Abstract
Background
This is an update of a Cochrane Review first published in Issue 3, 2004 and previously updated in 2007.
Ear surgery may be performed in the treatment of chronic otitis media, ossicular chain disorders, tympanic membrane perforations and otitis media with effusion. Postoperative infection in ear surgery may result in wound infections, infection of the middle ear or mastoid resulting in discharge from the ear canal, failure of the tympanic membrane to close, or labyrinthitis due to infection in, or adjacent to, the inner ear. These complications may be associated with discomfort and inconvenience for the patient, an increase in morbidity and an increase in the costs of medical care.
Objectives
To assess the effects of local and/or systemic antibiotics for preventing complications such as postoperative discharge, graft failure and labyrinthitis in patients undergoing clean or clean‐contaminated ear surgery.
Search methods
We searched the Cochrane Ear, Nose and Throat Disorders Group Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, EMBASE, CINAHL, Web of Science, BIOSIS Previews, Cambridge Scientific Abstracts, mRCT and additional sources for published and unpublished trials. The date of the most recent search was 31 August 2009.
Selection criteria
Randomised or quasi‐randomised trials involving patients undergoing clean or clean‐contaminated types of ear surgery. Skull base surgery was excluded. We included any regimen of local and/or systemic antibiotic prophylaxis administered at or around the time of surgery compared to placebo, no antibiotic or an alternative intervention group. Outcome measures were infection, discharge, graft failure, labyrinthitis and adverse effects of prophylaxis.
Data collection and analysis
When possible, we contacted investigators for additional information on data and methodological issues. At least two authors independently extracted data and assessed trial quality.
Main results
Eleven studies were included in the review. The methodological quality of the trials was fair to good. However, most studies presented insufficient methodological detail. Although definitions of outcome measures were heterogeneous, pooling of results was possible. There were no significant differences between antibiotic prophylaxis groups and control groups in terms of reduction of postoperative infections, graft failures, draining outer ear canals and adverse drug effects.
Authors' conclusions
There is no strong evidence that the large‐scale use of prophylactic antibiotics in clean and clean‐contaminated ear surgery is helpful in reducing postoperative complications such as wound infection, discharge from the outer ear canal, labyrinthitis and graft failure.
Plain language summary
Preventative antibiotics (prophylaxis) in clean and clean‐contaminated ear surgery
Ear surgery, as surgery in general, can be divided into several categories: clean, clean‐contaminated, contaminated and dirty surgery. Postoperative complications can include wound infection, discharge from the outer ear canal, labyrinthitis and graft failure. This review aimed to demonstrate whether the use of antibiotic prophylaxis in ear surgery can be helpful in reducing postoperative complications in clean or clean‐contaminated surgery. There is no current evidence from randomised controlled trials showing that there is any antibiotic substance, in any regime, which can contribute to reducing complications in any type of clean or clean‐contaminated surgical procedure in the ear.
Background
This is an update of a Cochrane Review first published in Issue 3, 2004 and previously updated in 2007.
Ear surgery may be performed in the treatment of a number of disorders including chronic otitis media (with or without cholesteatoma), ossicular chain disorders, tympanic membrane perforations and otitis media with effusion. Postoperative infection following ear surgery may result in:
wound infections;
infection of the middle ear or mastoid, resulting in discharge from the ear canal;
failure of the tympanic membrane to close; or
labyrinthitis due to infection in, or adjacent to, the inner ear.
These complications may be associated with discomfort and inconvenience for the patient, an increase in morbidity and an increase in the costs of medical care.
Surgical procedures have conventionally been classified as clean, clean‐contaminated, contaminated and dirty (Waddell 1994). A surgical operation is clean if there is no break in aseptic technique and the respiratory, gastrointestinal or genitourinary tracts are not entered. Procedures entering the oropharynx, sterile genitourinary or biliary tract, the gastrointestinal or respiratory tracts, or requiring a minor break in aseptic technique are considered to be clean‐contaminated surgery. Contaminated surgery is defined as the presence of acute inflammation, infected bile or urine, or gross spillage from the gastrointestinal tract. When established infection exists, it is classified as dirty surgery.
The principles of prophylaxis against post‐surgical infection were established in the laboratory in the early 1960s (Burke 1961). The administration of antibiotics prior to surgery is now widely used in certain types of operations (Kaiser 1986). Generally speaking, prophylaxis during clean operations without implantation of a foreign body is not recommended, due to the low incidence of postoperative infection (Garner 1988). Prophylaxis during clean‐contaminated or infected surgery can be useful in reducing postoperative infection. It has been claimed that antibiotic prophylaxis during ear surgery reduces infection rates (Govaerts 1998). As the pathogenesis of post‐surgical infection after ear surgery is similar to any other type of clean, clean‐contaminated or infected surgery, subgroup analysis is important with respect to bacterial contamination. Ear surgery and mode of contamination should be divided into the following distinct categories.
A. Any type of clean surgery. This includes all types of reconstructive tympanoplasties, such as myringoplasty, stapedotomy and stapedectomy. It also includes all types of canal wall up procedures in which the preoperative middle ear is not infected (i.e. a preoperative dry ear). Facial nerve decompression also falls into this category.
B. Any procedure in which preoperative discharge is present, i.e. a clean‐contaminated or infected ear. This includes any type of surgery for chronic otitis media (with or without cholesteatoma).
C. The insertion of ventilating tubes for otitis media with effusion (glue ear). This category can be subdivided into ears with no glue (clean), seromucous glue (clean/clean‐contaminated) and purulent glue (infected). The use of antibiotic prophylaxis in ventilation tube insertion will be considered in a separate review.
D. Any type of oto‐neurosurgical procedure in which there is a large exposure or even a breach of the dura: vestibular nerve section, translabyrinthine acoustic neuroma removal, decompression of the endolymphatic sac, etc. These types of operations are not considered in this review.
The incidence of postoperative infections in these groups varies. Estimates have ranged from less than 5% in clean procedures to more than 10% in contaminated and dirty procedures (Govaerts 1998). This supports the idea that different types of surgery should be considered separately, with regard to the risk of bacterial contamination. Other authors, however, suggest that combining data from similar prophylactic regimens used during different otologic surgical procedures is appropriate (Jackson 1988). Furthermore, there might be a difference in effectiveness between different antibiotic regimens, e.g. systemic or local antibiotics. A number of randomised controlled trials have compared the use of systemic antibiotic to the use of a placebo or no antibiotic (Bagger‐Sjoback 1987; Eschelman 1971; Jackson 1988; John 1988; Lildholdt 1986; Pirodda 1994; Winerman 1981). Others have compared the use of systemic antibiotics together with local antibiotics to the use of a local antibiotic alone (Donaldson 1966; Govaerts 1998; Hester 1998). Another study has compared the use of a local antibiotic alone to no antibiotic (Tong 2002).
Antibiotics given during mastoidectomy for acute mastoiditis are not considered to be prophylactic but therapeutic. Any study addressing this issue was not included.
A number of descriptive reviews of antibiotic prophylaxis in ear surgery have been published, in which some attempt has been made to assess methodological quality and include this in the interpretation of results (Govaerts 1998; Jackson 1988). However, there is sufficient persisting uncertainty about the effectiveness and cost‐effectiveness of antibiotic prophylaxis during the surgical treatment of ear diseases to justify a systematic review of the evidence from randomised trials.
Objectives
To determine whether the prophylactic administration of antibiotics in patients undergoing clean or clean‐contaminated otologic surgical procedures reduces the incidence of postoperative infection. This includes wound infections, any infection of the middle ear, mastoid or external ear itself, manifesting as postoperative discharge or inner ear infection (labyrinthitis).
The following hypotheses were tested:
the use of antibiotic prophylaxis leads to a reduction in the proportion of patients developing a postoperative infection, compared with those given a placebo, no prophylaxis, or an alternative antibiotic regime;
the use of antibiotic prophylaxis leads to a decrease in graft failure rates in patients undergoing any type of tympanic membrane or middle ear reconstruction.
Methods
Criteria for considering studies for this review
Types of studies
The predetermined inclusion criteria were broad so as to include any randomised controlled study testing a prophylactic antibiotic in ear surgery.
The study had to test some method of antibiotic prophylactic intervention aimed at reducing the wound infection rate in ear surgery and compare it with a placebo or alternative intervention group.
The study had to be a controlled study, randomised or quasi‐randomised.
The study population had to be defined to enable identification of the operative intervention, ideally with relevant subgroups given if more than one.
Postoperative infection had to be one of the primary outcome measures.
Types of participants
Any patient undergoing clean or clean‐contaminated types of ear surgery.
Types of interventions
Any regimen of systemic or local antibiotic prophylaxis administered at or around the time of surgery.
Types of outcome measures
We considered trials if the primary outcome of the study was postoperative infection, reported as one of the following:
wound infection;
discharge from the outer ear canal;
labyrinthitis;
graft failure;
adverse reaction to antibiotic (gastrointestinal symptoms, skin reactions);
length of hospital stay;
re‐operation due to infection.
Search methods for identification of studies
We conducted systematic searches for randomised controlled trials. There were no language, publication year or publication status restrictions. The last search update was 31 August 2009, following a previous search update in June 2007.
Electronic searches
We searched the following databases from their inception: the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library Issue 3, 2009); PubMed; EMBASE; CINAHL; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; BIOSIS Previews; CNKI; mRCT (Current Controlled Trials); ClinicalTrials.gov; ICTRP (International Clinical Trials Registry Platform); and Google.
We modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in The Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1, Box 6.4.b. (Handbook 2008)). Search strategies for key databases including CENTRAL are shown in Appendix 1.
Searching other resources
We scanned reference lists of identified studies for further trials. We searched PubMed, TRIPdatabase, NHS Evidence ‐ ENT and Audiology and Google to retrieve existing systematic reviews possibly relevant to this systematic review, in order to search their reference lists for additional trials. We sought abstracts from conference proceedings via the Cochrane Ear, Nose and Throat Disorders Group Trials Register.
Data collection and analysis
Selection of studies
Two authors independently used titles, keywords and (if available) abstracts of the identified citations to exclude trials which clearly did not meet the inclusion criteria of the review. Authors and journal names were blinded at this stage. If one of the authors concluded that the trial might possibly meet the criteria, the full paper was obtained for further study. We then assessed hard copies of the articles passing this initial screening to determine whether they met the inclusion criteria described previously for this review. From this stage on, blinding of authors and journal names was no longer feasible. We compared the results of the two independent selections. We resolved disagreements by discussion.
Data extraction and management
Two authors jointly extracted data. There was no blinding of journal and author names and affiliations. We resolved disagreements by discussion.
With regard to subgroup analysis, we extracted the following data if available:
range of patients' age;
basic hospital infection rate at time of study;
indication for surgery (otitis media with effusion, tympanic membrane perforation, cholesteatoma, conductive hearing loss);
surgical techniques (myringoplasty, ossiculoplasty, mastoidectomy, stapes surgery) performed in the study;
intraoperative condition, such as normal, clear secretion, inflamed, purulent;
onset of administration (preoperative, intraoperative, postoperative); and
duration of administration (single shot, repeated ≤ 24 hours, > 24 hours).
Assessment of risk of bias in included studies
Two authors independently assessed concealment of treatment allocation using the standard Cochrane criteria: adequate (A), unclear (B), inadequate (C) or allocation concealment was not used (D). There was no blinding of journal and author names and affiliations.
We resolved disagreement in the assessment by discussion. Two authors jointly extracted data according to methodological criteria which did not involve subjective interpretation:
loss to follow up: ≤ 10, > 10% and ≤ 20%, or > 20%;
range of length of follow up: ≥ 14 days, ≥ 7 and < 14 days, or < 7 days;
blinding of participants;
blinding of medical staff;
blinding of outcome assessors;
blinding of those responsible for data analysis;
study sponsored by pharmaceutical company;
results specified respective to different surgeons.
We conducted a sensitivity analysis by comparing the effect of inclusion and exclusion of studies of different qualities. No quality scale was used.
Data synthesis
We grouped antimicrobial agents used for therapy and/or prophylaxis by their molecular class. Each molecular class was divided into subgroups by main antimicrobial activity against intraaural pathogens, thus every antibiotic substance occurred in exactly one subgroup. We classified regimes in terms of subgroups and combinations of subgroups.
We classified onset of administration as preoperative or perioperative (including intraoperative and postoperative). We classified dosage as prophylaxis within 24 hours and treatment for more than 24 hours, thus each arm of each controlled study referred to a specific regime/dosage pattern. We pooled all studies where the antibiotics under comparison were assigned to the same set of regime/dosage pattern.
We grouped the antimicrobial agents used for prophylaxis by their way of administration, e.g. systemic, local or a combination of local and systemic, thus creating subgroups for the method of administration of the drug. If possible, we formed different subgroups for the type of surgical procedure, e.g. clean or clean‐contaminated. This resulted, where possible, in six different comparisons (clean or clean‐contaminated surgery; systemic antibiotics versus no antibiotics; systemic and local antibiotics versus local antibiotics; local antibiotics versus no antibiotics).
Tables of comparison included the following outcomes.
Postoperative fever ( > 37 °C (98 °F) for ≥ five days).
Postoperative wound infections (discharge of pus or necessity for additional interventions).
Postoperative change of antibiotic treatment (regime and/or dosage).
Adverse drug effects (minor symptoms such as laboratory findings, rash).
Adverse drug effects (major symptoms such as clinical/laboratory signs of organ dysfunction).
Graft failure rates.
Closure of the tympanic membrane.
Bacterial eradication (comparison of intra‐ and postoperative cultures).
Death from any cause.
We summarised outcomes 1) to 3) as "Postoperative infection (Yes/No)"; outcomes 4 and 5 as "Adverse effects (Yes/No)"; outcomes 6 and 7 as "Graft failure (Yes/No)"; and listed outcomes 8 and 9 separately.
We determined summary odds ratios (OR) with 95% confidence intervals (CI) using both a fixed‐effect model (Mantel‐Haenszel) and a random‐effects model (Der Simonian and Laird).
We also determined the risk differences (RD) between different regime/dosage patterns (difference in the failure rate) and a 95% confidence interval. In the event of a statistically significant risk difference, we determined the number of patients needed to be treated in order to prevent one failure (NNT) for every comparison of regime/dosage pattern.
We reviewed a test of heterogeneity and an appraisal of individual study odds ratio within each comparison of regime/dosage pattern to determine if similar results were obtained from each study. Where significant heterogeneity existed, we examined the trials for specific potential clinical differences.
We assessed potential effects of publication bias on the results of the meta‐analysis from a funnel graph of the sample size plotted against the odds ratio.
We planned to conduct subgroup analyses for:
children and adults (adults ≥ 16 to 17);
surgical techniques: intraoperative and/or histological diagnosis: normal, inflamed, purulent;
preoperative, intraoperative and postoperative onset of administration;
duration of administration (single shot, repeated ≤ 24 hours, repeated > 24 hours); and
mode of administration.
However, neither the original data nor additional data supplied by study authors allowed subgroup analysis for children and adults, surgical techniques, intra‐operative and/or histological diagnosis, onset of administration or duration of administration of the drug.
Results
Description of studies
The most recent update search (August 2009) identified no new studies. From previous searches (June 2007) thirteen publications regarding the use of any regimen of antibiotic prophylaxis in ear surgery appeared to meet the inclusion criteria. One study was presented as a randomised controlled trial, but appeared to be a case report (Leonard 1967). One study compared two different antibiotic regimens, and was therefore not suitable for this review (Liu 1983). Thus, we included 11 studies in the review.
We contacted authors, if possible. We asked questions about methodological issues and, when needed, we requested original data. Five authors responded. Details of the participants, interventions and outcomes in those studies are presented in the table 'Characteristics of included studies'. As is presented in this table, the studies were very heterogeneous with regard to the chosen antibiotic and dosage, and onset and duration of administration. Therefore, pooling of results by different regime/dosage pattern was impossible. Assuming the choice for an antibiotic is a rational one, we decided to make no distinction between different regime/dosage patterns, and just refer to an antibiotic as being used or not.
Seven studies compared the use of systemic antibiotics to the use of a placebo or no antibiotic (Bagger‐Sjoback 1987; Eschelman 1971; Jackson 1988; John 1988; Lildholdt 1986; Pirodda 1994; Winerman 1981). Three studies compared the use of systemic antibiotics together with local antibiotics to the use of a local antibiotic alone (Donaldson 1966; Govaerts 1998; Hester 1998). Finally, only one study compared the use of a local antibiotic alone to no antibiotic (Tong 2002).
Subgroup analysis was not possible for the type of surgical procedure, e.g. clean or clean‐contaminated, because most studies did not present their results in sufficient detail. Unfortunately, although the study authors were contacted, they could not provide us with more detailed results.
Subgroup analysis was also not possible for children and adults, because age was not assessed in sufficient detail in any study.
Bagger‐Sjoback 1987 focused on different clean and clean‐contaminated procedures, such as myringoplasties, stapedectomies and radical mastoidectomies (91 patients). The antibiotic used was phenoxymethylpenicillin. Both the antibiotic and placebo group received hydrocortisone soaked gauze in the outer ear canal after surgery. The most important outcome measure was postoperative infection one week after surgery.
Donaldson 1966 focused on different types of myringoplasties (96 patients). The antibiotic used was sulfamethoxazole. After surgery, both antibiotic and placebo group received polymyxin/neomycin/hydrocortisone soaked sponges in the outer ear canal. The outcome measures were postoperative infection after ten days and after six weeks, closure of the tympanic membrane, and (mild) adverse effects.
Eschelman 1971 designed a three‐arm trial. The authors used two different antibiotics, penicillin and ampicillin, compared to a placebo. Three different groups of patients were identified: 1) tympanomastoidectomies with cholesteatomas; 2) tympanoplasties and tympanomastoidectomies without cholesteatomas; and 3) stapedectomies and exploratory tympanotomies. The outcome measure was postoperative infection. A total of 107 patients were enrolled.
Govaerts 1998 presented a large study (750 patients were enrolled). Different procedures were carried out, such as tympanoplasties and radical mastoidectomies. The antibiotic used was cefuroxime. The most important outcome measure was postoperative infection within two weeks after surgery.
Hester 1998 used different antibiotics in a two‐arm trial, depending on the specific need of the patients (ampicillin/sulbactam/amoxicillin/clavulanate, cefazolin/cefalexin, erythromycin or clindamycin). Both antibiotic and placebo group received neomycin/polymyxin/hydrocortisone otic drops during the period following surgery. All procedures were tympanoplasties, tympanomastoidectomies or radical mastoidectomies. The outcome measures were graft failure within three weeks after surgery and after six months, and postoperative infection within three weeks after surgery. A total of 146 patients were enrolled in this study.
Jackson 1988 presented the largest study on this subject. He enrolled 3481 patients who underwent a broad variety of procedures, including some neurotological procedures. The antibiotics used were cephalotin and cefazolin. Unfortunately, he did not present subgroup analyses. Therefore, the only outcome measure suitable for this review was graft failure after three weeks of surgery. In 2136 of the 3481 patients a graft was used.
John 1988 investigated a total of 130 myringoplasties. The antibiotic used was a combination of ampicillin and flucloxacillin. The outcome measure was 'success', defined as closure of the tympanic membrane after eight weeks.
Lildholdt 1986 focused on patients undergoing surgery for chronic otitis media, who had positive pre‐operative cultures for Pseudomonas aeruginosa (26 patients). The antibiotic used was ceftazidime. The outcome measures were dryness of the outer ear canal postoperatively and after two months, and graft failure after two months.
Pirodda 1994 enrolled 100 patients in the study, who underwent myringoplasties, tympanoplasties or tympanomastoidectomies. Ceftriaxone was the antibiotic used. After one week, one month and two months, the status of the following outcome measures were assessed: 1) retro‐auricular incision; 2) outer ear canal; 3) tympanic membrane; and 4) mastoid cavity.
Tong 2002 was the only study which used only a local antibiotic (ofloxacin otic drops). In a three‐arm trial, two different dosages of otic drops were compared with each other and with a third group which received no antibiotic treatment. All 101 patients underwent tympanoplasties. The outcome measure was 'success', defined as closing of the tympanic membrane after eight weeks.
Winerman 1981 investigated a study population of 72 patients who all underwent closed cavity procedures. In the antibiotic group, 30 patients received clindamycin and gentamycin. Six patients received only clindamycin because preoperative cultures showed no need for the use of gentamycin. The most important outcome measure was the occurrence of infectious complications within three months after surgery, which was not specifically defined.
Risk of bias in included studies
The two authors initially agreed on about 90% of the assessments of methodological quality. All disagreements were dissolved by discussion. We designed a criteria list with validity items to score the methodological quality of the studies. We used a standard form for evaluating randomised controlled trials, made by the Evidence‐Based Guideline Development (EBRO) for this purpose (Table 1). Table 2 presents the scores on these validity items of the criteria list. The methodological quality of the included studies was fair to good. Many studies reported on randomised treatment allocation, but (a) failed to describe the exact procedure or (b) whether concealed allocation had been performed. Information on blinding the outcome assessor (e) was often not provided. Also the information on blinding of patients (c) and physician (d), and about loss to follow up (g) was often not provided. Although only two studies scored positive on all items of the criteria list, none of the studies were considered to contain 'fatal flaws'.
1. Criteria list for the assessment of methodological quality of included studies.
| Item ID | Description | Implementation |
| Patient selection | NOTE: all criteria were scored yes (+), no (‐) or don't know (?) | |
| a | Was an adequate method of randomisation applied? | A random (unpredictable) allocation sequence must have been applied. Methods of allocation using date of birth, date of admission, hospital numbers, or alternation are not considered to be appropriate. |
| b | Was the treatment allocation concealed? | Allocation should be performed by an independent person who is not responsible for determining eligibility for inclusion. This person has no information about the patients included in the trial and has no influence on the allocation sequence or the decision about eligibility for inclusion. |
| c | Were the patients blinded to the intervention? | Adequate information about blinding must be provided |
| d | Were the treating physicians blinded to the intervention? | Adequate information about blinding must be provided |
| e | Were the outcome assessors blinded to the intervention? | Adequate information about blinding must be provided |
| f | Were the groups similar at baseline with regard to the most important prognostic indicators? | Groups must be similar at baseline with regard to at least 3 of the 4 prognostic indicators of age, sex, duration of symptoms and value of main outcome measure(s) |
| g | Was the drop‐out/loss to follow‐up rate described and acceptable? | Included patients who did not complete the follow‐up period, or were not included in the analysis, must have been described. If the percentage of drop‐outs and loss to follow up is < 20% and the loss to follow up does not lead to substantial bias, a '+' is scored. (N.B. this percentage is arbitrary, and not supported by empirical evidence). |
| h | Did the analysis include an intention‐to‐treat analysis? | For all randomised patients, the most important moments of effect measurement should have been reported/analysed (minus missing values), irrespective of non‐compliance and co‐interventions |
| i | Were the index and control intervention(s) explicitly described? | Adequate description for index and control intervention(s), so that the treatment could be replicated |
2. Validity scores (a‐i).
| Reference | a | b | c | d | e | f | g | h | i |
| Adequate randomisation | Adequate blinding concealment | Adequate patient blinding | Adequate treating physician blinding | Adequate assessor blinding | Groups similar at baseline | Drop‐out rate described and acceptable | Intention‐to‐treat analysis | Index and control intervention explicit | |
| Bagger‐Sjoback 1987 | + | + | + | + | + | + | + | ‐ | + |
| Donaldson 1966 | ? | ? | ? | ? | ? | + | + | + | + |
| Eschelman 1971 | + | + | ? | + | ? | ? | ? | ? | + |
| Govaerts 1998 | + | + | + | + | + | + | + | + | + |
| Hester 1998 | ‐ | ? | ? | ? | ? | + | ? | + | + |
| Jackson 1988 | + | + | ? | + | ? | + | + | + | + |
| John 1988 | + | + | ? | ? | ? | ? | ? | + | + |
| Lildholdt 1986 | + | ‐ | ‐ | ‐ | ‐ | + | ? | + | + |
| Pirodda 1994 | + | ? | ? | ? | ? | + | + | + | + |
| Tong 2002 | + | + | ‐ | + | + | + | ? | + | + |
| Winerman 1981 | + | ? | ? | ? | ? | + | ? | + | + |
With regard to specific studies, we can mention the following.
Bagger‐Sjoback 1987 was a well‐designed and performed study. However, the authors withdrew four patients and addressed them as being lost to follow up. These patients developed clinical signs of infection and were withdrawn for proper treatment. These patients should, in fact, have been taken along in further analyses. In our analysis, we followed a 'worst‐case scenario'.
Donaldson 1966 offered very little information on methodological procedures. Moreover, the authors reported some patients not to be seen on every visit during follow up. Unfortunately they did not report to which arm of the trial these patients belonged. In our analyses, we followed a 'worst case scenario', assuming these patients were in the control group.
John 1988 used a questionable method of randomising patients: by flipping a coin. Moreover, the 110 patients were not equally divided (treatment:control rate was 55:75). After contacting them, the authors assured us that this was merely coincidence, and that the tossing of the coin had proven this to be an adequate method of randomisation.
Effects of interventions
It appeared to be impossible to present subgroup analyses for children and adults, surgical techniques, intra‐operative and/or histological diagnosis, onset of administration and duration of administration of the drug. The data in the studies were not presented in sufficient detail to make this possible. Subgroup analyses for different ways of administrating the drug, e.g. systemic or local, was possible.
Pooling of data was possible. However, it should be taken into consideration that most studies did not present subgroup analyses for specific procedures, or for the distinction between clean and clean‐contaminated procedures. Moreover, in some studies, outcome measures were not specified. Still, we believe that pooling of these data is appropriate.
1. Effect of antibiotic prophylaxis in clean and clean‐contaminated ear surgery
1.1. Effect of antibiotic prophylaxis on postoperative infection within three weeks after surgery
Six studies assessed the effect of antibiotic prophylaxis on the incidence of postoperative infection within three weeks from surgery (Bagger‐Sjoback 1987; Donaldson 1966; Eschelman 1971; Govaerts 1998; Hester 1998; Pirodda 1994). A total of 1291 patients was investigated. Infection occurred in 34 of the 671 patients who had received prophylaxis (5.1%), and in 38 of the 620 control patients (6.1%). The odds ratio (OR) (fixed‐effect) was 0.73 (confidence interval (CI) 0.45 to 1.20), which is not significant.
1.2. Effect of antibiotic prophylaxis on postoperative infection six weeks after surgery
Two studies assessed the effect of antibiotic prophylaxis on the incidence of postoperative infection after six weeks of surgery (Donaldson 1966; Pirodda 1994). A total of 196 patients was investigated. Infection occurred in eight of the 98 patients who had received prophylaxis (8.1%), and in 10 of the 98 control patients (10.2%). The odds ratio (fixed‐effect) was 0.78 (95% CI 0.30 to 2.07), which is not significant.
1.3. Effect of antibiotic prophylaxis on postoperative infection within three months after surgery
Two studies assessed the effect of antibiotic prophylaxis on the incidence of postoperative infection within three months from surgery (Pirodda 1994; Winerman 1981). A total of 172 patients was investigated. Infection occurred in nine of the 86 patients who had received prophylaxis (10.5%), and in nine of the 86 control patients (10.5 %). The odds ratio (fixed‐effect) was 1.00 (95% CI 0.38 to 2.66), which is not significant.
1.4. Effect of antibiotic prophylaxis on the postoperative status of the outer ear canal two months after surgery
Two studies assessed the effect of antibiotic prophylaxis on the postoperative status of the outer ear canal two months after surgery (Lildholdt 1986; Pirodda 1994). A total of 126 patients was investigated. Two months after surgery three of the 64 (4.7%) patients receiving prophylaxis, suffered from a draining outer ear canal. In the control group this was eight of the 62 patients (12.9%). The odds ratio (fixed‐effect) was 0.29 (95% CI 0.08 to 1.13), which is not significant.
1.5. Effect of antibiotic prophylaxis on graft failure rate within three weeks after surgery
Two studies assessed the effect of antibiotic prophylaxis on graft failure rates within three weeks from surgery (Hester 1998; Jackson 1988). A total of 2282 patients was investigated. Within three weeks after surgery, graft failure had occurred in 13 of the 1189 patients who had received prophylaxis (1.1%), and in 17 of the 1093 patients who were in the control group (1.6%). The odds ratio (fixed‐effect) was 0.71 (95% CI 0.35 to 1.45), which is not significant.
1.6. Effect of antibiotic prophylaxis on graft failure rate between six weeks and three months after surgery
Six studies assessed the effect of antibiotic prophylaxis on graft failure rates between six weeks and three months after surgery (Donaldson 1966; Hester 1998; John 1988; Lildholdt 1986; Pirodda 1994; Tong 2002). A total of 599 patients was investigated. Graft failure occurred in 42 of the 304 patients who had received prophylaxis (13.8%), and in 44 of the 295 patients who were in the control group (14.9%). The odds ratio (fixed‐effect) was 0.86 (95% CI 0.53 to 1.41), which is not significant.
1.7. Effect of antibiotic prophylaxis on graft failure rate within three months after surgery
A total of seven studies assessed the effect of antibiotic prophylaxis on graft failure at one point or another within three months (Donaldson 1966; Hester 1998; Jackson 1988; John 1988; Lildholdt 1986; Pirodda 1994; Tong 2002). A total of 2739 patients was investigated. Graft failure occurred in 55 of the 1426 (3.9%) patients who had received prophylaxis, and in 59 of the 1313 (4.5%) patients in the control group. The odds ratio (fixed‐effect) was 0.84 (95% CI 0.56 to 1.26), which is not significant.
1.8. Effect of antibiotic prophylaxis on adverse drug effects
Two studies assessed the incidence of adverse drug effects (Donaldson 1966; Govaerts 1998). A total of 841 patients was investigated. Only four of the 428 patients (0.9%) who had received an antibiotic substance suffered from adverse drug effects. On the other hand, five of the 413 patients (1.2%) who had not received an antibiotic suffered from adverse (drug) effects. The odds ratio (fixed‐effect) was 0.73 (95% CI 0.20 to 2.64), which is not significant.
1.9. Effect of antibiotic prophylaxis on bacterial eradication
No study compared intra‐ and postoperative cultures.
1.10. Effect of antibiotic prophylaxis on death from any cause
No study reported death from any cause.
Subgroup analyses were then made for different types of administration of the antibiotic prophylaxis:
2. Effect of systemic antibiotic prophylaxis versus no antibiotics in clean and clean‐contaminated ear surgery
2.1. Effect of systemic antibiotic prophylaxis versus no antibiotics on postoperative infection within three weeks after surgery
Three studies assessed the effect of systemic antibiotic prophylaxis versus no antibiotics on the incidence of postoperative infection within three weeks from surgery (Bagger‐Sjoback 1987; Eschelman 1971; Pirodda 1994). A total of 299 patients was investigated. Infection occurred in 20 of the 172 patients who had received prophylaxis (11.6%), and in 14 of the 127 control patients (11.0%). The odds ratio (fixed‐effect) was 1.02 (confidence interval (CI) 0.49 to 2.15), which is not significant.
2.2. Effect of systemic antibiotic prophylaxis versus no antibiotics on postoperative infection six weeks after surgery
One study assessed the effect of systemic antibiotic prophylaxis versus no antibiotics on the incidence of postoperative infection after six weeks of surgery (Pirodda 1994). A total of 100 patients was investigated. Infection occurred in five of the 50 patients who had received prophylaxis (10.0%), and in four of the 50 control patients (8.0%). The odds ratio (fixed‐effect) was 1.28 (95% CI 0.32 to 5.07), which is not significant.
2.3. Effect of systemic antibiotic prophylaxis versus no antibiotics on postoperative infection within three months after surgery
Two studies assessed the effect of systemic antibiotic prophylaxis versus no antibiotics on the incidence of postoperative infection within three months from surgery (Pirodda 1994; Winerman 1981). A total of 172 patients was investigated. Infection occurred in nine of the 86 patients who had received prophylaxis (10.5%), and in nine of the 86 control patients (10.5 %). The odds ratio (fixed‐effect) was 1.00 (95% CI 0.38 to 2.66), which is not significant.
2.4. Effect of systemic antibiotic prophylaxis versus no antibiotics on the postoperative status of the outer ear canal two months after surgery
Two studies assessed the effect of systemic antibiotic prophylaxis versus no antibiotics on the postoperative status of the outer ear canal two months after surgery (Lildholdt 1986; Pirodda 1994). A total of 126 patients was investigated. Two months after surgery three of the 64 (4.7%) patients receiving prophylaxis, suffered from a draining outer ear canal. In the control group this was eight of the 62 patients (12.9%). The odds ratio (fixed‐effect) was 0.29 (95% CI 0.08 to 1.13), which is not significant.
2.5. Effect of systemic antibiotic prophylaxis versus no antibiotics on graft failure rate within three weeks after surgery
One study assessed the effect of systemic antibiotic prophylaxis versus no antibiotics on graft failure rates within three weeks from surgery (Jackson 1988). A total of 2136 patients was investigated. Within three weeks after surgery, graft failure had occurred in 13 of the 1118 patients who had received prophylaxis (1.2%), and in 15 of the 1018 patients who were in the control group (1.5%). The odds ratio (fixed‐effect) was 0.79 (95% CI 0.37 to 1.66), which is not significant.
2.6. Effect of systemic antibiotic prophylaxis versus no antibiotics on graft failure rate between six weeks and three months after surgery
Three studies assessed the effect of systemic antibiotic prophylaxis versus no antibiotics on graft failure rates between six weeks and three months after surgery (John 1988; Lildholdt 1986; Pirodda 1994). A total of 256 patients was investigated. Graft failure occurred in 18 of the 119 patients who had received prophylaxis (15.1%), and in 18 of the 137 patients who were in the control group (13.1%). The odds ratio (fixed‐effect) was 1.13 (95% CI 0.52 to 2.49), which is not significant.
2.7. Effect of systemic antibiotic prophylaxis versus no antibiotics on graft failure rate within three months after surgery
A total of four studies assessed the effect of systemic antibiotic prophylaxis versus no antibiotics on graft failure at one point or another within three months (Jackson 1988; John 1988; Lildholdt 1986; Pirodda 1994). A total of 2392 patients was investigated. Graft failure occurred in 31 of the 1237 (2.5%) patients who had received prophylaxis, and in 33 of the 1155 (2.9%) patients in the control group. The odds ratio (fixed‐effect) was 0.93 (95% CI 0.54 to 1.61), which is not significant.
2.8. Effect of systemic antibiotic prophylaxis versus no antibiotics on adverse drug effects
No study assessed the effect of systemic antibiotic prophylaxis versus no antibiotics on the incidence of adverse drug effects.
2.9. Effect of systemic antibiotic prophylaxis versus no antibiotics on bacterial eradication
No study compared intra‐ and postoperative cultures.
2.10. Effect of systemic antibiotic prophylaxis versus no antibiotics on death from any cause
No study reported death from any cause.
3. Effect of systemic and local antibiotic prophylaxis versus local antibiotics in clean and clean‐contaminated ear surgery
3.1. Effect of systemic and local antibiotic prophylaxis versus local antibiotics on postoperative infection within three weeks after surgery
Three studies assessed the effect of systemic and local antibiotic prophylaxis versus local antibiotics on the incidence of postoperative infection within three weeks from surgery (Donaldson 1966; Govaerts 1998; Hester 1998). A total of 992 patients was investigated. Infection occurred in 14 of the 499 patients who had received systemic and local prophylaxis (2.8%), and in 24 of the 493 control patients (4.9%). The odds ratio (fixed‐effect) was 0.56 (confidence interval (CI) 0.29 to 1.10), which is not significant.
3.2. Effect of systemic and local antibiotic prophylaxis versus local antibiotics on postoperative infection six weeks after surgery
One study assessed the effect of systemic and local antibiotic prophylaxis versus local antibiotics on the incidence of postoperative infection after six weeks of surgery (Donaldson 1966). A total of 96 patients was investigated. Infection occurred in three of the 48 patients who had received systemic and local prophylaxis (6.3%), and in six of the 48 control patients (12.5%). The odds ratio (fixed‐effect) was 0.47 (95% CI 0.11 to 1.99), which is not significant.
3.3. Effect of systemic and local antibiotic prophylaxis versus local antibiotics on postoperative infection within three months after surgery
No study assessed the effect of systemic and local antibiotic prophylaxis versus local antibiotics on the incidence of postoperative infection within three months from surgery.
3.4. Effect of systemic and local antibiotic prophylaxis versus local antibiotics on the postoperative status of the outer ear canal two months after surgery
No study assessed the effect of systemic and local antibiotic prophylaxis versus local antibiotics on the postoperative status of the outer ear canal two months after surgery.
3.5. Effect of systemic and local antibiotic prophylaxis versus local antibiotics on graft failure rate within three weeks after surgery
One study assessed the effect of systemic and local antibiotic prophylaxis versus local antibiotics on graft failure rates within three weeks from surgery (Hester 1998). A total of 146 patients was investigated. Within three weeks after surgery, graft failure had occurred in none of the 71 patients who had received systemic and local prophylaxis (0%), and in two of the 75 patients who were in the control group (2.7%). This was, however, not significant. The odds ratio (fixed‐effect) was 0.21 (95% CI 0.01 to 4.36).
3.6. Effect of systemic and local antibiotic prophylaxis versus local antibiotics on graft failure rate between six weeks and three months after surgery
Two studies assessed the effect of systemic and local antibiotic prophylaxis versus local antibiotics on graft failure rates between six weeks and three months after surgery (Donaldson 1966; Hester 1998). A total of 242 patients was investigated. Graft failure occurred in 13 of the 119 patients who had received systemic and local prophylaxis (10.9%), and in 22 of the 123 patients who were in the control group (17.9%). The odds ratio (fixed‐effect) was 0.54 (95% CI 0.25 to 1.15), which is not significant.
3.7. Effect of systemic and local antibiotic prophylaxis versus local antibiotics on graft failure rate within three months after surgery
These results are the same as 3.6.
3.8. Effect of systemic and local antibiotic prophylaxis versus local antibiotics on adverse drug effects
Two studies assessed the effect of systemic and local antibiotic prophylaxis versus local antibiotics on the incidence of adverse drug effects (Donaldson 1966; Govaerts 1998). A total of 841 patients was investigated. Only four of the 428 patients (0.9%) who had received systemic and local antibiotic prophylaxis suffered from adverse drug effects. On the other hand, five of the 413 patients (1.2%) who had received only a local antibiotic suffered from adverse (drug) effects. The odds ratio (fixed‐effect) was 0.73 (95% CI 0.20 to 2.64), which is not significant.
3.9. Effect of systemic and local antibiotic prophylaxis versus local antibiotics on bacterial eradication
No study compared intra‐ and postoperative cultures.
3.10. Effect of systemic and local antibiotic prophylaxis versus local antibiotics on death from any cause
No study reported death from any cause.
4. Effect of local antibiotic prophylaxis versus no antibiotics in clean and clean‐contaminated ear surgery
Only one study compared the use of local antibiotic prophylaxis alone to no antibiotics (Tong 2002). This study only assessed the effect on graft failure rate within three months after surgery (comparisons 4.6 and 4.7). A total of 101 patients was investigated. Graft failure occurred in 11 of the 66 patients who had received prophylaxis (13.8%), and in four of the 35 patients who were in the control group (11.4%). The odds ratio (fixed‐effect) was 1.55 (95% CI 0.45 to 5.28), which is not significant.
Discussion
This review included 11 randomised controlled trials comparing a certain antibiotic in a certain regime/dosage pattern with a control group. These patterns were investigated during different otologic surgical procedures (clean as well as contaminated types of surgery). Often no subgroup analysis was presented with regard to type of surgery, e.g. clean or clean‐contaminated. This is also the reason why, unfortunately, we could not perform any sub‐analysis with regard to type of surgery. This is specifically the case in Jackson 1988. This well‐designed and performed study investigated 3481 patients and included some procedures which we did not investigate. The authors' most important conclusion, however, is that there is no need at all for the use of antibiotic prophylaxis in ear surgery in terms of reducing postoperative complications such as infection and graft failure. We have, however, been able to assess subgroup analyses with regard to the method of administration of the antibiotic, e.g. systemic and/or local.
The methodological quality of the included studies, scored by the use of a list of validity criteria, was fair to good. Often insufficient detail was given on methodological procedures. This could, of course, enhance bias. Nevertheless, it was the authors' opinion that none of the included studies contained fatal flaws.
Many studies differed in the definition of certain outcome measures, or did not define them at all. This made the pooling of results difficult. However, the authors believed that the character of the subject of this review allows more differences in defining these measures than other subjects, therefore allowing the pooling of results. One has to realise, however, that when we pooled results to evaluate an effect within three weeks, some studies had an evaluation after two weeks, while another study could have evaluated after two and a half weeks.
None of the trials in itself showed any significant difference in any of their outcome measures, independent of the quality of the study. Pooling of results did not change any of those views. Therefore, on the basis of the data available, there seems no significant contribution for antibiotic prophylaxis in ear surgery, in term of reduction of postoperative complications such as infection and graft failure.
Authors' conclusions
Implications for practice.
There is no evidence that the use of prophylactic antibiotics in clean or clean‐contaminated ear surgery, in any regimen, is helpful in reducing postoperative complications such as wound infection, discharge from the outer ear canal, labyrinthitis and graft failure.
Implications for research.
More than ten different studies all concluded that there is no need for antibiotic prophylaxis in clean and clean‐contaminated ear surgery. This review supports this conclusion. Therefore, there seems to be no need for an additional randomised controlled trial on this subject. However, there has never been a large study focusing on either clean or clean‐contaminated procedures alone. Taking into account the fact that there might be a difference in a priori risk of postoperative infection between clean and clean‐contaminated procedures, a large study focusing merely on clean or clean‐contaminated ear surgery, could be beneficial. Furthermore, a study focusing on different populations of patients, e.g. the elderly or children, could be interesting. It should also be noted that when the low incidence of postoperative infections is taken into consideration, for a new randomised controlled trial to have enough statistical power it would have to include a very large number of patients.
What's new
| Date | Event | Description |
|---|---|---|
| 31 August 2009 | New search has been performed | Update searches conducted 31 August 2009 identified no new studies matching the inclusion criteria |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 3, 2004
| Date | Event | Description |
|---|---|---|
| 28 August 2008 | Amended | Converted to new review format |
| 5 June 2007 | New search has been performed | An extensive update of the literature search was performed in June 2007. No new studies were found in addition to the studies described in the original review. No textual changes have been made to the results, conclusions or recommendations. |
Acknowledgements
We would like to thank Rob Scholten and Jan Schoones for their help and Martin Offringa for his moral support. Furthermore, we would like to thank the doctors Bagger‐Sjoback, Govaerts, John, Lildholdt and Tong, for being so kind as to reply to requests for more details.
Appendices
Appendix 1. Search strategies
| PubMed | EMBASE (Ovid) | CINAHL (EBSCO) |
| #1 "Ear" [MeSH] OR EAR* [ti] OR TYMPANIC [ti] OR STAPES [ti] OR EUSTACHIAN [ti] OR OTOLOGIC* [ti] OR ENDOLYMPHATIC [ti] OR AURICULAR [ti] #2 DISEASE* [ti] OR DISORDER* [ti] #3 #1 AND #2 #4 "Ear Diseases" [Mesh] OR (OTITIS [tiab] OR OTOSCLEROSIS [tiab] OR CHOLESTEATOMA [tiab] OR (TYMPANIC [tiab] AND MEMBRANE [tiab] AND PERFORAT* [tiab]) OR (TYMPANIC [tiab] AND MEMBRANE [tiab] AND RUPTURE* [tiab]) OR (OSSICULAR [tiab] AND CHAIN [tiab] AND DISORDER [tiab]) OR (GLUE [tiab] AND EAR [tiab]) #5 "Surgical Procedures, Operative" [Mesh] OR SURG* [ti] OR OPERAT* [ti] #6 (POST [tiab] AND OPERAT* [tiab]) OR (POST [tiab] AND SURG* [tiab]) OR ((POSTSURG* [tiab] OR POSTOPERATIVE* [tiab]) AND INFECTION* [tiab]) #7 (#3 OR #4) AND (#5 OR #6) #8 "Otologic Surgical Procedures" [Mesh] #9 TYMPANOPLAST* [tiab] OR TYMPANOTOM* [tiab] OR TYMPANOMASTOIDECTOM* [tiab] OR "TYMPANOMASTOID SURGERY" [tiab] OR MASTOIDECTOM* [tiab] OR STAPEDECTOM* [tiab] OR STAPEDOTOM* [tiab] OR MYRINGOPLAST* [tiab] OR (MIDDLE [tiab] AND EAR [tiab] AND RECONSTRUCT* [tiab]) OR (MIDDLE [tiab] AND EAR [tiab] AND VENTILAT* [tiab]) OR (GROMMET* [tiab] AND INSERT* [tiab]) OR (TYMPANIC [tiab] AND MEMBRANE [tiab] AND SURG* [tiab]) OR (EAR [tiab] AND SURG* [tiab]) OR (AURICULAR [tiab] AND SURG* [tiab]) OR OSSICULOPLAST* [tiab] OR (FACIAL [tiab] AND NERVE [tiab] AND DECOMPRESSION* [tiab]) OR (STAPES [tiab] AND SURG* [tiab]) OR (OSSICULAR [tiab] AND REPLACE* [tiab]) OR (OSSICULAR [tiab] AND IMPLANT* [tiab]) OR (COCHLEAR [tiab] AND IMPLANT* [tiab]) OR (ENDOLYMPHATIC [tiab] AND SHUNT* [tiab]) OR (ENDOLYMPHATIC* [tiab] AND SURG* [tiab]) OR (LABYRINTH [tiab] AND SURG* [tiab]) OR (STAPES [tiab] AND SURG* [tiab]) #10 "Ear/surgery"[Mesh] #11 "Ear Diseases/surgery"[Mesh] #12 #7 OR #8 OR #9 OR #10 OR #11 #13 "ANTI BACTERIAL AGENTS" [Mesh] OR "ANTIBIOTIC PROPHYLAXIS" [Mesh] OR "Lactams" [Mesh] OR "QUINOLONES" [Mesh] OR "Macrolides" [Mesh] #14 ANTIBIOT* [tiab] OR (ANTI [tiab] AND BIOT* [tiab]) OR ANTIMICROBIAL* [tiab] OR (ANTI [tiab] AND MICROBIAL* [tiab]) OR BACTERIOCID* [tiab] OR ANTIBACTERIAL* [tiab] OR (ANTI [tiab] AND BACTERIAL* [tiab]) OR CHEMOTHERAPY [tiab] OR PENICILLIN [tiab] OR AMOXICILLIN [tiab] OR AMPICILLIN [tiab] OR "CLAVULANIC ACID" [tiab] OR AMOXICLAV [tiab] OR AUGMENTIN [tiab] OR TICARCILLIN [tiab] OR TIMENTIN [tiab] OR FLUCLOXACILLIN [tiab] OR FLUAMPICIL [tiab] OR MAGNAPEN [tiab] OR PIPERACILLIN [tiab] OR TAZOCIN [tiab] OR CEPHALOSPORIN* [tiab] OR CEFACLOR [tiab] OR DISTACLOR [tiab] OR CEFADROXIL [tiab] OR BAXAN [tiab] OR CEFALEXIN [tiab] OR CEPOREX [tiab] OR KEFLEX [tiab] OR CEFAMANDOLE [tiab] OR KEFADOL [tiab] OR CEFAZOLIN* [tiab] OR KEFZOL [tiab] OR CEFIXIME [tiab] OR SUPRAX [tiab] OR CEFOTAXIME [tiab] OR CLAFORAN [tiab] OR CEFOXITIN [tiab] OR MEFOXIN [tiab] OR CEFPIROME [tiab] OR CEFROM [tiab] OR CEFPODOXIME [tiab] OR ORELOX [tiab] OR CEFPROZIL [tiab] OR CEFZIL [tiab] OR CEFRADINE [tiab] OR VELOSEL [tiab] OR CEFTAZIDIM [tiab] OR ORTUM [tiab] OR KEFADIM [tiab] OR CEFTRIAXONE [tiab] OR ROCEPHIN [tiab] OR CEFUROXIME [tiab] OR ZINACEF [tiab] OR ZINNAT [tiab] OR CEFONICID [tiab] OR AZTREONAM [tiab] OR AZACTAM [tiab] OR IMIPENEM [tiab] OR ILASTATIN [tiab] OR PRIMAXIN [tiab] OR MEROPENEM [tiab] #15 TETRACYCLINE* [tiab] OR DETECLO [tiab] OR DEMECLEOCYCLIN [tiab] OR LEDERMYCIN [tiab] OR DOXYCYCLINE [tiab] OR VIBRAMYCIN [tiab] OR MINOCYCLINE [tiab] OR MINOCINE [tiab] OR OXYTETRACYCLINE [tiab] OR TERRAMYCIN [tiab] OR MACROLIDE* [tiab] OR ERYTHROMYCIN [tiab] OR ERYMAX [tiab] OR ERYTHROCIN [tiab] OR ERYTHROPED [tiab] OR AZITHROMYCIN [tiab] OR ZITHROMAX [tiab] OR CLARITHROMYCIN [tiab] OR KLARICID [tiab] OR TELITHROMYCIN [tiab] OR KETEK [tiab] OR TRIMOXAZOLE [tiab] OR SEPTRIN [tiab] OR TRIMETHOPRIM [tiab] OR MONOTRIM [tiab] OR TRIMOPAN [tiab] OR METRONIDAZOLE [tiab] OR FLAGYL [tiab] OR METROLYL [tiab] OR PHENOXYMETHYLPENICILLIN [tiab] OR SULFAMETHOXAZOLE [tiab] OR OXACILLIN [tiab] OR CEPHALOTHIN [tiab] OR SULBACTAM [tiab] OR OFLOXACIN [tiab] OR CLINDAMYCIN [tiab] OR GENTAMYCIN [tiab] OR VANCOMYCIN [tiab] #16 #13 OR #14 OR #15 #17 #12 AND #16 | 1 exp Ear/ or (EAR* or TYMPANIC or STAPES or EUSTACHIAN or OTOLOGIC* or ENDOLYMPHATIC or AURICULAR).ti. 2 (disease* or disorder*).ti. 3 exp ear disease/ or (OTITIS or OTOSCLEROSIS or CHOLESTEATOMA or (TYMPANIC and MEMBRANE and PERFORAT*) or (TYMPANIC and MEMBRANE and RUPTURE*) or (OSSICULAR and CHAIN and DISORDER) or (GLUE and EAR)).tw. 4 exp surgery/ or (surg* or operat* or (POST and OPERAT*) or (POST and SURG*) or ((POSTSURG* or POSTOPERATIVE*) and INFECTION*)).tw. 5 1 and 2 6 3 or 5 7 6 and 4 8 exp ear surgery/ or (TYMPANOPLAST* or TYMPANOTOM* or TYMPANOMASTOIDECTOM* or "TYMPANOMASTOID SURGERY" or MASTOIDECTOM* or STAPEDECTOM* or STAPEDOTOM* or MYRINGOPLAST* or (MIDDLE and EAR and RECONSTRUCT*) or (MIDDLE and EAR and VENTILAT*) or (GROMMET* and INSERT*) or (TYMPANIC and MEMBRANE and SURG*) or (EAR and SURG*) or (AURICULAR and SURG*) or OSSICULOPLAST* or (FACIAL and NERVE and DECOMPRESSION*) or (STAPES and SURG*) or (OSSICULAR and REPLACE*) or (OSSICULAR and IMPLANT*) or (COCHLEAR and IMPLANT*) or (ENDOLYMPHATIC and SHUNT*) or (ENDOLYMPHATIC* and SURG*) or (LABYRINTH and SURG*) or (STAPES and SURG*)).tw. 9 exp ear disease/su [Surgery] 10 8 or 7 or 9 11 exp Antibiotic Agent/ or exp Antibiotic Prophylaxis/ or exp lactam/ or exp quinolone derivative/ 12 (ANTIBIOT* or (ANTI and BIOT*) or ANTIMICROBIAL* or (ANTI and MICROBIAL*) or BACTERIOCID* or ANTIBACTERIAL* or (ANTI and BACTERIAL*) or CHEMOTHERAPY or PENICILLIN or AMOXICILLIN or AMPICILLIN or "CLAVULANIC ACID" or AMOXICLAV or AUGMENTIN or TICARCILLIN or TIMENTIN or FLUCLOXACILLIN or FLUAMPICIL or MAGNAPEN or PIPERACILLIN or TAZOCIN or CEPHALOSPORIN* or CEFACLOR or DISTACLOR or CEFADROXIL or BAXAN or CEFALEXIN or CEPOREX or KEFLEX or CEFAMANDOLE or KEFADOL or CEFAZOLIN* or KEFZOL or CEFIXIME or SUPRAX or CEFOTAXIME or CLAFORAN or CEFOXITIN or MEFOXIN or CEFPIROME or CEFROM or CEFPODOXIME or ORELOX or CEFPROZIL or CEFZIL or CEFRADINE or VELOSEL or CEFTAZIDIM or ORTUM or KEFADIM or CEFTRIAXONE or ROCEPHIN or CEFUROXIME or ZINACEF or ZINNAT or CEFONICID or AZTREONAM or AZACTAM or IMIPENEM or ILASTATIN or PRIMAXIN or MEROPENEM).tw. 13 (TETRACYCLINE* or DETECLO or DEMECLEOCYCLIN or LEDERMYCIN or DOXYCYCLINE or VIBRAMYCIN or MINOCYCLINE or MINOCINE or OXYTETRACYCLINE or TERRAMYCIN or MACROLIDE* or ERYTHROMYCIN or ERYMAX or ERYTHROCIN or ERYTHROPED or AZITHROMYCIN or ZITHROMAX or CLARITHROMYCIN or KLARICID or TELITHROMYCIN or KETEK or TRIMOXAZOLE or SEPTRIN or TRIMETHOPRIM or MONOTRIM or TRIMOPAN or METRONIDAZOLE or FLAGYL or METROLYL or PHENOXYMETHYLPENICILLIN or SULFAMETHOXAZOLE or OXACILLIN or CEPHALOTHIN or SULBACTAM or OFLOXACIN or CLINDAMYCIN or GENTAMYCIN or VANCOMYCIN).tw. 14 11 or 13 or 12 15 10 and 14 | S1 (MH "Ear") S2 TI EAR* or TYMPANIC or STAPES or EUSTACHIAN or OTOLOGIC* or ENDOLYMPHATIC or AURICULAR S3 TI disease* OR disorder* S4 S1 or S2 S5 S3 and S4 S6 (MH "Ear Diseases+") S7 TX OTITIS or OTOSCLEROSIS or CHOLESTEATOMA S8 S5 or S6 or S7 S9 (MH "Surgery, Operative+") S10 TX surg* or operat* or POSTSURG* or POSTOPERATIVE* S11 S9 or S10 S12 S8 and S11 S13 (MH "Ear Surgery+") S14 S12 or S13 S15 (MH "Antibiotics+") or (MH "Antibiotic Prophylaxis") S16 TX ANTIBIOT* or ANTIMICROBIAL* or BACTERIOCID* or ANTIBACTERIAL* or CHEMOTHERAPY or PENICILLIN or AMOXICILLIN or AMPICILLIN or "CLAVULANIC ACID" or AMOXICLAV or AUGMENTIN or TICARCILLIN or TIMENTIN or FLUCLOXACILLIN or FLUAMPICIL or MAGNAPEN or PIPERACILLIN or TAZOCIN or CEPHALOSPORIN* or CEFACLOR or DISTACLOR or CEFADROXIL or BAXAN or CEFALEXIN or CEPOREX or KEFLEX or CEFAMANDOLE or KEFADOL or CEFAZOLIN* or KEFZOL or CEFIXIME or SUPRAX or CEFOTAXIME or CLAFORAN or CEFOXITIN or MEFOXIN or CEFPIROME or CEFROM or CEFPODOXIME or ORELOX or CEFPROZIL or CEFZIL or CEFRADINE or VELOSEL or CEFTAZIDIM or ORTUM or KEFADIM or CEFTRIAXONE or ROCEPHIN or CEFUROXIME or ZINACEF or ZINNAT or CEFONICID or AZTREONAM or AZACTAM or IMIPENEM or ILASTATIN or PRIMAXIN or MEROPENEMTX ANTIBIOT* or ANTIMICROBIAL* or BACTERIOCID* or ANTIBACTERIAL* or CHEMOTHERAPY or PENICILLIN or AMOXICILLIN or AMPICILLIN or "CLAVULANIC ACID" or AMOXICLAV or AUGMENTIN or TICARCILLIN or TIMENTIN or FLUCLOXACILLIN or FLUAMPICIL or MAGNAPEN or PIPERACILLIN or TAZOCIN or CEPHALOSPORIN* or CEFACLOR or DISTACLOR or CEFADROXIL or BAXAN or CEFALEXIN or CEPOREX or KEFLEX or CEFAMANDOLE or KEFADOL or CEFAZOLIN* or KEFZOL or CEFIXIME or SUPRAX or CEFOTAXIME or CLAFORAN or CEFOXITIN or MEFOXIN or CEFPIROME or CEFROM or CEFPODOXIME or ORELOX or CEFPROZIL or CEFZIL or CEFRADINE or VELOSEL or CEFTAZIDIM or ORTUM or KEFADIM or CEFTRIAXONE or ROCEPHIN or CEFUROXIME or ZINACEF or ZINNAT or CEFONICID or AZTREONAM or AZACTAM or IMIPENEM or ILASTATIN or PRIMAXIN or MEROPENEM S17 TX TETRACYCLINE* or DETECLO or DEMECLEOCYCLIN or LEDERMYCIN or DOXYCYCLINE or VIBRAMYCIN or MINOCYCLINE or MINOCINE or OXYTETRACYCLINE or TERRAMYCIN or MACROLIDE* or ERYTHROMYCIN or ERYMAX or ERYTHROCIN or ERYTHROPED or AZITHROMYCIN or ZITHROMAX or CLARITHROMYCIN or KLARICID or TELITHROMYCIN or KETEK or TRIMOXAZOLE or SEPTRIN or TRIMETHOPRIM or MONOTRIM or TRIMOPAN or METRONIDAZOLE or FLAGYL or METROLYL or PHENOXYMETHYLPENICILLIN or SULFAMETHOXAZOLE or OXACILLIN or CEPHALOTHIN or SULBACTAM or OFLOXACIN or CLINDAMYCIN or GENTAMYCIN or VANCOMYCINTX TETRACYCLINE* or DETECLO or DEMECLEOCYCLIN or LEDERMYCIN or DOXYCYCLINE or VIBRAMYCIN or MINOCYCLINE or MINOCINE or OXYTETRACYCLINE or TERRAMYCIN or MACROLIDE* or ERYTHROMYCIN or ERYMAX or ERYTHROCIN or ERYTHROPED or AZITHROMYCIN or ZITHROMAX or CLARITHROMYCIN or KLARICID or TELITHROMYCIN or KETEK or TRIMOXAZOLE or SEPTRIN or TRIMETHOPRIM or MONOTRIM or TRIMOPAN or METRONIDAZOLE or FLAGYL or METROLYL or PHENOXYMETHYLPENICILLIN or SULFAMETHOXAZOLE or OXACILLIN or CEPHALOTHIN or SULBACTAM or OFLOXACIN or CLINDAMYCIN or GENTAMYCIN or VANCOMYCIN S18 S15 or S16 or S17 S19 S14 and S18 |
| Web of Science | BIOSIS Previews/ CAB Abstracts (Ovid) | CENTRAL |
| #1 TI=((EAR* or TYMPANIC or STAPES or EUSTACHIAN or OTOLOGIC* or ENDOLYMPHATIC or AURICULAR) AND (disease* OR disorder*)) #2 TS=(surg* or operat* or POSTSURG* or POSTOPERATIVE*) #3 #2 AND #1 #4 TS=(TYMPANOPLAST* or TYMPANOTOM* or TYMPANOMASTOIDECTOM* or "TYMPANOMASTOID SURGERY" or MASTOIDECTOM* or STAPEDECTOM* or STAPEDOTOM* or MYRINGOPLAST* or (MIDDLE and EAR and RECONSTRUCT*) or (MIDDLE and EAR and VENTILAT*) or (GROMMET* and INSERT*) or (TYMPANIC and MEMBRANE and SURG*) or (EAR and SURG*) or (AURICULAR and SURG*) or OSSICULOPLAST* or (FACIAL and NERVE and DECOMPRESSION*) or (STAPES and SURG*) or (OSSICULAR and REPLACE*) or (OSSICULAR and IMPLANT*) or (COCHLEAR and IMPLANT*) or (ENDOLYMPHATIC and SHUNT*) or (ENDOLYMPHATIC* and SURG*) or (LABYRINTH and SURG*) or (STAPES and SURG*)) #5 #4 OR #3 #6 TS=(ANTIBIOT* or (ANTI and BIOT*) or ANTIMICROBIAL* or (ANTI and MICROBIAL*) or BACTERIOCID* or ANTIBACTERIAL* or (ANTI and BACTERIAL*) or CHEMOTHERAPY or PENICILLIN or AMOXICILLIN or AMPICILLIN or "CLAVULANIC ACID" or AMOXICLAV or AUGMENTIN or TICARCILLIN or TIMENTIN or FLUCLOXACILLIN or FLUAMPICIL or MAGNAPEN or PIPERACILLIN or TAZOCIN or CEPHALOSPORIN* or CEFACLOR or DISTACLOR or CEFADROXIL or BAXAN or CEFALEXIN or CEPOREX or KEFLEX or CEFAMANDOLE or KEFADOL or CEFAZOLIN* or KEFZOL or CEFIXIME or SUPRAX or CEFOTAXIME or CLAFORAN or CEFOXITIN or MEFOXIN or CEFPIROME or CEFROM) #7 TS=(CEFPODOXIME or ORELOX or CEFPROZIL or CEFZIL or CEFRADINE or VELOSEL or CEFTAZIDIM or ORTUM or KEFADIM or CEFTRIAXONE or ROCEPHIN or CEFUROXIME or ZINACEF or ZINNAT or CEFONICID or AZTREONAM or AZACTAM or IMIPENEM or ILASTATIN or PRIMAXIN or MEROPENEM) #8 TS=(TETRACYCLINE* or DETECLO or DEMECLEOCYCLIN or LEDERMYCIN or DOXYCYCLINE or VIBRAMYCIN or MINOCYCLINE or MINOCINE or OXYTETRACYCLINE or TERRAMYCIN or MACROLIDE* or ERYTHROMYCIN or ERYMAX or ERYTHROCIN or ERYTHROPED or AZITHROMYCIN or ZITHROMAX or CLARITHROMYCIN or KLARICID or TELITHROMYCIN or KETEK or TRIMOXAZOLE or SEPTRIN or TRIMETHOPRIM or MONOTRIM or TRIMOPAN or METRONIDAZOLE or FLAGYL or METROLYL or PHENOXYMETHYLPENICILLIN or SULFAMETHOXAZOLE or OXACILLIN or CEPHALOTHIN or SULBACTAM or OFLOXACIN or CLINDAMYCIN or GENTAMYCIN or VANCOMYCIN) #9 #8 OR #7 OR #6 #10 #9 AND #5 | 1 exp Ear/ or (EAR* or TYMPANIC or STAPES or EUSTACHIAN or OTOLOGIC* or ENDOLYMPHATIC or AURICULAR).ti. 2 (disease* or disorder*).ti. 3 exp ear disease/ or (OTITIS or OTOSCLEROSIS or CHOLESTEATOMA or (TYMPANIC and MEMBRANE and PERFORAT*) or (TYMPANIC and MEMBRANE and RUPTURE*) or (OSSICULAR and CHAIN and DISORDER) or (GLUE and EAR)).tw. 4 exp surgery/ or (surg* or operat* or (POST and OPERAT*) or (POST and SURG*) or ((POSTSURG* or POSTOPERATIVE*) and INFECTION*)).tw. 5 1 and 2 6 3 or 5 7 6 and 4 8 exp ear surgery/ or (TYMPANOPLAST* or TYMPANOTOM* or TYMPANOMASTOIDECTOM* or "TYMPANOMASTOID SURGERY" or MASTOIDECTOM* or STAPEDECTOM* or STAPEDOTOM* or MYRINGOPLAST* or (MIDDLE and EAR and RECONSTRUCT*) or (MIDDLE and EAR and VENTILAT*) or (GROMMET* and INSERT*) or (TYMPANIC and MEMBRANE and SURG*) or (EAR and SURG*) or (AURICULAR and SURG*) or OSSICULOPLAST* or (FACIAL and NERVE and DECOMPRESSION*) or (STAPES and SURG*) or (OSSICULAR and REPLACE*) or (OSSICULAR and IMPLANT*) or (COCHLEAR and IMPLANT*) or (ENDOLYMPHATIC and SHUNT*) or (ENDOLYMPHATIC* and SURG*) or (LABYRINTH and SURG*) or (STAPES and SURG*)).tw. (15601) 9 8 or 7 10 exp Antibiotic Agent/ or exp Antibiotic Prophylaxis/ or exp lactam/ or exp quinolone derivative/ (0) 11 (ANTIBIOT* or (ANTI and BIOT*) or ANTIMICROBIAL* or (ANTI and MICROBIAL*) or BACTERIOCID* or ANTIBACTERIAL* or (ANTI and BACTERIAL*) or CHEMOTHERAPY or PENICILLIN or AMOXICILLIN or AMPICILLIN or "CLAVULANIC ACID" or AMOXICLAV or AUGMENTIN or TICARCILLIN or TIMENTIN or FLUCLOXACILLIN or FLUAMPICIL or MAGNAPEN or PIPERACILLIN or TAZOCIN or CEPHALOSPORIN* or CEFACLOR or DISTACLOR or CEFADROXIL or BAXAN or CEFALEXIN or CEPOREX or KEFLEX or CEFAMANDOLE or KEFADOL or CEFAZOLIN* or KEFZOL or CEFIXIME or SUPRAX or CEFOTAXIME or CLAFORAN or CEFOXITIN or MEFOXIN or CEFPIROME or CEFROM or CEFPODOXIME or ORELOX or CEFPROZIL or CEFZIL or CEFRADINE or VELOSEL or CEFTAZIDIM or ORTUM or KEFADIM or CEFTRIAXONE or ROCEPHIN or CEFUROXIME or ZINACEF or ZINNAT or CEFONICID or AZTREONAM or AZACTAM or IMIPENEM or ILASTATIN or PRIMAXIN or MEROPENEM).tw. (910130) 12 (TETRACYCLINE* or DETECLO or DEMECLEOCYCLIN or LEDERMYCIN or DOXYCYCLINE or VIBRAMYCIN or MINOCYCLINE or MINOCINE or OXYTETRACYCLINE or TERRAMYCIN or MACROLIDE* or ERYTHROMYCIN or ERYMAX or ERYTHROCIN or ERYTHROPED or AZITHROMYCIN or ZITHROMAX or CLARITHROMYCIN or KLARICID or TELITHROMYCIN or KETEK or TRIMOXAZOLE or SEPTRIN or TRIMETHOPRIM or MONOTRIM or TRIMOPAN or METRONIDAZOLE or FLAGYL or METROLYL or PHENOXYMETHYLPENICILLIN or SULFAMETHOXAZOLE or OXACILLIN or CEPHALOTHIN or SULBACTAM or OFLOXACIN or CLINDAMYCIN or GENTAMYCIN or VANCOMYCIN).tw. (116296) 13 10 or 11 or 12 14 9 and 13 | #1 EAR explode all trees (MeSH) #2 (EAR* OR TYMPANIC OR STAPES OR EUSTACHIAN OR OTOLOGIC* OR ENDOLYMPHATIC OR AURICULAR).ti. #3 #1 OR #2 #4 (DISEASE* OR DISORDER*).ti. #5 #3 AND #4 #6 EAR DISEASES explode all trees (MeSH) #7 OTITIS OR OTOSCLEROSIS OR CHOLESTEATOMA OR TYMPANIC NEXT MEMBRANE NEAR PERFORAT* OR TYMPANIC NEXT MEMBRANE NEXT RUPTURE* OR OSSICULAR NEXT CHAIN NEAR DISORDER OR GLUE NEXT EAR #8 #5 OR #6 OR#7 #9 SURGICAL PROCEDURES OPERATIVE explode all trees (MeSH) #10 (SURG* OR OPERAT*).ti. #11 ((POST NEXT OPERAT* OR POST NEXT SURG* OR POSTSURG* OR POSTOPERATIVE*) NEXT INFECTION*) #12 #9 OR #10 OR #11 #13 #8 AND #12 #14 OTOLOGIC SURGICAL PROCEDURES explode all trees (MeSH) #15 TYMPANOPLAST* OR TYMPANOTOM* OR TYMPANOMASTOIDECTOM* OR TYMPANOMASTOID NEXT SURGERY OR MASTOIDECTOM* OR STAPEDECTOM* OR STAPEDOTOM* OR MYRINGOPLAST* #16 MIDDLE NEXT EAR NEAR RECONSTRUCT* OR MIDDLE NEXT EAR NEAR VENTILAT* OR GROMMET* NEAR INSERT* OR TYMPANIC NEXT MEMBRANE NEAR SURG* OR EAR NEAR SURG* OR AURICULAR NEAR SURG* OR OSSICULOPLAST* OR FACIAL NEXT NERVE NEAR DECOMPRESSION* #17 STAPES NEXT SURG* OR OSSICULAR NEAR REPLACE* OR OSSICULAR NEAR IMPLANT* OR COCHLEAR NEAR IMPLANT* OR ENDOLYMPHATIC NEAR SHUNT* OR ENDOLYMPHATIC NEAR SURG* OR LABYRINTH NEAR SURG* OR STAPES NEAR SURG* #18 EAR [su] explode all trees (MeSH) #19 EAR DISEASES [su] explode all trees (MeSH) #20 #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 #21 ANTI‐BACTERIAL AGENTS explode all trees (MeSH) #22 ANTIBIOTIC PROPHYLAXIS single term (MeSH) #23 LACTAMS explode all trees (MeSH) #24 QUINOLONES explode all trees (MeSH) #25 MACROLIDES explode all trees (MeSH) #26 ANTIBIOT* OR ANTI NEXT BIOT* OR ANTIMICROBIAL* OR ANTI NEXT MICROBIAL* OR BACTERIOCID* OR ANTIBACTERIAL* OR ANTI NEXT BACTERIAL* #27 PENICILLIN* OR AMOXICILLIN OR AMPICILLIN OR CLAVULANIC NEXT ACID OR AMOXICLAV OR AUGMENTIN OR TICARCILLIN OR TIMENTIN OR FLUCLOXACILLIN OR FLUAMPICIL OR MAGNAPEN OR PIPERACILLIN OR TAZOCIN #28 CEPHALOSPORIN* OR CEFACLOR OR DISTACLOR OR CEFADROXIL OR BAXAN OR CEFALEXIN OR CEPOREX OR KEFLEX OR CEFAMANDOLE OR KEFADOL OR CEFAZOLIN* OR KEFZOL OR CEFIXIME OR SUPRAX OR CEFOTAXIME OR CLAFORAN OR CEFOXITIN OR MEFOXIN OR CEFPIROME OR CEFROM OR CEFPODOXIME OR ORELOX OR CEFPROZIL OR CEFZIL OR CEFRADINE OR VELOSEL OR CEFTAZIDIM OR FORTUM OR KEFADIM OR CEFTRIAXONE OR ROCEPHIN OR CEFUROXIME OR ZINACEF OR ZINNAT OR CEFONICID OR AZTREONAM OR AZACTAM OR IMIPENEM OR CILASTATIN OR PRIMAXIN OR MEROPENEM #29 TETRACYCLINE* OR DETECLO OR DEMECLEOCYCLIN OR LEDERMYCIN OR DOXYCYCLINE OR VIBRAMYCIN OR MINOCYCLINE OR MINOCINE OR OXYTETRACYCLINE OR TERRAMYCIN #30 MACROLIDE* OR ERYTHROMYCIN OR ERYMAX OR ERYTHROCIN OR ERYTHROPED OR AZITHROMYCIN OR ZITHROMAX OR CLARITHROMYCIN OR KLARICID OR TELITHROMYCIN OR KETEK OR TRIMOXAZOLE OR SEPTRIN OR TRIMETHOPRIM OR MONOTRIM OR TRIMOPAN OR METRONIDAZOLE OR FLAGYL OR METROLYL #31 PHENOXYMETHYLPENICILLIN OR SULFAMETHOXAZOLE OR OXACILLIN OR CEPHALOTHIN OR SULBACTAM OR OFLOXACIN OR CLINDAMYCIN OR GENTAMYCIN OR VANCOMYCIN #32 #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 #33 #20 AND #32 |
Data and analyses
Comparison 1. Antibiotics in clean and clean‐contaminated ear surgery.
1.1. Analysis.
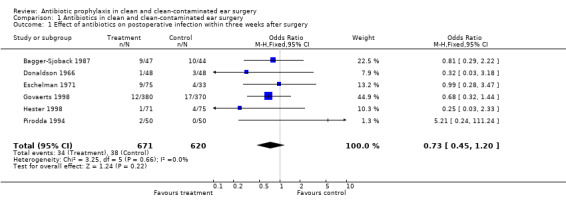
Comparison 1 Antibiotics in clean and clean‐contaminated ear surgery, Outcome 1 Effect of antibiotics on postoperative infection within three weeks after surgery.
1.2. Analysis.
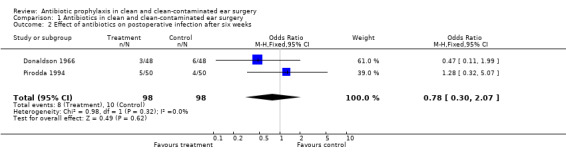
Comparison 1 Antibiotics in clean and clean‐contaminated ear surgery, Outcome 2 Effect of antibiotics on postoperative infection after six weeks.
1.3. Analysis.
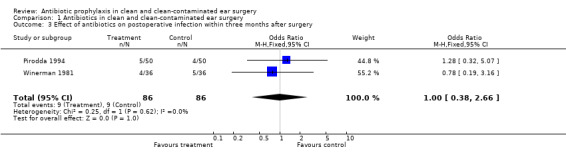
Comparison 1 Antibiotics in clean and clean‐contaminated ear surgery, Outcome 3 Effect of antibiotics on postoperative infection within three months after surgery.
1.4. Analysis.
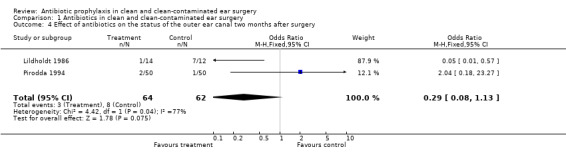
Comparison 1 Antibiotics in clean and clean‐contaminated ear surgery, Outcome 4 Effect of antibiotics on the status of the outer ear canal two months after surgery.
1.5. Analysis.
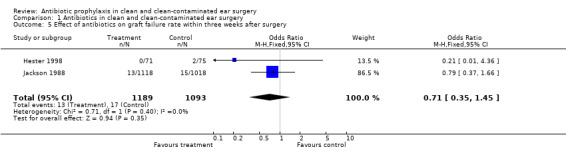
Comparison 1 Antibiotics in clean and clean‐contaminated ear surgery, Outcome 5 Effect of antibiotics on graft failure rate within three weeks after surgery.
1.6. Analysis.
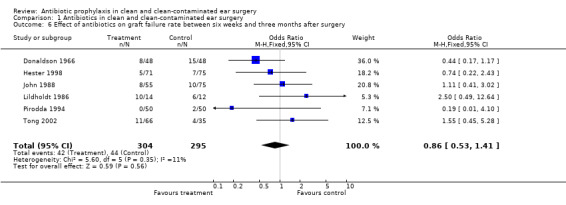
Comparison 1 Antibiotics in clean and clean‐contaminated ear surgery, Outcome 6 Effect of antibiotics on graft failure rate between six weeks and three months after surgery.
1.7. Analysis.
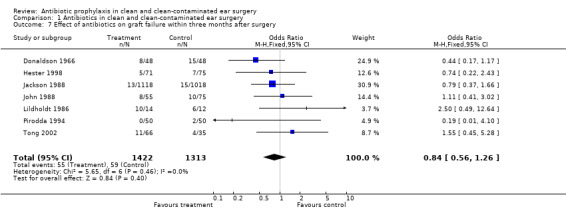
Comparison 1 Antibiotics in clean and clean‐contaminated ear surgery, Outcome 7 Effect of antibiotics on graft failure within three months after surgery.
1.8. Analysis.
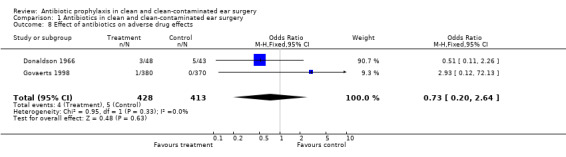
Comparison 1 Antibiotics in clean and clean‐contaminated ear surgery, Outcome 8 Effect of antibiotics on adverse drug effects.
Comparison 2. Systemic antibiotics versus no antibiotics in clean and clean‐contaminated ear surgery.
2.1. Analysis.
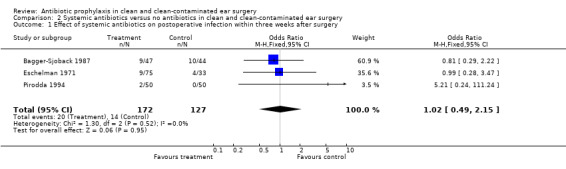
Comparison 2 Systemic antibiotics versus no antibiotics in clean and clean‐contaminated ear surgery, Outcome 1 Effect of systemic antibiotics on postoperative infection within three weeks after surgery.
2.2. Analysis.
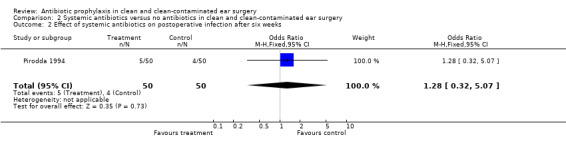
Comparison 2 Systemic antibiotics versus no antibiotics in clean and clean‐contaminated ear surgery, Outcome 2 Effect of systemic antibiotics on postoperative infection after six weeks.
2.3. Analysis.
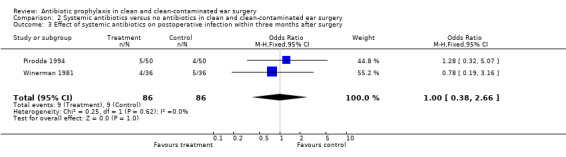
Comparison 2 Systemic antibiotics versus no antibiotics in clean and clean‐contaminated ear surgery, Outcome 3 Effect of systemic antibiotics on postoperative infection within three months after surgery.
2.4. Analysis.
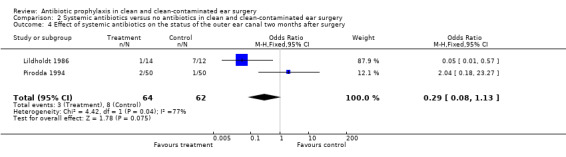
Comparison 2 Systemic antibiotics versus no antibiotics in clean and clean‐contaminated ear surgery, Outcome 4 Effect of systemic antibiotics on the status of the outer ear canal two months after surgery.
2.5. Analysis.
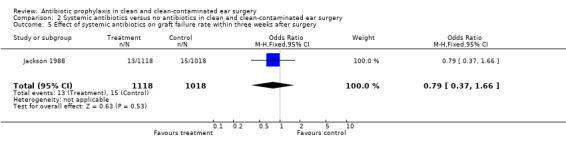
Comparison 2 Systemic antibiotics versus no antibiotics in clean and clean‐contaminated ear surgery, Outcome 5 Effect of systemic antibiotics on graft failure rate within three weeks after surgery.
2.6. Analysis.
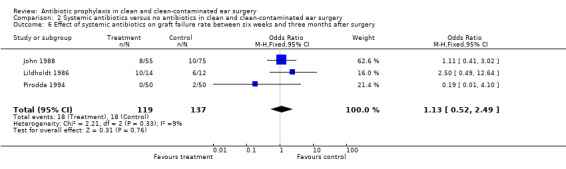
Comparison 2 Systemic antibiotics versus no antibiotics in clean and clean‐contaminated ear surgery, Outcome 6 Effect of systemic antibiotics on graft failure rate between six weeks and three months after surgery.
2.7. Analysis.
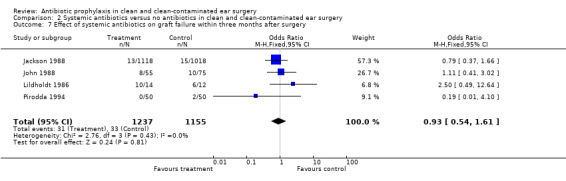
Comparison 2 Systemic antibiotics versus no antibiotics in clean and clean‐contaminated ear surgery, Outcome 7 Effect of systemic antibiotics on graft failure within three months after surgery.
Comparison 3. Systemic and local antibiotics versus local antibiotics in clean and clean‐contaminated ear surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Effect of systemic and local antibiotics on postoperative infection within three weeks after surgery | 3 | 992 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.29, 1.10] |
| 2 Effect of systemic and local antibiotics on postoperative infection after six weeks | 1 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.11, 1.99] |
| 3 Effect of systemic and local antibiotics on postoperative infection within three months after surgery | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Effect of systemic and local antibiotics on the status of the outer ear canal two months after surgery | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Effect of systemic and local antibiotics on graft failure rate within three weeks after surgery | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.36] |
| 6 Effect of systemic and local antibiotics on graft failure rate between six weeks and three months after surgery | 2 | 242 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.25, 1.15] |
| 7 Effect of systemic and local antibiotics on graft failure within three months after surgery | 2 | 242 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.25, 1.15] |
| 8 Effect of systemic and local antibiotics on adverse drug effects | 2 | 841 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.20, 2.64] |
3.1. Analysis.
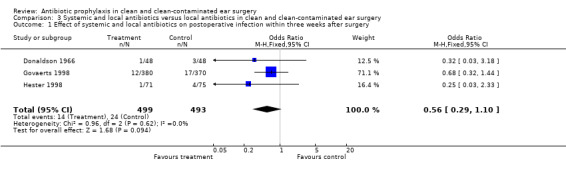
Comparison 3 Systemic and local antibiotics versus local antibiotics in clean and clean‐contaminated ear surgery, Outcome 1 Effect of systemic and local antibiotics on postoperative infection within three weeks after surgery.
3.2. Analysis.
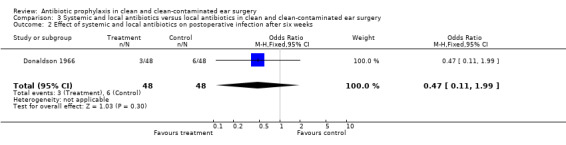
Comparison 3 Systemic and local antibiotics versus local antibiotics in clean and clean‐contaminated ear surgery, Outcome 2 Effect of systemic and local antibiotics on postoperative infection after six weeks.
3.5. Analysis.
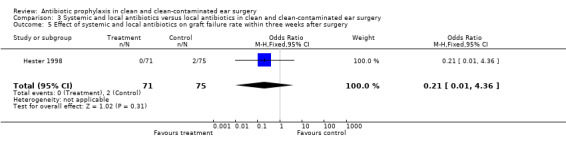
Comparison 3 Systemic and local antibiotics versus local antibiotics in clean and clean‐contaminated ear surgery, Outcome 5 Effect of systemic and local antibiotics on graft failure rate within three weeks after surgery.
3.6. Analysis.
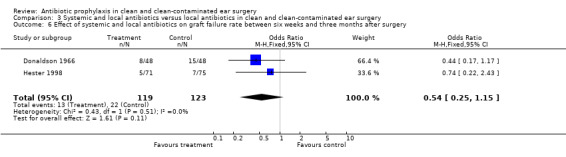
Comparison 3 Systemic and local antibiotics versus local antibiotics in clean and clean‐contaminated ear surgery, Outcome 6 Effect of systemic and local antibiotics on graft failure rate between six weeks and three months after surgery.
3.7. Analysis.
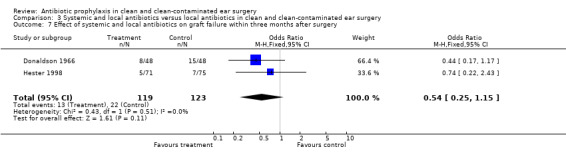
Comparison 3 Systemic and local antibiotics versus local antibiotics in clean and clean‐contaminated ear surgery, Outcome 7 Effect of systemic and local antibiotics on graft failure within three months after surgery.
3.8. Analysis.
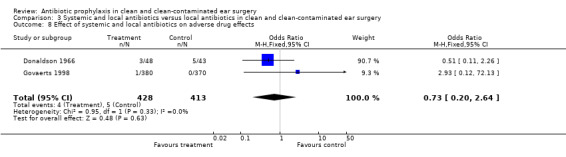
Comparison 3 Systemic and local antibiotics versus local antibiotics in clean and clean‐contaminated ear surgery, Outcome 8 Effect of systemic and local antibiotics on adverse drug effects.
Comparison 4. Local antibiotics versus no antibiotics in clean and clean‐contaminated ear surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Effect of local antibiotics on postoperative infection within three weeks after surgery | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Effect of local antibiotics on postoperative infection after six weeks | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Effect of local antibiotics on postoperative infection within three months after surgery | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Effect of local antibiotics on the status of the outer ear canal two months after surgery | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Effect of local antibiotics on graft failure rate within three weeks after surgery | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Effect of local antibiotics on graft failure rate between six weeks and three months after surgery | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.45, 5.28] |
| 7 Effect of local antibiotics on graft failure within three months after surgery | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.45, 5.28] |
| 8 Effect of local antibiotics on adverse drug effects | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.6. Analysis.
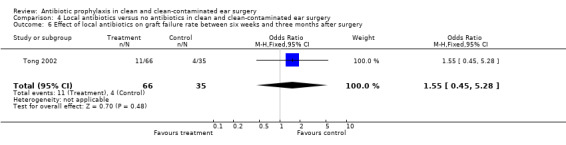
Comparison 4 Local antibiotics versus no antibiotics in clean and clean‐contaminated ear surgery, Outcome 6 Effect of local antibiotics on graft failure rate between six weeks and three months after surgery.
4.7. Analysis.
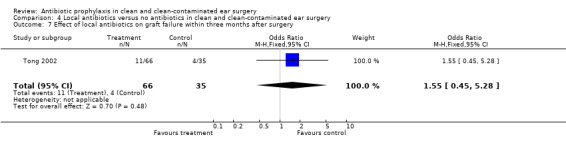
Comparison 4 Local antibiotics versus no antibiotics in clean and clean‐contaminated ear surgery, Outcome 7 Effect of local antibiotics on graft failure within three months after surgery.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bagger‐Sjoback 1987.
| Methods | Randomised controlled trial | |
| Participants | I: ‐ myringoplasty ‐ ossiculoplasty ‐ combined approach tympanoplasty ‐ radical mastoidectomy ‐ revision surgery ‐ stapedectomy ‐ others | |
| Interventions | (1) phenoxymethylpenicillin + hydrocortisone impregnated gauze (2) hydrocortisone impregnated gauze | |
| Outcomes | ‐ Postoperative infection after 6 to 8 days | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Donaldson 1966.
| Methods | Randomised controlled trial | |
| Participants | I: ‐ myringoplasty (different types) | |
| Interventions | (1) sulfamethoxazole + polymyxin B ‐ neomycin ‐ hydrocortisone impregnated sponge (2) polymyxin B ‐ neomycin ‐ hydrocortisone impregnated sponge | |
| Outcomes | ‐ Postoperative infection after 10 days ‐ Postoperative infection after 6 weeks ‐ Adverse effects of antibiotics ‐ Closure of tympanic membrane | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Eschelman 1971.
| Methods | Randomised controlled trial | |
| Participants | I: ‐ tympanomastoidectomy (with cholesteatoma) II: ‐ tympanoplasty ‐ tympanomastoidectomy (without cholesteatoma) III: ‐ stapedectomy ‐ exploratory tympanotomy | |
| Interventions | (1) penicillin (2) ampicillin (3) placebo | |
| Outcomes | ‐ Postoperative infection | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Govaerts 1998.
| Methods | Randomised controlled trial | |
| Participants | I: ‐ surgery for otosclerosis ‐ tympanoplasty (different types) ‐ radical mastoidectomy ‐ tympanotomy without reconstruction | |
| Interventions | (1) cefuroxime + oxytetracycline ‐ polymyxin B ‐ postoperative packing (2) oxytetracycline ‐ polymyxin B ‐ postoperative packing | |
| Outcomes | ‐ Postoperative infection within 2 weeks after surgery ‐ Adverse effects | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hester 1998.
| Methods | Randomised controlled trial | |
| Participants | I: ‐ tympanoplasty ‐ tympanomastoidectomy ‐ radical mastoidectomy | |
| Interventions | (1) ampicillin/sulbactam/Augmentin or cefazolin/cephalexin or erythromycin or clindamycin + bacitracin gauze + neomycin ‐ polymyxin ‐ hydrocortisone drops (2) bacitracin gauze + neomycin ‐ polymyxin ‐ hydrocortisone drops | |
| Outcomes | ‐ Postoperative infection within 3 weeks ‐ Graft failure within 3 weeks ‐ Graft failure after 3 months | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Jackson 1988.
| Methods | Randomised controlled trial | |
| Participants | I: ‐ a wide variety of surgical procedures, including neurotological surgery | |
| Interventions | (1) cephalothin or cefazolin or oxacillin or vancomycin (2) no antibiotic | |
| Outcomes | ‐ Graft failure after 3 weeks | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
John 1988.
| Methods | Randomised controlled trial | |
| Participants | I: ‐ tympanoplasty | |
| Interventions | (1) ampicillin + flucloxacillin (2) no antibiotic | |
| Outcomes | ‐ Closure of tympanic membrane after 8 weeks | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Lildholdt 1986.
| Methods | Randomised controlled trial | |
| Participants | I: ‐ surgery for chronic otitis media | |
| Interventions | (1) ceftazidim (2) no antibiotic | |
| Outcomes | ‐ Postoperative discharge ‐ Discharge after 2 months ‐ Graft failure after 2 months | |
| Notes | All patients had positive pre‐operative Pseudomonas swabs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Pirodda 1994.
| Methods | Randomised controlled trial | |
| Participants | I: ‐ mastoidectomy (different types) II: ‐ tympanoplasty (different types) | |
| Interventions | (1) ceftriaxone (2) no antibiotic | |
| Outcomes | ‐ Discharge after 1 week, 1 month and 2 months ‐ Perforation of the tympanic membrane after 1 week, 1 month and 2 months ‐ Stable situation after 1 week, 1 month and 2 months | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tong 2002.
| Methods | Randomised controlled trial | |
| Participants | I: ‐ tympanoplasty | |
| Interventions | (1) pre‐operative ofloxacin drops, 10 minutes a day, for two weeks (2) pre‐operative ofloxacin drops, 3 minutes a day, for two weeks (3) no antibiotic | |
| Outcomes | ‐ Closure of tympanic membrane after 8 weeks | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Winerman 1981.
| Methods | Randomised controlled trial | |
| Participants | I: ‐ tympanomastoid surgery (closed cavity procedure) | |
| Interventions | (1) clindamycin + gentamycin (2) placebo | |
| Outcomes | ‐ Infectious complications within 3 months | |
| Notes | 6 of the 36 patients in the antibiotic group received only clindamycin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Leonard 1967 | This study was presented as a randomised controlled trial, but turned out to be a case report |
| Liu 1983 | This study compared two different antibiotic regime/dosage patterns (penicillin versus penicillin + gentamycin), and could therefore not be used |
Contributions of authors
All authors contributed equally to searching for studies, initial screening and study selection, quality assessment, writing to study authors, data extraction and data analysis.
Hendrik Verschuur drafted protocol and review text.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bagger‐Sjoback 1987 {published data only}
- Bagger‐Sjoback D, Mendel L, Nord CE. The role of prophylactic antibiotics in middle ear surgery. A study on phenoxymethylpenicillin prophylaxis. American Journal of Otology 1987;8(6):519‐23. [MEDLINE: ] [PubMed] [Google Scholar]
Donaldson 1966 {published data only}
- Donaldson JA, Snyder IS. Prophylactic chemotherapy in myringoplasty surgery. Laryngoscope 1966;76:1201‐14. [Google Scholar]
Eschelman 1971 {published data only}
- Eschelman LT, Schleuning AJ 2nd, Brummett RE. Prophylactic antibiotics in otolaryngologic surgery: a double‐blind study. Transactions ‐ American Academy of Ophthalmology and Otolaryngology 1971;75(2):387‐94. [MEDLINE: ] [PubMed] [Google Scholar]
Govaerts 1998 {published data only}
- Govaerts PJ, Raemaekers J, Verlinden A, Kalai M, Somers T, Offeciers FE. Use of antibiotic prophylaxis in ear surgery. Laryngoscope 1998;108(1 Pt 1):107‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hester 1998 {published data only}
- Hester TO, Jones RO. Prophylactic antibiotics in surgery for chronic ear disease. Laryngoscope 1998;108(9):1334‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jackson 1988 {published data only}
- Jackson CG. Antimicrobial prophylaxis in ear surgery. Laryngoscope 1988;98(10):1116‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
John 1988 {published data only}
- John DG, Carlin WV, Lesser HJ, Carrick DG, Fielder C. Tympanoplasty surgery and prophylactic antibiotics: surgical results. Clinical Otolaryngology 1998;13(3):205‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lildholdt 1986 {published data only}
- Lildholdt T, Felding JU, Juul A, Kristensen S, Schouenborg P. Efficacy of perioperative ceftazidime in the surgical treatment of chronic otitis media due to Pseudomonas aeruginosa. Preliminary report of a prospective, controlled study. Archives of Oto‐rhino‐laryngology 1986;243(3):167‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pirodda 1994 {published data only}
- Pirodda A, Saggese D, Farneti G. Antibiotic prophylaxis in otological and otoneurological surgery [L'antibioticoprofilassi nella chirurgia otologica e otoneurologica]. Acta Otorhinolaryngologica Italica 1994;14(3):249‐55. [MEDLINE: ] [PubMed] [Google Scholar]
Tong 2002 {published data only}
- Tong MC, Yue V, Ku PK, Hasselt CA. Preoperative topical ofloxacin solution for tympanoplasty: a randomized, controlled study. Otology and Neurotology 2002;23(1):18‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Winerman 1981 {published data only}
- Winerman I, Segal S, Man A. Effectiveness of prophylactic antibiotic treatment in mastoid surgery. American Journal of Otology 1981;3(1):65‐7. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Leonard 1967 {published data only}
- Leonard JR. Prophylactic antibiotics in human stapedectomy. Laryngoscope 1967;77:663‐80. [DOI] [PubMed] [Google Scholar]
Liu 1983 {published data only}
- Liu C, Su WY. A therapeutic trial of aminoglycoside antibiotic in surgical treatment of chronic otitis media. Chinese Medical Journal 1983;31:167‐9. [Google Scholar]
Additional references
Burke 1961
- Burke. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery 1961;50:161‐8. [PubMed] [Google Scholar]
Garner 1988
- Garner. CDC definitions for nosocomial infections. American Journal of Infections Control 1988;16:128‐40. [DOI] [PubMed] [Google Scholar]
Handbook 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Kaiser 1986
- Kaiser AB. Antimicrobial prophylaxis in surgery. New England Journal of Medicine 1986;315:1129‐38. [DOI] [PubMed] [Google Scholar]
Waddell 1994
- Waddell TK, Rotstein ODSO. Antimicrobial prophylaxis in surgery. Committee on Antimicrobial Agents, Canadian Infectious Diseases Society. Canadian Medical Association Journal 1994;151(7):925‐31. [PMC free article] [PubMed] [Google Scholar]


