ABSTRACT
It is important to know the safety and efficacy of vaccination in immunocompromised people living with HIV (PLWH), but currently, there is limited data on the inactivated SARS-CoV-2 vaccines’ safety and immune responses in PLWH. In this prospective observational study, 139 PLWH and 120 healthy controls were enrolled and monitored for 21–105 days after a two-dose vaccination. The safety, anti-receptor binding domain IgG (anti-RBD-IgG) and anti-spike-IgG responses, and RBD-specific memory B cell (MBC) responses were evaluated. The overall adverse events within seven days were reported in 12.9% (18/139) of PLWH and 13.3% (16/120) of healthy controls. No serious adverse events occurred in both groups. Overall, the seroprevalence of anti-RBD-IgG in PLWH was significantly decreased (87.1% vs. 99.2%; p<0.001). The geometric mean end-point titer (GMT) of anti-RBD-IgG in PLWH was also reduced, especially in patients with CD4 counts <200 cells/µL, regardless of age, gender, or HIV viral load. GMTs of anti-RBD-IgG in both PLWH and healthy controls declined gradually over time. Similar results were also observed in the anti-spike-IgG response. The frequency of RBD-specific MBCs in PLWH decreased (p<0.05), and then remained stable over time. Lastly, through multivariate analysis, we found the factors that predicted a less robust response to inactivated vaccines in PLWH were a low CD4 count and long time interval after vaccination. In conclusion, inactivated vaccines are well-tolerated in PLWH but with low immunogenicity. Therefore, SARS-CoV-2 vaccines and booster doses should be given priority in PLWH, especially in patients with low CD4 counts.
Trial registration: ClinicalTrials.gov identifier: NCT05043129..
KEYWORDS: SARS-CoV-2 vaccine, COVID-19, PLWH, safety, humoral immune response
Introduction
The coronavirus disease 2019 (COVID-19) epidemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) began in 2019 and has continued to rage worldwide, bringing a heavy burden to global public health [1]. People living with HIV (PLWH) may be at increased risk for severe COVID-19 due to immunosuppression and higher rates of multimorbidity; therefore, these individuals may benefit from SARS-CoV-2 vaccination [2,3]. Even though several vaccines have been recommended for PLWH [4,5], their safety and efficacy in PLWH is controversial.
Several studies indicated that PLWH have good tolerance to the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2, and PLWH with well-controlled antiretroviral therapy (ART) have a similar immune response as healthy controls [6,7]. However, another study reported a suboptimal immune response in PLWH who received the Moderna vaccine [8]. PLWH with CD4 counts <200 cells/µL have diminished SARS-CoV-2 antibody production after an acute SARS-CoV-2 infection [9]. However, other studies have shown contrasting results indicating that this group can also elicit an antibody response after being vaccinated with a SARS-CoV-2 mRNA vaccine [10–12]. In addition, a case of viral activation and CD4+ T cell loss after receiving an inactivated COVID-19 vaccine in a treatment-naïve HIV-positive patient was reported by a recent study [13]. Therefore, the safety and immunogenicity of inactivated vaccines in PLWH are still unclear.
After vaccination, long-lasting humoral immunity is mediated by the antibody-secreting cells (ASCs) residing in the bone marrow and memory B cells (MBCs). When a secondary infection occurs, MBCs rapidly proliferate and differentiate into ASCs, protecting against severe disease or death [14,15]. However, no relevant data about the response of SARS-CoV-2-specific MBCs in PLWH after vaccination has been reported to date.
This study recruited 139 PLWH on stable ART and 120 healthy controls to evaluate the safety and SARS-CoV-2 RBD-IgG and spike-specific IgG response 21–105 days after receiving the inactivated vaccine. The response of MBCs and its four subpopulations were detected by flow cytometry.
Materials and methods
Participants and study design
In this prospective observational study, PLWH on stable ART from the People’s Hospital of Tongliang District, Chongqing City, and healthy controls from the health management centre of the Second Affiliated Hospital of Chongqing Medical University were recruited consecutively between 1 September 2021 and 30 November 2021. The key inclusion criteria for all individuals were: (i) 21–105 days after full-course vaccination (BBIBP-CorV [16] or Corona Vac [17]), (ii) age ≥18 years. Key exclusion criteria were: (i) history of COVID-19 infection, (ii) use of immunosuppressants within 6 months, (iii) autoimmune disease, and (iv) pregnancy.
Firstly, we conducted a cross-sectional analysis. Considering the different index dates for each vaccine recipient, we defined 21–45, 46–75, and 76–105 days as 1, 2, and 3 months, respectively, to study antibody and B cell responses over time. For the participants at 1-month, we continued follow-up until 6 months.
This study was approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University and conformed with the ethical guidelines of the Declaration of Helsinki. Written informed consent was obtained from all participants. This study has been registered at www.ClinicalTrials.gov (NCT05043129) and chictr.org.cn (ChiCTR2100050922).
Adverse events monitoring
Adverse events within 7–30 days after vaccination were self-reported by questionnaires. The classification of adverse events was based on a scale issued by the National Medical Products Administration (2019 version).
SARS-CoV-2 antibody testing
Serum samples of all participants were taken 21–105 days after full-course vaccination. The IgG antibody against spike protein receptor-binding domain (anti-RBD-IgG) was detected by indirect ELISA using a SARS-CoV-2 RBD antibody detection kit (Sino Biological, Beijing, China). The IgG antibody against spike protein (anti-spike-IgG) was detected using a SARS-CoV-2 spike protein detection kit (Sino Biological, Beijing, China). According to the manufacturer's instruction, serum was considered seropositive for IgG binding antibodies when the OD value ≥2.1 times the mean absorbance value of negative controls at 1:50 dilutions, and the antibody titers were presented as the highest serum dilution showing a positive result. In addition, the positive result for the anti-spike-IgG was >1.495 AU/mL. The detailed procedure of each antibody is shown in the Supplementary Materials.
Detection of SARS-CoV-2-specific B cells by flow cytometry
In order to detect SARS-CoV-2-specific B cells, biotin-labeled SARS-CoV-2 spike RBD protein (40592-V08H2-B; Sino Biological, Beijing, China) was mixed with Streptavidin BV421-11 (405225; Biolegend, California, CA, USA) at a molar ratio of 4:1 for 1 h to obtain an antigen probe. According to the manufacturer's instructions, peripheral blood mononuclear cells were isolated from whole heparinized blood by Histopaque (10771; Sigma-Aldrich, St Louis, MO, USA) density gradient centrifugation. After washing with flow cytometry staining (FACS) buffer (phosphate-buffered saline with 2% fetal bovine serum), staining was performed for 30 min at 4°C with an antigen probe (1:33.3) and the following binding antibodies at 1:50 dilution: anti-human CD3 (300430), anti-human CD19 (302212), anti-human CD21 (354918), and anti-human CD27 (356406) all purchased from Biolegend. After staining, the cells were re-washed and suspended in 200 µL FACS buffer. Samples were evaluated using a CytoFLEX cytometer (Beckman Coulter, Inc., Brea, CA, USA), and FlowJo software version 10.0.7r2 (Treestar Inc., Ashland, OR, USA) was used for data analysis.
Statistical analysis
Statistical analysis was performed according to the type of data. Normality assumption was checked for all continuous variables. Chi-square test and Fisher's exact test were used for categorical variables. Continuous variables were compared with Mann–Whitney U-test (for unpaired data) or Wilcoxon test (for paired data) for two groups and Kruskal–Wallis test for three groups. The results of multiple comparisons were corrected with Bonferroni. Statistical analysis was performed with IBM SPSS(version 25.0). GraphPad Prism(version 9.0.0) was used for plotting. p<0.05 was statistically different.
Results
Baseline characteristics of all participants
The median ages of PLWH and healthy controls were 55 (range: 23–81 yrs) and 54 (range: 21–83 yrs) yrs, respectively. More than half of the participants were male (64.0% [89/139] in PLWH and 60% [72/120] in healthy controls). The median time for vaccine safety and immunogenicity analysis post-vaccination was 40 (range: 23–102 days) and 41 (range: 21–105 days) days for PLWH and healthy controls, respectively. There were no significant differences between the two groups after the second dose of vaccination at 1, 2, and 3 months. Of note, PLWH received combined ART for more than one year. Most PLWH (92.8%) used one non-nucleoside reverse transcriptase inhibitor in combination with two nucleoside reverse transcriptase inhibitors (NRTIs), while the remainder received a protease inhibitor-based booster regimen, with either one (4.3%) or two (2.9%) NRTIs. Of the 139 PLWH, 18 (13%) patients had a CD4 count of <200 cells/µL and 109 (78.4%) patients had an HIV viral load of <20 copies/mL. Notably, the levels of alanine aminotransferase (23.0 U/L vs. 19.0 U/L; p<0.001) and aspartate aminotransferase (24.4 U/L vs. 20 U/L; p<0.001) were significantly higher in PLWH than those in healthy controls (Table 1).
Table 1.
Characteristics of participants.
| Variable | PLWH(n=139) | HC(n=120) | P value |
|---|---|---|---|
| Age(years)median(range) | 55(23-81) | 54(21-83) | 0.591 |
| 18-49, n (%) | 46(33.1%) | 47(39.2%) | 0.188 |
| ≥50, n (%) | 93(66.1%) | 73(60.8%) | |
| Gender | |||
| Male, n (%) | 89(64.0%) | 72(60.0%) | 0.523 |
| Female, n (%) | 50(36.0%) | 48(40.0%) | |
|
Days after 2nd dose Vaccination, median(range) |
40(23-102) | 41(21-105) | 0.148 |
| 1 month (21-45 days) (n, %) 2 month (46-75 days) (n, %) 3 month (76-105 days) (n, %) |
96(69.0%) 13(9.4%) 30(21.6%) |
70(58.3%) 23(19.2%) 27(22.5%) |
0.059 |
| ART use# | |||
| NNRTI + two NRTIs | 129(92.8%) | / | / |
| Boosted PI + one NRTI | 6(4.3%) | / | / |
| Boosted PI + two NRTIs | 4(2.9%) | / | / |
| COVID-19 vaccine | |||
| BBIBP-CorV, n (%) | 34(24.5%) | 53(44.2%) | 0.000 |
| Corona Vac, n (%) | 67(48.2%) | 61(50.8%) | |
| BBIBP-CorV + Corona Vac, n (%) | 38(27.3%) | 6(5%) | |
| CD4 count(cells/µL) | |||
| >500, n (%) | 47(33.8%) | / | / |
| 200-500, n (%) | 74(53.2%) | / | / |
| <200, n (%) | 18(13.0%) | / | / |
| Plasma HIV viral load | |||
| >20 copies/mL, n (%) | 30(21.6%) | / | / |
| <20 copies/mL, n (%) | 109(78.4%) | / | / |
| Laboratory examination | |||
| White blood cell count (10^9/L) | 5.97(3.35-11.92) | 5.46(3.52-9.86) | 0.106 |
| Lymphocyte count (10^9/L) | 1.81(0.48-7.16) | 1.84(0.97-3.48) | 0.574 |
| Platelet count (10^9/L) | 211(91-713) | 198(107-478) | 0.385 |
| Alanine aminotransferase (U/L) | 23(8.8-116.4) | 19(5-4) | 0.000 |
| Aspartate aminotransferase (U/L) | 24.4(11.4-147.9) | 20(9-38) | 0.000 |
ART, antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside or nucleotide reverse transcriptase inhibitor; PI, protease inhibitors; #The majority (92.8%) of the patients were on the efavirenz regimen, while the remainder received a protease inhibitor-based booster regimen, lopinavir plus ritonavir, and one or two NRTIs, including lamivudine, zidovudine, abacavir, or tenofovir.
Safety of inactivated SARS-CoV-2 vaccines in PLWH
As shown in Table 2, the overall incidence of adverse events within 7 days after vaccination in PLWH was 12.9% (18/139), which was similar to that of healthy controls (13.3%, [16/120]; p = 0.927). Injection site pain was the most common local adverse reaction, occurring in 8.6% (12/139) of PLWH and 7.5% (9/120) of healthy controls. Swelling, redness, and itch were uncommon in both PLWH and healthy controls (all <5%). Systemic adverse events in PLWH included fatigue and rash which were reported in three and two patients, respectively. Likewise, the healthy controls had scant systemic adverse reactions, including fatigue (0.8%, 1/120), drowsiness (2.5%, 3/120), cough (0.8%, 1/120), and abdominal pain (0.8%, 1/120). All adverse events were mild (grade 1 and 2) and resolved spontaneously within 7 days, according to the self-reports from all participants. After 30 days of observation, no new adverse events occurred in either group (Table 2).
Table 2.
Adverse events of COVID-19 vaccination in participants.
| Variable | PLWH(n=139) | HC (n=120) | P value |
|---|---|---|---|
| Overall adverse events within 7 days | 18(12.9%) | 16(13.3%) | 0.927 |
| Overall adverse events within 30 days | 18(12.9%) | 16(13.3%) | 0.927 |
| Local adverse events | |||
| Pain | 12(8.6%) | 9(7.5%) | 0.739 |
| Swelling | 1(0.7%) | 4(3.3%) | 0.284 |
| Redness | 1(0.7%) | 1(0.8%) | 1.000 |
| Itch | 1(0.7%) | 1(0.8%) | 1.000 |
| Induration | / | / | 1.000 |
| Systemic adverse events | |||
| Muscle pain | / | / | 1.000 |
| Pruritus | / | / | 1.000 |
| Rash | 2(1.4%) | / | 0.501 |
| Fatigue | 3(2.2%) | 1(0.8%) | 0.721 |
| Drowsiness | / | 3(2.5%) | 0.098 |
| Headache | / | / | 1.000 |
| Rhinorrhea | / | / | 1.000 |
| Laryngeal pain | / | / | 1.000 |
| Fever | / | / | 1.000 |
| Chill | / | / | 1.000 |
| Cough | / | 1(0.8%) | 0.463 |
| Inappetence | / | / | 1.000 |
| Abdominal pain | / | 1(0.8%) | 0.463 |
| Abdominal distension | / | / | 1.000 |
| Diarrhea | / | / | 1.000 |
| Nausea | / | / | 1.000 |
| Chest distress | / | / | 1.000 |
| Constipation | / | / | 1.000 |
| Decreased hemoglobin | / | / | 1.000 |
| Decreased platelet count | / | / | 1.000 |
| Elevated liver enzymes | / | / | 1.000 |
| Decreased albumin | / | / | 1.000 |
| Grade 3 and 4 adverse events | / | / | 1.000 |
Antibody responses to inactivated SARS-CoV-2 vaccines in PLWH
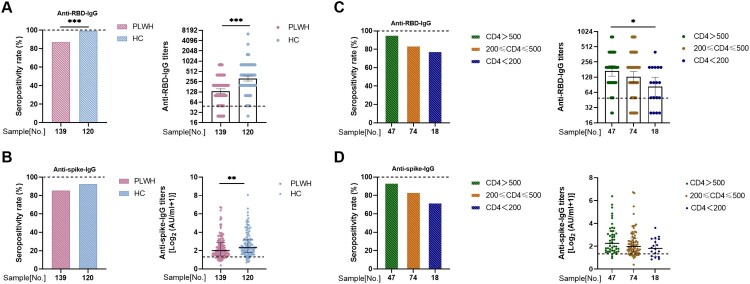
Blood samples from all subjects were collected at a single time point, between 21 and 105 days after full-course vaccination for cross-sectional analysis. Overall, the seroprevalence of anti-RBD-IgG in PLWH was significantly lower than that of healthy controls (87.1% vs. 99.2%; p<0.001). Similarly, the geometric mean end-point titers (GMTs) of anti-RBD-IgG were also significantly reduced in PLWH than in healthy controls (134.2 [95% CI: 114.0–158.0] vs. 317.5 [95% CI: 267.1–377.4]; p<0.001; Figure 1(A)). A similar trend was observed in seropositivity rate and titers of anti-spike-IgG. PLWH had lower anti-spike-IgG titers than healthy controls (2 log2 AU/mL; interquartile range [IQR: 1.51–2.85 log2 AU/mL] vs. 2.32 log2 AU/mL; IQR [1.79–3.25 log2 AU/mL]; p<0.01; Figure 1(B)). Subgroup analysis of gender and age showed that there were no differences in both seropositivity and GMTs of anti-RBD-IgG and anti-spike-IgG in PLWH (Figure S1 and S2). Further analysis of different vaccination regimens (BBIBP-CorV, Corona Vac, and BBIBP-CorV+Corona Vac) revealed a similar trend (Figure S3).
Figure 1.
Antibody responses to inactivated vaccines in people living with HIV (PLWH). The seropositivity rate and titers of (A) anti-receptor binding domain (RBD)-IgG and (B) anti-spike-IgG in PLWH and healthy controls. The seropositivity rate and titers of (C) anti-RBD-IgG and (D) anti-spike-IgG in PLWH with different CD4 count levels. The horizontal dotted lines represent the limit of detection.
To understand whether HIV CD4 counts or viral load can influence vaccine-induced antibody responses in PLWH, we performed a subgroup analysis. Among the 139 PLWH, 18, 74, and 47 patients had CD4 counts of <200, 200–500, and >500 cells/µL, respectively. PLWH with CD4 counts <200 cells/µL elicited an antibody response to the inactivated vaccines (Figure 1(C)). However, GMTs of anti-RBD-IgG in PLWH gradually decreased as CD4 counts declined, especially in the group with <200 cells/µL (170.0 [95% CI: 133.7–216.4] vs. 82.49 [95% CI: 53.2–128.0]; p<0.05). A similar trend was observed in the anti-spike-IgG response (Figure 1(D)). As shown in Figure S4, there were no differences in the GMTs of anti-RBD-IgG between different HIV viral load groups (158.7 [95% CI: 112.7–223.5] vs. 128.1 [95% CI: 106.2–154.6]; p = 0.26). Similarly, there were no significant differences in the titers of anti-spike-IgG (1.87 log2 AU/mL; IQR: 1.50–2.75 log2 AU/mL vs. 2.03 log2 AU/mL; IQR 1.54–2.90 log2 AU/mL; p = 0.44; Figure S4).
Taken together, these results indicated that the antibody response to inactivated SARS-CoV-2 vaccines in PLWH was inferior, especially in PLWH with a lower CD4 count level.
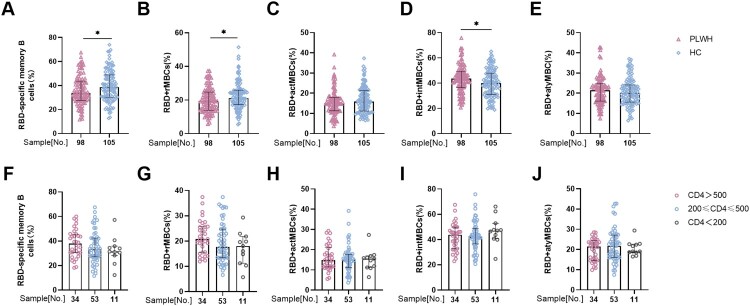
RBD-specific MBC responses to inactivated SARS-CoV-2 vaccines in PLWH
Since we proved the suboptimal antibody response in PLWH, we hypothesized that durable MBC responses were impaired in PLWH. Notably, the overall frequency of RBD-specific MBCs in PLWH was obviously lower than that in healthy controls (33.7% vs. 38.6%; p<0.05; Figure 2(A)). To further understand the function of MBCs, four subsets of MBCs, namely, activated MBCs (actMBCs), resting MBCs (rMBCs), intermediate MBCs (intMBCs), and atypical MBCs (atyMBCs), were analyzed in both groups. Interestingly, we found that the percentages of rMBCs (19.35% vs. 21.2%; p<0.05) and actMBCs (14.95 vs. 15.90; p>0.05) were lower in PLWH than those in healthy controls. In contrast, the percentages of intMBCs (43.5% vs. 39.9%; p<0.05) and atyMBCs (21.4% vs 20.1%; p>0.05) were higher in PLWH than in healthy controls (Figure 2(B–E)). The gating strategy and representative flow cytometric results are shown in Figure S5.
Figure 2.
Specific memory B cell (MBC) responses to inactivated vaccines in people living with HIV (PLWH). The frequencies of (A) receptor binding domain (RBD)-specific MBCs, (B) resting MBCs, (C) activated MBCs, (D) intermediate MBCs, and (E) atypical MBCs in PLWH and healthy controls. The frequencies of (F) RBD-specific MBCs, (G) rMBCs, (H) actMBCs, (I) intMBCs, and (J) atyMBCs in PLWH with different CD4 count levels.
In addition, we further analyzed the response of MBCs in patients with different CD4 counts. The overall frequency of MBCs in patients with CD4 <200 cells/µL was lower than in the other two groups, but the difference was not statistically significant (Figure 2(F)). In addition, the percentages of the four MBC subsets fluctuated among the three groups (Figure 2(G–J)). In summary, RBD-specific MBC responses in PLWH were also compromised.
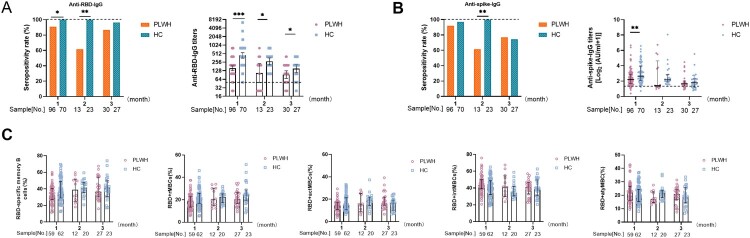
Humoral immune responses to inactivated SARS-CoV-2 vaccines in PLWH over time
To better understand the variation of humoral immune responses with passing time, we stratified three groups by time interval after full-course vaccination in the cross-sectional analysis. As expected, GMTs of anti-RBD-IgG gradually decreased over time in both PLWH and healthy controls. However, GMTs of anti-RBD-IgG in PLWH were sharply lower than that of healthy controls at every time point (1 month: 155.3 [95% CI: 128.3–188.1] vs. 441.6 [95% CI: 355–549.5], p<0.001; 2 months: 105.5 [95% CI: 48.67–228.6] vs. 278.6 [95% CI: 204.1–380.4], p<0.05; 3 months: 93.3 [95% CI: 68.66–126.8] vs. 150.8 [95% CI: 109.9–206.8], p<0.05; Figure 3(A)). Similarly, the titers of anti-spike-IgG in PLWH were lower than the titers in healthy controls at every time point (1 month: 2.21 log2 AU/mL, IQR [1.67–2.90 log2 AU/mL] vs. 2.64 log2 AU/mL, IQR [2.07–3.94 log2 AU/mL], p<0.01; 2 months: 1.42 log2 AU/mL, IQR [1.23–4.63 log2 AU/mL] vs. 2.17 log2 AU/mL, IQR [1.88–2.86 log2 AU/mL], p = 0.169; 3 months: 1.65 log2 AU/mL, IQR [1.38–2.01 log2 AU/mL] vs. 1.78 log2 AU/mL, IQR [1.21–2.29 log2 AU/mL], p = 0.786; Figure 3(B)) For RBD-specific MBCs, the frequency of MBCs at every time point was slightly lower in PLWH than in healthy controls, although this was not statistically significant. Furthermore, the frequency of MBCs was relatively stable in both PLWH and healthy controls over time. Similarly, the frequencies of the four MBC subsets persisted over time (Figure 3(C)).
Figure 3.
Antibody responses and specific memory B cell (MBC) responses to inactivated vaccines over time. The seropositivity rate and titers of anti-receptor binding domain (RBD)-IgG (A) and anti-spike-IgG (B) after 1, 2, and 3 months in people living with HIV (PLWH) and healthy controls. (C)The frequencies of RBD-specific MBCs, rMBCs, actMBCs, intMBCs, and atyMBCs after 1, 2, and 3 months in PLWH and healthy controls. The horizontal dotted lines represent the limit of detection.
In summary, the weakened antibody response in PLWH kept on falling over time, whereas the impaired MBC response did not change over time.
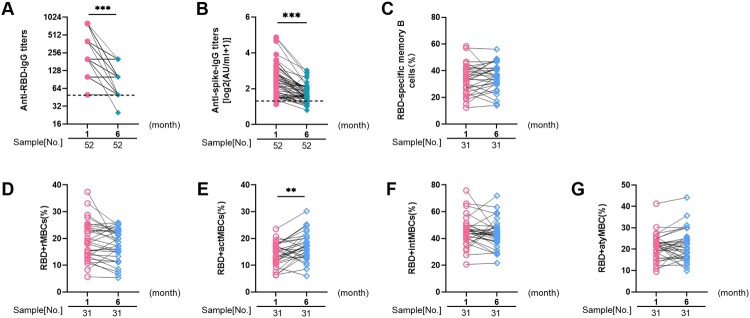
The longitudinal dynamic changes of humoral responses to inactivated SARS-CoV-2 vaccines in PLWH
To further explore the dynamic changes of antibody levels in PLWH, a longitudinal analysis was conducted in PLWH. Of the 96 PLWH observed after 1 month, 52 were followed up to the 6th month. As expected, GMTs of anti-RBD-IgG showed a clear downward trend in the 6th month compared with the 1st month (219.6 [95% CI: 179.3–268.8] vs. 97.37 [95% CI: 83.99–112.9]; p<0.001; Figure 4(A)). Similarly, the titers of anti-spike-IgG also declined (2.38 log2 AU/mL, IQR [1.72–2.98 log2 AU/mL] vs. 1.61 log2 AU/mL, IQR [1.41–2.03 log2 AU/mL]; p<0.001; Figure 4(B)). The response of longitudinal RBD-specific MBC was similar to that of cross-sectional analysis. (Figure 4(C–G)).
Figure 4.
The longitudinal dynamic changes of humoral responses to inactivated SARS-CoV-2 vaccines in people living with HIV (PLWH). The dynamic changes of anti-receptor binding domain (RBD)-IgG (A) and anti-spike-IgG (B) titers in PLWH from month 1 to month 6 post-vaccination. The dynamic changes of frequencies of (C) RBD-specific MBCs, (D) rMBCs, (E) actMBCs, (F) intMBCs, and (G) atyMBCs in PLWH from month 1 to month 6 after vaccination. The horizontal dotted lines represent the limit of detection.
Factors related to poor responses to inactivated SARS-CoV-2 vaccines in PLWH
Lastly, we wanted to investigate the risk factors related to inferior response to anti-RBD-IgG in PLWH. As shown in Table 3, factors significantly related to the poor response of anti-RBD-IgG were the time interval after full-course vaccination and a low CD4 count level.
Table 3.
Univariate and multivariate analyses for anti-RBD-IgG in PLWH.
| Variable | Univariate OR (95% CI) | P value | Multivariate OR (95% CI) | P value |
|---|---|---|---|---|
| Age (years) | 0.995 (0.970-1.020) | 0.696 | 0.985 (0.954-1.018) | 0.382 |
| Gender (male) | 0.712 (0.384-1.319) | 0.280 | 0.963 (0.422-2.197) | 0.928 |
| Days after 2nd dose Vaccination | 0.978 (0.966-0.991) | 0.001 | 0.969 (0.953-0.983) | 0.000 |
| Plasma HIV viral load | ||||
| >20 copies/mL | 1.498 (0.728-3.083) | 0.272 | 2.104 (0.861-5.140) | 0.103 |
| CD4 count (cells/µL) | ||||
| <200 | 0.284 (0.106-0.758) | 0.012 | 0.206 (0.053-0.797) | 0.022 |
| 200–500 | 0.640 (0.332-1.230) | 0.180 | 0.517 (0.231-1.160) | 0.109 |
| White blood cell count (10^9/L) | 1.013 (0.829-1.237) | 0.903 | ||
| Lymphocyte count (10^9/L) | 0.979 (0.944-1.016) | 0.266 | 0.972 (0.918-1.031) | 0.350 |
| Platelet count (10^9/L) | 1.002 (0.998-1.006) | 0.378 | ||
| Alanine aminotransferase (U/L) | 0.990 (0.969-1.010) | 0.333 | ||
| Aspartate aminotransferase (U/L) | 0.990 (0.967-1.014) | 0.403 | ||
| B cells (% of lymphocytes) | 0.989 (0.884-1.105) | 0.841 | ||
| RBD-specific B cells (%) | 0.984 (0.919-1.053) | 0.638 | ||
| RBD-specific MBCs (%) | 0.985 (0.959-1.013) | 0.294 | 0.161 (0.023-1.138) | 0.067 |
| RBD+ rMBCs (%) | 0.973 (0.929-1.020) | 0.264 | 0.163 (0.0001-241.290) | 0.626 |
| RBD+ actMBCs (%) | 0.982 (0.932-1.035) | 0.491 | 0.184 (0.0001-274.513) | 0.650 |
| RBD+ atyMBCs (%) | 1.002 (0.953-1.053) | 0.936 | 0.027 (0.00001-53.250) | 0.351 |
| RBD+ intMBCs (%) | 1.017 (0.986-1.049) | 0.285 | 0.028 (0.00001-55.202) | 0.355 |
CI, confidential interval; OR, odds ratio.
Discussion
In this prospective study, we evaluated the safety, antibody responses, and RBD-specific MBC responses of inactivated SARS-CoV-2 vaccines in PLWH and healthy controls. Our results showed that inactivated vaccines were safe and well-tolerated in PLWH. The antibody and MBC responses waned in PLWH, especially in PLWH with CD4 counts <200 cells/µL. Therefore, PLWH should be vaccinated ahead of the healthy population.
PLWH with SARS-CoV-2 have poor clinical outcomes, especially those who are immunosuppressed or do not receive ART [18,19]. However, the registration trials of the inactivated SARS-CoV-2 vaccines on the safety and humoral immune response in HIV-infected populations are limited. Hence, we first assessed the safety of inactivated vaccines in PLWH. The overall incidence of adverse events within 7 days in PLWH was 12.9%, which was similar to healthy controls (13.3%), but it was lower than phase 1/2 trials of BBIBP-CorV in China [16] (23–29%) and phase 3 trials of Corona Vac in Turkey [17] (18.9%). Notably, the proportion of adverse reactions in PLWH who received the inactivated vaccines is significantly lower than that in PLWH who received mRNA vaccines (60%) [10]. This discrepancy may be attributed to the different types of vaccines. No serious adverse events occurred after inoculation of the inactivated vaccines in PLWH, suggesting that inactivated vaccines are safe and reliable.
Our results showed that PLWH had lower anti-RBD-IgG and anti-spike-IgG titers than healthy controls 21–105 days after vaccination, which is consistent with previous studies showing that PLWH had lower immune responses to the vaccine than healthy individuals [20–22]. Previous studies also showed that 3–4 weeks after the first dose of vaccination, the production of anti-RBD antibodies in PLWH was lower than in HIV-negative controls. However, after the second dose of vaccination, the antibody levels did not differ significantly between the two groups [11,23,24]. This difference may be due to the type of vaccine, the population included, and the duration of ART. Thus, more data will be needed to clarify this in the future.
The level of immunosuppression is usually reflected by the CD4 cell counts [25]. Usually, HIV infection will cause the gradual loss of CD4+ T cells and a series of immune abnormalities [26]. However, after highly active ART, the CD4 counts of some patients can gradually increase [27]. In this study, PLWH with CD4 counts <200 cells/µL had significantly lower anti-RBD-IgG levels, similar to previous studies [10,11]. This suggests that effective ART for PLWH is needed to strengthen immunity against SARS-CoV-2. In addition, both cross-sectional and longitudinal analyses showed that the antibody titers in PLWH declined over time, which is similar to the results of previous longitudinal studies using mRNA vaccines [28–30]. Of note, anti-RBD-IgG and anti-spike-IgG titers in PLWH were lower than the titers in healthy controls at every time point in the cross-sectional analysis. Considering the occurrence of breakthrough infections with SARS-CoV-2 was correlated with low neutralizing antibody titers [31], hence, more concern should be taken on this special population.
The MBCs produced during primary infection are quickly reactivated after a secondary infection, thus, preventing severe disease or death [14,15]. Hence, we focused on the response of MBCs to inactivated vaccines. In the cross-sectional analysis, the frequency of RBD-specific MBCs in PLWH was lower than that in healthy controls, but it was relatively stable over time, which was corroborative in the longitudinal analysis. This suggests that the durable humoral immunity may be dysfunctional. In addition, the frequency of actMBCs was reduced in PLWH. ActMBCs are cells that recently left germinal centres and are already primed to become antibody-secreting plasma cells [32]. This suggests that the immune reactivation after inactivated SARS-CoV-2 vaccination may be impaired in PLWH.
There are some limitations in this study. First, few subjects participated in the follow-up until month 6 after full vaccination because the sporadic localized outbreaks of COVID-19 partially impeded travel. Second, the early stages of B and T cell responses were not evaluated, as we were mainly focusing on the significance of durable humoral immune responses. Therefore, further studies about the early stages of B and T cell responses in PLWH are needed. Third, the best correlation of antibody titers and vaccine efficacy in PLWH is currently unknown, and more large-scale population studies are needed. Nonetheless, we believe our results are particularly important and meaningful to clinicians.
In conclusion, the inactivated SARS-CoV-2 vaccines are safe and well-tolerated in people living with HIV, with no serious adverse events reported. However, the antibody response and RBD-specific MBC response were weak, especially in PLWH with a low CD4 count. Therefore, SARS-CoV-2 vaccines and booster doses should be prioritized for this special population.
Supplementary Material
Acknowledgements
We thank the health management centre and department of clinical laboratory of the Second Affiliated Hospital, Chongqing Medical University for their support.
Funding Statement
This work is supported by the National Science and Technology Major Project of China (2017ZX10202203-007, 2017ZX10202203-008, 2018ZX10302206-003) and a pilot project of clinical cooperation between traditional Chinese and western medicine for significant and complicated diseases of National Administration of Traditional Chinese Medicine: hepatic fibrosis. We also acknowledge the support of the National Natural Science Foundation of China (81772198), the Natural Science Foundation of Chongqing, China (cstc2020jcyj-msxmX0389).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Declaration of interest statement
The authors declare no conflicts of interest.
References
- 1.Cooper TJ, Woodward BL, Alom S, et al. . Coronavirus disease 2019 (COVID-19) outcomes in HIV/AIDS patients: a systematic review. HIV Med. 2020 Oct;21(9):567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tesoriero JM, Swain CE, Pierce JL, et al. . COVID-19 Outcomes Among persons living With or without diagnosed HIV infection in New York state. JAMA Netw Open. 2021 Feb 1;4(2):e2037069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirzaei H, McFarland W, Karamouzian M, et al. . COVID-19 Among People living with HIV: A systematic review. AIDS Behav. 2021 Jan;25(1):85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Interim clinical considerations . for use of COVID-19 vaccines currently authorized in the United States. Centers for Disease Control and Prevention. 2021. Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html.
- 5.Statement on COVID-19 . vaccines for patients who are immunocompromised or immunosuppressed. British Society for Immunology. 2021. Available from: https://www.immunology.org/news/bsi-statement-covid-19-vaccines-for-patients-immunocompromised-immunosuppressed.
- 6.Frater J, Ewer KJ, Ogbe A, et al. . Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. The Lancet HIV. 2021 Aug;8(8):e474–e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madhi SA, Koen AL, Izu A, et al. . Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: an interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial. The Lancet HIV. 2021 Sep;8(9):e568–e580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spinelli MA, Peluso MJ, Lynch KL, et al. . Differences in post-mRNA vaccination SARS-CoV-2 IgG concentrations and surrogate virus neutralization test response by HIV status and type of vaccine: a matched case-control observational study. Clin Inf Dis. 2021 Dec 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mondi A, Cimini E, Colavita F, et al. . COVID-19 in people living with HIV: Clinical implications of dynamics of the immune response to SARS-CoV-2. J Med Virol. 2021 Mar;93(3):1796–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy I, Wieder-Finesod A, Litchevsky V, et al. . Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin Microbiol Infect. 2021 Dec;27(12):1851–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruddy JA, Boyarsky BJ, Bailey JR, et al. . Safety and antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in persons with HIV. AIDS (London, England). 2021 Nov 15;35(14):2399–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nault L, Marchitto L, Goyette G, et al. Covid-19 vaccine immunogenicity in people living with HIV-1. bioRxiv. 2021. Available from: https://www.biorxiv.org/content/biorxiv/early/2021/08/13/2021.08.13.456258.full.pdf. [DOI] [PMC free article] [PubMed]
- 13.Gong C, Song X, Li X, et al. . Immunological changes after COVID-19 vaccination in an HIV-positive patient. Int J Infect Dis. 2022 Apr;117:230–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogega CO, Skinner NE, Blair PW, et al. . Durable SARS-CoV-2 B cell immunity after mild or severe disease. J Clin Invest. 2021 Apr 1;131(7):e145516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakharkar M, Rappazzo CG, Wieland-Alter WF, et al. . Prolonged evolution of the human B cell response to SARS-CoV-2 infection. Sci Immunol. 2021 Feb 23;6(56):eabg6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia S, Zhang Y, Wang Y, et al. . Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021 Jan;21(1):39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanriover MD, Doğanay HL, Akova M, et al. . Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet (London, England). 2021 Jul 17;398(10296):213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Risk Factors for Coronavirus Disease 2019 . (COVID-19) death in a population cohort study from the western cape province, South Africa. Clin Infect Dis. 2021 Oct 5;73(7):e2005–e2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geretti AM, Stockdale AJ, Kelly SH, et al. . Outcomes of Coronavirus disease 2019 (COVID-19) related hospitalization Among People With human immunodeficiency virus (HIV) in the ISARIC world health organization (WHO) Clinical characterization protocol (UK): A prospective observational study. Clin Infect Dis. 2021 Oct 5;73(7):e2095–e2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroon FP, van Dissel JT, Labadie J, et al. . Antibody response to diphtheria, tetanus, and poliomyelitis vaccines in relation to the number of CD4+ T lymphocytes in adults infected with human immunodeficiency virus. Clin Infect Dis. 1995 Nov;21(5):1197–1203. [DOI] [PubMed] [Google Scholar]
- 21.Avelino-Silva VI, Miyaji KT, Hunt PW, et al. . CD4/CD8 ratio and KT ratio predict yellow Fever vaccine immunogenicity in HIV-infected patients. PLoS Negl Trop Dis. 2016 Dec;10(12):e0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van den Berg R, van Hoogstraten I, van Agtmael M.. Non-responsiveness to hepatitis B vaccination in HIV seropositive patients; possible causes and solutions. AIDS Rev. 2009 Jul-Sep;11(3):157–164. [PubMed] [Google Scholar]
- 23.Feng Y, Zhang Y, He Z, et al. . Immunogenicity of an inactivated SARS-CoV-2 vaccine in people living with HIV-1: a non-randomized cohort study. EClinicalMedicine. 2022 Jan;43:101226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brumme ZL, Mwimanzi F, Lapointe HR, et al. . Humoral immune responses to COVID-19 vaccination in people living with HIV receiving suppressive antiretroviral therapy. medRxiv. 2021 Oct. doi:15:2021.10.03.21264320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duro R, Rocha-Pereira N, Figueiredo C, et al. . Routine CD4 monitoring in HIV patients with viral suppression: Is it really necessary? A Portuguese cohort. J Microbiol Immunol Infect. 2018 Oct;51(5):593–597. [DOI] [PubMed] [Google Scholar]
- 26.Deeks SG, Overbaugh J, Phillips A, et al. . HIV infection. Nat Rev Dis Primers. 2015 Oct 1;1:15035. [DOI] [PubMed] [Google Scholar]
- 27.Moore RD, Keruly JC.. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007 Feb 1;44(3):441–446. [DOI] [PubMed] [Google Scholar]
- 28.Campo F, Venuti A, Pimpinelli F, et al. . Antibody Persistence 6 months post-vaccination with BNT162b2 among Health Care workers. Vaccines (Basel). 2021 Oct 3;9(10):1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levin EG, Lustig Y, Cohen C, et al. . Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N Engl J Med. 2021 Dec 9;385(24):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doria-Rose N, Suthar MS, Makowski M, et al. . Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for COVID-19. N Engl J Med. 2021 Jun 10;384(23):2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergwerk M, Gonen T, Lustig Y, et al. . COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021 Oct 14;385(16):1474–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau D, Lan LY, Andrews SF, et al. . Low CD21 expression defines a population of recent germinal center graduates primed for plasma cell differentiation. Sci Immunol. 2017 Jan 27;2(7):eaai8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.