Abstract
A novel series of cyclopentane derivatives have been found to exhibit potent and selective inhibitory effects on influenza virus neuraminidase. These compounds, designated RWJ-270201, BCX-1827, BCX-1898, and BCX-1923, were tested in parallel with zanamivir and oseltamivir carboxylate against a spectrum of influenza A (H1N1, H3N2, and H5N1) and influenza B viruses in MDCK cells. Inhibition of viral cytopathic effect ascertained visually and by neutral red dye uptake was used, with 50% effective (virus-inhibitory) concentrations (EC50) determined. Against the H1N1 viruses A/Bayern/07/95, A/Beijing/262/95, A/PR/8/34, and A/Texas/36/91, EC50s (determined by neutral red assay) of the novel compounds were ≤1.5 μM. Twelve strains of H3N2 and two strains of avian H5N1 viruses were inhibited at <0.3 μM. Influenza B/Beijing/184/93 and B/Harbin/07/94 viruses were inhibited at <0.2 μM, with three other B virus strains inhibited at 0.8 to 8 μM. The novel inhibitors were comparable in potency to (or slightly more potent than) zanamivir and oseltamivir carboxylate. No cytotoxicity was seen with the compounds at concentrations of ≤1 mM in cell proliferation assays. The antiviral activity of RWJ-270201, chosen for clinical development, was studied in greater detail. Its potency and that of oseltamivir carboxylate decreased with increasing multiplicity of virus infection. Time-of-addition studies indicated that treatment with either compound needed to begin 0 to 12 h after virus exposure for optimal activity. Exposure of cells to RWJ-270201 caused most of the virus to remain cell associated, with extracellular virus decreasing in a concentration-dependent manner. This is consistent with its effect as a neuraminidase inhibitor. RWJ-270201 shows promise in the treatment of human influenza virus infections.
Influenza has continued to be a significant public health concern, with annual epidemics responsible for serious morbidity and mortality (1, 13). Much attention has consequently been given to the development of antiviral drugs for the treatment of this disease. Amantadine and rimantadine both have been approved for prophylaxis of influenza A virus infection (6). Ribavirin was shown to be effective against experimental influenza virus infections in mice (9), and was studied in humans by small-particle aerosol delivery against severe influenza virus infections (11). However, it was not effective enough to receive drug approval. As early as 1976 Palese and Compans (19) reported an inhibitor of influenza virus neuraminidase. This research was largely ignored for many years, and it was not until recently that the search for more potent neuraminidase inhibitors has intensified. From these investigations, zanamivir (GG167) and oseltamivir carboxylate (GS4071) emerged; these compounds were found to be highly active against both influenza A and B viruses (10, 27). Zanamivir, a topical agent approved for clinical use, is effective prophylactically and therapeutically for the treatment of influenza (16, 17). Oseltamivir, the orally active prodrug form of oseltamivir carboxylate (22), is also clinically approved and has been found to be effective for both prophylaxis and treatment of influenza in humans (7, 18).
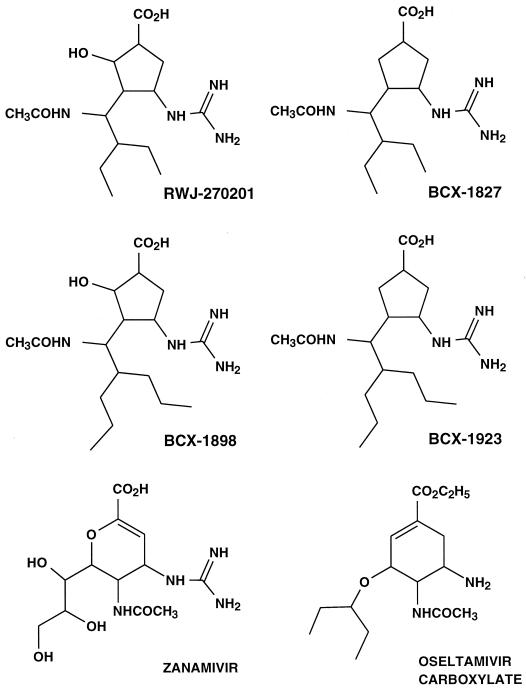
Structure-activity analyses with the purified influenza virus neuraminidase enzyme and knowledge of its three-dimensional structure (26) have led to the identification of new inhibitors. A series of cyclopentane derivatives was found to cause potent and selective inhibition of influenza virus neuraminidase (2). The chemical structures of the more potent antiviral compounds (Fig. 1) have features in common with both zanamivir and oseltamivir carboxylate but differ in having a five-membered-ring structure. In this report the activities of these novel compounds in vitro against various strains of influenza virus are presented. Compound RWJ-270201 was evaluated in greater detail in secondary assays, since it has been selected for clinical development.
FIG. 1.
Chemical structures of cyclopentane derivatives, zanamivir, and oseltamivir carboxylate.
MATERIALS AND METHODS
Compounds.
RWJ-270201, BCX-1827, BCX-1898, BCX-1923, zanamivir, and oseltamivir carboxylate were synthesized at BioCryst Pharmaceuticals (Birmingham, Ala.). Ribavirin was obtained from ICN Pharmaceuticals (Costa Mesa, Calif).
Viruses.
The following viruses were provided by H. Regnery of the Influenza Branch of the Centers for Disease Control and Prevention (Atlanta, Ga.): A/Texas/36/91 (H1N1), A/Bayern/07/95 (H1N1), A/Beijing/262/95 (H1N1), A/Washington/05/96 (H3N2), A/Johannesburg/33/94 (H3N2), A/Sydney/05/97 (H3N2), A/Shangdong/09/93 (H3N2), A/Beijing/32/92 (H3N2), B/Beijing/184/93, B/Panama/45/90, and B/Harbin/07/94. A/NWS/33 (H1N1) was provided by K. Cochran of the University of Michigan (Ann Arbor). A/PR/8/34 (H1N1) was obtained from F. Schabel, Jr., Southern Research Institute (Birmingham, Ala.). A/Victoria/3/75 (H3N2), A/Port Chalmers/1/73 (H3N2), B/Hong Kong/5/72, and B/Lee/40 were obtained from the American Type Culture Collection (Manassas, Va.). A/Los Angeles/2/87 (H3N2) and A/Washington/897/80 (H3N2) were from Program Resources, Inc. (Rockville, Md.). A/X-31 (H3N2), a reassortment virus containing hemagglutinin and neuraminidase genes from A/Aichi/2/68 (H3N2) and the remainder of the genes from A/PR/8/34 (H1N1), was obtained from E. Kilbourne, Mount Sinai School of Medicine, New York Medical College, City University of New York (New York, N.Y.). A/Port Chalmers/1/73r (H3N2), an amantadine-resistant virus, was prepared from the wild-type virus by serial passage in the presence of the drug in this laboratory. A/Virginia/2/88r (H3N2), a clinically isolated amantadine-resistant virus, was provided by F. Hayden, University of Virginia School of Medicine (Charlottesville). A/Duck/MN/1525/81 (H5N1) and A/Gull/PA/4175/83 (H5N1) were obtained from R. Webster of the St. Jude Children's Research Hospital (Memphis, Tenn.). All viruses were passaged in cells to prepare pools for use in these experiments.
Cells and media.
Madin-Darby canine kidney (MDCK) cells were grown in antibiotic-free minimum essential medium with nonessential amino acids (Gibco, Long Island, N.Y.) containing 5% fetal bovine serum (HyClone Laboratories, Logan, Utah) and 0.1% NaHCO3. Test medium consisted of minimum essential medium 0.18% NaHCO3, 10 U of trypsin per ml, 1 μg of EDTA per ml, and 50 μg of gentamicin/ml.
Cell culture assays.
Three methods were used to assay antiviral activity in vitro: inhibition of virus-induced cytopathic effect (CPE) determined by visual (microscopic) examination of the cells, increase in neutral red (NR) dye uptake into cells, and virus yield reduction. In the CPE inhibition method, which was reported previously by Sidwell and Huffman (21), seven concentrations of test drug were evaluated against each virus in 96-well flat-bottomed microplates. The compounds were added 5 to 10 min prior to virus, which was used at a concentration of approximately 50 cell culture 50% infections doses per well. This virus challenge dose equated to a multiplicity of infection (MOI) of approximately 0.001 infectious particle per cell. The tests were read after incubation at 37°C for 72 h. In the NR uptake assay, dye (0.34% concentration in medium) was added to the same set of plates used to obtain the visual scores. After 2 h, the color intensity of the dye absorbed by and subsequently eluted from the cells was determined by the method of Finter (5), using a computerized EL-309 microplate autoreader (Bio-Tek Instruments, Winooski, Vt.). Antiviral activity was expressed as the 50% effective (virus-inhibitory) concentration (EC50) determined by plotting compound concentration versus percent inhibition on semilogarithmic graph paper. Although the CPE and NR methods were both used for calculating EC50 against all of the influenza virus strains, for brevity only data obtained from the NR assays are reported. In general, the EC50 determined by NR assay were two- to fourfold higher than those obtained by the CPE method.
Cytotoxicity of compounds was assessed in parallel with the antiviral determinations in the same microplates, except in the absence of virus. From these results, 50% cytotoxic end points (50% cell-inhibitory concentrations [IC50s]) were determined. Later, the compounds were assayed for toxicity in actively proliferating MDCK cells. This was done by seeding 96-well microplates with 2 × 104 cells per well. Compounds were diluted in medium containing 5% fetal bovine serum and then were placed into the wells following cell attachment. After 3 days, the percent inhibition of cell proliferation was assessed by NR assay as described above.
Virus yield reduction assays were performed by a method which separated and quantified extracellular (supernatant) from cell-associated virus. These tests, using A/Texas/36/91 (H1N1), A/Sydney/05/97 (H3N2), and B/Beijing/184/93 viruses, were initiated in 24-well plates of MDCK cells infected at a virus MOI of 0.001. A visual determination of viral CPE was made after 72 h of incubation, when cell destruction in untreated cultures was maximum, at which time the extracellular medium was removed and placed in test tubes. The plates were refed fresh medium. The tubes containing extracellular virus and cell debris were centrifuged at 3,200 × g for 5 min, and most of the supernatant fluid was removed and transferred to unused tubes. This procedure was carefully done to avoid collecting any of the cell pellets. The remainder of the medium from each tube was discarded, and the resulting pellets were recombined with the fresh medium (and adhered cells) from wells where they originated. Eight wells were used per concentration of compound. Samples from the eight wells were paired, yielding a total of four samples for titration. The plates of cells and tubes of extracellular virus were frozen and thawed, sonicated for 30 s each, and then assayed for virus titer. Titrations were conducted by adding the serially diluted samples to four wells each (0.1 ml/well) in 96-well plates of MDCK cells. After 2 h of virus adsorption, the medium was replaced with fresh medium to remove residual compound present in the original samples. The plates were checked for virus-induced CPE on days 3 and 6. Quantitation of virus yield titers was by the end point method of Reed and Muench (20), and the titers were expressed as log10 50% cell culture infectious doses per 0.1 ml of medium assayed.
Effects of MOI and delay of treatment initiation on antiviral activity.
Experiments using CPE inhibition and confirmed by NR uptake were done to ascertain the effects of various viral challenge doses on antiviral, potency using MOIs of 0.00018, 0.0009, 0.0045, and 0.0225. To examine the influence of delay of treatment initiation on antiviral activity. MOI of approximately 0.001 was used. Compounds were added to the cells at 24 h pre-virus exposure and then rinsed off, added at 5 min pre-virus exposure (time zero), or added at 2, 4, 6, 8, 12, or 24 h post-virus exposure. EC50 were calculated from the NR assay results as described above. Influenza A/Sydney/05/97 (H3N2) virus was used for these studies.
RESULTS
Antiviral activities against influenza A and B virus strains.
Six neuraminidase inhibitors were evaluated for activity against several strains of influenza A (H1N1), influenza A (H3N2), influenza A (H5N1), and influenza B viruses in MDCK cell culture by the NR method (Table 1). Of the influenza A (H1N1) viruses, the A/Texas/36/91 strain was the most sensitive to inhibition by the compounds, with EC50s ranging from 0.06 to 0.22 μM. The A/Bayern/07/95, A/Beijing/262/95, and A/PR/8/34 viruses were sensitive to inhibition in the 0.18 to 3.4 μM range. Activities against the A/NWS/33 virus were up to an order of magnitude less, at 19 to >100 μM. Overall, zanamivir and oseltamivir carboxylate were slightly less potent (usually threefold or less) than the cyclopentane derivatives against the H1N1 viruses.
TABLE 1.
Activities of cyclopentane derivatives, zanamivir, and oseltamivir carboxylate on influenza virus replication in MDCK cells as determined by NR assay
| Virus | RWJ-270201
|
BCX-1827
|
BCX-1898
|
BCX-1923
|
Zanamivir
|
Oseltamivir carboxylate
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC50a(μM) | SIb | EC50 | SI | EC50 | SI | EC50 | SI | EC50 | SI | EC50 | SI | |
| H1N1 | ||||||||||||
| A/Bayern/07/95 | 1.0 | >1,000 | 0.64 | >1,560 | 0.36 | >2,770 | 0.72 | >1,390 | 3.4 | >290 | 2.7 | >370 |
| A/Beijing/262/95 | 0.36 | >2,770 | 0.56 | >1,780 | 0.18 | >5,550 | 0.37 | >2,700 | 2.6 | >380 | 2.4 | >410 |
| A/NWS/33 | 21 | >47 | 19 | >52 | 21 | >47 | 23 | >43 | >100 | —c | >100 | — |
| A/PR/8/34 | 1.5 | >660 | 1.0 | >1,000 | 0.7 | >1,420 | 0.7 | >1,420 | 0.42 | >2,380 | 0.22 | >4,540 |
| A/Texas/36/91 | 0.09 | >11,110 | 0.10 | >10,000 | 0.06 | >16,660 | 0.11 | >9,090 | 0.22 | >4,540 | 0.17 | >5,880 |
| H3N2 | ||||||||||||
| A/Beijing/32/92 | 0.07 | >14,280 | 0.14 | >7,140 | 0.05 | >20,000 | 0.05 | >20,000 | 0.65 | >1,530 | 0.23 | >4,340 |
| A/Johannesburg/33/94 | 0.16 | >6,200 | 0.1 | >10,000 | 0.09 | >11,110 | 0.05 | >20,000 | 0.62 | >1,610 | 0.50 | >2,000 |
| A/Los Angeles/2/87 | 0.07 | >14,280 | 0.07 | >14,280 | 0.04 | >25,000 | 0.06 | >16,660 | 0.18 | >5,550 | 0.38 | >2,630 |
| A/Port Chalmers/1/73 | 0.07 | >14,280 | 0.02 | >50,000 | 0.06 | >16,660 | 0.03 | >33,330 | 0.15 | >6,660 | 0.07 | >14,280 |
| A/Port Chalmers/1/73r | 0.08 | >12,500 | 0.10 | >10,000 | 0.1 | >1,800 | 0.1 | >10,000 | 0.15 | >6,660 | 0.2 | >5,000 |
| A/Shangdong/09/93 | 0.17 | >5,880 | 0.18 | >5,550 | 0.23 | >4,340 | 0.28 | >3,570 | 0.50 | >2,000 | 0.32 | >3,120 |
| A/Sydney/05/97 | 0.19 | >5,260 | 0.13 | >7,690 | 0.10 | >1,000 | 0.22 | >4,540 | 0.45 | >2,220 | 0.31 | >3,220 |
| A/Victoria/3/75 | 0.10 | >10,000 | 0.06 | >16,660 | 0.07 | >14,280 | 0.06 | >16,660 | 0.41 | >2,440 | 0.06 | >16,660 |
| A/Virginia/2/88r | <0.01 | >100,000 | <0.01 | >100,000 | 0.03 | >33,330 | 0.05 | >20,000 | 0.02 | >50,000 | <0.01 | >100,000 |
| A/Washington/897/80 | <0.01 | >100,000 | <0.01 | >100,000 | <0.01 | >100,000 | <0.01 | >100,000 | <0.01 | >100,000 | <0.01 | >100,000 |
| A/Washington/05/96 | 0.02 | >50,000 | 0.01 | >100,000 | <0.01 | >100,000 | 0.01 | >100,000 | 0.2 | >50,000 | 0.08 | >12,500 |
| A/X-31 | 0.01 | >100,000 | <0.01 | >100,000 | <0.01 | >100,000 | <0.01 | >100,000 | 0.4 | >2,500 | 0.12 | >8,330 |
| H5N1 | ||||||||||||
| A/Duck/MN/1525/81 | 0.01 | >100,000 | 0.022 | >45,450 | 0.02 | >50,000 | 0.01 | >100,000 | 0.20 | >5,000 | 0.22 | >4,540 |
| A/Gull/PA/4175/83 | 0.02 | >50,000 | 0.03 | >33,330 | 0.02 | >50,000 | 0.02 | >50,000 | 0.22 | >4,540 | 0.26 | >3,840 |
| B | ||||||||||||
| Beijing/184/93 | 0.06 | >16,660 | 0.09 | >11,110 | 0.02 | >50,000 | 0.02 | >50,000 | 0.03 | >33,330 | 0.11 | >9,090 |
| Harbin/07/94 | 0.12 | >8,330 | 0.16 | >6,250 | 0.06 | >16,660 | 0.06 | >16,660 | 0.20 | >5,000 | 0.26 | >3,840 |
| Hong Kong/5/72 | 2.2 | >450 | 2.4 | >410 | 2.0 | >500 | 1.8 | >550 | 1.0 | >1,000 | 2.5 | >400 |
| Lee/40 | 3.2 | >310 | 8.0 | >125 | 8.0 | >125 | 1.7 | >580 | 0.6 | >1,660 | 3.0 | >330 |
| Panama/45/90 | 2.3 | >430 | 2.8 | >350 | 0.8 | >1,250 | 1.6 | >620 | 1.3 | >770 | 1.5 | >660 |
Results are means from two or three independent assays. Assay variability ranged from 20 to 50%.
Determined by dividing the IC50 (which was >1,000 μM for all of these compounds) by the EC50.
—, the SI cannot be calculated, since the highest concentration tested (100 μM) was less than the IC50.
Twelve influenza A (H3N2) strains were inhibited by the cyclopentane derivatives at <0.3 μM. The activities of zanamivir and oseltamivir carboxylate were similar against these viruses, with 50% inhibition at 0.65 μM or less. The A/Washington/897/80, A/Washington/05/96, and A/X-31 strains were uniformly more sensitive to inhibition by the cyclopentane inhibitors than were the other viruses.
Because of the recent emergence of an influenza A (H5N1) virus from chickens that was transmitted to humans and resulted in lethal consequences (25), the neuraminidase inhibitors were evaluated against two strains of influenza A (H5N1) virus. Both the A/duck/MN/1525/81 and A/gull/PA/4175/83 viruses were markedly inhibited by the cyclopentane derivatives at 0.01 to 0.03 μM. Zanamivir and oseltamivir carboxylate were 10-fold less potent (0.2 to 0.26 μM) than the cyclopentane derivatives but were still highly active inhibitors of these viruses.
The influenza B/Beijing/262/95 and B/Harbin 07/94 strain were sensitive to inhibition by these compounds (EC50s ranging from 0.02 to 0.26 μM). However, three other strains (Hong Kong/5/72, Panama/45/90, and Lee/40) of influenza B virus were inhibited at 30- to 400-fold-higher concentrations (0.6 to 8 μM). Zanamivir and oseltamivir carboxylate were as potent as the cyclopentane derivatives against the influenza B virus strains.
Antiviral selectivities.
None of the compounds exhibited cytotoxicity in MDCK cells as determined by visual and NR assay methods at concentrations of up to 1,000 μM. In addition, actively dividing cells were not inhibited in their growth at a 1,000 μM concentration of each compound. By dividing this concentration (1,000 μM) by the EC50s, selectivity indices (SIs) were obtained, and these are reported in Table 1. For highly sensitive viruses such as influenza A/Washington/897/80 (H3N2), A/Virginia/2/88r (H3N2), and A/duck/MN/1525/81 (H5N1), SIs were >100,000. SIs against most other viruses were less (>125 to >50,000) but were still high compared to those of most nucleoside-type antiviral agents. Antiviral selectivities were least against the A/NWS/33 (H1N1) virus (>43 to >52 or less) due to lower potencies.
Time-of-addition studies.
Compounds have a certain span of time when they are most active; this is related to the life cycle of the virus and the step in the cycle where the compounds act. The neuraminidase inhibitors RWJ-270201 and oseltamivir carboxylate were compared with ribavirin (a nucleoside analog) for inhibition of influenza A/Sydney/05/97 (H3N2) virus when added at a different times relative to virus infection (Table 2). Treatment of cells starting at the time of infection required a <0.01 μM concentration of the neuraminidase inhibitors and 5.7 μM ribavirin for inhibition of viral CPE. Treatment of cells prior to infection required much higher concentrations of the compounds, indicating a lack of persistence following their removal from the culture medium. Treatments with the three compounds could begin at any time from 2 to 12 h post-virus infection and still require about the same concentration to achieve 50% inhibition as was effective for the 0-h time point of treatment initiation. However, by 24 h, higher concentrations were required to inhibit virus-induced cytopathology. These studies were done with a low input MOI, indicating that early treatments were necessary to suppress or contain the later rounds of virus replication.
TABLE 2.
Effect of time of treatment initiation on the anti-influenza A/Sydney/05/97 (H3N2) virus activities of RWJ-270201, oseltamivir carboxylate, and ribavirin in MDCK cells
| Time of treatment relative to virus infection (h) | EC50a (μM)
|
||
|---|---|---|---|
| RWJ-270201 | Oseltamivir carboxylate | Ribavirin | |
| −24–0b | 27 ± 4 | 100 ± 27 | 490 ± 102 |
| 0–72 | 0.01 ± 0.006 | 0.02 ± 0.003 | 8 ± 2 |
| 2–72 | 0.03 ± 0.006 | 0.02 ± 0.004 | 20 ± 6 |
| 4–72 | 0.02 ± 0.004 | 0.04 ± 0.014 | 9 ± 2 |
| 6–72 | 0.06 ± 0.02 | 0.02 ± 0.003 | 22 ± 4 |
| 8–72 | 0.02 ± 0.004 | 0.03 ± 0.004 | 26 ± 6 |
| 12–72 | 0.2 ± 0.03 | 0.06 ± 0.006 | 53 ± 8 |
| 24–72 | 65 ± 36 | >100 | 530 ± 120 |
Determined by NR assay. Results are means and standard deviations from four replicates.
The inhibitor was removed and cells were rinsed twice prior to infection.
Effect of virus MOI.
Certain compounds which inhibit virus in cell cultures infected at low MOI, often are less active (or even inactive) at higher virus-to-cell ratios. To explore this possibility with these compounds, antiviral activity was determined over a range of infecting MOIs differing five-fold from each other (Table 3). RWJ-270201 and oseltamivir carboxylate were most potent when virus infections were initiated at low MOIs, and activities decreased with increasing viral challenge dose. In contrast, the efficacy of ribavirin was not influenced by increasing the MOI. This phenomenon has been previously reported for ribavirin against other viruses (24).
TABLE 3.
Effect of virus MOI on the anti-influenza A/Sydney/05/97 (H3N2) virus activities of RWJ-270201, oseltamivir carboxylate, and ribavirin in MDCK cells
| MOI | EC50a (μM)
|
||
|---|---|---|---|
| RWJ-270201 | Oseltamivir carboxylate | Ribavirin | |
| 0.00018 | 0.01 ± 0.006 | 0.01 ± 0.003 | 20 ± 2 |
| 0.0009 | 0.1 ± 0.02 | 0.07 ± 0.05 | 22 ± 2 |
| 0.0045 | 1.0 ± 0.2 | 0.9 ± 0.2 | 24 ± 3 |
| 0.0225 | >10 | >10 | 24 ± 4 |
Determined by NR assay. Results are means and standard deviations from four replicates.
Virus yield reduction studies.
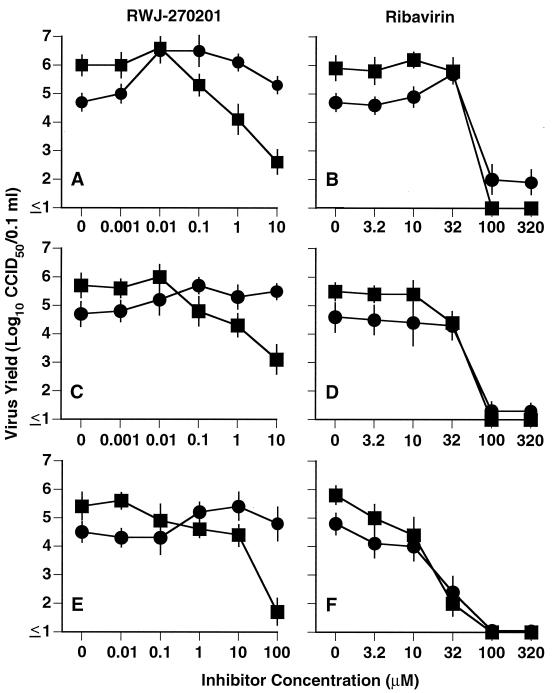
Because neuraminidase is involved in the efficient release of mature viruses from cells, mutant viruses lacking neuraminidase activity aggregate and remain at the cell surface (12, 14). Treatment with a neuraminidase inhibitor should produce the same effect. To demonstrate this, three influenza viruses were exposed to RWJ-270201 for 3 days, followed by assay of extracellular and cell-associated virus yields (Fig. 2). Ribavirin was evaluated in parallel, it being an influenza virus inhibitor with different modes of action (4, 28) unrelated to inhibition of viral neuraminidase. The A/Texas/36/91 (H1N1), A/Sydney/05/97 (H3N2), and B/Beijing/184/93 viruses showed a similar pattern, in that more virus was found extracellularly than cell associated in untreated cultures. RWJ-270201 blocked the production of extracellular virus in a dose-dependent manner. As expected, large amounts of cell-associated virus were present. EC90s for A/Texas, A/Sydney, and B/Beijing were 0.15, 0.1, and 10 μM, respectively. These values approximated the EC50s obtained against these viruses from the NR assays (Table 1). In contrast, cell-associated virus yields were not reduced at >10, >10, and >100 μM, respectively, against the three viruses. Ribavirin caused dose-dependent inhibition of both extracellular and cell-associated virus yields. The EC90s of ribavirin against extracellular A/Texas, A/Sydney, and B/Beijing viruses were 45, 32, and 5.5 μM, respectively. EC90s of this compound against cell-associated virus yields were 60, 32, and 12 μM, respectively. This is consistent with virus yield reduction results using ribavirin against other types of viruses (8).
FIG. 2.
Cell-associated (●) and extracellular (■) virus yields produced in the presence of RWJ-270201 and ribavirin. (A and B) Influenza A/Texas/36/91 (H1N1) virus; (C and D) influenza A/Sydney/05/97 (H3N2) virus; (E and F) influenza B/Beijing/184/93 virus. Data points represent means from four samples ± standard deviations.
DISCUSSION
The results of this study show that the cyclopentane derivatives were active against a large number of influenza A and B virus strains in cell culture at nontoxic concentrations. The potencies of these compounds were similar to or slightly greater than those of zanamivir and oseltamivir carboxylate. Only the A/NWS/33 (H1N1) virus was clearly less sensitive to inhibition by these compounds. Viruses with greater resistance to inhibition are in the process of being prepared by cell culture passage in the presence of RWJ-270201. These viruses will be examined to determine whether the insensitivity is due to a resistant neuraminidase protein, altered hemagglutinin or both. Resistance to neuraminidase inhibitors has been the subject of considerable investigation, as recently reviewed (15). Some of the viruses resistant in vitro are inhibited in mice and ferrets by these types of compounds (3), indicating dependence on the enzyme for spread and disease progression in vivo. Even the influenza A/NWS/33 (H1N1) virus, which we found to be relatively insensitive to inhibition in vitro compared to other viral strains, is highly sensitive to treatment with zanamivir and oseltamivir in mice (22).
High levels of cell-associated virus were produced in the presence of RWJ-270201. Yet, considerably less infectious virus was detected in the extracellular medium relative to virus yields from inhibitor-free cultures. It was shown with neuraminidase-deficient influenza virus mutants that the viruses are not fully released from cells but remain clumped at the cell surface (12, 14). An analogous situation should occur with wild-type virus replication in the presence of a neuraminidase inhibitor. Clumping is caused because viruses have picked up cell antigens that attract other viruses, resulting in their aggregation. The function of neuraminidase is to cleave off these cellular residues so that the virus particles are not attracted to one another.
In cell culture studies, the potencies of RWJ-270201 and oseltamivir carboxylate were dependent upon the time of initiation of treatment and the virus MOI. In order to prevent neuraminidase activity leading to inefficient virus release (or to cause virus aggregation as described above), treatments with the compound needed to be initiated within 12 h of infection. Increasing the MOI increased the number of cells initially infected. These cells were not spared by treatment, and high levels of cell-associated virus were produced. Treatment would protect neighboring cells during the secondary infection. Thus, under low-MOI conditions, a larger number of cells were not initially infected and would have escaped later infection by antiviral treatment. These results suggest that patients would most benefit by treatment early in the course of influenza illness prior to developing high respiratory tract virus titers.
RWJ-270201, which has been selected for clinical development, may prove to be effective in humans based upon recent results of animal studies. Our experiments with this compound, which are being published separately (23), indicate efficacy in treating influenza virus infections in mice when administered orally. RJW-270201 is well tolerated in mice, as are the Food and Drug Administration-approved neuraminidase inhibitors zanamivir and oseltamivir (22). RWJ-270201 appears to be a viable candidate for the treatment of influenza infections in humans.
ACKNOWLEDGMENTS
This work was supported by contract N01-AI-85348 from the Virology Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by a grant from The R. W. Johnson Pharmaceutical Research Institute.
REFERENCES
- 1.Anonymous. Update: influenza activity—worldwide, May–September 1999. Morb Mortal Wkly Rep. 1999;48:883–886. [PubMed] [Google Scholar]
- 2.Babu Y S, Chand P, Bantia S, Kotian P, Dehghani A, El-Kattan Y, Lin T H, Hutchison T L, Elliott A J, Parker C D, Ananth S L, Horn L L, Laver G W, Montgomery J A. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J Med Chem. 2000;43:3482–3486. doi: 10.1021/jm0002679. [DOI] [PubMed] [Google Scholar]
- 3.Blick T J, Sahasrabudhe A, McDonald M, Owens I J, Morley P J, Fenton R J, McKimm-Breschkin J L. The interaction of neuraminidase and hemagglutinin mutations in influenza virus in resistance to 4-guanidino-Neu5Ac2en. Virology. 1998;246:95–103. doi: 10.1006/viro.1998.9194. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson B, Helgstrand E, Johansson N G, Larsson A, Misiorny A, Noren J O, Philipson L, Stenberg K, Stening G, Stridh S, Oberg B. Inhibition of influenza virus ribonucleic acid polymerase by ribavirin triphosphate. Antimicrob Agents Chemother. 1977;11:946–951. doi: 10.1128/aac.11.6.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finter N B. Dye uptake methods for assessing viral cytopathogenicity and their application to interferon assays. J Gen Virol. 1969;5:419–427. [Google Scholar]
- 6.Hayden F G. Antivirals for pandemic influenza. J Infect Dis. 1997;176(Suppl. 1):S56–S61. doi: 10.1086/514177. [DOI] [PubMed] [Google Scholar]
- 7.Hayden F G, Treanor J J, Fritz R S, Lobo M, Betts R F, Miller M, Kinnersley N, Mills R G, Ward P, Straus S E. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA. 1999;282:1240–1246. doi: 10.1001/jama.282.13.1240. [DOI] [PubMed] [Google Scholar]
- 8.Huffman J H, Sidwell R W, Khare G P, Witkowski J T, Allen L B, Robins R K. In vitro effect of 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole, ICN 1229) on deoxyribonucleic acid and ribonucleic acid viruses. Antimicrob Agents Chemother. 1973;3:235–241. doi: 10.1128/aac.3.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khare G P, Sidwell R W, Witkowski J T, Simon L N, Robins R K. Suppression by 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole, ICN 1229) of influenza virus-induced infections in mice. Antimicrob Agents Chemother. 1973;3:517–522. doi: 10.1128/aac.3.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim C U, Lew W, Williams M A, Liu H, Zhang L, Swaminathan S, Bischofberger N, Chen M S, Mendel D B, Tai C Y, Laver W G, Stevens R C. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: design, synthesis, and structural analysis of carbocyclic sialic acid analogue with potent anti-influenza activity. J Am Chem Soc. 1997;119:681–690. doi: 10.1021/ja963036t. [DOI] [PubMed] [Google Scholar]
- 11.Knight V, Wilson S Z, Quarles J M, Greggs S E, McClung H W, Waters B K, Cameron R W, Zerwas J M, Couch R B. Ribavirin small-particle aerosol treatment of influenza. Lancet. 1981;ii:945–949. doi: 10.1016/s0140-6736(81)91152-1. [DOI] [PubMed] [Google Scholar]
- 12.Liu C, Eichelberger M C, Compans R W, Air G M. Influenza type A virus neuraminidase does not play a role in viral entry, replication, assembly, or budding. J Virol. 1995;69:1099–1106. doi: 10.1128/jvi.69.2.1099-1106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lui K J, Kendal A P. Impact of influenza epidemics on mortality in the United States from October 1972 to May 1985. Am J Public Health. 1987;77:712–716. doi: 10.2105/ajph.77.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo G, Chung J, Palese P. Alterations of the stalk of the influenza virus neuraminidase: deletions and insertions. Virus Res. 1993;29:141–153. doi: 10.1016/0168-1702(93)90055-r. [DOI] [PubMed] [Google Scholar]
- 15.McKimm-Breschkin J L. Resistance of influenza viruses to neuraminidase inhibitors—a review. Antiviral Res. 2000;47:1–17. doi: 10.1016/s0166-3542(00)00103-0. [DOI] [PubMed] [Google Scholar]
- 16.Monto A S, Fleming D M, Henry D, de Groot R, Makela M, Klein T, Elliott M, Keene O N, Man C Y. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza A and B virus infections. J Infect Dis. 1999;180:254–261. doi: 10.1086/314904. [DOI] [PubMed] [Google Scholar]
- 17.Monto, A. S., D. P. Robinson, M. L. Herlocher, J. M. Hinson Jr., M. J. Elliott, and A. Crisp. Zanamivir in the prevention of influenza among healthy adults: a randomized controlled trial. JAMA 282:31–35. [DOI] [PubMed]
- 18.Nicholson K G, Aoki F Y, Osterhaus A D, Trottier S, Carewicz O, Mercier C H, Rode A, Kinnersley N, Ward P. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet. 2000;355:1845–1850. doi: 10.1016/s0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- 19.Palese P, Compans R W. Inhibition of influenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA): mechanism of action. J Gen Virol. 1976;33:159–163. doi: 10.1099/0022-1317-33-1-159. [DOI] [PubMed] [Google Scholar]
- 20.Reed L J, Muench M. A simple method of estimating fifty percent end points. Am J Hyg. 1938;27:493–498. [Google Scholar]
- 21.Sidwell R W, Huffman J H. Use of disposable micro tissue culture plates for antiviral and interferon induction studies. Appl Microbiol. 1971;22:797–801. doi: 10.1128/am.22.5.797-801.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sidwell R W, Huffman J H, Barnard D L, Bailey K W, Wong M-H, Morrison A, Syndergaard T, Kim C U. Inhibition of influenza virus infections in mice by GS4104, an orally effective influenza virus neuraminidase inhibitor. Antiviral Res. 1998;37:107–120. doi: 10.1016/s0166-3542(97)00065-x. [DOI] [PubMed] [Google Scholar]
- 23.Sidwell R W, Smee D F, Huffman J H, Barnard D L, Bailey K W, Morrey J D, Babu Y S. In vivo influenza virus-inhibitory effects of the cyclopentane neuraminidase inhibitor RWJ-270201. Antimicrob Agents Chemother. 2001;45:749–757. doi: 10.1128/AAC.45.3.749-757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smee D F, Sidwell R W, Barnett B B, Spendlove R S. Inhibition of rotaviruses by selected antiviral substances. In: Acres S D, Forman S D, Fast H, editors. Proceedings of the Third International Symposium of Neonatal Diarrhea. Saskatoon, Saskatchewan, Canada: Veterinary Infectious Disease Organization; 1981. pp. 123–138. [Google Scholar]
- 25.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X, Fukuda K, Cox N. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 26.Von Itzstein M, Wu W-Y, Kok G B, Pegg M S, Cyason J C, Jin B, Phan T V, Smythe M L, White H F, Oliver S W, Colman P M, Varghese J N, Ryan D M, Woods J M, Bethell R C, Hotham V J, Cameron J M, Penn C R. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363:418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- 27.Woods J M, Bethell R C, Coates J A V, Healy N, Hiscox S A, Pearson B A, Ryan D M, Ticehurst J, Tilling J, Walcott S M, Penn C R. 4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid is a highly effective inhibitor both of the sialidase (neuraminidase) and of growth of a wide range of influenza A and B viruses in vitro. Antimicrob Agents Chemother. 1993;37:1473–1479. doi: 10.1128/aac.37.7.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wray S K, Gilbert B E, Noall M W, Knight V. Mode of action of ribavirin: effect of nucleotide pool alterations on influenza virus ribonucleoprotein synthesis. Antiviral Res. 1985;5:29–37. doi: 10.1016/0166-3542(85)90012-9. [DOI] [PubMed] [Google Scholar]