 [DOI] [PubMed] [Google Scholar]
- 15.Xian Y, Navar AM, Li S, et al. Intensity of lipid lowering with statin therapy in patients with cerebrovascular disease versus coronary artery disease: insights from the palm registry. J Am Heart Assoc 2019;8:e013229. 10.1161/JAHA.119.013229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko DT, Mamdani M, Alter DA. Lipid-Lowering therapy with statins in high-risk elderly patients: the treatment-risk paradox. JAMA 2004;291:1864–70. 10.1001/jama.291.15.1864 [DOI] [PubMed] [Google Scholar]
- 17.Kaasenbrood L, Bhatt DL, Dorresteijn JAN, et al. Estimated life expectancy without recurrent cardiovascular events in patients with vascular disease: the SMART-REACH model. J Am Heart Assoc 2018;7:e009217. 10.1161/JAHA.118.009217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thingstad P, Askim T, Beyer MK, et al. The Norwegian cognitive impairment after stroke study (Nor-COAST): study protocol of a multicentre, prospective cohort study. BMC Neurol 2018;18:193. 10.1186/s12883-018-1198-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO . World Health organization (who) collaborating centre for drug statistics methodology, 2020. Available: http://www.whocc.no/atcdddindex
- 20.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67–74. 10.1097/00005650-198601000-00007 [DOI] [PubMed] [Google Scholar]
- 21.Cannon CP, Khan I, Klimchak AC, et al. Simulation of lipid-lowering therapy intensification in a population with atherosclerotic cardiovascular disease. JAMA Cardiol 2017;2:959–66. 10.1001/jamacardio.2017.2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canavero I, Cavallini A, Perrone P, et al. Clinical factors associated with statins prescription in acute ischemic stroke patients: findings from the Lombardia stroke Registry. BMC Neurol 2014;14:53. 10.1186/1471-2377-14-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heuschmann PU, Kircher J, Nowe T, et al. Control of main risk factors after ischaemic stroke across Europe: data from the stroke-specific module of the EUROASPIRE III survey. Eur J Prev Cardiol 2015;22:1354–62. 10.1177/2047487314546825 [DOI] [PubMed] [Google Scholar]
- 24.Ní Chróinín D, Ní Chróinín C, Akijian L, et al. Suboptimal lipid management before and after ischaemic stroke and TIA-the North Dublin population stroke study. Ir J Med Sci 2018;187:739–46. 10.1007/s11845-018-1739-8 [DOI] [PubMed] [Google Scholar]
- 25.Munkhaugen J, Sverre E, Peersen K. Is the novel LDL-cholesterol goal <1.4 mmol/L achievable without a PCSK9 inhibitor in a chronic coronary population from clinical practice?. Eur J Prev Cardiol 2020:2047487320923187. 10.1177/2047487320923187 [DOI] [PubMed] [Google Scholar]
- 26.National guideline for treatment and rehabilitation in stroke . The Norwegian Directorate of health, 2010. Available: https://www.helsedirektoratet.no/retningslinjer/hjerneslag
- 27.De Backer G, Jankowski P, Kotseva K, et al. Management of dyslipidaemia in patients with coronary heart disease: results from the ESC-EORP EUROASPIRE V survey in 27 countries. Atherosclerosis 2019;285:135–46. 10.1016/j.atherosclerosis.2019.03.014 [DOI] [PubMed] [Google Scholar]
- 28.Sirimarco G, Lavallée PC, Labreuche J, et al. Overlap of diseases underlying ischemic stroke. Stroke 2013;44:2427–33. 10.1161/STROKEAHA.113.001363 [DOI] [PubMed] [Google Scholar]
- 29.Park H-K, Lee JS, Hong K-S, et al. Statin therapy in acute cardioembolic stroke with no guidance-based indication. Neurology 2020;94:e1984. 10.1212/WNL.0000000000009397 [DOI] [PubMed] [Google Scholar]
- 30.Hughes LD, McMurdo MET, Guthrie B. Guidelines for people not for diseases: the challenges of applying UK clinical guidelines to people with multimorbidity. Age Ageing 2013;42:62–9. 10.1093/ageing/afs100 [DOI] [PubMed] [Google Scholar]
- 31.Jaspers NEM, Visseren FLJ, Numans ME, et al. Variation in minimum desired cardiovascular disease-free longevity benefit from statin and antihypertensive medications: a cross-sectional study of patient and primary care physician perspectives. BMJ Open 2018;8:e021309. 10.1136/bmjopen-2017-021309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuvås KR, Saltvedt I, Aam S, et al. The risk of selection bias in a clinical multi-center cohort study. results from the Norwegian cognitive impairment after stroke (Nor-COAST) study. Clin Epidemiol 2020;12:1327–36. 10.2147/CLEP.S276631 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2022-001972supp001.pdf (383.6KB, pdf)
openhrt-2022-001972supp002.pdf (700.1KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. The data that support the findings of this study are available from the corresponding author upon reasonable request.