Abstract
Background
There is pre‐clinical evidence, involving several animal species, suggesting that opioid peptides play a role in the physiopathology of shock (endotoxic, hypovolemic, cardiogenic, spinal, anaphylactic). Many case reports have suggested that naloxone (an opiate antagonist) might be an effective treatment for shock in humans, but others have not supported such a point of view. This controversy led us to undertake a meta‐analysis of the available evidence on the efficacy of naloxone as a treatment measure for shock in humans.
Objectives
To evaluate the effectiveness and safety of naloxone in human shock and to estimate the methodological quality of the clinical trials.
Search methods
We searched the Cochrane Injuries Group Specialised Register, CENTRAL (The Cochrane Library), MEDLINE (Ovid SP), PubMed, EMBASE (Ovid SP), ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED), and ISI Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S) (to December 2008). In order to identify further studies the reference lists of all included papers were examined and the primary investigators of eligible studies were contacted.
Selection criteria
Randomized controlled trials evaluating naloxone in human shock, regardless of the patient's age (adult, child, or neonate).
Data collection and analysis
Three independent review authors extracted data on study design, intervention, outcomes, and methodological quality.
Main results
Three independent readers reviewed 120 publications and selected six clinical trials. Overall agreement on study selection was perfect (concordance: 100%). The meta‐analysis includes six studies involving 126 patients with septic, cardiogenic, hemorrhagic, or spinal shock.
Naloxone therapy was associated with statistically significant hemodynamic improvement (odds ratio 0.24; 95% confidence interval (CI) 0.09 to 0.68). The mean arterial pressure was significantly higher in the naloxone groups than in the placebo groups (weighted mean difference +9.33 mm Hg; 95% CI 7.07 to 11.59). No heterogeneity was found for this outcome. The death rate was lower in the naloxone group (odds ratio 0.59; 95% CI 0.21 was 1.67) but this was consistent with the play of chance. A significant heterogeneity was detected for the latter outcome (P < 0.05).
Authors' conclusions
Naloxone improves blood pressure, especially mean arterial blood pressure. However, the clinical usefulness of naloxone to treat shock remains to be determined and additional randomized controlled trials are needed to assess its usefulness.
Plain language summary
Naloxone may improve blood pressure in people who are in shock but more trials are needed to show whether this reduces deaths
When people go into shock, their blood pressure drops and may be too low to sustain life. One theory about the cause of this is the effect of the opiates that the body produces after major blood loss or trauma. Naloxone is a drug that counteracts the effects of opiates. It has been tried as a treatment to reduce the impact of shock. This review of trials found that giving naloxone to people in shock improves their blood pressure. It is not clear whether or not this improves their overall condition or reduces their chances of dying. More trials are needed.
Background
The discovery, in 1975, of endogenous opioid peptides (Hughes 1975) has generated abundant research on the potential functional role of opiate receptors and their peptide ligands. Holladay and Faden provided the first experimental evidence for opioid peptide involvement in the physiopathology of circulatory shock (Holaday 1978).
Subsequent research supported the therapeutic efficacy of opiate antagonists used in different experimental shock states (endotoxic, hypovolemic, cardiogenic, spinal, anaphylactic) in several animal species. Since then, many papers have been published on the use of an opiate antagonist (naloxone) in human shock. In most publications naloxone therapy was reported to be effective, but in eight reports it failed to show any kind of hemodynamic improvement (Allolio 1987; Bonnet 1985; Cabrera 1986; DeMaria 1985; Gerad 1983; Montastruc 1985; Rock 1985; Valdiviels 1984). This controversy, and the need to reconsider some of the less expensive technologies given that the immunotherapies have been so disappointing, prompted us to undertake this systematic review.
Objectives
This systematic review addressed the following questions.
What is the quality of the clinical trials dealing with this topic?
Is naloxone an effective therapy for shock in human patients?
Is there heterogeneity among the pooled studies?
How strong is the evidence?
Is naloxone a safe and useful therapy for shock in humans?
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Types of participants
Human participants only. Patients were considered to be in shock if they met the criteria as defined in each trial. No uniform definition of shock was used to redefine or reclassify the participants in each trial.
Types of interventions
Dose of naloxone of at least 0.01 mg/kg/dose in one or more bolus injections, or a continuous infusion of at least 0.01 mg/kg/hour for 60 minutes or longer.
Types of outcome measures
Change in death rate, reduction in doses of vasoactive drugs, mean arterial pressure, systolic blood pressure, and heart rate.
Search methods for identification of studies
Searches were not restricted by date, language, or publication status.
Electronic searches
We searched the following electronic databases:
Cochrane Injuries Group Specialised Register (searched 5 December 2008);
CENTRAL (The Cochrane Library 2008, Issue 4);
MEDLINE (Ovid SP) (1950 to week 3, November 2008);
Embase (Ovid SP) (1980 to November (week 49) 2008);
ISI Web of Science:Science Citation Index Expanded (SCI‐EXPANDED) (1970 to December 2008);
ISI Web of Science:Conference Proceedings Citation Index‐Science (CPCI‐S) (1990 to December 2008);
PubMed (searched 5 December 2008; added to PubMed in the last 180 days).
The search strategy can be found in Appendix 1.
Searching other resources
All search results were examined. These included reviews, overviews, editorials, monographs, symposia, book chapters, and clinical studies. We also searched the reference lists of relevant material. Primary investigators of eligible studies were contacted and asked whether they knew of any other study or systematic review on the same topic.
Data collection and analysis
Selection of studies
The literature was independently reviewed by three review authors (Catherine Ann Farrell, France Gauvin, Anne‐Marie Guerguerian) who selected studies according to the agreed inclusion criteria, defined a priori. Disagreement regarding inclusion was resolved by a consensus of at least two review authors. Agreement on the decision to include a study was assessed by the percentage of concordance and kappa score (Kramer 1981).
Data extraction and management
Data on the effectiveness of naloxone to treat shock were extracted from the included studies. First, they were displayed in two‐by‐two contingency tables. Two contingency tables were constructed. The outcome considered in the first was better blood pressure control; the second comprised data on death. We mailed these tables to each primary investigator and requested them to verify the data. Secondly, we extracted data on the three outcomes that were continuous (systolic blood pressure, mean arterial pressure, and heart rate). Following this, we made three new comparison tables.
Assessment of risk of bias in included studies
For all studies included in this review, the completeness of the information required to determine quality (see Chalmers 1981) was assessed independently by three review authors (Jacques Lacroix, Véronique Poirier, Chantal Roy). Disagreements were settled by consensus, which was defined as the agreement of at least two authors. The scoring system used to estimate the quality of the clinical trials is detailed elsewhere (Boeuf 1998). It included the following information: description of patient selection, number of patients assessed and rejected for eligibility, adequate description of therapeutic regimen given, allocation concealment, blinding of patients, blinding of physician to therapy, blinding of physician and patients to results, prior estimate of sample size, stopping rules, testing the adequacy of randomization, testing blinding, testing compliance, biological equivalence, endpoint duplicate variable, dates of starting and stopping accession, results pre‐randomization, statistical tests performed and results detailed, posterior power estimate of observed difference for negative trial, confidence interval calculated, life‐table or time‐series or repeated measures, timing of events, regression or correlation, appropriate statistical tests used, withdrawals, handling of withdrawals, adequate side effect discussion, proper retrospective analysis.
Data synthesis
For the two main outcomes, we combined data to estimate the odds ratio (OR) and its 95% confidence interval (95% CI) across the studies using a fixed‐effect model (Mantel 1959). The cumulative evidence of the pooled studies was evaluated by the method suggested by Collins and Langman (Collins 1987). A correction factor of 0.5 was attributed to zero cells (Roberts 1988). The heterogeneity of treatment effects across the studies was ascertained by a Chi2 analysis. For some outcomes, we combined continuous data to estimate a weighted mean reduction and its 95% CI across the studies using a fixed‐effect model. Following this, a Student t‐test was carried out to compare the mean differences.
Results
Description of studies
Study identification and selection
We found 120 papers (published to December 2008) dealing (at first glance) with naloxone and shock; 114 were excluded by adjudicators for the following reasons: a) animal studies (n = 39); b) case report, editorial, or review (n = 56); c) case series (n = 14) (Bone 1982; Bonnet 1985; Canady 1989; Duarte 1992; Gerad 1983; Groeger 1983; Hackshaw 1990; Hughes 1983; Martinon 1982; Peters 1981; Putterman 1986; Rock 1985; Tarelkina 1989; VelizPintos 1985); d) the cases or controls, or both, were not in shock (n = 5) (Allolio 1987; Desmonts 1978; Estilo 1982; Lightfoot 2000; Oldroyd 1995) (see Characteristics of excluded studies).
Six clinical trials were found that fulfilled the inclusion criteria. Overall agreement on selection of studies was perfect (concordance: 100%). The primary outcome was an increase in blood pressure in five reports (Hughes 1984; DeMaria 1985; Lu 1995; Montastruc 1985; Safani 1989). A decrease in the amount of vasopressor, inotrope, or both was used once (Roberts 1988), and death once (DeMaria 1985). The secondary outcomes considered were changes in blood catecholamine levels (Hughes 1984; Lu 1995) and blood lactic acid levels (Lu 1995). The definitions of shock used by the authors of the selected studies were not homogenous and did not follow the current American College of Chest Physicians (ACCP) definition (ACCP/SCCM 1992). Only one author (Roberts 1988) assessed the illness severity score at baseline: mean APACHE II score of 16.8 in the naloxone group and 18.9 in the control group.
Risk of bias in included studies
Overall agreement for the evaluation of the quality of each study was good (intraclass correlation coefficient 0.70). Scores were averaged between the three review authors. The maximum score of quality would be 104; the score was 61.1 for DeMaria 1985, 43 for Lu 1995, 60.2 for Roberts 1988, and 47.8 for Safani 1989. These are fair‐to‐good quality scores.
Effects of interventions
Death rate
Death rates ranged from 0% to 60% in the three naloxone groups, and from 45.4% to 66.6% in the three control groups. The pooled odds ratio (OR) comparing naloxone with the control groups was 0.59 (95% CI 0.21 to 1.67). A significant heterogeneity was found for this outcome (P < 0.05).
Reduction in dose of vasoactive drugs
In one study that included only 22 patients, the primary outcome measure was a reduction in dose of vasoactive drug (Roberts 1988). The OR was 0.12 (95% CI 0.12 to 1.29).
Mean arterial blood pressure
The mean arterial blood pressure changed from 87.5 ± 8.7 to 99.1 ± 9.1 mm Hg with naloxone, and from 84.3 ± 8.2 mm Hg to 93.8 ± 10.7 mm Hg with placebo in DeMaria 1985; from 64.1 ± 7.7 to 81.2 ± 9.8 mm Hg with naloxone, and from 77.2 ± 10.1 to 66.8 ± 6.3 mm Hg with placebo in Lu 1995; and from 61.3 ± 3 to 74 ± 4 mm Hg with naloxone, and from 62 ± 4 mm Hg to 65 ± 3 mm Hg with placebo in Safani 1989. Overall, the mean arterial blood pressure increased from 70.9 to 84.9 mm Hg with naloxone, and from 74.5 to 75.3 mm Hg with placebo. We were unable to obtain the standard deviations of these results at baseline, thus no statistical analysis was done on the difference between mean arterial blood pressure at baseline and after treatment. However, we were able to compare the mean arterial blood pressure after treatment in the two groups for these three studies: it was statistically higher in the naloxone than in the placebo group (weighted mean difference +9.33 mm Hg; 95% CI 7.07 to 11.59). No significant heterogeneity was found.
Systolic blood pressure
The systolic blood pressure increased from 87.5 ± 8.5 to 95 ± 10.8 mm Hg with naloxone, and from 89 ± 7.7 mm Hg to 99 ± 9.3 mm Hg with placebo in Hughes 1984; from 75.5 to 83.8 mm Hg with naloxone, and from 77.4 to 82.8 mm Hg with placebo in Montastruc 1985; and from 86 ± 4 to 102 ± 7 mm Hg with naloxone, and from 90 ± 4 mm Hg to 100 ± 6 mm Hg with placebo in Safani 1989. On average, the systolic blood pressure increased from 82.8 to 93.6 mm Hg with naloxone, and from 85.5 to 93.9 mm Hg with placebo. We were unable to obtain the standard deviations of these results at baseline, thus no statistical analysis was done on the difference between systolic blood pressure at baseline and after treatment. We compared the systolic blood pressure after treatment in these three studies: it was similar in the naloxone and placebo groups. No significant heterogeneity was found.
Heart rate
The heart rate changed from 109 ± 8 to 106 ± 7 beats/minute with naloxone, and from 102 ± 10 to 106 ± 15 beats/min with placebo in Hughes 1984; from 113 ± 32 to 110 ± 20 beats/min with naloxone, and from 108 ± 14 to 101 ± 19 beats/min with placebo in Lu 1995; from 80 to 75 beats/min with naloxone, and from 87 to 91 beats/min with placebo in Montastruc 1985; and from 114 ± 5 to 112 ± 4 beats/min with naloxone, and from 126 ± 4 to 115 ± 5 beats/min with placebo in Safani 1989. On average, the heart rate decreased from 103.9 to 100.7 beats/min with naloxone, and from 105.8 to 103.35 beats/min with placebo. We were unable to obtain the standard deviations of these results at baseline, thus no statistical analysis was done. We compared the heart rate after treatment in these three studies: it was similar in the naloxone and placebo groups. No significant heterogeneity was found.
Adverse effects
Adverse effects were monitored in two clinical trials (Roberts 1988; Safani 1989). No serious adverse effects were observed, although four of the 11 patients who received naloxone in Safani 1989 experienced a "mild to moderate degree of agitation" a few minutes after naloxone was given, which was not reported in the placebo group.
Discussion
Principal findings
This systematic review shows that naloxone can increase blood pressure in patients with shock; this was statistically significant (DeMaria 1985; Lu 1995; Safani 1989). Participants receiving continuous infusion of naloxone over a prolonged period appeared to have a lower death rate but this was not statistically significant (DeMaria 1985; Roberts 1988; Safani 1989).
Strengths and weaknesses of the review
The part of this systematic review considering survival as the outcome measure includes a limited number of patients, 59 patients from three clinical trials (DeMaria 1985; Roberts 1988; Safani 1989). The positive trend that we found could easily be reversed if there has been publication bias. This also holds true for the mean arterial pressure outcome (66 patients from three clinical trials) (DeMaria 1985; Lu 1995; Safani 1989).
Strengths and weaknesses in relation to other studies
The literature on the effectiveness of naloxone in treating shock in humans is abundant but controversial, and the methodological quality of the publications is frequently weak. We found 80 publications where 27 were letters or narrative reviews, 28 were case reports, 14 were cases series, five were non‐controlled clinical trials, and only six were double‐blind randomized studies. Many papers reported positive results. However, almost all the positive papers were case reports (28/28) (Accettelli 1982; Bagrov 1993; Campos 1986; Cattani 1982; Christensen 1986; Cocchi 1984; Cohen 1983; De Groot 1983; Dirksen 1980; Duarte 1992; Gaudette 1986; Gullo 1983; Furman 1984; Higgins 1981; Higgins 1983; Jolivet 1984; Lenz 1981; Parisot 1986; Pourriat 1981; Safani 1985; Siram 1984; Siram 1984; Swinburn 1982; Tiengo 1980; Unzueta 1987; Wright 1980; Xing 1990; Yeston 1983). Among 14 case series, one was negative (Rock 1985) and 13 were positive (Bone 1982; Bonnet 1985; Canady 1989; Duarte 1992; Gerad 1983; Groeger 1983; Hackshaw 1990; Hughes 1983; Martinon 1982; Peters 1981; Putterman 1986; Tarelkina 1989; VelizPintos 1985). Among the six double‐blind studies, four reported positive results (Hughes 1984; Lu 1995; Roberts 1988; Safani 1989) while results were not statistically significant in two (Montastruc 1985; DeMaria 1985). Clearly a systematic review was necessary to determine whether naloxone should be considered as a treatment for shock.
No serious adverse events or complications were reported in the six randomized clinical trials included in this systematic review. Most case reports did not prospectively monitor adverse events. In spite of this, there are data suggesting that the use of naloxone is not without risks. Naloxone caused anxiety in septic patients who received 0.3 mg/kg of naloxone (Groeger 1983). It can also be expected that giving naloxone could block the analgesic effect of endogenous and exogenous opioids. Adverse drug reactions such as hypotension, pulmonary edema, and grand mal seizures have been reported with a high‐dose regimen of naloxone (Prough 1984; Rock 1985). In healthy volunteers, naloxone decreases tolerance to hypotensive, hypovolemic stress (Lightfoot 2000). A deep level of anesthesia and postoperative analgesia attenuates the physiologic responses to stress (ACCP/SCCM 1992). Naloxone modulates the inflammatory process; blocking these effects could be a double‐edged sword. For example, naloxone may increase the risk of acquiring or worsening multiple organ dysfunction syndrome, or contracting nosocomial infections.
Meaning of the review
All types of shock states are associated with an over‐production of endorphins, and naloxone might improve shock by its antagonist activity on beta‐endorphins. The source of the beta‐endorphins involved in shock is a matter of debate. It was thought for a while that beta‐endorphins are released into the blood circulation exclusively by the central nervous system, but it was later shown that they are also released locally into inflamed tissue by lymphocytes and macrophages (Jessop 1998). Endorphins mediate the release of nitric oxide, which might cause systemic and local vasodilation. Endorphins can also depress heart function. The exact mechanism involved is unknown, but endorphins enhance the release of cytokines which are cardiac depressants, like interleukin‐1.
The data reported in human beings are supported by experimental data. In 1978, a study (Holaday 1978) reported that naloxone rapidly reversed the hemodynamic effects of endotoxic shock in rats. The improvement in hemodynamic status was dose‐related, with a minimal dose of 0.1 mg/kg. Several months later the same authors (Faden 1979) obtained the same results with a single dose of 1 mg/kg in a model of hemorrhagic shock (50% blood volume loss) in rats. Many subsequent experimental studies confirmed the therapeutic efficacy of naloxone in various animal species.
Authors' conclusions
Implications for practice.
The results of this systematic review suggest that naloxone may be an effective treatment of shock. However, the power of these results is weak because the number of patients is small. Therefore, the clinical inferences that can be drawn from this review are limited. Naloxone is not approved by the Food and Drugs Administration (USA) to treat shock. It might be beneficial in some patients but the available evidence does not support the use of naloxone as a standard treatment of shock.
Implications for research.
More clinical trials with positive results are needed before one can recommend naloxone as a standard treatment of shock. In these future trials the population selected should only include patients in shock. The patients' baseline status should be assessed by the Acute Physiology and Chronic Health Evaluation (APACHE) score, or the Pediatric Risk of Mortality (PRISM) score (Pollack 1996). Naloxone should be administered early, for a prolonged period, and with a continuous infusion of at least 0.1 mg/kg/h. The outcome under scrutiny should be death. Finally, clinical research concerning naloxone should also determine the dose‐effect curve and the optimal frequency of administration. A study of at least 340 patients in each group is needed to demonstrate a significant difference in mortality for a power of 80%.
In future studies, it would be useful to monitor surrogate outcomes (like blood pressure and heart rate) and secondary outcomes (such as the rate of nosocomial infections or the Multiple Organ Dysfunction score) in the experimental and control groups (Leteurtre 2003; Marshall 1995). Adverse effects, such as anxiety and pain, must be carefully recorded. Adverse drug reactions such as hypotension, pulmonary edema, and grand mal seizures must be reported. Moreover, a cost‐benefit analysis should be performed to determine whether the benefits of naloxone outweigh its complications and whether naloxone should be routinely used to treat shock in humans.
What's new
| Date | Event | Description |
|---|---|---|
| 20 April 2009 | New search has been performed | The search was updated to 5 December 2008. No new trials were identified. The conclusions remain the same. |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 11 September 2008 | Amended | Converted to new review format. |
Acknowledgements
Dr Chenchen Wang translated one paper from Chinese to English.
Appendices
Appendix 1. Search strategy
Cochrane Injuries Group Specialised Register (searched 5 December 2008) (intensive or critical or emergency or shock* or sepsis) and (naloxon* or narcan* or maloxone or nalone* or narcon or narvcam) CENTRAL (The Cochrane Library 2008, Issue 4) #1MeSH descriptor Shock explode all trees #2MeSH descriptor Shock, Cardiogenic explode all trees #3MeSH descriptor Shock, Hemorrhagic explode all trees #4MeSH descriptor Shock, Septic explode all trees #5MeSH descriptor Shock, Traumatic explode all trees #6MeSH descriptor Emergency Medicine explode all trees #7MeSH descriptor Emergency Treatment explode all trees #8MeSH descriptor Intensive Care explode all trees #9MeSH descriptor Critical Illness explode all trees #10MeSH descriptor Electric Injuries explode all trees #11(intensive or critical or emergency or shock* or sepsis):ab,ti #12(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11) #13MeSH descriptor Naloxone explode all trees #14(naloxon* or narcan* or maloxone or nalone* or narcon or narvcam) #15(#13 OR #14) #16(#12 AND #15) MEDLINE (Ovid SP) (1950 to week 3 November 2008) 1.exp SHOCK/ 2.exp Cardiogenic Shock/ 3.exp Hemorrhagic Shock/ 4.exp Septic Shock/ 5.exp Traumatic Shock/ 6.exp Emergency Medicine/ 7.exp Emergency Treatment/ 8.exp Intensive Care/ 9.exp Critical Illness/ 10.(intensive or critical or emergency or shock* or sepsis).ab,ti. 11.exp Electric Injuries/ 12.or/1‐11 13.exp Naloxone/ 14.(naloxon* or narcan* or maloxone or nalone* or narcon or narvcam).ab,ti. 15.13 or 14 16.12 and 15 17.randomi?ed.ab,ti. 18.randomized controlled trial.pt. 19.controlled clinical trial.pt. 20.placebo.ab. 21.clinical trials as topic.sh. 22.randomly.ab. 23.trial.ti. 24.or/17‐23 25.exp animals/ 26.exp humans/ 27.25 not (25 and 26) 28.24 not 27 29.28 and 16 EMBASE (Ovid SP) (1980 to November (week 49) 2008) 1.exp SHOCK/ 2.exp Cardiogenic Shock/ 3.exp Hemorrhagic Shock/ 4.exp Septic Shock/ 5.exp Traumatic Shock/ 6.exp Burn Shock/ 7.exp ELECTRIC SHOCK/ 8.(intensive or critical or emergency or shock* or sepsis).ab,ti. 9.exp Emergency Medicine/ 10.exp Emergency Treatment/ 11.exp Intensive Care/ 12.exp Critical Illness/ 13.or/1‐12 14.(naloxon* or narcan* or maloxone or nalone* or narcon or narvcam).ab,ti. 15.exp NALOXONE/ 16.exp NALOXONE BENZOYLHYDRAZONE/ 17.14 or 15 or 16 18.13 and 17 19.exp Randomized Controlled Trial/ 20.exp controlled clinical trial/ 21.randomi?ed.ab. 22.placebo.ab. 23.exp Clinical Trial/ 24.randomly.ab. 25.trial.ti. 26.19 or 20 or 21 or 22 or 23 or 24 or 25 27.exp animal/ not (exp human/ and exp animal/) 28.26 not 27 29.28 and 18 ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (1970 to December 2008) ISI Web of Science: Conference Proceedings Citation Index‐ Science (CPCI‐S) (1990 to December 2008) #1Topic=(intensive or critical or emergency or shock* or sepsis) AND Topic=(naloxon* or narcan* or maloxone or nalone* or narcon or narvcam) #2Topic=(random OR placebo OR randomised OR randomized OR randomly OR random order OR random sequence OR random allocation OR randomly allocated OR at random) AND Title=(trial* or group* or study or studies or placebo or controlled) #3Title=(random OR placebo OR randomised OR randomized OR randomly OR random order OR random sequence OR random allocation OR randomly allocated OR at random) AND Topic=(trial* or group* or study or studies or placebo or controlled) #4#1 and #2 #5#1 and #3 #6#4 or #5 PubMed (searched 5 December 2008; added to PubMed in the last 180 days) Search (intensive or critical or emergency or shock* or sepsis) AND (naloxon* or narcan* or maloxone or nalone* or narcon or narvcam)
Data and analyses
Comparison 1. Naloxone versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death rate | 3 | 59 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.21, 1.67] |
| 2 Reduction in dose of vasoactive drug | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 1.29] |
1.1. Analysis.
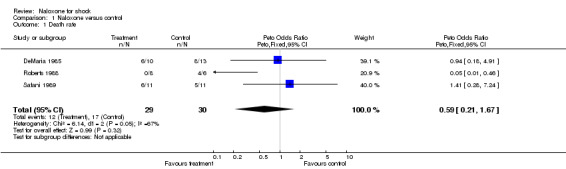
Comparison 1 Naloxone versus control, Outcome 1 Death rate.
1.2. Analysis.
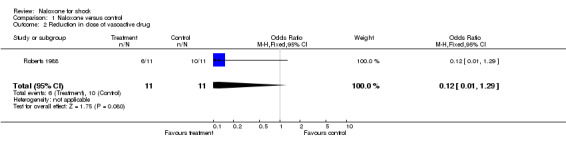
Comparison 1 Naloxone versus control, Outcome 2 Reduction in dose of vasoactive drug.
Comparison 2. After treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean Arterial Blood Pressure (MAP) | 3 | 66 | Mean Difference (IV, Fixed, 95% CI) | 9.33 [7.07, 11.59] |
| 2 Systolic Blood Pressure (SBP) | 2 | 36 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [‐4.10, 5.58] |
| 3 Heart Rate (HR) | 3 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐2.23 [‐5.77, 1.30] |
2.1. Analysis.
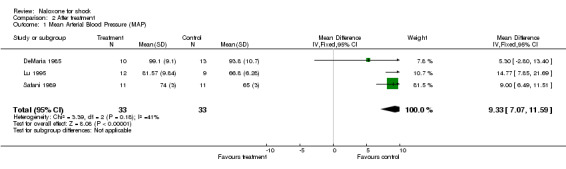
Comparison 2 After treatment, Outcome 1 Mean Arterial Blood Pressure (MAP).
2.2. Analysis.
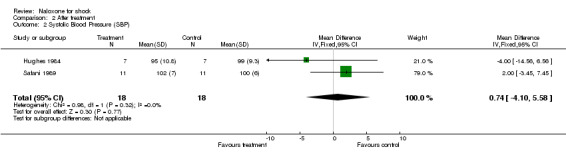
Comparison 2 After treatment, Outcome 2 Systolic Blood Pressure (SBP).
2.3. Analysis.
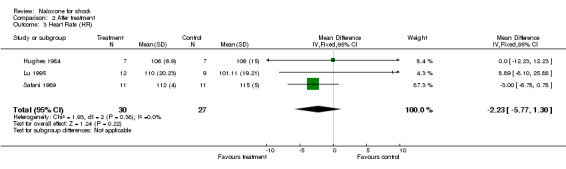
Comparison 2 After treatment, Outcome 3 Heart Rate (HR).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
DeMaria 1985.
| Methods | A placebo‐controlled, randomized, double‐blind trial of naloxone in patients with septic shock to estimate clinical efficacy. | |
| Participants | Criteria for inclusion (28 patients, and 38 episodes of shock):
The patient's general medical care was not changed by participation. Excluded (6 patients, and 15 episodes of shock):
In several patients substantial changes in doses of vasopressor agents or rates of intravenous infusion were made during the study period which rendered analysis of blood pressure changes impossible. Such patients were excluded from the study before the treatment code was broken. |
|
| Interventions | Naloxone group (n=10): bolus of 0.4 mg of naloxone every 5 min x 3 doses. Control group (n=13): bolus of sterile vehicle for injection every 5 min x 3 doses. |
|
| Outcomes | Primary outcomes
|
|
| Notes | Medical and surgical intensive care units at Boston City Hospital from March 1982 to March 1983. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hughes 1984.
| Methods | A placebo‐controlled, randomized, double‐blind trial on naloxone in patients with septic shock to evaluate its clinical efficacy. | |
| Participants | Criteria for inclusion: clinical evidence of septic shock with:
Conventional therapy of septic shock for every patient: consisting of antibiotics, intravenous fluids to maintain PWP of 12 mmHg and CVP of 11 mmHg, dopamine, and ventilatory assistance. |
|
| Interventions | Naloxone group (n=7): bolus dose of naloxone, 30 μg/kg followed by 30 μg/kg/hr for one hour plus a single dose of methyl‐ prednisolone, 30 mg/kg. Control group: conventional therapy alone (n=7). |
|
| Outcomes |
All outcomes were checked for 60 minutes. |
|
| Notes | Section of General Medicine, East Carolina University School of Medicine in Greenville, North Carolina. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Lu 1995.
| Methods | A controlled randomized trial of naloxone on hemorrhagic shock. | |
| Participants | 21 patients with moderate shock included:
No hypertension and no coronary heart disease in both groups. |
|
| Interventions | Naloxone group (n=12): 200 ml of normal saline infused in 15 min and bolus of 0.02 mg/kg of naloxone. Control group (n=9): 200 ml of normal saline infused in 15 min. |
|
| Outcomes |
All outcomes were measured during 15 minutes. |
|
| Notes | Department of Anesthesiology at first affiliated hospital of China Medical University in Senyang (China). No comment of when the study was conducted. Case fatality unspecified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Montastruc 1985.
| Methods | A placebo‐controlled, randomized, double‐blind trial of single dose of naloxone in patients with hypovolemic, cardiogenic, septic, or spinal shock to evaluate the hemodynamic effects. | |
| Participants | 30 patients (16 men and 14 women from 8 to 87 years; mean age: 51 +/‐ 4.9 years) suffering from hypovolemic (17 cases), cardiogenic (9 cases), septic (3 cases) and spinal shock (1 case) with SBP < 85 mmHg. Usual treatment of shock for every patient: blood products and plasma substitutes in hypovolemic, septic, and spinal shock; and catecholamines (dopamine and/or dobutamine) in cardiogenic shock. | |
| Interventions | Naloxone group (n=15): bolus of 0.8 mg of naloxone in 2 ml. Control group (n=15): bolus of an equivalent volume of normal saline. | |
| Outcomes | Primary outcome
|
|
| Notes | Department of Anesthesiology and Intensive Care Medicine at Purpan Hospital in Toulouse (France). No comment on when the study was conducted. Case fatality unspecified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Roberts 1988.
| Methods | A controlled, randomized, double‐blind trial of a longer continuous intravenous infusion of naloxone in patients with septic shock to evaluate the hemodynamic effects. | |
| Participants | Criteria for inclusion (n=16):
Exclusion criteria:
|
|
| Interventions | Bolus of 30 μg/kg of naloxone followed by continuous infusion of 30 μg/kg/hr for 8 to 16 hrs. Control: bolus of an equivalent volume of normal saline. |
|
| Outcomes | Primary outcome
|
|
| Notes | Medical and surgical intensive care units at the Health Sciences Center in Winnipeg, Manitoba. No comment on when the study was conducted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Safani 1989.
| Methods | A controlled, randomized trial of naloxone infusion in early hyperdynamic septic shock. | |
| Participants | Criteria for inclusion (n=22):
Excluded:
|
|
| Interventions | Naloxone group: bolus of 30 μg/kg of naloxone over 3 to 5 min followed by continuous infusion of 60 μg/kg/hr for 1 h. If hemodynamic improvement was observed within the first hour, the infusion was continued at the same rate for an additional 4 to 24 h. Control group: infusion of dextrose 5% in water. If deterioration of hemodynamic status was observed, the infusion of naloxone or dextrose 5% in water was resumed immediately. |
|
| Outcomes | Primary outcome Increase in MAP >15%, as recorded up to 24 hours after the end of the perfusion of naloxone or dextrose 5%. | |
| Notes | Division of Respiratory and Critical Care Medicine at Memorial Medical Center of Long Beach, CA. No comment on when the study was conducted. Case fatality unspecified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
BP: blood pressure MAP: mean arterial pressure SBP: systolic blood pressure PWP: pulmonary wedge pressure CI: cardiac index SVR: systemic vascular resistance CVP: central venous pressure HR: heart rate
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allolio 1986 | Not a randomised controlled trial. |
| Allolio 1987 | Ten cases, 10 controls. Inappropriate control group (patients were not in shock). No randomization. |
| Bone 1982 | No control (case series of 10 patients). |
| Bonnet 1985 | No control (case series of 7 patients). |
| Canady 1989 | No control (case series of 5 patients). |
| Desmonts 1978 | Fourteen cases, 11 controls. Cases and control were not in shock. No randomization. |
| Duarte 1992 | No control (case series of 5 patients). |
| Estilo 1982 | Six cases, 6 controls. Cases and controls were not in shock. No randomization. |
| Gerad 1983 | No control (case series of 5 patients). |
| Groeger 1983 | No control (case series of 10 patients). |
| Hackshaw 1990 | No control (case series of 13 patients). |
| Hughes 1983 | No control (case series of 8 patients). |
| ILCOR 2006 | Not a randomized controlled trial. |
| Lightfoot 2000 | Repeated measures design in 8 healthy male subjects. No randomization. |
| Martinon 1982 | No control (case series of 6 patients). |
| Oldroyd 1995 | Ten patients with heart failure were randomized to study the effects of naloxone or placebo on cardiopulmonary exercise performance (outcome measure not considered in this systematic review). |
| Peters 1981 | No control (case series of 13 patients). |
| Putterman 1986 | No control (case series of 10 patients). |
| Rock 1985 | No control (case series of 12 patients). |
| Tarelkina 1989 | No control (case series of 12 patients). |
| VelizPintos 1985 | No control (case series of 15 patients). |
Contributions of authors
Jacques Lacroix wrote the prococol of the review and wrote to authors of randomized clinical trials to check whether the data had been correctly extracted. Catherine Ann Farrell, France Gauvin and Anne‐Marie Guerguerian selected the studies. Jacques Lacroix, Véronique Poirier, and Chantal Roy extracted the data of the retained studies and assessed their quality. Jacques Lacroix, Benoit Boeuf, and Véronique Poirier did the meta‐analysis and wrote the final report of the systematic review. Chantal Roy helped to organize the systematic review and to edit the paper in RevMan.
Sources of support
Internal sources
No sources of support supplied
External sources
Canadian Institutes of Health Research, Canada.
Agence d'Évaluation des technologies et des modes d'intervention en santé, Canada.
Declarations of interest
None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
DeMaria 1985 {published data only}
- Maria A, Craven DE, Heffernan JJ, McIntosh TK, Grindlinger GA, McCabe WR. Naloxone versus placebo in treatment of septic shock. Lancet 1985;1:1363‐5. [DOI] [PubMed] [Google Scholar]
Hughes 1984 {published data only}
- Hughes GS. Naloxone and methylprednisalone sodium succinate enhance sympathomedullary in patients with septic shock. Life Sciences 1984;35:2319‐26. [DOI] [PubMed] [Google Scholar]
Lu 1995 {published data only}
- Lu H, Xu G, Sheng Z. Clinical effects of naloxone on hemorrhagic shock. Chung Hua Wai Ko Tsa Chih 1995;33(6):355‐8. [PubMed] [Google Scholar]
Montastruc 1985 {published data only}
- Montastruc JL, Richard J, Cathala B. Naloxone fails to reverse blood pressure in shock: A double blind study in man. Journal de Pharmacologie 1985;16(2):313‐4. [PubMed] [Google Scholar]
Roberts 1988 {published data only}
- Roberts DE, Dobson KE, Hall KW, Light RB. Effects of prolonged naloxone infusion in septic shock. Lancet 1988;2:699‐702. [DOI] [PubMed] [Google Scholar]
Safani 1989 {published data only}
- Safani M, Blair J, Ross D, Waki R, Li C, Libby G. Prospective, controlled, randomized trial of naloxone infusion in early hyperdynamic septic shock. Critical Care Medicine 1989;17:1004‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allolio 1986 {published data only}
- Allolio B, Fischer H, Kalen D, Deub U, Winkelmann W. Naloxon by therapy refractory shock [Naloxon im therapierefraktären Schock]. Klinische Wochenschrift 1986;64:16. [Google Scholar]
Allolio 1987 {published data only}
- Allolio B, Fischer H, Kaulen U, Deus U, Winkelmann W. Naloxone in treatment of circulatory shock resistant to conventional therapy. Klinische Wochenschrift 1987;65:213‐17. [DOI] [PubMed] [Google Scholar]
Bone 1982 {published data only}
- Bone R, Jacobs E, Wilson F, Hiller F. Naloxone reversal of hypotension: A clinical study. American Review of Respiratory Disease 1982;125:93. [Google Scholar]
Bonnet 1985 {published data only}
- Bonnet F, Bilaine J, Lhoste F, Mankijian B, Kerdelhue B, Rapin M. Naloxone therapy of human septic shock. Critical Care Medicine 1985;13:972‐5. [DOI] [PubMed] [Google Scholar]
Canady 1989 {published data only}
- Canady J, Williams W, Thompson I, Vincent GS, Hoover E. Use of the naloxone in septic shock. Journal of the National Medical Association 1989;81:669‐73. [PMC free article] [PubMed] [Google Scholar]
Desmonts 1978 {published data only}
- Desmonts JM, Bohm G, Couderc E. Hemodynamic responses to low doses of naloxone after narcotic‐nitrous oxide anesthesia. Anesthesiology 1978;49:12‐6. [DOI] [PubMed] [Google Scholar]
Duarte 1992 {published data only}
- Duarte RR, Mumtaz M, Youssef N, Nayak P, Grace BW. Effects of naloxone infusion in patients with septic shock and renal failure: A limited experience. American Journal of Nephrology 1992;12:431‐6. [DOI] [PubMed] [Google Scholar]
Estilo 1982 {published data only}
- Estilo AE, Cottrell JE. Hemodynamic and catecholamine changes after administration of naloxone. Anesthesia and Analgesia 1982;61:349‐53. [PubMed] [Google Scholar]
Gerad 1983 {published data only}
- Gerad H, Schimpff SC, Kaplan R. Naloxone for refractory hypotension in septic shock. Clinical Research 1983;31:257A. [Google Scholar]
Groeger 1983 {published data only}
- Groeger JS, Carlon GC, Howland WS. Naloxone in septic shock. Critical Care Medicine 1983;11:650‐4. [DOI] [PubMed] [Google Scholar]
Hackshaw 1990 {published data only}
- Hackshaw KV, Parker GA, Roberts JW. Naloxone in septic shock. Critical Care Medicine 1990;18:47‐51. [DOI] [PubMed] [Google Scholar]
Hughes 1983 {published data only}
- Hughes GS, Porter RS, Mars R, Harker CC. Naloxone and septic shock. Annals of Internal Medicine 1983;98:559. [DOI] [PubMed] [Google Scholar]
ILCOR 2006 {published data only}
- The International Liaison Committee on Resuscitation. The International Liaison Committee on Resuscitation (ILCOR) Consensus on Science With Treatment Recommendations for Pediatric and Neonatal Patients: Pediatric Basic and Advanced Life Support. Pediatrics 2006;117(5):e955‐977. [DOI: 10.1542/peds.2006-0206] [DOI] [PubMed] [Google Scholar]
Lightfoot 2000 {published data only}
- Lightfoot JT, Katz L, DeBate K. Naloxone decreases tolerance to hypotensive, hypovolemic stress in healthy humans. Critical Care Medicine 2000;28:684‐91. [DOI] [PubMed] [Google Scholar]
Martinon 1982 {published data only}
- Martinon JM, Franco A, Garcia JL, Fuster M, Bao A, Martinon F, Pena J. Naloxone hydrochloride in the therapy of endotoxic shock following meningococcal disease. Initial results. Anales Espanoles de Pediatria 1982;17:457‐60. [PubMed] [Google Scholar]
Oldroyd 1995 {published data only}
- Oldroyd KG, Gray CE, Carter R, Harvey K, Borland W, Beastall G, Cobbe SM. Activation and inhibition of the endogenous opioid system in human heart failure. British Heart Journal 1995;73:41‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peters 1981 {published data only}
- Peters WP, Johnson MW, Friedman PA, Mitch WE. Pressor effect of naloxone in septic shock. Lancet 1981;1:529‐32. [DOI] [PubMed] [Google Scholar]
Putterman 1986 {published data only}
- Putterman C, Halperin P, Leykin Y, Sorkine P, Geller B, Bursztein S. Early use of naloxone in shock ‐ A clinical trial. Resuscitation 1986;13:185‐90. [DOI] [PubMed] [Google Scholar]
Rock 1985 {published data only}
- Rock P, Silverman H, Pump D, Kecala Z, Smith P, Michael JR, Summer W. Efficacy and safety of naloxone in septic shock. Critical Care Medicine 1985;13:28‐33. [DOI] [PubMed] [Google Scholar]
Tarelkina 1989 {published data only}
- Tarelkina MN, Shirokov, Tsibin IN. Experience with using narcanti (naloxone) in patients with traumatic shock. Vestnik Khirurgii Imou I I Grekova 1989;143:110‐2. [PubMed] [Google Scholar]
VelizPintos 1985 {published data only}
- Veliz Pintos RA, Torres Bedoya MA, Forero Gomez J, Olvera Hidalgo C, Orozco Veraztegui O. Clinical evaluation of the effect of the administration of naloxone in comparison with methylprednisolone in infants with septic shock. Boletin Medico Del Hospital Infantil de Mexico 1985;42:531‐40. [PubMed] [Google Scholar]
Additional references
Accettelli 1982
- Accettelli U, Ambrosi F, Russo L, Petris U, Stagnitti F. Use of naloxone in first aid. Clinica Terapeutica 1982;102:197‐201. [PubMed] [Google Scholar]
ACCP/SCCM 1992
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Critical Care Medicine 1992;20:864‐74. [PubMed] [Google Scholar]
Bagrov 1993
- Bagrov AY. Plasma beta‐endorphin levels in acute myocardial infarction. Annals of Emergency Medicine 1993;22:268‐9. [DOI] [PubMed] [Google Scholar]
Cabrera 1986
- Cabrera Sorrosal JE, Montejo Gonzalez JC, Suarez Alvarez JR, Blesa Alpica A, Ferrero Zorita J. Failure of naloxone in septic shock. Critical Care Medicine 1986;14:79‐80. [DOI] [PubMed] [Google Scholar]
Campos 1986
- Campos JM, Zueras R, Escolano F, Villar Landeira JM. Use of naloxone in the therapy of septic shock. Revista Espanola de Anestesiologia 1986;33:451‐3. [PubMed] [Google Scholar]
Cattani 1982
- Cattani F, Grillanda G. Use of naloxone in refractory shock. Recenti Progressi in Medicina 1982;72:647‐52. [PubMed] [Google Scholar]
Chalmers 1981
- Chalmers TC, Smith H, Blackburn B, Silverman B, Schroeder B, Reitman D, Ambroz A. A method for assessing the quality of a randomized control trial. Controlled Clinical Trials 1981;2:31‐49. [DOI] [PubMed] [Google Scholar]
Christensen 1986
- Christensen MA, Gresch JJ. Use of naloxone in septic shock: A case report and review of current literature. Journal of Burn Care and Rehabilitation 1986;7:122‐6. [DOI] [PubMed] [Google Scholar]
Cocchi 1984
- Cocchi P, Silenzi M, Calabri G, Salvi G. Naloxone in fulminant meningococcemia. Pediatric Infectious Diseases 1984;3:187. [DOI] [PubMed] [Google Scholar]
Cohen 1983
- Cohen KR, Emmons KM, Goldstein MF. Naloxone treatment of toxic shock syndrome. Archives of Internal Medicine 1983;143:1072. [PubMed] [Google Scholar]
Collins 1987
- Collins R, Gray R, Godwin J, Peto R. Avoidance of large biases and large random errors in the assessment of moderate treatment effects: The need for systematic overviews. Statistics in Medicine 1987;6:245‐50. [DOI] [PubMed] [Google Scholar]
De Groot 1983
- Groot GH, Schalm SW, Dirksen R, Valk KC, Boks AL. Effect of naloxone on shock in a patient with fulminant hepatic failure. Netherlands Journal of Medicine 1983;26:77‐9. [PubMed] [Google Scholar]
Dirksen 1980
- Dirksen R, Otten M H, Wood GJ, Verbaan CJ, Haalebos MM, Verdouw PV, Nijhuis GMM. Naloxone in shock. Lancet 1980;1:1360‐1. [Google Scholar]
Einarson 1985
- Einarson TR, McGhan WF, Bootman JL, Sabers DL. Meta‐analysis: Quantitative integration of independent research results. American Journal of Hospital Pharmacy 1985;42:1957‐64. [PubMed] [Google Scholar]
Faden 1979
- Faden AI, Holaday JW. Opioate antagonists: A role in the treatment of hypovolemic shock. Science 1979;205:317‐8. [DOI] [PubMed] [Google Scholar]
Fleiss 1986
- Fleiss JL. Reliability of measurement. In: Fleiss JL editor(s). The Design and Analysis of Clinical Experiments. New York: John Wiley & Sons, 1986:17‐31. [Google Scholar]
Furman 1984
- Furman WL, Menke JA, Barson WJ, Miller RR. Continuous naloxone infusion in two neonates with septic shock. Journal of Pediatrics 1984;105:649‐51. [DOI] [PubMed] [Google Scholar]
Gaudette 1986
- Gaudette RR, Browne BJ. Use of naloxone in septic shock. Journal of Emergency Nursing 1986;12:81‐4. [PubMed] [Google Scholar]
Gullo 1983
- Gullo A, Romano E. Naloxone and anaphylactic shock. Lancet 1983;1:819. [DOI] [PubMed] [Google Scholar]
Higgins 1981
- Higgins TL, Sivak ED. Reversal of hypotension with naloxone. Cleveland Clinic Quarterly 1981;48:283‐8. [DOI] [PubMed] [Google Scholar]
Higgins 1983
- Higgins TL, Sivak ED, O'Neil DM, Grave JW, Foutch DG. Reversal of hypotension by continuous naloxone infusion in a ventilator dependent patient. Annals of Internal Medicine 1983;98:47‐8. [DOI] [PubMed] [Google Scholar]
Holaday 1978
- Holiday JW, Faden AI. Naloxone reversal of endotoxin hypotension suggests role of endorphins in shock. Nature 1978;275:450‐1. [DOI] [PubMed] [Google Scholar]
Hughes 1975
- Hughes J. Isolation of an endogenous compound from the brain with pharmacological properties similar to morphine. Brain Research 1975;88:295‐308. [DOI] [PubMed] [Google Scholar]
Jessop 1998
- Jessop DS. ß‐endorphin in the immune system ‐ mediator of pain and stress. Lancet 1998;351:1828‐9. [DOI] [PubMed] [Google Scholar]
Jolivet 1984
- Jolivet M, Laurion M. Shock and the administration of naloxone [Le choc et l'administration de naloxone]. Union Medicale Du Canada 1984;113:783‐5. [PubMed] [Google Scholar]
Kramer 1981
- Kramer MS, Feinstein AR. Clinical biostatistic LIV : The biostatistics of concordance. Clinical Pharmacology and Therapeutics 1981;29:111‐23. [DOI] [PubMed] [Google Scholar]
Lefebvre 2000
- Lefebvre C, Tasiemski A, Salzet M. Opioid peptides, opiated substances and immune response [Peptides opioïdes, substances opiacées et réponse immunitaire]. Médecine/Sciences 2000;16:235‐42. [Google Scholar]
Lenz 1981
- Lenz K, Druml W, Gassner A, Hruby K, Kleinberger G, Laggner A. Naloxone in shock. Lancet 1981;1:834. [DOI] [PubMed] [Google Scholar]
Leteurtre 2003
- Leteurtre S, Martinot A, Duhamel A, Proulx F, Grandbastien B, Cotting J, et al. Validation of the pediatric logistic organ dysfunction (PELOD) score. A prospective multicenter study. Lancet 2003:in press. [DOI] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of diseases. Journal of the National Cancer Institute 1959;22:719‐48. [PubMed] [Google Scholar]
Marshall 1995
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple Organ Dysfunction Score: A reliable descriptor of a complex clinical outcome. Critical Care Medicine 1995;23:1638‐52. [DOI] [PubMed] [Google Scholar]
Parisot 1986
- Parisot S, Pinaud P, Marchal C. Early septic shock and the use of naloxone in premature newborn infants [Choc septique précoce et emploi de la naloxone chez le nouveau‐né prématuré]. Archives Francaises de Pediatrie 1986;43:149. [PubMed] [Google Scholar]
Pollack 1996
- Pollack MM, Patel KM, Ruttimann UE. PRISM III: An updated Pediatric Risk of Mortility score. Critical Care Medicine 1996;24:743‐52. [DOI] [PubMed] [Google Scholar]
Pourriat 1981
- Pourriat JL, Pierrot M, Lapandry M, Cupa M. Naloxone in septic shock [La naloxone dans le choc septique]. Nouvelle Presse Medicale 1981;10:3793‐4. [Google Scholar]
Prough 1984
- Prough DS, Roy R, Bumgarner J, Shannon G. Acute pulmonary edema in healthy teenagers following conservative doses of intravenous naloxone. Anesthesiology 1984;600:485‐6. [DOI] [PubMed] [Google Scholar]
Putterman 1987
- Putterman C, Halpern P. Naloxone in circulatory shock. Klinische Wochenschrift 1987;65:926. [DOI] [PubMed] [Google Scholar]
Safani 1985
- Safani M, Libby G. Naloxone therapy of septic shock. Critical Care Medicine 1985;13:875‐6. [DOI] [PubMed] [Google Scholar]
Siram 1984
- Siram S, Park SY. Naloxone in septic shock: Report of two cases. Journal of the National Medical Association 1984;76:713‐6. [PMC free article] [PubMed] [Google Scholar]
Swinburn 1982
- Swinburn WR, Phelan P. Response to naloxone in septic shock. Lancet 1982;1:167. [DOI] [PubMed] [Google Scholar]
Tarelkina 1989
- Tarelkina MN, Shirokov, Tsibin IN. Experience with using narcanti (naloxone) in patients with traumatic shock. Vestnik Khirurgii Imeni I I Grekova 1989;143:110‐2. [PubMed] [Google Scholar]
Tiengo 1980
- Tiengo M. Naloxone in irreversible shock. Lancet 1980;2:690. [DOI] [PubMed] [Google Scholar]
Unzueta 1987
- Unzueta Merino MC, Bonnin O, Cabrera Ruiz JC, Villar Landeira JM. Naloxone and cardiogenic shock. Revista Espanola de Anestesiologia Y Reanimacion 1987;34:446‐9. [PubMed] [Google Scholar]
Valdiviels 1984
- Valdivielso A, Casado Flores J, Ruiz Beltran A, Garcia Perez J, Garcia Onieva M, Hidalgo I. Naloxone and endotoxic shock: A wonder drug?. Annales Espaňoles de Pediatría 1984;20:85‐90. [PubMed] [Google Scholar]
Wichman 2000
- Wichman MW, Zellweger R, Ayala A, Chaudry IH. Effect of naloxone on immune responses after hemorrhagic shock. Critical Care Medicine 2000;28:184‐9. [DOI] [PubMed] [Google Scholar]
Wright 1980
- Wright DJ, Phillips M, Weller MPI. Naloxone in shock. Lancet 1980;2:1361. [Google Scholar]
Xing 1990
- Xing R, Yi X, Guang‐Min L, Yuan‐Wen W. Naloxone for haemorrhagic fever shock?. Tropical Doctor 1990;20:72‐3. [PubMed] [Google Scholar]
Yeston 1983
- Yeston NS, Grasberger RC, McIntosh TK. Naloxone in reversal of hypotension in septic shock. JAMA 1983;250:2287. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Boeuf 1998
- Boeuf B, Gauvin F, Guerguerian AM, Farrell C, Lacroix J, Jenicek M. Therapy of shock with naloxone: A meta‐analysis. Critical Care Medicine 1998;26(11):1910‐6. [DOI] [PubMed] [Google Scholar]


