Abstract
The cyclopentane influenza virus neuraminidase inhibitor RWJ-270201 was evaluated against influenza A/NWS/33 (H1N1), A/Shangdong/09/93 (H3N2), A/Victoria/3/75 (H3N2), and B/Hong Kong/05/72 virus infections in mice. Treatment was by oral gavage twice daily for 5 days beginning 4 h pre-virus exposure. The influenza virus inhibitor oseltamivir was run in parallel, and ribavirin was included in studies with the A/Shangdong and B/Hong Kong viruses. RWJ-270201 was inhibitory to all infections using doses as low as 1 mg/kg/day. Oseltamivir was generally up to 10-fold less effective than RWJ-270201. Ribavirin was also inhibitory but was less tolerated by the mice at the 75-mg/kg/day dose used. Disease-inhibitory effects included prevention of death, lessening of decline of arterial oxygen saturation, inhibition of lung consolidation, and reduction in lung virus titers. RWJ-270201 and oseltamivir, at doses of 10 and 1 mg/kg/day each, were compared with regard to their effects on daily lung parameters in influenza A/Shangdong/09/93 virus-infected mice. Maximum virus titer inhibition was seen on day 1, with RWJ-270201 exhibiting the greater inhibitory effect, a titer reduction of >104 cell culture 50% infective doses (CCID50)/g. By day 8, the lung virus titers in mice treated with RWJ-270201 had declined to 101.2 CCID50/g, whereas titers from oseltamivir-treated animals were >103 CCID50/g. Mean lung consolidation was also higher in the oseltamivir-treated animals on day 8. Both neuraminidase inhibitors were well tolerated by the mice. RWJ-270201 was nontoxic at doses as high as 1,000 mg/kg/day. These data indicate potential for the oral use of RWJ-270201 in the treatment of influenza virus infections in humans.
A major public health goal has been the control of human influenza virus infections. The recent reports of the clinical efficacy of two influenza virus neuraminidase inhibitors, zanamivir administered by oral powder inhalation (5–7, 11–14) and oseltamivir taken orally (4, 8, 15, 22), have provided hope that treatment of this significant viral disease may provide a means for its effective containment. The search for additional, possibly more potent, influenza virus inhibitors has continued, and as reported by Babu et al. (1), a series of cyclopentane derivatives which exhibit striking and selective inhibition of influenza A and B virus neuraminidase have been developed. This enzyme-inhibitory activity has been followed up with a series of experiments demonstrating that these compounds also have potent in vitro anti-influenza virus activity (21), with the efficacy of these cyclopentane derivatives being comparable to or greater than that exerted by zanamavir and GS4071, the parent compound of oseltamivir.
This report describes initial in vivo experiments with RWJ-270201, one of the cyclopentane compounds considered to have the greatest promise based on in vitro activity. In the absence of complete pharmacokinetic data for mice, it was decided to utilize the same treatment route and schedule found to be effective for oseltamivir (10, 20). A comparison with oseltamivir was made in each experiment, with ribavirin included in some studies as an additional positive control (17). These studies have focused on influenza A (H1N1), A (H3N2), and B viruses, since these serotypes have been primarily responsible for current influenza epidemics (2).
MATERIALS AND METHODS
Animals.
Female specific-pathogen-free BALB/c mice weighing 18 to 21 g from B & K Universal (Fremont, Calif.) were used. Housing and care of the animals were as described previously (20).
Compounds.
RWJ-270201 (Fig. 1) and oseltamivir (designated BCX-1812 and RW-2, respectively) were synthesized by BioCryst Pharmaceuticals (Birmingham, Ala.). Ribavirin was obtained from ICN Pharmaceuticals, Inc. (Costa Mesa, Calif.). All of these compounds were dissolved in sterile physiological saline (PSS) and stored at 4°C for use in these studies.
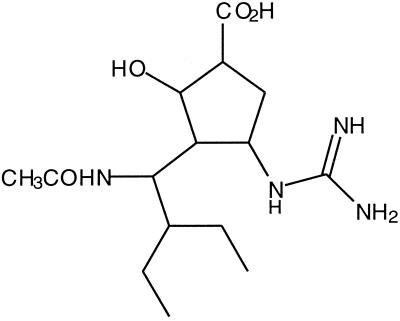
FIG. 1.
RWJ-270201 [(1S,2S,3S,4R)-3-[(1S)-1-(acetylamino)]-2-hydroxy-cyclopentane carboxylic acid].
Viruses.
Influenza A/Shangdong/09/93 (H3N2) virus was obtained from H. Regnery (Centers for Disease Control and Prevention, Atlanta, Ga.); A/NWS/33 (H1N1) virus was provided by K. Cochran (University of Michigan, Ann Arbor), and B/Hong Kong/05/72 and A/Victoria/3/75 (H3N2) viruses were obtained from the American Type Culture Collection (Manassas, Va.). The Shangdong virus was passaged seven times through mice, and then a pool was prepared in MDCK cells. The other viruses were passaged a single time intranasally through mice and used as a mouse lung homogenate. All were stored at −80°C; each was pretitrated in mice before being used in these experiments.
SaO2 determinations.
Arterial oxygen saturation (SaO2) was determined using the Biox 3740 pulse oximeter (Ohmeda, Louisville, Ohio). The ear probe attachment was used, with probe placed on the thigh of the animal, and the slow instrument mode selected. Readings were made after a 30-s stabilization time for each animal. The use of this device for measuring effects of influenza virus on SaO2 has been described by us previously (19).
Lung virus titer determinations.
Each lung was homogenized to a 10% (wt/vol) suspension in minimum essential medium containing 0.18% NaHCO3 and 50 μg of gentamicin/ml. Each homogenate was assayed in MDCK cells in 96-well microplates using triplicate wells for each 10-fold dilution, with viral cytopathic effect determined visually as an end point as described previously (18).
General procedure for in vivo antiviral experiments.
Mice were anesthetized by intraperitoneal injection of 100 mg of ketamine (Ft. Dodge Animal Health, Ft. Dodge, Iowa) per kg, and 90 μl of virus was administered intranasally to each. Treatments with test compound were by oral gavage (p.o.) twice daily for 5 days beginning 4 h pre-virus exposure. In each infected, drug-treated group, 8 to 10 mice were used per drug dosage, with 16 to 20 infected animals treated with PSS only. Three to five mice were used in toxicity control groups and as normal controls. These animals were weighed immediately prior to initial treatment and again 18 h after final treatment and observed for 21 days for signs of toxicity (lethargy, prostration, hunching, hyperactivity, and ruffled fur), and deaths were recorded. The SaO2 levels in the normal controls were also determined in parallel to 1 those in the infected mice. Parameters for evaluation of antiviral activity included prevention of death, prolongation of mean day to death, and reduced decline in SaO2 determined on days 3 through 11 of the infection. In addition, groups of three to five mice were killed at various times after infection, and their lungs were removed, weighed, assigned a consolidation score ranging from 0 (normal) to 4 (maximal plum coloration), and assayed for virus titer.
Procedure for comparison of the effects of RWJ-270201 and oseltamivir therapy on influenza A/Shangdong/09/93 (H3N2), A/Victoria/3/75 (H3N2), A/NWS/33 (H1N1), and B/Hong Kong/05/72 virus infections in mice.
Mice infected with each virus were treated p.o. with RWJ-270201 at doses of 1, 10, and, in some cases, 100 mg/kg/day; with oseltamivir at 1 and 10 mg/kg/day (10 mg/kg/day only with Victoria virus); with ribavirin at 75 mg/kg/day (with A/Shangdong and B/Hong Kong viruses); or with PSS twice daily for 5 days beginning 4 h pre-virus exposure. Ten mice in each drug-treated group and 20 PSS-treated controls were observed for death daily for 21 days, and SaO2 levels were determined. In the A/Shangdong and B/Hong Kong virus experiments, 5 additional mice in each high-dose drug-treated group and 10 saline-treated controls were killed on day 3, and 5 additional mice at all doses (10 saline-treated controls) were killed on day 6. In the A/Victoria virus experiment, five mice in each treatment group were killed on days 3, 6, and 9 for determination of lung consolidation and virus titer. The A/NWS virus experiment compared 10- and 1-mg/kg/day doses of both RWJ-270201 and oseltamivir, with lung parameters evaluated on days 3 and 6 using three drug-treated mice and five PSS-treated animals at each time of sacrifice. The A/Shangdong virus experiment was performed twice. Oseltamivir could not be tested at higher doses because of a shortage of the drug.
Procedure for comparison of the effects of RWJ-270201 and oseltamivir therapies on daily lung parameters in influenza virus-infected mice.
Groups of 34 influenza A/Shangdong virus-infected mice were treated p.o. with RWJ-270201 or oseltamivir at doses of 10 or 1 mg/kg/day. A group of 80 infected mice were treated with PSS as controls. Treatment was twice daily for 5 days beginning 4 h pre-virus exposure. Three animals in each drug treatment group and 10 mice from the placebo-treated controls were randomly selected on days 1 through 8 and sacrificed, and their lungs were assigned a consolidation score, weighed, and assayed for virus titer. To provide background data, two uninfected, untreated mice were killed on days 4 and 8 in parallel with those described above, and their lungs processed as described above.
RESULTS
Comparison of RWJ-270201, oseltamivir, and ribavirin therapy on influenza A (H3N2) virus infections.
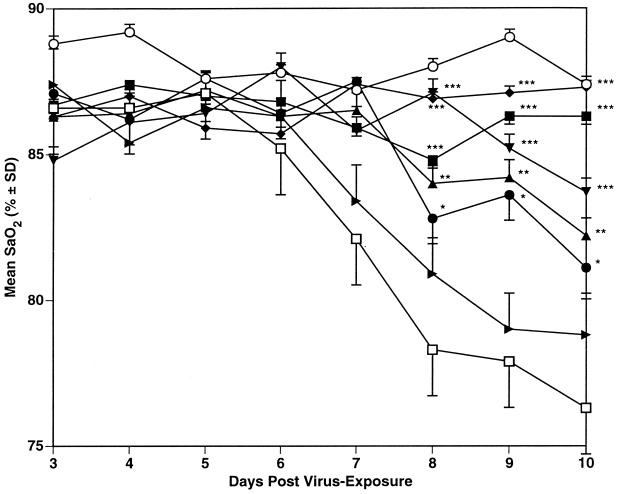
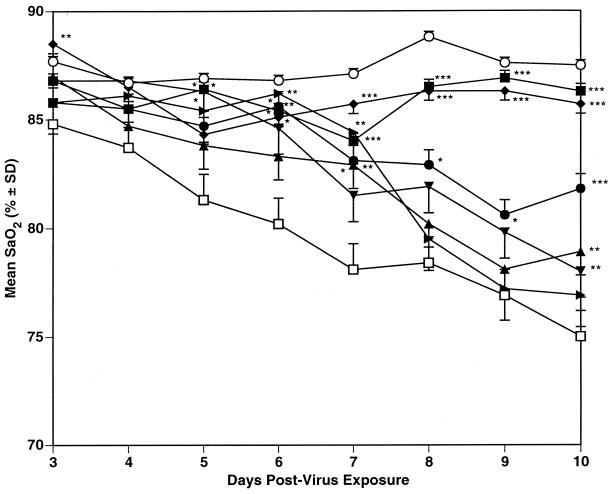
The results of the two experiments with influenza A/Shangdong virus are summarized in Table 1, with daily mean SaO2 values shown in Fig. 2 for experiment 1. Treatment with RWJ-270201 was protective to the mice at all dosages used, as seen by increased survivors, lessened SaO2 decline (data when time of maximum SaO2 decline was seen [day 11] are shown in Table 1), inhibition of lung consolidation, and inhibition of lung virus titers. Oseltamivir was significantly inhibitory to this infection as seen by prevention of death at a dose of 10 mg/kg/day only. At 1 mg/kg/day, only a delay in the mean day to death was seen, with moderate lessening of SaO2 decline and inhibition of lung consolidation. In experiment 1, a significant inhibition of lung virus titer was seen at the 1-mg/kg/day dose of oseltamivir, but this inhibition was not seen in experiment 2. Ribavirin was significantly inhibitory to the infection in both experiments.
TABLE 1.
Comparison of the effects of p.o. treatmenta with RWJ-270201, oseltamivir, and ribavirin on influenza A/Shangdong/09/93 (H3N2) virus infections in mice
| Expt and compound | Dosage (mg/kg/day) | No. of survivors/totalb | Mean day to deathc ± SDb | Day 11 mean SaO2 (%) ± SDb | Mean lung parameters ± SDb on day 6 (day 3)
|
||
|---|---|---|---|---|---|---|---|
| Score | Wt (mg) | Virus titer (log10/g) | |||||
| 1 | |||||||
| RWJ-270201 | 100 | 10/10*** | >21.0 ± 0.0*** | 86.2 ± 2.3*** | 1.8 ± 0.4*** (0.1 ± 0.2***) | 248 ± 19*** (116 ± 17***) | 4.2 ± 0.6 (<1.8 ± 0.0***) |
| 10 | 9/10*** | 8.0 ± 0.0 | 85.9 ± 4.6*** | 2.2 ± 0.4** | 262 ± 18*** | 3.9 ± 0.8 | |
| 1 | 5/10*** | 11.4 ± 2.7** | 83.3 ± 5.1*** | 2.4 ± 0.2** | 286 ± 18*** | 3.6 ± 1.2* | |
| Oseltamivir | 10 | 5/10*** | 12.6 ± 1.9*** | 88.9 ± 1.8*** | 1.1 ± 0.7*** (0.1 ± 0.2***) | 246 ± 36*** (174 ± 11***) | 1.8 ± 0.0*** (<1.8 ± 0.0***) |
| 1 | 0/10 | 10.7 ± 1.9** | 80.5 ± 6.9 | 2.6 ± 0.4* | 326 ± 40 | 2.1 ± 0.6*** | |
| Ribavirin | 75 | 10/10*** | >21.0 ± 0.0*** | 85.9 ± 1.9*** | 1.2 ± 0.6*** (0.3 ± 0.4**) | 196 ± 27*** (128 ± 36***) | 1.8 ± 0.0*** (2.6 ± 1.1***) |
| PSS | 0/20 | 8.3 ± 2.0 | 76.9 ± 4.6 | 3.4 ± 0.5 (1.9 ± 0.8) | 385 ± 36 (217 ± 20) | 4.6 ± 0.6 (4.3 ± 0.6) | |
| 2 | |||||||
| RWJ-270201 | 100 | 10/10*** | >21.0 ± 0.0*** | 86.3 ± 3.3*** | 0.6 ± 0.5*** (0.3 ± 0.3***) | 216 ± 15** (152 ± 18*) | 5.5 ± 0.5 (6.0 ± 0.7**) |
| 10 | 5/10* | 9.0 ± 0.8 | 81.1 ± 6.6* | 1.9 ± 0.2*** | 234 ± 18* | 5.7 ± 0.4 | |
| 1 | 4/10 | 10.1 ± 1.2 | 82.2 ± 6.3** | 2.6 ± 0.4* | 296 ± 62* | 5.7 ± 0.5 | |
| Oseltamivir | 10 | 8/9*** | 14.0 ± 0.0 | 83.7 ± 3.2*** | 2.7 ± 0.5 (0.2 ± 0.3***) | 270 ± 13 (158 ± 87*) | 6.3 ± 0.9 (6.5 ± 0.4) |
| 1 | 0/10 | 11.7 ± 3.3 | 78.8 ± 4.0 | 2.7 ± 0.3 | 276 ± 18 | 6.4 ± 0.4 | |
| Ribavirin | 75 | 9/9*** | >21.0 ± 0.0*** | 87.3 ± 2.2*** | 0.6 ± 0.4*** (0.2 ± 0.3***) | 196 ± 21*** (144 ± 5**) | 5.5 ± 0.8 (5.0 ± 0.6***) |
| PSS | 2/20 | 9.0 ± 1.1 | 76.3 ± 4.2 | 3.4 ± 0.5 (1.0 ± 0.2) | 326 ± 56 (177 ± 21) | 6.0 ± 0.4 (7.0 ± 0.4) | |
Twice a day for 5 days beginning 4 h pre-virus exposure.
*, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared to PSS-treated controls).
Animals dying prior to day 21.
FIG. 2.
Effect of p.o. treatment (twice a day for 5 days beginning 4 h pre-virus exposure) with RWJ-270201 and oseltamivir on SaO2 decline in influenza A/Shangdong/09/93 (H3N2) virus-infected mice. ■, RWJ-270201, 100 mg/kg/day; ●, RWJ-270201, 10 mg/kg/day; ▴, RWJ-270201, 1 mg/kg/day; ▾, oseltamivir, 10 mg/kg/day; ▸, oseltamivir, 1 mg/kg/day; ⧫, ribavirin, 75 mg/kg/day; □ PSS; ○, normal controls. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 (compared to PSS-treated controls). SD, standard deviation.
Toxicity control mice used in this study all survived; those treated with RWJ-270201 and oseltamivir gained weight at a rate comparable to that for normal controls. Those receiving ribavirin lost a mean of 0.7 g during therapy (data not shown). In a separate experiment, RWJ-270201 was evaluated for induction of toxicity in uninfected mice at a dose of 1000 mg/kg/day using the treatment schedule of p.o. twice daily for 5 days. The animals gained 0.7 g during the period of treatment, and no adverse effects were seen (data not shown).
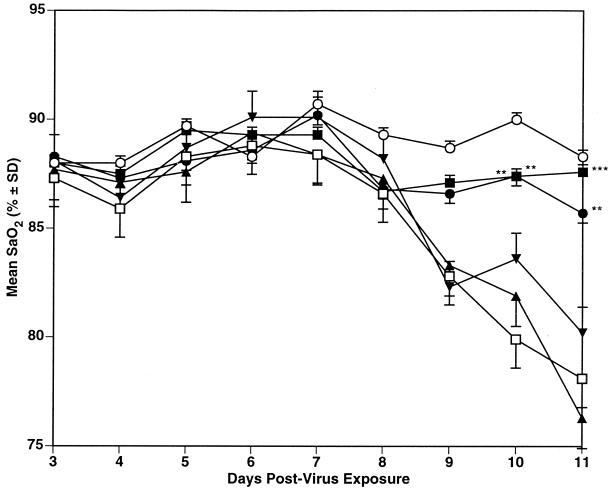
The results using these compounds in mice infected with influenza A/Victoria virus are shown in Table 2, with daily mean SaO2 values shown in Figure 3. RWJ-270201 significantly prevented deaths of the mice at 100 and 10 mg/kg/day. This antiviral effect was also reflected by significant inhibition of lung consolidation and lung virus titers, with the greatest virus titer inhibition seen at the day 9 sampling time. Oseltamivir, run in parallel at a dose of 10 mg/kg/day, was less effective against this virus infection, with only significant inhibition of lung score seen. The SaO2 data (Fig. 3) also indicate that RWJ-270201 inhibited SaO2 decline at the 100- and 10-mg/kg/day doses, but at 1 mg/kg/day, a drop in SaO2 level began to occur by day 9. Oseltamivir at the 10-mg/kg/day dose was similarly inhibitory to SaO2 decline until day 9. Both compounds were again well tolerated by the toxicity control animals (data not shown).
TABLE 2.
Comparison of the effects of p.o. treatmenta with RWJ-270201 and oseltamivir on an influenza A/Victoria/3/75 (H3N2) virus infection in mice
| Compound | Dosage (mg/kg/day) | No. of survivors/ totalb | Mean day to deathc ± SDb | Day 11 mean SaO2 (%) ± SDb | Mean lung parameters ± SDb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Score
|
Wt (mg)
|
Virus titer (log10/g)
|
|||||||||||
| Day 3 | Day 6 | Day 9 | Day 3 | Day 6 | Day 9 | Day 3 | Day 6 | Day 9 | |||||
| RWJ-270201 | 100 | 10/10*** | >21.0 ± 0.0*** | 87.6 ± 2.1*** | 0.0 ± 0.0 | 1.2 ± 0.3** | 0.8 ± 0.6* | 117 ± 15** | 157 ± 21** | 150 ± 35** | 6.0 ± 0.4 | 4.5 ± 0.8** | 0.0 ± 0.0*** |
| 10 | 7/10** | 12.7 ± 2.1 | 85.7 ± 4.6** | 0.0 ± 0.0 | 1.5 ± 0.0** | 1.2 ± 0.3* | 137 ± 6 | 187 ± 15 | 223 ± 12* | 5.9 ± 0.3 | 5.3 ± 0.5 | 0.0 ± 0.0*** | |
| 1 | 0/10 | 10.9 ± 0.7 | 76.3 ± 4.1 | 0.2 ± 0.3 | 2.8 ± 0.3 | 3.5 ± 0.0 | 140 ± 36 | 187 ± 21 | 257 ± 12 | 5.9 ± 0.5 | 5.4 ± 0.4 | 4.3 ± 0.4 | |
| Oseltamivir | 10 | 1/9 | 11.6 ± 1.6 | 80.2 ± 6.3 | 0.0 ± 0.0 | 1.3 ± 1.3** | 1.8 ± 0.8 | 177 ± 6 | 203 ± 45 | 240 ± 36 | 6.1 ± 0.4 | 5.3 ± 0.7 | 3.0 ± 1.0 |
| PSS | 2/16 | 10.6 ± 2.1 | 78.1 ± 5.6 | 0.3 ± 0.3 | 3.3 ± 0.4 | 2.6 ± 0.4 | 148 ± 11 | 208 ± 19 | 282 ± 34 | 6.2 ± 0.5 | 5.7 ± 0.5 | 3.7 ± 0.8 | |
Twice a day for 5 days beginning 4 h pre-virus exposure.
**, P < 0.01; ***, P < 0.001 (compared to PSS-treated controls).
Animals dying before day 21.
FIG. 3.
Comparison of p.o. treatment with RWJ-270201 and oseltamivir on SaO2 decline in influenza A/Victoria/3/75 (H3N2) virus-infected mice. ■, RWJ-270201, 100 mg/kg/day; ●, RWJ-270201, 10 mg/kg/day; ▴, RWJ-270201, 1 mg/kg/day; ▾, oseltamivir, 10 mg/kg/day; □, PSS; ○, normal controls. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 (compared to PSS-treated controls). SD, standard deviation.
Comparison of RWJ-270201 and oseltamivir therapies for influenza A (H1N1) virus infections.
The results of the study with influenza A (H1N1) virus are shown in Table 3. Each compound at the 10-mg/kg/day dose prevented deaths from occurring in the infected mice, lessened SaO2 decline (Fig. 4), and inhibited lung score development and lung weight increases. Lung virus titers were reduced by at least 0.6 log10 unit by both doses of each compound on day 3; by day 6, the high doses of each compound still significantly inhibited the lung virus titers. A lesser lung virus-inhibitory effect was seen using the 1-mg/kg/day doses of each compound. In this experiment, oseltamivir appeared to be slightly more inhibitory to lung virus titers than RWJ-270201. At the 1-mg/kg/day dose, all of the mice receiving RWJ-270201 survived the infection, but 30% of those treated with this dose of oseltamivir died. The lung parameters also reflected this difference in efficacy.
TABLE 3.
Comparison of the effects of p.o. treatmenta with RWJ-270201 and oseltamivir on an influenza A/NWS/33 (H1N1) virus infection in mice
| Compound | Dosage (mg/kg/day) | No. of survivors/ totalb | Mean day to deathc ± SDb | Day 11 mean SaO2 (%) ± SDb | Mean lung parameters ± SDb
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Score
|
Wt (mg)
|
Virus titer (log10/g)
|
||||||||
| Day 3 | Day 6 | Day 3 | Day 6 | Day 3 | Day 6 | |||||
| RWJ-270201 | 10 | 10/10*** | >21.0 ± 0.0 | 88.3 ± 2.0*** | 0.0 ± 0.0 | 0.0 ± 0.0* | 107 ± 11** | 117 ± 6*** | 5.4 ± 0.1*** | 4.9 ± 0.6** |
| 1 | 10/10*** | >21.0 ± 0.0 | 87.1 ± 2.9*** | 0.0 ± 0.0 | 0.5 ± 0.0* | 130 ± 10* | 143 ± 25** | 6.1 ± 0.6 | 6.2 ± 0.4 | |
| Oseltamivir | 10 | 10/10*** | >21.0 ± 0.0 | 87.2 ± 1.3*** | 0.3 ± 0.3 | 0.2 ± 0.0* | 130 ± 10* | 130 ± 12*** | 4.9 ± 0.4*** | 4.8 ± 0.6** |
| 1 | 7/10** | 13.0 ± 0.0 | 87.8 ± 1.8*** | 0.0 ± 0.0 | 0.8 ± 0.6* | 160 ± 10 | 230 ± 70 | 5.8 ± 0.1*** | 5.8 ± 0.4 | |
| PSS | 3/20 | 11.0 ± 3.1 | 78.7 ± 5.2 | 0.2 ± 0.3 | 2.0 ± 0.0 | 168 ± 22 | 232 ± 19 | 6.7 ± 0.1 | 6.4 ± 0.3 | |
Twice a day for 5 days beginning 4 h pre-virus exposure.
*, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared to PSS-treated controls).
Animals dying prior to day 21
FIG. 4.
Comparison of the effect of p.o. treatment with RWJ-270201 and oseltamivir on SaO2 decline in influenza A/NWS/33 (H1N1) virus-infected mice. ●, RWJ-270201, 10 mg/kg/day; ▴, RWJ-270201, 1 mg/kg/day; ▾ oseltamivir, 10 mg/kg/day; ▸, oseltamivir, 1 mg/kg/day; □, PSS; ○, normal controls. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 (compared to PSS-treated controls). SD, standard deviation.
Comparison of effects of p.o.-administered RWJ-270201 and oseltamivir on influenza B virus infections.
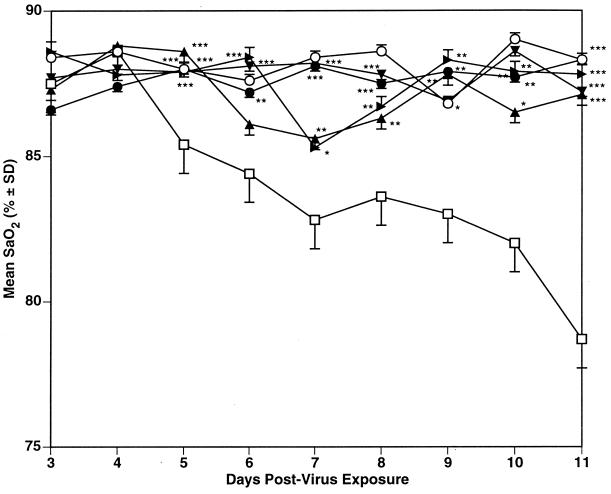
As seen in Table 4, RWJ-270201 therapy significantly prevented influenza B/Hong Kong virus-induced deaths in the mice at all three dosages. This antiviral effect was also seen by inhibition of SaO2 decline (Fig. 5), lung consolidation, and lung virus titers on day 6. Oseltamivir treatment at the 10- and 1-mg/kg/day dosages did not prevent death of the mice but did significantly prolong the mean day to death and lessen lung consolidation. Both doses also reduced SaO2 decline, but to a lesser extent than RWJ-270201 at the same doses. The 10-mg/kg/day dose of oseltamivir also significantly inhibited day 6 lung virus titers. Ribavirin was highly active against this virus infection by all parameters studied. Toxicity control animals treated with all compounds survived and gained weight (data not shown).
TABLE 4.
Comparison of the effects of p.o. treatmenta with RWJ-270201, oseltamivir, and ribavirin on influenza B/Hong Kong/05/72 virus infections in mice
| Compound | Dosage (mg/kg/day) | No. of survivors/ totalb | Mean day to deathc ± SDb | Day 11 mean SaO2 (%) ± SDb | Mean lung parameters ± SDb on day 6 (day 3)
|
||
|---|---|---|---|---|---|---|---|
| Score | Wt (mg) | Virus titer (log10/g) | |||||
| RWJ-270201 | 100 | 10/10*** | >21.0 ± 0.0*** | 86.3 ± 2.6*** | 2.4 ± 2.0*** (0.8 ± 0.8**) | 248 ± 26*** (172 ± 24) | 0.0 ± 0.0** (4.0 ± 0.5) |
| 10 | 6/10*** | 10.8 ± 3.4** | 81.8 ± 5.1*** | 2.4 ± 0.2** | 242 ± 43*** | 0.4 ± 0.8* | |
| 1 | 4/10** | 7.5 ± 2.0 | 78.9 ± 5.6** | 2.9 ± 0.7* | 278 ± 76 | 1.8 ± 2.0 | |
| Oseltamivir | 10 | 1/10 | 9.3 ± 1.7** | 78.0 ± 4.1** | 2.3 ± 0.0*** (1.3 ± 1.2) | 232 ± 29*** (166 ± 29*) | 0.4 ± 0.8* (4.4 ± 0.4) |
| 1 | 1/10 | 8.6 ± 1.7* | 76.9 ± 4.2 | 2.4 ± 1.2** | 260 ± 35*** | 2.1 ± 1.7 | |
| Ribavirin | 75 | 9/10*** | 5.0 ± 0.0 | 85.7 ± 4.1*** | 1.6 ± 1.0*** (0.7 ± 0.4***) | 206 ± 53*** (168 ± 24*) | 0.0 ± 0.0** (1.4 ± 1.6***) |
| PSS | 0/19 | 7.1 ± 1.6 | 75.0 ± 0.0 | 3.7 ± 0.4 (1.8 ± 0.4) | 334 ± 28 (202 ± 30) | 3.2 ± 1.5 (3.9 ± 0.6) | |
Twice a day for 5 days beginning 4 h pre-virus exposure.
*, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared to PSS-treated controls).
Animals dying before day 21.
FIG. 5.
Comparison of the effect of p.o. treatment with RWJ-270201 and oseltamivir on SaO2 decline in influenza B/Hong Kong/05/72 virus-infected mice. ■, RWJ-270201, 100 mg/kg/day; ●, RWJ-270201, 10 mg/kg/day; ▴, RWJ-270201, 1 mg/kg/day; ▾, oseltamivir, 10 mg/kg/day; ▸, oseltamivir, 1 mg/kg/day; ⧫, ribavirin, 75 mg/kg/day; □, PSS; ○ normal controls. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 (compared to PSS-treated controls). SD, standard deviation.
Effect of treatment on daily lung parameters.
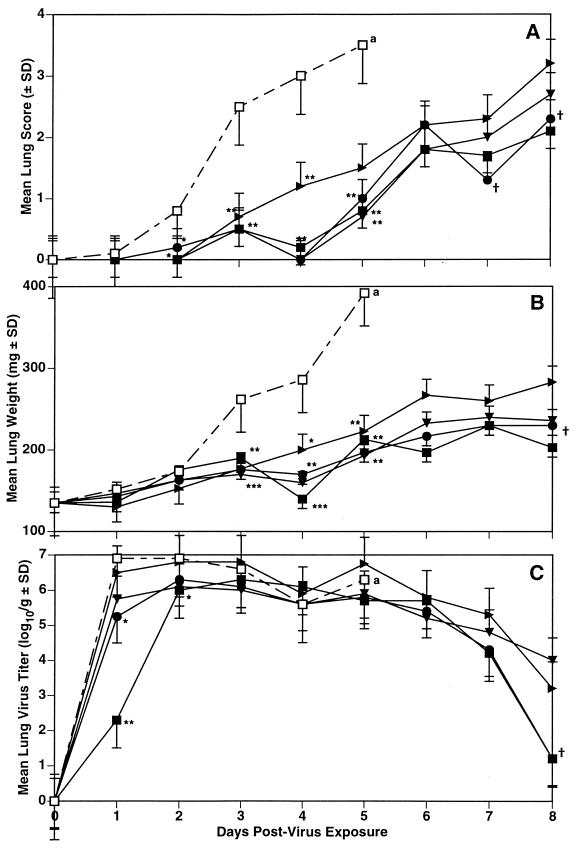
The daily lung scores, weights, and virus titers on infected animals treated with each compound are summarized in Fig. 6. In this experiment, treatment with both compounds significantly inhibited development of lung consolidation, although by day 6, 2 days after termination of therapy, the lung weights in the oseltamivir-treated groups were 18 to 23% higher than those in the mice treated with RWJ-270201. The day 7 and 8 lung data were also higher in the oseltamivir-treated mice. By day 7, the placebo-treated animals had all died in this experiment. The lung virus titers were particularly inhibited on day 1 of the infection, with the 10-mg/kg/day dose of RWJ-270201 inhibiting the mean virus titers by nearly 5 log10 units at this time. Oseltamivir treatment at the same dose lessened this titer from 106.9 cell culture 50% infective doses (CCID50)/g in the PSS-treated animals to 105.75 CCID50/g; the lower dose of RWJ-270201 yielded a lung virus titer of 105.25 CCID50/g, whereas the same dose of oseltamivir resulted in a mean lung titer of 106.5 CCID50/g at this same time. The later assay times indicated markedly less differences in lung virus titers between the treated and the control groups. It is noteworthy that by day 8, the mean lung virus titers in mice treated with both doses of RWJ-270201 were reduced to 101.2 CCID50/g whereas the virus titers in the mice receiving oseltamivir were 104.0 and 103.2 CCID50/g in the groups receiving dosages of 10 and 1 mg/kg/day, respectively. Statistical analysis of the differences between the equivalent doses of each drug showed a significant difference (P < 0.05) in all lung parameters on day 8.
FIG. 6.
Comparison of the effect of p.o. treatment with RWJ-270201 and oseltamivir on daily lung scores (A), lung weights (B), and virus titers (C) in mice infected with influenza A/Shangdong/09/93 (H3N2) virus. Treatment was twice a day for 5 days beginning 4 h pre-virus exposure. ■, RWJ-270201, 10 mg/kg/day; ●, RWJ-270201, 1 mg/kg/day; ▾, oseltamivir, 10 mg/kg/day; ▸, oseltamivir, 1 mg/kg/day; □, PSS. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 (compared to PSS-treated controls). †, P < 0.05 compared to the equivalent dose of oseltamivir. a, all PSS-treated mice died before day 6.
DISCUSSION
These data indicate that the orally administered influenza virus neuraminidase inhibitor RWJ-270201 is highly effective against experimentally induced influenza A (H1N1), A (H3N2), and B virus infections in mice. This in vivo activity appeared to often be more efficacious than that of oseltamivir when the effects of the same dosages of each compound were compared, although such differences were not seen in every experiment. The data showing a decline in SaO2 level in the infected mice treated with oseltamivir late in the infection, which also correlated with increased lung weights at this same time (i.e. approximately 2 days after termination of treatment), suggests that the latter compound may clear from the host sooner than RWJ-270201. Li et al. (9) have reported oseltamivir to persist as the parent compound, GS4071 (oseltamivir carboxylate), for approximately 24 h in rat, dog, and ferret plasma. Pharmacokinetics studies on orally administered RWJ-270201 indicate a similar profile in mice (G. Drusano, Albany Medical College, personal communication). No prodrug is involved with this compound. However, as shown by Eisenberg et al. (3), the levels in plasma may be misleading, since the concentration of GS4071 in the bronchoalveolar fluid in rats treated p.o. with oseltamivir declined at a lower rate than that in the plasma; such data are not yet available for RWJ-270201.
RWJ-270201 was well tolerated in a dose of up to 1,000 mg/kg/day by the toxicity control mice. Acute-toxicity studies with rats have shown no untoward effects using doses as high as 3,000 mg/kg/day, and multiple-dose treatments for 5 days using doses of 1,000 mg/kg/day in rats were similarly well tolerated (T. Coogan, The R. W. Johnson Pharmaceutical Institute, Raritan, N.J. personal communication). The 1,000-mg/kg/day dose in rats is equivalent, on a milligram-per-square meter basis, to a dose of 2,000 mg/kg/day in the mouse. This lack of toxicity indicates a high in vivo margin of safety for the use of RWJ-270201 against influenza virus infections in mice.
The treatment schedule used in all of these experiments was twice daily for 5 days beginning 4 h pre-virus exposure. A study has also been done in which p.o. treatment twice daily for 5 days using RWJ-270201 was delayed for up to 60 h after influenza A/NWS virus exposure, with all infected mice still surviving (R. W. Sidwell and D. F. Smee, unpublished data). These data were similar to those we reported using oseltamivir (20) and indicate the potential for RWJ-270201 to be used in a therapeutic, and not just prophylactic, manner. Additionally, the antiviral effects of other dosing schedules, including once, twice, and three times daily, are currently under investigation to determine the pharmacokinetic parameter that drives efficacy.
The results using oseltamivir in this study correlate quite well with those reported previously by us (20), where the A/Shangdong virus infections were strongly inhibited with 100- and 10-mg/kg/day doses given on the same treatment schedule; in those studies, a 1-mg/kg/day dose was marginally inhibitory. Previously, we found oseltamivir at 10 mg/kg/day to be highly protective to mice infected with influenza A/Victoria virus; in that experiment, however, the viral challenge dose was such that 5 of 16 placebo-treated mice survived (69% lethality), whereas in the present experiment the viral challenge was increased, killing 14 of 16 placebo controls (87.5%). In our earlier studies using influenza B/Hong Kong virus infection, efficacy was seen using 10- and 3.2-mg/kg/day doses of oseltamivir but no effect was seen at 1 mg/kg/day; in that study, however, the influenza B virus infection was lethal to 16 of 18 mice (88.8%) among the placebo-treated controls, with a mean day to death of 10.5 days. In the present experiment using this virus, the viral challenge was greater, killing all placebo-treated mice, and the mean day to death of those control animals was 7.1 days. It has been well established that the severity of the viral challenge dose will influence the antiviral activities of test compounds (16). It would be informative to test and compare the efficacies of these drugs against even higher viral challenge doses. Studies to investigate this effect are under way.
Efficacy using RWJ-270201 was demonstrated using all disease evaluation parameters. Inhibition of SaO2 decline was particularly useful, since this parameter could definitively indicate, on a daily basis, the disease condition of the mouse. As seen in the experiment where lungs were taken daily for determination of consolidation score, lung weight, and virus titer, the increasing consolidation, especially seen using the more objective lung weight, correlated well with SaO2 decline. Virus titers were highest (mean = 106.5 CCID50/g) in the placebo-treated mice 1 day after virus exposure; since the viral challenge dose was 105.1 CCID50/g, this was an approximate 2-log10-unit increase in a 24-h period. Treatment with either RWJ-270201 or oseltamivir inhibited this initial virus replication by 0.4 to 4.6 log10 units at this time, depending on the compound and dose used. In our experience, a reduction of viral challenge dose of 0.5 log10 unit is sufficient to prevent the majority of challenged mice from dying, so it would be expected that the infected mice treated with these compounds would survive the infection if such an initial lung virus inhibition was seen. It is noted that the mice receiving the 1-mg/kg/day dose of oseltamivir, where a maximum titer reduction of 0.4 log10 unit was seen, did not survive the infection (Table 1). Maximal lung virus inhibition was also seen relatively late (day 8 or 9) in the infection; this time point was not routinely selected for lung assay because often the placebo-treated animals had all died by this time.
As reported by Smee et al. (21), the A/Shangdong and A/Victoria viruses were highly sensitive to RWJ-270201 in vitro, with 50% effective concentrations ranging from 0.03 to 0.17 μM, depending on the assay system. The A/NWS and B/Hong Kong viruses appeared to be less sensitive in vitro, with 50% effective concentrations of 2.0 to 21 μM. All the virus infections responded to RWJ-270201 therapy to approximately the same extent in the present in vivo experiments, however. These differences between in vitro and in vivo viral sensitivities to antiviral compounds have been observed by us previously using oseltamivir (20).
As indicated earlier, the influenza virus serotypes A (H1N1), A (H3N2), and B represent those viral serotypes currently responsible for the influenza outbreaks in the world today. These data indicate that the orally administered cyclopentane influenza virus neuraminidase inhibitor RWJ-270201 is an effective inhibitor of in vivo infections caused by representatives of each serotype; these data, coupled with the broad therapeutic window of the compound, indicate it to be of potential for human use.
ACKNOWLEDGMENTS
This work was supported by contract N01-AI-65291 from the Virology Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by a grant from the R. W. Johnson Pharmaceutical Research Institute, Raritan, N.J.
REFERENCES
- 1.Babu Y S, Chand D, Bantia S, Kotian P, Dehghani A, El-Kattan Y, Lin T N, Littutchinson T, Elliott A J, Parker C D, Ananth S L, Horn L L, Laver G W, Montgomery J A. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J Med Chem. 2000;43:3482–3486. doi: 10.1021/jm0002679. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Update: influenza activity—United States, 1999–2000 season. Morb Mortal Wkly Rep. 2000;49:53–57. [PubMed] [Google Scholar]
- 3.Eisenberg E J, Bidgood A, Cundy K C. Penetration of GS4071, a novel neuraminidase inhibitor, into rat broncho-alveolar lining fluid following oral administration of the prodrug GS4104. Antimicrob Agents Chemother. 1997;41:1949–1952. doi: 10.1128/aac.41.9.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayden F G, Atmar R L, Schilling M, Johnson C, Poretz D, Paar D, Huson L, Ward P, Mills R G. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med. 1999;341:1336–1343. doi: 10.1056/NEJM199910283411802. [DOI] [PubMed] [Google Scholar]
- 5.Hayden F G, Lobo M, Hussey E K, Eason C U. Efficacy of intranasal GG167 in experimental human influenza A and B virus infection. In: Brown L E E, Hampson A W, Webster R G, editors. Options for the control of influenza III. New York, N.Y: Elsevier Science Publishing Inc.; 1996. pp. 718–725. [Google Scholar]
- 6.Hayden F G, Osterhaus A D, Treanor J J, Fleming D U, Aoki F Y, Nicholson M D, Bdinen A M, Hirst H M, Keene O, Wightman K. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza virus infections. N Engl J Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- 7.Hayden F G, Treanor J J, Betts R F, Lobo M, Esinhart J D, Hussey E K. Safety and efficacy of the neuraminidase inhibitor GG167 in experimental human influenza. JAMA. 1996;275:295–299. [PubMed] [Google Scholar]
- 8.Hayden F G, Treanor J J, Fritz R S, Lobo M, Betts R F, Miller M, Kinnersley N, Mills R G, Ward D, Straus S E. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA. 1999;282:1240–1246. doi: 10.1001/jama.282.13.1240. [DOI] [PubMed] [Google Scholar]
- 9.Li W, Escarpe P A, Eisenberg E J, Cundy K C, Sweet C, Jakeman K J, Merson J, Lew W, Williams M, Zhang L, Kim C U, Bischofberger N, Chen M S, Mendel D B. Identification of GS4104 as an orally bioavailable prodrug of the influenza virus neuraminidase inhibitor GS4071. Antimicrob Agents Chemother. 1998;42:647–653. doi: 10.1128/aac.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendel D B, Tai C Y, Escarpe P A, Li W, Sidwell R W, Huffman J H, Sweet C, Jakeman K J, Merson J, Lacy S A, Lew W, Williams M A, Zhang L, Chen M S, Bischofberger N, Kim C U. Oral administration of a prodrug of the influenza virus neuraminidase inhibitor GS4071 protects mice and ferrets against influenza infection. Antimicrob Agents Chemother. 1998;42:640–646. doi: 10.1128/aac.42.3.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mist Study Group. Randomized trial of efficacy and safety of inhaled zanamivir in treatment of influenza A and B virus infections. Lancet. 1998;352:1877–1881. [PubMed] [Google Scholar]
- 12.Monto A S, Fleming D M, Henry D, Groot R de,, Makela M, Klein T, Elliott M, Keene O N, Man C J. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza A and B virus infections. J Infect Dis. 1999;180:254–261. doi: 10.1086/314904. [DOI] [PubMed] [Google Scholar]
- 13.Monto A S, Robinson D P, Herlocher M L, Hinson J M, Jr, Elliott M J, Crisp A. Zanamivir in the prevention of influenza among healthy adults: a randomized controlled trial. JAMA. 1999;282:31–35. doi: 10.1001/jama.282.1.31. [DOI] [PubMed] [Google Scholar]
- 14.Monto A S, Webster A, Keene O. Randomized, placebo-controlled studies of inhaled zanamivir in the treatment of influenza A and B: pooled efficacy studies. J Antimicrob Chemother. 1999;44:23–29. doi: 10.1093/jac/44.suppl_2.23. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson K G, Aoki F Y, Osterhaus A D, Trottier S, Carewicz O, Mercier C H, Rode A, Kinnersley N, Ward P. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet. 2000;355:1845–1850. doi: 10.1016/s0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- 16.Sidwell R W. Determination of antiviral activity. In: Aszalos A, editor. Modern analysis of antibiotics. New York, N.Y: Marcel Dekker Inc.; 1986. pp. 434–480. [Google Scholar]
- 17.Sidwell R W, Huffman J H, Khare G P, Allen L B, Witkowski J T, Robins R K. Broad-spectrum antiviral activity of virazole: 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science. 1972;177:705–706. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- 18.Sidwell R W, Huffman J H, Call E W, Alaghamandan H, Cook P D, Robins R K. Effect of selenazofurin on influenza A and B virus infections in mice. Antiviral Res. 1986;6:343–353. doi: 10.1016/0166-3542(86)90016-1. [DOI] [PubMed] [Google Scholar]
- 19.Sidwell R W, Huffman J H, Gilbert J, Moscon B, Pedersen G, Burger R, Warren R P. Utilization of pulse oximetery for the study of the inhibitory effects of antiviral agents on influenza virus in mice. Antimicrob Agents Chemother. 1992;36:473–476. doi: 10.1128/aac.36.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sidwell R W, Huffman J H, Barnard D L, Bailey K W, Wong M-H, Morrison A, Syndergaard T, Kim C U. Inhibition of influenza virus infections in mice by GS4104, an orally effective influenza virus neuraminidase inhibitor. Antiviral Res. 1998;37:107–120. doi: 10.1016/s0166-3542(97)00065-x. [DOI] [PubMed] [Google Scholar]
- 21.Smee D F, Huffman J H, Morrison A C, Barnard D L, Sidwell R W. Cyclopentane neuraminidase inhibitors with potent in vitro anti-influenza virus activities. Antimicrob Agents Chemother. 2001;45:743–748. doi: 10.1128/AAC.45.3.743-748.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Treanor J J, Hayden F G, Vrooman P S, Barbarash R, Bettis R, Riff D, Singh S, Kinnersley N, Ward P, Mills R G. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. JAMA. 2000;283:1016–1024. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]