Abstract
The in vitro activities of ciprofloxacin, trovafloxacin, moxifloxacin, and grepafloxacin against 174 strains of Neisseria gonorrhoeae isolated in Sydney, Australia, were determined. The strains included 84 quinolone-less-sensitive and -resistant N. gonorrhoeae (QRNG) strains for which ciprofloxacin MICs were in the range of 0.12 to 16 μg/ml. The QRNG included strains isolated from patients whose infections were acquired in a number of countries, mostly in Southeast Asia. The gyrA and parC quinolone resistance-determining regions (QRDR) of 18 selected QRNG strains were sequenced, and the amino acid mutations observed were related to the MICs obtained. The activities of moxifloxacin and grepafloxacin against QRNG were comparable to that of ciprofloxacin. Trovafloxacin was more active than the other quinolones against some but not all of the QRNG strains. Increments in ciprofloxacin resistance occurred in a step-wise manner with point mutations initiated in gyrA resulting in amino acid alterations Ser91-to-Phe, Ser91-to-Tyr, Asp95-to-Gly, and Asp95-to-Asn. Single gyrA changes correlated with ciprofloxacin MICs in the range 0.12 to 1 μg/ml. The Ser91 changes in GyrA were associated with higher MICs and further QRDR changes. QRNG strains for which ciprofloxacin MICs were greater than 1 μg/ml had both gyrA and parC QRDR point mutations. ParC alterations were seen in these isolates only in the presence of GyrA changes and comprised amino acid changes Asp86-to-Asn, Ser87-to-Asn, Ser87-to-Arg, Ser88-to-Pro, Glu91-to-Lys, and Glu91-to-Gln. QRNG strains for which MICs were in the higher ranges had double GyrA mutations, but again only with accompanying ParC alterations. Not only did the nature and combination of GyrA and ParC changes influence the incremental increases in ciprofloxacin MICs, but they seemingly also altered the differential activity of trovafloxacin. Our findings suggest that the newer quinolones of the type examined are unlikely to be useful replacements for ciprofloxacin in the treatment of gonorrhea, particularly where ciprofloxacin MICs are high or where resistance is widespread.
Quinolone antibiotics have enjoyed considerable success in the treatment of gonococcal infections because of their directed activity against bacterial topoisomerases and ease of oral administration. However, their widespread use and often misuse, coupled with emerging resistance, have gradually compromised their utility (6, 18). Quinolone-resistant Neisseria gonorrhoeae (QRNG) strains first appeared in Sydney, in Australia, in 1984; they initially possessed only low-level resistance (ciprofloxacin MIC, 0.06 to 0.5 μg/ml), comprised multiple subtypes imported from Southeast Asia, and accounted for 2 to 3% of all gonococci isolated (14). This rate and pattern of isolation of low-level QRNG remained unchanged until 1991, when isolates with a higher level of resistance (MIC ≥ 1 μg/ml) were detected (13). In 1995, rates of isolation of QRNG for which ciprofloxacin MICs were 4 μg/ml or more increased, and treatment failures often ensued (11). Currently in Sydney, ciprofloxacin MICs have reached 16 μg/ml, and QRNG strains represent 20% of isolates and are present in all prominent cohort groups. As a consequence, quinolones are no longer a recommended treatment for gonococcal infections in Sydney.
The quinolones directly target two bacterial topoisomerases in N. gonorrhoeae, gyrase and topoisomerase IV. Topoisomerases are responsible for maintaining the correct topology of DNA in the cell. In this two-step process, gyrase, an ATP-dependent enzyme, negatively supercoils the DNA, allowing topoisomerase IV to separate the replicated DNA molecules. The lethal effect of the quinolones occurs when an intermediary complex of drug-enzyme-DNA blocks bacterial replication (1). The principal genes encoding these target enzymes for quinolone antibiotics are gyrA (gyrase) and parC (topoisomerase IV). GyrA is the primary target in gonococci, with ParC changes secondary but associated with high-level QRNG (2, 15). The quinolone resistance-determining region (QRDR), modelled on the analogous region in Escherichia coli, was first described by Belland et al. (2) using in vitro-generated mutants. Further changes in naturally occurring isolates were described by Deguchi et al. (4) and Tanaka et al. (9). Additional parC alterations were recently reported by Tanaka et al. (10) and Trees et al. (15). Gyrase B subunit (gyrB) changes and other influences may also be present and increase quinolone MICs, but they are of an additive nature rather than being a major influence.
Newer quinolones with differential activities against GyrA and ParC and overall enhanced activities have been developed (3). For example, moxifloxacin and grepafloxacin have greater activity against topoisomerase IV, while trovafloxacin targets gyrase activity. It has been suggested that they could provide more suitable treatments for infections caused by ciprofloxacin-resistant gonococci (17). Earlier studies of the QRDR were restricted to low-level resistance, and QRDR changes were related to ciprofloxacin only (4, 9, 16). Subsequently, more-resistant strains have been investigated with QRDR changes related to newer quinolones (10). In the present study, we also explored the activities of moxifloxacin, grepafloxacin, and trovafloxacin against phenotypically distinct QRNG strains with a wide range of resistance from various geographic sources, and we sequenced the QRDR of 18 of these isolates. Differences in activity were related to alterations in GyrA and/or ParC. The evolution and nature of these alterations, their differential effects on quinolone activity, and their implications for use of the newer quinolone antibiotics for the treatment of gonorrhea are discussed.
MATERIALS AND METHODS
Sources and typing of bacterial isolates.
Gonococci isolated from private- and public-sector laboratories in the State of New South Wales, Australia, were referred to the Neisseria Reference Laboratory of the Prince of Wales Hospital, Sydney, Australia, for confirmation of identity and susceptibility testing. Isolates were maintained in 20% glycerol broth at −70°C for long-term storage. Isolates were serotyped by coagglutination using a panel of 12 monoclonal antibodies (Syva, Palo Alto, Calif., kindly supplied by Julia Griffith, University of Melbourne) according to the nomenclature of Knapp et al. (7). Auxotyping was performed by the method of La Scolea and Young (8). The geographic locations of acquisition of antibiotic-resistant isolates were determined wherever possible by callback to the primary physicians. One hundred seventy-four gonococcal isolates were tested, of which 90 were sequentially isolated quinolone-susceptible strains from 1998 and 84 were QRNG organisms randomly selected from isolates stored between 1991 and 1998. N. gonorrhoeae WHO C and E were used as controls.
Susceptibility testing.
Quantitative tests of susceptibility to a variety of antibiotics, including ciprofloxacin and trovafloxacin, were routinely performed using the agar dilution techniques of the Australian Gonococcal Surveillance Programme (18). The test medium was Isosensitest agar (Oxoid, Basingstoke, United Kingdom) containing 8% saponin-lysed horse blood and an inoculum of 104 CFU per spot (12). Altered ciprofloxacin susceptibility (for QRNG) was defined as a MIC of ≥0.06 μg/ml. Strains were categorized as less sensitive when MICs were 0.06 to 0.5 μg/ml, as resistant when MICs were 1 or 2 μg/ml, and as displaying high-level resistance when MICs were ≥4 μg/ml. A subset of 18 phenotypically different strains for which ciprofloxacin MICs ranged from 0.12 to 16 μg/ml, as determined by multiple-repeat MIC determinations, were selected for further study. These were isolated from infections acquired in a number of different geographic regions over the period 1991 to 1998 (see Table 2). Tests of quantitative susceptibilities to moxifloxacin and grepafloxacin were performed, and gyrA and parC QRDRs of these 18 gonococci were sequenced. Antibiotic powders were of defined potency and were gifts from their manufacturers.
TABLE 2.
MICs for 18 strains of N. gonorrhoeae with altered quinolone resistance with details of phenotype, GyrA and ParC changes, and geographic source
| Strain no. | Yr | MICa (μg/ml) of:
|
Typeb (A/S class) | Sequence change(s)c
|
Geographic sourced | ||||
|---|---|---|---|---|---|---|---|---|---|
| CIP | GRX | MXF | TVA | GyrA | ParC | ||||
| 1 | 1993 | 0.12 | 0.12 | 0.12 | 0.03 | Pro/A6 | D95N | O | |
| 2 | 1997 | 0.12 | 0.12 | 0.25 | 0.03 | Nr/B4 | D95G | U | |
| 3 | 1991 | 0.12 | 0.25 | 0.25 | 0.12 | Nr/A4 | D95N | P | |
| 4 | 1998 | 0.25 | 0.5 | 0.5 | 0.06 | Nr/B3 | D95N | T | |
| 5 | 1991 | 0.5 | 0.5 | 0.5 | 0.12 | Pro/B8 | S91F | T | |
| 6 | 1992 | 0.5 | 0.5 | 0.5 | 0.25 | Nr/B3 | S91Y | P | |
| 7 | 1998 | 1 | 1 | 0.5 | 0.12 | Nr/B4 | S91F | T | |
| 8 | 1996 | 1 | 0.5 | 0.5 | 0.5 | Pro/B2 | S91Y | E91Q | C |
| 9 | 1997 | 2 | 1 | 2 | 0.12 | Nr/B6 | S91F, D95G | S88P | U |
| 10 | 1997 | 2 | 2 | 2 | 1 | Nr/A6 | S91F, D95G | D86N | P |
| 11 | 1997 | 4 | 2 | 4 | 0.12 | Nr/B3 | S91F, D95G | S88P | A |
| 12 | 1996 | 4 | 4 | 2 | 2 | Pro/A6 | S91F, D95G | D86N | U |
| 13 | 1998 | 8 | 4 | 2 | 1 | Pro/A6 | S91F, D95G | S87R | C |
| 14 | 1998 | 8 | 8 | 4 | 4 | Nr/A6 | S91F, D95N | E91K | C |
| 15 | 1995 | 8 | 16 | 4 | 4 | Nr/B7 | S91F, D95G | E91K, S87N | U |
| 16 | 1995 | 16 | 32 | 8 | 8 | ProArg/B3 | S91F, D95G | E91K, S87N | B |
| 17 | 1995 | 16 | 32 | 8 | 8 | Nr/B7 | S91F, D95G | E91K, S87N | U |
| 18 | 1995 | 16 | 16 | 8 | 2 | Arg/B4 | S91F, D95N | S87R | A |
CIP, ciprofloxacin; GRX, grepafloxacin; MXF, moxifloxacin; TVA, trovafloxacin.
Nr, nonrequirer; Pro, proline requirer; Arg, arginine requirer; A/S, auxotype/serotype.
D, aspartate; N, asparagine; G, glycine; S, serine; F, phenylalanine; Y, tyrosine; E, glutamine; Q, glutamate; P, proline; R, arginine; K; lysine.
O, overseas, unknown; U, unknown; P, Philippines; T, Thailand; C, China; A, Australia; B, Bali, Indonesia.
DNA extraction and PCR.
DNA extraction was performed by the routine procedure of alkali-sodium dodecyl sulfate lysis followed by phenol-chloroform extraction and precipitation with ethanol. Primers for gyrA QRDR were those of the design of Deguchi et al. (4) and yielded a 225-bp fragment. The 225-bp parC QRDR was amplified using a forward primer described previously by Belland et al. (2) and a reverse primer based on the work of Trees et al. (16). PCR was performed with 30 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 45 s.
DNA sequencing and computer analysis.
PCR products were purified by polyethylene glycol precipitation and washed with 70% ethanol. Products were sequenced directly using 4 μl of template (∼100 ng), 1 μl (10 pmol) of primer, 10 μl of water, 2.5 μl of CSA buffer (0.05 M Tris [pH 9.0], 1M MgCl2), and 2.5 μl of dye terminator premix (Perkin-Elmer). Following thermal cycling, reaction products were precipitated and analyzed using an ABI 377 DNA sequencer (Perkin-Elmer Applied Biosystems). Database searches were conducted using BLAST, and pair-wise alignments of DNA sequences were carried out using the Genetics Computer Group program GAP.
RESULTS
Comparative activity of quinolone antibiotics.
The relative activities of trovafloxacin and ciprofloxacin were compared by testing 174 isolates of N. gonorrhoeae, of which 90 were sensitive, 36 were less sensitive, 13 were resistant, and 35 demonstrated high-level resistance to ciprofloxacin (Table 1). Ciprofloxacin and trovafloxacin had similar activities against 126 isolates categorized as sensitive and less sensitive to ciprofloxacin. Gonococci for which ciprofloxacin MICs were greater than or equal to 1 μg/ml were more susceptible to trovafloxacin, exhibiting MIC reductions of up to 5 doubling dilutions with some strains.
TABLE 1.
Comparison of ciprofloxacin and trovafloxacin activities against 174 strains of N. gonorrhoeae isolated in Sydney, Australia, from 1991 to 1998
| Drug | MIC (μg/ml) range for strains in CIPa resistance category
|
|||
|---|---|---|---|---|
| Sensitive (90 isolates) | Less sensitive (36 isolates) | Resistant (13 isolates) | High-level resistance (35 isolates) | |
| Ciprofloxacin | ≤0.03 | 0.06–0.5 | 1.0–2.0 | 4–16 |
| Trovafloxacin | ≤0.03 | 0.03–1.0 | 0.12–1.0 | 0.12–8 |
CIP, ciprofloxacin.
When the activities of trovafloxacin, moxifloxacin, and grepafloxacin against the subset of 18 QRNG isolates for which ciprofloxacin MICs ranged from 0.12 to 16 μg/ml were compared, trovafloxacin was the most active, followed by moxifloxacin and grepafloxacin, and then ciprofloxacin (Table 2). However, reductions in trovafloxacin MICs were not uniform. When trovafloxacin MICs were compared with those of ciprofloxacin the MICs for strains 7, 9, 11, 13, and 18 showed pronounced reductions (8 to 32-fold), whereas for other isolates the MICs showed no reduction or single-tube reductions only (strains 3, 6, 8, 10, 12, 14, 15, 16, and 17). Activities of grepafloxacin, moxifloxacin, and ciprofloxacin were similar, with moxifloxacin slightly more active against high-level QRNG and gatifloxacin less active (Table 2).
Sequence changes in the QRDR of 18 selected QRNG strains.
The most common alterations in the gyrA QRDR resulted in serine exchanged for either a tyrosine or phenylalanine residue at position 91 (Ser91-to-Tyr or Ser91-to-Phe, respectively) and aspartate for either an asparagine or glycine residue at position 95 (Asp95-to-Asn or Asp95-to-Gly). GyrA changes were confined to amino acid positions 91 and 95. parC point mutations encoded amino acid changes to asparagine at position 86 (Asp86-to-Asn), arginine or asparagine at position 87 (Ser87-to-Arg or Ser87-to-Asn), proline at position 88 (Ser88-to-Pro), and glutamine or lysine at position 91 (Glu91-to-Gln or Glu91-to-Lys) (Table 2).
Correlation of ciprofloxacin MICs with QRDR sequence changes.
The alteration in the gyrA QRDR that produced the least effect on ciprofloxacin MICs encoded a conservative amino acid change of aspartate to asparagine in strains 1, 3, and 4, giving rise to ciprofloxacin MICs of 0.125, 0.125, and 0.25 μg/ml respectively. As ciprofloxacin MICs increased to 0.5 and 1 μg/ml (strains 5, 6, and 7), serine was exchanged for either phenylalanine or tyrosine in GyrA. This serine substitution in GyrA was a consistent feature in resistant strains (ciprofloxacin MIC, ≥1 μg/ml). Ciprofloxacin MICs of 1 and 2 μg/ml resulted from a number of possible QRDR changes: a single GyrA change (strain 7), changes to both GyrA and ParC (strain 8), or a double GyrA change accompanied by a single ParC change (strains 9 and 10) (Table 2). Strains demonstrating high-level quinolone resistance (ciprofloxacin MIC, ≥4 μg/ml) all had double mutations in GyrA combined with either a single (strains 11, 12, 13, 14, and 18) or double (strains 15, 16, and 17) ParC mutation (Table 2).
Ciprofloxacin and trovafloxacin differential resistance correlated to QRDR mutations.
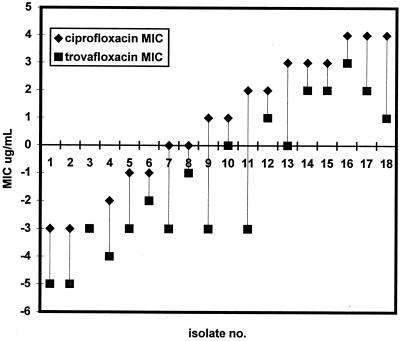
Ciprofloxacin and trovafloxacin MICs were nonrelated, with marked reductions in trovafloxacin MICs for some isolates and minimal changes for others (Fig. 1). For example, trovafloxacin and ciprofloxacin MICs were both high with the ParC alterations Asp86-to-Asn, Glu91-to-Lys/Gln, or Ser87-to-Asn, whereas for strains 9, 11, 13, and 18, which had Ser87-to-Arg or Ser88-to-Pro changes, trovafloxacin MICs were 8- to 32-fold lower than those of ciprofloxacin (Fig 1). These four strains had the same GyrA changes as the rest of the subset.
FIG. 1.
Relationship between ciprofloxacin and trovafloxacin MICs for 18 N. gonorrhoeae isolates with GyrA and ParC QRDR sequence changes. (Data for MICs have been linearized.)
DISCUSSION
Resistance in N. gonorrhoeae to the fluoroquinolone antibiotics ciprofloxacin and ofloxacin has caused their use to decline in the treatment of gonorrhea (6). Newer fluoroquinolones which have enhanced activity due to targeting of the enzymes gyrase and topoisomerase IV have been released (16) and may provide useful alternatives for treating gonococcal disease (17). The results of this study indicate that the newer agents trovafloxacin, moxifloxacin, and grepafloxacin have increased in vitro activities compared with that of ciprofloxacin against gonococci categorized as sensitive or less sensitive. However, this increased in vitro efficacy was not uniformly evident when 18 selected QRNG isolates were investigated. Trovafloxacin was the most active of the newer agents tested, whereas moxifloxacin and grepafloxacin had marginal increases or no increase in activity compared with the results observed for ciprofloxacin-resistant strains. For 4 of 18 QRNG isolates with high-level ciprofloxacin resistance, the MIC of trovafloxacin showed a pronounced reduction, while for others the reduction was minimal or nonexistent (Fig. 1). These results differ from those of Jones et al., who found a uniform loss of potency in trovafloxacin against resistant strains (5). However, this discrepancy may have been because in this investigation isolates with a greater range of resistance (ciprofloxacin MICs, 0.12 to 16 μg/ml) were tested. Our findings suggest that the newer quinolones of the type examined are unlikely to be useful replacements for ciprofloxacin in the treatment of gonorrhea, particularly where ciprofloxacin MICs are high or where resistance is widespread.
The QRDR changes were characterized in a subset of 18 phenotypically distinct QRNG strains from diverse sources and isolated between 1991 and 1998. Analysis of point mutations confirmed observations made in earlier studies (2, 4, 9, 15). Deguchi et al. (4) were the first to describe point mutations of gyrA and parC QRDR in clinical isolates from Japan, mostly with low-level ciprofloxacin resistance (MICs, ≤2 μg/ml). Some of these strains were later examined in the study of Jones et al. (5), but trovafloxacin MICs were not directly related to QRDR changes. In the present investigation, amino acid changes in the QRDR were directly related to differential quinolone activity. For four QRNG strains for which ciprofloxacin MICs were high, 8- to 32-fold reductions in trovafloxacin MICs were observed. These QRNG isolates had common GyrA combinations but harbored unique ParC alterations of Ser87-to-Arg and Ser88-to-Pro. Trovafloxacin and ciprofloxacin had comparable activity levels against QRNG strains with the other ParC alterations, namely, Asp86-to-Asn, Ser87-toAsn, Glu91-to-Lys, and Glu91-to-Gln. Serine changes in the ParC QRDR therefore provide greater protection against trovafloxacin than against ciprofloxacin.
The relationship between ciprofloxacin MICs and alterations to the QRDR has been reported in other studies on clinical isolates of N. gonorrhoeae. Tanaka et al. (10) further expanded the list of parC mutations present in QRNG and analyzed strains from Japan for which the MICs reached 16 μg/ml. In the recent report of Trees et al. (15), the ciprofloxacin MIC was directly related to ParC changes in isolates with high-level resistance. In that report, an apparent temporal progression in QRDR changes was noted in QRNG strains from the Philippines in the period 1994 to 1996. However, further analyses of the correlation between MIC level and QRDR changes were confounded by the apparent concomitant presence of other mechanisms of quinolone resistance, presumably including alterations due to efflux mechanisms. A wide range of MICs was recorded for isolates with the same QRDR changes.
In the present study of diverse strains, ciprofloxacin resistance was ranked and related to changes in the QRDR. A possible sequence of events in gyrA and parC eventuating in high-level resistance was observed. Changes were initiated in the primary target, gyrase (gyrA), and subsequent mutations oscillated between target genes: gyrA-parC, gyrA-parC-gyrA, and gyrA-parC-gyrA-parC. This organization appears to be important, as parC changes were never seen in isolation in QRNG, and equally, double gyrA mutations were never identified without an accompanying parC change. Each sequence of QRDR changes examined here was restricted to a narrow MIC range in contrast to the situation in the study of Trees et al. (15). A single gyrA change was seen in strains for which ciprofloxacin MICs ranged from 0.12 to 0.5 μg/ml. Changes in ciprofloxacin-resistant gonococci for which MICs were greater than 1.0 μg/ml involved both gyrA and parC mutations. gyrA changes were confined to positions 91 and 95, while parC, the more variable QRDR, had mutations at position 86, 87, 88, or 91. Within this framework, an alteration in ParC was always accompanied by a serine substitution in GyrA, suggesting that this amino acid predisposes to further ParC QRDR changes. The corresponding change in E. coli has also been associated with resistance (5). These observations and those suggesting that parC changes Ser87-to-Arg and Ser88-to-Pro were associated with the significant MIC differential between trovafloxacin and ciprofloxacin in four isolates imply that both the nature and combination of gyrA and parC alterations help determine the activities and differential actions of quinolone agents against gonococci.
The QRNG strains examined were from infections acquired from different geographical sources. Trees et al. (15) noted different distributions of GyrA and ParC changes in QRNG isolates from Cambodia, the Philippines, and Thailand. The geographic distribution of various QRDR changes was further extended by the results of this study, although this was not systematically examined. The recently reported Ser87-to-Asn ParC change (15) was also found in gonococci from infections acquired in Bali, Indonesia (strain 16). Point mutations require alteration in only a single nucleotide, and thus it is possible that more changes will be found once larger and more diverse gonococcal populations are examined. The possibility of additional point mutations with additive deleterious effects on gonococcal susceptibility would suggest that a further decrease in the clinical efficacy of this group of quinolones in treating gonorrhea is likely.
REFERENCES
- 1.Ball P. The quinolones, history and overview. In: Andriole V T, editor. The quinolones. 2nd ed. San Diego, Calif: Academic Press; 1998. pp. 1–28. [Google Scholar]
- 2.Belland R J, Morrison S G, Ison C, Huang W M. Neisseria gonorrhoeae acquires mutations in analogous regions of gyrA and parC in fluoroquinolone-resistant isolates. Mol Microbiol. 1994;14:371–380. doi: 10.1111/j.1365-2958.1994.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 3.Chu D T W, Fernandes P B. Structure-activity relationships of the fluoroquinolones. Antimicrob Agents Chemother. 1989;33:131–135. doi: 10.1128/aac.33.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deguchi T, Yasuda M, Asano M, Tada K, Iwata H, Komeda H, Ezaki T, Saito I, Kawada Y. DNA gyrase mutations in quinolone-resistant clinical isolates of Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1995;39:561–563. doi: 10.1128/aac.39.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones R N, Barrett M S, Deguchi T. Antimicrobial activity of trovafloxacin tested against ciprofloxacin-susceptible and resistant Neisseria gonorrhoeae. Interpretive criteria and comparisons with E-test results. Diagn Microbiol Infect Dis. 1997;28:193–200. doi: 10.1016/s0732-8893(97)00042-4. [DOI] [PubMed] [Google Scholar]
- 6.Kam K M, Lo K K, Chong L Y, Au W F, Wong P Y, Cheung M M. Correlation between in vitro quinolone susceptibility of Neisseria gonorrhoeae and outcome of treatment of gonococcal urethritis with single-dose ofloxacin. Clin Infect Dis. 1999;28:1165–1166. doi: 10.1086/517766. [DOI] [PubMed] [Google Scholar]
- 7.Knapp J S, Tam M R, Nowinski R C, Holmes K K, Sandstrom E G. Serological classification of Neisseria gonorrhoeae with use of monoclonal antibodies to gonococcal outer membrane protein I. J Infect Dis. 1984;150:44–48. doi: 10.1093/infdis/150.1.44. [DOI] [PubMed] [Google Scholar]
- 8.La Scolea L J, Jr, Young F E. Development of a defined minimal medium for the growth of Neisseria gonorrhoeae. Appl Microbiol. 1974;28:70–76. doi: 10.1128/am.28.1.70-76.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka M, Matsumoto T, Sakumoto M, Takahashi K, Saika T, Kabayashi I, Kumazawa J The Pazufloxacin STD Group. Reduced clinical efficacy of pazufloxacin against gonorrhea due to high prevalence of quinolone-resistant isolates with the GyrA mutation. Antimicrob Agents Chemother. 1998;42:579–582. doi: 10.1128/aac.42.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka M, Nakayama H, Haraoka M, Saika T, Kobayashi I, Naito S. Susceptibilities of Neisseria gonorrhoeae isolates containing amino acid substitutions in GyrA, with or without substitutions in ParC, to newer fluoroquinolones and other antibiotics. Antimicrob Agents Chemother. 2000;44:192–195. doi: 10.1128/aac.44.1.192-195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tapsall J W, Limnios E A, Shultz T R. Continuing evolution of the pattern of quinolone resistance in Neisseria gonorrhoeae isolated in Sydney, Australia. Sex Transm Dis. 1998;25:415–417. doi: 10.1097/00007435-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Tapsall J W, Phillips E A, Cossins Y M. Penicillin sensitivity of gonococci in Australia: the development of an Australian Gonococcal Surveillance Programme. Br J Vener Dis. 1984;60:226–230. doi: 10.1136/sti.60.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tapsall J W, Phillips E A, Shultz T R, Thacker C. Quinolone-resistant Neisseria gonorrhoeae isolated in Sydney, Australia, 1991 to 1995. Sex Transm Dis. 1996;23:425–428. doi: 10.1097/00007435-199609000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Tapsall J W, Shultz T R, Phillips E A. Characteristics of Neisseria gonorrhoeae isolated in Australia showing decreased sensitivity to quinolone antibiotics. Pathology. 1992;24:27–31. doi: 10.3109/00313029209063616. [DOI] [PubMed] [Google Scholar]
- 15.Trees D L, Sandul A L, Peto-Mesola V, Aplasca M R, Leng H B, Whittington W L, Knapp J S. Alterations within the quinolone resistance-determining regions of GyrA and ParC of Neisseria gonorrhoeae isolated in the Far East and the United States. Int J Antimicrob Agents. 1999;12:325–332. doi: 10.1016/s0924-8579(99)00081-3. [DOI] [PubMed] [Google Scholar]
- 16.Trees D L, Sandul A L, Whittington W L, Knapp J S. Identification of novel mutation patterns in the parC gene of ciprofloxacin-resistant isolates of Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1998;42:2103–2105. doi: 10.1128/aac.42.8.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisfuse I B. Gonorrhoea control and antimicrobial resistance. Lancet. 1998;351:928. doi: 10.1016/S0140-6736(05)60601-0. [DOI] [PubMed] [Google Scholar]
- 18.WHO Western Pacific Region Gonococcal Antimicrobial Surveillance Programme. Surveillance of antibiotic susceptibility of Neisseria gonorrhoeae in the WHO Western Pacific Region 1992–1994. Genitourin Med. 1997;73:355–361. doi: 10.1136/sti.73.5.355. [DOI] [PMC free article] [PubMed] [Google Scholar]