Abstract
Inflammation is a major contributor to the pathogenesis of almost all liver diseases. Low-molecular-weight proteins called chemokines are the main drivers of liver infiltration by immune cells such as macrophages, neutrophils and others during an inflammatory response. During the past 25 years, tremendous progress has been made in understanding the regulation and functions of chemokines in the liver. This Review summarizes three main aspects of the latest advances in the study of chemokine function in liver diseases. First, we provide an overview of chemokine biology, with a particular focus on the genetic and epigenetic regulation of chemokine transcription as well as on the cell type-specific production of chemokines by liver cells and liver-associated immune cells. Second, we highlight the functional roles of chemokines in liver homeostasis and their involvement in progression to disease in both human and animal models. Third, we discuss the therapeutic opportunities targeting chemokine production and signalling in the treatment of liver diseases, such as alcohol-associated liver disease and nonalcoholic steatohepatitis, including the relevant preclinical studies and ongoing clinical trials.
Chemokines are small, basic, heparin-binding and chemotactic cytokines that were discovered in the late 1970s and early 1980s1–3. In 1999, a systematic nomenclature was established and they were divided into four families: CXCLs, CCLs, CX3CLs and XCLs4. Chemokines are a family of more than 50 small cytokine-like proteins that chemoattract and activate immune cells during inflammation and in diseases such as cancer. In human liver diseases and experimental animal liver injury models, chemokines can be produced from different cell types in response to various external stimuli. These small molecules have been well known to regulate immune cell infiltration and modulate the activation and proliferation of a variety of liver cells, including hepatocytes, hepatic stellate cells (HSCs) and endothelial cells5. Through interactions with 19 chemokine receptors expressed on both immune cells and liver-resident cells, chemokines have been shown to have crucial roles in a wide range of biological processes both in health and disease6. With the advances in functional genomics, the transcriptional regulation of chemokines has been enthusiastically studied. These studies might lead to new therapeutic targets for acute and chronic liver diseases such as alcoholic hepatitis, cirrhosis and hepatocellular carcinoma (HCC).
Chemokines interact through cell surface-specific receptors7, signalling to cells and changing cell behaviour such as cell migration. In addition, chemokines can lead to signalling pathway activation by binding to glycosaminoglycans (GAGs) on endothelial cells, epithelial cells and the extracellular matrix. GAG interactions can result in the generation of soluble chemotactic and tissue-bound hepatic gradients for neutrophils or monocytes8,9.
In this Review, we discuss the function and regulation of chemokines as well as their associated signalling pathways. In particular, we address the genetic and epigenetic regulation of chemokine transcription and the cell-type specificity of chemokine production involving liver cells such as hepatocytes, liver sinusoidal endothelial cells (LSECs), HSCs and liver-associated immune cells (mainly macrophages (Kupffer cells), neutrophils, natural killer (NK) cells and natural killer T (NKT) cells) in healthy and disease conditions. The multiple functional roles of chemokines in homeostasis, in animal disease models (particularly mouse experimental models, including acute and chronic liver injury) and in human liver diseases are discussed in detail. Finally, several novel therapeutics targeting chemokine signalling have shown promising results in preclinical studies of animal liver disease models and in clinical trials. We highlight some of these agents, with an emphasis on anti-inflammatory agents and epigenetic-targeting drugs.
Chemokine biology and liver-specific chemokines
The chemokine superfamily is a group of 8–10 kDa, positively charged, secreted proteins with 20–50% sequence homology, which is reflected in their shared structural characteristics10,11. All chemokines have a highly conserved, three-stranded β-sheet or α-helix tertiary structural fold12,13. Their quaternary structures can vary. The sequential positions of the first two cysteines divide the chemokines into four families: CC-chemokines, CXC-chemokines, C-chemokines and CX3C-chemokines. All chemokine families have genes encoding 50 chemokine ligands in humans and there are similar protein structures but different numbers of chemokines in mice. The connections between original chemokine names and modern classification are described in TABLE 1.
Table 1 |.
List of chemokines and their receptors in the liver
| Standard name of chemokine ligand | Alternate name | Associated receptor | Key immunoregulatory functions |
|---|---|---|---|
| CXC | |||
| CXCL1 | Human: GROa, MGSA Mouse: KC |
CXCR2 | Neutrophil trafficking |
| CXCL2 | Human: GROb, MIP2α Mouse: MIP2 |
CXCR2 | Neutrophil trafficking and transendothelial migration |
| CXCL5 | Human: ENA78 Mouse: LIX |
CXCR2 | Neutrophil trafficking |
| CXCL6 | GCP2 | CXCR1 and CXCR2 | Neutrophil–monocyte trafficking |
| CXCL8 | IL-8 | CXCR1 and CXCR2 | Neutrophil–monocyte trafficking |
| CXCL9 | MIG | CXCR3 | TH1 immune response |
| CXCL10 | IP-10 | CXCR3 | TH1 immune response |
| CXCL11 | ITAC | CXCR3 | TH1 immune response |
| CXCL12 | SDF1 | CXCR4 and CXCR7 | Hepatic stellate cells, liver sinusoidal endothelial cells, hepatocellular carcinomas |
| CXCL13 | BLC, BCA1 | CXCR5 | B cells |
| CXCL16 | SRPSOX | CXCR6 | Natural killer T cells |
| CC | |||
| CCL1 | I-309 | CCR8 | TH2 immune response |
| CCL2 | MCP1 | CCR2 | Monocytes–macrophages |
| CCL3 | MIP1α | CCR1 and CCR5 | T cells and natural killer cells |
| CCL4 | MIP1β | CCR1 and CCR5 | T cells and natural killer cells |
| CCL5 | RANTES | CCR1 and CCR5 | T cells and natural killer cells |
| CCL7 | MCP3 | CCR1, CCR2 and CCR3 | TH2 immune response |
| CCL8 | MCP2 | CCR2 and CCR3 | TH2 immune response |
| CCL17 | TARC | CCR4 | TH2 immune response |
| CCL19 | ELC | CCR7 | T cells |
| CCL20 | MIP3α | CCR6 | TH17 cell response |
| CCL21 | SLC | CCR7 | T cells and dendritic cells |
| CCL22 | MDC | CCR4 | T regulatory cells |
| CCL25 | TECK | CCR9 | T cells and monocytes |
TH, T helper.
Chemokine receptors are classic G-protein-coupled transmembrane proteins with seven domains and are embedded in the lipid bilayer of the cell surface14. These chemokine receptors are divided into four families that are named after their ligands (TABLE 1): CCR1–10 (R for receptor) bind to CC chemokines, CXCR1–7 bind to CXC chemokines, and so on. The selectivity of different chemokines to the receptors is largely determined by the ligands. Receptor expression also varies in different cell types (Supplementary Box 1). For instance, while pro-inflammatory CXCR2 is the most common receptor on neutrophils15, its ligands are primarily CXCL1 and CXCL2 in liver. The anti-fibrotic and anti-angiogenic CXCR3 is the key receptor on T cells, NK cells and possibly HSCs, with CXCL9 and CXCL10 as its ligands in the liver16,17. Another important receptor, CCR2, is distributed mostly on inflammatory monocytes; CCL2, known to be pro-inflammatory and pro-fibrotic, is the ligand18,19.
The advancement of genetic and epigenetic technologies in the past decade has opened up new possibilities for liver disease research. Here, we focus on the genetic-mediated and epigenetic-mediated regulation of chemokine ligands and receptors and on the cell-type specificity of chemokine production in liver homeostasis and inflammation.
Regulation of chemokine ligands and receptors
Genetic regulation.
The discovery of allelic variants of HIV co-receptors (such as CCR5 and CXCR4) that protect against virus entry into usually permissive cells led to increased interest in chemokine polymorphisms20–24. Since then, investigations have searched for associations between allelic variants and phenotypes in both sterile and septic inflammatory diseases. One of the most studied is the CCL2-2518-A/G polymorphism, which has been shown to be a risk factor for Alzheimer disease25,26. CCL2 has also been shown to be highly upregulated in patients with hepatitis C virus (HCV) infection and fibrosis with these single-nucleotide polymorphisms (SNPs). Other SNPs in CCR clusters have been studied in 465 patients with HCV infection and in 370 matched healthy individuals as controls27. This study examined six SNPs and a 32 bp deletion in the genes encoding CCR3, CCR2 and CCR5 (all of which are located in a cluster on chromosome 3), including the G190A polymorphism (variant allele Ile64) in the first transmembrane domain of CCR2. However, the researchers found that host genetic factors in the CCR cluster only had a modest influence on the natural course of the infection, stage of fibrosis or response to therapy. Another example is CXCL9–11: a strong relationship was found between CXCL9–11 polymorphisms (specifically, CXCL9 rs10336, CXCL10 rs3921 and CXCL11 rs4619915) and liver fibrosis in patients with HCV infection28. The three CXCL9–11 SNPs are located at the 3′ untranslated region (UTR) of their respective genes and are targeted by microRNAs. The role of microRNAs on chemokine production has been reviewed previously29. It is thought that the combination of CXCL9–11 polymorphisms and other biomarkers (plasmatic, genetic and so on) could improve the prediction of the development of advanced liver fibrosis. Additionally, most of the SNPs for these chemokines are located in non-coding regulatory regions, such as promoters and enhancers, suggesting that transcriptional regulation is indeed critical for the functional role of chemokines.
Epigenetic regulation.
Given the importance of chemokines in biological homeostasis and disease, the regulation of their expression has been a focus of liver research in the past decade. Chemokines are closely regulated in a dose-dependent and time-dependent manner30. Some of the upstream regulators are the inflammatory cytokines tumour necrosis factor (TNF), IL-1, interferon-γ (IFNγ), IL-17 and IL-6 (REF.31). TNF stimulation, for both short and long periods, can activate nuclear factor-κB (NF-κB)32. In addition to NF-κB, other transcription factors, such as activator protein 1 (AP-1), signal transducer and activator of transcription 1 (STAT1) and ELK1, are also involved in stimulating the transcription of chemokines induced by the aforementioned inflammatory cytokines33,34.
Enhancers, a type of cis-regulatory element, are short DNA sequences that control gene expression by recruiting sequence-specific transcription factors and co-activators35–37. Data show that enhancers have a crucial role in determining the cell type-specific transcriptome38. In the past decade, the rapid application of next-generation sequencing has provided great insight into the regulatory roles of epigenetic modifications such as DNA methylation, histone modifications, non-coding RNA processes and chromosome conformation changes. Advances in epigenetics have fuelled the accelerated discovery of distal enhancers and revealed the importance of enhancers in the regulation of cell type-specific gene expression39,40. For example, not only do the post-transcriptional modifications (such as methylation, acetylation and ubiquitination) of histone tails correlate with the activated or repressed chromatin state of associated genes, they can also identify the presence of enhancers. The acetylation of histone H3 at lysine 27 (H3K27ac) and trimethylation of histone H3 at lysine 27 (H3K27me3) are commonly associated with gene enhancer and promoter regions41. Meanwhile, many studies have demonstrated the importance of super-enhancers, or large enhancer clusters, for the cell-specific regulation of genes. The expression of genes associated with super-enhancers is sensitive to external stimuli, including inflammatory cytokines such as TNF. TNF induces NF-κB signalling through the activation of cis-regulatory elements, such as proximal promoters and distal super-enhancers, in a context-dependent manner to activate gene expression42,43. NF-κB binding is crucial for the dynamic remodelling of the super-enhancer epigenetic landscape and chromatin structure, which enables rapid changes to clusters of gene expression. It has been well documented in human endothelial cells, such as human umbilical vein endothelial cells (HUVECs) and lung fibroblasts, that TNF-responsive super-enhancers are present in many chemokines, intronic or intergenic regions and support a very high occupancy of NF-κB, activation histone marks (such as H3K27ac and H3K4me1) and the epigenetic reader protein bromodomain-containing protein 4 (BRD4)32.
The development of chromosome conformation capture (3C)-based technologies, circularized chromosome conformation capture (4C) and other chromatin interaction techniques, such as chromatin interaction analysis by paired-end tag sequencing (ChIA-PET) and Hi-ChIP (which can map factor-directed chromatin conformation)44, have enabled the discovery of the relevance of the 3D genome organization for the transcriptional control of inflammatory chemokines32,41. Numerous studies using these new assays have suggested a role for chromatin loops (such as those formed by promoter–enhancer interactions) in chemokine expression programmes44. A genome-wide 3C analysis method (Hi-C) has also determined that the genome is organized into various topologically associated domains45 that have important roles in genome organization and control of gene expression, including genes encoding chemokines in HUVECs and lung fibroblasts32,41. It is well established that alcoholic hepatitis is associated with hepatic neutrophil infiltration, partly through the activation of cytokine pathways such as TNF signalling, leading to the increased expression of chemokines31,46. Data have demonstrated the role of super-enhancers in linking TNF expression with the amplification of chemokine expression in patients with alcoholic hepatitis (published in an abstract47). The integration of RNA sequencing (RNA-seq) and histone modification chromatin immunoprecipitation followed by sequencing (ChIP–seq) of human liver explants showed the upregulation of multiple CXCL chemokines in alcoholic hepatitis livers compared with healthy livers along with dramatic histone mark changes. Published databases have identified LSECs as a dominant source of CXCL production in mice and humans48. By using ChIP–seq and 4C-seq49 and analysing published databases, a putative super-enhancer for multiple CXCLs located 20 kb upstream from the CXCL gene loci was identified47 (Supplementary Fig. 1a). Owing to their broad activity against a large number of inflammatory genes and their specificity for their target genes, super-enhancers make attractive candidates for pharmacological intervention50.
Additional studies44,51 using ChIA-PET and Hi-ChIP technologies have found that super-enhancers that regulate inflammatory genes, such as those encoding CXCLs, IL-6 or macrophage colony-stimulating factor 1 (CSF1), can be modulated by long non-coding RNAs (lncRNAs) and histone demethylation (Supplementary Fig. 1b). For example, Fanucchi et al. found that a subset of lncRNAs called immune gene-priming lncRNAs (IP-lncRNAs or IPLs) can act in cis in human cells to direct the WD repeat-containing protein 5 (WDR5)–mixed lineage leukaemia protein 1 (MLL1) complex across the chemokine promoters (IL-8, CXCL1, CXCL2 and CXCL3), facilitating their H3K4me3 epigenetic priming44. This mechanism was also shared among dozens of regulators of trained immunity (for example, IL-6 and CSF1), which refers to the ability of innate immune cells to acquire a non-specific enhanced response to pathogen reinfection. The researchers demonstrated that β-glucan, a classic inducer of trained immunity, can increase the transcription of several IPLs. As mentioned in Supplementary Boxes 2, 3, the mouse CXCL gene locus is different to that of humans (Supplementary Fig. 1a) — the mouse locus lacks IPLs in the chemokine super-enhancers, so trained immune responses are absent at the CXCL locus. However, when an IPL was inserted into the mouse chemokine super-enhancer, it resulted in the training of CXCL genes. This study provides strong evidence that IPL-mediated changes to the epigenetic status of immune gene promoters are important for trained immune responses. Meanwhile, a 2020 study43 proposed a model in which lysine demethylases 7A (KDM7A) and 6A (also known as UTX) have vital roles in TNF signalling, where they are regulated by a novel TNF-responsive microRNA — miR-3679-5p. This work highlighted the role of histone methylation (such as H3K9me2 and H3K27me3) in regulating super-enhancer activity. The researchers showed that TNF rapidly induced the co-occupancy of KDM7A and UTX at NF-κB binding sites in HUVECs. KDM7A and UTX were then found to demethylate H3K9me2 and H3K27me3, respectively. H3K9me2 and H3K27me3 are histone-repressing marks required for the activation of NF-κB-dependent inflammatory genes, such as those encoding CXCL1 and CXCL8, and of other important genes such as those encoding vascular cell adhesion molecule 1 (VCAM1) and E-selectin. In addition, this study identified that increased interactions between TNF-induced super-enhancers and NF-κB-responsive target loci coincided with the recruitment of KDM7A and UTX by chromosome conformation capture-based methods. The pharmacological inhibition of KDM7A and UTX significantly reduced leukocyte adhesion in mice. The investigators concluded that the rapid reduction of repressive histone marks is essential for the NF-κB-dependent regulation of super-enhancers that control inflammatory gene expressions.
Chemokines and receptors in liver cells
The liver has a key role in the immune surveillance of enteric pathogens. However, the enteric circulation also carries large amounts of innocuous molecules, such as food antigens, to the liver, which requires the liver to have immunotolerant properties. Thus, a balance is needed between immunotolerance in health and mounting a rapid immunological response to enteric pathogens in disease; upsetting this balance can lead to allergic conditions, sterile inflammation, organ injury, chronic infections and carcinogenesis52. Chemokines have an important role in tissue homeostasis and pro-inflammatory processes in many organs, including the liver. Chemokines also contribute to immune surveillance — they direct immune cells via specific chemokine receptors into the liver6. Many factors can drive chemokines away from maintaining homeostasis and towards becoming more pro-inflammatory in different liver injuries. These injuries include viral infections, pathogen invasion, drug toxicity, metabolic dysfunctions, and acute or chronic alcohol intake. The functional roles of chemokines change during this process; it is surely context-dependent and continues to be the focus of much research. In general, many liver-resident cells as well as infiltrating cells can secrete chemokines in response to such injuries and can release cytokines, such as TNF, IL-6 and IL-1β, in addition to lipopolysaccharide6. These cytokines are common upstream regulators that can stimulate a huge production of chemokines that provoke immune cell infiltration into the liver and trigger inflammation. Meanwhile, the differential chemokine receptor expression of the targeted immune cells can shape the immune response (FIG. 1). Interestingly, the chemokine receptors primarily involved in modulating inflammatory responses are CCR1, CCR2, CCR5 and CXCR3, which ligate numerous chemokines, whereas ‘homeostatic’ receptors include CXCR4 and CXCR5, which are more specific and bind to only one or two ligands53. A distinct chemokine receptor can be expressed by various leukocyte subsets with divergent functions to define the outcome of the immune response. This ligand–receptor diversity demonstrates the complex nature of the chemokine network in liver homeostasis and in the transition to inflammation. Although most chemokines are pro-inflammatory, such as CXCL1, CXCL8 and CCL2, some chemokines are thought to be more important for homeostasis such as CCL12 (REF.54). According to in vivo studies, CXCL9, CXCL10 and CXCL11 can have anti-inflammatory and angiostatic effects as they target CXCR3 on endothelial cells, resulting in an inhibitory effect on cell proliferation55–57 (FIG. 1).
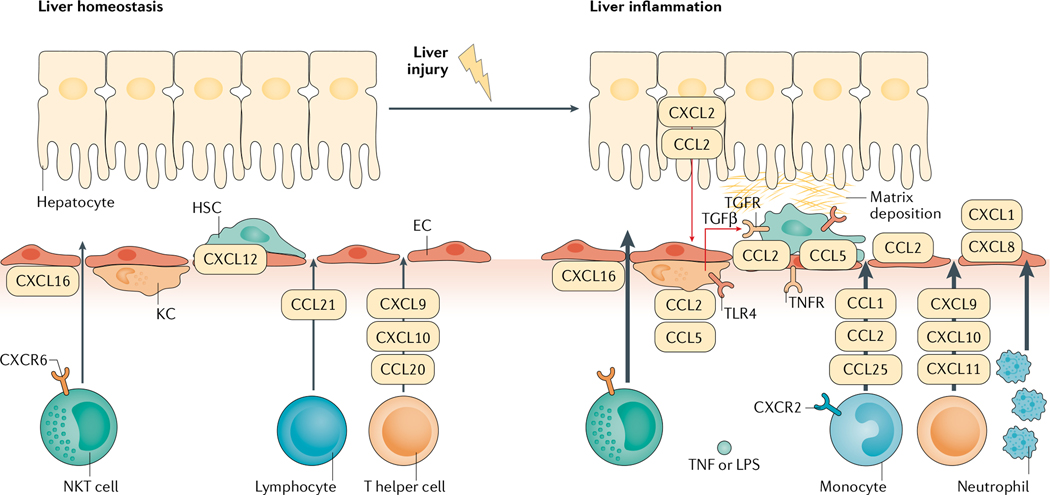
Fig. 1 |. Chemokine pathways in liver homeostasis and inflammation.
In liver homeostasis, Kupffer cells (KCs), liver sinusoidal endothelial cells (ECs) and hepatic stellate cells (HSCs) are in control of surveillance of tissue stress, pathogens and other disturbances. Local immune cells are first responders to chemokines such as CXCL12, maintaining liver homeostasis. When injury escalates, it can cause the release of damage-associated molecular motifs, which activate KCs. This activation induces the production of chemokines and cytokines. Transforming growth factor-β (TGFβ) is produced by KCs and induces the activation of HSCs. CCL2 and CCL5 are important in the process of activation of KCs and HSCs. Tumour necrosis factor (TNF) and lipopolysaccharide (LPS)-induced CXCL1 and CXCL8 are important chemokines that chemoattract neutrophil adhesion and invasion into liver inflammatory sites. Other chemokines, such as CCL1, CCL2 and CCL25, promote the infiltration of monocytes, CXCL9, CXCL10 and CXCL11 promote the infiltration of T helper cells, and CXCL16 promotes the infiltration of natural killer T (NKT) cells. These immune cells also contribute to the local inflammatory milieu. The activation of HSCs by immune cells and chemokines leads to the deposition of collagen in the liver, causing fibrosis. TLR4, Toll-like receptor 4.
In the liver, homeostatic processes control hemodynamic changes, capillary permeability, leukocyte migration into tissues and balance the secretion of inflammatory mediators such as chemokines to avoid pathological and excessive inflammation. Liver sinusoids support a low-pressure blood flow with a fenestrated endothelium, which lacks a basement membrane and allows for increased interactions between resident immune cells and non-haematopoietic hepatic cells. The sub-endothelial compartment, the space of Disse, drains into lymphatic vessels that run parallel to the portal tract. Resident cells, including macrophages, reside within this space of Disse and liver sinusoids58. Kupffer cells, LSECs, HSCs and local immune cells constituting the non-parenchymal liver cells, together with hepatocytes and cholangiocytes constituting the parenchymal liver cells, are all important sources of as well as responders to chemokines59–61. In the inactivated state, liver is enriched for innate immune cells such as Kupffer cells, NKs and NKTs. Upon immune activation, mediated largely by chemokines and cytokines, large influxes of innate and adaptive immune cells infiltrate the liver, which include bone-marrow derived monocytes, neutrophils, T cells and B cells62
The sources of different chemokines in health and disease are likely different6,58,63 and these differences are poorly understood. For example, CXCL1, CXCL2 and CXCL8 are key chemokines that attract neutrophils, mainly via the chemokine receptors CXCR1 and CXCR2 (REF.6). Our screening of public databases such as the FANTOM5 (functional annotation of the mammalian genome 5; see Related links) consortium identified LSECs as a predominant cell type in normal liver tissue that generate CXCL1, CXCL3, CXCL5, CXCL6 and CXCL8 but appear to be a relatively poor source of CXCL2 compared with hepatocytes (S.C., M.L., T.S.S. and V.H.S., unpublished observations)64. These points are important as LSECs represent a specialized and integral part of the local immune system owing to their roles in antigen capture and presentation and in directing immune cell extravasation65. A study performed on HUVECs demonstrated that CXCL1 and CXCL2 have distinct roles in neutrophil diapedesis66. Endothelial cell and pericyte-derived CXCL1 mediate neutrophil crawling, while CXCL2 is neutrophil-derived and retained at endothelial cell junctions by ACKR1, which is an atypical chemokine receptor. The ACKR1–CXCL2 axis is critical for unidirectional paracellular neutrophil transendothelial migration in mice in vivo. It is unclear whether the divergent functions of CXCL1 and CXCL2 are related to their varied production by liver cells. Further studies capitalizing on advances in single-cell sequencing technology have the potential to transform the field by identifying the cellular sources of chemokine production. A 2018 single-cell RNA-seq (scRNA-seq) analysis of normal human and mouse liver tissue was the first to show that a TNF receptor (TNFR1A; encoded by Tnfrsf1a) distributed to LSECs the most, and to hepatocytes to a lesser extent, suggesting that LSECs might be the main cellular sources of various chemokines in response to TNF67,68. The next challenge is performing scRNA-seq studies using diseased liver tissues, which would lend valuable insight into the sources and, perhaps, functions of chemokines in liver diseases.
Related links.
FANTOM5 database: https://fantom.gsc.riken.jp/zenbu/gLyphs/#config=2Z4giXeUDRQulVO5sw_x8C;loc=hg19::chr4:74734631..74737497
In summary, chemokines are variably expressed in different types of cells in the liver in both physiological and pathological conditions. Their functions vary widely, from pro-inflammatory and angiogenic to anti-inflammatory and angiostatic; they can be regulated by multiple mechanisms, including by epigenetic regulation of enhancer and promoter interactions.
Chemokines in liver diseases
The role of chemokines in the coordination of immune responses in liver diseases has been well established. The balance of various chemokines regulates the constitution of the inflammatory milieu in many acute and chronic liver diseases. Liver immune activation might be conducive to combating diseases, as in the case of viral hepatitis and HCC, but can be detrimental in other contexts such as in alcohol-associated liver disease (ALD), nonalcoholic fatty liver disease (NAFLD) and ischaemia–reperfusion injury (IRI). The paradigm of progression from inflammation to fibrosis to carcinogenesis is well recognized in biology and has particular relevance in the liver. Over 90% of liver cirrhosis cases around the world are caused by ALD, NAFLD and viral hepatitis69 and, according to an Italian cohort, 70–90% of cases of HCC occur in patients with cirrhosis70,71. Given the importance of inflammation in liver disease pathogenesis, it is therefore important to address the role of chemokines in inducing and maintaining the liver inflammatory response.
Many chemokine ligand–receptor pairs have been shown to have important functions in perpetuating the inflammatory cascade and to be important in the pathogenesis of multiple liver conditions. For example, CCL2 and its receptor CCR2 are well characterized in many hepatic diseases, including ALD, NAFLD, viral hepatitis, cirrhosis and HCC. CCL2 is expressed by liver-resident cells, such as Kupffer cells, HSCs and LSECs6, and CCL2 acts on CCR2 expressed on bone marrow-derived monocytes (BMDMs) to promote liver chemotaxis as shown in mice studies72. Activation of the CCL2–CCR2 axis has been shown in animal studies to promote macrophage accumulation, inflammatory response, fibrosis and steatosis in the liver as well as in adipose tissue in NAFLD73. CCR2-deficient mice have been shown to have a decreased accumulation of inflammatory cells in the liver and the inhibition of the CCL2–CCR2 axis reduces hepatic fibrosis in experimental models74–76. RNA-seq of human cirrhotic livers found that CCL2 is one of the key chemokines upregulated by scar-associated macrophages after the differentiation from monocytes and is important in fibrotic niche formation77. The fibrotic niche organized by these scar-associated macrophages enables cell–cell interactions between macrophages, fibroblasts, endothelial cells and parenchymal cells, leading to fibrogenesis.
Although many chemokine ligand–receptor pairs share similar cellular functions in different liver diseases, the same chemokine can have variable effects on disease progression according to specific pathogenic mechanisms. Certain chemokines or chemokine receptors have functions other than promoting immune cell chemotaxis in disease states. For example, CCL2 has been demonstrated in a mouse model to have a direct effect on lipogenesis gene expression in hepatocytes, thus impeding fatty acid oxidation and promoting steatosis, which might be independent of its actions on the CCR2 receptor78. Given these disease-specific properties of chemokines, it is important to explore chemokine functions in the context of inflammatory responses and liver diseases. In this section, we discuss the role of chemokines in several common liver diseases and liver cancer. We also provide a general overview of chemokine function in these disease processes (TABLE 2) and include references to relevant reviews discussing these diseases and others here, which readers can refer to for further details53,73,79–87.
Table 2 |.
Role of chemokines in human liver diseases
| Chemokine | Receptor | Proposed role in disease | Function in animal models |
|---|---|---|---|
| ALD | |||
| CXCL1, CXCL4, CXCL5, CXCL6, CXCL8 | CXCR1, CXCR2 | Neutrophil chemoattractant; levels increased in ALD, elevated levels of CXCL1, CXCL5, CXCL6 and CXCL8 correlate with poorer clinical outcomes in alcoholic hepatitis46 | Neutralizing CXCL1 by antibodies or genetic deletion or CXCR1-CXCR2 blockade ameliorated liver inflammation in alcohol-fed mice5,103 |
| CCL2 | CCR2 | Monocyte chemoattractant; levels increased in ALD2; plasma levels and hepatic expression of CCL2 were associated with disease severity in alcoholic hepatitis99 | CCL2-knockout mice showed attenuated liver inflammation and steatosis78; pharmacological inhibition of CCL2 ameliorated steatosis development207; CCL2 might have direct effects on steatosis via PPARα78 |
| Nonalcoholic fatty liver disease | |||
| CCL2 | CCR2 | Monocyte chemoattractant; levels elevated in NASH and correlated with fibrosis in NASH217 | CCL2 overexpression in adipose tissue led to insulin resistance and hepatic steatosis116; CCR2 inhibition decreased liver macrophage infiltration and fibrosis in NASH model218,219; CCL2 directly affects adipocytes favouring lipogenesis114,115 |
| CCL5 | CCR1, CCR5 | Recruits T cells and NK cells; levels elevated in patients with NASH122 | Levels elevated in animal models of NAFLD and CCL5 pharmacological inhibitors attenuated liver steatosis122,123 |
| CXCL9, CXCL10, CXCL11 | CXCR3 | Regulate and activate T cells; elevated in NASH125; promote lipid accumulation and induce endoplasmic reticulum stress125; CXCL9 and CXCL10 levels correlate with fibrosis55,125; circulating CXCL10 levels correlated with the degree of lobular inflammation and was an independent risk factor for NASH in patients125 | Mice with CXCR3-knockout or blockade of CXCR3 showed reduced steatohepatitis in NASH model125,220; mice with CXCL10-knockout or blockade of CXCL10 were protected against diet-induced NASH125,126; CXCL10-bearing hepatocyte extracellular vesicles were chemotactic for macrophages221 |
| Cirrhosis | |||
| CCL2 | CCR2 | Monocyte chemoattractant; contributes to inflammatory responses leading to steatosis and fibrosis; CCL2 might promote hepatic stellate cell migration132 | CCL2-knockout mice showed attenuated liver injury in CCl4 injection model133 |
| CCL3, CCL4, CCL5 | CCR1, CCR3, CCR5 | Recruits T cells and NK cells; promotes liver fibrogenesis; elevated in patients with cirrhosis136,222; CCR5 may be important for hepatic stellate cell activation136 | Deletion of CCL3, CCL5, CCR1, CCR5 or CCL5 inhibition are protective against fibrosis in CCl4 injection model122,137 |
| CXCL9, CXCL10, CXCL11 | CXCR3 | Recruit T helper cells, particularly TH17 and Treg cells; elevated in patients with cirrhosis55,138; CXCL9 has antifibrogenic and CXCL10 has profibrogenic effects on hepatic stellate ceils55,140 | CXCR3-knockout mice showed increased fibrosis in a CCl4 injection model55; CXCR3 inhibition is associated with reduced fibrosis in congenital hepatic fibrosis model223 |
| CXCL16 | CXCR6 | Survival and maturation factor for NKT cells and promote NKT cell accumulation at injury site142; CXCR6 and CXCL16 expression was increased in the livers of patients with cirrhosis142 | CXCR6-knockout mice showed attenuated inflammation and fibrosis in CCl4 injection model142 |
| CX3CL1 | CX3CR1 | Promotes macrophage survival; levels elevated in cirrhosis; favours the development of anti-inflammatory macrophages143 | CX3CR1-knockout mice showed increased fibrosis in CCl4 injection model144 |
| HCC | |||
| CCL1 | CCR8 | Monocyte chemoattractant; recruit myeloid-derived suppressor cells, which inhibit anti-tumour immune surveillance147, but might also recruit anti-tumour immune cells, including CD4+ TH1 cells, CD8+ T cells and NK cells148; CCL1 level was found to be elevated in HCC and even higher in peritumoural liver tissue224; CCL2 is highly expressed in patients with HCC and is a prognostic factor for poor outcome225 | CCR2-knockout or blockade inhibits tumour growth and host survival in murine HCC model; inhibiting CCR2+ tumour-associated macrophage infiltration using pharmacological CCL2 antagonist substantially reduced tumour growth226 |
| CCL2 | CCR2 | ||
| CCL17,CCL20, CCL22 | CCR6 | Recruits Treg cells; elevated levels correlate with poorer survival in patients with HCC227; CCL20 might recruit TH17 cells as well, opposing the action of Treg cells139 | CCL20 neutralization suppressed tumour growth and metastasis in murine HCC model228 |
| CXCL12 | CXCR4, CXCR7 | Immune activation; CXCL12 was shown to have an angiogenic effect; CXCL12 can act on HCC cells and promote cell proliferation, survival and invasion160; CXCR4 and CXCR7 both implicated in tumour growth and metastasis156–161 | CXCR7 inhibition decreases angiogenesis and tumour growth in murine HCC model156; the inhibition of CXCR4 decreases angiogenesis in animal models, HCC can be stimulated to produce vascular endothelial growth factor in the presence of CXCL12 (REFS156,157) |
| CXCL16 | CXCR6 | Elevated numbers of CXCR6+ cells were seen in patients with HCC164 | Elimination of CXCR6+ NKT cells resulted in worsened HCC tumour growth in vivo162,163 |
This tabel is an abbreviated version of Supplementary Table 1. ALD, alcohol-associated liver disease; CCl4, carbon tetrachloride; HCC, hepatocellular carcinoma; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; NK cells, natural killer cells; NKT cells, natural killer T cells; PPARα, peroxisome proliferator-activated receptor-α; TH, T helper; Treg, regulatory T.
Alcohol-associated liver disease
Chronic alcohol use induces a spectrum of pathologies in the liver, ranging from simple steatosis (accumulation of fat) to steatohepatitis (steatosis concurrent with inflammation) to cirrhosis88. Cytokines and chemokines have been well recognized to have important roles in the pathogenesis of ALD, largely by promoting inflammation. This effect is most evident in the development of alcoholic hepatitis, which is a highly morbid form of alcoholic steatohepatitis characterized by dense inflammatory cell infiltration, mostly neutrophilic, and hepatocellular injury89. The pathogenesis of alcoholic hepatitis is complex and involves the crosstalk between multiple organ systems. Ethanol metabolism in the liver causes oxidative stress and alterations to lipogenesis and fatty acid metabolism, leading to hepatic steatosis90. Alcohol not only causes direct hepatocyte toxicity but also increases gut permeability, resulting in the translocation of bacterial products and endotoxaemia31,91. The activation of immune cells in the human liver, particularly Kupffer cells, by pattern recognition receptor pathways leads to the release of many pro-inflammatory factors, including TNF, IL-1β, IL-6, chemokines and others92–94. TNF and other cytokines stimulate further chemokine release from immune and non-immune liver cells95–97 (FiG. 2). This release of chemokines generates chemokine gradients that promote transendothelial migration of immune cells into the liver, which further exacerbates hepatocyte damage by immune-mediated cytotoxicity31. Indeed, elevated levels of CCL2, CXCL8 and CXCL5 have been shown to positively correlate with increased neutrophilic infiltration and higher mortality in patients with alcoholic hepatitis46. CCL20, a CCR6 ligand that is chemotactic for lymphocytes, has also been shown to positively correlate with disease severity in patients with alcoholic hepatitis and it was suggested to act as a link between inflammation and fibrosis, not only through chemotactic functions but also by acting directly on HSCs98. Genome-wide association studies have identified polymorphisms in the genes encoding CCL2, CCR2 and CXCL1 that are associated with the higher serum levels of these chemokines. These polymorphisms were found at higher frequencies in patients with ALD than in healthy individuals, providing correlative evidence of the importance of these molecules99.
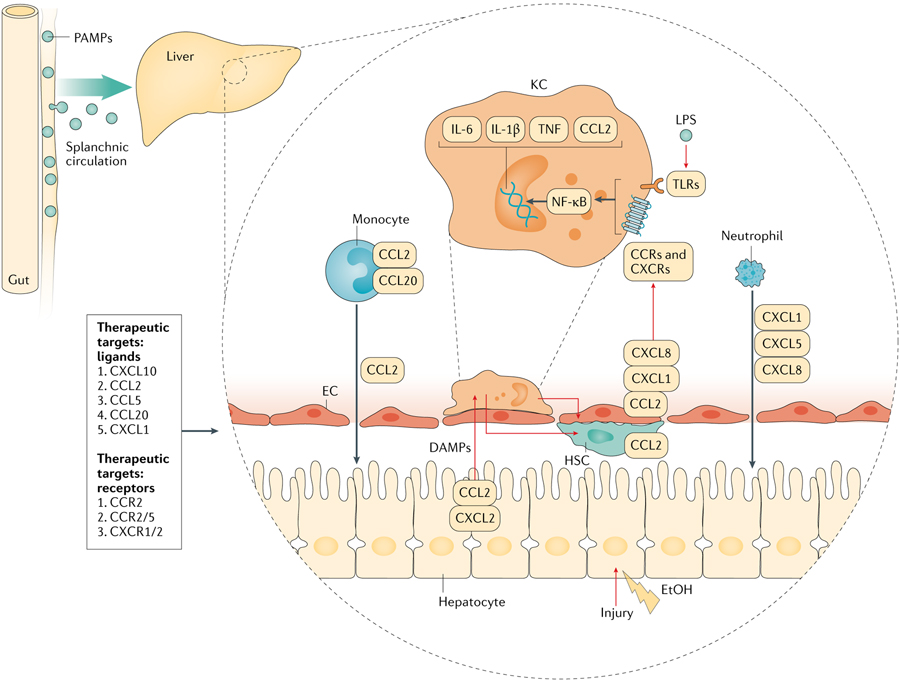
Fig. 2 |. Chemokines in the pathogenesis of alcohol-related injury.
Lipopolysaccharide (LPS) and bacterial products brought in via splanchnic circulation along with damage-associated molecular patterns (DAMPs) released by injured hepatocytes stimulate Kupffer cells (KCs) to release pro-inflammatory cytokines and chemokines. Immune cells and liver-resident cells respond to this paracrine stimulation by the production of chemokines. CXC chemokines are critical in the recruitment of neutrophils and CCL2 and CCL20 are important for the recruitment of circulating monocytes. Invading immune cells perpetuate injury to hepatocytes by a cell-mediated inflammatory response. CCRs, chemokine receptors; EC, endothelial cell; HSC, hepatic stellate cell; NF-kB, nuclear factor-kB; PAMPs, pathogen-associated molecular patterns; TLRs, Toll-Like receptors; TNF, tumour necrosis factor.
Many rodent models have been developed to study ALD100. The Lieber–DeCarli chronic–binge alcohol-feeding model is one of the most widely used but, despite being a reliable model of alcoholic steatosis, it generates only a modest degree of liver inflammation101. Continuous intragastric alcohol-feeding models generate more liver inflammation but they are also more invasive and require technical expertise102. There is a continued need for better animal models of human alcoholic hepatitis to further advance the field. Despite these shortcomings, alcohol-feeding models have assisted in shedding light on the role of chemokines in ALD, particularly in alcohol-induced hepatic steatosis. In the chronic–binge alcohol-feeding model, compared with controls, alcohol-fed mice had higher liver and serum levels of inflammatory markers, including CXCL1, which is a murine homologue of human CXCL8 (REF.5). Blockade of CXCL1 by neutralizing antibodies or by G-protein-specific pepducins targeting CXCR1 and CXCR2 ameliorated liver inflammation in alcohol-fed mice5,103. Alcohol-fed mice deficient in CCL2 (or the pharmacological inhibition of CCR2 and CCR5 signalling) attenuated liver inflammation and steatosis, likely by reducing the infiltration of pro-inflammatory macrophages78. Interestingly, in mice, CCL2 might also exacerbate steatosis through the suppression of peroxisome proliferator-activated receptor-α (PPARα)-related pathways in hepatocytes, independent of cognate receptor CCR2 (REF.78).
However, the infiltration of immune cells into the liver that occurs in ALD is not purely detrimental. One study found that the increased infiltration of neutrophils in liver biopsy samples was positively correlated with better clinical outcomes in patients with alcoholic hepatitis104. Neutrophils have important functions in debris removal and release of growth factors, which might promote tissue regeneration105. In an animal model of alcoholic liver injury, the activation of Kupffer cells through Toll-like receptor 3 (TLR3) was shown to favour an anti-inflammatory phenotype and to induce the release of IL-10, which is an important anti-inflammatory cytokine associated with improvement in inflammation and steatosis106. Hence, it is unclear whether the elevated chemokine levels seen in patients with ALD are a biomarker reflective of the degree of underlying injury or rather a driver of disease through the promotion of increased immune cell-mediated cytotoxicity. Further research in this area is needed to better delineate the role of chemokines and immune cells in ALD.
Nonalcoholic fatty liver disease
NAFLD is the most common form of liver disease in the United States and its prevalence has continued to increase over the past several decades107. NAFLD is characterized by increased fat accumulation in the liver and the histological characteristics range from asymptomatic steatosis to nonalcoholic steatohepatitis (NASH) to cirrhosis, sharing many features with alcoholic steatohepatitis108. Animal models of NASH have been fairly successful in replicating human liver disease and the two most commonly used are the methionine choline-deficient diet and the high-fat and high-cholesterol Western diet, with the latter perhaps better simulating physiology109. As in alcoholic steatohepatitis, chronic inflammation has an important role in the pathogenesis of NASH but advances in obesity research have highlighted other pathogenic factors. In particular, the crosstalk of the adipose–liver axis has been recognized to have important roles in the development of insulin resistance and NAFLD110. Human adipose tissue macrophages secrete pro-inflammatory cytokines, including TNF and IL-6, which contribute to chronic inflammation111–113. Multiple chemokines, many of which are upregulated in the livers and adipose tissues of patients with NAFLD, are crucial in macrophage trafficking, both within adipose tissue and into the liver. For example, in mice, the overexpression of CCL2 in adipose tissue leads to the development of insulin resistance and hepatic steatosis and the pharmaceutical inhibition of CCR2 decreases macrophage infiltration and reduces liver inflammation and fibrosis114,115. In animal models, CCL2 might also have direct effects on adipocytes: it has been shown to decrease insulin-stimulated glucose uptake and to affect the expression of adipogenic genes leading to adipocyte dedifferentiation116.
Within the liver, Kupffer cells are major sources of cytokine and chemokine production in NAFLD. In animal models of NAFLD, the depletion of Kupffer cells was protective against the development of NASH117. However, Kupffer cells and BMDMs in NASH are not universally pro-inflammatory. Single-cell transcriptomics data have shed light on the diversity of macrophage and monocyte populations in NASH and the interplay between these diverse macrophage populations modulates liver inflammation in NASH pathogenesis118–120. In particular, the emergence of NASH-associated macrophages in the setting of metabolic derangement seems to be instrumental in regulating downstream pro-inflammatory signalling in NASH118,119. These NASH-associated macrophages have a high expression of TREM2, are found in the liver and adipose tissue of mice and humans with NASH, are positively correlated with disease severity, and are responsive to dietary and pharmacological treatments for NASH in mice118,119. These have been proposed to undertake an adaptive and protective response in NASH as the deletion of TREM2 (leading to NASH-associated macrophage depletion) in mice induced metabolic dysregulations and exacerbated immune-mediated liver injury118,121. Similarly, the adoptive transfer of BMDMs from mice fed a normal chow diet to mice fed a Western diet resulted in an exaggerated response to acute sterile liver injury, suggesting the presence of a similar adaptive response to limit the excessive inflammatory response to liver injury in NASH120.
In addition to macrophages, lymphocytes, NK cells and neutrophils have also been examined in NAFLD. CCL5, which regulates the recruitment of T cells by interacting with CCR5 and of NK cells by interacting with CCR1, was shown to be elevated in animal models of NAFLD and in livers of patients with NASH122. Furthermore, the use of pharmacological inhibitors of CCL5 in mouse models of NAFLD was shown to attenuate liver steatosis122,123, likely mediated by the inhibition of lymphocyte chemotaxis124. Cenicriviroc, a dual CCR2–CCR5 antagonist, garnered much attention owing to its simultaneous effect on two important chemokine pathways — it was shown to be effective in reducing inflammation, steatosis and fibrosis in vivo74. Other T cell chemoattractants include CXCL9, CXCL10 and CXCL11, which interact with a common receptor, CXCR3, and have been shown to promote cholesterol-induced steatohepatitis, facilitate lipid accumulation and induce endoplasmic reticulum stress125. The pharmacological blockade of CXCR3 in mice can prevent the development of as well as reverse established steatohepatitis. Serum levels of CXCL9 are associated with liver fibrosis in patients55 and CXCL10 has been identified as an independent risk factor for NASH125. CXCL10 not only induces inflammation but also directly promotes steatosis by stimulating lipogenesis and promoting macrophage-associated liver injury in mouse models of NASH126. Neutrophil-attracting chemokines might be upregulated in NAFLD as well. For example, CXCL2 expression was higher in the adipose tissue of patients with obesity127 and serum CXCL8 levels were elevated in patients with NASH128.
Cirrhosis
Chronic liver inflammation from a variety of injuries leads to the development of liver cirrhosis. Advanced cirrhosis can lead to portal hypertension, liver dysfunction, and multi-organ failure and it predisposes the development of liver cancer. Many cell types can contribute to the progression of liver fibrosis but activated HSCs are considered the most important source of fibrogenic myofibroblasts that deposit collagen and extracellular matrix as part of the pathogenesis of cirrhosis129. Chemokines are important factors in this chronic process as they regulate the degree and composition of immune cell infiltration that drives HSC activation129. Macrophages, in particular, are the major inflammatory cell type responsible for HSC activation by the production of cytokines, including transforming growth factor-β (TGFβ)129. In addition, several chemokines have been shown to act directly on liver parenchymal cells, such as HSCs, to exert profibrogenic or antifibrogenic effects130.
The CCL2–CCR2 axis is one of the best-described chemokine ligand–receptor systems in liver fibrogenesis. The binding of CCL2 to CCR2 recruits and activates BMDMs to join the CD14+CD16+ inflammatory macrophage pool in fibrotic mouse livers, leading to further inflammatory responses and resulting in steatosis and fibrosis131. Additionally, CCL2 might promote the migration of HSCs via a CCR2-independent mechanism in vitro132. The importance of CCL2–CCR2 signalling has been confirmed by various models of liver injury using knockout mice. CCL2-knockout mice have attenuated liver injury after the administration of chronic carbon tetrachloride (CCl4) injections, a well-established model of liver fibrosis133. Other chemokines in the same subfamily, including CCL25 and its cognate receptor CCR9, have also been implicated as important pathways of BMDM recruitment in acute liver injury as well as in chronic liver fibrosis130,134. These recruited monocytes are important producers of TNF and were shown to activate HSCs in vitro130. CCR9-knockout mice showed attenuated liver fibrosis after CCl4-induced liver injury130.
CCL5 is another chemokine that is important in liver fibrogenesis. CCL5 binds to CCR1, CCR3, and CCR5 and is produced by HSCs and immune cells135. CCL5, along with the related chemokines CCL3 and CCL4, is elevated in patients with cirrhosis in the liver136. CCR1 is present on bone marrow-derived macrophages and CCR5 can be found on HSCs and seems to be necessary for their activation in animal models136. In animal models, deletion of the genes encoding CCL3, CCL5, CCR1, or CCR5 or the pharmacological inhibition of CCL5 are protective against fibrosis122,137. Another group of chemokines, CXCL9, CXCL10, CXCL11 and their receptor CXCR3, have also attracted attention. These chemokines are elevated in the livers of patients with cirrhosis and are positively correlated with fibrosis55,138. Functionally, these chemokines recruit T helper (TH) cells, particularly IL-17-producing TH17 and IL-10-producing regulatory T (Treg) cells in the liver139. In animal models, CXCR3 deletion increases the susceptibility to fibrosis in mice55. Interestingly, CXCL9 and CXCL10 might have opposing roles in fibrogenesis, with CXCL9 being antifibrogenic and CXCL10 being profibrogenic, both through effects on HSCs, as described earlier55,140. In addition to traditional αβ T cells, γδ T cells were also shown to suppress fibrosis through CCL20–CCR6 interactions. Additionally, CCL20 and CCR6 were shown to be upregulated in the livers of patients with cirrhosis and in mice after chronic CCl4 injury141. CCR6-knockout mice showed increased fibrosis after CCl4 injection, which was rescued by the adoptive transfer of wild-type mouse γδ T cells141.
Also worthy of discussion is CXCL16, which is a survival and maturation factor for hepatic NKT cells. CXCL16 is produced early in the fibrotic process by macrophages, resulting in an accumulation of NKT cells at sites of injury142. Knockout of CXCR6 (the CXCL16 receptor) in mice resulted in attenuated inflammation and fibrosis in models of fibrosis142. CX3C chemokines have also been studied in fibrogenesis. CX3CL1 is widely expressed on immune and non-immune cells in the body and its serum level is elevated in a mouse model of fibrosis143. Although CX3CL1–CX3CR1 signalling has been shown to promote macrophage survival, it might help to attenuate inflammation by skewing macrophages to an anti-inflammatory phenotype and CX3CR1-knockout mice demonstrated increased fibrosis after chronic CCl4 injury143,144.
Hepatocellular carcinoma
HCC is the most common primary liver cancer. It primarily arises from cirrhotic livers and is a significant contributor to mortality in patients with cirrhosis145. Chemokines have important roles in the pathogenesis of HCC. Chemokines not only contribute to the inflammatory conditions leading to tumorigenesis and regulate the stromal microenvironment of cancer cells with either pro-tumour or anti-tumour effects but they can also directly interact with chemokine receptors on cancer cells and modulate cell behaviour related to migration, invasion, growth and survival6. These chemokines come from a variety of cell sources. HCC cells are known to produce multiple chemokines and non-tumour cells, including immune cells, HSCs and other stromal cells, also contribute to chemokine production146.
Many chemokines contribute to the pro-inflammatory environment that favours tumorigenesis, as discussed in previous sections (FIG. 3). After a tumour arises in the liver, abnormal aggregates of immune cells are often found surrounding or infiltrating tumour tissue. Chemokines are key regulators of this process. For example, the CCL2–CCR2 axis is important in the recruitment of monocytes. In addition, this axis can also recruit myeloid-derived suppressor cells, which has an inhibitory effect on anti-tumour immune surveillance147. At the same time, CCL2 levels in the liver positively correlate with increased anti-tumour immune cells, including CD4+ TH1 cells, CD8+ T cells and NK cells, illustrating the potential anti-tumour role of this chemokine148. Another important chemokine that promotes the growth of HCC cells is CCL20. The CCL20–CCR6 axis recruits Treg cells into tumour tissue, an increased number of which is correlated with poorer survival in patients with HCC149. CCL17 and CCL22 might have similar effects in recruiting Treg cells150. However, increased levels of CCL20 might recruit TH17 cells as well, thereby opposing the action of Treg cells139. Neutrophilic infiltration in HCC is also commonly seen. Of this process, the CXCL8 and CXCL5–CXCR2 axes are the best characterized. In patients with HCC, higher levels of CXCL1, CXCL8 and CXCL5 are associated with increased neutrophil infiltration and poorer prognosis151–153.
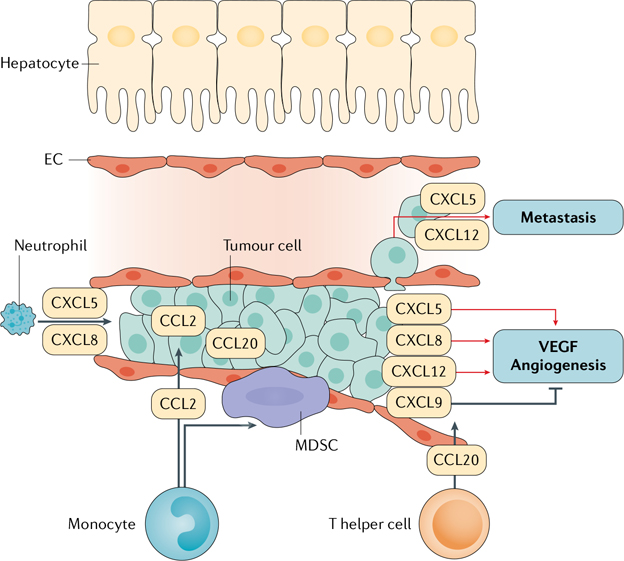
Fig. 3 |. Chemokine actions in HCC.
Hepatocellular carcinoma (HCC) tumour cells produce a large number of chemokines. Among them, CXCL5 and CXCL8 are known to directly stimulate tumour cell growth. They also promote neutrophil infiltration into the liver. CCL2 promotes monocyte recruitment, a subpopulation of which give rise to myeloid-derived suppressor cells (MDSCs) that suppress the anti-tumour immune response. CCL20 can promote the recruitment of T helper cells. These chemokines also have important roles in angiogenesis: multiple chemokines, such as CXCL5, CXCL8 and CXCL12, promote angiogenesis, whereas CXCL9 suppresses it. CXCL5 and CXCL12 also promote tumour invasion and metastasis. These specific enhancers provide a unique angle with which to study the activation of transcriptional responses to signals such as tumour necrosis factor (TNF) in liver diseases32,41. EC, endothelial cell; VEGF, vascular endothelial growth factor.
HCC is a hypervascularized tumour that relies on angiogenesis for its growth and survival154. Many chemokine pathways are directly involved in this process. According to preclinical studies, the CXCL12–CXCR4/CXCR7 pathway has pro-angiogenic effects on HCC155. The inhibition of CXCR4 decreases angiogenesis in animal models of HCC and HCC can be stimulated to produce vascular endothelial growth factor (VEGF) in the presence of CXCL12 (REFS156,157). CXCL8 is another chemokine that has pro-angiogenetic effects. The neutralization of CXCL8 can interfere with tumour endothelial cell capillary formation in vitro158. However, not all chemokines have pro-angiogenic effects and CXCL9 is a prominent example of a chemokine with anti-angiogenic effects that can be produced by HCC cells159.
Chemokines can also influence HCC growth by directly binding to tumour cells. CXCL12 can act on human HCC cells and promote cell proliferation, survival and invasion in vitro160. Clinically, the presence of CXCR4 (the receptor of CXCL12) correlates with an increased risk of metastasis and poor prognosis161. CXCR7 is also highly involved in the migration and invasion of tumour cells — its knockdown decreases tumour growth in vitro156.
The role of the gut microbiota has also been recognized in anti-tumour immunity in relation to the action of chemokines. Intestinal flora is involved in the process of bile conjugation and in converting primary bile acids into secondary bile acids. Secondary bile acids have long been shown to have important tumorigenic properties162. Work by Ma et al. showed in a mouse model that an accumulation of specific gut microorganisms, such as Clostridium species, leads to increased secondary bile acid production and the suppression of these microorganisms with antibiotics leads to an increase in hepatic CXCR6+ NKT cells mediated by increased CXCL16 secreted by LSECs163. The CXCL16–CXCR6 axis has also been implicated in tumorigenesis through the removal of senescent cells. Adoptive transfer experiments by Mossanen et al. demonstrated that CXCR6+ NKT and CD4+ T cells are involved in the removal of senescent hepatocytes, which prevents tumorigenesis164. This interaction again showcases the importance of chemokines in orchestrating the anti-tumorigenic immune response and illustrates that chemokines are mediators that enable other organs to directly affect liver anti-tumorigenic immunity146,165,166.
Viral hepatitis and chemokines
Chronic viral hepatitis due to HCV or hepatitis B virus (HBV) infections are important causes of liver disease around the world. Cytokines and chemokines have vital roles in the clearance or persistence of hepatitis viruses and these viruses can modulate the expression of chemokines and their receptors, which in turn hampers effective host immune responses167. Research on hepatitis viruses is hindered by the lack of widely available animal models due to the species-specific tropism of human hepatitis viruses. Chimpanzees, woodchucks, ducks, and humanized or transgenic mice have been used to study hepatitis virus infections, each with its own limitations168,169. Despite these difficulties, research on hepatitis viruses, particularly HCV and HBV, has made tremendous progress and has greatly advanced our understanding of liver antiviral immunity. Upon infection with HCV, infected hepatocytes and sentinel cells of the immune system, including dendritic cells and macrophages, are stimulated to produce cytokines and chemokines by the activation of pattern recognition receptor pathways167. Dendritic cells and macrophages also serve as antigen-presenting cells to activate the adaptive immune system167. Strong CD4+ TH1 helper and CD8+ effector T cell responses have been found to be critical in successful HCV resolution in humans, whereas virus-specific T cell responses are suppressed in chronic HCV infection170. A myriad of pro-inflammatory chemokines are produced in response to IFNγ and TNF and have important roles in HCV immune responses. For example, the chemokines CXCL9, CXCL10 and CXCL11 are produced by infected liver cells and activate CXCR3 on T helper cells140 and CCL3, CCL4 and CCL5 are similarly upregulated and bind to CCR5 on cytotoxic CD8+ T cells, promoting a TH1 immune response171. These chemokines are found to be substantially elevated in patients with acute HCV infections compared with healthy individuals and several chemokines have been suggested to be useful biomarkers. In particular, CXCL10 levels were associated with HCV-induced liver fibrosis as well as with fibrosis after liver transplantation172,173. The innate immune effector cells NK and NKT cells are also important in the HCV immune defence. These cells exert non-MHC-restricted cytotoxicity and produce abundant IFNγ and TNF167. However, in patients with chronic HCV infection, NK cell dysfunction can occur, with impairments in both degranulation and IFNγ production167. In the age of direct-acting antiviral agents, increasing numbers of patients are treated and have achieved sustained viral response or virological cure. After achieving virological cure, cytokine and chemokine levels are significantly decreased, although some data suggest that the clearance of HCV might not result in complete immunological restitution, as certain cytokine levels might remain elevated compared with healthy controls and CD8+ T cell dysfunction might persist after clearance of the virus174–176. Many chemokines are also significantly elevated in patients with HBV infection compared with healthy individuals, including CXCL9, CXCL10, CXCL11, CXCL13 and CCL4 (REFS177,178). However, the role of chemokines in disease clearance is less clear. In animal models, the neutralization of chemokines (such as CXCL9 and CXCL10) resulted in a reduction of liver injury but not in changes in HBV-specific CD8+ T cell activity179. Given that HBV-specific CD8+ T cells are thought to be most crucial in the clearance of the virus180, it seems that the chemokine-mediated inflammatory response contributes to liver injury but perhaps not to viral clearance in HBV. Consistent with this theory, serum levels of CXCL8, CXCL9 and CXCL10 are increased in patients with chronic HBV flare without changes in levels of HBV-specific CD8+ T cells181.
IRI and chemokines
Ischaemia is an important mechanism of liver injury, particularly during surgical procedures such as liver resection or transplantation. Cellular injury incurred during periods of ischaemia is amplified when perfusion is restored, in a process termed IRI182. In liver transplantation, minimizing IRI is important for allograft survival, not only in the immediate post-operative period but also for long-term graft function183. IRI has also been shown to correlate with the likelihood of transplant rejection184. Cytokines and chemokines are critical in the pathogenesis of IRI. The initial ischaemic insult causes the release of endogenous danger-associated molecular patterns from damaged cells that activate pattern recognition receptor pathways in liver-resident cells, particularly Kupffer cells, in the reperfusion phase. Kupffer cells produce large amounts of pro-inflammatory molecules, including cytokines and chemokines, which can be elevated by hundred-fold to thousand-fold above baseline185. This chemokine rush produces a very robust chemotactic gradient for the recruitment of immune cells, especially neutrophils, into injured tissue, leading to immune cell-mediated toxicity186. In humans, CXC chemokines are among the most important chemokines upregulated in IRI186. In mice, the blockade of CXCR2 with a pharmacological antagonist attenuates liver injury in a partial hepatic ischaemia model and, in rats, the neutralization of the homologue of human CXCL5 and CXCL6 resulted in decreased neutrophil sequestration and serum ALT levels187,188. Interestingly, in mice models, there appears to be a temporal pattern to the upregulation of certain chemokines. For example, CXCL2 appears to be elevated later in the course of injury and was also elevated in the non-ischaemic lobe189. The upregulation of these chemokines is inducible by TNF as shown in experimental models of TNF or lipopolysaccharide accentuating liver injury in the setting of IRI182. Therefore, it is possible that allografts that have sustained a prior insult that activated the TNF signalling pathway, resulting in elevated pro-inflammatory cytokines, might be more at risk for IRI as is the case for steatotic allografts190.
Therapeutic opportunities
Chemokines are instrumental in organizing immune cell movement and recruitment through both spatial and temporal differential expression190. As described earlier, different chemokines can have pro-angiogenic or anti-angiogenic and proliferative properties, making them potential therapeutic targets for immunotherapy against liver disease and cancers58,191 (TABLE 3; FiGS 1–3). Some chemokines might also act as biomarkers for liver diseases. Intrahepatic and serum levels of CXCL10 and its receptor, CXCR3, positively correlate with the severity of HCV-related liver disease in patients192–194. Similarly, CXCL10, along with CXCL8 and CXCL9, has shown potential as a biomarker for diagnosing HBV in patients177,195. CXCL1 and CCL2 levels have been shown to correlate with the disease process in NAFLD and ALD; however, the relevance of these observations needs to be further tested in larger studies196,197. Chemokines can provide insight into liver cancer activity as well. CXCL8 levels have been associated with increased tumour burden and disease aggressiveness in patients with HCC151. Multiple chemokines have been implicated in the activation of angiogenesis in HCC via neutrophil aggregation and can provide information about tumour invasion and progression198. The overexpression of CXCR2 and CXCL7 has been shown, in a small study (n = 51), to be correlated with shorter disease-free (15.4 ± 2.0 versus 31.8 ± 2.6 months, P = 0.002) and overall (29.8 ± 1.7 versus 65.4 ± 3.3 months, P = 0.012) survival in patients with liver metastasis199.
Table 3 |.
List of chemokine and chemokine receptor inhibitors in clinical trials
| Chemokines and receptors | Drugs | Current status |
|---|---|---|
| Ligands | ||
| CXCL1 | – | Important role in pathophysiology of multiple stress and inflammation-associated liver diseases, making it a potential target229 |
| CCL2 | mNOS-E36 | Shown to be of importance in alcohol-associated liver disease, a potential therapeutic target; compound shown to have benefit against inflammation in murine models of NASH and CCl4–induced hepatic injury207 |
| CCL5 | – | Antagonism shown to have benefit against liver fibrosis in animal models and is a potential new target122 |
| CCL20 | – | miR-590–5p-associated downregulation of CCL20 reduced fibrosis in NASH animal models, making it a novel therapeutic target203 |
| CXCL10 | NI-0801 (mAb) | Phase IIa studies show a good safety profile in patients with primary biliary cholangitis but failed to show clinical benefit204 |
| Receptors | ||
| CCR2 | CCX872 | Demonstrated good safety profile in phase II trials; these studies are based on promising effects in NASH murine models205,206 |
| CXCR1/CXCR2 | Reparixin | After demonstration of good safety profile in phase II studies, trials are under way for ischaemia-reperfusion injury prevention in liver transplant recipients208 |
| CCR2/CCR5 | Cenicriviroc | Multicentre phase II trials showed safety and efficacy signals; reduction of fibrosis at year 1 and maintenance at year 2 were noted in the phase IIb CENTAUR trial in patients with NASH compared with placebo; follow-up phase III trial is under way in patients with NASH and results are eagerly awaited209,210 (AURORA; NCT03028740) |
‘–’ in the Drugs column denotes no current named compounds in trials. CCl4, carbon tetrachloride; mAb, monoclonal antibody; NASH, nonalcoholic steatohepatitis.
Preclinical studies
Serum levels of CCL2 are increased in patients with alcoholic hepatitis, which is thought to be involved in the pathogenesis of ALD. A study showed that CCL2 levels are higher in patients with severe alcoholic hepatitis and biopsy-proven ALD compared with healthy individuals as controls. It also showed that patients who consumed the same amount of alcohol were more likely to develop severe alcoholic hepatitis with the G allele for the −2518A>G CCL2 polymorphism99,200,201. Studies in mouse models have previously also shown that CCL2 deficiency protects against alcohol-induced liver injury, independent of CCR2, possibly by the inhibition of pro-inflammatory cytokines78.
Similarly, CCR5 haplotypes and mRNA expression in livers were found to be associated with severe liver fibrosis in patients. CCR5 was also shown to be expressed on the surface of HSCs136,202. CCR5 antagonism has since been shown to reduce fibrosis in multiple animal models of fibrosis122. This observation carries potential therapeutic implications for NASH, alcoholic hepatitis and chronic HCV, among others.
CXCL1 is another chemokine that could be a potential therapeutic target, especially for primary inflammatory conditions such as alcoholic hepatitis and liver fibrosis. An earlier study, from 2016, showed that CXCL1 was released by hepatocytes in mouse liver5,47. Later evidence, including our own data, suggests that endothelial cells are one of the main sources of this chemokine in the human liver47. Its role is also being investigated in similar pathophysiological conditions such as lung fibrosis and atherosclerosis.
CCL20 ligand levels have been shown to correlate with worsening severity of liver histology in patients with NAFLD. A potential therapeutic benefit of exogenous miR-590-5p, which functionally interacts with the CCL20 3′ UTR region to downregulate its expression, has also been demonstrated. This observation points to a novel mechanism of CCL20 and HSC interactions promoting fibrogenesis in NAFLD203.
Clinical trials
Encouraged by the positive results of in vitro studies, an open-label phase IIa trial was conducted for NI-0801, a selective fully human monoclonal antibody against CXCL10, in ursodeoxycholic acid non-responder patients with primary biliary cholangitis. Even though the drug demonstrated a good safety profile, it was unfortunately unable to provide clinical benefit to study participants204.
The selective inhibition of CCR2 was found to be effective in NASH. The drug CCX872 has demonstrated a good safety profile in phase I studies as well as improvement in NASH animal models205. It has also been tested in a phase II trial as a combination therapy for pancreatic cancer, again demonstrating a good safety profile as well as some improvement in overall survival206. Another way to disrupt CCR2 signalling is to inhibit its ligand, CCL2. The CCL2 inhibitor mNOX-E36 was shown to reduce intrahepatic macrophage accumulation and steatosis development in NASH and toxic CCl4 mouse models207.
After showing a good tolerability and safety profile in multiple phase I and II studies, a small molecular inhibitor of CXCR1 and CXCR2, reparixin, is currently undergoing a phase II trial for IRI in liver transplantation and early allograft dysfunction208.
Cenicriviroc, an oral and dual CCR2–CCR5 antagonist, has been tested in multiple phase II studies (NCT01092104, NCT01338883) conducted in patients infected with HIV and has shown CCR2 blockade (with a reciprocal increase in CCL2 levels) and CCR5 blockade (as demonstrated by a reduction in HIV1 RNA levels). It has also demonstrated a good safety and tolerability profile. Notably, lower levels of soluble CD14, which is a circulating biomarker of monocyte activation, were observed in adults with NASH and liver fibrosis in the recent CENTAUR study after 48–52 weeks of therapy (n = 289; a randomized double-blinded phase IIb study)209. This was further analysed and aspartate aminotransferase-to-platelet ratio index (APRI) and fibrosis-4 index (FiB-4) were lower at 48 weeks, indicating a reduction in fibrosis. Preliminary analysis (1 year) of the 2-year CENTAUR study affirmed the safety of cenicriviroc and resulted in double the number of individuals achieving at least a one-stage fibrosis reduction compared with placebo. Furthermore, they noted twice the number of individuals showing no steatohepatitis worsening versus placebo. The final analysis of the CENTAUR study, after 2 years of therapy, has shown that, even though cenicriviroc is not associated with further improvement in fibrosis after year 1, the durability of the fibrosis benefit was higher compared with the placebo group. This was most remarkable in the advanced (stage 3) fibrosis group. The safety profile of the drug was well maintained throughout year 2 (REF.210). Cenicriviroc is currently undergoing a phase III evaluation for the treatment of liver fibrosis in adults with NASH (AURORA; NCT03028740). At the time of writing this Review, cenicriviroc is regarded as one of the most promising therapeutics under investigation for NASH.
One major drawback in limiting the translational potential of chemokine and chemokine receptor inhibitors is the redundancy in the function of inflammatory chemokines. Rather than targeting any specific chemokine, a more efficient approach might be using chemokine receptor antagonists with specificity for multiple targets or modulating the epigenetic regulation of many chemokine targets such as using a suppressor of chemokine super-enhancers. One study demonstrated that a pharmacological inhibitor of BET proteins, GSK620, attenuated liver steatosis and inflammation in a mouse model of NAFLD along with decreased CCL2 expression211. As discussed earlier, BET proteins, such as BRD4, are required for the function of super-enhancers and pharmacological BET inhibitors modulate super-enhancer-regulated genes, such as those encoding chemokines, with high specificity and provide a novel approach to modulating the inflammatory response212,213. The pan-BET inhibitors are effective in mouse models of hepatic steatosis and fibrosis211,212. Even though pan-BET inhibitors have shown clinical effectiveness in humans, they are not close to being used in clinical practice owing to their potential adverse effects. Phase I trials have noted grade 1/2 adverse effects, such as dysgeusia and vomiting, as well as grade 3/4 adverse effects, including elevated liver enzymes and bilirubin214,215. Studies currently aim to delineate the functions of the BD1 and BD2 bromodomains that are present in each of the four human BET proteins and they are leading to the development of next-generation epigenetic inhibitors for more specific inflammation or cancer BET-targeting therapies212.
To further advance chemokines as therapeutics, there needs to be an impetus towards translating animal studies to human clinical trials. Challenges include a mismatch in functions of chemokines between different species and an ineffectiveness to determine the correct dose to stay within the safety window but still impart therapeutic effect. Another limitation of current clinical trials is the standalone use of chemokine inhibitors to elicit a meaningful response in patients with liver diseases. As advanced liver disease is characterized not only by inflammation but also by fibrosis and other hallmark features, future clinical trials can be designed to test combination therapies of chemokines with other pathophysiological targets of the specific type of liver disease.
Conclusions
Chemokines are expressed in a temporal and spatial manner in the pathogenesis of several acute and chronic liver diseases and have many different functions (pro-inflammatory, anti-inflammatory, angiogenic, angiostatic, liver remodelling). Many cell types can express and secrete chemokines in damaged livers, including most liver-resident cells and infiltrating immune cells. To clarify what cell types are the main sources of chemokines in human or animal models is challenging. In addition, their interactions with G-protein-coupled receptors are highly relevant to the clinic. Likewise, many liver and immune cells also express different chemokine receptors. Altogether, the local concentrations of chemokines and their interactions with receptors determine the progression and outcome of associated liver damage.
With the rapid development of next-generation sequencing and the development of 3D chromatin capture technology and single-cell sequencing, more light will be shed on the multiple cell type-specific sources and roles of chemokines in the liver (BOX 1). For instance, a 2019 study profiled the transcriptomes of more than 100,000 single human cells in healthy and cirrhotic human liver by scRNA-seq77. This study identified a scar-associated TREM2+CD9+ subpopulation of macrophages that expands in liver fibrosis and defined ACKR1+ and PLVAP+ endothelial cells216 that are topographically restricted to the fibrotic niche in cirrhosis and enhance the transmigration of leukocytes. ACKR1 is an atypical receptor for CXCL2, which has been indicated to be critical for unidirectional paracellular neutrophil trans-endothelium migration in vivo66. All of these studies support the notion that different CXCLs can act in a sequential manner to guide neutrophils through blood vessel walls as regulated by their distinct cellular sources. Nevertheless, more new insights and discoveries on chemokines and their interactions with receptors are warranted and many more studies are necessary before those new findings can be translated into clinical targets. Altogether, it is conceivable that this work, by interfering with the production of pro-inflammatory or angiogenic chemokines and/or their receptors, might effectively reduce or eliminate chronic inflammation and angiogenesis in the treatment of liver diseases.
Box 1. Open questions and future directions.
It is critical to clarify the cellular sources of chemokines and their target receptors. With the application of single-cell RNA sequencing technology in the field of liver diseases, we will be able to answer this question with increased precision in the future.
The single-cell technique has also been adopted in genomic functional studies such as single-cell Assay for Transposase-Accessible Chromatin using sequencing (ATAC-seq) and single-cell ChIP–seq, which hold tremendous potential. These techniques will enable the investigation of gene transcriptional regulation with single-cell precision, which can help refine our understanding of cell type-specific and context-specific gene expression.
The majority of current therapeutics being studied in clinical trials are receptor inhibitors, ligand antagonists or neutralizing antibodies directed to either the chemokine or its receptor. More specific approaches to affect chemokine-mediated signalling pathways using CRISPR–dCas delivery or with extracellular vesicles as drug loading carriers have the potential to transform the field.
In addition to treating liver diseases by targeting specific chemokines or their receptors, chemokines might be important biomarkers to enable the early detection of liver damage in certain liver diseases.
Chemokines and their receptors have long been identified as promising pharmacological targetable molecules. With the fast-paced developments in single-cell technology and 3D chromatin genomic functional studies, we will be ready to embrace these bioinformatics-driven technologies and new insights will be gained from them in the understanding and treatment of liver diseases.
Supplementary Material
Key points.
Chemokines are secreted mediators that regulate the infiltration of immune cells into the liver and modulate the activation and proliferation of almost all liver cell types.
Individual chemokines can interact with their corresponding receptors based on their structural characteristics and local context and can contribute to liver homeostasis by interacting with immune and non-immune liver cells.
Tumour necrosis factor (TNF), IL-6 and many other cytokines can rapidly stimulate the transcription of pro-inflammatory chemokines in a robust and co-regulated manner through the epigenetically primed chromatin 3D conformation of enhancer–promoter interactions.
Chemokines are associated with liver disease and it is critical to identify different major players that provide the similarities and dissimilarities between mouse and human genomic arrangement.
Current drug development based on chemokines and receptors in liver diseases is limited and can be greatly improved with novel therapeutic strategies.
Acknowledgements
Work on chemokines in the laboratory of V.H.S. is supported by NIH grants AA21171 and DK59615.
Footnotes
Competing interests
The authors declare no competing interests.
Supplementary information
The online version contains supplementary material available at https://doi.org/10.1038/s41575-021-00444-2.
References
- 1.Deuel TF, Keim PS, Farmer M & Heinrikson RL Amino acid sequence of human platelet factor 4. Proc. Natl Acad. Sci. USA 74, 2256–2258 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anisowicz A, Bardwell L & Sager R Constitutive overexpression of a growth-regulated gene in transformed Chinese hamster and human cells. Proc. Natl Acad. Sci. USA 84, 7188–7192(1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugano S, Stoeckle MY & Hanafusa H Transformation by Rous sarcoma virus induces a novel gene with homology to a mitogenic platelet protein. Cell 49, 321–328 (1987). [DOI] [PubMed] [Google Scholar]
- 4.Vinader V & Afarinkia K A beginner’s guide to chemokines. Future Med. Chem. 4, 845–852 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Chang B et al. Short- or long-term high-fat diet feeding plus acute ethanol binge synergistically induce acute liver injury in mice: an important role for CXCL1. Hepatology 62, 1070–1085 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marra F & Tacke F Roles for chemokines in liver disease. Gastroenterology 147, 577–594.e1 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Hughes CE & Nibbs RJB A guide to chemokines and their receptors. FEBS J. 285, 2944–2971 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rot A Chemokine patterning by glycosaminoglycans and interceptors. Front. Biosci. 15, 645–660 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Joseph PRB et al. Lysines and arginines play non-redundant roles in mediating chemokine-glycosaminoglycan interactions. Sci. Rep. 8, 12289 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luster AD Chemokines-chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 338, 436–445 (1998). This is one of the early reviews that introduced the burgeoning family of cytokines, with special emphasis on their role in the pathophysiology of disease and their potential as targets for therapy.
- 11.Charo IF & Ransohoff RM The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354, 610–621 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Laing KJ & Secombes CJ Trout CC chemokines: comparison of their sequences and expression patterns. Mol. Immunol. 41, 793–808 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Raman D, Sobolik-Delmaire T & Richmond A Chemokines in health and disease. Exp. Cell Res. 317, 575–589 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sahin H, Trautwein C & Wasmuth HE Functional role of chemokines in liver disease models. Nat. Rev. Gastroenterol. Hepatol. 7, 682–690 (2010). This paper summarized the chemokines in experimental liver disease models, the advances that might lead to preclinical applications and the roles of chemokine receptors as promising pharmacologically targetable molecules.
- 15.Gollomp K et al. Neutrophil accumulation and NET release contribute to thrombosis in HIT. JCI Insight 3, e99445 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neumann K et al. Chemokine transfer by liver sinusoidal endothelial cells contributes to the recruitment of CD4+ T cells into the murine liver. PLoS ONE 10, e0123867 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yano T, Ohira M, Nakano R, Tanaka Y & Ohdan H Hepatectomy leads to loss of TRAIL-expressing liver NK cells via downregulation of the CXCL9-CXCR3 axis in mice. PLoS ONE 12, e0186997 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambade A et al. Pharmacological inhibition of CCR2/5 signaling prevents and reverses alcohol-induced liver damage, steatosis, and inflammation in mice. Hepatology 69, 1105–1121 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krenkel O et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology 67, 1270–1283 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Gavegnano C, Savarino A, Owanikoko T & Marconi VC Crossroads of cancer and HIV-1: pathways to a cure for HIV. Front. Immunol. 10, 2267 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng H et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381, 661–666 (1996). [DOI] [PubMed] [Google Scholar]
- 22.Dragic T et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381, 667–673 (1996). [DOI] [PubMed] [Google Scholar]
- 23.Oberlin E et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature 382, 833–835 (1996). [DOI] [PubMed] [Google Scholar]
- 24.Bleul CC et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382, 829–833 (1996). [DOI] [PubMed] [Google Scholar]
- 25.Pola R et al. Monocyte chemoattractant protein-1 (MCP-1) gene polymorphism and risk of Alzheimer’s disease in Italians. Exp. Gerontol. 39, 1249–1252 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Pham MH et al. The rs1024611 regulatory region polymorphism is associated with CCL2 allelic expression imbalance. PLoS ONE 7, e49498 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascheretti S et al. Genetic variants in the CCR gene cluster and spontaneous viral elimination in hepatitis C-infected patients. Clin. Exp. Immunol. 136, 328–333 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pineda-Tenor D et al. Single nucleotide polymorphisms of CXCL9–11 chemokines are associated with liver fibrosis in HIV/HCV-coinfected patients. J. Acquir. Immune Defic. Syndr. 68, 386–395 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Hsu SH & Ghoshal K MicroRNAs in liver health and disease. Curr. Pathobiol. Rep. 1, 53–62 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saiman Y & Friedman SL The role of chemokines in acute liver injury. Front. Physiol. 3, 213 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gao B, Ahmad MF, Nagy LE & Tsukamoto H Inflammatory pathways in alcoholic steatohepatitis. J. Hepatol. 70, 249–259 (2019). An important review about the roles of multiple cell types that are involved in inflammation in alcoholic steatohepatitis, including resident macrophages and infiltrating monocytes, as well as other cell types in the innate and adaptive immune system.
- 32. Brown JD et al. NF-κB directs dynamic super enhancer formation in inflammation and atherogenesis. Mol. Cell 56, 219–231 (2014). This is one of the early studies about how BET bromodomain inhibition abrogates super-enhancer- mediated inflammatory transcription, atherogenic endothelial responses and atherosclerosis both in vitro and in vivo.
- 33.Ohmori Y, Schreiber RD & Hamilton TA Synergy between interferon-gamma and tumor necrosis factor-alpha in transcriptional activation is mediated by cooperation between signal transducer and activator of transcription 1 and nuclear factor kappaB. J. Biol. Chem. 272, 14899–14907 (1997). [DOI] [PubMed] [Google Scholar]
- 34.Li QJ et al. MAP kinase phosphorylation-dependent activation of EIk-1 leads to activation of the co-activator p300. EMBO J. 22, 281–291 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanyal A, Lajoie BR, Jain G & Dekker J The long-range interaction landscape of gene promoters. Nature 489, 109–113 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carey M The enhanceosome and transcriptional synergy. Cell 92, 5–8 (1998). [DOI] [PubMed] [Google Scholar]
- 37.Bulger M & Groudine M Functional and mechanistic diversity of distal transcription enhancers. Cell 144, 327–339 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heinz S, Romanoski CE, Benner C & Glass CK The selection and function of cell type-specific enhancers. Nat. Rev. Mol. Cell Biol. 16, 144–154 (2015). Enhancers are genetic elements that have major roles in determining cell type-specific gene expression patterns and responses to internal and external signals. An understanding of the mechanisms underlying the cell type-specific selection and function of enhancers will improve our understanding of the effects of natural genetic variation on complex phenotypes and diseases.
- 39.Hnisz D et al. Super-enhancers in the control of cell identity and disease. Cell 155, 934–947 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dekker J et al. The 4D nucleome project. Nature 549, 219–226 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jin F et al. A high-resolution map of the three-dimensional chromatin interactome in human cells. Nature 503, 290–294 (2013). This is one of the important studies about the use of 3C-based techniques to cover a whole-genome, unbiased view of chromatin interactions, including the characterization on the dynamics of promoter-enhancer contacts after TNF signalling in cells.
- 42.Barnes PJ & Karin M Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336, 1066–1071 (1997). [DOI] [PubMed] [Google Scholar]
- 43.Higashijima Y et al. Coordinated demethylation of H3K9 and H3K27 is required for rapid inflammatory responses of endothelial cells. EMBO J. 39, e103949 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fanucchi S et al. Immune genes are primed for robust transcription by proximal long noncoding RNAs located in nuclear compartments. Nat. Genet. 51, 138–150 (2019). This study shows that 3D chromatin topology enables CXCL family genes to engage in chromosomal contacts with a subset of long non-coding RNAs under a powerful enhancer.
- 45.Tarjan DR, Flavahan WA & Bernstein BE Epigenome editing strategies for the functional annotation of CTCF insulators. Nat. Commun. 10, 4258 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dominguez M et al. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology 136, 1639–1650 (2009). A prospective study about hepatic expression of the CXC subfamily of chemokines, including CXCL1 and CXCL8, with histological findings to correlate with the prognosis of patients with alcoholic hepatitis.
- 47.Liu M et al. Cytokine induced inflammatory chemokine production is under super enhancer regulation in alcoholic hepatitis hepatology. Hepatology 70, 186A–187A (2019). [Google Scholar]
- 48.The FANTOM Consortium and the RIKEN PMI and CLST (DGT). A promoter-level mammalian expression atlas. Nature 507, 462–470 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van de Werken HJ et al. 4C technology: protocols and data analysis. Methods Enzymol. 513, 89–112 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Nicodeme E et al. Suppression of inflammation by a synthetic histone mimic. Nature 468, 1119–1123 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fok ET, Davignon L, Fanucchi S & Mhlanga MM The lncRNA connection between cellular metabolism and epigenetics in trained immunity. Front. Immunol. 9, 3184 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kubes P & Jenne C Immune responses in the liver. Annu. Rev. Immunol. 36, 247–277 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Oo YH, Shetty S & Adams DH The role of chemokines in the recruitment of lymphocytes to the liver. Dig. Dis. 28, 31–44 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zlotnik A, Yoshie O & Nomiyama H The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 7, 243 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wasmuth HE et al. Antifibrotic effects of CXCL9 and its receptor CXCR3 in livers of mice and humans. Gastroenterology 137, 309–319 319.e1–3 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sahin H et al. Chemokine Cxcl9 attenuates liver fibrosis-associated angiogenesis in mice. Hepatology 55, 1610–1619 (2012). [DOI] [PubMed] [Google Scholar]
- 57.Shiraha H, Glading A, Gupta K & Wells A IP-10 inhibits epidermal growth factor-induced motility by decreasing epidermal growth factor receptor-mediated calpain activity. J. Cell Biol. 146, 243–254 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zimmermann HW & Tacke F Modification of chemokine pathways and immune cell infiltration as a novel therapeutic approach in liver inflammation and fibrosis. Inflamm. Allergy Drug Targets 10, 509–536 (2011). [DOI] [PubMed] [Google Scholar]
- 59.Bigorgne AE et al. TLR4-dependent secretion by hepatic stellate cells of the neutrophil-chemoattractant CXCL1 mediates liver response to gut microbiota. PLoS ONE 11, e0151063 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aguilar-Bravo B et al. Ductular reaction cells display an inflammatory profile and recruit neutrophils in alcoholic hepatitis. Hepatology 69, 2180–2195 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roh YS, Zhang B, Loomba R & Seki E TLR2 and TLR9 contribute to alcohol-mediated liver injury through induction of CXCL1 and neutrophil infiltration. Am. J. Physiol. Gastrointest. Liver Physiol 309, G30–G41 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robinson MW, Harmon C & O’Farrelly C Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 13, 267–276 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sahin H, Berres ML & Wasmuth HE Therapeutic potential of chemokine receptor antagonists for liver disease. Expert Rev. Clin. Pharmacol. 4, 503–513 (2011). [DOI] [PubMed] [Google Scholar]
- 64.Kanamori-Katayama M et al. Unamplified cap analysis of gene expression on a single-molecule sequencer. Genome Res. 21, 1150–1159 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Krausgruber T et al. Structural cells are key regulators of organ-specific immune responses. Nature 583, 296–302 (2020). This in vivo bulk RNA-seq study shows that, within 2 hours of TNF treatment in mice, liver endothelial cells are the only structural cell type in liver to have a significant CXCL1 mRNA level increase compared with other cell types such as hepatocytes and fibroblasts.
- 66.Girbl T et al. Distinct compartmentalization of the chemokines CXCL1 and CXCL2 and the atypical receptor ACKR1 determine discrete stages of neutrophil diapedesis. Immunity 49, 1062–1076.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.MacParland SA et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat. Commun. 9, 4383 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tabula Muris C et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562, 367–372 (2018). An early paper on mouse single-cell transcriptomic data comprising >100,000 cells from 20 organs and tissues.
- 69.GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 5, 245–266 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Colombo M et al. Hepatocellular carcinoma in Italian patients with cirrhosis. N. Engl. J. Med. 325, 675–680 (1991). [DOI] [PubMed] [Google Scholar]
- 71.El–Serag HB & Rudolph KL Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132, 2557–2576 (2007) [DOI] [PubMed] [Google Scholar]
- 72.Guicciardi ME et al. Macrophages contribute to the pathogenesis of sclerosing cholangitis in mice. J. Hepatol. 69, 676–686 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tacke F Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 66, 1300–1312 (2017). [DOI] [PubMed] [Google Scholar]
- 74.Lefebvre E et al. Antifibrotic effects of the dual CCR2/CCR5 antagonist cenicriviroc in animal models of liver and kidney fibrosis. PLoS ONE 11, e0158156 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramachandran P et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc. Natl Acad. Sci. USA 109, E3186–E3195 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mitchell C et al. Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice. Am. J. Pathol. 174, 1766–1775 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ramachandran P et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 575, 512–518 (2019). Latest scRNA-seq paper from human cirrhotic liver dissects that unanticipated aspects of the cellular and molecular basis of liver fibrosis at a single-cell level.
- 78.Mandrekar P, Ambade A, Lim A, Szabo G & Catalano D An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology 54, 2185–2197 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dhanda AD & Collins PL Immune dysfunction in acute alcoholic hepatitis. World J. Gastroenterol. 21, 11904–11913 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gao B & Xu M Chemokines and alcoholic hepatitis: are chemokines good therapeutic targets? Gut 63, 1683–1684 (2014). This is a well-written review summarizing the roles played by chemokines in the pathogenesis of alcoholic hepatitis.
- 81. Wasmuth HE, Tacke F & Trautwein C Chemokines in liver inflammation and fibrosis. Semin. Liver Dis. 30, 215–225 (2010). This is a nice review about the role of chemokines in the pathogenesis of liver cirrhosis.
- 82. Chen W, Zhang J, Fan HN & Zhu JS Function and therapeutic advances of chemokine and its receptor in nonalcoholic fatty liver disease. Ther. Adv. Gastroenterol. 11, 1756284818815184 (2018). This review is on the role of chemokines in the pathogenesis of NAFLD and the potential for therapeutic targeting of these chemokine pathways for the treatment of NAFLD.
- 83.Braunersreuther V, Viviani GL, Mach F & Montecucco F Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J. Gastroenterol. 18, 727–735 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feng D The alteration of immune cells in the pathogenesis of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Liver Res. 4, 23–27 (2020). [Google Scholar]
- 85.Fahey S, Dempsey E & Long A The role of chemokines in acute and chronic hepatitis C infection. Cell Mol. Immunol. 11, 25–40 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fallahi P et al. Chemokines in the pathogenesis and as therapeutical markers and targets of HCV chronic infection and HCV extrahepatic manifestations. Curr. Drug Targets 18, 786–793 (2017). [DOI] [PubMed] [Google Scholar]
- 87.Zhai Y, Petrowsky H, Hong JC, Busuttil RW & Kupiec-Weglinski JW Ischaemia-reperfusion injury in liver transplantation — from bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 10, 79–89 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yip WW & Burt AD Alcoholic liver disease. Semin. Diagn. Pathol. 23, 149–160 (2006). [DOI] [PubMed] [Google Scholar]
- 89. Singal AK & Shah VH Current trials and novel therapeutic targets for alcoholic hepatitis. J. Hepatol. 70, 305–313 (2019). A review summarizing the novel therapeutic agents targeting various pathways in the pathophysiology of alcoholic hepatitis and the ongoing clinical trials in which some of these agents are being studied.
- 90.Xu M-J, Zhou Z, Parker R & Gao B Targeting inflammation for the treatment of alcoholic liver disease. Pharmacol. Ther. 180, 77–89 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rao R Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology 50, 638–644 (2009). This review discusses a key concept of inter-organ crosstalk in the setting of alcoholic liver disease. A similar inter-organ crosstalk also exists between liver and other organs, such as adipose tissue, and in other disease conditions, such as NAFLD.
- 92.Bird GL, Sheron N, Goka AK, Alexander GJ & Williams RS Increased plasma tumor necrosis factor in severe alcoholic hepatitis. Ann. Intern. Med. 112, 917–920 (1990). [DOI] [PubMed] [Google Scholar]
- 93.Sheron N et al. Circulating and tissue levels of the neutrophil chemotaxin interleukin-8 are elevated in severe acute alcoholic hepatitis, and tissue levels correlate with neutrophil infiltration. Hepatology 18, 41–46 (1993). [PubMed] [Google Scholar]
- 94.Khoruts A, Stahnke L, McClain CJ, Logan G & Allen JI Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology 13, 267–276 (1991). [PubMed] [Google Scholar]
- 95.Fujita T & Narumiya S Roles of hepatic stellate cells in liver inflammation: a new perspective. Inflamm. Regen. 36, 1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seo W & Jeong WI Hepatic non-parenchymal cells: master regulators of alcoholic liver disease? World J. Gastroenterol. 22, 1348–1356 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cohen JI & Nagy LE Pathogenesis of alcoholic liver disease: interactions between parenchymal and non-parenchymal cells. J. Dig. Dis. 12, 3–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Affo S et al. CCL20 mediates lipopolysaccharide induced liver injury and is a potential driver of inflammation and fibrosis in alcoholic hepatitis. Gut 63, 1782 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Degre D, Lemmers A & Gustot T Hepatic expression of CCL2 in alcoholic liver disease is associated with disease severity and neutrophil infiltrates. Clin. Exp. Immunol. 169, 302–310 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ghosh Dastidar S, Warner JB, Warner DR, McClain CJ & Kirpich IA Rodent models of alcoholic liver disease: role of binge ethanol administration. Biomolecules 8, 3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bertola A, Mathews S, Ki SH, Wang H & Gao B Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 8, 627–637 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lazaro R et al. Osteopontin deficiency does not prevent but promotes alcoholic neutrophilic hepatitis in mice. Hepatology 61, 129–140 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wieser V et al. Reversal of murine alcoholic steatohepatitis by pepducin-based functional blockade of interleukin-8 receptors. Gut 66, 930–938 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Altamirano J et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology 146, 1231–1239.e1–6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Taieb J et al. Polymorphonuclear neutrophils are a source of hepatocyte growth factor in patients with severe alcoholic hepatitis. J. Hepatol. 36, 342–348 (2002). [DOI] [PubMed] [Google Scholar]
- 106.Byun J-S, Suh Y-G, Yi H-S, Lee Y-S & Jeong W-I Activation of toll-like receptor 3 attenuates alcoholic liver injury by stimulating Kupffer cells and stellate cells to produce interleukin-10 in mice. J. Hepatol. 58, 342–349 (2013). [DOI] [PubMed] [Google Scholar]
- 107.Younossi Z et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 15, 11 (2017). [DOI] [PubMed] [Google Scholar]
- 108.Brunt EM Pathology of fatty liver disease. Mod. Pathol. 20 (Suppl. 1), S40–S48 (2007). [DOI] [PubMed] [Google Scholar]
- 109.Stephenson K et al. Updates on dietary models of nonalcoholic fatty liver disease: current studies and insights. Gene Expr. 18, 5–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kanneganti TD & Dixit VD Immunological complications of obesity. Nat. Immunol. 13, 707–712 (2012). [DOI] [PubMed] [Google Scholar]
- 111.Xu H, Barnes GT & Yang Q Chronic inflammation in fat plays a crucial role in the development of obesityrelated insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Makki K, Froguel P & Wolowczuk I Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm 2013, 139239–139239 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Coppack SW Pro-inflammatory cytokines and adipose tissue. Proc. Nutr. Soc. 60, 349–356 (2001). [DOI] [PubMed] [Google Scholar]
- 114.Kanda H, Tateya S & Tamori Y MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116, 1494–1505 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tamura Y, Sugimoto M & Murayama T C-C chemokine receptor 2 inhibitor improves diet-induced development of insulin resistance and hepatic steatosis in mice. J. Atheroscler. Thromb. 17, 219–228 (2010). [DOI] [PubMed] [Google Scholar]
- 116.Sartipy P & Loskutoff DJ Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl Acad. Sci. USA 100, 7265–7270 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reid DT et al. Kupffer cells undergo fundamental changes during the development of experimental NASH and are critical in initiating liver damage and inflammation. PLoS ONE 11, e0159524 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xiong X et al. Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis. Mol. Cell 75, 644–660.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jaitin DA et al. Lipid-associated macrophages control metabolic homeostasis in a trem2-dependent manner. Cell 178, 686–698.e14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Krenkel O et al. Myeloid cells in liver and bone marrow acquire a functionally distinct inflammatory phenotype during obesity-related steatohepatitis. Gut 69, 551–563 (2020). [DOI] [PubMed] [Google Scholar]
- 121.Perugorria MJ et al. Non-parenchymal TREM-2 protects the liver from immune-mediated hepatocellular damage. Gut 68, 533–546 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Berres ML et al. Antagonism of the chemokine CCL5 ameliorates experimental liver fibrosis in mice. J. Clin. Invest. 120, 4129–4140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Perez-Martinez L et al. Maraviroc, a CCR5 antagonist, ameliorates the development of hepatic steatosis in a mouse model of non-alcoholic fatty liver disease (NAFLD). J. Antimicrob. Chemother. 69, 1903–1910 (2014). [DOI] [PubMed] [Google Scholar]
- 124.Hu Y et al. Gut-derived lymphocyte recruitment to liver and induce liver injury in non-alcoholic fatty liver disease mouse model. J. Gastroenterol. Hepatol. 31, 676–684 (2016). [DOI] [PubMed] [Google Scholar]
- 125.Zhang X et al. CXCL10 plays a key role as an inflammatory mediator and a non-invasive biomarker of non-alcoholic steatohepatitis. J. Hepatol. 61, 1365–1375 (2014). [DOI] [PubMed] [Google Scholar]
- 126.Tomita K et al. CXCL10-mediates macrophage, but not other innate immune cells-associated inflammation in murine nonalcoholic steatohepatitis. Sci. Rep. 6, 28786 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rouault C, Pellegrinelli V & Schilch R Roles of chemokine ligand-2 (CXCL2) and neutrophils in influencing endothelial cell function and inflammation of human adipose tissue. Endocrinology 154, 1608–1614 (2013). [DOI] [PubMed] [Google Scholar]
- 128.Jarrar MH et al. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 27, 412–421 (2008). [DOI] [PubMed] [Google Scholar]
- 129.Tsuchida T & Friedman SL Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 14, 397 (2017). [DOI] [PubMed] [Google Scholar]
- 130.Chu PS, Nakamoto N & Ebinuma H C-C motif chemokine receptor 9 positive macrophages activate hepatic stellate cells and promote liver fibrosis in mice. Hepatology 58, 337–350 (2013). [DOI] [PubMed] [Google Scholar]
- 131.Ehling J et al. CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut 63, 1960 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Marra F, Romanelli RG & Giannini C Monocyte chemotactic protein-1 as a chemoattractant for human hepatic stellate cells. Hepatology 29, 140–148 (1999). [DOI] [PubMed] [Google Scholar]
- 133.Seki E, de Minicis S & Inokuchi S CCR2 promotes hepatic fibrosis in mice. Hepatology 50, 185–197 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nakamoto N et al. CCR9+ macrophages are required for acute liver inflammation in mouse models of hepatitis. Gastroenterology 142, 366–376 (2012). [DOI] [PubMed] [Google Scholar]
- 135.Chen L, Zhang Q, Yu C, Wang F & Kong X Functional roles of CCL5/RANTES in liver disease. Liver Res. 4, 28–34 (2020). [Google Scholar]
- 136.Seki E, De Minicis S & Gwak GY CCR1 and CCR5 promote hepatic fibrosis in mice. J. Clin. Invest. 119, 1858–1870 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Heinrichs D, Berres ML & Nellen A The chemokine CCL3 promotes experimental liver fibrosis in mice. PLoS ONE 8, 66106 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tacke F & Zimmermann HW Macrophage heterogeneity in liver injury and fibrosis. J. Hepatol. 60, 1090–1096 (2014). [DOI] [PubMed] [Google Scholar]
- 139.Oo YH, Banz V & Kavanagh D CXCR3-dependent recruitment and CCR6-mediated positioning of Th-17 cells in the inflamed liver. J. Hepatol. 57, 1044–1051 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hintermann E, Bayer M & Pfeilschifter JM CXCL10 promotes liver fibrosis by prevention of NK cell mediated hepatic stellate cell inactivation. J. Autoimmun. 35, 424–435 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hammerich L et al. Chemokine receptor CCR6-dependent accumulation of γδ T cells in injured liver restricts hepatic inflammation and fibrosis. Hepatology 59, 630–642 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wehr A, Baeck C & Heymann F Chemokine receptor CXCR6-dependent hepatic NK T Cell accumulation promotes inflammation and liver fibrosis. J. Immunol. 190, 5226–5236 (2013). [DOI] [PubMed] [Google Scholar]
- 143.Karlmark KR, Zimmermann HW & Roderburg C The fractalkine receptor CX(3)CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology 52, 1769–1782 (2010). [DOI] [PubMed] [Google Scholar]
- 144.Aoyama T, Inokuchi S, Brenner DA & Seki E CX3CL1-CX3CR1 interaction prevents carbon tetrachloride-induced liver inflammation and fibrosis in mice. Hepatology 52, 1390–1400 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Llovet JM et al. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2, 16018 (2016). [DOI] [PubMed] [Google Scholar]
- 146. Hou J, Zhang H, Sun B & Karin M The immunobiology of hepatocellular carcinoma in humans and mice: basic concepts and therapeutic implications. J. Hepatol. 72, 167–182 (2020). This review summarizes the current understanding of HCC onco-immunology with an emphasis on how these mechanisms might be a basis for HCC-targeting immunotherapy.
- 147.Lim SY, Yuzhalin AE, Gordon-Weeks AN & Muschel RJ Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget 7, 28697–28710 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chew V et al. Inflammatory tumour microenvironment is associated with superior survival in hepatocellular carcinoma patients. J. Hepatol. 52, 370–379 (2010). [DOI] [PubMed] [Google Scholar]
- 149.Chen KJ, Lin SZ & Zhou L Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS ONE 6, 24671 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Oo YH et al. Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. J. Immunol. 184, 2886–2898 (2010). [DOI] [PubMed] [Google Scholar]
- 151.Ren Y, Poon RT & Tsui HT Interleukin-8 serum levels in patients with hepatocellular carcinoma: correlations with clinicopathological features and prognosis. Clin. Cancer Res. 9, 5996–6001 (2003). [PubMed] [Google Scholar]
- 152.Zhou SL, Dai Z & Zhou ZJ Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology 56, 2242–2254 (2012). [DOI] [PubMed] [Google Scholar]
- 153.Li L et al. CXCR2–CXCL1 axis is correlated with neutrophil infiltration and predicts a poor prognosis in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 34, 129 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu K et al. Targeting the vasculature in hepatocellular carcinoma treatment: Starving versus normalizing blood supply. Clin. Transl Gastroenterol. 8, e98 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yamada K et al. CXCL12–CXCR7 axis is important for tumor endothelial cell angiogenic property. Int. J. Cancer 137, 2825–2836 (2015). [DOI] [PubMed] [Google Scholar]
- 156.Zheng K et al. Chemokine receptor CXCR7 regulates the invasion, angiogenesis and tumor growth of human hepatocellular carcinoma cells. J. Exp. Clin. Cancer Res. 29, 31 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu J-Y et al. Delivery of siRNA using CXCR4-targeted nanoparticles modulates tumor microenvironment and achieves a potent antitumor response in liver cancer. Mol. Ther. 23, 1772–1782 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhu B et al. Activated hepatic stellate cells promote angiogenesis via interleukin-8 in hepatocellular carcinoma. J. Transl Med 13, 365 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lasagni L et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J. Exp. Med. 197, 1537–1549 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sutton A, Friand V & Brule-Donneger S Stromal cell derived factor-1/chemokine (C-X-C motif) ligand 12 stimulates human hepatoma cell growth, migration, and invasion. Mol. Cancer Res. 5, 21–33 (2007). [DOI] [PubMed] [Google Scholar]
- 161.Xiang ZL, Zeng ZC & Tang ZY Chemokine receptor CXCR4 expression in hepatocellular carcinoma patients increases the risk of bone metastases and poor survival. BMC Cancer 9, 176 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Armstrong D & Cameron RG Comparison of liver cancer and nodules induced in rats by deoxycholic acid diet with or without prior initiation. Cancer Lett. 57, 153–157 (1991). [DOI] [PubMed] [Google Scholar]
- 163.Ma C et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360, eaan5931 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mossanen JC et al. CXCR6 inhibits hepatocarcinogenesis by promoting natural killer T- and CD4+ T-cell-dependent control of senescence. Gastroenterology 156, 1877–1889.e4 (2019). [DOI] [PubMed] [Google Scholar]
- 165.Ehling J & Tacke F Role of chemokine pathways in hepatobiliary cancer. Cancer Lett. 379, 173–183 (2016). [DOI] [PubMed] [Google Scholar]
- 166.Park SH & Rehermann B Immune responses to HCV and other hepatitis viruses. Immunity 40, 13–24 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hu J, Lin YY, Chen PJ, Watashi K & Wakita T Cell and animal models for studying hepatitis B virus infection and drug development. Gastroenterology 156, 338–354 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Burm R, Collignon L, Mesalam AA & Meuleman P Animal models to study hepatitis C virus infection. Front. Immunol. 9, 1032 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rehermann B Pathogenesis of chronic viral hepatitis: differential roles of T cells and NK cells. Nat. Med. 19, 859–868 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Riezu-Boj JI, Larrea E & Aldabe R Hepatitis C virus induces the expression of CCL17 and CCL22 chemokines that attract regulatory T cells to the site of infection. J. Hepatol. 54, 422–431 (2011). [DOI] [PubMed] [Google Scholar]
- 171.Berres ML, Trautwein C & Schmeding M Serum chemokine CXC ligand 10 (CXCL10) predicts fibrosis progression after liver transplantation for hepatitis C infection. Hepatology 53, 596–603 (2011). [DOI] [PubMed] [Google Scholar]
- 172.Tacke F, Zimmermann HW & Berres ML Serum chemokine receptor CXCR3 ligands are associated with progression, organ dysfunction and complications of chronic liver diseases. Liver Int 31, 840–849 (2011). [DOI] [PubMed] [Google Scholar]
- 173.Vranjkovic A et al. Direct-acting antiviral treatment of HCV infection does not resolve the dysfunction of circulating CD8+ T-cells in advanced liver disease. Front. Immunol. 10.3389/fimmu.2019.01926 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Umemura T et al. Quantitative analysis of serum chemokines associated with treatment failure of direct-acting antivirals in chronic hepatitis C. Cytokine 111, 357–363 (2018). [DOI] [PubMed] [Google Scholar]
- 175.Hengst J et al. Direct-acting antiviral-induced hepatitis C virus clearance does not completely restore the altered cytokine and chemokine milieu in patients with chronic hepatitis C. J. Infect. Dis. 214, 1965–1974 (2016). [DOI] [PubMed] [Google Scholar]
- 176.Yoshio S et al. Cytokine and chemokine signatures associated with hepatitis B surface antigen loss in hepatitis B patients. JCI Insight 3, e122268 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Keating SM, Heitman JD & Wu S Cytokine and chemokine responses in the acute phase of hepatitis B virus replication in naive and previously vaccinated blood and plasma donors. J. Infect. Dis. 209, 845–854 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kakimi K et al. Blocking chemokine responsive to gamma-2/interferon (IFN)-gamma inducible protein and monokine induced by IFN-gamma activity in vivo reduces the pathogenetic but not the antiviral potential of hepatitis B virus-specific cytotoxic T lymphocytes. J. Exp. Med. 194, 1755–1766 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Maini MK et al. Direct ex vivo analysis of hepatitis B virus-specific CD8+ T cells associated with the control of infection. Gastroenterology 117, 1386–1396 (1999). [DOI] [PubMed] [Google Scholar]
- 180.Tan AT, Koh S & Goh W A longitudinal analysis of innate and adaptive immune profile during hepatic flares in chronic hepatitis B. J. Hepatol. 52, 330–339 (2010). [DOI] [PubMed] [Google Scholar]
- 181.Brass A & Brenndorfer ED The role of chemokines in hepatitis C virus-mediated liver disease. Int. J. Mol. Sci. 15, 4747–477 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ali JM et al. Analysis of ischemia/reperfusion injury in time-zero biopsies predicts liver allograft outcomes. Liver Transplant. 21,487–499 (2015). [DOI] [PubMed] [Google Scholar]
- 183.Degli Esposti D et al. Ischemic preconditioning induces autophagy and limits necrosis in human recipients of fatty liver grafts, decreasing the incidence of rejection episodes. Cell Death Dis 2, e111 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wilson GC et al. CXC chemokines function as a rheostat for hepatocyte proliferation and liver regeneration. PLoS ONE 10, e0120092 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Oliveira THCD, Marques PE, Proost P & Teixeira MMM Neutrophils: a cornerstone of liver ischemia and reperfusion injury. Lab. Invest. 98, 51–62 (2018). [DOI] [PubMed] [Google Scholar]
- 186.Bertini R et al. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc. Natl Acad. Sci. USA 101, 11791–11796 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Colletti LM et al. The role of cytokine networks in the local liver injury following hepatic ischemia/reperfusion in the rat. Hepatology 23, 506–514 (1996). [DOI] [PubMed] [Google Scholar]
- 188.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN & Edwards MJ Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and Kupffer cells. Hepatology 27, 507–512 (1998). [DOI] [PubMed] [Google Scholar]
- 189.Selzner N et al. Mouse livers with macrosteatosis are more susceptible to normothermic ischemic injury than those with microsteatosis. J. Hepatol. 44, 694–701 (2006). [DOI] [PubMed] [Google Scholar]
- 190.Mollica Poeta V, Massara M, Capucetti A & Bonecchi R Chemokines and chemokine receptors: new targets for cancer immunotherapy. Front. Immunol. 10, 379 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Balkwill F Cancer and the chemokine network. Nat. Rev. Cancer 4, 540–550 (2004). [DOI] [PubMed] [Google Scholar]
- 192.Sahin H et al. Proapoptotic effects of the chemokine, CXCL 10 are mediated by the noncognate receptor TLR4 in hepatocytes. Hepatology 57, 797–805 (2013). [DOI] [PubMed] [Google Scholar]
- 193.Zeremski M et al. Peripheral CXCR3-associated chemokines as biomarkers of fibrosis in chronic hepatitis C virus infection. J. Infect. Dis. 200, 1774–1780 (2009). [DOI] [PubMed] [Google Scholar]
- 194.Zeremski M et al. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology 48, 1440–1450 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhou Y et al. Hepatitis B virus protein X-induced expression of the CXC chemokine IP-10 is mediated through activation of NF-κB and increases migration of leukocytes. J. Biol. Chem. 285, 12159–12168 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Haukeland JW et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J. Hepatol. 44, 1167–1174 (2006). [DOI] [PubMed] [Google Scholar]
- 197.Sehrawat TS, Liu M & Shah VH The knowns and unknowns of treatment for alcoholic hepatitis. Lancet Gastroenterol. Hepatol. 5, 494–506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kuang DM et al. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J. Hepatol. 54, 948–955 (2011). [DOI] [PubMed] [Google Scholar]
- 199.Desurmont T et al. Overexpression of chemokine receptor CXCR2 and ligand CXCL7 in liver metastases from colon cancer is correlated to shorter disease-free and overall survival. Cancer Sci. 106, 262–269 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fisher NC, Neil DA, Williams A & Adams DH Serum concentrations and peripheral secretion of the beta chemokines monocyte chemoattractant protein 1 and macrophage inflammatory protein 1alpha in alcoholic liver disease. Gut 45, 416–420 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Devalaraja MN, McClain CJ, Barve S, Vaddi K & Hill DB Increased monocyte MCP-1 production in acute alcoholic hepatitis. Cytokine 11, 875–881 (1999). [DOI] [PubMed] [Google Scholar]
- 202.Schwabe RF, Bataller R & Brenner DA Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. Am. J. Physiol. Gastrointest. Liver Physiol 285, G949–G958 (2003). [DOI] [PubMed] [Google Scholar]
- 203.Hanson A et al. Chemokine ligand 20 (CCL20) expression increases with NAFLD stage and hepatic stellate cell activation and is regulated by miR-590–5p. Cytokine 123, 154789 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.de Graaf KL et al. NI-0801, an anti-chemokine (C-X-C motif) ligand 10 antibody, in patients with primary biliary cholangitis and an incomplete response to ursodeoxycholic acid. Hepatol. Commun. 2, 492–503 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Richard Parker MW et al. Therapeutic use of a clinical stage CCR2 inhibitor, CCX872, in obesity-associated steatohepatitis. Lancet 10.1016/S0140-6736(14)60341-X (2014). [DOI] [Google Scholar]
- 206.Linehan D et al. Overall survival in a trial of orally administered CCR2 inhibitor CCX872 in locally advanced/metastatic pancreatic cancer: correlation with blood monocyte counts. J. Clin. Oncol. 36, 92–92 (2018). [Google Scholar]
- 207. Baeck C et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut 61,416–426 (2012). This study demonstrated the blocking of monocyte–macrophage infiltration post-liver injury via MCP1 inhibition in vivo. Such early studies helped develop interest in studying chemokine inhibition as a novel therapeutic approach.
- 208.Cheng Y, Ma XL, Wei YQ & Wei XW Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochim. Biophys. Acta Rev. Cancer 1871, 289–312 (2019). [DOI] [PubMed] [Google Scholar]
- 209. Friedman SL et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 67, 1754–1767 (2018). A landmark double-blinded and multicentre RCT for the potential therapeutic cenicriviroc. Based on positive efficacy signal and safety profile, this trial has led to the establishment of a phase III trial.
- 210. Ratziu V et al. Cenicriviroc treatment for adults with nonalcoholic steatohepatitis and fibrosis: final analysis of the phase 2b CENTAUR study. Hepatology 72, 892–905 (2020). This is a nice paper about a landmark clinical trial for recent NASH therapeutics.
- 211. Gilan O et al. Selective targeting of BD1 and BD2 of the BET proteins in cancer and immunoinflammation. Science 368, 387–394 (2020). This study identifies the specificity of blocking BD1 and BD2 domains. iBET-BD1 and iBET-BD2 were both shown to have immunomodulatory activity but iBET-BD2 inhibition with GSK620 was shown to ameliorate inflammatory injury.
- 212. Filippakopoulos P & Knapp S Next-generation epigenetic inhibitors. Science 368, 367–368 (2020). A comprehensive and current review of new advances in promising epigenetic inhibitors; it describes roles for bromodomains in transcription and various ways that they can be targeted.
- 213.Qi J & Shi Y Selective targeting of different bromodomains by small molecules. Cancer Cell 37, 764–766 (2020). [DOI] [PubMed] [Google Scholar]
- 214.Berthon C et al. Bromodomain inhibitor OTX015 in patients with acute leukaemia: a dose-escalation, phase 1 study. Lancet Haematol. 3, e186–e195 (2016). [DOI] [PubMed] [Google Scholar]
- 215.Dawson M et al. A phase I study of GSK525762, a selective bromodomain (BRD) and extra terminal protein (BET) inhibitor: results from part 1 of Phase I/II open label single agent study in patients with acute myeloid leukemia (AML). Blood 130, 1377 (2017).28667012 [Google Scholar]
- 216.Jophlin LL, Cao S & Shah VH The transcriptome of hepatic fibrosis revealed by single cell RNA sequencing. Hepatology 71, 1865–1867 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kar S, Paglialunga S, Jaycox SH, Islam R & Paredes AH Assay validation and clinical performance of chronic inflammatory and chemokine biomarkers of NASH fibrosis. PLoS ONE 14, e0217263 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nio Y, Yamauchi T & Iwabu M Monocyte chemoattractant protein-1 (MCP-1) deficiency enhances alternatively activated M2 macrophages and ameliorates insulin resistance and fatty liver in lipoatrophic diabetic A-ZIP transgenic mice. Diabetologia 55, 3350–3358 (2012). [DOI] [PubMed] [Google Scholar]
- 219.Kitade H, Sawamoto K & Nagashimada M CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes 61, 1680–1690 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang X et al. CXC chemokine receptor 3 promotes steatohepatitis in mice through mediating inflammatory cytokines, macrophages and autophagy. J. Hepatol. 64, 160–170 (2016). [DOI] [PubMed] [Google Scholar]
- 221. Ibrahim SH et al. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology 63, 731–744 (2016). An elegant study describing chemokine-bearing extracellular vesicles as chemoattractants for macrophages in liver injury models; extracellular vesicles are also currently being explored both as biomarkers and as therapeutic delivery agents.
- 222.Mohs A et al. Functional role of CCL5/RANTES for HCC progression during chronic liver disease. J. Hepatol. 66, 743–753 (2017). [DOI] [PubMed] [Google Scholar]
- 223.Kaffe E et al. β-Catenin and interleukin-1 β-dependent chemokine (C-X-C motif) ligand 10 production drives progression of disease in a mouse model of congenital hepatic fibrosis. Hepatology 67, 1903–1919 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wiedemann GM et al. Peritumoural CCL1 and CCL22 expressing cells in hepatocellular carcinomas shape the tumour immune infiltrate. Pathology 51, 586–592 (2019). [DOI] [PubMed] [Google Scholar]
- 225.Li X et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut 66, 157–167 (2017). [DOI] [PubMed] [Google Scholar]
- 226.Bartneck M et al. The CCR2+ macrophage subset promotes pathogenic angiogenesis for tumor vascularization in fibrotic livers. Cell Mol. Gastroenterol. Hepatol. 7, 371–390 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhu F et al. Tumor-associated macrophage or chemokine ligand CCL17 positively regulates the tumorigenesis of hepatocellular carcinoma. Med. Oncol. 33, 17 (2016). [DOI] [PubMed] [Google Scholar]
- 228.He H et al. CCR6+ B lymphocytes responding to tumor cell-derived CCL20 support hepatocellular carcinoma progression via enhancing angiogenesis. Am. J. Cancer Res. 7, 1151–1163 (2017). [PMC free article] [PubMed] [Google Scholar]
- 229. Hilscher MB et al. Mechanical stretch increases expression of CXCL1 in liver sinusoidal endothelial cells to recruit neutrophils, generate sinusoidal microthombi, and promote portal hypertension. Gastroenterology 157, 193–209.e9 (2019). Although liver diseases such as Budd–Chiari syndrome are mostly considered to be pressure-induced injuries, this study explains how chemokines such as CXCL1 might have a role in the development of portal hypertension in vivo as well as in patients with cardiac cirrhosis.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.