ABSTRACT
Diabetes mellitus (DM) is one of the most common underlying diseases that may aggravates COVID-19. In the present study, we explored islet function, the presence of SARS-CoV-2 and pathological changes in the pancreas of patients with COVID-19. Oral glucose tolerance tests (OGTTs) and the C-peptide release test demonstrated a decrease in glucose-stimulated C-peptide secretory capacity and an increase in HbA1c levels in patients with COVID-19. The prediabetic conditions appeared to be more significant in the severe group than in the moderate group. SARS-CoV-2 receptors (ACE2, CD147, TMPRSS2 and neuropilin-1) were expressed in pancreatic tissue. In addition to SARS-CoV-2 virus spike protein and virus RNA, coronavirus-like particles were present in the autophagolysosomes of pancreatic acinar cells of a patient with COVID-19. Furthermore, the expression and distribution of various proteins in pancreatic islets of patients with COVID-19 were altered. These data suggest that SARS-CoV-2 in the pancreas may directly or indirectly impair islet function.
KEYWORDS: Covid-19, sARS-CoV-2, pancreas, islet, diabetes
Introduction
The outbreak of coronavirus disease 2019 (COVID-19) has raised tremendous challenges. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) shares similarities with the SARS coronavirus [1]. SARS-CoV was reported to infect multiple organs in humans [2]. Similarly, SARS-CoV-2 was detected in the lung, pharynx, heart, liver, brain, and kidney of infected patients [3].
Diabetes mellitus (DM) is a chronic disease affecting millions of people. Concerns have been raised those diabetic patients may be at high risk for COVID-19 [4,5]. ACE2, the putative receptor for SARS-CoV-2, is rarely detected in pancreatic endocrine cells [6,7], leading to the hypothesis that SARS-CoV-2 is unlikely to directly infect pancreatic β cells in vivo in an ACE-2-dependent manner. In contrast, in vitro studies have provided evidence that human pancreatic α and β cells are susceptible to SARS-CoV-2 infection [8], implying that SARS-CoV-2 may directly target the pancreas and impair islet function. Moreover, contradictory data have shown that the SARS-CoV-2 receptors ACE2 and TMPRSS2 are expressed in pancreatic islets [9]. Although SARS-CoV-2 has been postulated to promote the occurrence of DM [10], the direct evidence linking SARS-CoV-2 with DM is still inadequate.
Hyperglycemia is commonly observed in patients with SARS [11]. Limited retrospective studies [12,13] have shown that elevation of blood glucose levels might also occur in patients with COVID-19. It is speculated that the systemic inflammatory response may contribute to the onset of DM [14,15]. SARS-CoV-2 has been detected in respiratory system[16] and kidney [3] specimens. However, the existence of SARS-CoV-2 in the pancreas and the islet function of patients with COVID-19 have not been well documented. To explore the effects of SARS-CoV-2 infection on islet function, an oral glucose tolerance test (OGTT) and C-peptide release test were performed in SARS-CoV-2-infected patients without a history of diabetes or impaired glucose tolerance. Autopsy specimens from the pancreas of patients with COVID-19 were also analyzed with immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), and transmission electron microscopy (TEM). We found that islet function was compromised in patients with COVID-19 and that SARS-CoV-2 was present in the pancreas, suggesting that SARS-CoV-2 may directly target the pancreas and contribute to the initiation of DM.
Materials and methods
Study design and participants
We recruited patients with COVID-19 from March 1st to April 12th, 2020, at Wuhan No. 1. Hospital and Wuhan Jinyintan Hospital, Wuhan China. All the patients were confirmed to have SARS-CoV-2 infection with a real-time reverse transcriptase-polymerase chain reaction (RT–PCR) test. The exclusion criteria of this study included (1) a history of diabetes, prediabetes, or taking medicine to control blood sugar before COVID-19; (2) cancer; (3) pancreatic diseases (acute pancreatitis, chronic pancreatitis or pancreatic injury); (4) autoimmune disease; (5) immunodeficiency; (6) glucocorticoid treatment within 6 months before admission; and (7) pregnancy or breastfeeding. None of the patients received glucocorticoid treatment during hospitalization. All patients were provided with enough carbohydrate intake for a balanced diet, and none were prescribed parenteral nutrition or nasal feeding.
Study approval
The study was approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University, Wuhan No. 1 Hospital, Wuhan Jinyintan Hospital and Tongji Medical College of Huazhong University of Science and Technology (2020-SR-134, KY-2020-15.01 and KY-2020-52.01). Written informed consent was obtained from all patients.
Clinical procedures
Epidemiological, demographic, and baseline characteristics and laboratory results were obtained from patients’ medical records. Inflammatory factors, including C-reactive protein (CRP) and IL-6, were routinely measured. The 75-g OGTT was performed. Briefly, after at least 8 h of fasting, the patients donated blood to measure fasting plasma glucose and glycosylated hemoglobin A1c (HbA1c) levels. Water-free glucose powder (75 g) was dissolved in 200 ml of drinking water and was consumed in 5 min. The timer was set as 0 min when the patient drank the first sip. Then, blood samples were collected at 30-, 60-, 120-, and 180-min post-glucose consumption. Plasma glucose and C-peptide were measured to determine glucose tolerance and the secretory capacity of pancreatic islets. According to the glucose metabolism levels announced by the World Health Organization (WHO) in 1999 [17], subjects with fasting blood glucose (FBG) < 6.1 mmol/L and 2-h blood glucose (2hBG) < 7.8 mmol/L were grouped into normal glucose tolerance; those with FBG ≥ 7.0 mmol/L and 2hBG ≥11.1 mmol/L were in the diabetes group; and those with blood levels not fitting in the above two groups were in the prediabetes group.
Opal immunofluorescence staining in pancreatic samples
Autopsy samples were collected from four patients with COVID-10, and opal multiplex immunofluorescence staining was processed as described previously [18,19]. Briefly, formalin-fixed paraffin-embedded (FFPE) pancreatic tissue samples were cut into 3-μm-thick serial sections and further stained for simultaneous detection and quantitation of ACE2 (Ab108252, 1:200, Opal 570 channel, pseudo-yellow), NKX6.1a (CST#54551S, 1:100, Opal 520 channel, pseudo-green), CD147 (Ab10830, 1:500, Opal 690 channel, pseudo-magenta), neuropilin-1 (ab81321, 1:100, Opal 620 channel, pseudo-red), TMPRSS2 (Abcolonal, A9126, 1:400, Opal 780 channel, pseudo-white) and nucleus (DAPI, pseudo-blue) by using an Opal Polaris 7 Color Automation IHC Detection Kit (Akoya Biosciences, Menlo Park, CA). The slides were observed and imaged by a Vectra Polaris automated quantitative pathology imaging system. The images were sequentially spectrally unmixed by Akoya phenoptics inForm software (inform 2.4.8).
Immunohistochemistry (IHC) of pancreatic samples
Autopsy samples were collected from four patients with COVID-19 and processed as described previously [16]. Briefly, FFPE pancreatic tissue samples were cut into 3-μm-thick serial sections. Following antigen retrieval in EDTA (pH: 9.0), the sections were incubated overnight at 4 °C with primary antibodies against SARS-CoV-2 spike (1:200, GTX632604, Genetex), ACE2 (1:200, GB11267, Servicebio), CD147 (1:200, GB11390-1, Servicebio), PDX1 (1:200, 20989-1-ap, Proteintech), PPAR (1:200, BS-4590R, BIOSS), CD36 (1:200, 18836-1-ap, Proteintech), GLUT2 (1:200, bs-051r, BIOSS), IRS1 (1:200, AF6273, Affinity), or IRS2 (1:200, bs-0173r, BIOSS). After extensive washing, the sections were further stained with the corresponding secondary antibodies and visualized using the Dako REAL™ EnVision™ Detection System. Dilutants without primary antibodies were used as negative controls.
Fluorescence in situ hybridization (FISH) of pancreatic samples
Pancreatic samples obtained from autopsy were fixed with 4% PFA in diethyl pyrocarbonate (DEPC) for 8 h. After dehydration, the fixed tissue sample was cut into 3-μm-thick serial sections. Following digestion in proteinase K (20 μg/ml) at 37 °C for 20 min, the sections were incubated with 6 ng/μl SARS-CoV-2 probe (5’-CY3-CCGUC UGCGG UAUGU GGAAA GGUUA UGG-3’) at 37 °C overnight. After washing, the FISH preparations were counterstained with DAPI and observed by confocal microscopy with appropriate fluorescence filter sets (Nikon, Japan).
Transmission electron microscopy (TEM)
TEM was utilized to detect changes in the ultrastructure and structure of the SARS-CoV-2 virus particles [16]. Briefly, fresh pancreatic tissues (approximately 1 mm×1 mm×1 mm in size) were fixed in 3% buffered glutaraldehyde in 0.1 M phosphoric buffer (pH: 7.4) for 2∼4 h and 1% (w/v) osmic acid for 2 h, dehydrated with gradient alcohol, and embedded in Epon 812 (SPI, PA, USA). After polymerization of the resin at 60 °C for 48 h, ultrathin sections were cut at 70-nm thickness with an ultramicrotome using a diamond knife, stained with 5% uranyl acetate and lead citrate, and observed under a Hitachi HT7700 transmission electron microscope.
Statistical analysis
All data, as appropriate, are expressed as the mean ± standard deviation or percentages. The mean and percentages between groups were compared using ANOVA and the chi-square test in SPSS (version 22.0). The calculation formulas for evaluating insulin resistance are listed as follows: C-peptide index = fasting C-peptide (mmol/L)/fasting blood glucose (mmol/L)*100[20]; 20/(fasting C-peptide (nmol/L) fasting glucose (mmol/L))[21].
Results
Islet function was compromised in patients with covid-19
A total of 42 patients with COVID-19, 21 males and 21 females, were recruited from two medical centers in Wuhan for the study (Table 1). The patients ranged from 23 to 93 years old. Of the 42 patients, the classification of COVID-19 disease severity from the Clinical Criteria of the WHO [22] was as follows: 1 was mild, 22 were moderate, 18 were severe, and 1 was critical. We divided the patients into two groups based on their disease severity: the 23 mild and moderate patients were named the moderate group, while the 19 severe and critical patients were named the severe group (Table 2). To explore whether SARS-CoV-2 infection impaired systemic glucose tolerance and islet function, we performed OGTT tests and C-peptide secretion tests on patients with COVID-19. The patients in the severe group were older than the patients in the moderate group (65.79 ± 14.73 vs. 52.78 ± 15.37, p = 0.008), reflecting that age was a risk factor for the disease severity of COVID-19. A higher body mass index (BMI) is a common risk factor for type 2 diabetes mellitus (T2DM) [23], and it was comparable between the two groups (21.70 ± 2.67 vs. 23.22 ± 3.84, p = 0.202).
Table 1.
Clinical characteristics and laboratory findings in patients with COVID-19.
| Wuhan No.1 | Jinyintan | Total | Reference range | |
|---|---|---|---|---|
| Gender(M/F) | 11/6 | 10/15 | 21/21 | |
| Age (years old) | 68.6 ± 12.7 | 52.28 ± 15.57 | 58.66 ± 16.48 | |
| Underlying diseases | ||||
| hypertension | 0 | 2 | 2 | |
| hyperuricemia | 0 | 1 | 1 | |
| No potential comorbidities | 17 | 22 | 39 | |
| Disease Severity | ||||
| mild disease | 0 | 1 | 1 | |
| moderate disease | 1 | 21 | 22 | |
| severe disease | 15 | 3 | 18 | |
| critical disease | 1 | 0 | 1 | |
| Hospitalization days1 | ||||
| <30 days | 2 | 0 | 2 | |
| 30∼60 days | 14 | 12 | 27 | |
| >60 days | 1 | 13 | 13 | |
| Blood routine | ||||
| Leukocyte (×109/L) | 7.01 ± 0.58 | 5.49 ± 0.32 | 6.13 ± 0.32 | 3.50-9.50 |
| Neutrophils (×109/L) | 4.43 ± 0.63 | 3.05 ± 0.23 | 3.63 ± 0.31 | 1.80-6.30 |
| Lymphocytes (×109/L) | 1.58 ± 0.21 | 1.85 ± 0.12 | 1.74 ± 0.11 | 1.10-3.20 |
| Blood chemistry | ||||
| TBIL (μmol/L) | ND | 10.9 ± 3.5 | NA | 1.71-21 |
| Albumin (g/L) | 36.06 ± 8.11 | 39.8 ± 3.71 | 38.46 ± 5.87 | 40–55 |
| Globulin (g/L) | 28.00 ± 6.16 | 27.08 ± 2.55 | 27.44 ± 4.28 | 20–40 |
| ALT (IU/L) | 28.7 ± 3.6 | 29.4 ± 4.3 | 29.1 ± 2.9 | 7–45 |
| AST (IU/L) | 24.3 ± 2.0 | 27.3 ± 2.5 | 26.1 ± 1.7 | 13–35 |
| Creatinine (μmol/L) | 70.4 ± 5.6 | 60.3 ± 4.1 | 64.5 ± 3.4 | 44–97 |
| BUN (mmol/L) | 5.7 ± 0.9 | 4.5 ± 0.2 | 5.0 ± 0.4 | 1.8-7.3 |
| Inflammation parameters | ||||
| CRP (mg/L) | 17.38 ± 24.92 | 1.58 ± 2.03 | 7.75 ± 17.21 | ≤5 |
| IL-6 (pg/ml) | 9.50 ± 10.67 | 6.74 ± 2.53 | 7.66 ± 6.46 | ≤5 |
| Treatment | ||||
| antiviral2 | 17 | 0 | ||
| antibiotics3 | 16 | 1 |
The infection history for patients in Jingyintan Hospital cohort study was over 30 days.
Patients were treated with Arbidol, Oseltamivir, Kaletra, or Hydroxychloroquine.
Patients were treated with Meropenem, Moxifloxacin, Tegafycline or Azithromycin.
Abbreviation: NLR, neutrophil-to-lymphocyte ratio; TBIL, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CRP, C-reactive protein; IL-6, interleukin-6; ND, not done; NA, not available
Table 2.
Results of the oral glucose tolerance test in patients with COVID-19.
| Moderate group (n = 23) | Severe group (n = 19) | P value | Reference range | |
|---|---|---|---|---|
| Age (years) | 52.78 ± 15.37 | 65.79 ± 14.73 | 0.008 | |
| BMI (kg/m2) | 23.22 ± 3.84 | 21.70 ± 2.67 | 0.202 | |
| Glucose (mmol/L) | ||||
| 0 min | 4.95 ± 0.50 | 5.39 ± 1.02 | 0.238 | 3.9-6.1 |
| 30 min | 8.80 ± 2.09 | 9.40 ± 2.09 | 0.433 | 6.1-9.4 |
| 60 min | 9.09 ± 2.22 | 9.92 ± 2.92 | 0.463 | 6.7-9.4 |
| 120 min | 7.63 ± 1.96 | 8.97 ± 4.38 | 0.595 | 3.9-7.8 |
| 180 min | 5.68 ± 1.70 | 7.82 ± 5.41 | 0.390 | 3.9-6.1 |
| C-peptide (ng/ml) | ||||
| 0 min | 1.97 ± 0.95 | 2.37 ± 1.01 | 0.060 | 0.30-0.61 |
| 30 min | 7.76 ± 4.52 | 6.45 ± 3.07 | 0.536 | 1.5-6.1 |
| 60 min | 8.65 ± 3.34 | 9.30 ± 5.69 | 0.791 | 1.5-6.1 |
| 120 min | 9.71 ± 3.77 | 9.75 ± 6.38 | 0.640 | |
| 180 min | 7.72 ± 3.83 | 7.32 ± 4.71 | 0.471 | 0.30-0.61 |
| HbA1c (%) | 5.15 ± 0.37 | 6.12 ± 1.60 | 0.001 | 4∼6% |
| Glu_AUC | 1375.24 ± 272.38 | 1582.18 ± 588.38 | 0.587 | |
| Cp_AUC | 1543.73 ± 547.45 | 1451.97 ± 822.95 | 0.411 | |
| CPI | 1.32 E-4 ± 6.98 E-5 | 1.51 E-4 ± 7.09 E-5 | 0.153 | |
| 20/(FCp*FBG) | 7.45 ± 2.94 | 6.19 ± 4.07 | 0.063 | |
| Cpmax/FCp | 6.14 ± 1.79 | 4.78 ± 1.63 | 0.014 |
Abbreviations: HbA1c, hemoglobin A1c; Glu_AUC, area under the curve of glucose in the OGTT; Cp_AUC, area under the curve of C-peptide in the OGTT; Cp, C-peptide; CPI, C-peptide index; FCp, fasting C-peptide; FBG, fasting blood glucose; Cpmax, the maximum value of C-peptide during the OGTT.
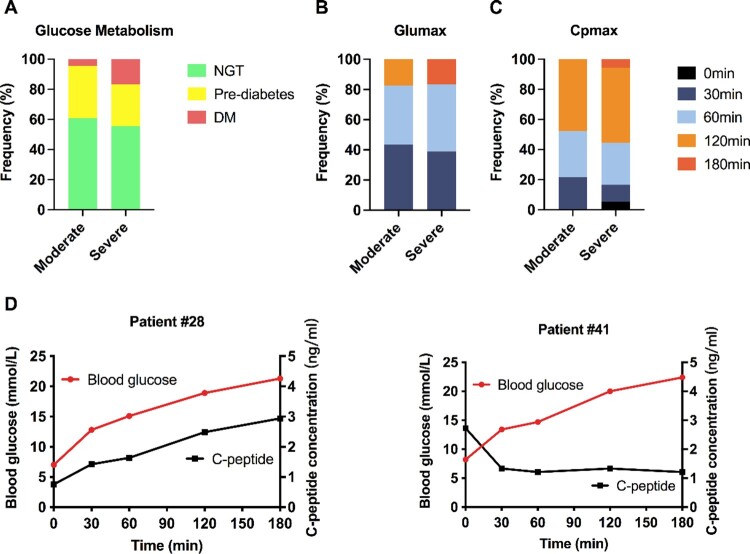
The HbA1c level was significantly higher in the severe group than in the moderate group (6.1 ± 1.6 vs. 5.1 ± 0.4, p = 0.001). In the OGTT test, the average fasting blood glucose (FBG) concentration in the severe group was slightly higher than that in the moderate group (5.4 ± 1.0 vs. 4.9 ± 0.5, p = 0.238), but was not statistically significant. Similar results were found for the blood glucose concentrations 30-, 60-, 120-, and 180-min post-glucose consumption. Accordingly, 14 patients (60.9%) in the moderate group had normal glucose tolerance (NGT), 9 showed characteristics of prediabetes, and 1 was newly diagnosed with DM. In contrast, 3 patients were diagnosed with new-onset diabetes, 5 patients suffered from prediabetes, and 10 patients in the severe group had NGT (Figure 1A). The chi-square test did not show a significant difference in the composition ratio between the mild-moderate and severe-critical groups according to glucose tolerance levels. Collectively, 18 of the 42 patients with COVID-19 (18/42 = 42.86%) developed prediabetes or diabetes. The peak time of blood glucose or C-peptide concentration indicated that the patients in the severe group seemed to respond slower than those in the moderate group (Figure 1B and C), although the difference did not reach statistical significance. To our surprise, the level of C-peptide was very low in 1 patient (patient #41) diagnosed with new-onset diabetes. The second patient (patient #28) with newly diagnosed diabetes had impaired insulin secretion and failed to lower glucose levels to a normal range (Figure 1D), indicating an insulin resistance. These data suggest that SARS-CoV-2 might promote the development of diabetes.
Figure 1.
Distribution of normal glucose tolerance, prediabetes and diabetes after COVID-19. (A) Distribution of patients with COVID-19 into normal glucose tolerance (NGT), prediabetes or diabetes mellitus (DM) groups. During the OGTT, the peak time distributions of blood glucose (B) and C-peptide (C) in the mild-moderate group and the severe-critical group were analyzed. (D) OGTT results of 2 representative patients with COVID-19 and new-onset diabetes. In patient #29, C-peptide (black curve, right axis, ng/ml) was released in response to the elevation of blood glucose levels (red curve, left axis, mmol/L). In patient #42, the blood glucose level was increased, but the C-peptide level was very low throughout the entire test.
Similar to blood glucose, C-peptide is a surrogate marker for pancreatic islet function, reflecting insulin secretion. As shown in Table 2, the average baseline level of C-peptide (0 min) was slightly higher in the severe group than in the moderate group, indicating a higher fasting insulin concentration, an indicator of systemic insulin resistance. Insulin is a rapid-acting hormone with a short half-life of approximately 4–6 min. Moreover, C-peptide measurements will be significantly influenced by sample hemolysis, which means that the repeatability and accuracy may vary in different labs. Alternatively, C-peptide is simultaneously released with insulin, remaining stable for 24 hours in separated serum and plasma samples, thus becoming a more reliable marker for indicating insulin secretion capacity. Two indices based on serum C-peptide contents were applied in the current study, including the C-peptide index (CPI) and the 20/(fasting C-peptide*fasting blood glucose) ratio (20/FCp*FBP), which are indicators for beta-cell function and insulin sensitivity, respectively. Moreover, it has even been proven that 20/FCp*FBP performs better than the widely used index HOMA-IR, especially in those with mild insulin resistance [21]. As shown in Table 2, 20/FCP*FBP showed a reduction tendency in the severe group (7.45 ± 2.94 vs. 6.19 ± 4.07, p = 0.063), while CPI was comparable between the two groups. Moreover, the maximum levels of C-peptide during the OGTT/FCp (Cpmax/FCp) was significantly decreased in patients with severe COVID-19 (6.14 ± 1.79 vs. 4.78 ± 1.63, p = 0.014). Collectively, these data suggested that SARS-CoV-2 infection may impair islet functions, especially in patients with severe COVID-19.
SARS-CoV-2 virus was detected in the pancreas of patients with covid-19
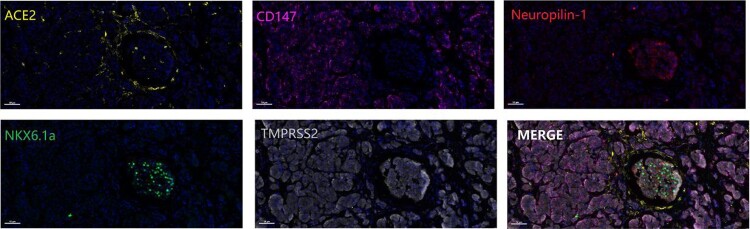
ACE2 [24], CD147 [25], neuropilin-1 [26] and TMPRSS2 [27] are receptors for SARS-CoV-2. To explore the possibility that SARS-CoV-2 may infect pancreatic cells directly, we first examined the expression of SARS-CoV-2 receptors in pancreatic autopsy samples. As shown in Figure 2, ACE2, CD147 and TMPRSS2 were widely expressed on the cell membrane in the pancreas. NKX6.1a, as a critical regulator of pancreatic β cells [28], was clearly detected only in islets. In contrast, neuropilin-1 was weakly expressed in islets. The expression of SARS-CoV-2 receptors in pancreatic tissues and islets suggested that islets may be susceptible to SARS-CoV-2 infection.
Figure 2.
Opal immunofluorescence staining of SARS-CoV-2 receptors in pancreatic samples. The SARS-CoV-2 receptors ACE2 (pseudo-yellow), CD147 (pseudo-magenta), neuropilin-1 (pseudo-red), and TMPRSS2 (pseudo-white) and the pancreatic β cell maker NKX6.1a (pseudo-green) were detected in the pancreatic samples. Scale bar, 50 μm.
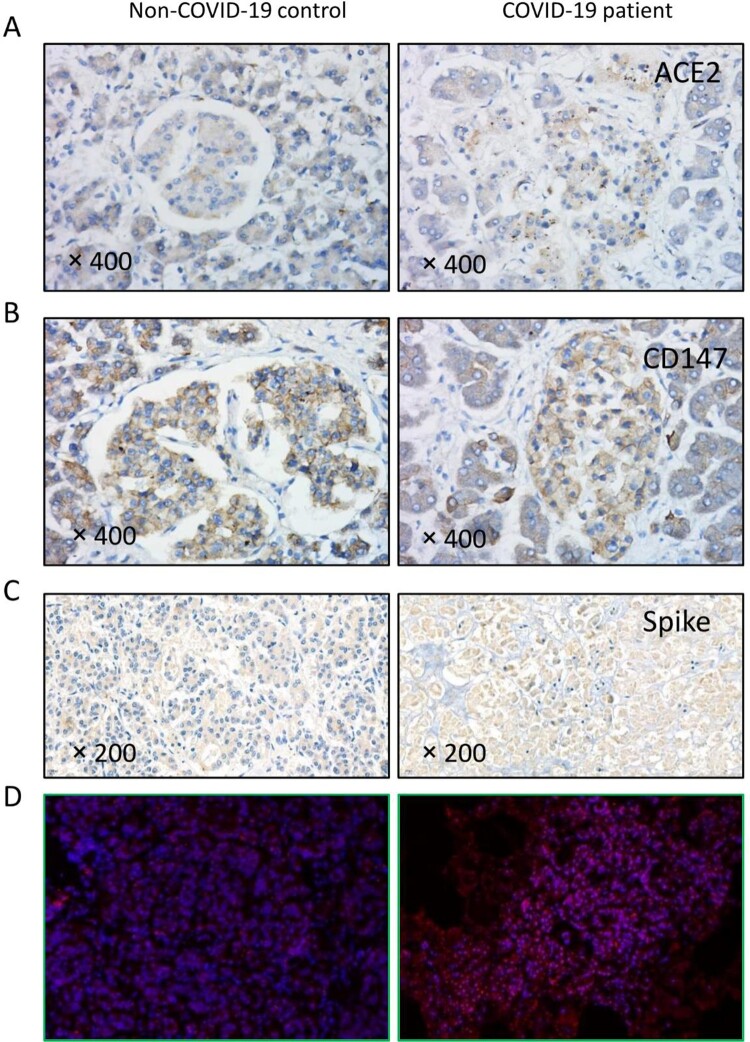
To further compare the difference between non-COVID-19 and COVID-19 autopsy samples, we performed immunohistochemical analysis in patients with COVID-19, and the expression of ACE2 and CD147 seemed to be higher in the endocrine gland (islet). SARS-CoV-2 spike proteins were widely distributed in the parenchyma of the pancreas of patients with COVID-19 but not in the non-COVID-19 control samples. To further confirm this observation, we performed fluorescence in situ hybridization (FISH) analyses using a nucleic acid probe specific for SARS-CoV-2 RNA. We detected positive fluorescent signals in the cytoplasm of pancreatic cells of the patients with COVID-19 but not in the non-COVID-19 controls (Figure 3).
Figure 3.
SARS-CoV-2 in the pancreas of COVID-19 patients. (A-B) SARS-CoV-2 receptors ACE2 and CD147 were diffusely expressed in the pancreas. In the non-COVID-19 control patient, ACE2 expression was higher in the exocrine gland, and CD147 was evenly expressed. In patients with COVID-19, ACE2 and CD147 expression was much higher in the endocrine gland. (C) SARS-CoV-2 spike antibody staining showed diffuse positive signals (brown) in the sample from the patient with COVID-19 but not in the non-COVID-19 control. (D) The SARS-CoV-2 nucleic acid probe showed positive staining (red particles) in the cytoplasm of pancreatic cells of a patient with COVID-19 but not in that of the non-COVID-19 control.
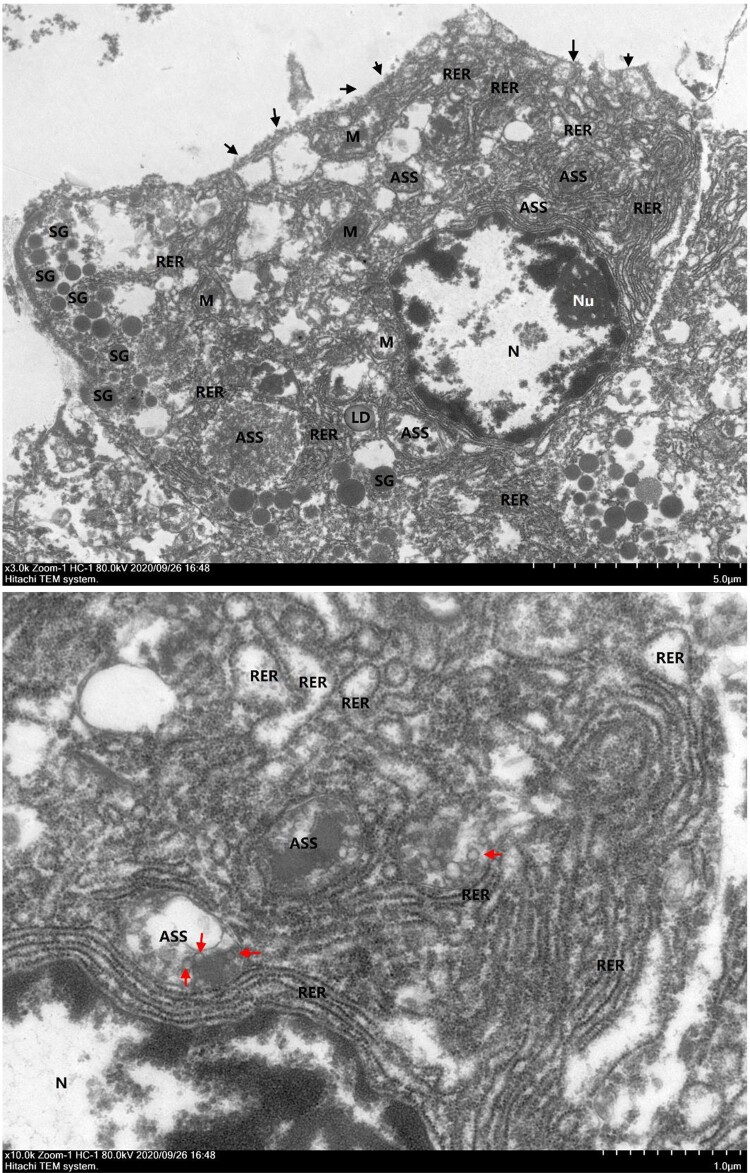
With the aid of transmission electron microscopy, we directly observed cell damage and virus-like particles in the pancreas (Figure 4). The overall structure of acinar epithelial cells was significantly edema and disintegrated, a large area of cell membrane was damaged and dissolved, intracytoplasmic organelles were swollen, vacuolated and transformed nuclei were irregularly shaped with local pits, euchromatin dissolved heterochromatin edge set, nucleoli were large, and the nuclear membrane was clear. The mitochondria were obviously swollen, most of them were enlarged, and the stromal lysis ridge disappeared. The coarse endoplasmic reticulum was abundant, and some parts of the ER were obviously expanded and degranulated. There were fewer secretory granules, the uniform density of cell connection disappeared, and the cell gap was significantly widened. Moreover, a small number of circular structures could be seen in the autophagolysosomes, which were suspected to be virus-like particles. Collectively, these data suggest that SARS-CoV-2 may directly infect islet cells.
Figure 4.
Transmission electron microscopic examination of pancreatic samples. Black solid triangles (▴) indicate cell membrane rupture. Red solid triangles (▴) indicate virus-like particles in the autophagolysosome (ASS). M, mitochondria; N, nucleus; Nu, nucleoli; RER, rough endoplasmic reticulum; SG, secretory granules.
Altered expression patterns of islet function-related molecules in the pancreas of patients with covid-19
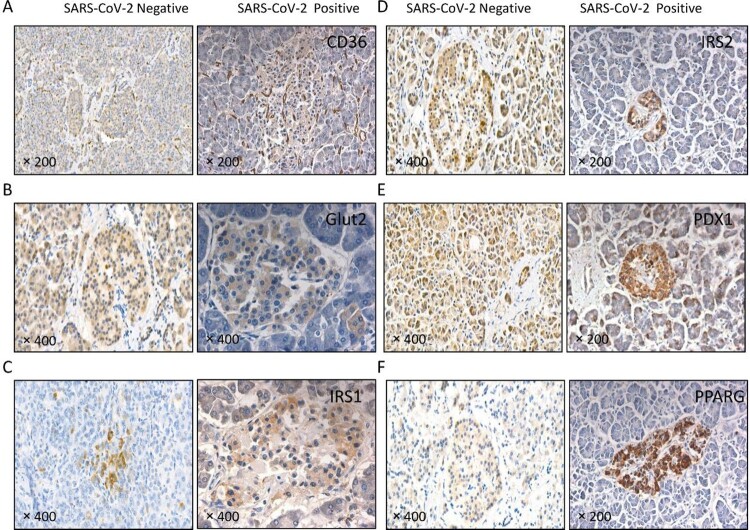
Many molecules are involved in islet function and the pathogenesis of diabetes, including CD36, glucose transporters (GLUT) 2, insulin receptor substrate (IRS) 1, IRS2, pancreatic and duodenal homeobox 1 (PDX1), and peroxisome proliferator activated receptor gamma (PPARG) [29–33]. In the COVID-19 patient without SARS-CoV-2 virus expression in the pancreas (SARS-CoV-2 negative), CD36, GLUT2, IRS2, and PDX1 were widely distributed in the pancreatic, serous acini, duct (exocrine gland) and islet (endocrine gland). PPARG was mainly expressed in the islet, while IRS1 was barely detected. In the patient with SARS-CoV-2 virus expression in the pancreas (SARS-CoV-2 positive), CD36, GLUT2, IRS1, IRS2, PDX1, and PPARG were mainly detected in the islet. CD36, IRS1, and PDX1 were also weakly expressed in the exocrine gland. Of note, CD36 was abnormally expressed in the small vasculature of pancreatic interstitial tissue of SARS-CoV-2-infected patients accompanied by SARS-CoV-2 virus in the pancreas. In summary, the expression patterns of CD36, GLUT2, IRS1, IRS2, PDX1, and PPARG in the pancreas were altered upon SARS-CoV-2 infection (Figure 5), implying that islet function may be compromised in patients with COVID-19.
Figure 5.
The expression of islet function-related molecules was altered in patients with COVID-19. Immunochemical staining using antibodies against (A) CD36, (B) GLUT2, (C) IRS1, (D) IRS2, (E) PDX1 and (F) PPARG. In the patient with COVID-19 but without SARS-CoV-2 virus in the pancreas (SARS-CoV-2 negative), CD36, GLUT2, IRS2 and PDX1 were widely distributed in the pancreatic serous acinus, the duct (exocrine gland) and the islet (endocrine gland); PPARG was mainly in the islet; and IRS1 was barely detected. In the patient with COVID-19 accompanied by SARS-CoV-2 virus in the pancreas (SARS-CoV-2 positive), CD36, GLUT2, IRS1, IRS2, PDX1 and PPARG were mainly detected in the islets. CD36 was also present in the small vasculature of pancreatic interstitial tissue.
Discussion
The mortality rate of COVID-19 is estimated to be 1-4%, which is significantly lower than that of SARS (∼9%) [34] and MERS (∼35%) [35]. However, COVID-19 may become more fatal for patients with diabetes and other underlying diseases, e.g. hypertension and chronic obstructive pulmonary disease (COPD). The COVID-19 case fatality rate accompanied by diabetes was 7% [36]. The increased mortality rate may be caused by the prevalence of diabetes in elderly patients or the interactions between SARS-CoV-2 and diabetes. In this pilot study, SARS-CoV-2 was found in the pancreas, and islet function was impaired in patients with COVID-19, suggesting that SARS-CoV-2 infection may promote the occurrence of DM.
SARS-CoV-2 virus particles have been found in the lungs [16], kidney [37,38], brain [39], and feces [40]. A cohort autopsy study identified SARS-CoV-2 in multiple organs, including the pancreas [41]. In the present study, we detected SARS-CoV-2 receptors (ACE2 and CD147), spike protein, viral nucleic acids, and intact coronavirus-like particles in the pancreas of patients with COVID-19. Our observation strengthened the previous finding that pancreatic cells in organoid culture were permissive to SARS-CoV-2 infection [8]. Moreover, SARS-CoV-2 virus particles were mainly present in the autophagolysosomes of infected acinar cells, indicating that autophagy may be involved in SARS-CoV-2 infection of the pancreas. Indeed, SARS-CoV-2 infection may cause the accumulation of autophagosomes [42]. The mechanisms and potential roles of autophagy in SARS-CoV-2 infection of the pancreas need further investigation.
As a scavenger receptor for free fatty acids, CD36 is widely expressed on β cells and α cells in pancreatic islets and contributes to insulin resistance and diabetes [29]. GLUT2 is required for glucose-stimulated insulin secretion in pancreatic islet β cells [30, 43]. IRS1 and IRS2 mediate the growth and function of pancreatic islet β cells [31]. IRS2 is especially crucial in insulin sensitivity and is responsible for initiating the progression of T2DM [44]. Similarly, PDX1 regulates pancreatic development and pancreatic islet β cell function [45]. PPARG plays diverse roles in the pathogenesis of diabetes, regulating adipogenesis, lipid metabolism, insulin sensitivity, and inflammation [33]. We found that SARS-CoV-2 infection in the pancreas enriched the expression of CD36, GLUT2, IRS2, and PDX1 in the pancreatic islets, which were widely distributed in the SARS-CoV-2-negative pancreas. IRS1 was found to be expressed in the pancreatic islets of the SARS-CoV-2-positive pancreas but was barely detectable in the SARS-CoV-2-negative pancreas. Moreover, CD36 was abnormally expressed in the small vasculature of pancreatic interstitial tissue of SARS-CoV-2-infected patients accompanied by SARS-CoV-2 expression in the pancreas, which might damage endothelial cells. All of these molecules are closely associated with pancreatic islet β cell function, implying that SARS-CoV-2 infection may directly or indirectly alter pancreatic islet function.
To evaluate the islet function in patients with COVID-19, we performed OGTT and C-peptide release tests. C-peptide is usually released simultaneously with the secretion of insulin. C-peptide levels are normally rather low in patients with type 1 diabetes mellitus (T1DM), and its secretion is slow in reaction to acute glucose stimulation in patients with T2DM. Patient #42 in our study had low C-peptide levels not only at baseline but also after OGTT, which were similar to the phenotypes of subjects with T1DM [46]. On the other hand, patient #29 developed insulin resistance, which is the hallmark of T2DM. However, we were unable to subtype these two patients into either T1DM or T2DM without other clinical indices, such as islet autoantibodies, and responses to insulin treatment. HbA1c provides a reliable measure of chronic glycemia in patients from the previous 2–3 months of treatment and is largely influenced by glucose levels during the last month of treatment. In our study, most of the patients were hospitalized for approximately 1 month. Thus, HbA1c was regarded as a good indicator of overall glucose metabolism during COVID-19 infection. The level of HbA1c was increased, especially in patients with severe or critical COVID-19, and was in line with the observation that the blood glucose level was higher in patients with severe or critical COVID-19. Although HbA1c is routinely detected in diabetic patients [47], some other conditions may also lead to elevated HbA1c in the absence of long-term increase in blood glucose levels [48]. In line with our observations, a case report described the occurrence of DM following SARS-CoV-2 infection [49]. Emerging evidence supports that SARS-CoV-2 may induce hyperglycemia [50] and new-onset insulin resistance [51]. More studies are needed to better understand the roles and mechanisms of SARS-CoV-2 infection and COVID-19 in the initiation and progression of diabetes.
Our study has some limitations. 1) In the Wuhan outbreak of SARS-CoV-2, most hospitals received only COVID-19 patients. Therefore, COVID-19 patients alone were recruited in the study, and non-COVID-19 controls were lacking in the OGTT. 2) Absence of evidence is not evidence of absence. The islet conditions in recovered COVID-19 patients were clear, which may limit the effects of SARS-CoV-2 infection on the pancreas. 3) We followed up with 6 patients for up to 2 years. Considering that abnormal glycemia reverts to normal in recovered COVID-19 patients [52], it is unexpected that five survivors still had impaired glucose tolerance. More patients should be followed up for a longer time. 4) Due to the limited number of autopsies, we could not convincingly correlate virus proteins and inflammation markers in pancreatic tissue with disease severity and prognosis.
In this pilot study, OGTT and C-peptide release tests demonstrated that pancreatic islet function was impaired in patients with COVID-19, especially patients with severe or critical disease. SARS-CoV-2 virus particles were detected in the pancreas of patients with COVID-19, accompanied by altered expression of CD36 and other molecules potentially contributing to the pathogenesis of diabetes. Collectively, our data suggested that SARS-CoV-2 was present in the pancreas and that islet function was compromised in patients with COVID-19. The controversial relationship between the occurrence of diabetes in patients with COVID-19 and SARS-CoV-2 infection [53] warrants further study.
Acknowledgments
This study was funded by the Jiangsu Provincial Key Research and Development Program BE2020616 and the Ministry of Science and Technology of China No. 2020YFC0844700. We deeply appreciate the valuable advice and assistance from Xu Qi, Hui Kong, Ke Jin, Gang Yang, Jun Wu, Xin Yao, Kaisheng Yin from the First Affiliated Hospital of Nanjing Medical University and Ganzhu Feng from the Second Affiliated Hospital of Nanjing Medical University.
Funding Statement
This work was supported by Jiangsu Provincial Key Research and Development Program: [Grant Number BE2020616]; Ministry of Science and Technology of China: [Grant Number 2020YFC0844700].
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
MH, LL, YL and JT designed the study. NJ, LR, YW, BH, JX, CW, GQ, WD, ZY, SL, ZW, LZ, XC, and YM collected the data. NJ, MZ, and YG performed the statistical analysis and drafted the manuscript. LR, YW, JX and GQ revised the manuscript for critical content.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puelles VG, Lutgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383:590–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta R, Ghosh A, Singh AK, et al. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metab Syndr. 2020;14:211–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020;36:e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kusmartseva I, Wu W, Syed F, et al. Expression of SARS-CoV-2 entry factors in the pancreas of normal organ donors and individuals with COVID-19. Cell Metab. 2020;32:1041–1051.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coate KC, Cha J, Shrestha S, et al. SARS-CoV-2 cell entry factors ACE2 and TMPRSS2 Are expressed in the microvasculature and ducts of human pancreas but Are Not enriched in β cells. Cell Metab. 2020;32:1028–1040.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27:125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller JA, Gross R, Conzelmann C, et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3:149–165. [DOI] [PubMed] [Google Scholar]
- 10.Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang JK, Feng Y, Yuan MY, et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabetic Med. 2006;23:623–628. [DOI] [PubMed] [Google Scholar]
- 12.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in wuhan, China: a descriptive study. Lancet. 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin J-M, Bai P, He W, et al. Gender differences in patients with COVID-19: Focus on severity and mortality. medRxiv. 2020. doi: 10.1101/2020.02.23.20026864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michalakis K, Ilias I.. COVID-19 and hyperglycemia/diabetes. World J Diabetes. 2021;12:642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubino F, Amiel SA, Zimmet P, et al. New-Onset diabetes in COVID-19. N Engl J Med. 2020;383:789–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao XH, He ZC, Li TY, et al. Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient. Cell Res. 2020;30:541–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization . Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1, Diagnosis and classification of diabetes mellitus. . 1999.
- 18.Toth ZE, Mezey E.. Simultaneous visualization of multiple antigens with tyramide signal amplification using antibodies from the same species. J Histochem Cytochem. 2007;55:545–554. [DOI] [PubMed] [Google Scholar]
- 19.Stack EC, Wang C, Roman KA, et al. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of tyramide signal amplification, multispectral imaging and multiplex analysis. Methods. 2014;70:46–58. [DOI] [PubMed] [Google Scholar]
- 20.Iwata M, Maeda S, Kamura Y, et al. Genetic risk score constructed using 14 susceptibility alleles for type 2 diabetes is associated with the early onset of diabetes and may predict the future requirement of insulin injections among Japanese individuals. Diabetes Care. 2012;35:1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohkura T, Shiochi H, Fujioka Y, et al. 20/(fasting C-peptide x fasting plasma glucose) is a simple and effective index of insulin resistance in patients with type 2 diabetes mellitus: a preliminary report. Cardiovasc Diabetol. 2013;12:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization . Living guidance for clinical management of COVID-19. 2021. [PubMed]
- 23.Ma H, Wu X, Guo X, et al. Optimal body mass index cut-off points for prediction of incident diabetes in a Chinese population. J Diabetes. 2018;10:926–933. [DOI] [PubMed] [Google Scholar]
- 24.Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang K, Chen W, Zhou Y-S, et al. Signal Transduct Target Ther. 2020;5: 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantuti-Castelvetri L, Ojha R, Pedro LD, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aigha II, Abdelalim EM.. NKX6.1 transcription factor: a crucial regulator of pancreatic β cell development, identity, and proliferation. Stem Cell Res Ther. 2020;11:459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moon JS, Karunakaran U, Suma E, et al. The role of CD36 in type 2 diabetes mellitus: β-cell dysfunction and beyond. Diabetes Metab J. 2020;44:222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berger C, Zdzieblo D.. Glucose transporters in pancreatic islets. Pflügers Archiv - European Journal of Physiology. 2020;472:1249–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White MF. Irs proteins and the common path to diabetes. American Journal of Physiology-Endocrinology and Metabolism. 2002;283:E413–E422. [DOI] [PubMed] [Google Scholar]
- 32.Spaeth JM, Gupte M, Perelis M, et al. Defining a novel role for the Pdx1 transcription factor in islet β-cell maturation and proliferation during weaning. Diabetes. 2017;66:2830–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HI, Ahn YH.. Role of peroxisome proliferator-activated receptor-γ in the glucose-sensing apparatus of liver and β-cells. Diabetes. 2004;53(Suppl 1):S60–S65. [DOI] [PubMed] [Google Scholar]
- 34.Roper RL, Rehm KE.. Sars vaccines: where are we? Expert Rev Vaccines. 2009;8:887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arabi YM, Balkhy HH, Hayden FG, et al. Middle East respiratory syndrome. N Engl J Med. 2017;376:584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. [DOI] [PubMed] [Google Scholar]
- 37.Diao B, Wang C, Wang R, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat Commun 2021;12:2506–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paniz-Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol. 2020;92:699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao F, Sun J, Xu Y, et al. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerging Infect. Dis.. 2020;26:1920–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao XH, Luo T, Shi Y, et al. A cohort autopsy study defines COVID-19 systemic pathogenesis. Cell Res. 2021;31:836–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shojaei S, Suresh M, Klionsky DJ, et al. Autophagy and SARS-CoV-2 infection: A possible smart targeting of the autophagy pathway. Virulence. 2020;11:805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorens B. Glut2, glucose sensing and glucose homeostasis. Diabetologia. 2015;58:221–232. [DOI] [PubMed] [Google Scholar]
- 44.Brady MJ. Irs2 takes center stage in the development of type 2 diabetes. J Clin Invest. 2004;114:886–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spaeth JM, Walker EM, Stein R.. Impact of Pdx1-associated chromatin modifiers on islet β-cells. Diabetes, Obesity and Metabolism. 2016;18(Suppl 1):123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marchand L, Pecquet M, Luyton C.. Type 1 diabetes onset triggered by COVID-19. Acta Diabetol. 2020;57:1265–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sherwani SI, Khan HA, Ekhzaimy A, et al. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark Insights. 2016;11:BMI.S38440–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29:388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hollstein T, Schulte DM, Schulz J, et al. Autoantibody-negative insulin-dependent diabetes mellitus after SARS-CoV-2 infection: a case report. Nat Metab. 2020;2:1021–1024. [DOI] [PubMed] [Google Scholar]
- 50.Wan L, Gao Q, Deng Y, et al. Gp73 is a glucogenic hormone contributing to SARS-CoV-2-induced hyperglycemia. Nat Metab. 2022;4:29–43. [DOI] [PubMed] [Google Scholar]
- 51.He X, Liu C, Peng J, et al. COVID-19 induces new-onset insulin resistance and lipid metabolic dysregulation via regulation of secreted metabolic factors. Signal Transduction and Targeted Therapy. 2022;107:e1009–e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laurenzi A, Caretto A, Molinari C, et al. No evidence of long-term disruption of glycometabolic control after SARS-CoV-2 infection. J Clin Endocrinol Metab. 2022;107:e1009–e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drucker DJ. Diabetes, obesity, metabolism, and SARS-CoV-2 infection: the end of the beginning. Cell Metab. 2021;33:479–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.