Abstract
Estrogen is a critical hormone for bone homeostasis in men, but no information is available on the role of estrogen metabolism among men. The aim of this study was to evaluate the effect of estrogen hydroxylation on male bone mineral density (BMD). Participants consisted of 61 healthy Caucasian males (mean age 66.6 ± 1.0 years). Urinary estrogen metabolites were measured by enzyme-linked immunosorbent assay, serum estradiol by ultrasensitive radioimmunoassay, sex hormone binding globulin by radioimmunoassay, and BMD of the lumbar spine and the proximal femur by dual-energy X-ray absorptiometry. Active estrogen metabolites, 16α-hydroxyestrone (16αOHE1) and estriol (E3), positively correlated with adjusted BMD in all regions of the proximal femur (all P < 0.05) but not at the lumbar spine, and those in the highest tertile of urinary 16αOHE1 had the highest BMD. Free estradiol index (FEI) also positively correlated with BMD of the total hip, femoral neck, and intertrochanter (all P < 0.05), while there was no correlation between BMD with inactive metabolites (2)hydroxyestrone and 2-methoxyestrone) and serum testosterone. Multiple regression analysis showed 16αOHE1, FEI, and body mass index are important independent predictors of BMD in all regions of the proximal femur. Estrogen metabolism may modulate BMD in men. Increased urinary 16αOHE1 and E3 levels are associated with high BMD at the proximal femur, and 16αOHE1 appears to be a major determinant of BMD among the metabolites evaluated.
Keywords: Male osteoporosis, Estrogen metabolism, Bone mineral density
Recent studies have suggested estrogen to be the critical hormone for bone homeostasis in men, even more so than testosterone [1, 2]. These studies have demonstrated that in men the correlation between BMD and estradiol levels is even stronger than that between BMD and testosterone [1]. More importantly, the risk for fractures was found to be higher in men with reduced levels of circulating free estradiol [2], further highlighting the important role of estrogen status in bone health among males.
Circulating estrogen, which is predominantly in the form of estrone (E1) in men and in postmenopausal women [3], is metabolized primarily via two mutually exclusive pathways, the C2- and C16α-hydroxyl pathways [4]. Hydroxylation through the 2-hydroxyl pathway leads to formation of inactive metabolites, 2-hydroxyestrone (2OHE1) and 2-methoxyestrone (2MeOE1), while 16α-hydroxylation results in the formation of active metabolites, 16α-hydroxyestrone (16αOHE1) and estriol (E3) [5, 6]. The dominance of one pathway over the other contributes to the overall estrogenic state of an individual [5]. Women with preferential metabolism through the 16-hydroxyl pathway, as indicated by lower 2 to 16α ratios, are protected from bone loss over 1 year of follow-up [7]. Furthermore, women with increased 2-hydroxylation, as shown by higher levels of the inactive metabolite 2MeOE1, have lower bone mineral density (BMD) and vice versa. On the other hand, no information is available on the role of estrogen metabolism in skeletal health among men. We hypothesize that estrogen metabolism is also an important determinant of bone metabolism in men. The objective of this study, therefore, is to evaluate the role of estrogen metabolism in BMD in men.
Materials and Methods
Study Population
This is a cross-sectional study on community-dwelling, otherwise healthy men 50 years of age or older, living in St. Louis, MO, and its metropolitan area. Participants were recruited through advertisements or direct mailing. This study was conducted in accordance with the guidelines in the Declaration of Helsinki for the appropriate treatment of human subjects. The protocol was approved by the Washington University School of Medicine Institutional Review Board, and informed consent was obtained from each participant. Subjects taking any medication affecting bone metabolism, such as bisphosphonates (alendronate, risedronate, pamidronate, or zoledronate), gonadotropin-releasing hormone analogs, glucocorticoids (>5 mg of prednisone or equivalent for more than 1 month), or phenytoin, were excluded from the study. Participants who took medications known to affect estrogen hydroxylation, including phytoestrogens, cimetidine, thyroid hormones, monooxygenase inhibitors, and drugs known to affect cytochrome P-450 enzyme activity, were excluded, as were those with diseases or conditions known to interfere with bone metabolism, including hyperthyroidism, osteomalacia, chronic liver disease, renal failure, hypercortisolism, malabsorption, immobilization, and alcoholism. Current tobacco users were excluded, while past smokers who had stopped smoking for at least 6 months were allowed in the study. Subjects consuming more than one serving per day of vegetables containing high levels of phytochemicals known to preferentially enhance 2-hydroxylation of estrogen, such as cabbage, cauliflower, brussels sprouts, broccoli, and kale [8], were also excluded from participation.
Clinical, Dietary, and Anthropometric Data
Dietary calcium and vitamin D intakes were estimated from a 7-day dietary record, which was mailed to the participants at least 1 week prior to the study visit. The record contains a list and serving sizes of common dietary sources of calcium. The participants were asked to record daily intake of these food items, and the average daily intake was determined for 7 days. Diet history also included intake of vegetables such as cabbage, cauliflower, brussels sprouts, broccoli, and kale.
Alcohol intake was expressed as the average number of alcoholic drink equivalents consumed over a 1-week period. A can of beer (336 mL), a glass of wine (112 mL) and a heavy alcoholic beverage (28 mL) were considered one drink-equivalent. Previous smoking was expressed in pack-years and estimated as the number of 20-cigarette packs smoked per day multiplied by the number of years of smoking. Physical activity was expressed as a numerical score and defined as follows: 1, sedentary (sitting or lying most of the day); 2, moderately active (being on feet more than half a day; and 3, very active (engaging in regular physical exercise) [9].
Body mass index (BMI) was calculated as weight in kilograms divided by square of the height in meters (kg/m2). The waist-to-hip ratio was calculated as the ratio between the waist circumference, taken at the umbilical level, and the hip circumference, measured 6 inches below the anterior superior iliac spine.
Biochemical Data
Urinary estrogen metabolites were measured in a 24-hour urine specimen using the ESTRAMET™ immunoassay kits (Immuna Care, Bethlehem, PA). The ESTRAMET series of test kits are monoclonal antibody-based competitive enzyme immunoassays for estrogen metabolites in microtiter plate format. The antibodies and assays for urinary 2- and 16α-hydroxyestrogens have been described [10]. The monoclonal antibody to 2-hydroxyestrogens recognizes the 2-hydroxy forms of E1, estradiol, and E3 equivalently. Similarly, the monoclonal antibody to 2-methoxyestrogens recognizes the 2-methoxy forms of estrogen metabolites equivalently and exhibits < 0.1% cross-reactivity with any other estrogen, including 2-hydroxyestrogens. The monoclonal antibody to E3 exhibited < 2% cross-reactivity with any other estrogen. All urinary estrogen assays were performed according to methods described previously [7]. Briefly, urine samples were incubated with enzymes that deconjugated estrogen metabolite sulfates and glucuronides to their respective free forms. The amount of estrogen metabolite in the enzymic hydrolysate was determined by competition between deconjugated estrogen in the hydrolysate and estrogen-labeled alkaline phosphatase for binding to specific monoclonal antibodies attached to the microtiter plate. Greater than 90% of the metabolites in the urine exist as glucuronides and were recovered totally by this method. The inter- and intra-assay coefficients of variability for these enzyme-linked immunoassays were < 9% and 13%, respectively. Each urinary metabolite value was corrected for 24-hour urinary creatinine (mg/24 hour) and expressed in nanograms per milligram creatinine (ng/mg Cr).
Serum samples were collected in the nonfasting state. Serum estradiol (pmol/L) was measured by an ultrasensitive radioimmunoassay technique (Diagnostic Systems Laboratory, Webster, TX), sex-hormone-binding globulin (SHBG, nmol/L) by immunoradiometric assay (Diagnostic Systems Laboratory), and serum testosterone by enzyme-linked immunosorbent assay (Diagnostic Systems Laboratory). The inter- and intra-assay coefficients of variability for serum estradiol and SHBG were < 10%. The free estradiol index (FEI) was calculated as the molar ratio of total estradiol to SHBG [11, 12].
BMD
BMD and skeletal size of the lumbar spine and all regions of the proximal femur were measured by dual-energy X-ray absorptiometry (DXA) using the Hologic (Waltham, MA) QDR 4500. BMD of the lumbar spine was determined using anteroposterior projection and calculated as the average of L1 to L4 vertebrae. The nondominant hip was used for proximal femur scans, and values were calculated on the total femur, femoral neck, trochanter, and intertrochanteric areas. BMD values were expressed as grams per square centimeter. The coefficients of variability of this technique using the QDR 4500 densitometer are 1.09% for the lumbar spine and 1.2% for the total femur in our center.
Statistical Analysis
Results were expressed as means ± standard error (SE). P < 0.05 was considered statistically significant. The association between each urinary metabolite and each clinical variable was evaluated by simple regression analysis. The correlations between BMD in the different skeletal sites and each metabolite, metabolite ratios (2OHE1/16αOHE1 and 2MeOE1/16αOHE1), total metabolites (2OHE1 + 2MeOE1 + 16αOHE1 + E3), FEI, and testosterone were evaluated by partial correlation analysis adjusted for age and BMI. The contributions of clinical and biochemical variables as predictors of BMD in the different skeletal sites were analyzed by stepwise multiple regression analysis. Differences between BMD values among the different tertiles of 16αOHE1 were compared using analysis of covariance adjusted for age, BMI, and FEI. Data were managed using Excel 2000 (Microsoft, Redmond, WA) and analyzed using Statgraphic Plus 5.0 (Manugistic, Rockville, MD).
Results
Sixty-six men over 50 years old participated in the study. Because of the well-known ethnic differences in BMD and estrogen metabolism, we limited our analysis to 61 Caucasian subjects. The demographic and BMD values of the whole study population are presented in Table 1, while biochemical data are reported in Table 2.
Table 1.
Demographic and BMD data of the study subjects
| Clinical features | Mean ± SE | Range |
|---|---|---|
| Age (years) | 66.6 ± 1.0 | 50–87 |
| BMI | 28.1 ± 0.6 | 19.0–36.4 |
| Waist-to-hip ratio | 0.96 ± 0.01 | 0.87–1.78 |
| Past smoking (pack-years) | 11.5 ± 2.3 | 0–125 |
| Alcohol intake (oz-Eq/week) | 4.05 ± 0.8 | 0–34 |
| Calcium intake (mg/day) | 972 ± 91 | 102.6–5.035 |
| BMD (g/cm2) | ||
| Spine | 1.067 ± 0.02 | 0.780–1.419 |
| Total femur | 0.997 ± 0.01 | 0.713–1.225 |
| Neck | 0.786 ± 0.01 | 0.596–0.995 |
| Trochanter | 0.768 ± 0.01 | 0.529–0.982 |
| Intertrochanter | 1.177 ± 0.02 | 0.874–1.440 |
Table 2.
Biochemical data of the study subjects
| Urinary estrogen metabolites (ng/mg Cr) | Mean ± SE | Range |
|---|---|---|
| 2OHE1 | 6.39 ± 0.51 | 1.75–16.4 |
| 2MeOE1 | 4.15 ± 0.37 | 1.61–16.4 |
| 16αOHE1 | 4.26 ± 0.26 | 1.43–11.74 |
| E3 | 6.65 ± 0.49 | 1.90–20.08 |
| 2OHE1/16αOHE1 | 1.59 ± 0.08 | 0.36–0.42 |
| 2MeOE/16αOHE1 | 0.946 ± 0.04 | 0.46–1.89 |
| Total metabolites | 21.46 ± 1.1 | 8.6–45.5 |
| Testosterone (pmol/nmol) | 4.16 ± 0.43 | 1.33–19.5 |
| FEI (pmol/nmol) | 0.65 ± 0.06 | 0.27–1.35 |
Simple correlation analysis between urinary metabolites and clinical variables showed negative correlations between BMI and 2OHE1, 16αOHE1, and total metabolites (Table 3). None among the other clinical variables correlated with any metabolite. Dividing our study subjects according to physical activity score (1, sedentary; 2, moderately active; 3, very active) did not show any significant differences in the level of physical activity between the three groups.
Table 3.
Simple correlations between urinary metabolites and clinical variables
| Age (years) | BMI | Alcohol intake | Calcium intake | Smoking history | Waist-to-hip ratio | |
|---|---|---|---|---|---|---|
| 2OHE1 | −0.26 | −0.36* | −0.09 | −0.06 | −0.12 | −0.18 |
| 2MeOE1 | −0.17 | −0.20 | −0.07 | 0.1 | −0.03 | −0.07 |
| 16αOHE1 | −0.08 | −0.31* | −0.02 | −0.07 | 0.01 | −0.12 |
| E3 | −0.02 | −0.19 | 0.11 | −0.1 | −0.09 | −0.05 |
| 2OHE1/16αOHE1 | −0.20 | 0.05 | −0.07 | −0.05 | −0.18 | −0.10 |
| 2MeOE/16αOHE1 | −0.13 | −0.01 | −0.06 | 0.05 | −0.06 | −0.01 |
| Total metabolites | −0.19 | −0.36* | −0.02 | −0.05 | −0.14 | −0.14 |
P < 0.05
BMI and age-adjusted correlations between BMD and urinary metabolites revealed significant positive correlations between levels of active metabolites, 16αOHE1 and E3, and BMD in all regions of the proximal femur (Table 4). There were no correlations with levels of inactive metabolites (2OHE1 and 2MeOE1) and BMD in any skeletal sites, and no correlation between spine BMD and any urinary metabolite. Significant positive correlations were observed between FEI and BMD of the total hip, femoral neck, and intertrochanter. By contrast, no significant correlations were observed between testosterone levels and BMD in any of the skeletal sites examined.
Table 4.
Partial correlation analysis between BMD and urinary metabolites
| Spine | Total hip | Femoral neck | Trochanter | Intertrochanter | |
|---|---|---|---|---|---|
| 2OHE1 | 0.15 | 0.15 | 0.14 | 0.16 | 0.13 |
| 2MeOE1 | −0.08 | −0.13 | 0.11 | −0.13 | −0.15 |
| 16αOHE1 | 0.27 | 0.63** | 0.59** | 0.57** | 0.62** |
| E3 | −0.01 | 0.42* | 0.34* | 0.47* | 0.40* |
| 2OHE1/16αOHE1 | −0.05 | −0.08 | 0.01 | −0.08 | −0.11 |
| 2MeOE/16αOHE1 | −0.27 | −0.38* | −0.17 | −0.36* | −0.40* |
| Total metabolites | 0.08 | 0.35* | 0.38* | 0.35* | 0.32* |
| Testosterone | 0.10 | 0.06 | 0.07 | 0.16 | 0.03 |
| FEI | 0.20 | 0.38* | 0.42* | 0.26 | 0.42* |
P < 0.05,
P < 0.001; correlations adjusted for age and BMI
The best correlation for urinary metabolites and BMD was seen mostly with 16αOHE1. Dividing BMD in the different regions of the proximal femur according to tertiles of 16αOHE1 showed that men in the lowest tertile of 16αOHE1 had the lowest BMD in the femoral neck, total femur, trochanter, and intertrochanter (Fig. 1). BMD increased with increasing tertiles of 16αOHE1 and was highest for those in the highest tertile.
Fig. 1.
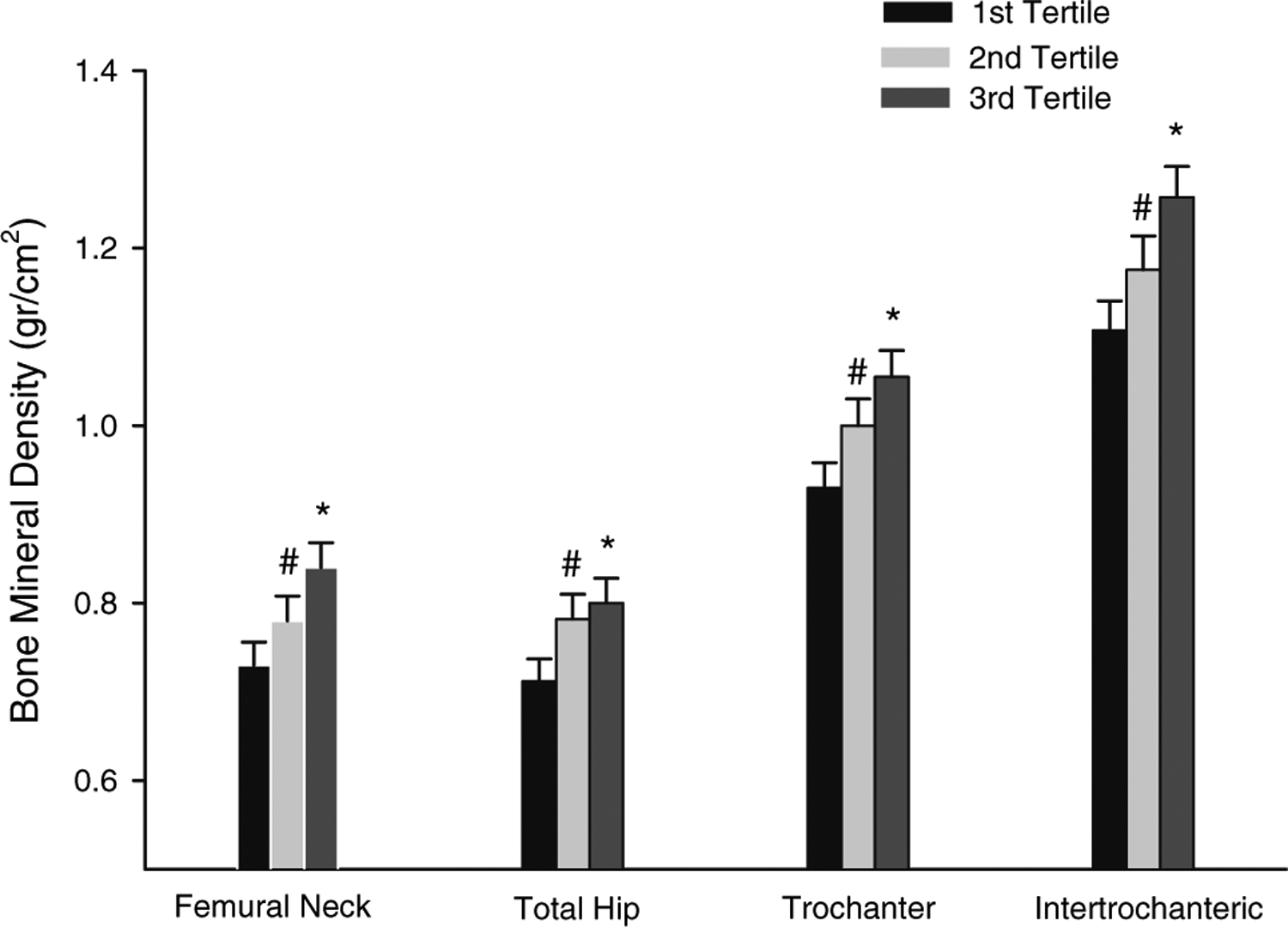
BMD in the different regions of the proximal femur stratified according to tertiles of 16OHE1 (ng/mg Cr). Each group represents the BMD for each skeletal site in the different tertiles, from the lowest to the highest (left to right). *P < 0.05, tertile 3 vs. tertiles 1 and 2; #P < 0.05, tertile 2 vs. tertile 1; analysis by covariance adjusted for age, BMI, and FEI.
Results from multiple regression analysis (Table 5) showed that 16αOHE1 by itself accounted for 17.7–28.2% of the variability in BMD at the different proximal femur sites. The combination of 16αOHE1 and BMI increased the correlation to 40–56% and adding FEI to the other variables further strengthened the correlation model, accounting for 50–67% (Table 5).
Table 5.
Multiple regression analysis of clinical predictors of BMD (r2)
| Skeletal site | 16αOHE1 | 16αOHE1 and BMI | 16αOHE1, BMI, and FEI |
|---|---|---|---|
| Total femur | 20.8 | 56.0 | 63.9 |
| Femoral neck | 28.2 | 41.1 | 60.9 |
| Trochanter | 20.9 | 49.8 | 51.0 |
| Intertrochanter | 17.7 | 54.8 | 67.1 |
All P values are significant (P < 0.05) except for FEI as predictor of trochanteric BMD
Discussion
The present data suggest that, similar to women, estrogen metabolism may modulate BMD in men. Increasing levels of active estrogen metabolites, 16αOHE1 and E3, are associated with increasing BMD; however, only 16αOHE1 appears to be a major determinant of BMD in men independent of estradiol levels and BMI. On the other hand, testosterone did not seem to have an important impact on BMD in our population of elderly men.
While the main effect of testosterone in stimulating periosteal apposition may be responsible for the larger size and thicker cortices in the male skeleton, studies have identified estradiol as a possible key hormone for the attainment and maintenance of peak bone mass in men. Observations of reduced bone mass in men with estrogen resistance resulting from a point mutation in the estrogen receptor α gene [13] and in those with undetectable estradiol levels resulting from aromatase enzyme deficiency despite an elevated testosterone level [14–16] have initiated this important concept. In fact, BMD of patients with the latter condition have been found to improve with estrogen replacement therapy [15, 16].
Estradiol level declines with age in both sexes [17], and its deficiency is the likely cause of age-related bone loss in men, as it is in women [17, 18]. Whereas younger men have lower bioavailable estradiol compared to age-matched premenopausal women, this relationship is reversed in the elderly, with men over 60 years of age having higher levels of bioavailable estradiol compared to age-matched women [19]. While a substantial body of evidence supports the relationship between estradiol and BMD in men, data from population studies are not consistent on the association between total testosterone and BMD [20–22]. In a group of elderly men, Slemenda and colleagues [20] found a negative association between total testosterone and BMD in the mainly trabecular areas of the lumbar spine and trochanter. In another study, although a positive correlation between bioavailable testosterone and BMD of total hip, lumbar spine, and ultradistal radius was observed [21], this relationship was comparably weaker than the association between bioavailable estradiol and BMD of every skeletal site in their population of male subjects. Additionally, a longitudinal follow-up study demonstrated that the rate of bone loss in the forearm correlates more strongly with levels of bioavailable estradiol than of bioavailable testosterone [19]. More importantly, while no association was found between fractures and testosterone levels, lower total and bioavailable estradiol levels were associated with a higher prevalence of vertebral fractures in male participants of the Rancho Bernardo Study [23]. In agreement with these studies, our results likewise demonstrated the absence of correlation between testosterone and BMD in every skeletal site, whereas positive correlations were observed between BMD in the different regions of the femur and circulating estradiol levels. Although this may be partly related to the limited number of subjects in our study, it supports the notion of a relatively minor role of testosterone in comparison to estrogen in the maintenance of bone density in elderly males.
Because estrogen activity is an important regulator of the male skeleton, we believe that estrogen metabolism is also an important determinant of BMD in men, as it is for women. We demonstrated in a previous study an inverse correlation between bone loss and the 2OHE1/16αOHE1 ratio in postmenopausal women [7]. Those with a higher ratio, suggesting preference of the active 2-hydroxyl pathway, have accelerated bone loss over a 1-year period of observation and vice versa. This ratio does not seem to have any impact on BMD among men, as suggested by the lack of correlation between this ratio and BMD. On the other hand, our results suggest that the level of active metabolites may have a stronger influence on male skeletal health as indicated by higher BMD among those with high levels of 16αOHE1 and E3, although 16αOHE1 remains the only important metabolite after adjusting for estradiol levels and BMI. As shown from the results of the multiple regression analysis, 16αOHE1 is an independent predictor of BMD by itself and, in conjunction with BMI and FEI, may account for approximately 50–67% of variability in BMD of the proximal femur sites.
So far the biological properties of the different estrogen metabolites have been investigated mostly in estrogen-dependent malignancies and in animal studies. Animal studies have demonstrated that metabolites resulting from16α-hydroxylation have estrogenic properties that are closer or even comparable to the parent compound, estradiol [24]. Administration of 16αOHE1 prevented ovariectomy-induced bone loss and reduced cholesterol levels to the same extent as 17β-estradiol. Results from cancer studies likewise showed that a lower 2OHE1/16αOHE1 ratio is associated with an increased risk for breast cancer, while the converse is true for those with a higher ratio [25, 26]. In fact, 16αOHE1 may have an augmented estrogenic property, resulting from its covalent attachment to estrogen receptors and its low affinity to SHBG [27].
There are several factors identified to modulate estrogen metabolism [28]. For instance, smoking [29] and increased consumption of indole-containing vegetables [8] have been found to enhance 2-hydroxylation of estrogen. Medications such as cimetidine may inhibit estrogen hydroxylation [30], while thyroid hormones increases 2-hydroxylation [31]. Obesity is associated with decreased 2-hydroxylation without any change in 16α-hydroxylation [32], and a family history of osteoporosis is associated with increased 2-hydroxylation [33]. In the current study, among the clinical variables examined, only BMI was found to impact estrogen metabolism in men. Increasing body weight is associated with inhibition of estrogen hydroxylation in men, as suggested by the inverse correlation between BMI and total metabolites.
Our study has some limitations. First, this is a retrospective cross-sectional study, and although we excluded subjects with current factors that may influence both estrogen metabolism and BMD, the present observations may not reflect the lifelong lifestyle and dietary habits of our participants. Second, the small number of subjects may limit our ability to fully evaluate the interaction between clinical variables and estrogen metabolism in men. Further, we did not analyze markers of bone turnover, and this lack may not allow us to determine if the role of the active estrogen metabolites on femoral BMD is modulated by a reduction in bone turnover. Finally, considering that most of our subjects were elderly (mean age > 60 years), the lack of association between spine BMD and urinary metabolites may reflect the effect of degenerative changes on DXA measurements in the spine [34].
In summary, our observations suggest that estrogen metabolism may modulate BMD in men; i.e., higher levels of the active metabolites 16αOHE1 and E3 are associated with higher BMD, a finding that supports our previous data in women. These results demonstrate a positive impact on bone metabolism when there is increased 16α-hydroxylation as contrasted to states where there is increased inactive 2-hydroxylation [7, 35]. These findings may be clinically relevant as they suggest that specifically stimulating the active pathway of estrogen hydroxylation may reduce the risk for low bone density and bone loss in both sexes. This model can be used for drug development targeted at inducing the activity of the CYP450 enzymes responsible for 16α-hydroxylation and inhibiting those involved in 2-hydroxylation to enhance circulating levels of active estrogens. Since estrogen metabolism is a lifelong process, this would lead not only to a reduction in the rate of age-related bone loss but perhaps also to a higher peak bone mass in younger individuals at risk for osteoporosis. However, the beneficial effects of inducing 16α-hydroxylation over 2-hydroxylation should be balanced by the possibility of increasing risk of prostate cancer, as indicated by recent findings [36].
Acknowledgment.
This work was supported by National Institutes of Health grants R03 AR049401 (to R. A.-V.) and K12 HD01459 (Building Interdisciplinary Research Careers in Women’s Health) and the General Clinical Research Center at Washington University.
References
- 1.Falahati-Nini A, Riggs BL, Atkinson EJ, O’Fallon WM, Eastell R, Khosla S (2000) Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest 106:1553–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett-Connor E, Mueller JE, von Muhlen DG, Laughlin GA, Schneider DL, Sartoris DJ (2000) Low levels of estradiol are associated with vertebral fractures in older men, but not women: the Rancho Bernardo Study. J Clin Endocrinol Metab 85:219–223 [DOI] [PubMed] [Google Scholar]
- 3.Grodin JM, Siiteri PK, MacDonald PC (1973) Source of estrogen production in postmenopausal women. J Clin Endocrinol Metab 36:207–214 [DOI] [PubMed] [Google Scholar]
- 4.Fishman J, Bradlow HL, Gallagher TF (1960) Oxidative metabolism of estradiol. J Biol Chem 235:3104–3107 [PubMed] [Google Scholar]
- 5.Martucci C, Fishman J (1977) Direction of estradiol metabolism as a control of its hormonal action - uterotropic activity of estradiol metabolites. Endocrinology 101:1709–1715 [DOI] [PubMed] [Google Scholar]
- 6.Fishman J, Martucci C (1980) Biological properties of 16a-hydroxyestrone: implications in estrogen physiology and pathophysiology. J Clin Endocrinol Metab 51:611–615 [DOI] [PubMed] [Google Scholar]
- 7.Leelawattana R, Ziambaras K, Roodman-Weiss J, et al. (2000) The oxidative metabolism of estradiol conditions postmenopausal bone density and bone loss. J Bone Miner Res 15:2513–2520 [DOI] [PubMed] [Google Scholar]
- 8.Michnovicz JJ, Adlercreutz H, Bradlow HL (1997) Changes in levels of urinary estrogen metabolites after oral indole-3-carbinol treatment in humans. J Natl Cancer Inst 89:718–723 [DOI] [PubMed] [Google Scholar]
- 9.Armamento-Villareal R, Villareal DT, Avioli LV, Civitelli R (1992) Estrogen status and heredity are major determinants of premenopausal bone mass. J Clin Invest 90:2464–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klug TL, Bradlow HL, Sepkovic DW (1994) Monoclonal antibody-based enzyme immunoassay for simultaneous quantitation of 2- and 16 alpha-hydroxyestrone in urine. Steroids 59:648–655 [DOI] [PubMed] [Google Scholar]
- 11.Szulc P, Hofbauer LC, Heufelder AE, Roth S, Delmas PD (2001) Osteoprotegerin serum levels in men: correlation with age, estrogen, and testosterone status. J Clin Endocrinol Metab 86:3162–3165 [DOI] [PubMed] [Google Scholar]
- 12.Napoli N, Villareal DT, Mumm S, et al. (2005) Effect of CYP1A1 gene polymorphisms on estrogen metabolism and bone density. J Bone Miner Res 20:232–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith EP, Boyd J, Frank GR, et al. (1994) Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331:1056–1061 [DOI] [PubMed] [Google Scholar]
- 14.Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K (1995) Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 80:3689–3698 [DOI] [PubMed] [Google Scholar]
- 15.Carani C, Qin K, Simoni M, et al. (1997) Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med 337:91–95 [DOI] [PubMed] [Google Scholar]
- 16.Bilezikian JP, Morishima A, Bell J, Grumbach MM (1998) Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. N Engl J Med 339:599–603 [DOI] [PubMed] [Google Scholar]
- 17.Khosla S, Melton LJ III, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL (1998) Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 83:2266–2274 [DOI] [PubMed] [Google Scholar]
- 18.Riggs BL, Khosla S, Melton LJ III (2002) Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302 [DOI] [PubMed] [Google Scholar]
- 19.Khosla S, Melton LJ III, Atkinson EJ, O’Fallon WM (2001) Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metab 86:3555–3561 [DOI] [PubMed] [Google Scholar]
- 20.Slemenda CW, Longcope C, Zhou L, Hui SL, Peacock M, Johnston CC (1997) Sex steroids and bone mass in older men. Positive associations with serum estrogens and negative associations with androgens. J Clin Invest 100:1755–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greendale GA, Edelstein S, Barrett-Connor E (1997) Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. J Bone Miner Res 12:1833–1843 [DOI] [PubMed] [Google Scholar]
- 22.Murphy S, Khaw KT, Cassidy A, Compston JE (1993) Sex hormones and bone-mineral density in elderly men. Bone Miner 20:133–140 [DOI] [PubMed] [Google Scholar]
- 23.Barrett-Connor E, Mueller JE, von Muhlen DG, Laughlin GA, Schneider DL, Sartoris DJ (2000) Low levels of estradiol are associated with vertebral fractures in older men, but not women: the Rancho Bernardo Study. J Clin Endocrinol Metab 85:219–223 [DOI] [PubMed] [Google Scholar]
- 24.Westerlind KC, Gibson KJ, Malone P, Evans GL, Turner RT (1998) Differential effects of estrogen metabolites on bone and reproductive tissues of ovariectomized rats. J Bone Miner Res 13:1023–1031 [DOI] [PubMed] [Google Scholar]
- 25.Muti P, Bradlow HL, Micheli A, et al. (2000) Estrogen metabolism and risk of breast cancer: a prospective study of the 2:16alpha-hydroxyestrone ratio in premenopausal and postmenopausal women. Epidemiology 11:635–640 [DOI] [PubMed] [Google Scholar]
- 26.Osborne MP, Bradlow HL, Wong GY, Telang NT (1993) Upregulation of estradiol C16 alpha-hydroxylation in human breast tissue: a potential biomarker of breast cancer risk. J Natl Cancer Inst 85:1917–1920 [DOI] [PubMed] [Google Scholar]
- 27.Swaneck GE, Fishman J (1988) Covalent binding of the endogenous estrogen 16 alpha-hydroxyestrone to estradiol receptor in human breast cancer cells: characterization and intranuclear localization. Proc Natl Acad Sci USA 85:7831–7835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michnovicz JJ, Bradlow HL (1990) Dietary and pharmacological control of estradiol metabolism in humans. Ann N Y Acad Sci 595:291–299 [DOI] [PubMed] [Google Scholar]
- 29.Michnovicz JJ, Hershcopf RJ, Naganuma H, Bradlow HL, Fishman J (1986) Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette smoking. N Engl J Med 315:1305–1309 [DOI] [PubMed] [Google Scholar]
- 30.Michnovicz JJ, Galbraith RA (1991) Cimetidine inhibits catechol estrogen metabolism in women. Metabolism 40:170–174 [DOI] [PubMed] [Google Scholar]
- 31.Michnovicz JJ, Galbraith RA (1990) Effects of exogenous thyroxine on C-2 and C-16 alpha hydroxylations of estradiol in humans. Steroids 55:22–26 [DOI] [PubMed] [Google Scholar]
- 32.Schneider J, Bradlow HL, Strain G, Levin J, Anderson K, Fishman J (1983) Effects of obesity on estradiol metabolism: decreased formation of nonuterotropic metabolites. J Clin Endocrinol Metab 56:973–978 [DOI] [PubMed] [Google Scholar]
- 33.Napoli N, Donepudi S, Sheikh S, Rini GB, Armamento-Villareal R (2005) Increased 2-hydroxylation of estrogen in women with family history of osteoporosis. J Clin Endocrinol Metab 90(4):2035–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu G, Peacock M, Eilam O, Dorulla G, Braunstein E, Johnston CC (1997) Effect of osteoarthritis in the lumbar spine and hip on bone mineral density and diagnosis of osteoporosis in elderly men and women. Osteoporos Int 7:564–569 [DOI] [PubMed] [Google Scholar]
- 35.Lim SK, Won YJ, Lee JH, et al. (1997) Altered hydroxylation of estrogen in patients with postmenopausal osteopenia. J Clin Endocrinol Metab 82:1001–1006 [DOI] [PubMed] [Google Scholar]
- 36.Muti P (2004) The role of endogenous hormones in the etiology and prevention of breast cancer: the epidemiological evidence. Ann N Y Acad Sci 1028:273–282 [DOI] [PubMed] [Google Scholar]


