Abstract
Background:
Childhood maltreatment types can co-occur and are associated with increased substance use during adolescence and early adulthood. There is also a strong genetic basis for substance use which interacts with environmental factors (e.g., childhood maltreatment) to influence substance use phenotype.
Objective:
This research aimed to identify childhood maltreatment sub-groups based on type and chronicity, and their association with substance use change from adolescence to early adulthood, while accounting for the influence of substance use polygenic risk (i.e., genetic risk based on the combined effects of multiple genes).
Participants:
We used a sample of unrelated European-origin Americans with genetic and childhood maltreatment data (n = 2,664) from the National Longitudinal Study of Adolescent to Adult Health.
Methods:
Latent profile analysis was used for sub-group identification and direct and interaction effects were tested for longitudinal trajectories of substance use utilizing generalized estimating equations.
Results:
Three sub-groups with co-occurring childhood maltreatment exposures were identified: a high sexual abuse sub-group, a high physical abuse sub-group, and a normative sub-group (with low maltreatment exposure). At high polygenic risk, the high physical abuse sub-group had faster increases in substance use over time. In comparison, the high sexual abuse sub-group had faster progression in substance use only at low and medium polygenic risk.
Conclusions:
Findings provide initial evidence for biological and environmental differences among maltreatment sub-groups on trajectories of substance use.
Keywords: Polygenic Risk Score, Substance Use, Longitudinal, Gene-environment interaction
Childhood maltreatment is associated with increased substance use frequency during adolescence and early adulthood (Traube, James, Zhang, & Landsverk, 2012), a time when substance use behaviors are most prevalent in terms of frequency of use (Feinstein, Richter, & Foster, 2012). Among adolescent victims of childhood maltreatment, substance use frequency is typically higher than among adolescents without maltreatment exposures and is linked to subsequent high frequency of substance use in early adulthood (Garner, Hunter, Smith, Smith, & Godley, 2014; Traube et al., 2012). However, there is significant inter-individual variability in exposure to childhood maltreatment (Debowska, Willmott, Boduszek, & Jones, 2017), and these differences in exposure may be differentially related to substance use outcomes. Research suggests that children with maltreatment exposure are likely to experience multiple maltreatment types with varying degrees of chronicity (Debowska et al., 2017). Therefore, it is important to understand the role of multi-type childhood maltreatment exposures based on differing degrees of chronicity (i.e., how often the exposure occurred in childhood) on substance use frequency change over time. Such an evaluation is important because different combinations of maltreatment exposures (based on type and chronicity) may have differing associations with substance use frequency at any given time and over time. In addition to social exposures, research also suggests genetic influences for high substance use frequency (Neiderhiser, Reiss, Hetherington, & Plomin, 1999). However, few studies have evaluated genetic risk for substance use on the development of substance use frequency from adolescence to early adulthood within the context of childhood maltreatment exposure.
The present study will bridge these gaps in current knowledge by evaluating substance use trajectories from ages 11 to 26 for sub-groups of individuals with different chronicity of co-occurring childhood maltreatment exposure types. Additionally, a substance use polygenic risk score (i.e., genetic risk based on a combination of multiple genetic markers) will be tested as a moderator of the association between childhood maltreatment sub-group membership and change over time in substance use frequency from adolescence to early adulthood (ages 11 to 26).
Childhood Maltreatment and Substance Use
Frequent substance use among adolescent survivors of childhood maltreatment is linked to high prevalence of substance use frequency during early adulthood (Dubowitz, Roesch, & Lewis, 2021; Garner et al., 2014). To illustrate, among youth exposed to childhood maltreatment, there was a 10% increase in substance use frequency during the transition from adolescence to adulthood (Narendorf & McMillen, 2010). The chronicity of maltreatment exposure during childhood can further exacerbate substance use frequency during adolescence and early adulthood (Garner et al., 2014; Narendorf & McMillen, 2010), and substance use may be a coping mechanism for alleviating stress following childhood maltreatment exposure (Oshri, Tubman, & Burnette, 2012). Studies of childhood maltreatment effects on trajectories of substance use frequency over time, however, are limited in number and somewhat mixed in their findings. Whereas some evidence supports that childhood maltreatment is associated with increases in substance use frequency during adolescence (Kosty, Seeley, Farmer, Stevens, & Lewinsohn, 2017), others demonstrate that childhood maltreatment is associated with high stable levels of substance use frequency but not increases during adolescence (Wilson, Samuelson, Staudenmeyer, & Widom, 2015).
Emotional abuse, physical abuse, and neglect types of childhood maltreatment in particular have emerged as impactful for substance use frequency during adolescence (Benedini & Fagan, 2020; Huang et al., 2011; Kisely, Strathearn, & Najman, 2021). Studies evaluating multi-type childhood maltreatment exposure reveal similar results and illustrate that all abuse types (physical abuse, emotional abuse, and sexual abuse) impact substance use frequency during adolescence and young adulthood (Kisely et al., 2021; Rogers, McKinney, & Asberg, 2018). However, few studies examine the association between multiple co-occurring childhood maltreatment exposures (based on both type and chronicity of exposure) and trajectories of substance use frequency from adolescence to early adulthood. Such an examination will allow us to understand the effects of multiple co-occurring childhood maltreatment on substance use frequency during critical life periods such as adolescence and early adulthood when the risk for substance use frequency is the highest (Park-lee & Tice, 2017).
Substance Use during Adolescence and Early Adulthood
Any substance use during adolescence is considered a problem behavior that has implications for continued use over time and is detrimental to health outcomes (Fishbein, Rose, Darcey, Belcher, & VanMeter, 2016; Johnston et al., 2021; Sawyer, Azzopardi, Wickremarathne, & Patton, 2018; Schulte & Hser, 2013). Substance use frequency among adolescents typically increases over time, and can persist well into early adulthood (Johnston et al., 2021). And, 90% of adults with substance use problems report first substance use experiences during adolescence (Feinstein et al., 2012; Johnston et al., 2021; Sawyer et al., 2018). Specifically, high substance use frequency during adolescence is associated with an even higher frequency of substance use during early adulthood (Johnston et al., 2016; Park-Lee & Tice, 2017). High frequency of substance use can have life-long detrimental effects on overall health and well-being such as heart disease, cancer, bipolar disorder, anxiety, depression, vehicular accidents (Schulte & Hser, 2013) as well as impact adolescent and early adult brain development (Fishbein et al., 2016).
Given the significance of substance use in adolescence for the continuation of substance use behaviors in early adulthood, it is important to evaluate the development of substance use frequency over time from adolescence to early adulthood. Moreover, childhood maltreatment experiences can be consequential for substance use as a stress-coping mechanism (Hall, 2016; McKinney & Renk, 2011; Paus, Keshavan, & Giedd, 2010). Taken together, it is likely that certain combinations of childhood maltreatment can result in greater stress and a higher likelihood that substance use increases over time from adolescence to early adulthood. Understanding these associations could lead to more targeted prevention and intervention efforts for sub-groups with specific constellations of childhood maltreatment exposures at greater risk for high substance use frequency or persistent substance use over time during these critical developmental periods.
Genetic Risk for Substance Use
In addition to social environment exposures, such as child maltreatment, research also suggests genetic influences for substance use frequency (Bierut, 2011; Iacono, Carlson, Taylor, Elkins, & McGue, 1999; Neiderhiser, Marceau, & Reiss, 2013; Rende & Slomkowski, 2008; Trucco, Madan, & Villar, 2019). Recent advances in molecular genetics and genome-wide association studies have demonstrated that genetic risk for most complex phenotypes such as substance use are polygenic in nature (i.e. combined effect of multiple genes; Maier, Visscher, Robinson, & Wray, 2017). A majority of genome-wide association studies have been conducted with individuals of European ancestry and it is recommended to conduct such analysis separately by ancestry due to population stratification or differences in allelic inheritance. Further, it has been shown that polygenic scores trained with a specific ancestry type do not always perform well for individuals of a different ancestry (Braudt & Harris, 2018; Dudbridge, 2013).
There is also evidence suggesting that genetic influences continually interact with environmental factors to predict substance use outcomes (e.g., G × E). Genetic studies of substance use have demonstrated the moderating effects of genetic risk for substance use on the association between environmental stress (e.g., harsh parenting, parental warmth, deviant peer affiliation) and substance use frequency particularly in samples of individuals of European descent (Creemers et al., 2011; Gorwood et al., 2012; Harden, Hill, Turkheimer, & Emery, 2008; Neiderhiser et al., 1999; Salvatore, 2018). Given the litany of problems, such as cardiovascular disease, poor mental health, injuries, accidents, and cancers, that are associated with early and frequent substance use (Schulte & Hser, 2013), the role of polygenic risk for substance use in the association between childhood maltreatment exposure and subsequent substance use frequency change over time needs further evaluation.
According to the diathesis-stress models (Ingram & Luxton, 2005; Sigelman & Rider, 2009), high biological risk and high environmental stress interact to synergistically result in negative outcomes such as high substance use frequency. For example, negative parenting and genetic risk can interact to produce stronger substance use phenotypes (Creemers et al., 2011). However, this theoretical model also posits that the presence of biological vulnerabilities alone is not sufficient for negative outcomes. It is the interaction of impoverished environments and biological predispositions that results in specific negative outcomes. Further, different environmental adversities interact differently with biological predispositions (Belsky & Pluess, 2009; Ingram & Luxton, 2005). Therefore, in this study, we expect that more chronic exposure to certain types of childhood maltreatment exposures (specifically physical abuse, emotional abuse, and neglect as identified by previous research; Huang et al., 2011) will interact with genetic risk for substance use to produce greater initial substance use frequency and increases over time in substance use frequency until early adulthood (i.e., until age 26).
Study Aims
The present study builds on and extends previous research and theory by addressing two primary aims. The first aim is to assess the association of childhood maltreatment sub-group membership - determined by the chronicity (frequency) of exposure to multiple types of maltreatment - with change over time (or trajectory) in substance use frequency from adolescence into early adulthood (from ages 11 to 26). It is hypothesized that childhood maltreatment sub-groups with more chronic physical abuse, emotional abuse, and neglect will have increases over time in substance use frequency from ages 11–26 consistent with prior literature.
The second aim is to examine substance use polygenic moderation of the association between childhood maltreatment sub-group membership with change over time in substance use frequency from adolescence into early adulthood (age 11–26). It is hypothesized that high polygenic risk for substance use will exacerbate change over time for all maltreatment exposures but will be most critical for sub-groups with more chronic exposures to physical abuse, emotional abuse, and neglect. To our knowledge, no previous study has evaluated the synergistic association of multiple childhood maltreatment sub-groups and genetic risk for substance use on substance use trajectories from adolescence to early adulthood.
Methods
Participants
The data for this study come from The National Longitudinal Study of Adolescent to Adult Health (Add Health; Harris, 2013). Add Health is a longitudinal panel study of adolescents (N = 20,743) between 7th and 12th grade at the first wave of data collection (1994–95). Three additional waves of data were conducted wave 2: 1996, wave 3: 2001–2002, and wave 4: 2008–2009. Wave 2 was conducted a year after Wave 1 completion and the sample for Wave 2 included 7th to 11th graders who were in the study at wave 1 and a special sub-sample of 12th graders who were part of the genetic/adoption studies. In subsequent waves, original wave 1 respondents were included in the sample. Data were collected using paper-based, face-to-face and computer-assisted in-person interviews. The Add Health sampling design is a multiple-stage, school-based (clustered), stratified design with unequal selection probabilities of observations (i.e. certain minority groups were oversampled). Out of the core sample, 12,234 participants agreed to the archival of DNA data (dbGaP accession phs001367.v1.p1; Braudt & Harris, 2018). The present study utilizes a sub-sample of 2,664 unrelated European Americans from the DNA archival data, who also had retrospective childhood maltreatment reports at waves 3 and 4. We restricted the sample to European Americans as prescribed by Add Health researchers and previous genetic studies due to differences in the inheritance of allele frequency based on ancestry and due to evidence that polygenic scores created from original studies of European ancestry do not perform well in samples of individuals from other different ancestry (Braudt & Harris, 2018; Dudbridge, 2013). Therefore, it will be important for future research to evaluate the aims of this study with more racially and ethnically diverse samples.
Measures
Child Maltreatment.
Retrospective measures assessing child maltreatment exposure: physical abuse (e.g., “How often had your parents or other adult care-givers slapped, hit, or kicked you?”), sexual abuse (e.g., “How often had one of your parents or other adult care-givers touched you in a sexual way, forced you to touch him or her in a sexual way, or forced you to have sexual relations?”), emotional abuse (e.g., “How often did a parent or other adult caregiver say things that really hurt your feelings or made you feel like you were not wanted or loved?”), and neglect (“How often had your parents or other adult care-givers not taken care of your basic needs, such as keeping you clean or providing food or clothing?”) prior to age 18 were administered at waves 3 and 4. Two items were used to assess neglect (at wave 3), one item assessed sexual abuse (at waves 3 and 4), one item assessed physical abuse (at waves 3 and 4), and one item assessed emotional abuse (at wave 4). All items were measured with a frequency count (i.e. measure of chronicity) for each maltreatment type and coded on intervals (e.g., 1 = one time, 2 = two times, 3 = three to five times, 6 = six to ten times, 11 = more than 10 time). Mean scores across items and waves for sexual and physical abuse and across items for neglect were created. These mean scores were subsequently used in a latent profile analysis to create sub-groups of individuals with exposure to similar types and chronicity of childhood maltreatment. Retrospective reports have been used in previous research to understand the impact of adverse childhood experiences, including child maltreatment, on health (Suglia, Clark, Boynton-Jarrett, Kressin, & Koenen, 2014) and health behaviors (i.e., substance use behaviors; Hughes et al., 2017).
Substance Use.
Self-reported use of marijuana and other drug use (LSD, PCP, ecstasy, mushrooms, speed, ice, heroin, and pills) within the last 30 days was reported at waves 1–3 wherein participants reported the number of times they used these substances. Alcohol use (waves 1–3) was assessed by 12-month use and re-scaled to 30-day use. Responses of 30 or more times were top-coded at 30. Alcohol use (waves 1–3) was originally assessed by 12-month use and were originally coded as: 0 = “never”, 1 = “once or twice”; 2 = “once a month”; 3 = “2–3 days a month”; 4 = “once or twice a week”, 5 = “3–5 days a week”; 6 = “nearly every day”. Alcohol items were re-coded as count variables (so that they would be on the same scale as marijuana and illicit drug) to assess monthly use. The revised coding included: 0 = “never”; 1 = “once a month”; 2 = “2–3 days a month”; 4 = “4–8 days a month” (recoded original coding of once or twice a week to approximate number of days in a month); 12 = “12–20 days a month” (recoded 3 to 5 days a week to reflect the approximate number of days in a week); and 30 = “30 plus days a month” (recoded all responses of nearly every day). The alcohol use scale was treated as a continuous variable and conservative values for monthly use were estimated so that alcohol use frequency would be similar to the other two substances (i.e., an estimation of the number of times on average the respondent used alcohol in a month). An average substance use scale was created to assess the average monthly substance use at each wave. The creation of this average substance use scale mimicked that from previous research used to evaluate overall substance use (Litwiller & Brausch, 2013; Park-lee & Tice, 2017), with higher frequency of use (assessed by number of times per month) indicating more substance use behaviors.
Substance Use Polygenic Risk Score.
The Add Health genetic data (dbGaP accession phs001367.v1.p1) at wave 3 were used in the present research (genotype platform used: Illumina HumanOmni1-Quad chip and the imputation data are from the HRC r1.1 2016 reference panel). Single nucleotide polymorphisms (SNPs) for genes related to substance use were used to create a substance use polygenic risk score. Specifically, genome-wide significance levels p < 5 × 10−8 (from the original genome-wide association studies), were used to identify SNPs for inclusion in the substance use polygenic risk score from multiple genome-wide association studies. SNPs were selected from 18 genome-wide studies of substance use, alcohol use, marijuana use, illicit drug use, and substance use biomarker related phenotypes to create one single genetic risk index for substance use (See supplemental Table S1 for a full list of studies used for the initial list of SNPs selected). Fifteen SNPs from 14 genes (and 5 genome-wide studies) were included after quality control steps were undertaken as prescribed in extant research (Marees et al., 2018). Quality control steps and computation of polygenic risk score are detailed in supplementary materials. The number of alleles for all SNPs that were indicated in the genome-wide association studies as related to higher frequency of substance use (i.e., effect allele) were multiplied with the corresponding Cohen’s D effect size estimates using the same procedure. Subsequently, estimates from all the SNPs were pooled (summed) to obtain a single risk score for substance use for each participant. See supplemental table S2 for a list of genes, SNPs, effect alleles, and corresponding effect size estimates used to create the polygenic risk score.
Covariates.
Biological sex (1 = Male, 0 = Female) and parental years of education were included as covariates. The respondent’s highest educational attainment (years of education) was also included as a covariate. Covariates were selected to account for confounding and only included those variables that were not causally linked from the predictor to the outcome variables (or mediators; Weng, Hsueh, Messam, & Hertz-Picciotto, 2009). Sample descriptive statistics are described in Table 1.
Table 1.
Sample Descriptive Statistics (n = 2,664)
| Key Variables | Mean | Std. Dev |
|---|---|---|
|
| ||
| Sexual abuse | 0.31 | 1.56 |
| Physical abuse | 1.47 | 3.13 |
| Emotional abuse | 2.22 | 3.61 |
| Neglect | 1.83 | 3.70 |
| Age Wave 1 | 16.10 | 1.69 |
| Age Wave 2 | 16.16 | 1.60 |
| Age Wave 3 | 21.93 | 1.75 |
| Substance Use Polygenic Score | 0.13 | 0.05 |
| Substance Use Frequency Wave 1 | 4.29 | 9.27 |
| Substance Use Frequency Wave 2 | 5.30 | 10.31 |
| Substance Use Frequency Wave 3 | 6.96 | 10.58 |
| Parent Education (in years) | 13.28 | 2.33 |
| Respondent Education (in years) | 14.27 | 2.10 |
|
| ||
| Percentages | Mean | Std. Dev |
|
| ||
| Gender: Male | 47.22% | 0.49 |
Analytic Strategy
Step 1 Latent Profile Analysis.
Latent profile analysis (Lanza & Cooper, 2016) was used to determine homogenous sub-groups experiencing similar combinations of childhood maltreatment. Respondents were categorized into subgroups based on similar exposures to emotional abuse, neglect, sexual abuse, and physical abuse. These subgroups were used as predictors of the substance use levels in early adolescence and change over time. Three sub-groups emerged from the latent profile analysis, including a normative sub-group with low maltreatment exposure, which was the reference sub-group across all subsequent analytic models (described in detail below in the results section). AIC, BIC, adjusted-BIC, and entropy were used to discern the best class solution as well as replication of models when the number of random starts were increased (i.e., if the models converged on a global solution).
Step 2 Substance Use Trajectory.
Substance use frequency change was assessed using linear trajectory models to obtain population-level trajectories for substance use. An intercept was centered at age 11 and the average annual change in substance use frequency was estimated from ages 11 to 26 (i.e., change in substance per year each year or slope; Model 1). Model constraints were used such that age in years was the unit of time. This was done to understand age related yearly development of substance use. For instance, if an individual started the study at age 14 and was age 23 at wave 3, their substance use trajectory was estimated using the ages at which they were observed. Therefore, each individual’s trajectory was based on their age at the beginning of the study until their age at wave 3 and then information across all individuals was pooled. Substance use trajectories were then conditioned on the maltreatment sub-groups that emerged from the latent profile analysis to get subpopulation estimates of change over time in substance use for each sub-group (unconditional model with no covariates).
Step 3 Hypothesis Testing.
In step three, we estimated the direct association of the childhood maltreatment sub-groups and substance use polygenic risk score, with substance use levels (at age 11) and average annual change over time until age 26 while accounting for the effects of all covariates on both levels and change of substance use over time (Model 2). Next, the substance use polygenic risk score was evaluated as a moderator of the association between sub-groups of childhood maltreatment exposures and substance use levels and change (Model 3) using an interaction between polygenic risk score and maltreatment subgroups. Maltreatment associations with substance use trajectories were probed at different levels of genetic risk: high (+1 SD above the mean), low (−1 SD below the mean) and medium (mean levels for the sample; Aiken, West, & Reno, 1991). All covariates were included for Model 3 as well.
SAS statistical software was used for data preparation, Plink v1.9 was used for cleaning and coding genomic data, and Mplus v.8 (Muthén & Muthén, 2020) statistical software was used for estimation of analytic models. A maximum likelihood estimator and an integration algorithm was used in Mplus (Muthén & Muthén, 2020) to obtain estimation with robust standard errors in the presence of missing data. Add Health created sampling weights were applied to correct for unequal selection bias and a cluster sandwich estimator was used to correct for school-level clustering of individuals (Binder, 1983; Chen & Chantala, 2014). All predictor variables and covariates were grand mean centered prior to analysis for the analytic model.
Post-hoc Models.
Model 3 was also tested separately in post-hoc models for two of the most commonly used substances in adolescence and early adulthood – alcohol and marijuana.
Results
Step 1: Latent Class Analysis.
A three sub-group solution was considered optimal for the latent profile analysis. Model fit statistics are summarized in Table S3. The three sub-group solution had better overall model fit statistics compared to the two sub-group solution and replicated when random starts were increased. The four sub-group solution did not replicate when random starts were increased. Sub-groups labels were applied for the three sub-group solution and were based on exposure to childhood maltreatment type and frequency. Sub-group 1: Sub-group with high levels of sexual abuse and moderate physical abuse, emotional abuse and neglect (n = 62 or 2.3%; high sexual abuse sub-group); Sub-group 2: Sub-group with high physical abuse and moderate levels of neglect and emotional abuse (n = 263 or 9.9%; high physical abuse sub-group); and Sub-group 3: Sub-group with low frequency of all maltreatment types (n = 2,339 or 87.8%; normative sub-group). Figure 1 shows the exposure frequency for each maltreatment type for the subgroups.
Figure 1.
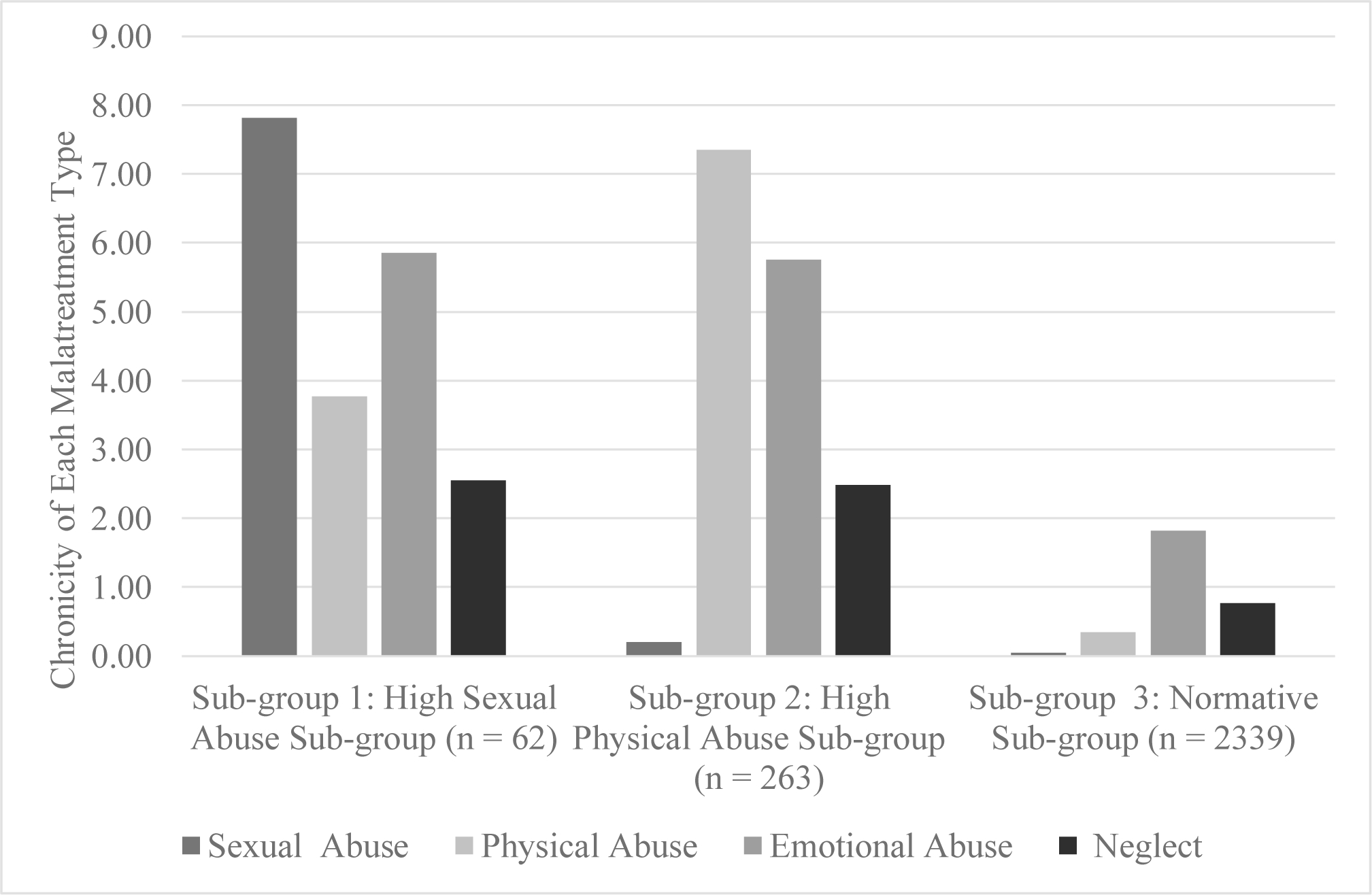
Subgroups of childhood maltreatment based on types and chronicity of exposure.
We also tested if exposure to different types of maltreatment frequencies differed across the three sub-groups. The results from this analysis are summarized in supplemental Table S4. The high sexual abuse sub-group and the high physical abuse sub-group had significantly higher frequency of all maltreatment types compared to the normative sub-group. The high sexual abuse sub-group had significantly higher frequency of childhood sexual abuse exposure and significantly lower frequency of childhood physical abuse exposure compared to the high physical abuse sub-group, but both these sub-groups had similar levels of childhood emotional abuse and neglect exposures. Correlations among study variables is summarized in supplemental Table S5.
Step 2: Substance Use Trajectory.
On average, adolescents (i.e., the entire sample) reported an increase in substance use frequency by 0.21 per year from age 11 to 26 (p = < 0.001). Based on this trajectory, by age 26, respondents were using substances 2.5 times on average every month. There were maltreatment sub-group specific differences in frequency of substance use trajectories over time. Substance use frequency trajectories by each sub-group is presented in Figure 2.
Figure 2.
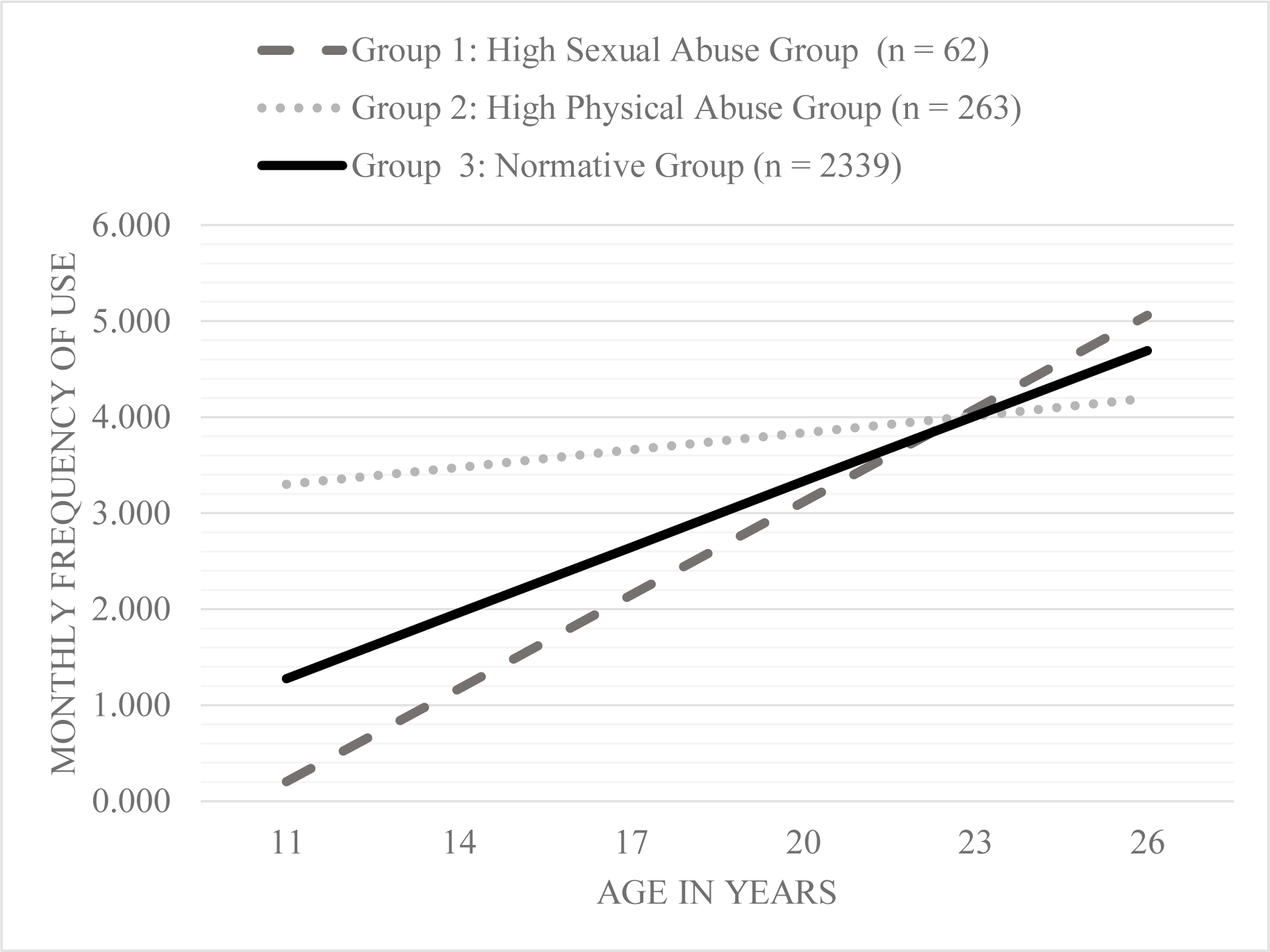
Average substance use frequency change over time by maltreatment sub-groups based on chronicity of four types of maltreatment.
On average, the normative sub-group (β = 0.16; p < 0.001) and the high sexual abuse sub-group (β = 0.29, p = 0.06) had increases over time in substance use frequency each year from adolescence to early adulthood in the unconditional model (i.e., model without any covariates). In contrast, on average, the high physical abuse sub-group did not have change in substance use frequency over time in the unconditional model (β = 0.03, p = 0.65). However, there was notable variance in substance use frequency change over time (or slopes) for the normative sub-group (σ = 20.74, p < 0.001), high sexual abuse sub-group (σ = 10.64, p = 0.001), and high physical abuse sub-group (σ = 30.71, p < 0.001).
Step 3: Hypothesis Testing.
Results from Models 2 and 3 for substance use frequency are summarized in Table 2. Neither maltreatment sub-group experienced change over time in substance use frequency that was different compared to the normative sub-group after controlling for substance use polygenic risk score and other covariates. However, in Model 3, there was an interaction between substance use polygenic risk score and high physical abuse sub-group as well as an interaction between substance use polygenic risk score and high sexual abuse sub-group for average substance use frequency trajectories.
Table 2.
Association of maltreatment sub-groups and genetic risk with initial frequency and change in frequency of substance use over time
| Substance Use Frequency at Age 11 | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Model 2 (substance use frequency) | Model 3 (substance use frequency) | |||||
| β | s.e | p | β | s.e | p | |
|
|
||||||
| High Sexual Abuse Sub-group | −0.04 | 0.04 | 0.23 | −0.19 | 0.03 | 0.45 |
| High Physical Abuse Sub-group | 0.10 | 0.07 | 0.15 | 0.12 | 0.07 | 0.11 |
| Substance Use Polygenic Risk Score | −0.09 | 0.04 | 0.03 | −0.05 | 0.05 | 0.26 |
| Respondent Education (in years) | −0.12 | 0.07 | 0.08 | −0.13 | 0.07 | 0.05 |
| Parent Education (in years) | −0.01 | 0.07 | 0.91 | 0.03 | 0.07 | 0.64 |
| Biological Sex | −0.07 | 0.05 | 0.19 | −0.07 | 0.05 | 0.14 |
| High Sexual Abuse Subg-roup*Substance Use Polygenic Risk Score | - | - | - | 0.06 | 0.01 | 0.00 |
| High Physical Abuse Sub-group*Substance Use Polygenic Risk Score | - | - | - | −0.15 | 0.01 | 0.00 |
| Substance Use Frequency Change Over time | β | s.e. | p | β | s.e. | p |
|
| ||||||
| High Sexual Abuse Sub-group | 0.05 | 0.04 | 0.22 | 0.03 | 0.03 | 0.34 |
| High Physical Abuse Sub-group | −0.06 | 0.06 | 0.30 | −0.07 | 0.06 | 0.24 |
| Substance Use Polygenic Risk Score | 0.05 | 0.04 | 0.23 | 0.01 | 0.04 | 0.90 |
| Respondent Education (in years) | 0.05 | 0.06 | 0.45 | 0.06 | 0.06 | 0.33 |
| Parent Education (in years) | 0.21 | 0.04 | 0.00 | 0.23 | 0.04 | 0.00 |
| Biological Sex | 0.03 | 0.06 | 0.64 | 0.00 | 0.05 | 0.97 |
| High Sexual Abuse Sub-group*Substance Use Polygenic Risk Score | - | - | - | −0.03 | 0.06 | 0.02 |
| High Physical Abuse Sub-group*Substance Use Polygenic Risk Score | - | - | - | 0.11 | 0.05 | 0.02 |
Specifically, at low (β = 0.21, p = 0.23), medium (β = 0.21, p = 0.89), and high (β = 0.21, p = 0.31) polygenic risk for substance use, members of the high sexual abuse sub-group did not have an initial substance use frequency different than 0. Similarly, at low (β = 0.35, p = 0.02; see Figure 2.4) and medium (β = 0.34, p = 0.01; see Figure 2.5) but not high (β = 0.34, p = 0.73; see Figure 2.5) polygenic risk, the high physical abuse sub-group had an initial substance use level that was different from 0.
At low (β = 0.21, p < 0.01), medium (β = 0.19, p = 0.01), and high levels (β = 0.17, p = 0.08 Figures 3–5) of polygenic risk for substance use, there was a positive association (albeit a marginal association at high levels) between membership in the high sexual abuse sub-group and substance use frequency change over time. Additionally, at high levels of substance use polygenic risk, there was a positive association between the high physical abuse sub-group members (β = 0.21, p < 0.01; Figure 5) and substance use frequency change over time.
Figure 3.
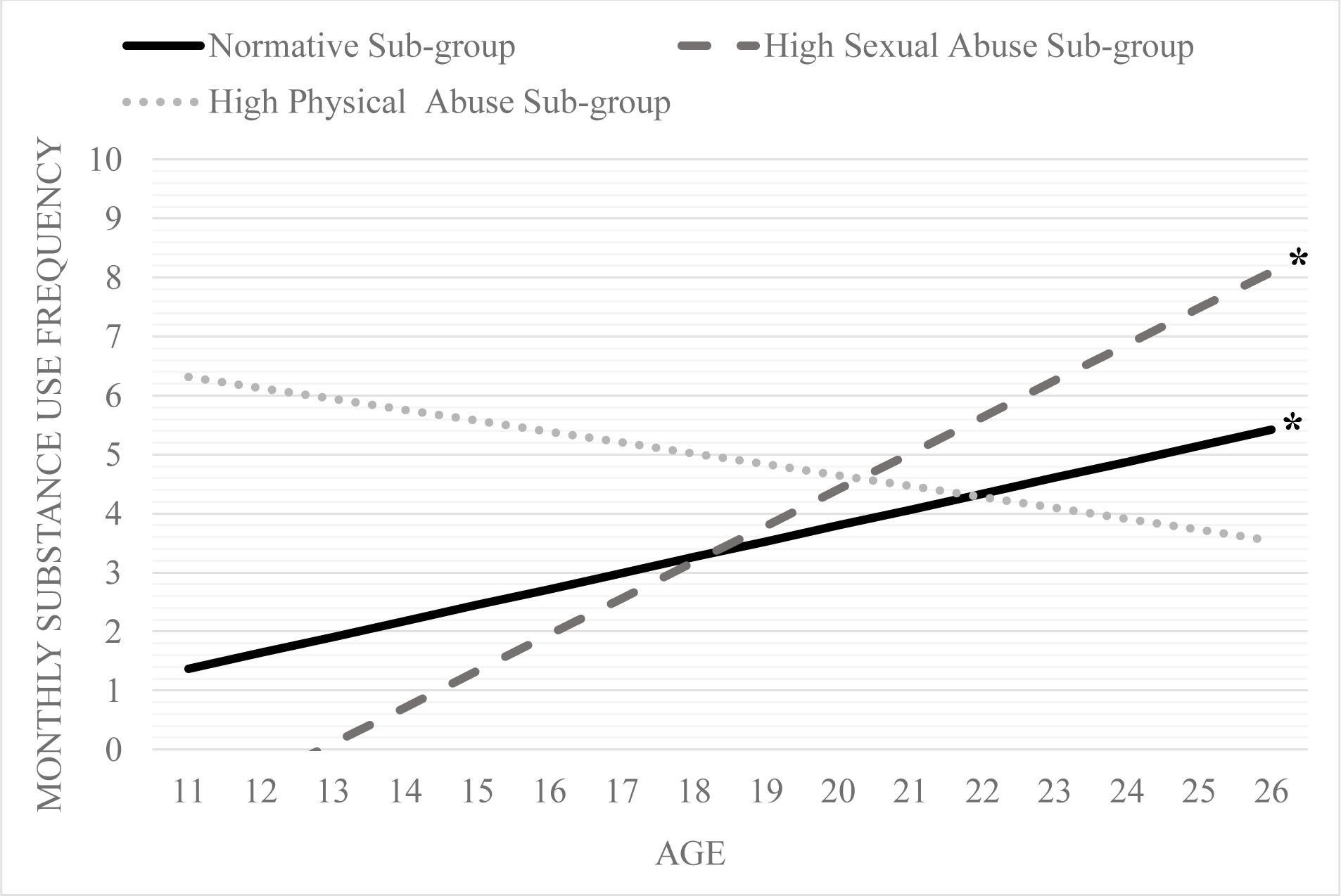
Change over time in substance use frequency for the three maltreatment sub-groups at a) low levels of substance use polygenic risk score (i.e., −1 SD).
Note: trajectories at α <= 0.05 are denoted by * and 0.10 are denoted by +
Figure 5.
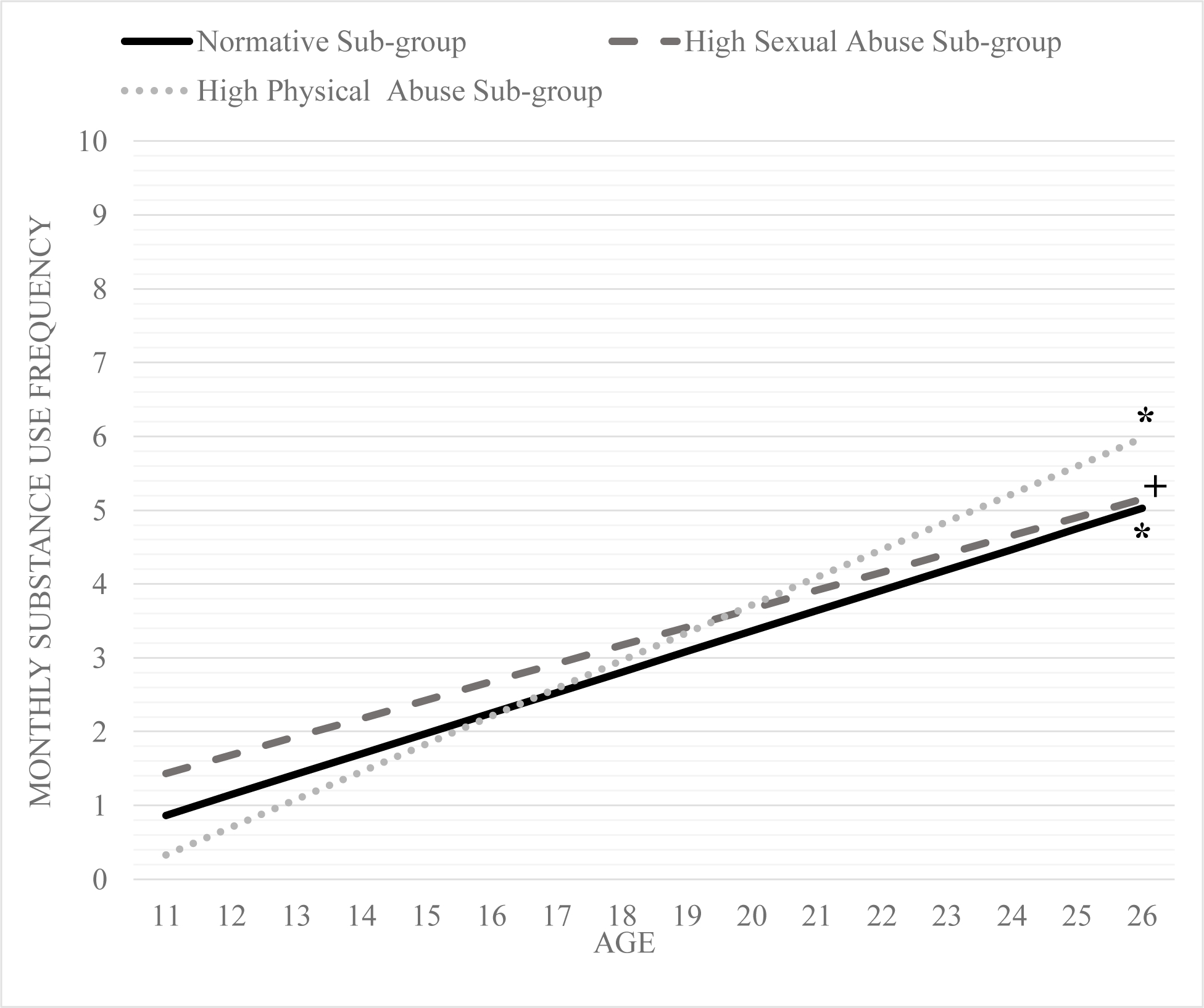
Change over time in substance use frequency for the three maltreatment sub-groups at high levels of substance use polygenic risk score (i.e.,+1 SD).
Note: trajectories at α <= 0.05 are denoted by * and 0.10 are denoted by +
To elaborate, at high, medium, and low levels of polygenic risk score, the high sexual abuse sub-group reported increases in average monthly substance use frequency over time. The high sexual abuse sub-group had the highest increases in average monthly substance use frequency at low levels of polygenic risk with an approximate 0.61 increase in average monthly use per year. In comparison, the high sexual abuse sub-group had slower increases in average monthly substance use frequency at high levels of polygenic risk score with approximately 0.25 increase in average monthly substance use frequency per year. At medium polygenic risk for substance use, the high sexual abuse sub-group had a 0.43 average monthly increase per year. Moreover, the rate of change in substance use frequency over time for the high sexual abuse sub-group was higher than the normative sub-group at low and medium levels of polygenic risk.
The high physical abuse sub-group demonstrated increases in substance use frequency only at high polygenic risk for substance use. The yearly increase in average monthly substance use frequency was 0.38. The high physical abuse sub-group had the strongest/highest increases in substance use frequency at high polygenic risk score compared to the other two sub-groups at high genetic risk. The effect size estimate for average monthly substance use frequency increases per year were small to medium for the high sexual abuse sub-group and medium for the high physical abuse sub-group.
Post-hoc Models
Results from Model 3 for the frequency of specific substance (i.e., alcohol and marijuana) use are summarized in supplementary materials as well as supplementary Tables S6–S7 and Figures S1–S2. There were increases in monthly alcohol use over time for the high sexual abuse sub-group at low and medium levels of polygenic risk but not at high levels of polygenic risk. At high polygenic risk for substance use, the high physical abuse sub-group had average monthly increases of 1.14 times in marijuana use frequency and the high sexual abuse group had average monthly decreases of 0.77 in marijuana use frequency per year.
Discussion
This study identified co-occurring maltreatment subgroups based on both type and chronicity of exposure in order to address two main hypotheses. First, it was hypothesized that maltreatment sub-groups with more chronic physical abuse, emotional abuse, and neglect would have increases over time in substance use frequency (Hypothesis 1). Second, it was hypothesized that a high substance use polygenic risk score – indicating greater risk for substance use phenotype – would exacerbate substance use change over time for all maltreatment exposures but would be most critical for sub-groups with more chronic exposures to physical abuse, emotional abuse, and neglect (Hypothesis 2).
Childhood Maltreatment Sub-groups
We identified three sub-groups of co-occurring multi-type childhood maltreatment (high physical abuse sub-group; high sexual abuse sub-group, and normative sub-group) and these findings map onto previous research with a different national sample of middle-aged adults that examined retrospective childhood maltreatment exposure based on both type and chronicity of exposure (Authors, 2019). In this study, we found sub-groups of co-occurring childhood maltreatment exposures based on retrospective reports in a national sample of young adults that were similar to previous studies.
The maltreatment sub-groups identified indicate that certain co-occurring maltreatment exposures may be recalled more frequently among individuals compared to others. A large body of literature on trauma-focused research indicates that events that are perceived as more traumatic are often remembered or recalled throughout life and can be more detrimental for lifelong outcomes such as substance use (Center for Substance Abuse Treatment, 2014). Therefore, it is likely that the recollection of specific co-occurring childhood maltreatment exposures may largely depend on the trauma induced by such experiences and could potentially be more detrimental for negative outcomes, even though other forms of childhood maltreatment (e.g., physical neglect) are more prevalent forms (Olson & Stroud, 2012).
Childhood Maltreatment and Substance Use Trajectory
In line with previous research, we also find overall increases in substance use frequency over time from adolescence to early adulthood in the unconditional model (L D Johnston et al., 2016). Specifically, adolescents in the full sample reported a substance use frequency of 1.44 times per month at age 11 and approximately 4.7 times by age 26. Given that high levels of substance use frequency during adolescence are associated with high levels of substance use frequency in early adulthood (Johnston et al., 2016; Park-Lee & Tice, 2017), our findings reiterate the importance of adolescence as a developmental period for substance use development over time. However, in model 2 neither maltreatment sub-group had an initial substance use frequency that was different than 0 or a change in substance use frequency over time. Therefore, hypothesis 1 was not supported. Nonetheless, there were significant interaction effects in support of hypothesis 2 which are discussed below.
Childhood Maltreatment x Substance Use Polygenic Risk Score
Findings for High Physical Abuse Sub-group
Based on results from previous research (Huang et al., 2011), it was anticipated that individuals who had chronic exposure to a combination of physical abuse, emotional abuse, and neglect would demonstrate the most detrimental substance use trajectory over time particularly at high genetic risk. We found that members of the high physical abuse sub-group who reported high chronicity of co-occurring physical abuse and moderate chronicity of emotional abuse and neglect exposures had the most detrimental substance use development over time at high (+1 SD) substance use polygenic risk. This finding is consistent with the diathesis-stress framework (Ingram & Luxton, 2005; Sigelman & Rider, 2009), and indicates that combined exposure to physical abuse, emotional abuse, and neglect, together with high genetic risk or biological predisposition towards substance use, act in an interactive manner to influence substance use development during critical developmental periods (i.e., from adolescence to early adulthood).
A closer examination of specific substances used helped further clarify the findings from hypothesis 2 for the high physical abuse sub-group. To illustrate, the high physical abuse sub-group at high genetic risk displayed similar patterns for marijuana use change over time (i.e., similar to the overall substance use trajectory at high genetic risk). Members of the high physical abuse sub-group demonstrated faster increases in marijuana use frequency at high genetic risk for substance use in comparison to the other sub-groups and marijuana use for this sub-group may be propelling the overall substance use trajectory at high genetic risk.
Drug use, including marijuana use, may require some personal affinity towards seeking out those substances. Even though recreational marijuana use is currently legal in a few states in the U.S., it remains illegal in a majority of states and at the federal level. Moreover, the three waves of data used in this study come from a time period when the recreational use of marijuana was illegal across all states. It can be speculated that seeking out illegal substances may be rooted in a biologically affinity or desire towards using such illicit substances. Therefore, a broader biosocial model of development is critical for understanding how maltreatment exposures such as those experienced by the high physical abuse sub-group transmit their influence on substance use frequency over time.
Findings for High Sexual Abuse Sub-group
Contrary to the findings for the high physical abuse sub-group, the findings for hypothesis 2 for the high sexual abuse sub-group largely point towards a stronger and more ubiquitous influence of the social environment on substance use development. Specifically, the high sexual abuse sub-group had increasing substance use frequency over time across all levels of polygenic risk and the overall substance use trajectories for this sub-group may largely be driven by their alcohol use frequency over time. Given, alcohol is more socially acceptable and is available for legal purchase, it may be used more frequently by members of the high sexual abuse sub-group to alleviate the trauma associated with sexual abuse experiences. Since the high sexual abuse sub-group demonstrated increases in substance use and alcohol use over time at all levels of genetic risk, their substance use behaviors may not depend as much on genetic risk but more on their exposure to sexual abuse - especially for individuals in the sexual abuse sub-group who had average or low genetic risk because these individuals had the fastest increases in substance use over time. It is important to keep in mind that the sample size for the high sexual abuse sub-group at all levels of genetic risk may be too small to capture the true association between the high-sexual abuse sub-group membership and substance use at levels of genetic risk. Replication of these findings will be necessary in larger samples with similar child maltreatment exposures as the high sexual abuse sub-group.
A potential explanation for such maltreatment-related substance and alcohol use change over time could be the presence of other psychological mechanisms. It has been well-established in previous literature that childhood sexual abuse is associated with high levels of trauma as well as internalizing and mental health problems throughout life (Murray, Nguyen, & Cohen, 2014). Victims of childhood sexual abuse may use alcohol as a coping mechanism to deal with the trauma, distress, and psychological burdens of sexual abuse. The easy availability of alcohol could further add to this problem. Additionally, as members of this sub-group develop a tolerance for alcohol, they may increase their alcohol use frequency over time to get the same feelings of “high” as before (Sinha, 2008). However, these mechanisms linking the high sexual abuse sub-group to substance use and alcohol use frequency increases over time are merely speculative and will need examination in future research.
It will also be imperative for future research to examine additional pathways to better understand the association between high sexual abuse sub-group membership and substance use (and use of specific substances) while accounting for genetic risk. Findings from previous research have shown that sexual abuse sub-group membership may be associated with depressive symptoms in adulthood via psycho-social pathways and in comparison, the high physical abuse sub-group was associated with depressive symptoms through biological pathways (Authors, 2019). In fact, we do find that biological (i.e., genetic) pathways were particularly salient for the high physical abuse sub-group and were associated with increases in substance use frequency over time. Similarly, it is likely that psychosocial factors may explain the associations between high sexual abuse sub-group membership and substance use frequency over time.
In summary, mitigation efforts for the high sexual abuse sub-group can be focused on prevention of substance use and alcohol use, and can be implemented uniformly for all individuals (Torkamani, Wineinger, & Topol, 2018) who report similar maltreatment exposures since increases over time for substance use in this sub-group are most likely linked to environmental risks. However, for the high physical abuse sub-group, future prevention efforts should increase the dosage of intervention for individuals who have high genetic risk (Torkamani et al., 2018) for substance use since this sub-group of individuals experience a double dose of risk (i.e., maltreatment × high genetic risk).
Limitations
It should be noted that these findings have several limitations. First, this study is restricted to those with European American ancestry. Although the large sample size of this study and the use of survey methods (weight and clustering) correct for selection bias and allow us to generalize findings for substance use patterns from adolescence to early adulthood among European Americans, replication of findings will be necessary with other ancestral groups. Several genome-wide association studies are currently being conducted to understand genes that may carry a risk for substance use in different ancestral groups and should be utilized in future research.
Second, we use SNPs from multiple genome-wide association studies to capture substance use genetic risk. It is still likely that the substance use polygenic risk score does not encompass genetic risk for substance use entirely (i.e., lack of coverage for substance use phenotype and may be underpowered). Therefore, replication of these findings with the same gene set in different samples and using extended gene sets is imperative. Third, we use retrospective childhood maltreatment data, which may suffer from under-reporting, does not provide information on timing of exposure, and does not indicate Child Protective Services substantiated childhood maltreatment exposure. However, previous research has shown a strong link between retrospective maltreatment reports and health outcomes throughout life (Suglia, Clark, Boynton-Jarrett, Kressin, & Koenen, 2014). Moreover, there is also a moderate association between prospective and retrospective reports of childhood maltreatment (Tajima, Herrenkohl, Huang, & Whitney, 2004), with retrospective reports typically downwardly biased (i.e., under-reported; Ferraro, Schafer, & Wilkinson, 2016). Nonetheless, future studies should use prospective childhood maltreatment exposure data and compare their findings to those of the present research. Finally, the use of secondary data does not allow for understanding more problematic substance use such as substance use disorders or addictions. Future research should evaluate the aims of the present study with clinical samples of individuals with more problematic substance use behaviors.
Conclusion
Despite these limitations, the present study has numerous strengths. This study provides an initial understanding of 1) how a combination of childhood maltreatment exposures interacts with genetic risk for substance use to influence the progression of substance use frequency over time, and 2) which combination of childhood maltreatment exposures by themselves may be detrimental for substance use over time even when genetic risk is low. The findings of this research are, therefore, unique and establish that maltreatment exposures similar to those of the high physical abuse sub-group may be more biologically linked to substance use progression over time. Whereas findings for the high sexual abuse sub-group illustrate a more pervasive influence of environmental factors over genetics.
With polygenic risk scores becoming more commonly used to predict susceptibility to specific negative phenotypes including substance use frequency (i.e., Torkamani et al., 2018), the inclusion of such a probabilistic model based on genetic susceptibility can be particularly critical for understanding their interactive influence with adversity on changes in outcomes over time and could lead to more tailored prevention efforts in the future.
Supplementary Material
Figure 4.
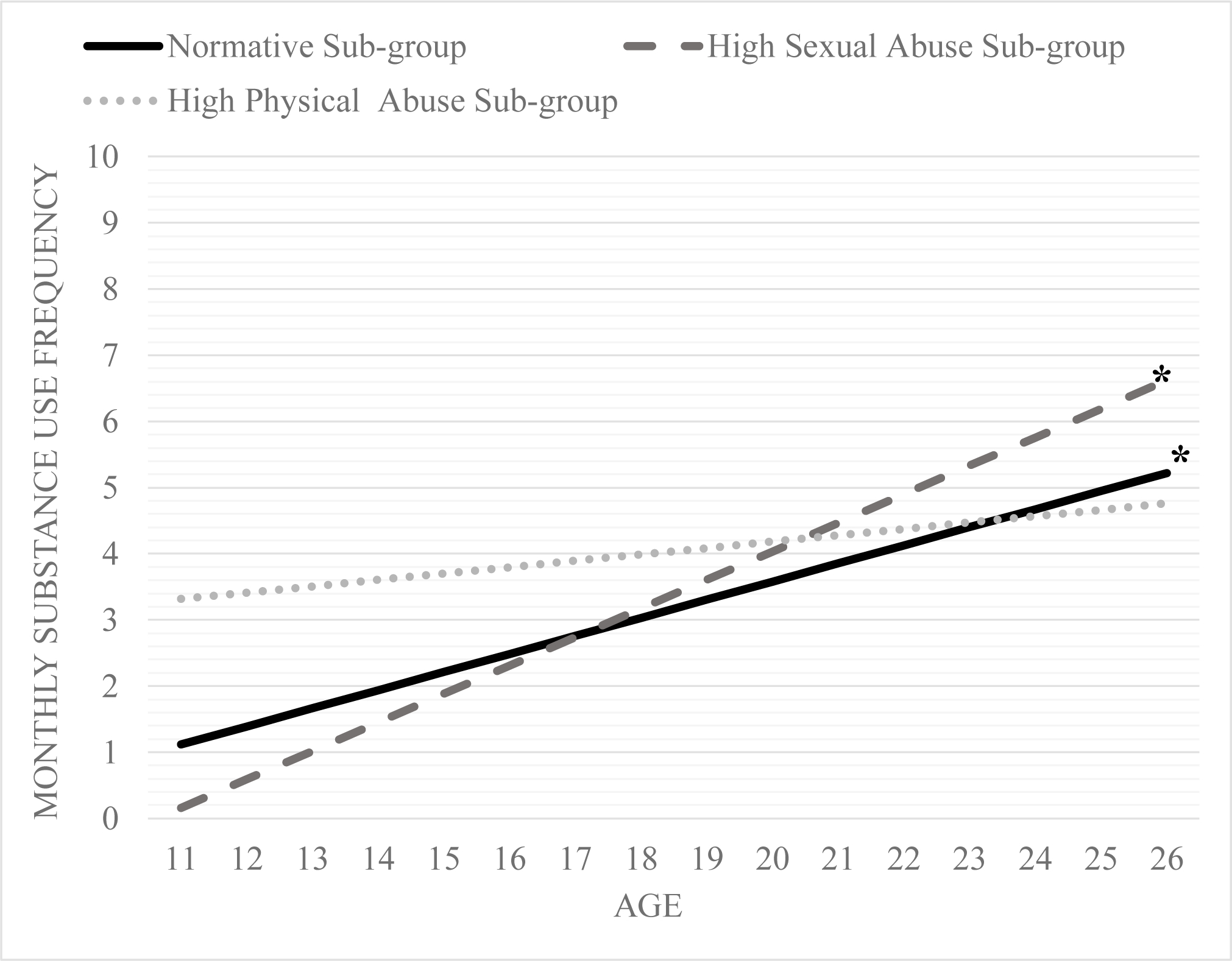
Change over time in substance use frequency for the three maltreatment sub-groups at average levels of substance use polygenic risk score (i.e., mean).
Note: trajectories at α <= 0.05 are denoted by * and 0.10 are denoted by +
Acknowledgment.
“Add Health is directed by Robert A. Hummer and funded by the National Institute on Aging cooperative agreements U01 AG071448 (Hummer) and U01AG071450 (Aiello and Hummer) at the University of North Carolina at Chapel Hill. Waves I-V data are from the Add Health Program Project, grant P01 HD31921 (Harris) from Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), with cooperative funding from 23 other federal agencies and foundations. Add Health was designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris at the University of North Carolina at Chapel Hill.
Add Health GWAS data were funded by NICHD Grants R01 HD073342 (Harris) and R01 HD060726 (Harris, Boardman, and McQueen). Investigators thank the staff and participants of the Add Health Study for their important contributions.”
This research was supported by the National Institute on Drug Abuse (R36DA047563, Mishra). Dr. Marceau was supported by the National Institute on Drug Abuse (K01DA039288, Marceau).
Footnotes
Disclosure Statement: The authors report no conflict of interest.
Data Statement.
Add Health Data can be obtained via contract from https://addhealth.cpc.unc.edu/
Genetic data available through dbGaP: study accession phs001367.v1.p1
Ethical approval: The study uses pre-existing data from The National Longitudinal Study of Adolescent to Adult Health (Add Health) study that was reviewed and deemed as an expedited category 5 for Human Subjects Research by the Purdue University IRB. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiken LS, West SG, & Reno RR (1991). Multiple regression: Testing and interpreting interactions. Sage. [Google Scholar]
- Benedini KM, & Fagan AA (2020). From child maltreatment to adolescent substance use: different pathways for males and females? Feminist Criminology, 15, 147–173. 10.1177/1557085118810426 [DOI] [Google Scholar]
- Bierut LJ (2011). Genetic vulnerability and susceptibility to substance dependence. Neuron, 69, 618–627. 10.1016/j.neuron.2011.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DA (1983). On the variances of asymptotically normal estimators from complex surveys. International Statistical Review/Revue Internationale de Statistique, 279–292. 10.2307/1402588 [DOI] [Google Scholar]
- Braudt D, & Harris K (2018). Polygenic Scores (PGSs) in the National Longitudinal Study of Adolescent to Adult Health (Add Health)–Release 1. Carolina Digital Repository. [Google Scholar]
- Chen P, & Chantala K (2014). Guidelines for analyzing Add Health data. Carolina Population Center, University of North Carolina at Chapel Hill, 1–53. [Google Scholar]
- Creemers HE, Harakeh Z, Dick DM, Meyers J, Vollebergh WAM, Ormel J, … Huizink AC (2011). DRD2 and DRD4 in relation to regular alcohol and cannabis use among adolescents: does parenting modify the impact of genetic vulnerability? The TRAILS study. Drug and Alcohol Dependence, 115, 35–42. 10.1016/j.drugalcdep.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debowska A, Willmott D, Boduszek D, & Jones AD (2017). What do we know about child abuse and neglect patterns of co-occurrence? A systematic review of profiling studies and recommendations for future research. Child Abuse and Neglect, 70, 100–111. 10.1016/j.chiabu.2017.06.014 [DOI] [PubMed] [Google Scholar]
- Dubowitz H, Roesch S, & Lewis T (2021). Child maltreatment, early adult substance use, and mediation by adolescent behavior problems. Child Maltreatment, 26, 238–248. 10.1177/1077559520941919 [DOI] [PubMed] [Google Scholar]
- Dudbridge F (2013). Power and Predictive Accuracy of Polygenic Risk Scores. 9. 10.1371/journal.pgen.1003348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein EC, Richter L, & Foster SE (2012). Addressing the Critical Health Problem of Adolescent Substance Use Through Health Care, Research, and Public Policy. Journal of Adolescent Health, 50, 431–436. 10.1016/j.jadohealth.2011.12.033 [DOI] [PubMed] [Google Scholar]
- Ferraro KF, Schafer MH, & Wilkinson LR (2016). Childhood Disadvantage and Health Problems in Middle and Later Life. American Sociological Review, 81, 107–133. 10.1177/0003122415619617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein DH, Rose EJ, Darcey VL, Belcher AM, & VanMeter JW (2016). Neurodevelopmental precursors and consequences of substance use during adolescence: Promises and pitfalls of longitudinal neuroimaging strategies. Frontiers in Human Neuroscience, 10, 296. 10.3389/fnhum.2016.00296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner BR, Hunter BD, Smith DC, Smith JE, & Godley MD (2014). The Relationship Between Child Maltreatment and Substance Abuse Treatment Outcomes Among Emerging Adults and Adolescents. Child Maltreatment, 19, 261–269. 10.1177/1077559514547264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorwood P, Le Strat Y, Ramoz N, Dubertret C, Moalic J-M, & Simonneau M (2012). Genetics of dopamine receptors and drug addiction. Human Genetics, 131, 803–822. 10.1007/s00439-012-1145-7 [DOI] [PubMed] [Google Scholar]
- Hall FS (2016). Reverse Translational Implications of Genome-Wide Association Studies for Addiction Genetics. In Neuropathology of Drug Addictions and Substance Misuse (Vol. 3). 10.1016/B978-0-12-800634-4.00016-0 [DOI] [Google Scholar]
- Harden KP, Hill JE, Turkheimer E, & Emery RE (2008). Gene-environment correlation and interaction in peer effects on adolescent alcohol and tobacco use. Behavior Genetics, 38, 339–347. 10.1007/s10519-008-9202-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Trapido E, Fleming L, Arheart K, Crandall L, French M, … Prado G (2011). The long-term effects of childhood maltreatment experiences on subsequent illicit drug use and drug-related problems in young adulthood. Addictive Behaviors, 36, 95–102. 10.1016/j.addbeh.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, … Dunne MP (2017). The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. The Lancet Public Health, 2, e356–e366. 10.1016/S2468-2667(17)30118-4 [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, & McGue M (1999). Behavioral disinhibition and the development of substance-use disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology, 11, 869–900. 10.1017/S0954579499002369 [DOI] [PubMed] [Google Scholar]
- Ingram RE, & Luxton DD (2005). Vulnerability-stress models. Development of Psychopathology: A Vulnerability-Stress Perspective, 32–46. [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, & Schulenberg JE (2016). Monitoring the Future results on drug use: 1975–2015: Overview, Key Findings on Adolescent Drug Use, 2015. Ann Arbor: Institute for Social Research, The University of Michigan. 10.1017/CBO9781107415324.004 [DOI] [Google Scholar]
- Johnston, Lloyd D, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, & Patrick ME (2021). Monitoring the Future National Survey Results on Drug Use, 1975–2020: Overview, Key Findings on Adolescent Drug Use. Institute for Social Research. [Google Scholar]
- Kisely S, Strathearn L, & Najman J (2021). The influence of child maltreatment on substance or alcohol use in 30-year-old adults: A birth cohort study. Drug and Alcohol Review, 40, 673–680. 10.1111/dar.13192 [DOI] [PubMed] [Google Scholar]
- Kosty DB, Seeley JR, Farmer RF, Stevens JJ, & Lewinsohn PM (2017). Trajectories of cannabis use disorder: risk factors, clinical characteristics and outcomes. Addiction, 112, 279–287. 10.1111/add.13557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwiller BJ, & Brausch AM (2013). Cyber Bullying and Physical Bullying in Adolescent Suicide : The Role of Violent Behavior and Substance Use. 675–684. 10.1007/s10964-013-9925-5 [DOI] [PubMed] [Google Scholar]
- Maier RM, Visscher PM, Robinson MR, & Wray NR (2017). Embracing polygenicity: a review of methods and tools for psychiatric genetics research. Psychological Medicine, 1–19. 10.1017/S0033291717002318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marees AT, de Kluiver H, Stringer S, Vorspan F, Curis E, Marie-Claire C, & Derks EM (2018). A tutorial on conducting genome-wide association studies: Quality control and statistical analysis. International Journal of Methods in Psychiatric Research, 27, 1–10. 10.1002/mpr.1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney C, & Renk K (2011). A multivariate model of parent–adolescent relationship variables in early adolescence. Child Psychiatry & Human Development, 42, 442–462. 10.1007/s10578-011-0228-3 [DOI] [PubMed] [Google Scholar]
- Mishra AA, Friedman EM, Mihalec-Adkins BP, Evich CD, Christ SL, & Marceau K (2019). Childhood maltreatment exposure and physical functional limitations in late adulthood: examining subjective sleep quality in midlife as a mediator. Psychology & Health, 0446, 1–20. 10.1080/08870446.2019.1657576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra AA, & Marceau K (2019). Co-occurring childhood maltreatment exposure and depressive symptoms in adulthood: Testing differential effects of stress dysregulation and perceived stress. Aging & Mental Health, 1–10. 10.1080/13607863.2019.1619166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray LK, Nguyen A, & Cohen JA (2014). Child sexual abuse. Child and Adolescent Psychiatric Clinics, 23, 321–337. 10.1016/j.chc.2014.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L, & Muthén B (2020). Mplus. The Comprehensive Modelling Program for Applied Researchers: User’s Guide, 5. [Google Scholar]
- Narendorf SC, & McMillen JC (2010). Substance use and substance use disorders as foster youth transition to adulthood. Children and Youth Services Review, 32, 113–119. 10.1016/j.childyouth.2009.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiderhiser JM, Marceau K, & Reiss D (2013). Four factors for the initiation of substance use by young adulthood: A 10-year follow-up twin and sibling study of marital conflict, monitoring, siblings, and peers. Development and Psychopathology, 25, 133–149. 10.1017/S0954579412000958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiderhiser JM, Reiss D, Hetherington EM, & Plomin R (1999). Relationships between parenting and adolescent adjustment over time: genetic and environmental contributions. Developmental Psychology, 35(3), 680–692. 10.1037/0012-1649.35.3.680 [DOI] [PubMed] [Google Scholar]
- Olson S, & Stroud C (2012). Decade : Workshop Summary Workshop Summary. In Child Maltreatment. [Google Scholar]
- Oshri A, Tubman JG, & Burnette ML (2012). Childhood maltreatment histories, alcohol and other drug use symptoms, and sexual risk behavior in a treatment sample of adolescents. American Journal of Public Health, 102, 250–257. 10.2105/AJPH.2011.300628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park-lee E, & Tice P (2017). Key Substance Use and Mental Health Indicators in the United States : Results from the 2017 National Survey on Drug Use and Health.
- Paus T, Keshavan M, & Giedd JN (2010). Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience, 9, 947–957. 10.1038/nrn2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rende R, & Slomkowski C (2008). Incorporating the family as a critical context in genetic studies of children: implications for understanding pathways to risky behavior and substance use. Journal of Pediatric Psychology, 34, 606–616. 10.1093/jpepsy/jsn053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MM, McKinney C, & Asberg K (2018). Substance use predicted by parental maltreatment, gender, and five-factor personality. Personality and Individual Differences, 128, 39–43. 10.1016/J.PAID.2018.02.030 [DOI] [Google Scholar]
- Salvatore C (2018). Emerging Adults and Illegal Drugs/Substance Use. In Sex, Crime, Drugs, and Just Plain Stupid Behaviors (pp. 65–72). Springer. [Google Scholar]
- Sawyer SM, Azzopardi PS, Wickremarathne D, & Patton GC (2018). The age of adolescence. The Lancet Child & Adolescent Health, 2, 223–228. 10.1016/S2352-4642(18)30022-1 [DOI] [PubMed] [Google Scholar]
- Schulte MT, & Hser Y-I (2013). Substance use and associated health conditions throughout the lifespan. Public Health Reviews, 35, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigelman CK, & Rider EA (2009). Developmental psychopathology. Life-Span Human Development, 468–495. [Google Scholar]
- Sinha R (2008). Chronic Stress, Drug Use, and Vulnerability to Addiction. Annals of the New York Academy of Sciences, 1141, 105–130. 10.1196/annals.1441.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima EA, Herrenkohl TI, Huang B, & Whitney SD (2004). Measuring child maltreatment: A comparison of prospective parent reports and retrospective adolescent reports. American Journal of Orthopsychiatry, 74, 424–435. 10.1037/0002-9432.74.4.424 [DOI] [PubMed] [Google Scholar]
- Torkamani A, Wineinger NE, & Topol EJ (2018). The personal and clinical utility of polygenic risk scores. Nature Reviews Genetics, 19, 581–590. 10.1038/s41576-018-0018-x [DOI] [PubMed] [Google Scholar]
- Traube DE, James S, Zhang J, & Landsverk J (2012). A national study of risk and protective factors for substance use among youth in the child welfare system. Addictive Behaviors, 37, 641–650. 10.1016/j.addbeh.2012.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco EM, Madan B, & Villar M (2019). The impact of genes on adolescent substance use: a developmental perspective. Current Addiction Reports, 6, 522–531. 10.1007/s40429-019-00273-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng H-Y, Hsueh Y-H, Messam LLM, & Hertz-Picciotto I (2009). Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. American Journal of Epidemiology, 169, 1182–1190. 10.1093/aje/kwp035 [DOI] [PubMed] [Google Scholar]
- Wilson HW, Samuelson SL, Staudenmeyer AH, & Widom CS (2015). Trajectories of psychopathology and risky behaviors associated with childhood abuse and neglect in low-income urban African American girls. Child Abuse and Neglect, 45, 108–121. 10.1016/j.chiabu.2015.02.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


