Abstract
Background
Seizures can present at any time before or after diagnosis of a brain tumor. The risk of seizures varies by tumor type and its location in the brain. For a long time we believed that preventing seizures with antiepileptic drugs (seizure prophylaxis) was effective and necessary, but the supporting evidence was little and mixed. Such evidence was the basis for previous reviews to conclude that seizure prophylaxis was ineffective in people with brain tumors.
Objectives
To estimate the effectiveness of seizure prophylaxis in people with brain tumors, and to estimate the adverse event rates in the identified clinical trials.
Search methods
A search strategy that included free‐text and MeSH terms in LILACS, EMBASE, PubMed, CENTRAL, and The Cochrane Library (1966 to 2007).
Selection criteria
Controlled clinical trials with random allocation, blinded or unblinded, and placebo or observation in the control groups.
Data collection and analysis
We screened the articles, extracted the data, and rated the validity of each trial to assess the risk of bias. Our primary outcome was the occurrence of a first seizure. The secondary outcome was adverse events. We pooled the aggregate data for each outcome into a random‐effects model meta‐analysis using the relative risk (RR). For adverse events, we also included the number needed to harm (NNH) using the absolute risk increase to compute the NNH.
Main results
There was no difference between the treatment interventions and the control groups in preventing a first seizure in participants with brain tumors. The risk of an adverse event was higher for those on antiepileptic drugs than for participants not on antiepileptic drugs (NNH 3; RR 6.10, 95% CI 1.10 to 34.63; P = 0.046).
Authors' conclusions
The evidence is neutral, neither for nor against seizure prophylaxis, in people with brain tumors. These conclusions apply only for the antiepileptic drugs phenytoin, phenobarbital, and divalproex sodium. The decision to start an antiepileptic drug for seizure prophylaxis is ultimately guided by assessment of individual risk factors and careful discussion with patients.
Plain language summary
Antiepileptic drugs for preventing seizures in people with brain tumors
Up to 60% of people with brain tumors may present with seizures, or may have a seizure for the first time after diagnosis or neurosurgery. The risk of a seizure varies with the tumor type and its location in the brain. Seizures are an added burden with a negative impact on quality of life, affecting activities of daily living, independence, work, and driving. Many doctors believe that antiepileptic drugs are effective and necessary to prevent seizures (seizure prophylaxis), but this practice has been put into question. Antiepileptic drugs can have adverse effects and they interact with steroids and chemotherapy.
The five randomised controlled trials identified by the review authors from the medical literature looked at the antiepileptic drugs phenytoin, phenobarbital, and divalproex sodium. There was no difference between treatment with these antiepileptic drugs and placebo, or observing the patient, in preventing a first seizure in 404 people with brain tumors. The risk of an adverse event was higher for those on antiepileptic drugs (number needed to be treated to cause a harm in one person (NNH) 3). The types of adverse effects when reported in these trials were nausea, skin rash, sore gums, myelosuppression, vertigo, blurred vision, tremor, and gait unsteadiness. The length of follow up was short in one study. No studies were identified for any of the newer antiepileptic drugs.
Background
Up to 60% of people with brain tumors may present with seizures or may have a seizure for the first time after diagnosis of the tumor. The risk of seizures varies according to the type of brain tumor, its grade and its location (Vecht 2003; Vecht 2006; Wen 2002). A seizure is a burden for persons with brain tumors as it has a negative impact on quality of life, including effects on activities of daily living, independence, work, and driving. Approximately 2% of people with gliomas (brain tumors of glial origin), and 3 to 16% of people with meningiomas (brain tumors arising from meningothelial cells) also seize after surgery (Ketz 1974). This range of seizure probability and the additional surgical trauma has made seizure prophylaxis, that is the prevention of new onset seizures with drugs used to treat epilepsy, a widely accepted practice despite potential adverse effects and drug interactions.
Due to the lack of benefit from seizure prophylaxis in several retrospective studies (Boarini 1985; Cohen 1988; Mahaley 1981) and the well‐known interaction of antiepileptic drugs with steroids and chemotherapy (Patsalos 2002; Vecht 2003), the question of seizure prophylaxis in people with brain tumors is important. For years there has been controversy about the indications and the effectiveness of seizure prophylaxis. Some authors have recommended prophylactic antiepileptics for cerebral metastatic melanoma and also for postoperative patients with specific conditions (Byrne 1983; Deutschman 1985) whereas others have questioned the value of such practice (Glantz 1997). Most published randomized controlled trials studied this issue in persons who had brain surgery because the postoperative cerebral edema from surgical manipulation and trauma predisposed them to seizures.
The Quality Standards Subcommittee of the American Academy of Neurology did not recommend the routine use of prophylactic antiepileptics in people with newly diagnosed brain tumors (AAN 2000), based on a meta‐analysis of four randomized controlled clinical trials. These guidelines have found support in the neuro‐oncology community as shown in reviews since published on this topic (Batchelor 2006; Vecht 2003; Vecht 2006; Wen 2002). However, the reality is that many physicians and particularly neurosurgeons in North America and Europe still prescribe antiepileptic drugs for people with brain tumors who have not had seizures (Brouwers 2003; De Santis 2002; Hildebrand 2005; Siomin 2005).
Objectives
(1) To determine if seizure prophylaxis with antiepileptic drugs is effective in people with brain tumors. (2) To estimate the adverse event rate from prophylactic antiepileptic drugs.
Methods
Criteria for considering studies for this review
Types of studies
Controlled clinical trials with random allocation, blinded or unblinded.
Types of participants
Participants with diagnosis of glioma, using the World Health Organization (WHO) classification of brain tumors (astrocytomas grades II, III, and IV; oligodendrogliomas grades II and III; ependymomas grades II and III); meningiomas; skull base tumors, and brain metastases from any primary tumor.
Types of interventions
Prophylactic antiepileptics (treatment intervention) compared with no prophylaxis or prophylaxis with a placebo (control intervention). Participants may have had surgery for the diagnosis or treatment of the underlying tumor. We excluded studies comparing two anticonvulsants.
Types of outcome measures
(1) Proportion of individuals in the treatment and control groups who were free from seizures at the time defined by the trialists as time of outcome measurement. (2) Adverse event rate: an adverse event is any untoward reaction attributed to the drug of interest regardless of dose and magnitude, causing or not causing withdrawal from the study. The drugs of interest in this review were phenytoin, carbamazepine, valproic acid, and phenobarbital. We also included in the search newer drugs such as gabapentin, pregabalin, zonisamide, lamotrigine, oxcarbazepine, levetiracetam, topiramate, vigabatrin, and tiagabine.
Search methods for identification of studies
Our search strategy included the electronic databases CENTRAL (The Cochrane Library, Issue 4/2007), PubMed, EMBASE, CancerLit (until October 2002), and LILACS (1966 to 2007). A list of the search terms we used is given in Appendix 1
We also handsearched conference proceedings, textbooks, original and review articles, and contacted clinical researchers who conducted or are conducting identified trials, if necessary. We screened non‐English articles, which were included if they were eligible for this review.
Data collection and analysis
Application of selected criteria Three of us (Ivo Tremont‐Lukats, Bernardo Ratilal, and Terri Armstrong) independently screened all titles and abstracts identified in the literature search. We resolved any disagreement after discussion with a fourth review author (Mark Gilbert) in order to reach a consensus. We were not blinded to the author names, affiliated institutions, journal of publication, or study results. We assessed methodological quality and validity by checking: (a) randomization and description of method of concealed random allocation; (b) blinding and methods used to ensure appropriate blinding; (c) description of sample size; and (d) description of adverse events, toxicity and study withdrawals.
Data collection and analysis All four of us collected data on participants, methods, interventions, outcome measurements, and adverse effects onto a spreadsheet from the original articles. We recorded outcome measurements as binary data (proportion of participants with seizures receiving or not receiving antiepileptic prophylaxis). We combined the aggregate data to obtain a pooled effect size for all included randomized trials.
Synthesis and presentation of data We analyzed the collected data using the analysis module for RevMan 4.2 (NCC 2006). We estimated the relative risk of seizures with 95% confidence intervals (CIs) between participants receiving antiepileptic prophylaxis and individuals treated with the control intervention. We explored heterogeneity using tests for statistical heterogeneity (the Q and I2 statistics) and a graphical display (funnel plots). For the analysis of adverse events we used binary data to estimate the relative risk and the number needed to harm (NNH). The NNH is equal to the inverse of the absolute risk increase (ARI), which in turn is the difference between the event rate of participants treated with antiepileptics and the control event rate. There was no opportunity to run sensitivity analyses in this systematic review.
Results
Description of studies
We found 1454 citations using the initial search strategy. Further screening narrowed the results to 74 articles. Five trials met our inclusion criteria for analysis. One of these studies was a multicenter trial in Canada and the United States (Forsyth 2003). The other trials were from Italy (Franceschetti 1990), Australia (North 1983), Taiwan (Lee 1989), and the United States (Glantz 1996). The treatment sequence of all trials had a classic parallel design. In three trials (Franceschetti 1990; Lee 1989; North 1983) the participants with brain tumors formed one of several subgroups that included several non‐neoplastic conditions. The eligibility criteria were uniform for all studies, overall. The main goal of two trials was to investigate whether antiepileptic drugs could prevent seizures in the early or late postoperative periods (Franceschetti 1990; Lee 1989) and one study followed participants for up to 12 months after craniotomy (North 1983). The remaining two studies assessed the value of seizure prevention without surgery as a potential confounding variable (Forsyth 2003; Glantz 1996). All the included trials enrolled participants with gliomas and brain metastases; three studies included participants with meningiomas (Franceschetti 1990; Lee 1989; North 1983) and three trials included patients with sellar tumors (Franceschetti 1990; Lee 1989; North 1983); one study included sellar tumors without specifying type (North 1983). The design of two trials included an estimate of how many participants were necessary to detect a difference in favor of the treatment intervention (Glantz 1996; Forsyth 2003). These two trials specified the subtype of glioma (glioblastoma, anaplastic astrocytoma, etc). The antiepileptic drugs tested were phenytoin alone (Lee 1989; North 1983) phenobarbital or phenytoin (Forsyth 2003; Franceschetti 1990), and divalproex sodium (Glantz 1996). All trials planned and described drug‐level monitoring.
We did not identify any prospective, controlled studies (randomized or nonrandomized) of seizure prophylaxis in adults or children with brain tumors using newer antiepileptic drugs.
We excluded the following studies and study reports.
Clinical trials with random allocation . One placebo‐controlled trial with random allocation published overall results for the treatment and control groups but did not include data for the subsets of participants with brain tumors (Foy 1992). Two trials were later published with the inclusion of more patients so we excluded the earlier articles (North 1980; Franceschetti 1988). One trial appeared as an abstract and did not contain data on participants with brain tumors (Holland 1995). Two trials evaluated seizure prevention using diazepam after the injection of contrast media in participants with brain metastases or with gliomas (Pagani 1983; Pagani 1984). Five other controlled trials with random allocation did not meet inclusion criteria for this review because they compared two antiepileptics: zonisamide with phenobarbital (Nakamura 1999); phenytoin with valproic acid (Beenen 1999; Zhang 2000); phenytoin as add‐on to phenobarbital or carbamazepine with either of these drugs alone (De Santis 2002); and different doses of phenytoin (Levati 1996).
Retrospective studies . We found nine studies: two were abstracts that have not been fully published (Dent 1996; Hung 1991); four articles dealt with seizure prevention in patients with gliomas (Boarini 1985; Mahaley 1981; Mauro 2007; Moots 1995); and three studies were on patients with brain metastases (Byrne 1983; Cohen 1988; Hagen 1990). None of these were clinical trials but rather reviews of retrospective data from clinical charts.
Prospective trials . One prospective study enrolled participants with meningiomas but there was no control group (Tsuji 1993).
Risk of bias in included studies
Risk of bias in included studies We rated and summarized study validity using a simple but effective approach outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2006) (Table 1). Three of the included trials were very vulnerable to bias: in one, the sequence generation and allocation concealment were unclear (North 1983); in another, the authors described the sequence generation but not allocation concealment (Lee 1989); in the remaining trial the sequence generation was unclear and there was no allocation concealment (Franceschetti 1990). One trial described sequence generation, allocation concealment, did not report results selectively, was free from other bias, included information on adverse events and withdrawals from the study, and had the lowest risk of bias (Glantz 1996). The fifth trial had a moderate risk of bias because it did not include a placebo intervention (Forsyth 2003) (Figure 1).
1. Categories of Risk of Bias and Their Meaning.
| Risk of Bias | Interpretation | Individual Criteria |
| Low Interpretation Relation to individual criteria Low Bias unlikely to alter results All criteria met Moderate Bias that raises some doubt about results One or more criteria partly met High Bias that seriously weakens confidence in the results | Bias unlikely to alter results Interpretation Relation to individual criteria Low Bias unlikely to alter results All criteria met Moderate Bias that raises some doubt about results One or more criteria partly met High Bias that seriously weakens confidence in the results One or more criteria not met | All criteria are met |
| Moderate | Bias that raises some questions about results | One or more criteria partly met |
| High | Bias that seriously weakens confidence in results | One or more criteria not met |
1.
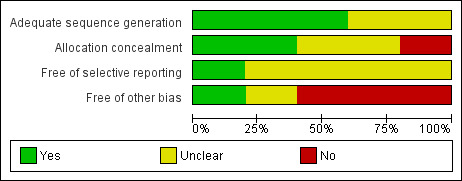
Risk‐of‐bias graph: Review authors' judgments about each risk‐of‐bias item presented as percentages across all included studies.
Effects of interventions
Effectiveness to prevent seizures . For this outcome there were 404 participants: 210 allocated to the group treated with antiepileptic drugs, and 194 allocated to the control group. Prophylaxis with the antiepileptic drugs phenytoin, phenobarbital, or divalproex sodium was no better than placebo or observation (RR 0.94, 95% CI 0.55 to 1.61; P = 0.82). The trials were ranked by weight in a forest plot. One trial was the least precise and contributed little to the meta‐analysis because participants were followed for 72 hours after surgery and there were no seizures in the treatment group (Lee 1989). Therefore, a sensitivity analysis without this trial was unnecessary. The model was reasonably homogeneous (I2 = 38.5%) and the funnel plot did not suggest publication bias (Figure 2).
Adverse events . One study did not report adverse events (Lee 1989) and another reported them but gave only overall results (North 1983). Franchescetti and collaborators reported that three patients in the group allocated to phenytoin and one participant in the group allocated to phenobarbital had neurological adverse effects in the first postoperative week without further detail (Franceschetti 1990). Two trials detailed the adverse events. In one trial 13 of 46 participants taking antiepileptic drugs had adverse events: nausea (4), rash (3), sore gums (1), myelosuppression (1), increased levels of lactate dehydrogenase (1), vertigo and blurred vision (1), tremor (1), and gait ataxia (1) (Forsyth 2003). Glantz and collaborators reported three patients who developed skin rash (two receiving divalproex sodium, one allocated to placebo) (Glantz 1996). Overall, there were 237 participants with data for this outcome: 124 allocated to the treatment group, and 113 to the control group. The participants who received antiepileptics were more likely to have adverse effects (19 events in the treatment group (15% of participants), and one in the control group (0.9%) (RR 6.10, 95% CI 1.10 to 34.63; P = 0.04). The number of participants taking antiepileptic drugs for one to experience an adverse effect (NNH) was three (Table 2). The model was homogeneous with a symmetric funnel plot (Figure 3).
2.
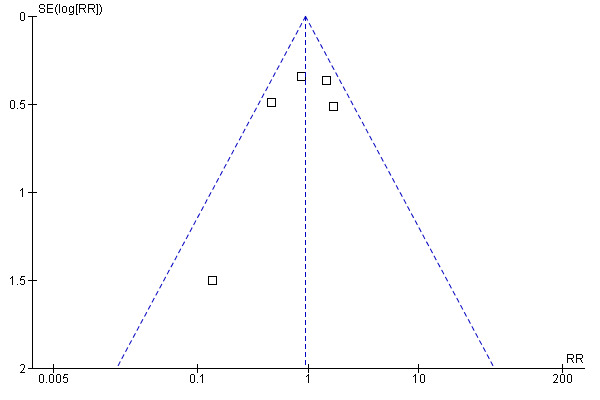
Funnel plot of comparison: 1 Prevention of seizures in participants with brain tumors, outcome: 1.1 Seizures.
2. Absolute Risk Increase (ARI) and Number Needed to Harm (NNH).
| Trial | ARI | NNH |
| Franceschetti 1990 | 4 | 25 |
| Glantz 1996 | 1 | 1 |
| Forsyth 2003 | 13 | 6 |
3.
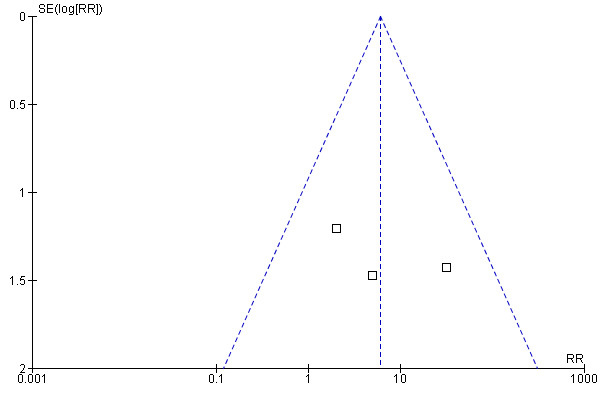
Funnel plot of comparison: 1 Prevention of seizures in participants with brain tumors, outcome: 1.2 Adverse Events.
Discussion
In these five clinical trials with random allocation phenobarbital, phenytoin, and divalproex sodium did not prevent seizures in people with brain tumors who had been seizure free before participation in the study. This conclusion is not new but we wished to perform a Cochrane review to address our reservations about how others had interpreted review results to formulate recommendations that influence health policies and medical decisions. A pioneer meta‐analysis that addressed the merit of seizure prophylaxis for supratentorial craniotomies found that "no empirical data supporting the attitude of using AEDs prophylactically with supratentorial intracranial surgery, have been presented on a scientific basis" (Kuijlen 1996). The scope of that meta‐analysis was more global and its conclusions did not apply to people with brain tumors.
At a meeting of the American Society of Clinical Oncology in 1998, an abstract (Glantz 1998) presented the results of a meta‐analysis that later evolved into the practice parameters endorsed by the American Academy of Neurology (AAN 2000). This meta‐analysis set out to answer a more specific question about the efficacy of antiepileptic drugs to prevent seizures in people with brain tumors. The focus was no longer on postoperative seizures. The AAN review concluded that seizure prophylaxis was not effective in patients with brain tumors. Therefore, the panel did not recommend its routine use and recommended that antiepileptic drugs be tapered off after the first postoperative week.
There are four pitfalls in the AAN review that weaken the strength of its conclusions. First, the reviewers misclassified eight studies as level II evidence (evidence provided by one or more well‐designed observational studies with concurrent controls) instead of level III evidence (evidence provided by studies with nonrandomized historical controls), since all those studies were retrospective chart reviews and not clinical trials. Secondly, there was no exploration of clinical or statistical heterogeneity despite acknowledging the importance of heterogeneity as background noise in the interpretation of meta‐analytic results. Randomized, controlled clinical trials are less susceptible, but not immune, to multiple sources of bias and can suffer from heterogeneity. Third, the adverse event rate quoted in the AAN review (23.8%) is the pooled result from three randomized trials (Forsyth 2003; Franceschetti 1990; Glantz 1996), and four retrospective studies with historical controls (Hagen 1990; Hung 1991; Mahaley 1981; Moots 1995). This indicates a selective bias in the reporting of outcomes. Finally, four of the main authors of the AAN review were the principal investigators or coauthors of two clinical trials included in the meta‐analysis. The clinical trials included in all these systematic reviews are of good quality but we have concerns about their extracting data from subgroups (brain tumors, aneurysms, arteriovenous malformations) and analyzing data from subsets of subgroups (brain metastases, sellar tumors, gliomas) with even fewer participants. Therefore, the probability of detecting a difference is zero. This flaw is applicable to another review (Sirven 2004) and we chose not to analyze subgroup data because of the imprecision of these meta‐analysis results.
The second group published their meta‐analysis with similar results to those of the AAN meta‐analysis (Sirven 2004) when our review was under development. These reviewers pointed out some of the methodological flaws of the earlier review, such as the presence of clinical heterogeneity and the confounding effect of surgery, yet they analyzed subsets of subgroups by examining three tumor types separately. The wide 95% confidence intervals attest to the large uncertainty and imprecision of these results. Based on these subset analyses, one conclusion of the review was that antiepileptic drugs were not effective in preventing seizures in people with gliomas, metastases, or meningiomas.
The best data we have is from a collection of different brain tumors with different seizure risks, each subgroup with few participants. Therefore, the strength of evidence showing that antiepileptic drugs are ineffective for seizure prophylaxis is not as solid as stated in previous reviews on this topic and is largely based on two trials, only one of which had enough statistical power (Glantz 1996). Evidence of this nature is inconclusive and hence we prefer to say that the best evidence available at present is neither in favor nor against seizure prophylaxis in brain tumors.
However, it is unlikely from these results that there is a clinically important effect of phenytoin, phenobarbital, and divalproex sodium in preventing seizures in the absence of careful drug‐level monitoring. Therefore, it is important to test the efficacy of newer antiepileptic drugs in this setting, beginning with phase II studies. Levetiracetam could be a promising candidate because it has an intravenous formulation and can be used preoperatively and in the immediate postoperative period. The design of these trials should include random allocation maintained throughout the trial, control with the use of placebo, double blinding, and follow up for one to three months to avoid the problem of high mortality rates present in at least one trial (Forsyth 2003) and no outcomes after a very short follow‐up (Lee 1989).
Adverse events As for the seizure outcome, an analysis of adverse effects is difficult and incomplete because some clinical trials did not routinely report adverse events. In our review we tried to compare the adverse event rate between treatment with antiepileptic drugs and control interventions. The risk of adverse effects was significantly higher in people taking antiepileptic drugs but not as high as presented in the AAN review, in which the data from retrospective studies could have inflated the estimate. However, we recognize that older antiepileptic drugs may have a higher rate of adverse events. Therefore, the risk‐to‐benefit analysis of seizure prophylaxis can improve by using newer antiepileptic drugs. We do not underestimate the toxicity of the older generations of antiepileptic drugs and decisions about seizure prophylaxis need to weigh up the side‐effect profile of these drugs.
Authors' conclusions
Implications for practice.
The evidence for seizure prophylaxis with phenobarbital, phenytoin, and divalproex sodium in people with brain tumors is inconclusive, at best. The clinical heterogeneity between and within trials limits any claim of effectiveness or ineffectiveness. Therefore, there are no data supporting the use of prophylactic antiepileptics and the risk of adverse events lessens their overall potential benefit. Use of these antiepileptic drugs is associated with a higher risk of adverse events than in a control group, which is a major factor to consider when deciding to start seizure prophylaxis.
Implications for research.
There is a need for trials using adaptive randomization methods that will allow us to test different newer antiepileptics. The active participation of neurosurgeons as investigators in these trials may enhance the impact of these trials in changing clinical management paradigms and longstanding dogmas.
What's new
| Date | Event | Description |
|---|---|---|
| 24 September 2008 | Amended | Converted to new review format. |
Acknowledgements
Vivien Liu, RN, Research Nurse Supervisor for clinical trials in the Neuro‐Oncology Department, and Weiming She, MD, Department of Neurosurgery, The University of Texas ‐ MD Anderson Cancer Center translated one trial published in Chinese (Zhang 2000).
Appendices
Appendix 1. Search strategy
We searched using the following free text or MeSH terms, modified to suit the different databases:
#1. Clinical trials #2. Controlled clinical trial #3. randomized clinical trial #4. Random allocation #5. Prospective study #6. Comparative study #7. Double blind #8. Placebo #9. Parallel design #10. Crossover design #11. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 #12. Brain neoplasms #13. Brain tumors #14. Primary brain tumors #15. Secondary brain tumors #16. Brain metasta* #17. Glioma* #18. Astrocytoma* #19. Oligodendroglioma* #20. Glioblastoma multiforme #21. Glial tumors #22. Ependymoma #23. Meningioma #24. #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 #25. Seizure* #26. Anticonvulsants #27. Antiepileptics #28. Phenytoin #29. Valproic acid #30. Sodium valproate #31. Phenobarbi* #32. Oxcarbazepine #33. Lamotrigine #34. Gabapentin #35. Pregabalin #36. Zonisamide #37. Topiramate #38. Vigabatrin #39. Tiagabine #40. Prophylaxis #41. Prevention #42. Craniotomy #43. Epilepsy #44. Neurosurgery #45. Neurooncology #46. #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 not animal
Data and analyses
Comparison 1. Prevention of seizures in participants with brain tumors.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure occurrence | 5 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.55, 1.61] |
| 2 Adverse event rate | 3 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 6.09 [1.07, 34.63] |
1.1. Analysis.
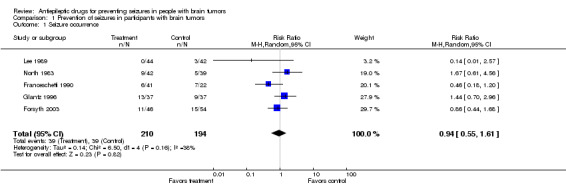
Comparison 1 Prevention of seizures in participants with brain tumors, Outcome 1 Seizure occurrence.
1.2. Analysis.
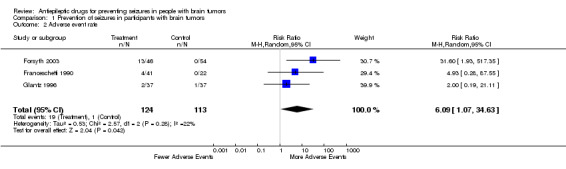
Comparison 1 Prevention of seizures in participants with brain tumors, Outcome 2 Adverse event rate.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Forsyth 2003.
| Methods | Random allocation, unmasked design | |
| Participants | 100; 60 had brain metastases (26 in the treatment arm, 34 controls), and 40 had gliomas (20 in each arm) | |
| Interventions | Treatment group: Phenytoin 15mg/kg oral loading in 3 divided doses, followed by 5mg/kg po qd (n=45). If intolerance, phenobarbital was used instead (n=1). The control group did not receive treatment | |
| Outcomes | For prevention of seizures after start of therapy, phenytoin was not better than placebo in brain metastases (P = 0.6, logrank), and in gliomas (P=0.95, logrank) | |
| Notes | Trial stopped at 100 pts because seizure and survival rates were lower than expected. The investigators had estimated the sample size for a statistical power of 80% to detect a 15% difference between groups. The trial ultimately had a power of 20% to detect a positive difference | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Franceschetti 1990.
| Methods | Random allocation, placebo‐controlled design, unblinded | |
| Participants | 63 of 128 participants had never seized. They had meningioma (n=27), malignant glioma (n=23) or metastases (n=13) | |
| Interventions | Group A: 65 pts with preoperative seizures (not considered in this review). Group B: 63 pts without preoperative seizures, randomized in three subgroups: no treatment (n=22); Phenobarbital 4 mg/kg/day x 5 days, then 2mg/kg/day orally (n=25); and phenytoin 10 mg/kg/d x 5 days, then 5 mg/kg/d orally once daily (n=16) | |
| Outcomes | Prophylaxis lowered early postop seizures compared with no treatment (7% vs. 18%) without reaching statistical significance. No effect in preventing late postoperative seizures. The authors recommended prevention with phenobarbital for the first postoperative wk 1, but not for later | |
| Notes | No trial size calculations. Unspecified adverse events in four participants in the treatment group during the first postoperative week. No mention of adverse effects in the late postoperative period and no mention of withdrawals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Glantz 1996.
| Methods | Random allocation, double‐blind, placebo‐controlled design with calculation of sample size | |
| Participants | Metastatic or primary brain tumors without history of seizures Placebo group: Lung cancer (n=28), non‐Hodgkin lymphoma (n=2), glioblastoma (n=4), melanoma (n=1), other (n=2). Treatment group: lung cancer (n=23), breast cancer (n=4), GBM (n=5), melanoma (n=1), and other tumors (n=4) |
|
| Interventions | Patients took placebo or valproic acid. Dose of valproic acid was adjusted to levels 50‐100 ug/mL | |
| Outcomes | There was no difference between valproic acid and placebo to prevent seizures (P=0.7, Fisher test) | |
| Notes | This trial had the highest methodological validity. It also avoided the confounding effect of surgery on seizures since participants entered the trial after 14 days of diagnosis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Lee 1989.
| Methods | Random allocation, double‐blind, placebo‐controlled design | |
| Participants | Adult eligible patients without history of seizures, who had meningioma (n=50), glioma (n=30), metastases (n=5) | |
| Interventions | Phenytoin: 15mg/kg intravenously before wound closure, then 5‐6 mg/kg/day intravenously, or placebo three times daily in the first three postoperative days | |
| Outcomes | Phenytoin was not more effective than placebo to prevent immediate and early postoperative seizures | |
| Notes | Study with no power to compare the subset with brain tumors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
North 1983.
| Methods | Random allocation, double‐blind design | |
| Participants | Participants with a supratentorial tumor, allocated to 1. Phenytoin: Meningioma (n=10), metastasis (n=6), sellar tumor (n=10), glioma (n=16) 2. Placebo group: meningioma (n=9), metastasis (n=7), sellar tumor (n=7), glioma (n=16) |
|
| Interventions | Phenytoin 250 mg twice daily iv, then 100 mg orally three times daily x 12 months. Serum levels once a month for inpatients, bimonthly for outpatients. Doses were adjusted accordingly | |
| Outcomes | 18 seizures in group treated with phenytoin, 26 in placebo group. By time‐to‐event analysis, group treated with PHT had significantly fewer seizures between days 7‐72 of study. The maximal protective effect was in postop week 2 | |
| Notes | Because 65% of seizures occurred within 3 mo from surgery, authors recommended prophylaxis for 2‐3 mo, starting 1 wk before surgery. Focal seizures in 7/9 pts with therapeutic levels | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Avanzini 1982 | Not a clinical trial |
| Beenen 1999 | Trial with random allocation that compared two anticonvulsants (phenytoin and valproic acid) |
| Boarini 1985 | Retrospective chart review |
| Byrne 1983 | Retrospective study |
| Cohen 1988 | Retrospective study |
| De Santis 2002 | Inclusion of participants with history of seizures |
| Dent 1996 | Retrospective study with historical controls |
| Foy 1992 | Outcome data not extractable for patients with brain tumors in the results section |
| Franceschetti 1988 | It is a duplicate of Franceschetti 1990 with fewer patients |
| Hagen 1990 | Retrospective chart review |
| Holland 1995 | A double‐blind, randomized trial using valproic acid for prevention of seizures after craniotomy or head injury, this trial has not been fully published, and no data about brain tumors are in the abstract |
| Hung 1991 | Retrospective chart review |
| Levati 1996 | Study with random allocation comparing three different doses of phenytoin for prevention of postoperatory seizures in participants with supratentorial brain tumors, there was no placebo or suitable control group |
| Mahaley 1981 | Not a controlled clinical trial, but a retrospective study |
| Mauro 2007 | Retrospective study |
| Moots 1995 | A retrospective review |
| Nakamura 1999 | Double‐blind trial with random allocation comparing zonisamide with phenobarbital |
| North 1980 | This trial was later expanded and published elsewhere, North 1983 |
| Pagani 1983 | Prevention of seizures induced by contrast media in patients with brain metastases |
| Pagani 1984 | Prevention of seizures induced by contrast media in patients with glioma |
| Tsuji 1993 | Observational study of 20 patients with meningioma who received valproic acid for seizure prevention, this study did not use a control group |
| Zhang 2000 | A controlled clinical trial with random allocation that compared phenytoin and valproic acid for the prevention of postoperatory seizures |
Contributions of authors
Ivo W Tremont‐Lukats: conception and planning of the protocol, literature search, data collection and analyis, and manuscript writing. Bernardo Ratilal: literature search, data collection and analysis, manuscript writing. Terri Armstrong: literature search, data collection, analysis and manuscript writing. Mark R Gilbert: typescript proofreading, editing, and advice on methodology.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Forsyth 2003 {published data only}
- Forsyth PA, Weaver S, Fulton D, Brasher PM, Sutherland G, Stewart D, et al. Prophylactic anticonvulsants in patients with brain tumour. The Canadian Journal of Neurological Sciences. Le Journal Canadien des Sciences Neurologiques 2003;30(2):106‐12. [DOI] [PubMed] [Google Scholar]
Franceschetti 1990 {published data only}
- Franceschetti S, Binelli S, Casazza M, Lodrini S, Panzica F, Pluchino F, et al. Influence of surgery and antiepileptic drugs on seizures symptomatic of cerebral tumours. Acta Neurochirurgica 1990;103(1‐2):47‐51. [DOI] [PubMed] [Google Scholar]
Glantz 1996 {published data only}
- Glantz MJ, Cole BF, Friedberg MH, Lathi E, Choy H, Furie K, et al. A randomized, blinded, placebo‐controlled trial of divalproex sodium prophylaxis in adults with newly diagnosed brain tumors. Neurology 1996;46(4):985‐91. [DOI] [PubMed] [Google Scholar]
Lee 1989 {published data only}
- Lee ST, Lui TN, Chang CN, Cheng WC, Wang DJ, Heimburger RF, Lin CG. Prophylactic anticonvulsants for prevention of immediate and early postcraniotomy seizures. Surgical Neurology 1989;31(5):361‐4. [DOI] [PubMed] [Google Scholar]
North 1983 {published data only}
- North JB, Penhall RK, Hanieh A, Frewin DB, Taylor WB. Phenytoin and postoperative epilepsy. A double‐blind study. Journal of Neurosurgery 1983;58(5):672‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Avanzini 1982 {published data only}
- Avanzini G, Boroni S, Franceschetti S, Solero CL, Spreafico R. Pharmacologic prophylaxis of epileptic seizures occurring after surgery for intracranial tumors [Crisi post‐operatorie e profilassi farmacologica in soggetti affetti da neoplasie cerebrali]. Bolletino Lega Italiana contro L'Epilessia 1982;39:53‐5. [Google Scholar]
Beenen 1999 {published data only}
- Beenen LF, Lindeboom J, Kasteleijn‐Nolst Trenite DG, Heimans JJ, Snoek FJ, et al. Comparative double blind clinical trial of phenytoin and sodium valproate as anticonvulsant prophylaxis after craniotomy: efficacy, tolerability, and cognitive effects. Journal of Neurology, Neurosurgery, and Psychiatry 1999;67(4):474‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boarini 1985 {published data only}
- Boarini DJ, Beck DW, VanGilder JC. Postoperative prophylactic anticonvulsant therapy in cerebral gliomas. Neurosurgery 1985;16(3):290‐2. [DOI] [PubMed] [Google Scholar]
Byrne 1983 {published data only}
- Byrne TN, Cascino TL, Posner JB. Brain metastasis from melanoma. Journal of Neuro‐oncology 1983;1(4):313‐7. [DOI] [PubMed] [Google Scholar]
Cohen 1988 {published data only}
- Cohen N, Strauss G, Lew R, Silver D, Recht L. Should prophylactic anticonvulsants be administered to patients with newly‐diagnosed cerebral metastases? A retrospective analysis. Journal of Clinical Oncology 1988;6(10):1621‐4. [DOI] [PubMed] [Google Scholar]
De Santis 2002 {published data only}
- Santis A, Villani R, Sinisi M, Stocchetti N, Perucca E. Add‐on phenytoin fails to prevent early seizures after surgery for supratentorial brain tumors: A randomized controlled study. Epilepsia 2002;43(2):175‐82. [DOI] [PubMed] [Google Scholar]
Dent 1996 {unpublished data only}
- Dent S, Bociek G. Prophylactic anticonvulsants for cancer patients with newly diagnosed brain metastases. American Society of Clinical Oncology Proceedings 1996;15:529. [Google Scholar]
Foy 1992 {published data only}
- Foy PM, Chadwick DW, Rajgopalan N, Johnson AL, Shaw MD. Do prophylactic anticonvulsant drugs alter the pattern of seizures after craniotomy?. Journal of Neurology, Neurosurgery, and Psychiatry 1992;55(9):753‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Franceschetti 1988 {published data only}
- Franceschetti S, Binelli S, Casazza M, Croci D, Lodrini S, Panzica F, et al. Postoperative seizures in patients operated on supratentorial neoplasms: A prospective study [Crisi postoperatorie in pazienti operati per neoplasie sopratentoriali: studio prospettico]. Bolletino della Lega Italiana contro L'Epilessia 1988;62‐63:413‐5. [Google Scholar]
Hagen 1990 {published data only}
- Hagen NA, Cirrincione C, Thaler HT, DeAngelis LM. The role of radiation therapy following resection of single brain metastasis from melanoma. Neurology 1990;40(1):158‐60. [DOI] [PubMed] [Google Scholar]
Holland 1995 {unpublished data only}
- Holland JP, Stapleton SR, Moore AJ, Marsh HT, Uttley D, Bell BA. A randomised double blind study of sodium valproate for the prevention of seizures in neurosurgical patients. Journal of Neurology, Neurosurgery, and Psychiatry 1995;58:116. [Google Scholar]
Hung 1991 {unpublished data only}
- Hung S, Hilsenbeck S, Feun L. Seizure prophylaxis with phenytoin in patients with brain metastases. American Society of Clinical Oncology Proceedings 1991;10:327. [Google Scholar]
Levati 1996 {published data only}
- Levati A, Savoia G, Zoppi F, Boselli L, Tommasino C. Peri‐operative prophylaxis with phenytoin: dosage and therapeutic plasma levels. Acta neurochirurgica. Acta Neurochirurgica 1996;138(3):274‐8. [DOI] [PubMed] [Google Scholar]
Mahaley 1981 {published data only}
- Mahaley MS Jr, Dudka L. The role of anticonvulsant medications in the management of patients with anaplastic gliomas. Surgical Neurology 1981;16(6):399‐401. [DOI] [PubMed] [Google Scholar]
Mauro 2007 {published data only}
- Mauro AM, Bomprezzi C, Morresi S, Provinciali L, Formica F, Iacoangeli M, Scerrati M. Prevention of early postoperative seizures in patients with primary brain tumors: preliminary experience with oxcarbazepine. Journal of Neuro‐oncology 2007;81(3):279‐85. [DOI] [PubMed] [Google Scholar]
Moots 1995 {published data only}
- Moots PL, Maciunas RJ, Eisert DR, Parker RA, Laporte K, Abou‐Khalil B. The course of seizure disorders in patients with malignant gliomas. Archives of Neurology 1995;52(7):717‐24. [DOI] [PubMed] [Google Scholar]
Nakamura 1999 {published data only}
- Nakamura N, Ishijima B, Mayanagi Y, Manaka S. A randomized controlled trial of zonisamide in postoperative epilepsy: A report of the Cooperative Group Study. Japanese Journal of Neurosurgery 1999;8(10):647‐56. [Google Scholar]
North 1980 {published data only}
- North JB, Penhall RK, Hanieh A, Hann CS, Challen RG, Frewin DB. Postoperative epilepsy: a double‐blind trial of phenytoin after craniotomy. Lancet 1980;1(8165):384‐6. [DOI] [PubMed] [Google Scholar]
Pagani 1983 {published data only}
- Pagani JJ, Hayman LA, Bigelow RH, Libshitz HI, Lepke RA, Wallace S. Diazepam prophylaxis of contrast media‐induced seizures during computed tomography of patients with brain metastases. American Journal of Roentgenology 1983;140(4):787‐92. [DOI] [PubMed] [Google Scholar]
Pagani 1984 {published data only}
- Pagani JJ, Hayman LA, Bigelow RH, Libshitz HI, Lepke RA. Prophylactic diazepam in prevention of contrast media‐associated seizures in glioma patients undergoing cerebral computed tomography. Cancer 1984;54(10):2200‐4. [DOI] [PubMed] [Google Scholar]
Tsuji 1993 {published data only}
- Tsuji M, Shinomiya S, Inoue R, Sato K. Prospective study of postoperative seizure in intracranial meningioma. The Japanese Journal of Psychiatry and Neurology 1993;47(2):331‐4. [DOI] [PubMed] [Google Scholar]
Zhang 2000 {published data only}
- Zhang Y, Zhou LF, Du GH, Gao L, Xu B, Xu J, Gu YX. Phenytoin or sodium valproate for prophylaxis of postoperative epilepsy: a randomized comparison. Chinese Journal of Nervous and Mental Diseases 2000;26(4):231‐3. [Google Scholar]
Additional references
AAN 2000
- Glantz MJ, Cole BF, Forsyth PA, Recht LD, Wen PY, Chamberlain MC, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000;54(10):1886‐93. [DOI] [PubMed] [Google Scholar]
Agbi 1993
- Agbi CB, Bernstein M. Seizure prophylaxis for brain tumour patients. Brief review and guide for family physicians. Canadian Family Physician Medecin de Famille Canadien 1993;39:1153‐6, 1159‐60, 1163‐4. [PMC free article] [PubMed] [Google Scholar]
Batchelor 2006
- Batchelor TT, Byrne TN. Supportive care of brain tumor patients. Hematology/Oncology Clinics of North America 2006;20(6):1337‐61. [DOI] [PubMed] [Google Scholar]
Brouwers 2003
- Brouwers MC, Chambers A, Perry J, The Neuro‐oncology Disease Site Group. Can surveying practitioners about their practices help identify priority clinical practice guideline topics?. BMC Health Services Research 2003;3(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cascino 2004
- Cascino GD. "Antiepileptic" not "antiepileptogenic" drug therapy: ineffective prophylaxis of seizures. Mayo Clinic Proceedings 2004;79(12):1487‐98. [DOI] [PubMed] [Google Scholar]
Deutschman 1985
- Deutschman CS, Haines SJ. Anticonvulsant prophylaxis in neurological surgery. Neurosurgery 1985;17(3):510‐7. [DOI] [PubMed] [Google Scholar]
Glantz 1997
- Glantz M, Recht LD. Epilepsy in the cancer patient. In: Vinken PJ, Bruyn GW editor(s). Handbook of Neurology. Vol. 25(69): Neuro‐oncology, Part III, Amsterdam: Elsevier, 1997:9‐18. [Google Scholar]
Glantz 1998
- Glantz MJ, Cole BF, Forsyth PA, Recht L. Meta‐analysis of anticonvulsant prophylaxis (ACP) in seizure‐naive patients (pts) with brain tumors. American Society of Clinical Oncology Proceedings 1998;17:388a. [Google Scholar]
Higgins 2006
- Higgins JPT, Green S, editors. Aproaches to summarising the validity of studies. Section 6.7. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. Cochrane Database of Systematic Reviews 2005, Issue 3. [Google Scholar]
Hildebrand 2005
- Hildebrand J, Lecaille C, Perennes J, Delattre JY. Epileptic seizures during follow‐up of patients treated for primary brain tumors. Neurology 2005;65:212‐5. [DOI] [PubMed] [Google Scholar]
Ketz 1974
- Ketz E. Brain tumours and epilepsy. In: Vinken PJ, Bruyn GW editor(s). Handbook of Clinical Neurology. Vol. 16, Amsterdam: North‐Holland Publishing Company, 1974:254‐69. [Google Scholar]
Kuijlen 1996
- Kuijlen JM, Teernstra OP, Kessels AG, Herpers MJ, Beuls EA. Effectiveness of antiepileptic prophylaxis used with supratentorial craniotomies: a meta‐analysis. Seizure 1996;5(4):291‐8. [DOI] [PubMed] [Google Scholar]
Kvam 1983
- Kvam DA, Loftus CM, Copeland B, Quest DO. Seizures during the immediate post‐operative period. Neurosurgery 1983;12(1):14‐7. [DOI] [PubMed] [Google Scholar]
Lackner 1991
- Lackner TE. Interaction of dexamethasone with phenytoin. Pharmacotherapy 1991;11(4):344‐7. [PubMed] [Google Scholar]
NCC 2006 [Computer program]
- The Cochrane Collaboration. RevMan Analyses [computer program]. Version 1.0 for Windows. In: Review Manager (RevMan) 4.2.10. Oxford, England: The Cochrane Collaboration, 2006.
Patsalos 2002
- Patsalos PN, Fröscher W, Pisani F, Rijn CM. The importance of drug interactions in epilepsy therapy. Epilepsia 2002;43(4):365‐85. [DOI] [PubMed] [Google Scholar]
Ruegg 2002
- Ruegg S. Dexamethasone/phenytoin interactions: neurooncological concerns. Swiss Medical Weekly 2002;132(29‐30):425‐6. [DOI] [PubMed] [Google Scholar]
Siomin 2005
- Siomin V, Angelov L, Vogelbaum MA. Results of a survey of neurosurgical practice patterns regarding the prophylactic use of anti‐epilepsy drugs in patients with brain tumors. Journal of Neuro‐oncology 2005;74(2):211‐5. [DOI] [PubMed] [Google Scholar]
Sirven 2004
- Sirven JI, Wingerchuk DM, Drazkowski JF, Lyons MK, Zimmerman RS. Seizure prophylaxis in patients with brain tumors: a meta‐analysis. Mayo Clinic Proceedings 2004;79(12):1489‐94. [DOI] [PubMed] [Google Scholar]
Vecht 2003
- Vecht CJ, Wagner GL, Wilms EB. Treating seizures in patients with brain tumors: Drug interactions between antiepileptic and chemotherapeutic agents. Seminars in Oncology 2003;30 Suppl 19(6):49‐52. [DOI] [PubMed] [Google Scholar]
Vecht 2006
- Vecht CJ, Breemen M. Optimizing therapy of seizures in patients with brain tumors. Neurology 2006;67 Suppl 4(12):10‐3. [DOI] [PubMed] [Google Scholar]
Wen 2002
- Wen PY, Marks PW. Medical management of patients with brain tumors. Current Opinions in Oncology 2002;14(3):299‐307. [DOI] [PubMed] [Google Scholar]


