Abstract
Buddleja lindleyana Fort., a traditional Chinese medicine, has demonstrated anti-inflammatory, immunomodulatory, antidementia, neuroprotective, antibacterial, and antioxidant effects. Its flowers, leaves, and roots have been used as traditional Chinese medicines. A simple and rapid high-performance liquid chromatography method coupled with mass spectrometry (HPLC-MS/MS) was applied in the multicomponent determination of Buddleja lindleyana Fort., and the discrepancies in the contents from ten different habitats were analyzed. The present study simultaneously determined the concentrations of seven chemical compounds of Buddleja lindleyana Fort. extract in rat plasma via HPLC-MS/MS, which was applied in the pharmacokinetic (PK) study of Buddleja lindleyana Fort. A C18 column was used for chromatographic separation, and ion acquisition was achieved by multiple-reaction monitoring (MRM) in negative ionization mode. The optimized mass transition ion-pairs (m/z) for quantization were 591.5/282.8 for linarin, 609.4/300.2 for rutin, 284.9/133.0 for luteolin, 300.6/151.0 for quercetin, 268.8/116.9 for apigenin, 283.0/267.9 for acacetin, 623.3/160.7 for acteoside, and 252.2/155.8 for sulfamethoxazole (IS). A double peak appeared in the drug–time curve of apigenin, which was associated with entero-hepatic recirculation. There were discrepancies in the contents of seven chemical compounds from 10 batches of Buddleja lindleyana Fort., which were associated with the growth environments. Herein, the pharmacokinetic parameters of seven analytes in Buddleja lindleyana Fort. extract are summarized. The maximum plasma concentration (Cmax) of linarin, rutin, luteolin, quercetin, apigenin, acacetin and acteoside were 894.12 ± 9.34 ng mL−1, 130.76 ± 18.33 ng mL−1, 77.37 ± 25.72 ng mL−1, 20.15 ± 24.85 ng mL−1, 146.42 ± 14.88 ng mL−1, 31.92 ± 17.58 ng mL−1, and 649.78 ± 16.42 ng mL−1, respectively. The time to reach Cmax for linarin, rutin, luteolin, quercetin, apigenin, acacetin, and acteoside were 10, 5, 5, 5, 180, 10 and 10 min, respectively. This is the first report on the simultaneous determination of seven active components for 10 different growing environments and the pharmacokinetic studies of seven active components in rat plasma after the oral administration of Buddleja lindleyana Fort. extract. This study lays the foundation for a better understanding of the absorption mechanism of Buddleja lindleyana Fort., and the evaluation of its clinical application.
Quality control and pharmacokinetics of Buddleja lindleyana Fort by HPLC-MS/MS.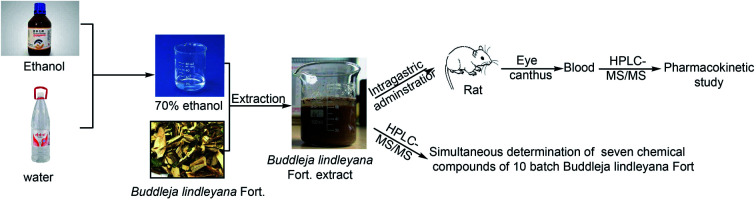
1. Introduction
Buddleja lindleyana Fort. is the general name of the Buddle-ja plants in the Loganiaceae family, which are distributed in tropical and subtropical areas such as South America, Asia and southern Africa.1 There are more than 100 species, with 20 species and hybrids found in China.2Buddleja lindleyana Fort. is famous for its toxicity to fish as well as their exotic flowers.3 Its flowers, leaves and roots have been used as traditional Chinese medicine, and have been applied in the treatment of rheumatism, cough, and blood stasis among other.4–8 The main chemical components of Buddleja lindleyana Fort. include triterpenoids,9,10 flavonoids,11 iridoid glycosides12,13 and phenylethaoids.14
In recent years, high-performance liquid chromatography coupled with mass spectrometry (HPLC-MS/MS) has been routinely used to determine the contents of chemical compounds in traditional chemical materials and also applied in pharmacokinetic studies in rats and humans.15,16 Moreover, it has been used to study drug metabolism,17–22 toxicokinetics,23,24 lipidomics,25,26 proteomics27,28 and metabolomics.29,30 The main advantage of mass spectrometry (MS/MS) is the ability to detect a broad range of drugs with high sensitivity and specificity in a single analytical run.31–34 Chemical compounds are recognized by comparing their retention times and parent-daughter ions with those of reference substances.
Pharmacokinetics (PK) is a discipline that quantitatively studies the absorption, distribution, metabolism and excretion in vivo, which uses mathematical theory and methods to make an exposition of the law for changing drug concentrations in blood over time. With the development of medicinal chemistry and the continuous improvement of human health, the requirements for the PK of drugs are getting higher and higher, which will determine the development trends of drugs, especially in the foreground of the market. The therapeutic effects of a drug must be strong, with few side effects and good PK parameters.
The contents of chemical compounds (linarin, luteolin, acacia-7-O-β-d-glucopyranoside, acacetin, rutin, quercetin, apigenin, clinoposaponin III, desrhamnoverbascosaponin and mimengoside I) were determined by HPLC and UPLC according to previous research.5,35,36 In this study, a sensitive and rapid HPLC-MS/MS method was developed for the simultaneous determination of seven compounds in different batches of medicinal materials and in rat plasma after oral treatment with Buddleja lindleyana Fort. extract. The HPLC-MS/MS technology demonstrated the advantages of being rapid and efficient. This is the first systematic multicomponent quantification and PK study of seven chemical constituents of Buddleja lindleyana Fort. Multiple-reaction monitoring (MRM) was employed and the ESI source was operated in negative mode. The contents of seven components of Buddleja lindleyana Fort. from ten different sources were compared and the PK parameters were summarized. These results laid the foundation for clinical application.
2. Materials and methods
2.1. Chemicals and materials
Linarin (137-200702), luteolin (137-200708), apigenin (137-200928), acacetin (137-200619) and acteoside (137-200901) were purchased from Nanjing Guangrun Biotechnology Co. Ltd (Nanjing, China). Rutin (100080-200707), quercetin (100081-200907) and sulfamethoxazole (100025-200904) were obtained from the National Institutes for Food and Drug Control. The purities of these standards were higher than 98%.
HPLC-grade methanol was purchased from J. T. Baker Chemical Company (Phillipsburg, NJ, USA). Formic acid (HPLC grade) was provided by Diamond Technology (Dikma Technologies Inc., Lake Forest, CA, USA). Ethanol (analytical grade) was provided by Tianjin Guangfu Technology Development Co. Ltd (Tianjin, China). Purified water was obtained from Guangzhou Watson's Food & Beverage Co. Ltd (Guangzhou, China).
The entire Buddleja lindleyana Fort. plant was collected from Anguo Chinese Medicinal Materials Wholesale Market and identified by Zengke Kong, Professor of Pharmacognosy, Hebei Province Institute for Drug Control. The sources of Buddleja lindleyana Fort. are listed in Table 1.
The list of Buddleja lindleyana Fort. samples.
| Sample number | Source |
|---|---|
| 1 | Guangxi |
| 2 | Xizang |
| 3 | Sichuan |
| 4 | Anhui-Bozhou |
| 5 | Zhejiang-Huzhou |
| 6 | Yunnan |
| 7 | Anhui-Bengbu |
| 8 | Zhejiang-Hangzhou |
| 9 | Jiangxi |
| 10 | Henan |
2.2. Instrumentation and conditions
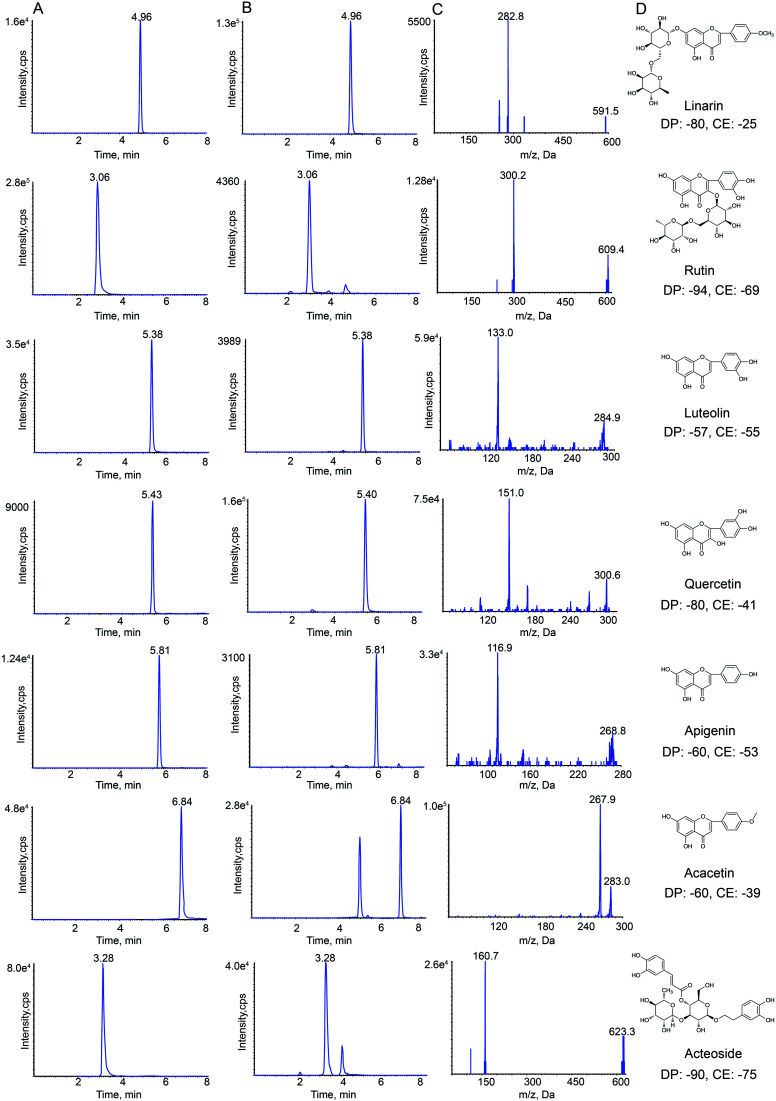
HPLC-MS/MS analysis was performed on a Shimadzu HPLC system (Shimadzu Corp., Kyoto, Japan) that was coupled with an API 4000+ MS/MS system (AB Sciex, CA, USA). Nitrogen was obtained from Shijiazhuang Fulite Gas Co. Ltd The chromatographic separation was conducted on a Symmetry® C18(4.6 × 150 mm, 3.5 μm) column with a SecurityGuard® HPLC C18 pre-column (Agilent Corp, Santa Clara, CA, USA). The column temperature was maintained at 25 °C. The mobile phase consisted of water containing 0.1% formic acid (A) and acetonitrile (B). The gradient elution program was optimized for the separation, and the program was as follows: 0–2 min, 25–40% B; 2–5 min, 40–90% B; 5–8 min, 90–95% B. The column was returned to its starting conditions in 1 min and gradient elution was carried out after pre-equilibration for 6 min. The flow rate of the mobile phase was set to 0.8 mL min−1, and the injection volume was 10 μL. Data acquisition was carried out using Analyst software (version 1.6.2) from Applied Biosystems/MDS Sciex. The typical extracted-ion chromatograms (XIC) of MRM chromatograms of standards and samples obtained are shown in Fig. 1.
Fig. 1. Representative extraction chromatograms (XIC) of multiple-reaction monitoring (MRM) chromatograms of linarin, rutin, luteolin, quercetin, apigenin, acacetin and acteoside (A) standard and (B) Buddleja lindleyana Fort. extract; (C) monitored MRM transitions of the seven standards; (D) chemical structure, declustering potential (DP) and collision energy (CE).
Negative electrospray ionization mode was used for detecting analytes by API 4000+ MS. The following MS/MS conditions were used: ion spray voltage, −4.5 kV; the turbo spray temperature, 550 °C. Nitrogen was used as the nebulizer and auxiliary gas. Furthermore, the flows of the nebulizer gas (gas 1), heater gas (gas 2) and curtain gas were set to 55, 50, and 25 psi, respectively. The declustering potential (DP) and collision energy (CE) of all analytes are summarized in Fig. 1.
2.3. Preparation of Buddleja lindleyana Fort. extract
For quantitative analysis in different batches of medical materials, the powdered Buddleja lindleyana Fort. (1.0 g, 60 mesh sieve) was approximately weighed and placed in a 50 mL conical flask with a cover, then, 25 mL of 70% ethanol was precisely added and both were accurately weighed. After ultrasound treatment for 40 min, the lost weight was made up using 70% ethanol. The resultant was filtered through a 0.22 μm millipore filter and 10 μL was used for HPLC-ESI-MS/MS analysis. Buddleja lindleyana Fort. materials from ten different sources were treated in the same way, which were used for quantitative analysis.
For pharmacokinetics study, Buddleja lindleyana Fort. (Xizang) was cut up and soaked for 12 h with 70% ethyl alcohol.37 The ratios of medicinal materials and 70% ethyl alcohol were 1 : 15, 1 : 10 and 1 : 10, respectively. The medicinal materials were extracted three times by heating under reflux. The extraction solutions were filtered and merged, and were concentrated by reducing the pressure. Finally, the residuary solution was concentrated to get the Buddleja lindleyana Fort. extract with a concentration equivalent to 2.5 g mL−1 of the raw Buddleja lindleyana Fort. material. The content of linarin, rutin, luteolin, quercetin, apigenin, acacetin and acteoside were 14 828.24, 422.1, 510.99, 336.45, 665.01, 1840.24 and 1555.87 ng mL−1, respectively. For the PK study, Buddleja lindleyana Fort. (Xizang) was treated with this method.
2.4. Preparation of standard solutions and quality control (QC) samples
The appropriate amounts of linarin, rutin, luteolin, quercetin, apigenin, acacetin, acteoside and sulfamethoxazole were accurately weighed and dissolved in methanol to make stock solutions. The concentrations of each standard in the stock solutions were 0.91, 1.05, 0.92, 1.08, 0.88, 0.65, 0.94 and 0.88 mg mL−1, which were diluted with methanol to plot the standard curve for quality control of Buddleja lindleyana Fort.
For the PK study, the mixed standard solution was made by mixing the seven stock solutions, which contained 2894.93 ng mL−1 of linarin, 410.28 ng mL−1 of rutin, 248.68 ng mL−1 of luteolin, 65.52 ng mL−1 of quercetin, 467.72 ng mL−1 of apigenin, 104.68 ng mL−1 of acacetin and 2077.28 ng mL−1 of acteoside. A series of standard mixture working solutions with concentrations in the range of 6.85–14 474.65 ng mL−1 for linarin, 6.05–2051.40 ng mL−1 for rutin, 7.90–1243.40 ng mL−1 for luteolin, 6.60–327.60 ng mL−1 for quercetin, 8.85–2338.60 ng mL−1 for apigenin, 11.60–523.40 ng mL−1 for acacetin, and 10.15–10 386.40 ng mL−1 for acteoside, were obtained by the attenuation of stock standard solutions with methanol. The concentration of the IS (sulfamethoxazole) standard solution was 1.18 μg mL−1, which was dissolved in methanol.
Working solutions of the corresponding concentrations (20 μL) and IS solution (20 μL) were added to 100 μL of blank rat plasma. The ranges of the final plasma concentrations were 1.37–2894.93 ng mL−1 for linarin, 1.21–410.28 ng mL−1 for rutin, 1.58–248.68 ng mL−1 for luteolin, 1.32–65.52 ng mL−1 for quercetin, 1.77–467.72 ng mL−1 for apigenin, 2.32–104.68 ng mL−1 for acacetin, and 2.03–2077.28 ng mL−1 for acteoside.
The quality control (QC) samples containing low, medium and high concentrations were prepared at the concentrations of 6.85, 723.73 and 2861.18 ng mL−1 for linarin, 6.05, 102.57 and 418.53 ng mL−1 for rutin, 6.32, 62.17 and 247.58 ng mL−1 for luteolin, 2.64, 16.38 and 64.48 ng mL−1 for quercetin, 7.08, 116.93 and 468.54 ng mL−1 for apigenin, 4.62, 26.17 and 102.14 ng mL−1 for acacetin, 2.03, 519.32 and 2027.29 ng mL−1 for acteoside.
2.5. Preparation of plasma samples
All rat plasma samples were prepared by protein precipitation with methanol. IS (20 μL) and methanol (20 μL, volume of the corresponding working solution for the calibration curve and QC sample) were spiked into the plasma samples (100 μL). After that, 500 μL of methanol was added to the mixture, followed by vortexing for 5 min and centrifugation at 12 726.2×g for 10 min. The supernatant was then transferred into a 1.5 mL tube and dried under nitrogen at 37 °C, then reconstituted with 100 μL of methanol. The mixture was then vortexed for 5 min and centrifuged at 12 726.2×g for 10 min. Finally, the supernate (10 μL) was injected for HPLC-MS/MS analysis.
2.6. Method validation for the quantitative study of Buddleja lindleyana Fort
The validation guidelines for the quantitative study of Buddleja lindleyana Fort. were taken from the Pharmacopoeia of the People's Republic of China, and other literature.38–40
2.6.1. Linearity, limit of detection (LOD) and limit of quantification (LOQ)
The mixed reference solution mentioned above was diluted by the method of multiple dilutions to obtain a series of 7 different concentrations of control solutions. The standard curve equations were obtained through linear regression using the concentration (X) of the reference substance as the abscissa and the peak area (Y) as the ordinate. The correlation coefficient (R) of the regression line should not be less than 0.999. The mixed reference solution was gradually diluted and when the signal-to-noise ratio (S/N) was 3 and 10, the amount of each reference substance was regarded as the detection limit (LOD) and the limit of quantification (LOQ).
2.6.2. Accuracy
Here, 0.5 g of powdered medicinal material (Xizang) was accurately weighed, and 80%, 100%, and 120% of the reference substance for each ingredient were accurately added to the medicinal materials, 3 parts for each level. The concentrations were as follows: linarin, 7629.24, 9536.55 and 11 443.86 μg; rutin, 24.29, 30.36 and 36.44 μg; luteolin, 208.35, 260.44 and 312.52 μg; quercetin, 5.79, 7.24 and 8.69 μg; apigenin, 174.86, 218.57 and 262.29 μg; acacetin, 3290.89, 4113.61 and 4936.34 μg; acteoside, 943.75, 1179.69 and 1415.62 μg. The average recoveries were calculated by the formula: recovery (%) = (detected values − original amount)/amount spiked × 100%, and RSD (%) = (SD/mean) × 100%. The range of sample recovery was 95–105%, and the RSD value was not more than 5%.
2.6.3. Precision and stability
The intra- and inter-day precisions of the method were validated by detecting the sample solution of Buddleja lindleyana Fort. (Xizang). The intra-day precision was carried out by continuously assaying for six times, and the inter-day precision was consecutively analyzed for three days. The concentrations of seven chemical components were obtained by the corresponding calibration curves.
In order to investigate the stability of the samples, they were analyzed at 0, 2, 4, 8, 12, and 24 h at room temperature, respectively.
The RSD values of precision and stability were not more than 5%.
2.7. Method validation of the pharmacokinetics study of Buddleja lindleyana Fort. extract
The validation guidelines adopted in this study were from the Guiding Principle of ICH M10 “Validation of Bioanalytical Methodology” 2019 edition.
2.7.1. Assay specificity
Blank plasma samples were prepared from six SD male rats given water. In order to investigate the interferences of the endogenous substances in the biological sample with the analyte and the internal standard, the detection signals of the blank biological matrix, the simulated biological sample (reference substance added to the blank biological matrix) and the actual biological sample after administration were compared.37
2.7.2. Linearity and LOQ
The plasma calibration curves consisted of seven different concentration levels, and a sample of each concentration was repeatedly assayed for three days. The standard curve was made up of the plasma drug concentration of the tested substance (abscissa) and the ratio of the peak area of the tested substance to the internal standard substance (ordinate), which was calculated using the weighted least squares method (W = 1/χ2) for linear regression.41 The correlation coefficient (R) of the regression line should not be less than 0.99.
The lower limit of quantification (LLOQ, S/N = 10) of the assay served as the lowest concentration of the standard curve.42 The LLOQ should be less than 10–5% of Cmax. The accuracy of six samples continuously determined should be 80–120%, and the RSD values should be less than 20%.
2.7.3. Accuracy and precision
The accuracy and precision of the method were evaluated by detecting different concentrations of the QC sample (low, medium, and high concentration levels).43 The QC sample and standard curve were continuously measured for three days. The precision and accuracy were expressed as the relative standard deviation (RSD) and relative error (RE), respectively. Accuracy was expressed in terms of the relative error (SE).SE = (measured value − actual value)/actual value × 100%RSD = SD/X × 100%; SD = Sqr (∑Xn − X)2/(n − 1) (SD – standard deviation; X – mean; n – number of samples)
The intra- and inter-day RSD of high and medium concentrations were less than 15%, and the intra- and inter-day RSD of the low concentration was less than 20%. The RE of high and medium concentrations were less than ±15%, and the low concentration was not more than ±20%.
2.7.4. Stability
Six replicate QC samples (low, medium and high concentration levels) exposed to different conditions were detected to evaluate the stability. The post-preparation stability tests were investigated by exposing the samples at room temperature for 4 h. The short-term stability tests, long-term stability tests and freeze-thaw stability tests were evaluated by exposing the samples at room temperature for 8 h, storing the samples for 21 days at −20 °C and three freeze (−20 °C)–thaw (room temperature) cycles on three consecutive days.37 The stability was expressed as the relative error (accuracy). The accuracy of the stability in high and medium concentrations should be less than ±15%, and the accuracy in low concentrations should not more than ±20%.
2.7.5. Extraction recovery and matrix effect
In order to evaluate the extraction recoveries and matrix effects, QC samples of different concentration levels (low, medium, and high) were analyzed.44 The peak area ratio of analyte to IS was selected as an indication of recovery. The peak area ratios of the analytes in the plasma sample (post-treatment) to that dissolved in the pre-treatment plasma samples were calculated to determine the extraction recoveries of the analytes. The matrix effects were detected by comparing the peak areas of the analytes dissolved in the pre-treatment blank plasma sample with that of pure standard solution containing equivalent amounts of the analytes. The extraction recovery and matrix effects are generally in the range of 85–115% (generally the deviation should be less than 15%), and it should be in the range of 80–120% when the deviation is more than 15%.
2.7.6. Pharmacokinetic studies in rat plasma
Eighteen male Sprague-Dawley (SD) rats (voucher number: 210726210100183037), 10–12 weeks of age and weighing 200–220 g, were purchased from Liaoning Changsheng Biotechnological Co. Ltd (Shandong, China). The rats were fed under the conditions of standard temperature (22 ± 2 °C), humidity (55 ± 5%), and light (12 h dark–light cycle) for 3 days. All animal experiments were performed according to the guidelines of the experimental animal management committee of Hebei Medical University (Shijiazhuang, China).41,42 This study was also performed in strict accordance with the NIH guidelines for the care and use of laboratory animals (NIH publication no. 85-23 Rev. 1985) and was approved by the Institutional Animal Care and Use Committee of the National Tissue Engineering Center (Shanghai, China).43 Before the experiment, the rats were fasted for 12 h but with free watering. Eighteen rats were randomly divided into three groups, which included a blank group (six rats), medication administration group I (six rats), and medicine administration group II (six rats). The dose of administration was 15 mL kg−1. Blank group rats were administered water, and Buddleja lindleyana Fort. extract was given to the administration groups I and II. The route of administration was by intragastric injection. Blood samples of the first medicine group were collected from the angulus oculi of rats at 0, 2, 5, 10, 15, 25, 60, 90, 180 and 240 min with a tube containing heparin, and the second medicine group at 0, 5, 10, 25, 60, 90, 180, 360, 720, 1140 and 1800 min after a single oral administration. After the blood samples were centrifuged at 1789.6×g for 5 min, the plasma layer was gained and stored at −20 °C. Three methanol samples were taken before each bio-sample was injected.
A non-compartmental model was used to calculate the pharmacokinetic parameters of analytes by using the DAS 3.0 software.44,45 The PK parameters calculated in this study included the maximum concentration (Cmax), time of achieving maximum concentration (Tmax), half-time (T1/2), area of the concentration–time curve at 0–24 h (AUC0–t), area of the concentration–time curve at 0–∞(AUC0–∞) and clearance rate (CL), which were expressed as mean ± standard deviation (mean ± SD).
3. Results and discussion
3.1. Optimization of the extraction procedure
Methanol (50%, 60%, 70% 80%, 90% and 100%) and alcohol (50%, 60%, 70% 80%, 90% and 100%) of different proportions were compared in this study, which displayed that the extraction efficiency of 70% alcohol was highest. This study also compared ultrasonication with refluxing, which revealed that ultrasonic extraction was simpler and more effective for seven analytes. Four sample-solvent ratios (1 : 15, 1 : 25, 1 : 35 and 1 : 60, w/v) were compared, and the sample-solvent ratio of 1 : 25 was chosen. Different ultrasonication times (15, 30, 40, 45 and 60 min) were also compared. The results displayed that the extraction efficiency would no longer increase when the time of extraction was higher than 40 min.
3.2. Optimization of bio-sample preparation
Sample preparation is a key step for the precise analysis of bio-samples by HPLC-MS/MS. In this study, solid-phase extraction (SPE), liquid–liquid extraction (LLE) and protein precipitation (PPT) methods for sample preparation were compared. The SPE method is complicated and expensive; PPT was used to prepare bio-samples for greater extraction recovery than LLE. This study also compared different precipitants (methanol and acetonitrile) and volumes of precipitant. The results showed that 500 μL of methanol serving as the protein precipitant for 5 min could obtain the highest extraction recovery.
3.3. LC-MS/MS optimization
The precursor and product ions of seven analytes and IS were detected by infusing 200 ng mL−1 standard solution under MRM mode, respectively. The results showed that the seven chemical components and IS had higher intensities under the negative ion mode, which could be associated with the fact that these analytes were flavonoid and phenylpropanoid glycosides. Because of the stability and high abundance, the deprotonated molecular ions [M–H]− were chosen as precursor ions for the MS/MS fragmentation analysis of analytes. The optimized mass transition ion-pairs (m/z) for quantitation were 591.5/282.8 for linarin, 609.4/300.2 for rutin, 284.9/133.0 for luteolin, 300.6/151.0 for quercetin, 268.8/116.9 for apigenin, 283.0/267.9 for acacetin, 623.3/160.7 for acteoside, and 252.2/155.8 for IS, which are shown in Fig. 1.
Different mobile phases (methanol–water and acetonitrile–water) were compared in order to obtain a better peak shape and shorter elution time, which showed that the effect of using acetonitrile–water as the mobile phase was better. Moreover, different concentrations of formic acid (0.1%, 0.2%, 0.5%, 0.01%, 0.02% and 0.05%) were compared. Finally, acetonitrile–water (0.1% formic acid) was chosen as the mobile phase in this study.
3.4. Method validation for the quantitative study of seven analytes
3.4.1. Linearity, LOD and LOQ
The linearity, LOD and LOQ of seven chemical compounds are presented in Table 2. The linearities were good, with coefficients of association over 0.9994.
Regression, LODs and LOQs for the investigated compounds.
| Component | Regression equation | r | Linear range/(μg mL−1) | LOD (ng mL−1) | LOQ (ng mL−1) |
|---|---|---|---|---|---|
| Linarin | Y = 1999X + 5742 | 0.999 8 | 10.24–655.38 | 0.40 | 1.30 |
| Rutin | Y = 14 952X − 2106 | 0.999 4 | 0.01875–2.360 | 0.37 | 1.02 |
| Luteolin | Y = 14 102X + 15 678 | 0.999 7 | 0.02625–26.75 | 0.27 | 0.96 |
| Quercetin | Y = 29 099X + 295.1 | 0.999 8 | 0.003875–2.47 | 0.38 | 1.00 |
| Apigenin | Y = 30 536X + 12 621 | 0.999 5 | 0.02625–13.62 | 0.34 | 1.02 |
| Acacetin | Y = 11 197X + 79 447 | 0.999 4 | 1.400–212.2 | 0.32 | 0.74 |
| Acteoside | Y = 32 671X + 19 740 | 0.999 6 | 2.750–352.5 | 0.27 | 0.66 |
3.4.2. Precision, stability and accuracy
The results for the precision, stability and accuracy are summarized in Table 3. The intra- and inter-day RSD values were in the range of 0.56–2.17% and 1.75–4.01%. The recoveries of analytes ranged from 98.02–101.5%, and the value of RSD was less than 1.31. Besides, the value of RSD for stability was less than 4.02%. These results indicated that the method possessed high sensitivity.
Precision, stability and recovery of seven analytes.
| Analyte | Precision (n = 6) | Accuracy (n = 3) | ||||||
|---|---|---|---|---|---|---|---|---|
| Intra-day RSD (%) | Inter-day RSD (%) | Stability (%) | Original quantity (μg) | Spiked quantity (μg) | Detected (μg) | Recovery (%) | RSD (%) | |
| Linarin | 1.82 | 2.39 | 2.19 | 9536.98 | 7629.24 | 17 141.04 | 99.67 | 1.09 |
| 9536.55 | 18 934.30 | 98.54 | 0.98 | |||||
| 11 443.86 | 20 909.89 | 99.38 | 0.82 | |||||
| Rutin | 1.03 | 3.39 | 3.02 | 30.38 | 24.29 | 54.53 | 99.43 | 0.79 |
| 30.36 | 60.31 | 98.57 | 0.48 | |||||
| 36.44 | 66.85 | 100.1 | 1.17 | |||||
| Luteolin | 1.79 | 2.94 | 1.55 | 260.52 | 208.35 | 466.23 | 98.73 | 0.56 |
| 260.44 | 520.41 | 99.79 | 0.64 | |||||
| 312.52 | 569.67 | 98.92 | 0.39 | |||||
| Quercetin | 0.56 | 1.75 | 3.68 | 7.24 | 5.79 | 13.12 | 101.5 | 0.84 |
| 7.24 | 14.34 | 98.02 | 1.03 | |||||
| 8.69 | 15.86 | 99.26 | 0.98 | |||||
| Apigenin | 2.17 | 3.88 | 3.79 | 218.59 | 174.86 | 391.79 | 99.05 | 0.67 |
| 218.57 | 438.92 | 100.8 | 1.13 | |||||
| 262.29 | 476.82 | 98.45 | 0.78 | |||||
| Acacetin | 1.98 | 4.01 | 4.02 | 4113.61 | 3290.89 | 7380.15 | 99.26 | 1.27 |
| 4113.61 | 8187.73 | 99.04 | 1.31 | |||||
| 4936.34 | 9049.95 | 100.0 | 0.59 | |||||
| Acteoside | 1.64 | 3.22 | 2.37 | 1179.68 | 943.75 | 2105.59 | 98.11 | 0.46 |
| 1179.69 | 2356.54 | 99.76 | 0.63 | |||||
| 1415.62 | 2580.44 | 98.95 | 0.86 | |||||
3.4.3. The determination of seven analytes in Buddleja lindleyana Fort. from different habitats
Buddleja lindleyana Fort. from different habitats, used as Chinese medicinal materials, were determined by the optimized method. The places of origin of Buddleja lindleyana Fort. are summarized in Table 1. Peak identity was established by both the retention time compared with that of reference compounds and the characteristic transitions (precursor and product ion pair). The quantitative analysis of seven compounds was performed by using the calibration curves. The contents of seven analytes from different places are displayed in Table 4. The results indicated that the content of linarin was the highest and the contents of seven chemical compounds were discrepant because they originated from different producing areas.
Contents of the seven analytes in compound samples.
| Content (μg g−1,n = 3) | Linarin | Rutin | Luteolin | Quercetin | Apigenin | Acacetin | Acteoside |
|---|---|---|---|---|---|---|---|
| 1 | 6581.903 | 15.080 | 79.359 | 2.563 | 12.660 | 2091.259 | 837.118 |
| 2 | 9536.981 | 30.377 | 260.522 | 7.238 | 218.592 | 4113.609 | 1179.681 |
| 3 | 7967.634 | 47.280 | 134.832 | 11.376 | 104.280 | 2705.533 | 3268.070 |
| 4 | 6962.231 | 16.364 | 167.611 | 36.405 | 67.599 | 2907.661 | 673.124 |
| 5 | 7700.850 | 19.088 | 462.755 | 2.710 | 120.592 | 2625.502 | 999.367 |
| 6 | 5341.558 | 6.743 | 1.071 | 0.825 | 2.441 | 1530.265 | 445.744 |
| 7 | 5363.830 | 6.263 | 1.723 | 0.525 | 2.690 | 102.032 | 981.599 |
| 8 | 5713.082 | 23.612 | 14.470 | 1.842 | 21.240 | 645.446 | 6949.254 |
| 9 | 5908.544 | 13.550 | 28.033 | 0.898 | 63.274 | 879.504 | 1571.813 |
| 10 | 6529.122 | 8.779 | 54.185 | 0.440 | 35.725 | 413.231 | 606.707 |
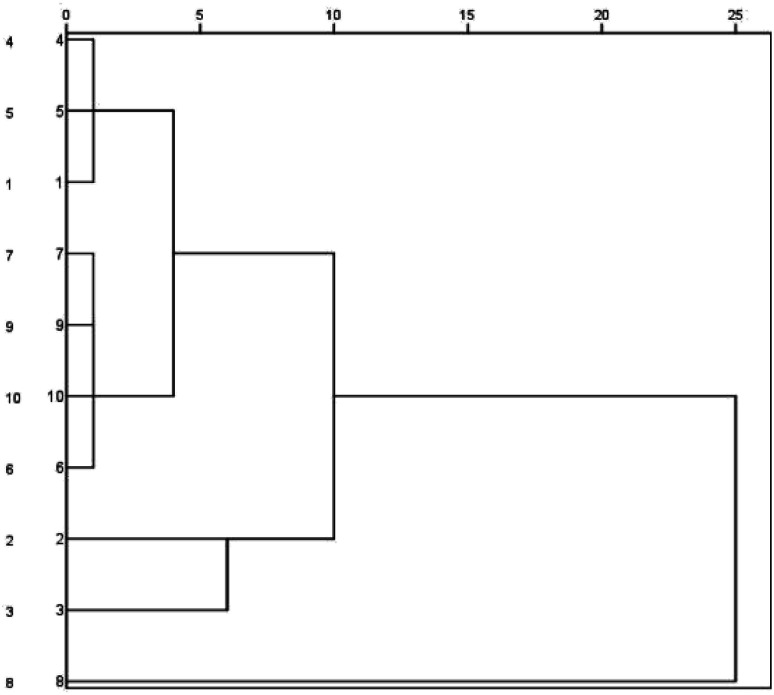
Cluster analysis was performed for Buddleja lindleyana Fort. from ten habitats, as displayed in Fig. 2. The results showed that Buddleja lindleyana Fort. from ten habitats were divided into five classes (Class I: 1, 4 and 5; Class II: 6, 7, 9 and 10, Class III: 2, Class IV: 3 and Class V: 8). Buddleja lindleyana Fort. from Tibet (Xizang) was used as an extract for intragastric administration to perform the PK study. Buddleja lindleyana Fort. material from Xizang was selected as the representative plant for pharmacokinetic study because of its highest content of the main compounds. The results revealed that the contents of compounds in Buddleja lindleyana Fort. were associated with their places of origin and planting environments, which would guide the safety and rationalization of clinical use of traditional Chinese medicine.
Fig. 2. Dendrograms of hierarchical clustering for the ten batches of samples of Buddleja lindleyana Fort. from different sources using the furthest neighbour method.
3.5. Method validation of PK study of Buddleja lindleyana Fort.
3.5.1. Specificity and selectivity
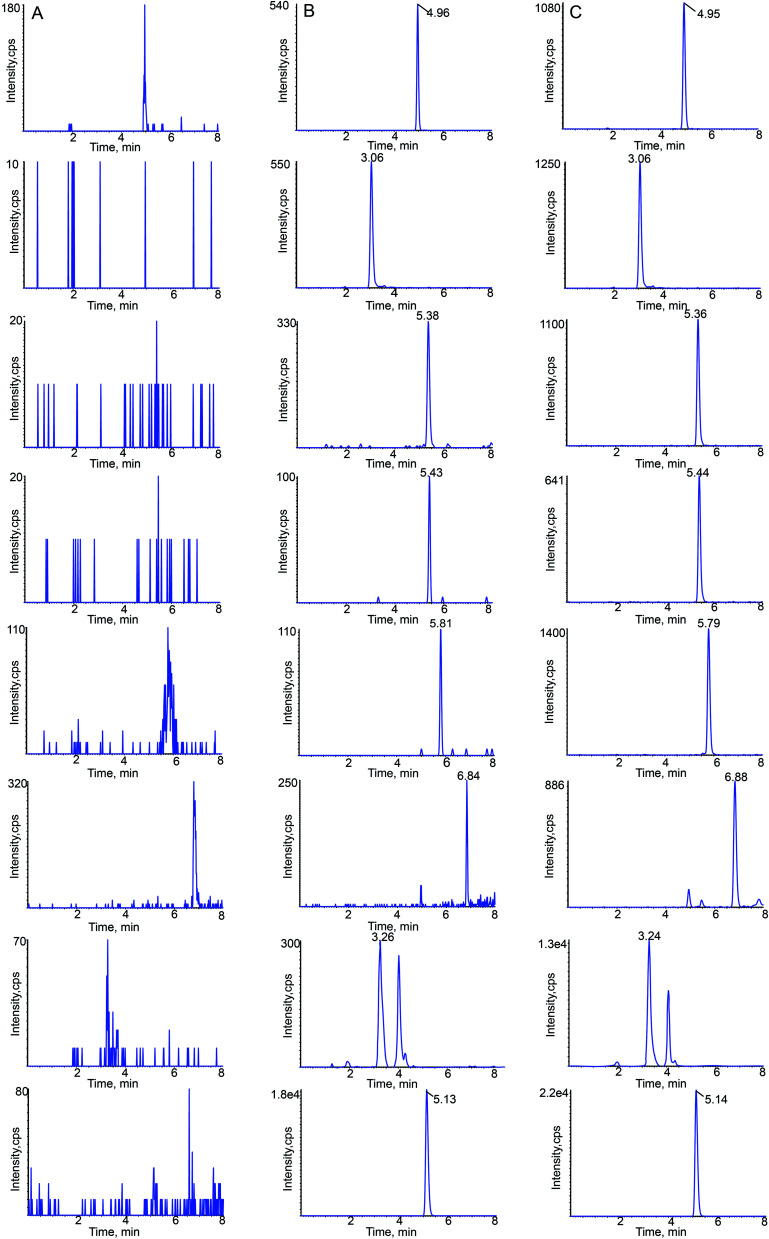
MRM chromatograms of blank rat plasma, blank plasma spiked with the analytes and IS and plasma samples at 15 min after oral administration of Buddleja lindleyana Fort. extract are shown in Fig. 3. The retention times of linarin, rutin, luteolin, quercetin, apigenin, acacetin, acteoside and IS were 4.96, 3.06, 5.38, 5.43, 5.81, 6.84, 3.26 and 5.13 min, respectively.
Fig. 3. Typical chromatograms of linarin, rutin, luteolin, quercetin, apigenin, acacetin, acteoside and sulfamethoxazole (IS) in rat plasma: (A) blank plasma; (B) blank plasma spiked with the seven analytes at LLOQ and IS; (C) 15 min sample plasma after a single oral administration of Buddleja lindleyana Fort. extract.
3.5.2. Linearity and LLOQ
The calibration curves and LLOQs of the analytes are summarized in Table 5. The calibration curves presented good linearities over the validated concentration ranges. The correlation coefficient of each canonical plot was higher than 0.9974.
The regression equations, linear range and LLOQs of seven components from Buddleja lindleyana Fort. for pharmacokinetic studies.
| Analyte | Regression equation | R 2 | Linear range (ng mL−1) | LLOQ (ng mL−1) |
|---|---|---|---|---|
| Linarin | Y = 0.0008X + 0.0034 | 0.9998 | 1.37–2894.93 | 1.37 |
| Rutin | Y = 0.0024X + 0.0001 | 0.9989 | 1.21–410.28 | 1.21 |
| Luteolin | Y = 0.2120X + 0.1311 | 0.9979 | 1.58–248.68 | 1.58 |
| Quercetin | Y = 0.0259X + 0.0007 | 0.9974 | 1.32–65.52 | 1.32 |
| Apigenin | Y = 0.0085X + 0.0002 | 0.9981 | 1.77–467.72 | 1.77 |
| Acacetin | Y = 0.0545X + 0.0232 | 0.9978 | 2.32–104.68 | 2.32 |
| Acteoside | Y = 0.0166X − 0.0056 | 0.9989 | 2.03–2077.28 | 2.03 |
3.5.3. Accuracy and precision
The intra- and inter-day precisions and accuracies of the seven analytes are shown in Table 6. The RSD values of intra- and inter-day precisions were in ranges of 3.47–9.16% and 3.19–8.75%, respectively. The accuracies of intra- and inter-day ranged from 1.39% to 5.68% and 1.65% to 8.63%, respectively. The results indicated that the method was accurate, reliable and reproducible.
The intra-day and inter-day accuracies and precisions of seven components in rat plasma at low, medium, and high concentration levels (n = 6).
| Compounds spiked concentration (ng mL−1) | Intra-day (n = 6) | Inter-day (n = 6) | ||||
|---|---|---|---|---|---|---|
| Measured concentration (ng mL−1) | Accuracy (%) | Precision (%) | Measured concentration (ng mL−1) | Accuracy (%) | Precision (%) | |
| Linarin | ||||||
| 6.85 | 6.97 ± 1.78 | 1.75 | 8.19 | 7.05 ± 3.36 | 2.92 | 4.57 |
| 723.73 | 735.74 ± 3.45 | 1.66 | 5.04 | 736.68 ± 4.34 | 1.79 | 3.19 |
| 2861.18 | 2969.33 ± 5.86 | 3.78 | 7.93 | 2958.17 ± 2.01 | 3.39 | 4.86 |
| Rutin | ||||||
| 6.05 | 6.20 ± 4.24 | 2.48 | 3.47 | 6.15 ± 6.19 | 1.65 | 3.67 |
| 102.57 | 106.55 ± 5.83 | 3.88 | 6.23 | 105.08 ± 7.42 | 2.45 | 8.25 |
| 418.53 | 424.81 ± 9.09 | 1.50 | 5.01 | 438.12 ± 4.89 | 4.68 | 5.62 |
| Luteolin | ||||||
| 6.32 | 6.60 ± 1.07 | 4.43 | 9.16 | 6.69 ± 6.09 | 5.85 | 7.47 |
| 62.17 | 64.11 ± 3.33 | 3.12 | 7.31 | 64.27 ± 5.12 | 3.38 | 4.85 |
| 247.58 | 252.68 ± 4.47 | 2.06 | 6.04 | 253.40 ± 8.48 | 2.35 | 5.79 |
| Quercetin | ||||||
| 2.64 | 2.79 ± 3.14 | 5.68 | 6.85 | 2.78 ± 7.04 | 5.30 | 8.74 |
| 16.38 | 16.84 ± 4.57 | 2.81 | 4.16 | 17.14 ± 6.46 | 4.64 | 7.88 |
| 64.48 | 65.38 ± 6.81 | 1.40 | 3.58 | 68.70 ± 8.43 | 6.54 | 6.89 |
| Apigenin | ||||||
| 7.08 | 7.27 ± 4.13 | 2.68 | 5.75 | 7.41 ± 6.44 | 4.66 | 4.69 |
| 116.93 | 121.70 ± 3.49 | 4.08 | 5.89 | 123.76 ± 8.66 | 5.84 | 6.59 |
| 468.54 | 485.55 ± 5.23 | 3.63 | 7.51 | 480.49 ± 5.18 | 2.55 | 5.86 |
| Acacetin | ||||||
| 4.62 | 4.71 ± 3.15 | 1.95 | 7.48 | 4.93 ± 7.14 | 6.71 | 6.46 |
| 26.17 | 27.24 ± 4.65 | 4.09 | 4.46 | 27.14 ± 4.68 | 3.71 | 4.61 |
| 102.14 | 105.46 ± 7.65 | 3.25 | 5.17 | 107.08 ± 6.23 | 4.84 | 3.83 |
| Acteoside | ||||||
| 2.03 | 2.14 ± 8.18 | 5.42 | 5.58 | 2.13 ± 8.02 | 4.93 | 5.79 |
| 519.32 | 536.20 ± 7.54 | 3.25 | 4.52 | 564.14 ± 9.98 | 8.63 | 7.98 |
| 2027.29 | 2167.78 ± 6.25 | 1.39 | 6.93 | 2100.48 ± 5.24 | 3.61 | 8.75 |
3.5.4. Stability
The results of post-preparative stability, short-term temperature stability, long-term stability and freeze-thaw stability are shown in Table 7, which suggest that all the bio-samples were stabilized under the conditions of existing storage.
The stability of the seven flavonoids in rat plasma (n = 3).
| Compounds spiked concentration (ng mL−1) | Short-tern stability (room temperature for 8 h) | Long-tern stability (−20 °C for 21 d) | Free-thaw stability (3 free thaw cycles) | Post-preparation stability (room temperature for 4 h) | ||||
|---|---|---|---|---|---|---|---|---|
| Measured concentration (ng mL−1) | Accuracy (%) | Measured concentration (ng mL−1) | Accuracy (%) | Measured concentration (ng mL−1) | Accuracy (%) | Measured concentration (ng mL−1) | Accuracy (%) | |
| Linarin | ||||||||
| 6.85 | 7.07 ± 1.76 | 3.21 | 7.10 ± 17.48 | 3.65 | 7.02 ± 0.73 | 2.48 | 7.22 ± 1.36 | 5.40 |
| 723.73 | 755.28 ± 10.77 | 4.36 | 766.72 ± 17.29 | 5.94 | 754.99 ± 16.54 | 4.32 | 740.38 ± 7.66 | 2.30 |
| 2861.18 | 2930.71 ± 17.78 | 2.43 | 3056.88 ± 21.55 | 6.84 | 2962.18 ± 18.46 | 3.53 | 2986.21 ± 12.59 | 4.37 |
| Rutin | ||||||||
| 6.05 | 6.36 ± 6.81 | 5.12 | 6.31 ± 5.88 | 4.30 | 6.2 ± 1.88 | 2.48 | 6.26 ± 0.48 | 3.47 |
| 102.57 | 108.48 ± 7.41 | 5.76 | 107.37 ± 9.98 | 4.68 | 110.30 ± 8.24 | 7.54 | 106.61 ± 13.32 | 3.94 |
| 418.53 | 426.82 ± 18.15 | 1.98 | 429.20 ± 19.79 | 2.55 | 437.7 ± 10.02 | 4.58 | 440.50 ± 16.46 | 5.25 |
| Luteolin | ||||||||
| 6.32 | 6.76 ± 0.18 | 6.96 | 6.7 ± 0.76 | 6.01 | 6.60 ± 0.18 | 4.43 | 6.50 ± 0.16 | 2.85 |
| 62.17 | 64.88 ± 0.92 | 4.36 | 67.34 ± 3.63 | 8.32 | 66.03 ± 2.64 | 6.21 | 64.29 ± 0.37 | 3.41 |
| 247.58 | 260.55 ± 0.49 | 5.24 | 261.02 ± 4.65 | 5.43 | 258.35 ± 4.86 | 4.35 | 253.42 ± 2.77 | 2.36 |
| Quercetin | ||||||||
| 2.64 | 2.80 ± 1.62 | 6.06 | 2.72 ± 0.32 | 3.03 | 2.78 ± 0.89 | 5.30 | 2.74 ± 0.32 | 3.79 |
| 16.38 | 17.12 ± 1.22 | 4.52 | 17.27 ± 2.69 | 5.43 | 17.48 ± 1.19 | 6.72 | 16.78 ± 1.77 | 2.44 |
| 64.48 | 66.98 ± 7.47 | 3.88 | 68.36 ± 3.68 | 6.02 | 67.46 ± 2.42 | 4.62 | 66.99 ± 4.58 | 3.89 |
| Apigenin | ||||||||
| 7.08 | 7.17 ± 1.91 | 1.27 | 7.58 ± 0.51 | 7.06 | 7.34 ± 0.32 | 3.67 | 7.33 ± 0.16 | 3.53 |
| 116.93 | 123.78 ± 2.47 | 5.86 | 124.51 ± 4.02 | 6.48 | 124.68 ± 6.33 | 6.63 | 122.61 ± 7.12 | 4.86 |
| 468.54 | 490.89 ± 7.31 | 4.77 | 484.98 ± 5.09 | 3.51 | 494.36 ± 4.99 | 5.51 | 485.92 ± 3.94 | 3.71 |
| Acacetin | ||||||||
| 4.62 | 4.69 ± 0.36 | 1.52 | 4.81 ± 0.27 | 4.11 | 4.81 ± 0.22 | 4.11 | 4.78 ± 0.18 | 3.46 |
| 26.17 | 27.31 ± 5.34 | 4.36 | 26.53 ± 3.21 | 1.38 | 26.99 ± 4.36 | 3.13 | 27.25 ± 3.96 | 4.13 |
| 102.14 | 107.02 ± 3.38 | 4.78 | 105.68 ± 9.65 | 3.46 | 108.48 ± 6.51 | 6.21 | 107.29 ± 6.25 | 5.04 |
| Acteoside | ||||||||
| 2.03 | 2.11 ± 0.13 | 3.94 | 2.07 ± 0.12 | 1.97 | 2.1 ± 0.14 | 3.45 | 2.16 ± 0.35 | 6.40 |
| 519.32 | 548.82 ± 2.92 | 5.68 | 540.92 ± 9.44 | 4.16 | 544.87 ± 4.54 | 4.92 | 542.64 ± 4.56 | 4.49 |
| 2027.29 | 2150.14 ± 11.92 | 6.06 | 2167.98 ± 18.52 | 6.94 | 2132.91 ± 11.91 | 5.21 | 2140.01 ± 9.32 | 5.56 |
3.5.5. The matrix effect and extraction recovery
The evaluation of the matrix effects of bio-samples by HPLC-MS/MS is an important step for pharmacokinetic study. When the protein precipitation method was used for sample preparation, ion suppression or enhancement caused by endogenous materials may be greater than other methods.46 The results of the recovery and matrix effect of seven chemical compounds in mice are summarized in Table 8. The average extraction recoveries (RSD values less than 7.32%) of the QC sample ranged from 83.43% to 96.89%, and the matrix effects (RSD values less than 7.97%) were in the range of 80.67–101.5%. These data proved that the matrix effect could be ignored.
Mean extraction recoveries and matrix effects of the seven flavonoids and IS in rat plasma (n = 6).
| Compounds spiked concentration (ng mL−1) | Extraction recovery | Matrix effect | ||
|---|---|---|---|---|
| Mean (%) | RSD (%) | Mean (%) | RSD (%) | |
| Linarin | ||||
| 6.85 | 89.02 | 3.16 | 80.67 | 3.67 |
| 723.73 | 85.42 | 5.94 | 85.34 | 7.97 |
| 2861.18 | 87.57 | 2.95 | 91.27 | 4.45 |
| Rutin | ||||
| 6.05 | 87.68 | 2.86 | 101.5 | 3.65 |
| 102.57 | 88.75 | 5.64 | 95.10 | 4.18 |
| 418.53 | 86.76 | 3.53 | 90.13 | 5.29 |
| Luteolin | ||||
| 6.32 | 83.43 | 4.42 | 83.56 | 3.13 |
| 62.17 | 96.21 | 6.57 | 85.86 | 6.71 |
| 247.58 | 88.84 | 7.32 | 87.47 | 7.16 |
| Quercetin | ||||
| 2.64 | 87.39 | 2.64 | 96.49 | 5.48 |
| 16.38 | 88.64 | 4.89 | 88.77 | 4.23 |
| 64.48 | 90.05 | 7.13 | 97.64 | 6.26 |
| Apigenin | ||||
| 7.08 | 89.77 | 2.39 | 101.2 | 5.87 |
| 116.93 | 87.16 | 3.42 | 93.28 | 2.16 |
| 468.54 | 96.89 | 4.08 | 90.09 | 1.93 |
| Acacetin | ||||
| 4.62 | 85.48 | 4.42 | 84.07 | 2,67 |
| 26.17 | 89.66 | 7.21 | 87.59 | 5.26 |
| 102.14 | 84.06 | 2.95 | 91.28 | 4.98 |
| Acteoside | ||||
| 2.03 | 89.10 | 3.95 | 87.31 | 5.18 |
| 519.32 | 90.69 | 4.78 | 84.23 | 7.34 |
| 2027.29 | 85.25 | 5.23 | 90.79 | 5.87 |
| Sulfamethoxazole (IS) | ||||
| 476 | 98.41 | 3.78 | 95.67 | 3.28 |
3.5.6. Pharmacokinetic study
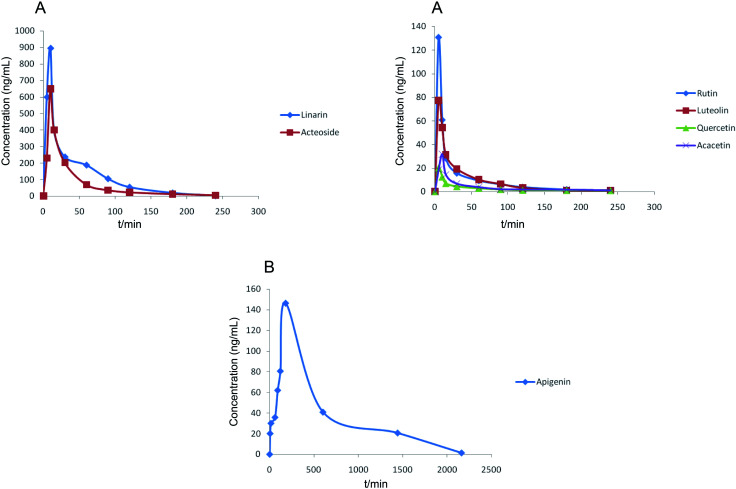
The developed and validated HPLC-MS/MS method was successfully used for the determination of seven chemical compounds in rat plasma after oral administration of Buddleja lindleyana Fort. extract. The mean plasma concentration–time profiles of the seven investigated components are shown in Fig. 4 and the pharmacokinetic parameters are listed in Table 9.
Fig. 4. Mean plasma concentration–time curves of seven analytes after the oral administration of Buddleja lindleyana Fort. extract. (A) Linarin, rutin, luteolin, quercetin, acacetin and acteoside, (B) apigenin.
Pharmacokinetic parameters of linarin, acacetin, rutin, hesperidin, luteolin, apigenin and protocatechuic acid in the plasma of six rats after a single oral administration of C. setosum extract (n = 6).
| Compounds | C max (ng mL−1) (mean ± SD) | T max (min) | T 1/2 (min) | AUC0–t (ng L−1 h−1) (mean ± SD) | AUC0–∞ (ng L−1 h−1) (mean ± SD) | CL (L h−1 kg−1) |
|---|---|---|---|---|---|---|
| Linarin | 894.12 ± 9.34 | 10 ± 0.45 | 36.49 ± 0.07 | 28 447.88 ± 11.16 | 28 770.18 ± 27.33 | 695.164 |
| Rutin | 130.76 ± 18.33 | 5 ± 0.35 | 49.57 ± 0.18 | 3039.40 ± 26.08 | 3039.62 ± 54.07 | 6464.93 |
| Luteolin | 77.37 ± 25.72 | 5 ± 0.41 | 36.08 ± 0.24 | 2106.15 ± 28.81 | 2122.72 ± 34.23 | 9421.89 |
| Quercetin | 20.15 ± 24.85 | 5 ± 0.33 | 46.03 ± 0.09 | 558.58 ± 33.33 | 1093.45 ± 55.91 | 18 290.81 |
| Apigenin | 146.42 ± 14.88 | 180 ± 0.38 | 298.50 ± 0.16 | 85 202.18 ± 58.54 | 85 759.09 ± 32.94 | 233.21 |
| Acacetin | 31.92 ± 17.58 | 10 ± 0.35 | 96.87 ± 0.12 | 916.00 ± 51.59 | 1062.19 ± 50.61 | 18 829.04 |
| Acteoside | 649.78 ± 16.42 | 10 ± 0.22 | 46.80 ± 0.35 | 18 381.44 ± 65.07 | 18 704.54 ± 59.21 | 1069.26 |
The results showed that the mean plasma concentration–time curves of the seven compounds were made up of two parts, which included (A) linarin, rutin, luteolin, quercetin, acacetin and acteoside and (B) apigenin. Linarin, rutin, luteolin, quercetin, acacetin and acteoside were rapidly absorbed, and the Tmax values were 10, 5, 5, 5, 10 and 10 min, respectively. However, the Tmax of apigenin was 180 min. The T1/2 of the seven chemical compounds were 36.49, 49.57, 36.08, 46.03, 298.50, 96.87 and 46.80 min.
The concentrations of linarin and acteoside were highest. Acteoside could be obtained by the hydrolysis of linarin after oral administration of Buddleja lindleyana Fort. extract, which indicated that the amount of acteoside absorbed in blood included the chemical compound acteoside and part of the hydrolysed linarin. A further study should be performed, such as on the metabolism and excretion of the linarin monomer. A double-peak phenomenon of apigenin appeared in Fig. 4, which was associated with entero-hepatic recirculation. The first peak of apigenin appeared at 5 min, and the second at 180 min, which is higher than the first. In general, the results of this study might be helpful for the study of metabolism, excretion and activity screening after oral administration of Buddleja lindleyana Fort. extract and monomer linarin, which would be beneficial for the application of Buddleja lindleyana Fort. in clinical therapy.
4. Conclusion
Herein, a powerful analytical and sensitive HPLC-MS/MS method was developed for the simultaneous determination of seven chemical compounds of Buddleja lindleyana Fort., and PK studies were conducted after oral administration of Buddleja lindleyana Fort. extract. This is the first quantitative and pharmacokinetic study of Buddleja lindleyana Fort via HPLC-MS/MS. The excellent selectivity, sensitivity, precision, accuracy and extraction recovery proved that the method is fast and highly efficient. This study revealed that the contents of seven chemical compounds were different because of different growth environments. In general, the highest contents of the seven chemical compounds were found in Buddleja lindleyana Fort. obtained from Xizang, so this was chosen for conducting the PK study. Linarin, rutin, luteolin, quercetin, acacetin and acteoside were rapidly absorbed in the blood, while apigenin was absorbed very slowly. The main absorption substances after the administration of Buddleja lindleyana Fort. extract were linarin and acteoside. The maximum plasma concentrations (Cmax) of linarin, rutin, luteolin, quercetin, apigenin, acacetin and acteoside were 894.12 ± 9.34 ng mL−1, 130.76 ± 18.33 ng mL−1, 77.37 ± 25.72 ng mL−1, 20.15 ± 24.85 ng mL−1, 146.42 ± 14.88 ng mL−1, 31.92 ± 17.58 ng mL−1 and 649.78 ± 16.42 ng mL−1, respectively. The times to reach Cmax of linarin, rutin, luteolin, quercetin, apigenin, acacetin and acteoside were 10, 5, 5, 5, 180, 10, and 10 min, respectively. A double-peak phenomenon appeared in the drug–time curve of apigenin. These results might be helpful for investigating the bioactive mechanism and clinical application of Buddleja lindleyana Fort.
Conflicts of interest
All the authors have declared no conflict of interest.
Supplementary Material
Acknowledgments
The work received financial support from Hebei Administration of Traditional Chinese Medicine (No. 2021147), and the Natural Science Foundation of Hebei Province of China (H2020206091).
Electronic supplementary information (ESI) available: The validation information on the content analysis. Table repeatability and matrix effect of seven analytes. See DOI: 10.1039/d1ra04154a
References
- Flora of China Editorial Committee, Flora Reipublicae Popularis Sinicae. Science Press, Beijing, 1992, vol. 61, p. 308 [Google Scholar]
- Chen G. Sun W. B. Sun H. Bot. J. Linn. Soc. 2007;154:305–312. doi: 10.1111/j.1095-8339.2007.00650.x. [DOI] [Google Scholar]
- Zhang W. Li Z. Xu F. Q. Ren Y. S. Xu S. W. Wang T. S. Liu J. S. Wu D. L. J. Asian Nat. Prod. Res. 2019;21(5):426–434. doi: 10.1080/10286020.2018.1516211. [DOI] [PubMed] [Google Scholar]
- Jiangsu new medical college, Traditional Chinese medicine dictionary. Vol II: Shanghai Scientific and Technical Publisher. Shanghai, 1986, p. 3575 [Google Scholar]
- Wu P. Y. Ren Y. S. Wu D. L. Qiao J. W. China J. Chin. Mater. Med. 2016;41:1218–1221. doi: 10.4268/cjcmm20160710. [DOI] [PubMed] [Google Scholar]
- Mensan A. Y. J. Nat. Prod. 2000;2000(63):1210–1213. doi: 10.1021/np0001023. [DOI] [PubMed] [Google Scholar]
- Lu J. Pu X. Li Y. Zhao Y. Tu G. Z. Naturforsch., B: J. Chem. Sci. 2005;60:211–214. doi: 10.1515/znb-2005-0214. [DOI] [Google Scholar]
- Yoshida T. Nobuhara J. Fuji N. Okuda T. Chem. Pharm. Bull. 1978;26:2543. doi: 10.1248/cpb.26.2543. [DOI] [Google Scholar]
- Guo H. Z. Kazuo K. Li W. Tadaaki S. Guo D. A. Tamotsu N. J. Nat. Prod. 2004;67:10–13. doi: 10.1021/np0300131. [DOI] [PubMed] [Google Scholar]
- Wu D. L. Wang Y. K. Liu J. S. Wang X. C. Zhang W. J. Asian Nat. Prod. Res. 2012;14:342–347. doi: 10.1080/10286020.2011.653783. [DOI] [PubMed] [Google Scholar]
- Emam A. M. Elias R. Moussa A. M. Faure R. Debrauwer L. Balansard G. Phytochemistry. 1998;48:739–742. doi: 10.1016/S0031-9422(97)01043-1. [DOI] [PubMed] [Google Scholar]
- Lu J. H. Pu X. P. Tu G. Z. Zhao Y. P. J. Chin. Pharm. Sci. 2004;13:151–154. [Google Scholar]
- Tai B. H. Nhiem N. X. Quang T. H. Ngan N. T. T. Tung N. H. Kim Y. Lee J. J. Myung C. S. Cuong N. M. Kim Y. H. Bioorg. Med. Chem. Lett. 2011;21:3462–3466. doi: 10.1016/j.bmcl.2011.03.078. [DOI] [PubMed] [Google Scholar]
- Acevedo J. G. A. Castaneda C. M. C. Benitez F. J. C. Duran D. A. Barroso V. R. Martinez C. G. Munoz L. J. L. Martinez C. A. De Vivar A. R. Fitoterapia. 2005;76:301–309. doi: 10.1016/j.fitote.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Moreira L. N. Feltrin C. Gonçalves J. E. de Castro W. V. Simoes C. M. O. de Pádua R. M. Cortes S. F. Braga F. C. Biomed. Pharmacother. 2020;132:110900. doi: 10.1016/j.biopha.2020.110900. doi: 10.1016/j.biopha.2020.110900. [DOI] [PubMed] [Google Scholar]
- Nijstad A. L. Tibben M. M. Gebretensae A. Rosing H. de Vos-Kerkhof E. Zwaan C. M. Huitema A. D. R. Beijnen J. H. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2021;1171:122639–122645. doi: 10.1016/j.jchromb.2021.122639. [DOI] [PubMed] [Google Scholar]
- Zhang X. Yin J. T. Liang C. J. Sun Y. P. Zhang L. T. J. Agric. Food Chem. 2017;65:10959–10972. doi: 10.1021/acs.jafc.7b04265. [DOI] [PubMed] [Google Scholar]
- Feng R. Zhang X. W. Yin J. T. Zhang Y. Q. Ma Y. L. Zhang X. Zhang L. T. Li D. Q. J. Pharm. Biomed. Sci. 2021;197:113905. doi: 10.1016/j.jpba.2021.113905. doi: 10.1016/j.jpba.2021.113905. [DOI] [PubMed] [Google Scholar]
- Zhang X. Liao M. Cheng X. Y. Liang C. J. Diao X. P. Zhang L. T. Rapid Commun. Mass Spectrom. 2018;32:1451–1461. doi: 10.1002/rcm.8161. [DOI] [PubMed] [Google Scholar]
- Liu M. Y. Zhao S. H. Wang Z. Q. Wang Y. F. Liu T. Li S. Wang C. C. Wang H. T. Tu P. F. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2014;949–950:115–126. doi: 10.1016/j.jchromb.2013.12.022. [DOI] [PubMed] [Google Scholar]
- Wang S. Qi P. C. Zhou N. Zhao M. M. Ding W. J. Li S. Liu M. Y. Wang Q. Jin S. M. Anal. Bioanal. Chem. 2016;408:7423–7436. doi: 10.1007/s00216-016-9828-x. [DOI] [PubMed] [Google Scholar]
- Shi X. W. Liu M. Zhang M. Zhang K. R. Liu S. C. Qian S. Shi R. Jiang X. J. Wang Q. Food Chem. 2013;141:357–365. doi: 10.1016/j.foodchem.2013.02.068. [DOI] [PubMed] [Google Scholar]
- Nie J. Yaro P. He K. F. Zeng S. J. Pharm. Biomed. Sci. 2019;72:78–85. doi: 10.1016/j.jpba.2019.04.004. [DOI] [PubMed] [Google Scholar]
- Yang C. P. Liu M. H. Zou W. Guan X. L. Lai L. Su W. W. J. Asian Nat. Prod. Res. 2012;14:68–75. doi: 10.1080/10286020.2011.632369. [DOI] [PubMed] [Google Scholar]
- Ge N. Kong L. Zhang A. H. Sun Y. Zhao M. Q. Zhang B. Xu L. Ke X. Sun H. Wang X. J. RSC Adv. 2021;11:5491–5505. doi: 10.1039/D0RA00343C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C. Cao J. Bao Y. Y. Sun Z. X. Liu Z. Y. Li C. Food Chem. 2021;347:129057. doi: 10.1016/j.foodchem.2021.129057. doi: 10.1016/j.foodchem.2021.129057. [DOI] [PubMed] [Google Scholar]
- Afzal M. Sielaff M. Curella V. Neerukonda M. Hassouni K. E. Schuppan D. Tenzer S. Longin C. F. H. Plants. 2021;10:424–442. doi: 10.3390/plants10030424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D. Fan W. L. Xu Y. Food Chem. 2021;353:129521. doi: 10.1016/j.foodchem.2021.129521. doi: 10.1016/j.foodchem.2021.129521. [DOI] [PubMed] [Google Scholar]
- Gao J. Shi N. Guo H. J. Gao J. F. Tang X. Yuan S. Y. Qian J. H. Wen B. Y. ACS Omega. 2021;6:5348–5358. doi: 10.1021/acsomega.0c05488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K. Jia B. J. Zhou L. P. Xing L. Wu L. Y. Li Y. Y. Lu J. G. Zhang L. Guan S. J. Proteome Res. 2021;20:1371–1381. doi: 10.1021/acs.jproteome.0c00757. [DOI] [PubMed] [Google Scholar]
- Wang X. Johansen S. S. Nielsen M. K. K. Linnet K. Drug Test. Anal. 2017;9:1137–1151. doi: 10.1002/dta.2130. [DOI] [PubMed] [Google Scholar]
- Cortese M. Delporte C. Dufour D. Noyon C. Chaumont M. De Becker B. Reye F. Rousseau A. Omer E. Neve J. Piagnerelli M. Boudjeltia K. Z. Robaye B. Van Antwerpen P. Talanta. 2019;193:206–214. doi: 10.1016/j.talanta.2018.10.003. [DOI] [PubMed] [Google Scholar]
- Parker S. L. Pandey S. Sime F. B. Lipman J. Roberts J. A. Wallis S. C. Anal. Bioanal. Chem. 2019;411:7831–7840. doi: 10.1007/s00216-019-02184-4. [DOI] [PubMed] [Google Scholar]
- Jungen H. Andresen- Streichert H. Muller A. Iwersen-Bergman S. J. Anal. Toxicol. 2017;41:22–31. doi: 10.1093/jat/bkw099. [DOI] [PubMed] [Google Scholar]
- Li Z. L Wu D. Zhao H. S. Xu F. Q. Zhang W. Chin. J. Exp. Tradit. Med. Formulae. 2017;23(6):74–77. [Google Scholar]
- Wang X. C. Lu J. H. Chin. J. Hosp. Pharm. 2018;38(10):1056–1058. [Google Scholar]
- Zhang Z. Y. Jia P. P. Zhang X. X. Zhang Q. Y. Yang H. T. Shi H. Zhang L. T. J. Ethnopharmacol. 2014;158:66–75. doi: 10.1016/j.jep.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Xu H. J. Shi X. W. Ji X. Du Y. F. Zhu H. Zhang L. T. J. Sep. Sci. 2011;34:308–316. doi: 10.1002/jssc.201000660. [DOI] [PubMed] [Google Scholar]
- Shi X. W. Wu Y. B. Lv T. Wang Y. F. Fu Y. Sun M. M. Shi Q. W. Huo C. H. Wang Q. Gu Y. C. Anal. Bioanal. Chem. 2017;409:4669–4679. doi: 10.1007/s00216-017-0413-8. [DOI] [PubMed] [Google Scholar]
- Zhang X. Liang C. J. Li C. L. Bu M. D. Bu L. Y. Xiao Y. D. Sun H. G. Zhang L. T. J. Chromatogr. Sci. 2018;56(7):582–594. doi: 10.1093/chromsci/bmy030. [DOI] [PubMed] [Google Scholar]
- Sun Y. Du Y. F. Yang K. Chang L. Cao L. Ren Y. P. Sun Q. Wang Q. Zhang L. T. Lv P. T. J. Pharm. Biomed. Sci. 2013;81:34–43. doi: 10.1016/j.jpba.2013.03.022. [DOI] [PubMed] [Google Scholar]
- Liang C. J. Zhang X. Diao X. P. Liao M. Sun Y. P. Zhang L. T. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2018;1084:69–79. doi: 10.1016/j.jchromb.2018.03.032. [DOI] [PubMed] [Google Scholar]
- Zhang X. Liang C. J. Yin J. T. Sun Y. P. Zhang L. T. RSC Adv. 2018;8:11813–11827. doi: 10.1039/C7RA13760E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L. J. Chen L. Bai C. F. Wu A. G. Liang S. C. Huang F. H. Cao S. S. Yang L. Zou W. J. Wu J. M. Biomed. Pharmacother. 2020;123:109756. doi: 10.1016/j.biopha.2019.109756. doi: 10.1016/j.biopha.2019.109756. [DOI] [PubMed] [Google Scholar]
- Du C. H. Yan Y. Shen C. X. Cui X. F. Pei X. P. Qin X. M. J. Pharm. Anal. 2020;10:385–395. doi: 10.1016/j.jpha.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai F. Xu W. Wei H. Sun L. N. Gao S. H. Yang Q. Feng J. Zhang F. Chen W. S. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2010;878:1845–1854. doi: 10.1016/j.jchromb.2010.05.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.