Abstract
Background:
Across the nation, growing numbers of individuals are exploring the use of cannabis for medical or recreational purposes, and the proportion of cannabis-positive drivers involved in fatal crashes increased from 8 percent in 2013 to 17 percent in 2014, raising concerns about the impact of cannabis use on driving. Previous studies have demonstrated that cannabis use is associated with impaired driving performance, but thus far, research has primarily focused on the effects of acute intoxication.
Methods:
The current study assessed the potential impact of cannabis use on driving performance using a customized driving simulator in non-intoxicated, heavy, recreational cannabis users and healthy controls (HCs) without a history of cannabis use.
Results:
Overall, cannabis users demonstrated impaired driving relative to HC participants with increased accidents, speed, and lateral movement, and reduced rule-following. Interestingly, however, when cannabis users were divided into groups based on age of onset of regular cannabis use, significant driving impairment was detected and completely localized to those with early onset (onset before age 16) relative to the late onset group (onset ≥16 years old). Further, covariate analyses suggest that impulsivity had a significant impact on performance differences.
Conclusions:
Chronic, heavy, recreational cannabis use was associated with worse driving performance in non-intoxicated drivers, and earlier onset of use was associated with greater impairment. These results may be related to other factors associated with early exposure such as increased impulsivity.
Keywords: Cannabis, Marijuana, Driving, Safety, Age of onset, Executive function, Impulsivity
1. Introduction
To date, several countries, including Canada and Uruguay have completely legalized cannabis, while in the United States, recreational cannabis use is legal for adults in 11 US states and Washington DC; an additional 33 states have fully legalized medical cannabis programs (National Conference of State Legislatures NCSL, 2019). In the US, national surveys indicate that approximately 123.9 million people aged 12 or older have tried cannabis at least once, and 27.7 million report past month use (Substance Abuse and Mental Health Services Administration SAMHSA, 2019). In addition, a recent Canadian survey indicated that approximately 4.4 million Canadians aged 15 or older reported using cannabis at least once in the past year (Canadian Tobacco, Alcohol and Drugs Survey CTADS, 2019). Further, the most recent US National Roadside Survey, which collected data from 2013 to 2014, reported that cannabis is the second most commonly detected substance (second only to alcohol) in randomized, voluntary assessments of drivers; 12.6 % of weekend, night-time drivers aged 16 or older tested positive for cannabis, representing a 48 % increase from the last national survey performed in 2007 (Berning et al., 2015). Additional data from the US National Survey of Drug Use and Health collected between 2002 and 2014 indicates that 3.2 % of individuals aged 16–25 reported driving while intoxicated with cannabis (Azofeifa et al., 2015). Similarly, the Canadian Road Safety Monitor survey, which has gathered information on drugged driving since 2002, indicated that approximately 2.9 % of Canadians reporting driving within two hours of using cannabis (Robertson et al., 2017).
Significant evidence suggests that acute cannabis intoxication, the result of exposure to Δ-9 tetrahydrocannabinol (THC), the primary psychoactive constituent of the plant, is associated with impaired driving (reviewed in Hartman and Huestis, 2013; Compton, 2017), and higher blood serum concentrations of THC-related metabolites are associated with greater impairment (e.g., Khiabani et al., 2006; Ramaekers et al., 2004), although results are somewhat heterogenous across studies. Specifically, driving under the influence of cannabis (DUIC) has been associated with slower driving in a number of investigations (e.g., Anderson et al., 2010; Lenné et al., 2010; Ronen et al., 2008, 2010); however, others did not observe this relationship (e.g., Liguori et al., 1998; Robbe, 1998). Acute cannabis intoxication has also been associated with increased lateral movement such as lane weaving in some studies (e.g., Lenné et al., 2010; Ronen et al., 2008; Robbe, 1998) but not others (e.g., Anderson et al., 2010; Ronen et al., 2010). Increased brake latency has been reported by Liguori et al. (1998), but a later study by this group did not replicate this finding (Liguori et al., 2002). In addition, some evidence suggests decreased response time while driving (e.g., Lenné et al., 2010), but this is not always observed (e.g., Liguori et al., 1998). Interestingly, in several studies, drivers demonstrated insight regarding their intoxication and reported adopting a slower driving style in an attempt to compensate for their impairment (reviewed in Sewell et al., 2009).
Further, several epidemiological studies have reported that DUIC significantly increases the odds of motor vehicle collisions (e.g., Asbridge et al., 2014; Bédard et al., 2007); however, some have suggested that once confounding variables (e.g., blood alcohol level) are controlled for, cannabis use no longer significantly impacts the odds of collision (e.g., Blows et al., 2005). Two meta-analyses reported that pooled odds ratios from these studies indicate a moderate to high risk of cannabis use on vehicle collisions (Asbridge et al., 2012; Li et al., 2012), but more recent meta-analyses indicate that publication bias may have inflated these numbers and that the increased risk may only be low to moderate, at an approximately 20–30 % higher likelihood (Elvik, 2013; Rogeberg and Elvik, 2016). Additionally, studies assessing driving after acute cannabis intoxication have reported increased collisions in some (e.g., Ronen et al., 2008, 2010) but not all studies (e.g., Anderson et al., 2010; Liguori et al., 1998).
Despite increasing numbers of cannabis consumers and research efforts focused on the relationship to driving performance, findings thus far remain limited. The cannabinoid metabolite examined in traditional assays (11-nor-9-carboxy-THC) has a long half-life, and the period of detection in biological samples can be several days or weeks after last use; accordingly, these assays do not provide accurate information regarding acute intoxication or recency of cannabis use. Further, in contrast to the alcohol per se limit in the US and Canada (≥0.08 % blood alcohol concentration), there is no currently accepted threshold for THC-related metabolites that indicates acute intoxication. Many roadside survey studies of DUIC continue to utilize these assays to determine intoxication status even though their limitations are well-known. Additionally, epidemiologic studies generally do not exclude individuals who use multiple substances, making it difficult to distinguish the specific impact of cannabis.
Experimental studies have primarily focused on the impact of acute cannabis intoxication on driving. Interestingly, while the effects of acute cannabis intoxication on driving performance have been studied extensively in regular cannabis users (e.g., Anderson et al., 2010; Lenné et al., 2010; Liguori et al., 1998, 2002; Robbe, 1998; Ronen et al., 2008, 2010) as well as in non-users (e.g., Robbe, 1998), to our knowledge, driving performance has never been assessed in cannabis users when they are not acutely intoxicated.
Further, previous studies examining the impact of recreational cannabis use on a range of outcome variables have emphasized the importance of thoroughly assessing cannabis use characteristics, particularly age of onset of cannabis use, as data suggest that adolescent onset of use is related to poorer cognitive performance and neurodevelopmental changes to both grey and white matter (reviewed in Gruber and Sagar, 2017; Lisdahl, 2013; Sagar and Gruber, 2018). Specifically, earlier onset of cannabis use is related to difficulties on tasks of executive function while those with later cannabis onset appear more similar to control subjects (e.g., Battisti et al., 2010; Fontes et al., 2011; Gruber et al., 2012a). Additionally, earlier age of cannabis onset and increased frequency and magnitude of cannabis use have also been shown to predict greater impairment of executive function and vice versa in recreational cannabis users (e.g., Dahlgren et al., 2016; Squeglia et al., 2014). Importantly, these studies have demonstrated poorer cognitive performance in chronic recreational cannabis users, even in the absence of acute intoxication, suggesting there may be residual cognitive impairment associated with heavy cannabis use.
Given the increasing numbers of recreational and medical cannabis users, it is imperative to assess the potential residual impact of chronic, heavy cannabis use on driving performance, which has not thoroughly been addressed, as previous studies have primarily focused on impairments related to acute intoxication. Consistent with previous research on cannabis use and cognitive function, we hypothesized that heavy, recreational cannabis users would demonstrate impairment on a driving simulator task relative to healthy control participants, even in the absence of acute cannabis intoxication, and that impaired performance would be primarily localized to early onset cannabis users.
2. Materials and methods
2.1. Participants
As part of a larger comprehensive study, cannabis users (n = 28) and non-cannabis using, healthy control (HC) subjects (n = 17) were recruited from the Greater Boston Metropolitan area. Prior to participation in this study, all procedures were thoroughly explained to participants, including the voluntary nature of the study. Additionally, all participants were required to read and sign an informed consent form approved by the Partners Healthcare Institutional Review Board.
Cannabis users were defined as chronic, heavy, recreational users who used at least five of the last seven days, reported at least 1500 lifetime uses, and tested positive for urinary cannabinoids. Importantly, cannabis users abstained from cannabis use for at least 12 h before their study visit to ensure that they were not acutely intoxicated at the time of assessment. If any participant endorsed cannabis use within the 12 -h abstinence period or appeared to be intoxicated, they were compensated for their time and rescheduled.
Upon arrival at the laboratory, all participants provided a urine sample that was tested using an in-house triage kit assessing for drugs of abuse (e.g., cocaine, opiates, cannabis, etc.). This procedure was required for three reasons: (a) to ensure negative drug status among all participants (except for THC-metabolites in the cannabis-using group); (b) to confirm the presence of THC-metabolites in the cannabis-using group; (c) and to encourage cannabis users to adhere to the 12-hr abstinence period, as cannabis users were led to believe that the urine sample could be used to determine compliance with the abstinence request, which is a method we have successfully utilized in the past (e.g., Gruber et al., 2014, 2012a; 2012b). A portion of the sample was sent to an outside laboratory for quantification of urinary THC-metabolite concentration with creatinine correction via gas chromatography-mass spectrometry.
In order to further explore the potential impact of age of onset of cannabis use on driving performance, the cannabis-using group was subdivided into two groups based on their age of onset of cannabis use, which was defined as the age of consistent, regular cannabis use (i.e., at least once per month). The early onset group included those with regular heavy cannabis use prior to or at age 16 (n = 14) while the late onset group included those with regular heavy cannabis use after age 16 (n = 14).
2.2. Assessments
Participants were administered the Structured Clinical Interview for DSM-IV, Patient Edition (SCID; First et al., 1994) to ensure that no Axis I pathology was present other than cannabis abuse or dependence in the cannabis-using groups. Participants also completed the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), which provides an estimate of IQ. Individuals were excluded from the study if they reported a significant head injury with loss of consciousness, history of any neurological disorder, estimated IQ < 75, current or previous use of psychotropic medication, current heavy alcohol use (regular consumption of > 20 drinks/week), binge drinking (defined by the National Institute on Alcohol Abuse and Addiction as when men consume ≥5 drinks in 2 h or when women consume ≥4 drinks in 2h), or a history of illicit substance use (> 20 lifetime uses). In addition, HC participants were excluded if they reported more than 20 lifetime uses of cannabis. All participants had to be in possession of a valid driver’s license and report driving regularly.
Cannabis use was assessed via a modified timeline follow-back procedure customized for cannabis use (TLFB; Sobell et al., 1998) to provide qualitative information regarding the type of products used (e.g., joint, blunt, edible, etc.) and mode of use (e.g., smoke, vape, ingest, etc.) as well as quantitative information regarding frequency (episodes/week) and magnitude (grams/week) of use. The SCID was used to confirm lifetime cannabis use information such as estimated lifetime uses and age of onset of regular use. Additionally, all cannabis users completed the Cannabis Use Disorder Identification Test-Revised (CUDIT-R; Adamson et al., 2010). As cannabis users were required to abstain from cannabis use for at least 12 h prior to completing the driving simulator paradigm, they completed a modified version of the Marijuana Withdrawal Checklist (MWC; Budney et al., 1999). The modified MWC is a 16-item self-report scale in which respondents rank symptoms associated with cannabis withdrawal (e.g., irritability, craving, etc.) as none, mild, moderate, or severe (numerically ranked from 0 to 3). The range of potential scores on this version of the MWC is 0–48.
Participants also completed the Barratt Impulsiveness Scale-11 (BIS; Patton et al., 1995), which is a self-report assessment that measures impulsivity across three domains: attention (e.g., “I don’t pay attention”); motor (e.g., “I act on impulse”); and non-planning (e.g., “I say things without thinking”). A total score of all subscales was also calculated to reflect general impulsivity.
2.3. Driving simulator
Driving simulator paradigms allow for measurement of driving performance while minimizing actual risk to participants and others. The driving simulator used in the current study included a GTR simulator racing seat, Logitech G27 racing kit (dual-motor force feedback mechanism with quiet helical gearing; a six-speed shifter with pushdown reverse gear; integrated RPM/shift indicator LEDs; an 11-inch leather-wrapped rim; and steel gas, brake, and clutch pedals), a 19-inch monitor, and surround sound speakers.
The driving simulator software and paradigm (STISIM Drive, Systems Technology Inc.) consists of 4.2 miles of simulated driving and takes approximately 10 min to complete. It includes both rural and urban driving conditions with road hazards, including stop signs, traffic lights, merges, turns, yielding to pedestrians, and reacting to other vehicles. Dependent variables on the task are grouped by subtype and include accidents (number of collisions, pedestrians hit, and off-road accidents); rule-following (number of missed stop signs, stops at red lights, and illegal turns); speed (number of speed exceedances, total run length, and percentage of distance driven over the speed limit); and lateral movement (number of centerline crossings, road edge excursions, and percentage of distance driven out of lane).
2.4. Statistical analyses
Driving simulator performance data was screened for outliers, defined as values beyond the 1.5 interquartile range. One HC participant was removed from the analyses due to outlier driving simulator performance (final HC n = 16). One-way analyses of variance (ANOVAs) were used to assess between-group differences for two different analyses: two-group (HC versus all cannabis users) and three-group (HC versus early onset cannabis users versus late onset cannabis users). Scheffé post hoc comparisons were used to assess between-group differences for the three-group analyses. Additionally, if significant between-group differences were observed for any demographic variable (e.g., IQ, impulsivity, etc.), analyses of covariance (ANCOVAs) were conducted in order to control for these variables and assess their impact on driving simulator performance. Lastly, bivariate correlation analyses were conducted to further assess the relationship between driving performance and age of cannabis onset. Given substantial scientific evidence demonstrating adolescent onset of cannabis use is associated with poorer cognitive performance even in the absence of acute intoxication (reviewed in Gruber and Sagar, 2017; Lisdahl, 2013; Sagar and Gruber, 2018), one-tailed analyses were used for the current study.
3. Results
3.1. Two-group analyses: healthy controls versus all cannabis users
HC and all cannabis users were well-matched for age, IQ, and alcohol use (Table 1). With regard to impulsivity, the cannabis-using group reported significantly higher BIS scores on the attention and non-planning subscales as well as overall total BIS score (all ps < .01) compared to the HC group. No significant between-group differences were noted for the BIS motor subscale.
Table 1.
Demographic Information: Healthy Control (HC) versus Cannabis Users (CU).
| Demographic Variables | HC n = 16 |
CU n = 28 |
ANOVA (2-tailed)a |
|
|---|---|---|---|---|
| F | p (ηp2) | |||
| Sex | 6M, 10F | 23 M, 5F | – | – |
| Handedness | 16R, 0L | 27R, 1L | – | – |
| Age | 22.94 ± 3.96 | 22.96 ± 5.56 | < 0.01 | .99 (< .01) |
| IQ: WASIb | 123.55 ± 6.85 | 117.71 ± 10.11 | 3.08 | .09 (.08) |
| Alcohol Use (days out of last 30)b | 4.96 ± 4.50 | 6.52 ± 4.48 | 0.96 | .33 (.03) |
|
| ||||
| Cannabis Use Variables | ||||
|
| ||||
| Age of Cannabis Onsetc | – | 15.95 ± 2.04 | – | – |
| Cannabis Use Episodes/Weekc | – | 14.76 ± 12.34 | – | – |
| Cannabis Grams Used/Weekc | – | 5.24 ± 6.42 | – | – |
| Duration of Cannabis Use (yr)c | – | 7.43 ± 6.45 | – | – |
| Urinary THC/Creatinine Ratio (ng/ml)d | – | 480.58 ± 663.56 | – | – |
| CUDIT-R Scorec | – | 15.57 ± 5.30 | – | – |
| MWCc | – | 7.32 ± 3.67 | – | – |
|
| ||||
| Impulsiveness: BISb | ||||
|
| ||||
| Attention | 13.00 ± 3.58 | 16.64 ± 3.12 | 9.93 | < .01 (.21) |
| Motor | 20.73 ± 4.92 | 23.14 ± 4.08 | 2.47 | .13 (.06) |
| Non-Planning | 18.46 ± 5.17 | 24.79 ± 5.02 | 12.36 | < .01 (.25) |
| Total | 52.18 ± 12.85 | 64.57 ± 9.23 | 11.35 | < .01 (.24) |
Abbreviations: Analysis of Variance (ANOVA); Barratt Impulsiveness Scale (BIS); Cannabis Use Disorder Identification Test-Revised (CUDIT-R); Marijuana Withdrawal Checklist (MWC); Tetrahydrocannabinol (THC); Wechsler Abbreviated Scale of Intelligence (WASI).
Notes:
Bold numbers are significant at p ≤ .05 (2-tailed).
Italicized numbers are significant at p ≤ .10 (2-tailed).
Degrees of Freedom (df) = 1,42 unless otherwise noted.
df = 1,37.
n = 28.
n = 26.
On the driving simulator task, the cannabis-using group exhibited significantly impaired performance compared to the HC group (Table 2; Fig. 1). Cannabis users hit significantly more pedestrians (p = .03), missed more stop signs (p = .04), made fewer stops at red lights (p = .02), had more speed exceedances (p = .03), drove a greater percentage of distance over the speed limit (p = .04), and made more centerline crossings (p = .05) relative to HC participants; there was also a non-significant trend for cannabis users to have a greater percentage of distance driven out of lane. The HC and cannabis-using groups had similar numbers of collisions, total run length, and road edge excursions. None of the participants in either the HC or cannabis-using groups had any off-road accidents or made any illegal turns.
Table 2.
Driving Simulator Performance: Healthy Control (HC) versus Cannabis Users (CU) Analyses of Variance (ANOVA) and Analyses of Covariance (ANCOVA) Controlling for Impulsivity.
| Driving Simulator Variables | HC n = 16 |
CU n = 28 |
ANOVA (1-tailed)a |
ANCOVA (1-tailed)b |
||
|---|---|---|---|---|---|---|
| F | p (ηp2) | F | p (ηp2) | |||
| Accidents | ||||||
| # Pedestrians Hit | 0.94 ± 0.93 | 1.57 ± 1.10 | 3.75 | .03 (.08) | 0.21 | .33 (.01) |
| # Collisions | 1.06 ± 0.85 | 1.21 ± 1.07 | 0.24 | .32 (.01) | 0.28 | .30 (.01) |
| # Off-Road Accidents | 0.00 ± 0.00 | 0.00 ± 0.00 | – | – | – | – |
| Rule-Following | ||||||
| # Missed Stop Signs | 0.56 ± 0.73 | 1.04 ± 0.92 | 2.28 | .04 (.07) | 0.01 | .47 (< .01) |
| # Stops at Red Lights | 0.75 ± 0.77 | 0.36 ± 0.49 | 4.28 | .02 (.09) | 2.57 | .06 (.07) |
| # Illegal Turns | 0.00 ± 0.00 | 0.00 ± 0.00 | – | – | – | – |
| Speed | ||||||
| # Speed Exceedances | 4.50 ± 3.33 | 6.64 ± 3.61 | 3.83 | .03 (.08) | .41 | .26 (.01) |
| Total Run Length (s) | 585.91 ± 89.90 | 552.24 ± 88.69 | 1.45 | .12 (.03) | < .01 | .48 (< .01) |
| % Distance Over Speed Limit | 7.50 ± 7.40 | 15.37 ± 16.66 | 3.18 | .04 (.07) | 0.48 | .25 (.01) |
| Lateral Movement | ||||||
| # Centerline Crossings | 4.88 ± 1.75 | 6.00 ± 2.23 | 3.01 | .05 (.07) | 0.21 | .33 (.01) |
| # Road Edge Excursions | 3.38 ± 2.00 | 3.39 ± 1.45 | < 0.01 | .49 (< .01) | 0.04 | .42 (< .01) |
| % Distance Out of Lane | 8.50 ± 3.01 | 9.30 ± 3.13 | 1.64 | .10 (.04) | 0.03 | .43 (< .01) |
Notes:
Bold numbers are significant at p ≤ .05 (1-tailed).
Italicized numbers are significant at p ≤ .10 (1-tailed).
Degrees of Freedom (df) = 1,42.
ANCOVA with Barratt Impulsiveness Scale (BIS) Total Score included as a covariate; df = 1,36.
Fig. 1. Driving Simulator Performance Analyses.
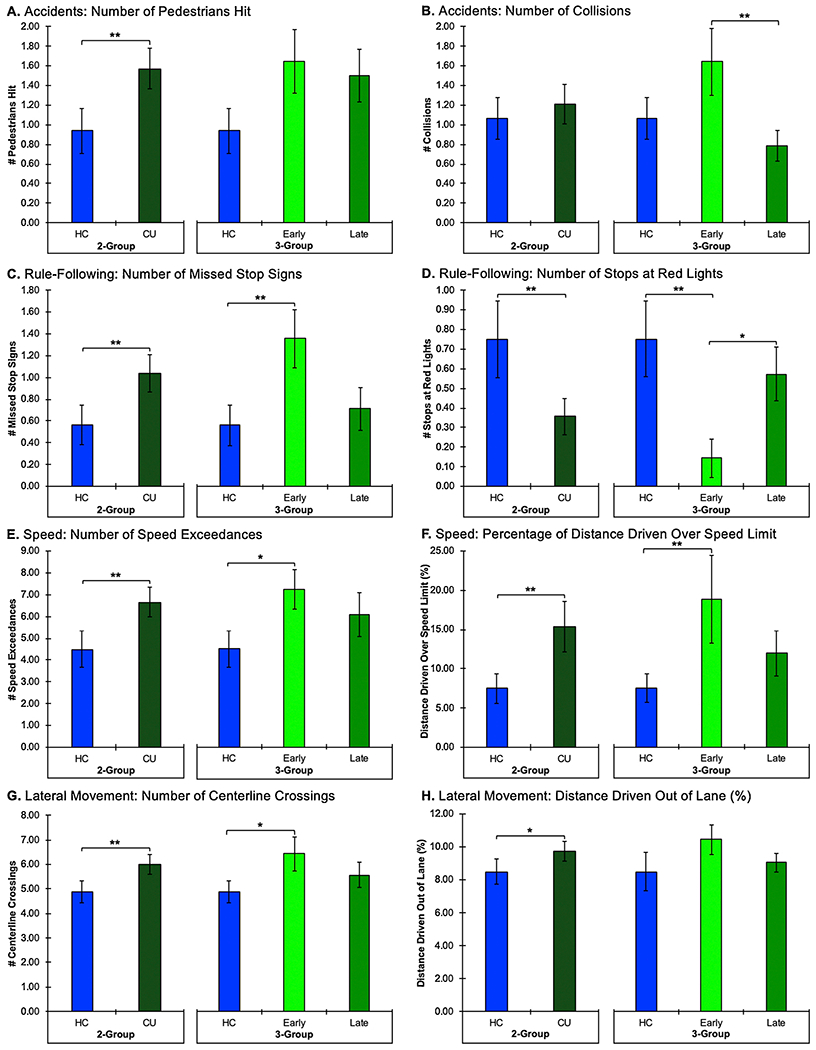
The two-group assessments comparing the healthy control (HC) participants to all chronic, heavy cannabis users (CU) are on the left side of the graphs. The three-group assessments comparing HC versus early onset cannabis users (Early) versus late onset cannabis users (Late) are on the right side of the graphs. Note: ** denotes significance at p ≤ .05 and * denotes significance at p ≤ .10 (1-tailed).
Given significant between-group differences for impulsivity between HCs and cannabis users, ANCOVAs were also performed for driving simulator performance data. Once total BIS score was controlled for in the analyses, no significant differences between the HC and cannabis users remained for driving performance, other than a non-significant trend for cannabis users to make fewer stops at red lights (p = .06).
3.2. Three-group analyses: healthy controls versus early onset cannabis users versus late onset cannabis users
The three-group comparisons revealed that HC participants, early onset cannabis users, and late onset cannabis users were well-matched for age, IQ, and alcohol use (Table 3). In terms of cannabis use patterns, no significant differences were noted between early and late onset users for number of use episodes per week, total grams used per week, duration of use (yrs), urinary THC/creatinine ratio, or CUDIT-R scores. As expected, age of onset of cannabis use distinguished the two groups (p < .01). Further, both early and late onset users reported similar levels of withdrawal symptoms on the 16-item MWC (M = 7.71 and M = 6.93, respectively). Given that the range of possible scores on this version of the MWC is from 0 to 48, the group averages were very low, suggesting that the cannabis users did not experience many withdrawal symptoms despite at least 12 h of abstinence. With regard to impulsivity, significant differences emerged between the three groups for the attention and non-planning subscales, as well as BIS total (all ps≤.01), but no significant differences were noted on the motor subscale. Scheffé post hoc tests indicated that the HC group reported significantly lower BIS scores relative to early and late onset groups for the attention (p = .03 and p = .04) and non-planning subscales (p = .01 and p = .07) as well as total BIS scores (p = .01 and p = .05). No significant differences were noted between the early and late cannabis onset groups on the BIS.
Table 3.
Demographic Information: Healthy Control (HC) versus Early Cannabis Onset versus Late Cannabis Onset.
| Demographic Variables | HC n = 16 |
Early Onset n = 14 |
Late Onset n = 14 |
ANOVA (2-tailed)a |
|
|---|---|---|---|---|---|
| F | p (ηp2) | ||||
| Sex | 6M, 10F | 13 M, 1F | 10 M, 4F | – | – |
| Handedness | 16R, 0L | 13R, 1L | 14R, 0L | – | – |
| Age | 22.94 ± 3.96 | 23.14 ± 7.07 | 22.79 ± 3.77 | 0.02 | .98 (< .01) |
| IQ: WASIb | 123.55 ± 6.85 | 116.00 ± 12.99 | 119.43 ± 6.11 | 2.01 | .15 (.10) |
| Alcohol Use (days out of last 30)b | 4.97 ± 4.50 | 5.82 ± 2.31 | 7.21 ± 5.94 | 0.81 | .45 (.04) |
|
| |||||
| Cannabis Use Variables | |||||
|
| |||||
| Age of Cannabis Onsetc | – | 14.00 ± 1.04 | 17.36 ± 1.08 | 70.21 | < .01 (.73) |
| Cannabis Use Episodes/Weekc | – | 11.88 ± 4.56 | 17.65 ± 16.66 | 1.56 | .22 (.06) |
| Cannabis Grams Used/Weekc | – | 4.43 ± 2.66 | 6.05 ± 8.77 | 0.44 | .51 (.02) |
| Duration of Cannabis Use (yr)c | – | 9.57 ± 7.98 | 5.29 ± 3.58 | 3.36 | .08 (.12) |
| Urinary THC/Creatinine Ratiod | – | 408.43 ± 594.23 | 564.75 ± 754.35 | 0.35 | .56 (.01) |
| CUDIT-R Scorec | – | 15.14 ± 4.59 | 16.00 ± 6.08 | 0.18 | .68 (.01) |
| MWC (16-item)c | – | 7.71 ± 4.14 | 6.93 ± 3.25 | 0.31 | .58 (.01) |
|
| |||||
| Impulsiveness: BIS | |||||
|
| |||||
| Attentione | 13.00 ± 3.58 * , * | 16.79 ± 3.17 * | 16.50 ± 3.18 * | 4.87 | .01 (.21) |
| Motore | 20.73 ± 4.92 | 23.00 ± 4.49 | 23.29 ± 3.79 | 1.22 | .31 (.06) |
| Non-Planninge | 18.46 ± 5.17 * , ** | 26.36 ± 4.27 * | 23.21 ± 5.37 ** | 7.91 | < .01 (.31) |
| Totale | 52.18 ± 12.85 * , * | 66.14 ± 7.83 * | 63.00 ± 10.50 * | 5.94 | .01 (.25) |
Abbreviations: Analysis of Variance (ANOVA); Barratt Impulsiveness Scale (BIS); Cannabis Use Disorder Identification Test-Revised (CUDIT-R); Marijuana Withdrawal Checklist (MWC); Tetrahydrocannabinol (THC); Wechsler Abbreviated Scale of Intelligence (WASI).
Notes:
Bold numbers are significant at p ≤ .05 (2-tailed).
Italicized numbers are significant at p ≤ .10 (2-tailed).
Scheffé Post Hoc Analyses indicated significant differences between pairs at p ≤ .10 (2-tailed).
Scheffé Post Hoc Analyses indicated significant differences between pairs at p ≤ .05 (2-tailed).
Degrees of Freedom (df) = 2,41 unless otherwise noted.
df = 2,35.
df = 1,26.
df = 1,24.
df = 2,36.
For the driving simulator data, omnibus ANOVAs revealed significant between-group differences for collisions (p = .03), missed stop signs (p = .02), stops at red lights (p = .01), and percentage of distance driven over the speed limit (p = .05; Table 4, Fig. 1). Additionally, there were non-significant trends for between-group differences for number of pedestrians hit (p = .08), number of speed exceedances (p = .06), and number of centerline crossings (p = .07). No significant between-group differences were detected for total run length, number of road edge excursions or percentage of distance driven out of lane. None of the participants in any of the groups had any off-road accidents or made any illegal turns.
Table 4.
Driving Simulator Performance: Healthy Control (HC) versus Early versus Late Onset Cannabis Users Analyses of Variance (ANOVA) and Analyses of Covariance (ANCOVA) Controlling for Impulsivity.
| Driving Simulator Variables | HC n = 16 |
Early Onset n = 14 |
Late Onset n = 14 |
ANOVA (1-tailed)a |
ANCOVA (1-tailed)b |
||
|---|---|---|---|---|---|---|---|
| F | p (ηp2) | F | p (ηp2) | ||||
| Accidents | |||||||
| # Pedestrians Hit | 0.94 ± 0.93 | 1.64 ± 1.22 | 1.50 ± 1.02 | 1.90 | .08 (.09) | 0.12 | .45 (.01) |
| # Collisions | 1.06 ± 0.85 | 1.64 ± 1.28 ** | 0.79 ± 0.58 ** | 3.02 | .03 (.13) | 3.01 | .03 (.15) |
| # Off-Road Accidents | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | – | – | – | – |
| Rule-Following | |||||||
| # Missed Stop Signs | 0.56 ± 0.73 ** | 1.36 ± 01.01 ** | 0.71 ± 0.73 | 3.79 | .02 (.16) | 1.65 | .10 (.09) |
| # Stops at Red Lights | 0.75 ± 0.78 ** | 0.14 ± 0.36 * , ** | 0.57 ± 0.51 * | 4.14 | .01 (.17) | 3.05 | .03 (.15) |
| # Illegal Turns | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | – | – | – | – |
| Speed | |||||||
| # Speed Exceedances | 4.50 ± 3.33 * | 7.21 ± 3.42 * | 6.07 ± 3.77 | 2.28 | .06 (.10) | 0.44 | .32 (.02) |
| Total Run Length (s) | 585.91 ± 89.90 | 539.00 ± 105.32 | 565.49 ± 69.75 | 1.03 | .18 (.05) | 0.24 | .40 (.01) |
| % Distance Over Speed Limit | 7.50 ± 7.40 ** | 18.79 ± 20.90 ** | 11.94 ± 10.68 | 2.46 | .05 (.11) | 0.87 | .22 (.05) |
| Lateral Movement | |||||||
| # Centerline Crossings | 4.88 ± 1.75 * | 6.43 ± 2.53 * | 5.57 ± 1.87 | 2.12 | .07 (.09) | 0.44 | .32 (.03) |
| # Road Edge Excursions | 3.38 ± 2.00 | 3.50 ± 1.56 | 3.29 ± 1.38 | 0.06 | .47 (< .01) | 0.10 | .45 (.01) |
| % Distance Out of Lane | 8.50 ± 3.01 | 10.44 ± 4.06 | 9.06 ± 1.78 | 1.54 | .11 (.07) | 0.51 | .30 (.03) |
Notes: Bold numbers are significant at p ≤ .05 (1-tailed).
Italicized numbers are significant at p ≤ .10 (1-tailed).
Scheffé Post Hoc Analyses indicated significant differences between pairs at p ≤ .10 (1-tailed).
Scheffé Post Hoc Analyses indicated significant differences between pairs at p ≤ .05 (1-tailed).
Degrees of Freedom (df) = 2,41.
ANCOVA with Barratt Impulsiveness Scale (BIS) Total Score included as a covariate; df = 1,36.
Scheffé post hoc tests indicated that poorer task performance was primarily localized to the early onset cannabis group (Fig. 1). Specifically, the early onset cannabis group had significantly more collisions (p = .03), more missed stop signs (p = .02), fewer stops at red lights (p = .01), and drove a greater percentage of distance over the speed limit (p = .05) relative to the HC group. Additionally, several non-significant trends were observed in which the early onset group had poorer performance relative to both the HC and late onset groups. The early onset cannabis group tended to make more speed exceedances (p = .06) and more centerline crossings (p = .07) than the HC group. Further, the early onset group tended to miss more stop signs (p = .07) and make fewer stops at red lights (p = .08) than the late cannabis onset group. While there was a non-significant trend for between-group differences on the number of pedestrians hit, none of the post hoc comparisons approached significance. Interestingly, no significant differences were noted between the HC and late cannabis onset groups for any of the driving simulator variables. Additionally, correlation analyses indicated that age of onset of cannabis use was negatively correlated with number of collisions (r(26) = −.40, p = .02) as well as missed stop signs (r(26) = −.32, p = .05), suggesting that earlier onset of cannabis use is associated with impaired driving skills, specifically increased collisions and missed stop signs; however, significant relationships were not detected between age of onset of cannabis use and other driving simulator variables. Interestingly, no significant relationships were observed between driving simulator performance and any of the other cannabis use variables.
Given significant between-group differences on the BIS, ANCOVAs controlling for total BIS impulsivity scores were completed. Interestingly, when total BIS score was included as a covariate, only the total number of collisions and stops at red lights (both ps = .03) remained significantly different between groups, with the early onset cannabis users demonstrating poorer performance; the remaining driving simulator variables were no longer significantly different after controlling for impulsivity.
4. Discussion
Data from the current study suggest that chronic cannabis use is associated with impaired driving performance even in the absence of acute intoxication. Poorer performance was observed in the cannabis-using group, with increased accidents, speeding, and lateral movement as well as decreased rule-following relative to HC participants. Importantly, when cannabis users were divided into those with early versus late onset, results revealed that impairment was primarily localized to the early onset group. These findings provide evidence that non-intoxicated early onset cannabis users demonstrate poorer driving performance, suggesting that there may be a residual impact of chronic, heavy, recreational cannabis use, particularly when regular use is initiated during early adolescence. However, it is important to note that when self-reported impulsivity was controlled for, most of the significant differences between the HC and cannabis users were no longer significant, suggesting that increased impulsivity within the cannabis users impacts, at least in part, the performance differences observed on the driving simulator.
The current study extends previous findings demonstrating that early cannabis onset is associated with a broad variety of cognitive decrements including poorer attention, visuospatial skills, verbal memory, and executive functioning (reviewed in Gruber and Sagar, 2017; Lisdahl, 2013; Sagar and Gruber, 2018) as well as alterations in functional brain activation (e.g., Hatchard et al., 2014; Sagar et al., 2015; Schweinsburg et al., 2008), and reduced integrity of white matter microstructure (e.g., Clark et al., 2012; Gruber et al., 2014). Further, findings related to increased number of collisions in non-intoxicated, early-onset cannabis users are similar to reports from acute intoxication studies demonstrating increased number of collisions (Ronen et al., 2008, 2010) as well as epidemiological studies indicating increased risk of motor vehicle collisions associated with DUIC (Asbridge et al., 2012; Elvik, 2013; Li et al., 2012; Rogeberg and Elvik, 2016). Additionally, earlier age of onset was associated with poorer driving simulator performance; previous studies have also demonstrated a link between earlier age of onset and poorer cognitive performance (e.g., Dahlgren et al., 2016; Ehrenreich et al., 1999; Squeglia et al., 2014).
Results from the current study indicate an impulsive style of driving performance in non-intoxicated cannabis users, particularly in those with early onset of use, characterized by increased accidents, speeding, and lateral movement, and decreased rule-following. This is further underscored by the BIS data, with cannabis users reporting significantly higher levels of impulsivity relative to controls. Studies of acutely intoxicated individuals as well as DUIC have also shown impaired driving performance, but with a slightly different characterization than observed in the current study. Acute cannabis intoxication is associated with slower driving (e.g., Anderson et al., 2010; Lenné et al., 2010; Ronen et al., 2008, 2010); increased number of collisions (Ronen et al., 2008, 2010); increased lateral movement such as lane weaving (e.g., Lenné et al., 2010; Ronen et al., 2008; Robbe, 1998), increased brake latency (Liguori et al., 1998), and slower reaction time (e.g., Lenné et al., 2010). Interestingly, across several studies, drivers demonstrated insight regarding their intoxication and reported adopting a slower driving style in an attempt to compensate for impairment (reviewed in Sewell et al., 2009). The main difference between acute impairment/DUIC findings and results of the current study is driving speed; acute intoxication is associated with slower driving, whereas chronic use without acute intoxication is associated with faster driving. Taken together, the discrepancy between these findings suggests that acute cannabis intoxication results in a different type of driving impairment relative to residual impairment in the absence of acute cannabis intoxication, which appears to be associated with an impulsive style of driving.
One important question raised by current study is whether the observed driving impairment is directly related to early onset cannabis use or other mediating factors. Given the extensive literature demonstrating that early onset cannabis users demonstrate cognitive decrements relative to HC participants (reviewed in Gruber and Sagar, 2017; Lisdahl, 2013; Sagar and Gruber, 2018) as well as findings indicating that late onset cannabis users appear more similar to HC participants (e.g., Ehrenreich et al., 1999; Fontes et al., 2011; Gruber et al., 2012a; Schuster et al., 2016), it is possible that cannabis use during critical periods of neurodevelopment may mediate observed cognitive deficits. However, it is also possible that confounding factors (e.g., impulsivity, sensation seeking, etc.) associated with earlier onset of cannabis use may mediate these deficits. Several longitudinal studies have observed that behavioral problems, which may be an indicator of lack of inhibitory control, often precede cannabis use in adolescents (e.g., King et al., 2004; Fergusson et al., 2007; Griffith-Lendering et al., 2011; Pedersen et al., 2001). However, although cannabis users typically demonstrate significantly higher levels of impulsivity on self-report measures such as the BIS relative to control participants (reviewed in Wrege et al., 2014), performance deficits on neuropsychological assessments of inhibitory control are not always consistently observed (e.g., Griffith-Lendering et al., 2012; Gruber et al., 2012b; Harding et al., 2012). With regard to driving, earlier onset of cannabis use (Le Strat et al., 2015) as well as increased risk-taking behaviors and sensation-seeking (Bergeron and Paquette, 2014; Bergeron et al., 2014; Richer and Bergeron, 2009) have been shown to be associated with DUIC, suggesting that each of these variables may contribute to an overall reckless style of driving. In the current study, after controlling for differences in impulsivity, HC participants and cannabis users generally displayed similar driving simulator performance, indicating that impulsivity significantly influences these differences. Future investigations employing longitudinal designs, larger sample sizes, and multivariate modeling should further assess these overlapping variables to attempt to clarify and model the specific impact of each factor.
4.1. Limitations and future directions
Several limitations regarding the current study should be considered. First, the current study utilized a driving simulator in order to assess driving performance and may not be completely generalizable to actual driving performance. While real-world driving track paradigms may have better ecological validity than driving simulator paradigms, they also involve increased risk to both the participants and the researchers. Additionally, due to the cross-sectional design of the current study, it is not possible to determine whether driving simulator impairment or increased impulsivity precede or are a consequence of early cannabis exposure; longitudinal studies assessing these factors before initiation of cannabis use are necessary to address this question. In order to more fully investigate the specific impact of age of cannabis onset and impulsivity on driving, multivariate modeling and mediation analyses should be utilized; these statistical analyses require larger sample sizes than provided by the current study.
In the current study, all cannabis users were chronic, heavy, recreational users, with most endorsing daily or near-daily use; accordingly, these results may not be generalizable to individuals who use cannabis less frequently or importantly, to individuals using cannabis for medical purposes. Recreational users and medical cannabis (MC) patients differ in their motives for cannabis use, as recreational users generally aim to get “high,” while MC patients primarily use cannabis to achieve symptom alleviation. Accordingly, recreational users and MC patients are likely to differ on several characteristics of cannabis use including, but not limited to goal of use, product selection, overall exposure to specific cannabinoids, and age of onset of use. In fact, preliminary data from an ongoing, observational, longitudinal study of MC patients revealed improvement in some aspects of cognitive performance and brain function after initiation of MC treatment (Gruber et al., 2016, 2018). These findings are in stark contrast to the cognitive decrements and brain alterations often observed in recreational cannabis users and suggest that cannabis use is likely to confer distinct effects in MC patients relative to recreational users. Thus far, driving performance has not been assessed in MC patients; future studies are needed to address this gap in the scientific literature.
Additionally, to our knowledge, no other studies have examined the impact of chronic, heavy use of any other substances on driving performance in the absence of acute intoxication. Given that approximately 16.6 million Americans aged 12 or older reported heavy alcohol use in the past month (Substance Abuse and Mental Health Services Administration SAMHSA, 2019), it is of particular interest to assess whether chronic, heavy alcohol use results in similar driving impairment observed in the current study. Further, many of our study participants reported onset of cannabis use before age 16 (when most American teenagers learn to drive); therefore, it would be interesting to explore the potential impact of state-dependent learning effects on driving. Given that learning to drive is a highly supervised activity legally required to be overseen by an adult (typically a teacher or parent), it is unlikely that participants were acutely intoxicated for the duration of their training. However, future studies should directly assess this research question by collecting specific information related to cannabis use during driver education programs and training as well as potential factors that may influence this outcome.
5. Conclusions
The current study demonstrates residual driving impairment in non-intoxicated cannabis users, which appears specific to those with early onset cannabis use. Previous studies of acutely intoxicated individuals and DUIC have observed a slower style of impaired driving behavior; conversely, the current study suggests that earlier onset users demonstrate an impulsive style of impaired driving, which may be related to characteristics inherent in individuals who engage in substance use at earlier ages such as increased impulsivity. Given increased legalization of cannabis, growing numbers of cannabis consumers, and increased prevalence of drivers testing positive for cannabis metabolites, results from the current study underscore concerns about the impact of cannabis use on driving as a potential public safety issue.
Acknowledgments
Funding
This work was supported by the National Institute on Drug Abuse (NIDA) [grant number 1R01-DA032646 awarded to Dr. Gruber] and the McLean Hospital Rossano Mind, Brain, and Behavior Pre-Doctoral Fellowship [awarded to Dr. Dahlgren].
Footnotes
Declaration of Competing Interest
None.
References
- Adamson SJ, Kay-Lambkin FJ, Baker AL, Lewin TJ, Thornton L, Kelly BJ, Sellman JD, 2010. An improved brief measure of cannabis Misuse: the cannabis use disorders identification test – revised (CUDIT-R). Drug Alcohol Depend. 110, 137–143. 10.1016/j.drugalcdep.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Anderson BM, Rizzo M, Block RI, Pearlson GD, O’Leary DS, 2010. Sex differences in the effects of marijuana on simulated driving performance. J. Psychoactive Drugs 42, 19–30. 10.1080/02791072.2010.10399782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbridge M, Hayden JA, Cartwright JL, 2012. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. Br. Med. J 344. 10.1136/bmj.e536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbridge M, Mann R, Cusimano MD, Trayling C, Roerecke M, Tallon JM, Whipp A, Rehm J, 2014. Cannabis and traffic collision risk: findings from a case-crossover study of injured drivers presenting to emergency departments. Int. J. Public Health 59, 395–404. 10.1007/s00038-013-0512-z. [DOI] [PubMed] [Google Scholar]
- Azofeifa A, Mattson ME, Lyerla R, 2015. Driving under the influence of alcohol, marijuana, and alcohol and marijuana combined among persons aged 16-25 years – United States, 2002-2014. Morb. Mortal. Wkly. Rep 64, 1325–1329. [DOI] [PubMed] [Google Scholar]
- Battisti R, Roodenrys A, Johnstone S, Pesa S, Hermens J, Solowij N, 2010. Chronic cannabis users show altered neurophysiological functioning on Stroop task conflict resolution. Psychopharmacology 212, 613–624. 10.1007/s00213-010-1988-3. [DOI] [PubMed] [Google Scholar]
- Bédard M, Dubois S, Weaver B, 2007. The impact of cannabis on driving. Can. J. Public Health 98, 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J, Paquette M, 2014. Relationships between frequency of driving under the influence of cannabis, self-reported reckless driving and risk-taking behavior observed in a driving simulator. J. Saf. Risk 49, 19. 10.1016/j.jsr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Bergeron J, Langlois J, Cheang HS, 2014. An examination of the relationships between cannabis use, driving under the influence of cannabis and risk-taking on the road. Eur. Rev. Appl. Psychol 64, 101. 10.1016/j.erap.2014.04.001. [DOI] [Google Scholar]
- Berning A, Compton R, Wochinger K, 2015. Results of the 2013–2014 National Roadside Survey of Alcohol and Drug Use by Drivers. (Traffic Safety Facts Research Note. Report No. DOT HS 812 118). National Highway Traffic Safety Administration, Washington, DC. [Google Scholar]
- Blows S, Ivers RQ, Connor J, Ameratunga S, Woodward M, Norton R, 2005. Marijuana use and car crash injury. Addiction 100, 605–611. 10.1111/j.1360-0443.2005.01100.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Novy PL, Hughes JR, 1999. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction 94, 1311–1322. 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- Canadian Tobacco, Alcohol and Drugs Survey [CTADS], 2019. Canadian Tobacco, Alcohol and Drugs Survey (CTADS): Summary of Results for 2017. 4 January 2019. Retrieved from https://www.canada.ca/en/health-canada/services/canadian-tobacco-alcohol-drugs-survey/2017-summary.html (Accessed on 14 August 2019). .
- Clark DB, Chung T, Thatcher DL, Pajtek S, Long EC, 2012. Psychological dysregulation, white matter disorganization and substance use disorders in adolescence. Addiction 107, 206–214. 10.1111/j.1360-0443.2011.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton R, 2017. Marijuana-Impaired Driving - a Report to Congress. (DOT HS 812 440). National Highway Traffic Safety Administration, Washington, DC. [Google Scholar]
- Dahlgren MK, Sagar KA, Racine MT, Dreman MW, Gruber SA, 2016. Marijuana use predicts cognitive performance on tasks of executive function. J. Stud. Alcohol Drugs 77, 298–308. 10.15288/jsad.2016.77.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Gigerenzer G, Hoehe MR, 1999. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology 142, 295–301. [DOI] [PubMed] [Google Scholar]
- Elvik R, 2013. Risk of road accident associated with the use of drugs: a systematic review and meta-analysis of evidence from epidemiological studies. Accid. Anal. Prev 60, 254–267. 10.1016/j.aap.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Ridder EM, 2007. Conduct and attentional problems in childhood and adolescence and later substance use, abuse and dependence: results of a 25-year longitudinal study. Drug Alcohol Depend. 88, 14–26. 10.1016/j.drugalcdep.2006.12.011. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 1994. Structured Clinical Interview for Axis I DSM-IV Disorders, Patient Edition (SCID-I/P) Version 2.0. Biometric Research Department, NY State Psychiatric Institute, New York. [Google Scholar]
- Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Jungerman F, Laranjeira RR, Bressan RA, Lacerda AL, 2011. Cannabis use before age 15 and subsequent executive functioning. Br. J. Psychiatry 198, 442–447. 10.1192/bjp.bp.110.077479. [DOI] [PubMed] [Google Scholar]
- Griffith-Lendering MFH, Huijbregts SCJ, Vollebergh WAM, Swaab H, 2012. Motivational and cognitive inhibitory control in recreational cannabis users. J. Clin. Exp. Neuropsychiatr 34, 688–697. 10.1080/13803395.2012.668874. [DOI] [PubMed] [Google Scholar]
- Griffith-Lendering MFH, Huijbregts SCJ, Mooijaart A, Vollebergh WAM, Swaab H, 2011. Cannabis use and development of externalizing and internalizing behaviour problems in early adolescence: a TRAILS study. Drug Alcohol Depend. 116, 11–17. 10.1016/j.drugalcdep.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Gonenc A, Smith RT, Lambros AM, Cabrera KB, Lukas SE, 2018. The grass might be greener: medical marijuana patients exhibit altered brain activity and improved executive function after 3 months of treatment. Front. Pharmacol 8. 10.3389/fphar.2017.00983.eCollection2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, 2017. Marijuana on the mind? The impact of marijuana on cognition, brain structure, and brain function, and related public policy implications. Policy Insights Behav. Brain Sci 4, 104–111. 10.1177/2372732216684851. [DOI] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine RT, Smith RT, Lukas SE, 2016. Splendor in the grass? A pilot study assessing the impact of medical marijuana on executive function. Front. Pharmacol 7. 10.3389/fphar.2016.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gonenc A, Lukas SE, 2014. Worth the wait: effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacology 231, 1455–1465. 10.1007/s00213-013-3326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE, 2012a. Age of onset of marijuana use and executive function. Psychol. Addict. Behav 26, 496–506. 10.1037/a0026269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gönenc A, Killgore WDS, 2012b. Age of onset of marijuana use impacts inhibitory processing. Neurosci. Lett 511, 89–94. 10.1016/j.neulet.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding IH, Solowij N, Harrison BJ, Takagi M, Lorenzetti V, Lubman DI, Seal ML, Pantelis C, Yüeel M, 2012. Functional connectivity in brain networks underlying cognitive control in chronic cannabis users. Neuropsychopharmacology 37, 1923–1933. 10.1038/npp.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman RL, Huestis MA, 2013. Cannabis effects on driving skills. Clin. Chem 59, 478–492. 10.1373/clinchem.2012.194381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchard T, Fried PA, Hogan MJ, Cameron I, Smith AM, 2014. Marijuana use impacts cognitive interference: an fMRI investigation in young adults performing the counting Stroop task. J. Addict. Res. Ther 5, 197–203. 10.4172/2155-6105.1000197. [DOI] [Google Scholar]
- Khiabani HZ, Bramness JG, Bjørneboe A, Mørland J, 2006. Relationship between THC concentration in blood and impairment in apprehended drivers. Traffic Inj. Prev 7, 111–116. 10.1080/15389580600550172. [DOI] [PubMed] [Google Scholar]
- King SM, Iacono WG, Mcgue M, 2004. Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction 99, 1548–1559. 10.1111/j.1360-0443.2004.00893.X. [DOI] [PubMed] [Google Scholar]
- Le Strat Y, Dubertret C, Le Foil B, 2015. Impact of age at onset of cannabis use on cannabis dependence and driving under the influence in the United States. Accid. Anal. Prev 76, 1–5. 10.1016/j.aap.2014.12.015. [DOI] [PubMed] [Google Scholar]
- Lenné MG, Dietze PM, Triggs TJ, Walmsley S, Murphy B, Redman JR, 2010. The effects of cannabis and alcohol on simulated arterial driving: influences of driving experience and task demand. Accid. Anal. Prev 42, 859–866. 10.1016/j.aap.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Li M, Brady JE, DiMaggio CJ, Lusardi AR, Tzong KY, Li G, 2012. Marijuana use and motor vehicle crashes. Epidemiol. Rev 34, 65–72. 10.1093/epirev/mxr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori A, Gatto CP, Jarrett DB, 2002. Separate and combined effects of marijuana and alcohol on mood, equilibrium and simulated driving. Psychopharmacology 163, 399–405. 10.1007/s00213-002-1124-0. [DOI] [PubMed] [Google Scholar]
- Liguori A, Gatto CP, Robinson JH, 1998. Effects of marijuana on equilibrium, psychomotor performance, and simulated driving. Behav. Pharmacol 9, 599–609. 10.1097/00008877-199811000-00015. [DOI] [PubMed] [Google Scholar]
- Lisdahl KM, 2013. Dare to delay? The impacts of adolescent alcohol and marijuana use onset of cognition, brain structure and function. Front. Psychiatry 4, 53. 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Conference of State Legislatures [NCSL], 2019. State Medical Marijuana Laws. 16 October 2019. Retrieved from http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx (Accessed on 18 December 2019). .
- Patton JH, Stanford MS, Barratt ES, 1995. Factor structure of the barratt impulsiveness scale. J. Clin. Psychol 51, 768–774. [DOI] [PubMed] [Google Scholar]
- Pedersen W, Mastekaasa A, Wichstrom L, 2001. Conduct problems and early cannabis initiation: a longitudinal study of gender differences. Addiction 96, 415–431. 10.1080/0965214002005392. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Berghaus G, Van Laar M, Drummer OH, 2004. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 73, 109–119. 10.1016/j.drugalcdep.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Richer I, Bergeron J, 2009. Driving under the influence of cannabis: links with dangerous driving, psychological predictors, and accident involvement. Accid. Anal. Prev 41, 299–307. 10.1016/j.aap.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Robbe H, 1998. Marijuana’s impairing effects on driving are moderate when taken alone but severe when combined with alcohol. Hum. Psychopharmacol. Clin. Exp 13, S70–S78. [Google Scholar]
- Robertson RD, Mainegra-Hing M, Woods-Fry H, Vanlaar WGM, 2017. Road Safety Monitor 2017 Drugs & Driving in Canada. Traffic Injury Research Foundation, Ottawa, Ontario. [Google Scholar]
- Rogeberg O, Elvik R, 2016. The effects of cannabis intoxication on motor vehicle collision revisited and revised: cannabis and motor vehicle collision risk. Addiction 111, 1348–1359. 10.1111/add.13347. [DOI] [PubMed] [Google Scholar]
- Ronen A, Chassidim HS, Gershon P, Parmet Y, Rabinovich A, Bar-Hamburger R, Cassuto Y, Shinar D, 2010. The effect of alcohol, THC and their combination on perceived effects, willingness to drive and performance of driving and non-driving tasks. Accid. Anal. Prev 42, 1855–1865. 10.1016/j.aap.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Ronen A, Gershon P, Drobiner H, Rabinovich A, Bar-Hamburger R, Mechoulam R, Cassuto Y, Shinar D, 2008. Effects of THC on driving performance, physiological state and subjective feelings relative to alcohol. Accid. Anal. Prev 40, 926–934. 10.1016/j.aap.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Sagar KA, Gruber SA, 2018. Marijuana matters: reviewing the impact of marijuana on cognition, brain structure and function, and exploring policy implications and barriers to research. Int. Rev. Psychiatry 30, 251–267. 10.1080/09540261.2018.1460334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar KA, Dahlgren MK, Gönenç A, Racine MT, Dreman MW, Gruber SA, 2015. The impact of initiation: early onset marijuana smokers demonstrate altered Stroop performance and brain activation. Dev. Cogn. Neurosci 16, 84–92. 10.1016/j.dcn.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster RM, Hoeppner SS, Evins AE, Gilman JM, 2016. Early onset marijuana use is associated with learning inefficiencies. Neuropsychology 30, 405–415. 10.1037/neu0000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF, 2008. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res. 163, 40–51. 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell R, Poling J, Sofuoglu M, 2009. The effect of cannabis compared with alcohol on driving. Am. J. Addict 18, 185–193. 10.1080/10550490902786934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GL, Caneilla A, 1998. Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br. J. Addict 83, 393–402. 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration [SAMHSA], 2019. Results from the 2018 National Survey on Drug Use and Health. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Squeglia LM, Jacobus J, Nguyen-Louie TT, Tapert SF, 2014. Inhibition during early adolescence predicts alcohol and marijuana use by late adolescence. Neuropsychology 28, 782–790. 10.1037/neu0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 1999. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Wrege J, Schmidt A, Walter A, Smieskova R, Bendfeldt K, Radude EW, Lang UE, Borgwardt S, 2014. Effects of cannabis on impulsivity: a systematic review of neuroimaging findings. Curr. Pharm. Des 20 10.2174/13816128113199990428.2167-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]


