Abstract
Vanadium is a ubiquitous environmental contaminant although there are limited data to assess potential adverse human health impact following oral exposure. In support of studies investigating the subchronic toxicity of vanadyl sulfate (V4+) and sodium metavanadate (V5+) following perinatal exposure via drinking water in male and female rats, we have determined the internal exposure and urinary excretion of total vanadium at the end of study. Water consumption decreased with increasing exposure concentration following exposure to both compounds. Plasma and urine vanadium concentration normalized to total vanadium consumed per day increased with the exposure concentration of vanadyl sulfate and sodium metavanadate suggesting absorption increased as the exposure concentration increased. Additionally, females had higher concentrations than males (in plasma only for vanadyl sulfate exposure). Animals exposed to sodium metavanadate had up to 3-fold higher vanadium concentration in plasma and urine compared to vanadyl sulfate exposed animals, when normalized to total vanadium consumed per day, demonstrating differential absorption, distribution, metabolism, and excretion properties between V5+ and V4+ compounds. These data will aid in the interpretation of animal toxicity data of V4+ and V5+ compounds and determine the relevance of animal toxicity findings to human exposures.
Keywords: vanadate, vanadyl, vanadyl sulfate, sodium metavanadate, plasma vanadium, urine vanadium
Introduction
Vanadium is a naturally occurring element and present in a variety of minerals, coal, and crude oils (ATSDR, 2012). The main source of anthropogenic release of vanadium to the environment is from fossil fuel combustion and mining for industrial applications such as steel production (ATSDR, 2012). Vanadium is used in unregulated dietary supplements (Trevino et al., 2019). In addition, vanadium-containing compounds are being considered as potential antidiabetic, anticancer, and anti-hypertensive agents (Maughan et al., 2004; NTP, 2008; Trevino et al., 2019). Low levels of vanadium are also detected in many foods and in drinking water. Vanadium was detected in 73.6% of samples collected from public water systems and 3.3% of the samples were found to contain levels above the reference concentration of 21 μg/L set by the US Environmental Protection Agency (EPA) (EPA, 2017). Collectively, these reports suggest that there is a potential for human exposure to vanadium. Due to its ubiquitous presence, vanadium was included in the Fourth Contaminant Candidate List by the U.S. EPA in 2016 (Richardson and Ternes, 2014; EPA, 2020). Recently, the EPA selected oral vanadium compounds to be assessed in the Integrated Risk Information System (IRIS) and put forth an assessment plan (EPA, 2021).
Vanadium can exist in several oxidation states (+2 to +5). In the environment, V4+ and V5+ are the most prevalent oxidation states (ATSDR, 2012; Trevino et al., 2019). The oxidation state and the vanadium species present vary depending on the concentration, pH, redox potential, and other factors making the chemistry of vanadium compounds complex. In low pH and suboxic and/or mildly reducing conditions, vanadyl (V4+) and corresponding oxo cations (VO2+) predominate. Under neutral to high pH and oxic conditions, vanadate (V+5) and corresponding oxoanions (e.g., H2VO4−, HVO42−) predominate— oligomers of H2VO4− and HVO42− (eg., dimers, trimers, tetramers) can be formed around neutral and lower pH at high vanadium concentrations (Crans et al., 2004; ATSDR, 2012; Mutlu et al., 2017; Gustafsson, 2019; Trevino et al., 2019).
There are limited data to assess the impact of oral vanadium exposure on human health. Data gaps identified by the Agency for Toxic Substances and Disease Registry (ATSDR) included subchronic and chronic toxicity of V4+ and V5+ compounds. Hence, the Division of the National Toxicology Program (DNTP) initiated toxicity studies following exposure to vanadyl sulfate (V4+) (Figure 1A) and sodium metavanadate (V5+) (Figure 1B) in rodents following exposure via drinking water. Concentration of vanadium in blood and excreta following exposure is important to put toxicological findings into context. In addition, such data allow for comparison of rodent exposures to human levels and subsequently to understand the relevance of animal toxicity findings to human exposures. We have previously demonstrated that speciation of vanadium in biological matrices is not feasible due to interconversion of oxidation states and that total vanadium measurement is a useful metric to determine internal exposure (Harrington et al., 2021b; Harrington et al., 2021c). In addition, using samples following exposure of adult rats to vanadyl sulfate or sodium metavanadate via drinking water, we demonstrated that plasma is a better matrix than blood for assessing the internal exposures (Harrington et al., 2021b; Harrington et al., 2021c) and subsequently validated analytical methods to quantify vanadium in plasma and urine (Harrington et al., 2021a). The current manuscript reports the plasma and urine concentrations of vanadium in F1 male and female Hsd:Sprague Dawley® SD® rats following exposure during the perinatal period (beginning at gestation day 6 through postnatal day 21) and for 3 months after weaning to vanadyl sulfate at 0 (control), 21.0, 41.9, 83.8, 168, and 335 mg/L or sodium metavanadate at 0 (control), 31.3, 62.5, 125 , 250, and 500 mg/L in drinking water. Current studies were conducted as a part of larger design evaluating the subchronic toxicity of sodium metavanadate and vanadyl sulfate following perinatal exposure in rats (NTP, 2022b; NTP, 2022a). The subchronic toxicity findings will be reported elsewhere.
Figure 1.
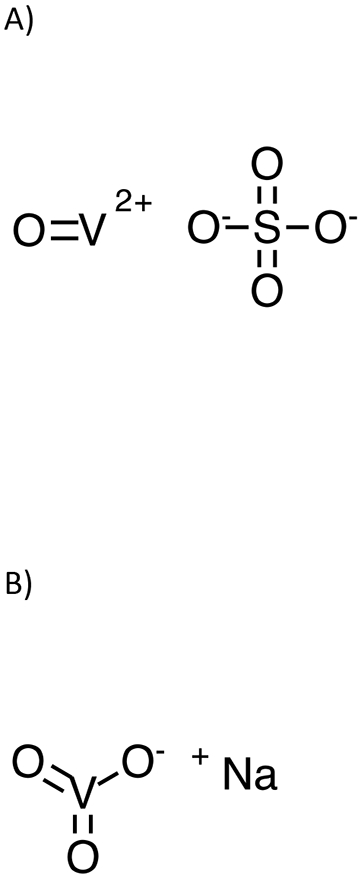
Structures of A) vanadyl sulfate and B) sodium metavanadate
Materials and Methods
Materials.
Sodium metavanadate (CASRN 13718-26-8, Lot No. 8579K) was purchased from MP Biomedicals (Santa Ana, CA). The identity and the purity (100%) were determined as described elsewhere (Roberts et al., 2016). Vanadyl sulfate (CASRN 27774-13-6, Lot No.0210324/1.1) was purchased from Noah Technologies Corporation (San Antonio, TX). The manufacturer identified the test article as vanadyl sulfate hydrate without specifying the number of water molecules but with the CASRN and molecular formula for the anhydrous form. The moisture content in vanadyl sulfate lot No.0210324/1.1 was determined by multiple techniques and found to be ~ 33%. The purity of vanadyl sulfate after excluding moisture content was 100%.
For determination of vanadium concentration in plasma and urine, stock solutions of vanadium (10 and 1,000 μg/mL) were purchased from two sources (High-Purity Standards, Charleston, SC and Inorganic Ventures, Christiansburg, VA). Praseodymium (1,000 μg/mL) to be used as the internal standard was also purchased from High-Purity Standards (Charleston, SC). Pooled male Sprague Dawley rat plasma isolated from blood collected using potassium ethylenediaminetetraacetic acid (K3EDTA) anticoagulant and urine were purchased from BioIVT (Westbury, NY). High-purity, 30% (v/v) non-stabilized hydrogen peroxide (Suprapur grade) was purchased from EMD Millipore (Billerica, MA) and concentrated nitric acid (Optima grade) was purchased from Thermo Fisher Scientific (Fair Lawn, NJ). Deionized water (18 MΩ cm−1) was produced using a purification system from Pure Water Solutions Inc. (Castle Rock, CO). Optima-grade hydrochloric acid was purchased from Fisher Scientific (Waltham, MA).
Animals and husbandry:
Animal studies were conducted at Battelle (West Jefferson, OH). Animal care and use were in accordance with the Public Health Service Policy on Humane Care and Use of Animals. All animal studies were conducted in an animal facility accredited by AAALAC International. Studies were approved by the Battelle Animal Care and Use Committee and conducted in accordance with all relevant National Institutes of Health animal care and use policies and applicable federal, state, and local regulations and guidelines. Animal procedures were in accordance with the “Guide for the Care and Use of Laboratory Animals” (NRC, 2011). The study was conducted in compliance with the Food and Drug Administration Good Laboratory Practice. Time-mated female Hsd:Sprague Dawley® SD® (HSD) rats were obtained from Envigo (Haslett, Ml); gestation day (GD) 0 was assigned as day of mating. Animals were 13-16 weeks old at arrival and had ad libitum access to feed and water. Time-mated rats and F1 offspring were provided certified, irradiated NIH-07 diet (Zeigler Brothers, Inc., Gardeners, PA) until postnatal day (PND) 28. After weaning on PND28, F1 animals were provided certified, irradiated NTP-2000 diet (Zeigler Brothers, Inc., Gardeners, PA). Feed was used within 180 days of milling. Animals were housed in rooms with the following settings for environmental conditions: room temperature 72 ± 3 °F (22 ± 2 °C), relative humidity 50% ± 15% and a 12-h light/dark cycle.
Study design and exposure.
Drinking water formulations of vanadyl sulfate were prepared in pH 3.5 deionized water (ASTM Type 1) at 0 (control), 21.0, 41.9, 83.8, 168, and 335 mg/L with respect to vanadyl sulfate after adjusting for water content of ~ 33%. Drinking water formulations of sodium metavanadate were prepared in pH 7 deionized water at 0 (control), 31.3, 62.5, 125 , 250, and 500 mg/L. Formulations were analyzed prior to exposing animals using a validated high performance liquid chromatography method (Roberts et al., 2016). All study formulations were within 10% of target concentration. Prior to study initiation, the stability of vanadyl sulfate (as V4+) and sodium metavanadate (as V5+) in drinking water formulations stored at refrigerated temperature up to 42 d was established (Mutlu et al., 2017). During formulation development work, in the lowest concentration of vanadyl sulfate formulation, a small peak corresponding to the retention time of vanadate was observed suggesting slight oxidation of vanadyl to vanadate. Vanadium was not detected in control water at or above the limit of quantitation of the analytical method (vanadyl sulfate 0.20 μg/mL; sodium metavanadate 0.70 μg/mL).
Studies were conducted as part of a larger design investigating the subchronic toxicity (including perinatal exposure) of vanadyl sulfate and sodium metavanadate in rodents. On GD3, body weights of animals were collected into the Provantis™ (Instem Life Sciences Systems, Ltd., Staffordshire, UK) electronic data collection system and animals were randomly assigned to exposure groups. Groups of pregnant animals (n=16/exposure group) were exposed ad libitum to control or dosed water (vanadyl sulfate: 0, 21.0, 41.9, 83.8, 168, and 335 mg/L; sodium metavanadate: 0, 31.3, 62.5, 125, 250, and 500 mg/L) from GD 6 until PND28. Pups (FI animals) were weaned on PND28. F1 animals were exposed to the same concentrations as respective dams for 13-weeks post weaning. To determine internal exposure and excretion, groups of male and female F1 animals (n=5/exposure group/sex where 1 animal/sex/litter) were selected from 5 separate litters. There were 2 male animals and 1 female animal removed from the study early in the 83.8 mg/L vanadyl sulfate group for reasons unrelated to test article exposure; the resulting number of animals per sex for this group was 3 and 4 for males and females, respectively. At the end of the 13-week exposure period, animals were placed individually in metabolism cages for collection of 24 h urine. While in metabolism cages all animals were given control (0 mg/L) drinking water. Following 24 h in metabolism cages, within 2 h of lights being turned on, animals were humanely euthanized by CO2 inhalation and blood was collected via cardiac puncture using K3EDTA as an anticoagulant. Blood from all animals were collected within 2.5 h and kept on wet ice until plasma isolation. Plasma and urine for vanadium analysis were immediately frozen at −70°C. Additional samples of urine were utilized for analysis of appearance, specific gravity, total protein, glucose, creatinine, calcium, phosphorus, N-acetyl-β-glucosaminidase, alkaline phosphorus, and aspartate aminotransferase but that data will not be reported here. The creatinine levels were used to normalize total vanadium concentration in urine.
All animals on study were observed at least twice daily for signs of moribundity and mortality. Body weights were recorded prior to study start and twice weekly thereafter. Water consumption was measured weekly. Chemical consumption was estimated from water consumption and dose was estimated from chemical consumption and body weights.
Quantitation of vanadium in plasma and urine.
Plasma and urine samples were analyzed using validated analytical methods (Harrington et al., 2021a). Briefly, standard spiking solutions of vanadium in the range 10-1000 ng/mL were prepared from commercially procured alternate stock solutions by diluting in nitric acid and water. Plasma and urine matrix standards in the range 5-1000 ng/mL were prepared by spiking appropriate volumes of vanadium spiking solutions in respective matrix. Quality control (QC) samples were prepared at 7.50 and 750 ng/mL in respective matrix. Method blanks were prepared similarly in each matrix except no vanadium standard was added.
Sample preparation was conducted in a clean plastic hood. Study samples, matrix calibration standards, QC samples, and method blanks were prepared for digestion by aliquoting 100 μL of each plasma or urine sample into a plastic tube and subjecting to a multistage digestion sequence in a graphite heating block (DigiPREP graphite heating block from SCP Science, Champlain, NY) at 65 °C in the presence of concentrated nitric acid and 30% hydrogen peroxide. Samples were cooled to room temperature, 100 μL of 100 ng/mL internal standard (praseodymium) was added following which samples were diluted to a final volume of 10 mL using deionized water. All samples were analyzed by inductively coupled plasma-mass spectrometry (ICP-MS) using a X-Series II quadrupole ICP-MS (Thermo Fisher Scientific, Bridgewater, NJ) equipped with a Peltier-cooled spray chamber and an ASX-500 autosampler (Teledyne CETAC Technologies, Omaha, NE) as described elsewhere (Harrington et al., 2021a).
Study samples that exceeded the matrix calibration range were diluted 5-fold using a matrix-matched blank solution to bring the concentration into the validated range. Each study sample set was bracketed by method blanks, matrix calibration standards, and QC samples. Unweighted linear least-squares regression was used to relate analyte signal to the standard concentration. Vanadium concentration in samples was calculated from the linear regression equation and the measured vanadium signal intensity in each sample corrected for instrument background vanadium signal, if present. The concentration in plasma was reported as ng vanadium/mL plasma and the concentration in urine was reported as both ng/mL urine and ng vanadium/mg creatinine. For purpose of this manuscript, the urine vanadium concentration normalized to creatinine was used. The limit of quantitation (LOQ) and limit of detection (LOD), respectively, for both plasma and urine were 5.00 and 0.155 ng/mL, respectively.
Data from study samples were considered valid if the following criteria were met: the matrix calibration curve linearity (r ≥ 0.99); at least 75% of matrix standards were within 15% of nominal values (except at the LOQ where it was 20%); at least 67% of the QC samples were within 15% of nominal values. All analytical runs met the criteria.
Statistical analysis.
Extreme values for F0 body weights, water and chemical consumption, urinalysis data, and vanadium concentration were identified by the outlier test of Dixon and Massey when sample size n < 20 (Dixon and Massey, 1957) and by Tukey’s outer fences method when sample size n≥20 . For F1 body weights, all observations across dose groups were fit to a linear mixed effects model with a random litter effect, and the residuals were tested by exposure group for outliers using Tukey’s outer fences method (Tukey, 1977). F0 body weights were analyzed using Jonckheere’s trend test (Jonckheere, 1954) and Williams’ or Dunnett’s (pairwise) test depending on detection of a significant trend at 0.01 level (Dunnett, 1955; Williams, 1971; Williams, 1972; Williams, 1986). F1 body weights were analyzed using mixed models with a random litter effect and a Dunnett-Hsu adjustment (Hsu, 1992) for both trend and pairwise analyses. Water and chemical consumption, urinalysis data, and vanadium concentration were analyzed using the nonparametric multiple comparison methods of Shirley (Shirley, 1977) as modified by Williams (Williams, 1986) or Dunn (Dunn, 1964). Jonckheere’s trend test was used to assess the significance of exposure-related trends (Jonckheere, 1954). Vanadium concentration above the LOD of the assay was detected in some samples from control exposure groups. In these cases, if at least 20% of the values within a control group were above the LOD, the samples that were below the LOD in those groups were replaced with ½ of LOD value prior to calculation of group average and standard error (SE). This was done to better characterize low exposures and allow for statistical comparison. When a control group did not have over 20% of values above the LOD, mean or SE was not calculated for that group and statistical analysis was not performed.
Results
All study data are available in the Chemical Effects of Biological Systems (CEBS) database and can be accessed using the following link (Please note that the reviewer link will be replaced with the public link when the manuscript is accepted for publication by the journal):https://manticore.niehs.nih.gov/cebssearch/paper/15267/private/ROCSS91XYZ.) Specific tables are: I04, Mean body weight summary; I07, Mean water consumption; I08, Mean compound consumption; PA44, Urinalysis data summary (urinary creatinine data and total urine vanadium concentration (ng/mg creatinine)); PA48, Summary of tissue (plasma) concentration; Plasma and urine vanadium concentration for individual animals.
Survival, body weights, water and chemical consumption following exposure to vanadyl sulfate.
Male and female rats were exposed via drinking water to 0 (control), 21.0, 41.9, 83.8, 168, and 335 mg/L vanadyl sulfate from in utero (GD 6) through PND 118-119 (90 d following weaning on PND 28). During the post-weaning period, there was no impact on F1 survival following exposure to vanadyl sulfate. Terminal body weights in male and female F1 rats exposed to vanadyl sulfate were within 5-10% of the respective control groups. Group average daily water consumption during the last week of study is given in Table 1. Water consumption significantly decreased in males at the two highest and in females at the highest exposure concentration likely due to decreasing palatability with increasing test material concentration. Doses of vanadyl sulfate during at the end of the study (PND 105-112) were estimated based on exposure concentration and water consumption and ranged from 1.2 to 15.8 and 1.5 to 20.5 mg/kg/day in males and females, respectively (Table 1).
Table 1.
Average daily water and vanadium consumption in rats following perinatal exposure to vanadyl sulfate (V4+) via drinking water
| Exposure Concentration (mg/L) |
Water Consumption (g/kg body weight/day)a |
Dose (mg vanadyl sulfate/kg body weight/day)b |
Water Consumption (g/animal/day)a |
Vanadium Concentration in exposure solution (mg/L)c |
Vanadium Consumption (mg/day)d |
|---|---|---|---|---|---|
| Male | |||||
| 0 | 63.0 ± 2.7** | 0 | 27.1 | 0 | 0 |
| 21.0 | 58.5 ± 1.9 | 1.2 ± 0.0 | 26.2 | 6.5 | 0.171 |
| 41.9 | 57.9 ± 1.8 | 2.4 ± 0.1 | 25.4 | 13.0 | 0.330 |
| 83.8 | 55.1 ± 2.0* | 4.6 ± 0.2 | 24.8 | 26.0 | 0.644 |
| 168 | 52.0 ± 1.3** | 8.7 ± 0.2 | 21.8 | 52.1 | 1.14 |
| 335 | 47.3 ± 0.5** | 15.8 ± 0.2 | 19.5 | 103.9 | 2.03 |
| Female | |||||
| 0 | 90.6 ± 10.6* | 0 | 24.4 | 0 | 0 |
| 21.0 | 72.8 ± 8.1 | 1.5 ± 0.2 | 18.9 | 6.5 | 0.123 |
| 41.9 | 81.2 ± 4.1 | 3.4 ± 0.2 | 22.7 | 13.0 | 0.295 |
| 83.8 | 87.6 ± 7.5 | 7.3 ± 0.6 | 23.4 | 26.0 | 0.608 |
| 168 | 75.4 ± 4.1 | 12.7 ± 0.7 | 20.6 | 52.1 | 1.07 |
| 335 | 61.2 ± 3.0* | 20.5 ± 1.0 | 16.3 | 103.9 | 1.69 |
Group average water consumption for all study animals (n=6-11 per exposure group) at the end of study (PND 105-112) are given as g/kg body weight/day or g/animal/day (CEBS Table I07)
Values given are group averages estimated from water consumption and subsequently the vanadyl sulfate consumption and animal body weights using data from the final week of study (PND105-112) (CEBS Table I08)
Calculated using exposure concentration and 31% (w/w) vanadium in vanadyl sulfate
Calculated as vanadium concentration (mg/L) *water consumed (g/animal/day) and a solution density of 1 g/mL
Statistically significant at P <= 0.05.
Statistically significant at P <= 0.01. Statistical significance for a treatment group indicates a significant pairwise test compared to the vehicle control group. Statistical significance for a control group indicates a significant trend test
Vanadium concentration (mg vanadium/L) in drinking water formulations of vanadyl sulfate was estimated using vanadium content of 31% (w/w) in vanadyl sulfate and vanadyl sulfate exposure concentration (Table 1). Using the average daily water consumed (g/animal/day) for a given exposure group during the last week of study and corresponding vanadium concentration (mg/L) in drinking water formulations, daily vanadium consumption (mg vanadium/day) by an animal was estimated (Table 1). Due to exposure concentration-related decreases in water consumption, vanadium consumption increased only 12- and 14-fold with a 16-fold increase in vanadyl sulfate exposure concentration in males and females, respectively (Table 1).
Survival, body weights, water and chemical consumption following exposure to sodium metavanadate.
Male and female rats were exposed via drinking water to 0 (control), 31.3, 62.5, 125, 250, and 500 mg/L sodium metavanadate from in utero (GD 6) through PND 118 (90 d following weaning on PND 28). There was reduced pup viability, and higher whole litter loss and F0 mortality that contributed to lower number of 500 mg/L F1 pups available for post-weaning exposure. During the post-weaning period, there was no impact on F1 survival following exposure to sodium metavanadate. Terminal body weights in male and female F1 rats exposed to sodium metavanadate were significantly reduced in the 500 mg/L group (−27% and −11.5% in male and female rats respectively) compared to controls. Male and female rats exposed to lower (<500 mg/L) concentrations had terminal body weights within 10% of their respective controls. Group average daily water consumption during the last week of study is given in Table 2. Water consumption significantly decreased in both male and female rats at the two highest exposure concentrations likely due to palatability issues. Doses of sodium metavanadate during the last week of the study (PND 112-119) were estimated using exposure concentration and water consumption and ranged from 1.9 to 22.4 and 2.5 to 27.0 mg/kg/day in males and females, respectively (Table 2).
Table 2.
Average water and vanadium consumption in rats following perinatal exposure to sodium metavanadate (V5+) via drinking water
| Exposure Concentration (mg/L) |
Water Consumption (g/kg body weight/day)a |
Dose (mg sodium metavanadate/kg body weight/day)b |
Water Consumption (g/animal/day)a |
Vanadium concentration in exposure solution (mg/L)c |
Vanadium Consumption (mg/day)d |
|---|---|---|---|---|---|
| Male | |||||
| 0 | 56.3 ± 3.2** | 0 | 25.7 | 0 | 0 |
| 31.3 | 59.6 ± 1.9 | 1.9 ± 0.1 | 27.0 | 12.8 | 0.346 |
| 62.5 | 57.0 ± 1.6 | 3.6 ± 0.1 | 26.2 | 25.6 | 0.671 |
| 125 | 56.5 ± 2.3 | 7.1 ± 0.3 | 23.8 | 51.3 | 1.22 |
| 250 | 45.5 ± 1.7* | 11.4 ± 0.4 | 18.7 | 102.5 | 1.92 |
| 500 | 44.8 ± 0.8** | 22.4 ± 0.4 | 15.0 | 205 | 3.08 |
| Female | |||||
| 0 | 78.2 ± 2.1** | 0 | 20.8 | 0 | 0 |
| 31.3 | 80.3 ± 9.0 | 2.5 ± 0.3 | 22.7 | 12.8 | 0.291 |
| 62.5 | 76.8 ± 5.4 | 4.8 ± 0.3 | 20.5 | 25.6 | 0.525 |
| 125 | 76.6 ± 3.2 | 9.6 ± 0.4 | 20.1 | 51.3 | 1.03 |
| 250 | 55.4 ± 2.8* | 13.8 ± 0.7 | 15.0 | 102.5 | 1.54 |
| 500 | 54.1 ± 3.9* | 27.0 ± 1.9 | 12.7 | 205 | 2.60 |
Group average water consumption for all study animals (n=3-7 per exposure group) at the end of study (PND 112-119) are given as g/kg body weight/day or g/animal/day (CEBS Table I07)
Values given are group averages estimated from water consumption and subsequently the vanadyl sulfate consumption and animal body weights using data from the final week of study (PND105-112) (CEBS Table I08)
Calculated using exposure concentration and 41% (w/w) vanadium in sodium metavanadate
Calculated as vanadium concentration (mg/L) *water consumed (g/animal/day) and a solution density of 1 g/mL
Statistically significant at P <= 0.05
Statistically significant at P <= 0.01. Statistical significance for a treatment group indicates a significant pairwise test compared to the vehicle control group. Statistical significance for a control group indicates a significant trend test
Vanadium concentration (mg vanadium/L) in drinking water formulations of sodium metavanadate was estimated using vanadium weight of 41% (w/w) in sodium metavanadate and exposure concentration (Table 2). Using the average daily water consumed (g/animal/day) during the last week of study for a given exposure group and corresponding vanadium concentration (mg/L) in drinking water formulations, daily vanadium consumption (mg vanadium/day) by an animal was estimated (Table 2). Due to exposure concentration-related decreases in water consumption, vanadium consumption increased only 9-fold with a 16-fold increase in sodium metavanadate exposure concentration in both males and females (Table 2).
Vanadium concentrations in plasma and urine following exposure to vanadyl sulfate.
Data given are for N=3-5 animals per exposure group per sex. Plasma and urine vanadium concentration following exposure to vanadyl sulfate is shown in Figures 2A and 2B, respectively. Plasma vanadium concentration in males and females increased with the external vanadyl sulfate exposure concentration. While the increase in males was proportional, in females the increase was more than proportional at the highest exposure concentration with a 25-fold increase in plasma vanadium concentration when the exposure concentration was increased 16-fold (21-335 mg vanadyl sulfate/L). Concentration of vanadium in urine was much higher than in plasma. Unlike plasma, urinary vanadium concentration in both males and females increased more than proportional to the exposure concentration with a 46- and 42-fold increase in males and females, respectively, with a 16-fold increase in exposure concentration. Although the source(s) for the observed low vanadium concentrations in some control animals (plasma 0.327 ng/mL; urine 0.3-2.6 ng/mg creatinine) were unknown, the measured concentrations were ≤16% of the concentration in the corresponding lowest exposure group.
Figure 2.
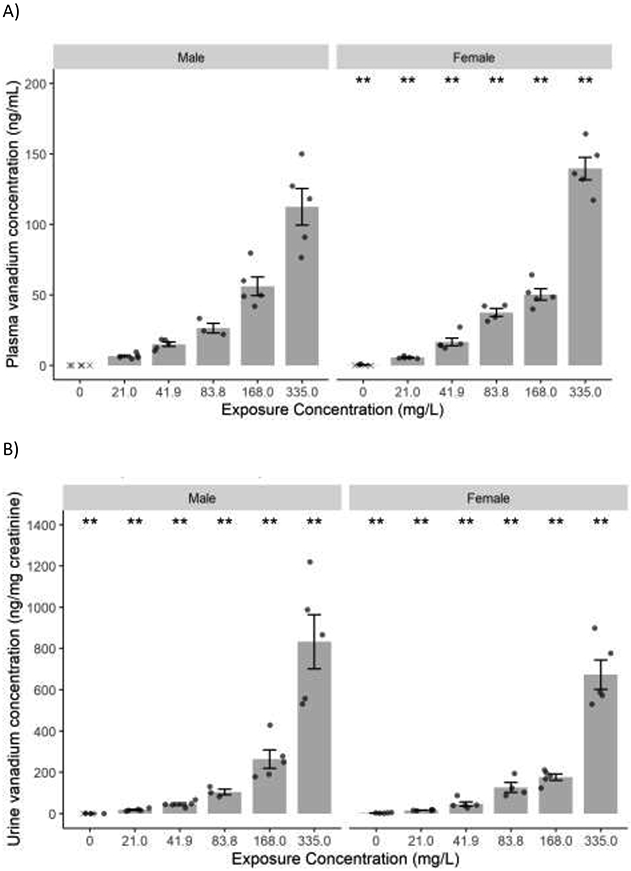
Mean concentration (± SE) of vanadium in A) plasma and B) urine following perinatal exposure of male and female rats to vanadyl sulfate (V4+) via drinking water. Data are shown for N=3-5 animals per exposure group per sex. Individual data values are shown with points, and values below the limit of detection (LOD) are marked with “x.” Statistical comparisons were not performed when 80% or more of the control values were below the LOD. Statistical significance for an exposure group indicates a significant pairwise test compared to the control group (** p<0.01). Statistical significance for a control group indicates a significant trend test (** p<0.01). Because values in control male group were below LOD, a statistical significance could not be established for males.
To allow for better comparisons across exposure concentrations and between sexes, and subsequently between compounds, plasma and urine concentrations were normalized to daily total vanadium consumed (i.e., plasma or urine vanadium concentration per unit daily total vanadium consumed) which accounts for the difference in water and subsequently the vanadium consumption between exposure groups, sexes, and compounds. Plasma vanadium concentration for a unit daily vanadium consumed following exposure to vanadyl sulfate increased with the exposure concentration in both males (38.5 to 55.3 (ng/mL)/(mg vanadium/day) and females (45.2 to 82.7 (ng/mL)/(mg vanadium/day)) with females, in general, having higher values than males (Figure 3 and Supplemental Table 1). A similar increase was observed with urine in both sexes (males, 106 to 411 (ng/mg creatinine)/(mg vanadium/day); females 129 to 398 (ng/mg creatinine)/(mg vanadium/day)) although there was no apparent sex difference (Figure 4, Supplemental Table 1).
Figure 3.
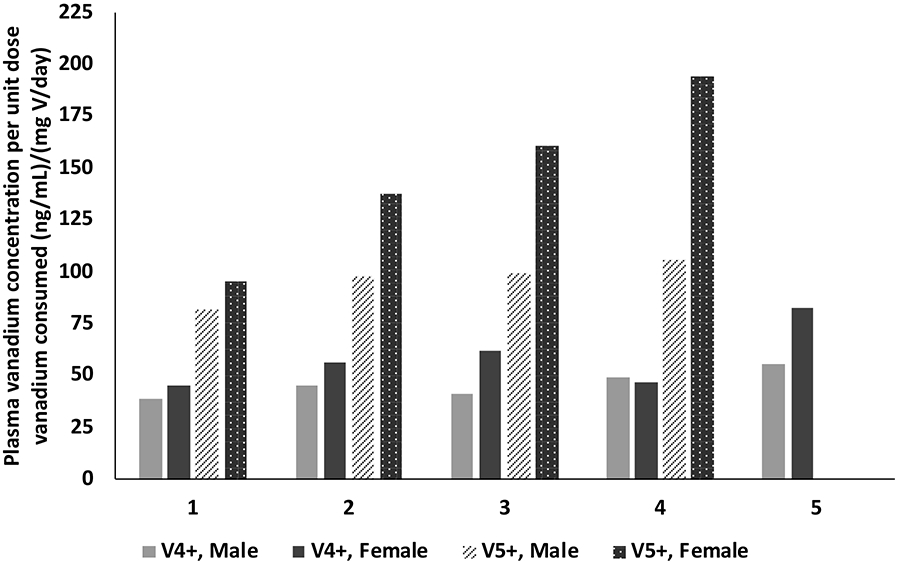
Plasma vanadium concentration normalized to vanadium consumed following perinatal exposure of male and female rats to vanadyl sulfate (V4+) and sodium metavanadate (V5+) via drinking water. Exposure groups 1, 2, 3, 4, and 5 (vanadyl sulfate only), respectively, for vanadyl sulfate are 21, 41.9, 83.8, 168, and 335 mg/L and for sodium metavanadate are 31.3, 62.5, 125, 250, and 500 mg/L. Plasma for 500 mg/L sodium metavanadate were not available for analysis.
Figure 4.
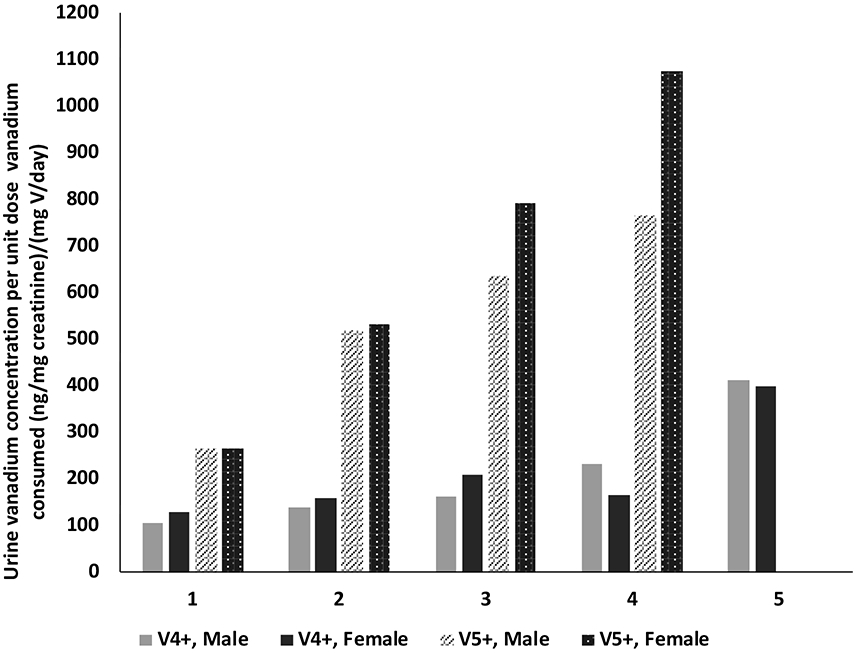
Urine vanadium concentration normalized to vanadium consumed following perinatal exposure of male and female rats to vanadyl sulfate (V4+) and sodium metavanadate (V5+) via drinking water. Exposure groups 1, 2, 3, 4, and 5 (vanadyl sulfate only), respectively, for vanadyl sulfate are 21, 41.9, 83.8, 168, and 335 mg/L and for sodium metavanadate are 31.3, 62.5, 125, 250, and 500 mg/L. Urine for 500 mg/L sodium metavanadate was not available for analysis.
Vanadium concentrations in plasma and urine following exposure via to sodium metavanadate.
Data given are for N=5 animals per exposure group per sex. Plasma and urine vanadium concentration in male and female rats following exposure to sodium metavanadate is presented in Figures 5A and 5B, respectively. Plasma and urine samples were not available for animals in the highest exposure group due high pup mortality during lactation. Plasma vanadium concentration in males and females increased with the sodium metavanadate external exposure concentration. In males the increase was proportional to the exposure concentration while in females, the plasma concentration increased 11-fold with 8-fold increase in exposure concentration (31.3-250 mg/L). Concentration of vanadium in urine was much higher than plasma; unlike plasma vanadium, urinary vanadium concentration in both males and females increased more than proportional to the exposure concentration with a 16- and 21-fold increase with 8-fold increase in exposure concentration, in males and females, respectively.
Figure 5.
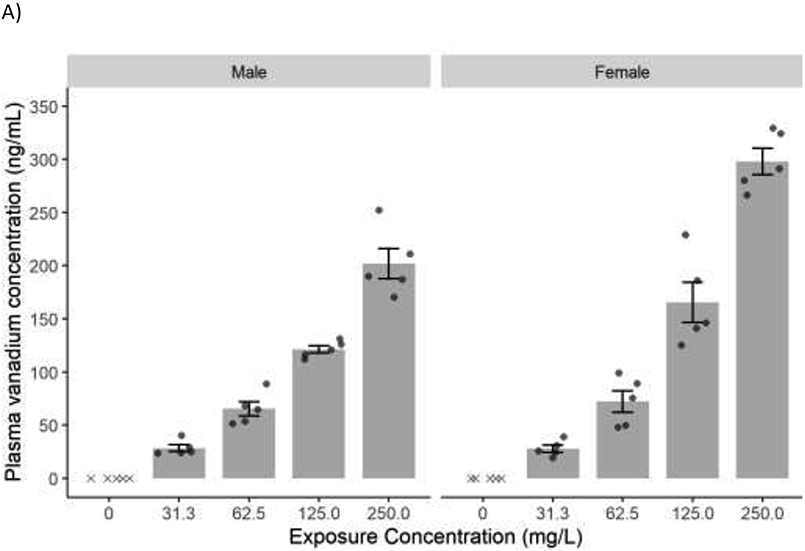
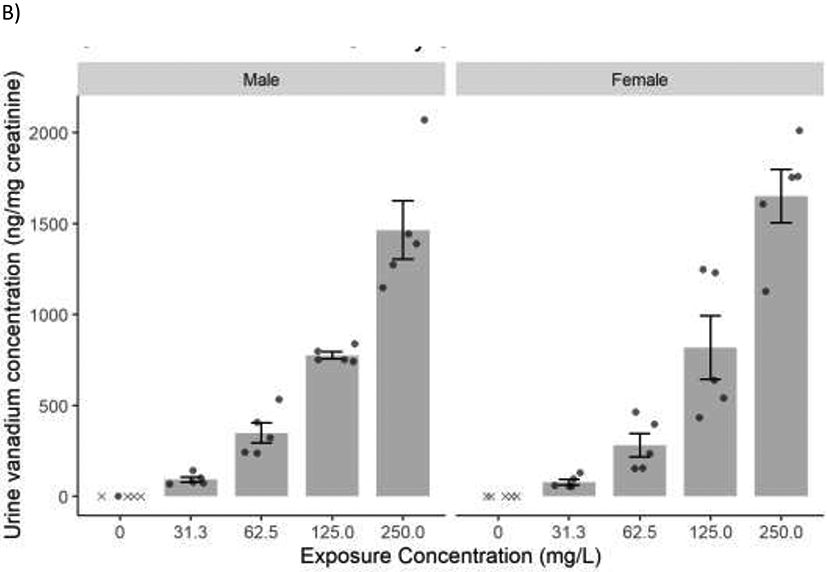
Mean concentration (± SE) of vanadium in A) plasma and B) urine following perinatal exposure of male and female rats to sodium metavanadate (V5+) via drinking water. Data shown are for N=3-5 animals per exposure group per sex. Individual data values are shown with points, and values below the limit of detection (LOD) are marked with “x.” Statistical comparisons were not performed when 80% or more of the control values were below the LOD. Because values in control groups were below LOD, a statistical significance between control and exposed groups could not be established.
Plasma and urine data were normalized to total daily vanadium consumed to compare across exposure concentrations and sexes, and subsequently between compounds. Plasma vanadium concentration for a unit daily vanadium consumed following exposure to sodium metavanadate increased with the exposure concentration in both males (82.0 to 105 (ng/mL)/(mg vanadium/day) and females (95.4 to 194 (ng/mL)/(mg vanadium/day) with females having higher values than males at all exposure concentrations (Figure 3 and Supplemental Table 2). A similar pattern was observed with urine (males, 266 to 764 (ng/mg creatinine)/(mg vanadium/day); females 265 to 1074 (ng/mg creatinine)/(mg vanadium/day)) (Figure 4, Supplemental Table 2).
Discussion
Because of concerns with potential adverse human health following exposure, the EPA has selected oral exposure to vanadium compounds assessed by the IRIS program (EPA, 2021). However, there are limited toxicity data available for these compounds (ATSDR, 2012). Hence, DNTP conducted a toxicity assessment of vanadyl sulfate and sodium metavanadate, representing V4+ and V5+ compounds, respectively, following exposure via drinking water in rodents. We have previously reported the short-term toxicity (Roberts et al., 2016; Roberts et al., 2018) and limited data on internal exposure (Harrington et al., 2021b; Harrington et al., 2021c) following drinking water exposure of adult animals to vanadyl sulfate and sodium metavanadate for 14 days. Subsequently, subchronic toxicity studies were conducted following perinatal exposure of rats via drinking water to vanadyl sulfate and sodium metavanadate. The subchronic toxicity data will be reported elsewhere. Here, we present the internal exposure and urinary excretion data of vanadium in male and female rats, conducted as a part of the subchronic toxicity study. The studies were designed to allow for direct comparisons between V4+ and V5+ compounds. To the best of our knowledge, there are no studies in the current literature comparing the internal exposure and excretion of vanadium following repeated oral exposure to V4+ and V5+ compounds in rodents. Additionally, similar data for humans following oral exposure could not be located at the current time.
Following exposure to both vanadyl sulfate and sodium metavanadate, water consumption decreased at higher exposure concentrations compared to concurrent controls in both sexes, likely stemming from decreased palatability with increasing test material concentration. Average daily consumption of vanadium estimated from water consumption following exposure to sodium metavanadate (0.291 to 3.08 mg/day) at exposure concentrations ranging from 31.3 to 500 mg/L was approximately 1.5- to 2.4-fold higher than that following exposure to vanadyl sulfate (0.123 to 2.03 mg/day) at exposure concentrations ranging from 21 to 335 mg/L (Tables 1 and 2) .
Because vanadium amount consumed following exposure to vanadyl sulfate and sodium metavanadate was different, plasma and urine vanadium concentrations were normalized for the daily total vanadium consumed to allow for direct comparison between exposure concentrations, sexes, and compounds. We used daily levels of vanadium consumed, without adjusting for body weight, to allow for direct comparisons between sexes. Plasma and urine vanadium concentrations for a unit of vanadium consumed following exposure to both compounds increased with the exposure concentration demonstrating changes in absorption, distribution, metabolism, and excretion (ADME) properties with increasing exposure to vanadium. The fact that the increase was observed for both plasma and urine suggest that the increase may have been due to increased absorption with increasing exposure concentration. Although a sex difference in normalized concentrations was found only in plasma concentration following exposure to vanadyl sulfate, a sex difference was apparent in both plasma and urine data following exposure to sodium metavanadate with females having a higher exposure than males (Supplemental Tables 1 and 2).
When compared between the two compounds using plasma concentrations, for a unit of vanadium consumed, male and female rats have 2-fold higher exposure to vanadium following exposure to sodium metavanadate than vanadyl sulfate. A similar comparison using vanadium excreted in urine showed 2- to 3- fold higher vanadium in sodium metavanadate exposed rats compared to vanadyl sulfate exposed animals. Similar findings were observed in our limited investigations following exposure of rodents to 14 days via drinking water (Harrington et al., 2021b; Harrington et al., 2021c). Taken collectively, these data demonstrate ADME differences between V4+ and V5+ compounds in rodents leading to higher internal exposure following exposure to V5+ compounds. Differential ADME properties of V4+ and V5+ compounds have been suggested by other investigators and is summarized in Trevino et al. (Trevino et al., 2019).
Interconversion between oxidation states and complex speciation under the conditions in the gastrointestinal tract as well as during transport in the systemic circulation makes understanding the ADME behavior of vanadium compounds difficult. Higher internal levels of vanadium following exposure to V5+ compounds have been attributed to higher absorption of V5+ compounds compared to V4+ compounds due to differential speciation in the gastrointestinal tract. For example, intestinal absorption of oxoanions (H2VO4−, HVO42−) of V5+ compounds are known to be 3- to 4-times higher than the oxo cation (VO2+) of V4+ compounds leading to higher internal vanadium concentrations following V5+ exposure (Trevino et al., 2019). This fold increase is in the same range as that observed with our data where plasma and urine concentrations normalized to vanadium consumed following exposure to sodium metavanadate (V5+) were 2- to 3-fold higher than those following exposure to vanadyl sulfate (V4+).
There are a few studies in the literature investigating the ADME properties of vanadium compounds following oral exposure in animals (Adachi et al., 2000; Edel et al., 2006; Trevino et al., 2019). Absorption of vanadium has been reported to be low and depending on the study, values between 1-16% have been observed. In one study, following exposure of rats for 7 d via feed to sodium metavanadate at 100 ppm, vanadium was distributed to tissues with the highest concentration found in the bone (Adachi et al., 2000). Vanadium in the urine and feces were estimated as 0.86 and 83.5%, respectively, of the vanadium intake. Based on the excretion data, the authors concluded that vanadium was poorly absorbed from the gastrointestinal tract and the absorption and retention was ~16%. In a study investigating the kinetic behavior and oral bioavailability of vanadyl sulfate in rats following a single gavage administration, authors reported absolute bioavailability of vanadium between 12.5 and 16.8% (Azay et al., 2001). The limited data available in humans also suggest that the oral absorption and retention of vanadium is ≤1% (Edel et al., 2006; Levina et al., 2014; Ma et al., 2018; Trevino et al., 2019). Hence, ADME and kinetics of oral vanadium compounds in animals and humans warrants further investigation. Nevertheless, the data presented here will aid in the interpretation of animal toxicity data of V4+ and V5+ compounds and to put animal toxicity findings into context with human exposures.
Conclusion
There are limited data to assess adverse human health impacts from oral exposure to soluble vanadium compounds. As a part of a larger study investigating the subchronic toxicity of these compounds, we have investigated the internal exposure and urinary excretion of vanadium in male and female rats following perinatal exposure to vanadyl sulfate or sodium metavanadate representing soluble V4+ and V5+ compounds, respectively. Both plasma and urine vanadium concentrations normalized to daily total vanadium consumed increased with increasing exposure concentration suggesting an increase in absorption with the exposure concentration. Based on the normalized data, females had higher plasma vanadium concentrations following exposure to vanadyl sulfate than males while higher values in females were observed for both plasma and urine following exposure to sodium metavanadate. For a unit of vanadium consumed, rats have up to 3-fold higher levels of vanadium following sodium metavanadate exposure than vanadyl sulfate exposure demonstrating difference in ADME properties between V4+ and V5+ compounds. The data presented here will aid in the interpretation of animal toxicity data of V4+ and V5+ and will help contextualize the relevance of animal toxicity data to human exposures.
Supplementary Material
Highlights.
Vanadium (V) is a ubiquitous environmental contaminant.
Internal exposure was determined in rodents after oral exposure to V4+ and V5+.
Plasma and urine V levels increased more than proportional to V4+ and V5+ dose.
Exposure to V5+ caused up to 3-fold higher plasma and urine V levels than V4+.
Data demonstrate differential ADME properties between V5+ and V4+ compounds.
Acknowledgements
The authors are grateful to Mr. Bradley Collins and Dr. Shannah Witchey for their review of this manuscript and Carol Co for generation of Figure 2 for the manuscript. This work was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, Intramural Research project ZIA ES103316-05, and performed for the Division of the National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health, U.S. Department of Health and Human Services, under contracts HHSN273201400015C (Animal study conduct, Battelle, Columbus, OH), HHSN273201400022C (Sample analysis, RTI International, RTP, NC), HHSN273201600011C (Statistical analysis, Social and Scientific Systems, Durham, NC), and HHSN316201200054W (CEBS data table preparation, ASRC Federal, RTP, NC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adachi A, Ogawa K, Tsushi Y, Nagao N, Okano T, 2000. Balance, excretion andtissue distribution of vanadium in rats after short-term ingestion. J. HealthSci. 46, 59–62. [Google Scholar]
- ATSDR, 2012. Agency for Toxic Substances and Disease Registry (ATSDR). A ToxicologicalProfile for Vanadium, U.S. Department of Health and Human Services, PublicHealth Service, ATSDR, Atlanta, GA. [Google Scholar]
- Azay J, Bres J, Krosniak M, Teissedre PL, Cabanis JC, Serrano JJ, Cros G, 2001. Vanadium pharmacokinetics and oral bioavailability upon single-dose administration of vanadyl sulfate to rats. Fundam Clin Pharmacol 15, 313–324. [DOI] [PubMed] [Google Scholar]
- Crans DC, Smee JJ, Gaidamauskas E, Yang L, 2004. The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem Rev 104, 849–902. [DOI] [PubMed] [Google Scholar]
- Dixon WJ, Massey FJ, 1957. Introduction to statistical analysis. New York, NY: McGraw Hill Book Company Inc. [Google Scholar]
- Dunn OJ, 1964. Multiple Comparisons Using Rank Sums. . Technometrics 6, 341–352. [Google Scholar]
- Dunnett CW, 1955. A Multiple Comparison Procedure for Comparing Several Treatments with a Control. J. Am. Stat. Assoc 50, 1096–1121. [Google Scholar]
- Edel AL, Kopilas M, Clark TA, Aguilar F, Ganguly PK, Heyliger CE, Pierce GN, 2006. Short-term bioaccumulation of vanadium when ingested with a tea decoction in streptozotocin-induced diabetic rats. Metabolism 55, 263–270. [DOI] [PubMed] [Google Scholar]
- EPA, 2017. The Third Unregulated Contaminant Monitoring Rule (UCMR 3): Data Summary, January 2017. https://www.epa.gov/sites/production/files/2017-02/documents/ucmr3-data-summary-january-2017.pdf. Last accessed July 2020.
- EPA, 2020. Contaminant Candidate List and Regulary Determination. https://www.epa.gov/ccl/contaminant-candidate-list-4-ccl-4-0. Last accessed July 2020.
- EPA, 2021. Systematic Review Protocol for the Vanadium and Compounds (Oral Exposure) IRIS Assessment (Preliminary Assessment Materials). U.S. Environmental Protection Agency, Washington, DC, EPA/635/R-21/047. [Google Scholar]
- Gustafsson JP, 2019. Vanadium geochemistry in the biogeosphere -speciation, solid-solution interactions, and ecotoxicity. Applied Geochemistry 102, 1–25. [Google Scholar]
- Harrington JM, Haines LG, Essader AS, Liyanapatirana C, Poitras E, Weber F, Levine KE, Fernando RA, Robinson VG, Waidyanatha S, 2021a. Quantitation of Total Vanadium in Rodent Plasma and Urine by Inductively Coupled Plasma–Mass Spectrometry (ICP-MS). Analytical Letters 54, 2777–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington JM, Haines LG, Levine KE, Liyanapatirana C, Essader AS, Fernando RA, Robinson VG, Roberts GK, Stout MD, Hooth MJ, Waidyanatha S, 2021b. Corrigendum to "Internal dose of vanadium in rats following repeated exposure to vanadyl sulfate and sodium orthovanadate via drinking water" [Toxicology and Applied Pharmacology 412 (2021) 115395]. Toxicol Appl Pharmacol 423, 115546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington JM, Haines LG, Levine KE, Liyanapatirana C, Essader AS, Fernando RA, Robinson VG, Roberts GK, Stout MD, Hooth MJ, Waidyanatha S, 2021c. Internal dose of vanadium in rats following repeated exposure to vanadyl sulfate and sodium orthovanadate via drinking water. Toxicol Appl Pharmacol 412, 115395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JC, 1992. The factor analytic approach to simultaneous inference in the general linear model. . J. Comput. Graph. Stat 1, 151–168. [Google Scholar]
- Jonckheere A, 1954. A distribution-free k-sample test against ordered alternatives. . Biometrika 41, 133–145. [Google Scholar]
- Levina A, McLeod AI, Kremer LE, Aitken JB, Glover CJ, Johannessen B, Lay PA, 2014. Reactivity-activity relationships of oral anti-diabetic vanadium complexes in gastrointestinal media: an X-ray absorption spectroscopic study. Metallomics 6, 1880–1888. [DOI] [PubMed] [Google Scholar]
- Ma J, Pan LB, Wang Q, Lin CY, Duan XL, Hou H, 2018. Estimation of the daily soil/dust (SD) ingestion rate of children from Gansu Province, China via hand-to-mouth contact using tracer elements. Environ Geochem Hlth 40, 295–301. [DOI] [PubMed] [Google Scholar]
- Maughan RJ, King DS, Lea T, 2004. Dietary supplements. J Sports Sci 22, 95–113. [DOI] [PubMed] [Google Scholar]
- Mutlu E, Cristy T, Graves SW, Hooth MJ, Waidyanatha S, 2017. Characterization of aqueous formulations of tetra- and pentavalent forms of vanadium in support of test article selection in toxicology studies. Environ Sci Pollut Res Int 24, 405–416. [DOI] [PubMed] [Google Scholar]
- NRC, 2011. Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press. [Google Scholar]
- NTP, 2008. Chemical Information Review Document for Oral Exposure to Tetravalent and Pentavalent Vanadium Compounds. Last accessed July 2020. [Google Scholar]
- NTP, 2022a. Testing Status of Sodium Metavanadate M940043. https://ntp.niehs.nih.gov/whatwestudy/testpgm/status/ts-m940043.html?utm_source=direct&utm_medium=prod&utm_campaign=ntpgolinks&utm_term=ts-m940043. Last accessed Feruary 2, 2022.
- NTP, 2022b. Testing Status of Vanadyl sulfate 08004. https://ntp.niehs.nih.gov/whatwestudy/testpgm/status/ts-08004.html?utm_source=direct&utm_medium=prod&utm_campaign=ntpgolinks&utm_term=ts-08004. Last accessed February 2, 2022. [Google Scholar]
- Richardson SD, Ternes TA, 2014. Water Analysis: Emerging Contaminants and Current Issues. Analytical Chemistry 86, 2813–2848. [DOI] [PubMed] [Google Scholar]
- Roberts GK, Stout MD, Sayers B, Fallacara DM, Hejtmancik MR, Waidyanatha S, Hooth MJ, 2016. 14-Day Toxicity Studies of Tetravalent and Pentavalent Vanadium Compounds in Harlan Sprague Dawley Rats and B6C3F1/N Mice via Drinking Water Exposure. Toxicol Rep 3, 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts GK, Stout MD, Sayers B, Fallacara DM, Hejtmancik MR, Waidyanatha S, Hooth MJ, 2018. Clarification and lessons learned for reporting studies with hydrates. Citation: Roberts et al., 2016. Toxicology Reports 3: 531-538. Toxicol Rep 5, 207–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley E, 1977. A non-parametric equivalent of Williams' test for contrasting increasing dose levels of a treatment. . Biometrics 33, 386–389. [PubMed] [Google Scholar]
- Trevino S, Diaz A, Sanchez-Lara E, Sanchez-Gaytan BL, Perez-Aguilar JM, Gonzalez-Vergara E, 2019. Vanadium in Biological Action: Chemical, Pharmacological Aspects, and Metabolic Implications in Diabetes Mellitus. Biol Trace Elem Res 188, 68–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey JW, 1977. Easy summaries – Numerical and graphical In Exploratory Data Analysis, Addison-Wesley, Reading, MA., 43–44. [Google Scholar]
- Williams DA, 1971. A test for differences between treatment means when several dose levels are compared with a zero dose control. Biometrics 27, 103–117. [PubMed] [Google Scholar]
- Williams DA, 1972. The comparison of several dose levels with a zero dose control. Biometrics 28, 519–531. [PubMed] [Google Scholar]
- Williams DA, 1986. A note on Shirley's nonparametric test for comparing several dose levels with a zero-dose control. . Biometrics 42, 183–186. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


