Abstract
The objective of the present experiment was to evaluate the effect of maternal supplementation with fatty acids (FAs) and methionine (Met) during late gestation on offspring growth, energy metabolism, plasma resolvin (RvD1) concentration, carcass characteristics, and hepatic mRNA expression. Ewes (5 pens/treatment; 3 ewes/pen) blocked by body weight (BW) were assigned to one of four treatments from day 100 of gestation until lambing. The treatments were: basal diet (NS) without FAs or Met supplementation; FA supplementation (FS; 1.01 % of Ca salts, containing n-3 FA); Met supplementation (MS; 0.1 % of rumen-protected methionine); and FS and MS (FS-MS). At birth (day 0), ewes and lambs were placed in a common pen. On day 60, lambs were weaned, sorted by sex, blocked by BW, and placed on a common finishing diet for 54 d (FP). A lamb per pen was used for a glucose tolerance test (GTT) after the FP. Carcass characteristics were recorded on day 56. Lamb data were analyzed as a randomized complete block design with a 2 × 2 × 2 factorial arrangement, with repeated measurements when needed (SAS 9.4). At weaning, lambs born to MS- or FS-fed ewes were heavier than lambs born from FS-MS ewes (FS × MS × Time; P = 0.02). A marginal significant FS × MS interaction (P = 0.09) was also observed on RvD1; lambs born to ewes in the NS and FS-MS treatments showed a lower RvD1 plasma concentration when compared with lambs born to FS- or MS-fed ewes. Lambs born to dams fed FA showed an increase (P = 0.05) in liver COX-2 mRNA relative expression. Lambs born to ewes supplemented with Met showed an increase (P = 0.03) in liver FABP4 mRNA expression. An FS × MS × Time interaction (P = 0.07) was observed in plasma glucose during the GTT; lambs born from FS-fed ewes showed lower plasma glucose concentration than lambs born to Met-supplemented ewes at 2 min after bolus administration. During the GTT, a marginal significant effect (P = 0.06) was observed for the lamb average insulin concentration due to maternal Met supplementation during late gestation, where these lambs had the lowest plasma concentration. Contrary to our hypothesis, the interaction of FA and Met supplementation during late gestation did not show a greater positive effect on offspring postnatal growth and metabolism. However, the individual supplementation of each nutrient has an effect on offspring development with a concomitant change in markers involved in the inflammatory response and energy metabolism.
Keywords: fatty acids, fetal programming, prepartum diet, rumen protected methionine, sheep
Lay Summary
Late gestation supplementation of omega-3 fatty acids (FAs) or methionine (Met) alters the offspring’s development. However, the effect of both nutrients on the physiology and growth of the progeny has not been explored. The experiment’s objective was to evaluate the effect of dam supplementation with a Met and the omega-3 FAs, such as eicosapentaenoic and docosahexaenoic acids, during late gestation on growth, energy metabolism, and inflammatory response markers of the lamb. Ewes received one of the four following treatments: basal diet without FA or Met, FA supplementation, Met supplementation, or FA and Met supplementation. Supplementation of omega-3 FAs and Met did not show a greater effect on postnatal growth and metabolism of the offspring compared with the supplementation of each nutrient individually. However, individual supplementation influences offspring development with a concomitant change in markers involved in the inflammatory response and energy metabolism. Results of the present experiment suggest that offspring born to mothers that were fed FAs or Met on late gestation could have the ability to better cope with inflammatory processes, which could improve their long-term growth performance. Moreover, maternal supplementation of Met during late gestation could modulate offspring’s glucose–insulin system, which may also affect offspring’s growth.
Maternal supplementation with omega-3 polyunsaturated fatty acids or methionine during late gestation can modulate offspring’s glucose–insulin system and markers involved in the inflammatory response, which may be responsible for changes in long-term growth performance.
Introduction
Extrinsic stimulus during gestation, such as nutrient availability, can have fetal programming effects, which may impact the physiology, health, and metabolism throughout the life of the offspring. Consequently, maternal nutrition management during gestation is important for food-producing animals because of the potential lifelong impact on an animal’s health and as a feasible alternative to enhanced offspring productivity (Garcia et al., 2014; Batistel et al., 2019). In ruminants, maternal supply of omega-3 polyunsaturated fatty acids (PUFAs; Coleman et al., 2018a; Nickles et al., 2019) or methionine (Met; Batistel et al., 2019) during late gestation alters postnatal growth and physiology of the offspring, which have been associated with alterations in mRNA expression (Coleman et al., 2018a; Batistel et al., 2019). Likewise, supplementing the diet of the dam with these nutrients in late gestation modified in utero development and the fetal mRNA expression of genes involved in the inflammatory response, lipid metabolism, and DNA methylation (Rosa-Velaquez et al., 2020).
Omega-3 PUFAs during gestation increased the growth of the offspring (Marques et al., 2017; Carranza-Martin et al., 2018; Nickles et al., 2019; Rosa-Velazquez et al., 2021). In sheep, treating the dam with omega-3 PUFAs during the last third of gestation increased offspring’s hot carcass weight (HCW; Rosa-Velazquez et al., 2021). These findings on growth in sheep are consistent with that reported in studies conducted in dairy (Santos et al., 2013) and beef cattle (Marques et al., 2017; Brandão et al., 2020), in which PUFA supplementation of dairy cows during late gestation increased the calf body weight (BW) at birth (Santos et al., 2013). Furthermore, feeder calves from cows supplemented with PUFAs had greater mean final BW, carcass weight, and marbling when compared with calves from the control beef cows supplemented with a mixture of saturated FAs and monounsaturated FA (MUFA; Marques et al., 2017). In addition, Longissimus muscularis area was greater in calves from cows treated with calcium salts of soybean oil (Brandão et al., 2020). The greater growth of offspring derived from cows supplemented with PUFA during late gestation has been associated with changes in mRNA expression of genes involved in lipid metabolism, immune response, and DNA methylation (Coleman et al., 2018a; Rosa-Velazquez et al., 2020). Furthermore, maternal PUFA supplementation has demonstrated changes in markers of energy metabolism such as in the glucose–insulin system (Nickles et al., 2019; Rosa-Velazquez et al., 2021) and molecules involved in the inflammatory response in the offspring such as haptoglobin and resolvin D1(RvD1; Marques et al., 2017 and Rosa-Velazquez et al., 2020, respectively).
Dairy cows treated with rumen-protected methionine (RPM) during late gestation had calves with a greater BW at birth compared with calves from cows allocated to the control treatment (Batistel et al., 2019). Furthermore, the greater birth BW in calves from RPM-treated cows in the study of Batistel et al. (2019) was associated with changes in mRNA expression of genes involved in DNA methylation. Supplementing multiparous Holstein cows with methyl donors during gestation affected the immune response of the dam by downregulating genes related to the pro-inflammatory response in follicular cells (Acosta et al., 2017).
Methylation of DNA has an effect on fetal programming because it is a significant mechanism for gene expression regulation (Van den Veyver, 2001). Methylation of DNA depends on the availability of methyl donors supplied such as Met; therefore, it is possible that dietary supply of Met can alter the gene expression on the individual (Van den Veyver, 2001). Methylation of DNA is carried out by the covalent addition of a methyl group from S-adenosylmethionine (SAM) to cytosine by the action of DNA methyltransferase (DNMT) enzymes (Osorio et al., 2014). An increase of methionine-adenosyltransferase-1A expression (MAT1A), the enzyme that starts the reaction that converts Met to SAM in the Met cycle, was observed in dairy cows supplemented with RPM during the peripartum period with a concomitant increase in the gene expression of catalytic DNMT (Osorio et al., 2014).
Previous studies conducted in ruminants observed that maternal supply of PUFAs or Met during late gestation altered growth, molecules involved in the immune response, and mRNA expression of the offspring. Previous studies conducted in sheep where the effect of maternal supplementation with omega-3 PUFA and Met during late gestation in utero development was studied, reported changes in fetal growth, and gene expression (Rosa-Velazquez et al., 2020). However, it is still unknown whether the maternal dietary supply of both nutrients will affect an offspring’s growth and physiology during the growing period. We hypothesized that lambs born from ewes supplemented with both nutrients (a source of omega-3 PUFAs and Met) in the last third of gestation would have an improved energy metabolism by increasing insulin sensitivity, a greater concentration of molecules involved in the anti-inflammatory response, and a greater postnatal growth than lambs born from ewes supplemented with one nutrient or the other. Hence, the main objective of this study was to evaluate the effects of supplementing a source of omega-3 PUFAs and Met to pregnant ewes during late gestation on offspring’s growth, energy metabolism, plasma RvD1 concentration, carcass characteristics, and hepatic mRNA expression.
Materials and Methods
Experimental design and sampling
This research study was conducted at the Sheep Research Center of the Ohio Agricultural Research and Development Center, Wooster, OH. All procedures involving animals were approved by The Ohio State University Institutional Animal Care and Use Committee (IACUC #2019A00000001). Sixty pregnant Dorset × Hampshire ewes (BW 96.2 ± 14.6 kg) between 3 and 4 yr of age were blocked by BW and housed in 20 pens (3 ewes per pen) and supplemented from day 100 of gestation until lambing (day −50 and 0, respectively). Five Dorset rams were used (1 ram per block, 4 blocks) for natural service. The sire was part of the block error. Day one of pregnancy was considered the day on which a standing estrus was confirmed by the use of rams. On days 45 to 50 of gestation, a pregnancy check was performed with the use of a rectal ultrasound, and only pregnant ewes were allocated to the experiment.
Dam treatments were arranged in a 2 × 2 factorial. The main factors were FA supplementation containing omega-3 PUFAs and Met supplementation. Ewes within each block were randomly assigned to one of four treatments: 1) Ewes were fed a basal diet (NS) to meet sheep nutrients requirements during late gestation (NRC, 2007), with no FA or Met supplementation, 2) Basal diet supplemented with FA containing omega-3 PUFA, particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA; FS; 1.01 % DMI of Ca salts; Strata G113, Virtus Nutrition), 3) Basal diet supplemented with Met (MS; 0.1 0% DMI of RPM, Smartamine M, Adisseo), and 4) Basal diet supplemented with FA containing omega-3 PUFAs and Met (FS-MS; same doses and sources described for the FS and MS treatments).
Ewes were fed 2.02 kg/d of the treatment diet (Table 1). The dose of FA supplementation was based on previous research in pregnant ewes with the same product (Table 2) and dose rate (Carranza-Martin et al., 2018; Coleman et al., 2018a; Rosa-Velazquez et al., 2020). The source of FA was chosen because it is rumen inert and commercially available. Commercially, there is no rumen undegradable FA that contains purified EPA and DHA. The dose of RPM supplemented was determined from the results observed in previous studies in pregnant cows (Ordway et al., 2009; Li et al., 2016) and pregnant ewes (Rosa-Velazquez et al., 2020). The RPM supplement contains 75% dl-methionine (w:w) of which 90% of the available methionine is stated to pass to the small intestine, and 90% is absorbed. Consequently, 60% of methionine from this product can be found in the bloodstream (Whitehouse, 2016). Methionine supplementation was added to the diet at a rate of 0.1%, and it was individually weighed, added, and mixed into the feed in a daily dose of 2.019 g/ewe. Feed samples were taken weekly and pooled for further analysis. No feed refusals were reported during the entire period of the study. At birth (day 0), the supplementation stopped and all ewes and lambs were placed in a common pen and given ad libitum access to a common diet until weaning (day 60).
Table 1.
Diet ingredients and chemical composition (expressed as % DM basis) of the dietary treatments fed to pregnant ewes at 2.02 kg/d during the last 50 d of gestation and to their offspring during the finishing period.
| Maternal treatment | Offspring | ||||
|---|---|---|---|---|---|
| NS1 | FS2 | MS3 | FS-MS | ||
| Ingredient | |||||
| Alfalfa haylage | 17.96 | 17.96 | 17.96 | 17.96 | — |
| Corn silage | 30.54 | 30.54 | 30.54 | 30.54 | — |
| Ground corn | 10.10 | 8.89 | 10.10 | 8.89 | — |
| Dry rolled corn | — | — | — | — | 62.59 |
| DDGS | 10.10 | 10.07 | 10.10 | 10.07 | — |
| Limestone | 0.44 | 0.48 | 0.44 | 0.48 | — |
| Soy Hulls | 30.65 | 30.88 | 30.65 | 30.88 | 24.07 |
| Soybean meal | — | — | — | — | 11.08 |
| PUFA Ca salts (EPA +DHA)4 | — | 1.01 | — | 1.01 | — |
| Rumen-protected methionine5 | — | — | 0.10 | 0.10 | — |
| Mineral and vitamin premix | 0.206 | 0.186 | 0.206 | 0.186 | 2.367 |
| Chemical composition | |||||
| Neutral detergent fiber | 40.70 | 41.79 | 40.70 | 41.79 | 16.24 |
| Crude protein | 15.46 | 17.99 | 15.46 | 17.99 | 15.60 |
| Ash | 5.87 | 6.40 | 5.87 | 6.40 | 4.78 |
| Ether extract | 3.68 | 4.37 | 3.68 | 4.37 | 3.45 |
| NEm8 | 1.74 | 1.76 | 1.74 | 1.76 | 1.81 |
NS, basal diet with no supplementation; dams were fed to meet sheep requirements of nutrients (NRC, 2007).
FS, fatty acid supplementation; dams were fed to meet sheep requirements of nutrients (NRC, 2007) and supplemented with 10 g/kg of Ca salts of polyunsaturated fatty acids containing EPA and DHA (FA; StrataG113, Virtus Nutrition LLC, Corcoran, CA).
MS, methionine supplementation; dams were fed to meet sheep requirements of nutrients (NRC, 2007) and supplemented with 1 g/kg (dry matter basis) of rumen-protected methionine (Smartamine M, Adisseo, Alpharetta, GA).
StrataG113, Virtus Nutrition LLC.
Smartamine M, Adisseo.
Vitaferm Concept-Aid Sheep (BioZyme, St. Joseph, MO). Contains 15.5% Ca, 5% P, 16% NaCl, 4% Mg, 2% K, 10 mg/kg Co, 70 mg/kg I, 2,850 mg/kg Mn, 16.4 mg/kg Se, 2,500 mg/kg Zn, 130,000 IU/kg vitamin A, 7,500 IU/kg vitamin D3, and 550 IU/kg vitamin E.
Contains 19.35 % urea, 38.76 % of limestone, 19.36 % of sodium chloride, 0.4 % of Vitamin A, 0.4 % of Vitamin D3, 1.96 % of Vitamin E, 3.76 % selenium, 0.49 % Bovatec 91, and 15.52 % of ammonium chloride.
NEm values, in Mcal/kg, were estimated using NEm values of the feed ingredients based on NRC (2007), except for the Ca salts that were used the values from FEDNA (2010).
Abbreviations: DDGS, distiller’s dried grains with solubles; DHA, docosahexaenoic acid; DM, dry matter; FS-MS, supplementation with fatty acids and methionine; EPA, eicosapentaenoic acid; NEm, net energy for maintenance; PUFA, polyunsaturated fatty acids.
Table 2.
Fatty acid profile (% of total fatty acid) of Ca salts used to supplement pregnant ewes during the last 50 d of gestation
| Fatty acid | Supplement1,2 |
|---|---|
| FS | |
| C8:00.110.00C10:00.020.00C12:0 | 0.12 |
| C14:0 | 5.99 |
| C16:0 | 22.01 |
| C16:1 | 7.40 |
| C18:0 | 7.47 |
| C18:1 c9 | 17.46 |
| C18:1 other | 4.51 |
| C18:2 | 2.69 |
| C20:0 | 0.34 |
| C20:1 | 0.84 |
| C18:3n-3 | 0.94 |
| C22:0 | 0.35 |
| C22:1 | 1.38 |
| C20:3n-3 | 0.51 |
| C20:4n-6 | 0.00 |
| C20:5n-3 | 9.19 |
| C22:6n-3 | 7.00 |
| Other | 12.15 |
Ca salts of polyunsaturated fatty acids containing eicosapentaenoic acid and docosahexaenoic acid (fatty acids; StrataG113, Virtus Nutrition LLC, Corcoran, CA).
Fatty acid profiles were evaluated using the methods of Weiss and Wyatt (2003).
Ewes were weighed and body condition scored (BCS) at the beginning of the experiment (day −50) and at day −9. BCS was assessed using a 5-point scale (Russel et al., 1969). Ewe blood samples were taken on days −50 and 0.
Blood samples from lambs were collected on day 0, between 8 and 16 h after birth depending on whether the delivery happened overnight or during the daytime. Therefore, we assume that lamb blood samples were collected after colostrum consumption. At birth (day 0), one lamb per ewe was blood sampled; when there was a set of twins (43 dams) or triplets (3 dams), one lamb was randomly selected from the set for sampling. The incidence of twins per treatment was NS = 0.63, MS = 0.77, FS = 0.78, and FS-MS = 0.64. The incidence of triplets per treatment was NS = 0.06, MS = 0.07, FS = 0, and FS-MS = 0.07. The number of lambs per ewe was included as a covariate for changes in weight. From the ewes used in this experiment, 100 lambs were born. After weaning and before starting the finishing period, three of these lambs died, two born to dams on the FS treatment died due to Listeria infection and one born to a dam from the MS treatment died of unknown reasons. Of the remaining lambs, only 72 were used in the rest of the experiment. There were no differences between treatments in early postnatal morbidity in lambs.
During the preweaning period, all lambs were weighed on days 0, 30, and 60. On weaning day (day 60), all lambs were blood sampled and blocked by BW into three groups (heavy, medium, and light blocks). After weaning, lambs had an adaptation to a pelleted finishing diet for 1 wk for the heavy, 2 wk for the medium, and 3 wk for the light blocks. After the adaptation to the pelleted diet, the finishing diet was fed for 54 d (Table 1). The first day of the finishing period was staggered a week between blocks. Starting days for the finishing period were days 67, 74, and 81 for the heavy, medium, and light blocks, respectively. On the initial day of the finishing period, lambs (n = 72) were weighed, allotted by sex, and placed in 24 pens with 3 lambs per pen and 6 pens per treatment. The pens were balanced for sex such that 3 pens per treatment were ewes and 3 pens per treatment were wethers. Lambs were blood sampled and weighed on the initial day of the finishing period, and 28 and 54 d after the lambs started to eat the finishing diet (fd 0, fd 28, and fd 54, respectively).
Blood samples from ewes (10 mL) and lambs (6 mL at birth and 10 mL for the remainder of the sampling) were taken from the jugular vein and transferred into 14 mL polypropylene tubes (VWR International, Radnor, Pa) containing disodium EDTA (1.6 mg/mL of blood) and benzamidine HCL (4.7 mg/mL of blood). The tubes were immediately placed on ice and centrifuged for 25 min at 1,800 × g and 4 °C. After centrifugation, plasma was aliquoted and stored in four individual 1.5 mL micro polypropylene tubes with snap caps (VWR International, Radnor, Pa, USA) at −80 °C until analysis. At birth, both dam and offspring’s blood samples were analyzed for plasma nonesterified fatty acids (NEFAs) and glucose concentration. Blood samples of the light blocks for the last sampling (fd 54) were not analyzed because of problems with the temperature after processing. Offspring blood samples at birth were also analyzed for plasma FAs and plasma RvD1 concentration.
On fd 55, 24 lambs (1 per pen and 6 per treatment) were randomly selected to conduct a GTT. Catheters (Milacath extended use # 1603, 16 g × 3.0 inches, Mila International, Inc., USA) were placed in the jugular vein of each lamb using aseptic procedures (Rosa-Velazquez et al., 2021). Once catheters were placed, lambs were moved into individual pens without feed for 24 h and provided ad libitum access to water.
Lambs were weighed 30 min before glucose administration to determine the glucose bolus size (0.25 g of glucose/kg of BW in a 50% wt/vol dextrose solution). Blood samples were collected 5 min prior to glucose administration and at 2, 5, 10 15, 20, 30, 60, and 90 min after glucose administration. Before each blood sample was collected and to remove all catheter content, blood (1 mL) was collected in a spare syringe, and the contents were discarded. Blood samples of 10 mL were collected, and after the blood was collected, 1 mL of heparin solution (10 IU of heparin/mL and 0.9% NaCl) was infused into the catheter to prevent clotting. Once 10 mL of blood was collected, 7 mL was transferred into a polypropylene tube (VWR International, Radnor, Pa) similar to the ones used for the ewe blood samples (containing solutions of disodium EDTA and benzamidine HCL), to measure plasma ghrelin and insulin concentration, and 3 mL was transferred into 4 mL BD Vacutainer plastic tubes with Fluoride (0268847, Fisher Scientific, Pittsburg, Pa, USA) to evaluate glucose plasma concentration. Both tubes were immediately placed in ice and centrifuged using the same protocol to separate plasma from blood as explained previously. Plasma from tubes was aliquoted and stored into micro polypropylene tubes with snap caps (1.5 mL, VWR International, Radnor, Pa, USA) at −80 °C until analyzed for glucose and insulin. The procedure used to determine plasma ghrelin concentration was similar; however, plasma was placed in similar micro tubes that were acidified with the addition of 50 µl of 1 N HCl and 10 μL of phenylmethylsulphonyl fluoride per 1 mL of plasma. Plasma glucose and insulin concentration were measured at 5 min prior to the bolus administration and at 2, 5, 10 15, 20, 30, 60, and 90 min after the glucose bolus administration, while plasma ghrelin concentration was measured 5 min prior to glucose administration and at 2 and 5 min relative to the glucose bolus administration.
On fd 56, another group of 24 lambs (one per pen was randomly selected, six per treatment) was harvested at the Meat laboratory (Department of Animal Sciences, Ohio State University, Columbus, OH, USA). Lambs were euthanized by captive bolt followed by exsanguination. At harvest, lamb liver samples were collected, and HCW was recorded. Liver samples were placed into labeled cryogenic vials (Thermo Fisher Scientific, Waltham, Ma, USA), flash-frozen in liquid nitrogen, and stored at –80 °C until further analysis. The time for lamb euthanasia until the liver sample was frozen was ≤5 min. Lamb carcasses were stored overnight in a walk-in cooler maintained at 4 °C prior to recording carcass data; a trained university employee determined rib eye area (REA), back fat thickness (BFT), body wall thickness (BWT), and marbling score.
Sample analysis
Pool feed sample was analyzed according to the AOAC (1990) for dry matter (DM; method number 981.10), crude protein (CP; method number 967.03), neutral detergent fiber (NDF), and acid detergent fiber (ADF) according to Van Soest et al. (1991) with a heat-stable amylase included in the NDF and expressed including residual ash. The total FA composition of Ca salts was determined using the methods described by Weiss and Wyatt (2003; Table 2).
Plasma total FAs were extracted as described by Folch et al. (1957), with few modifications (Coleman et al., 2018a). The extracted FAs were methylated as described by Doreau et al. (2007). All FA methyl esters were separated by gas chromatography (GC, model HP 5890) using a CP-SIL88 capillary column (film thickness: 100-m × 0.25-mm × 0.2-µm; Varian Inc., Palo Alto, CA, USA).
Resolvin D1 concentration in lamb’s plasma at birth was quantified with the use of a commercial kit (Resolvin D1 ELISA, Cayman Chemical, Ann Arbor, MI, USA). We validated the assay based on a parallel displacement of serial dilutions of ovine plasma compared with the RvD1 standard curve. The recovery for RvD1 was 101.03 ± 3.02%. The intraassay and interassay coefficients of variation were 5% and 14 %, respectively. The RvD1 assay was conducted according to the manufacturer’s protocol with slight modifications. Based on the parallel displacement results, it was decided not to extract the samples. Samples were diluted at a ratio of 1:1 RvD1 assay buffer to plasma to fit the values in the standard curve. Plasma glucose (1070 Glucose Trinder, Stanbio Laboratory, Boerne, TX, USA) and NEFA (NEFA Wako Pure Chemical 1, FUJIFILM Wako Diagnostics USA Corporation, Richmond, VA, USA) concentrations were measured using a commercial colorimetric assay as validated previously by Relling et al. (2010) for sheep. Intraassay and interassay coefficients of variation were 3.07% and 1.42% for glucose and 3.25% and 0.25% for NEFAs. Plasma insulin concentration was evaluated using a commercial kit (EMD Millipore Corporation, Billerica, MA, USA). This assay was validated for sheep using a parallel displacement as described previously for the RvD1 assay. This assay detected a concentration of insulin as low as 1.611 µU/mL when using a 100-µL sample size. The interassay coefficient of variation was 8.79%. Plasma acetylated ghrelin concentration was measured using a commercial assay, Linco’s Ghrelin (Active) Radioimmunoassay Kit (GHRA-88HK, Linco Research, St. Charles, MO, USA), which was validated previously for sheep (Relling et al., 2010). The intraassay coefficient of variation was 7.12%, and the minimum sensitivity was 7.8 pg/mL.
Lamb liver RNA was extracted using the procedure for total RNA Isolation from animal tissue using RNAzol RT (Molecular Research Center, Inc., Cincinnati, OH, USA) modified for liver tissue; RNAzol RT (1 mL) and 0.15 g of 0.1 mm Zirconia/Silica beads (Cat. No. 11079101z BioSpec Products, Bartlesville, OK) were added to a 2-mL tube (round bottom safe-lock, cat. No. 022363352 Eppendorf North America) and then chilled on ice. Frozen liver tissues were quickly weighed (0.05 g) into ice-cold RNAzol RT. The sample was homogenized for 1 min with a bead beater at 3450 RPM (MiniBeadBeater-16 Model 607, BioSpec Products, Bartlesville, OK), put on ice for 1 min, and then homogenized once again for 1 min. After homogenization, the manufacturer’s instructions were followed (RNAzol RT, Molecular Research Center, Inc., Cincinnati, OH). Samples of RNA were stored at −80°C until analysis. The concentration of RNA was measured using UV spectroscopy (Nanodrop Technologies). Sizing, quantitation, integrity, and purity from RNA were assessed using a BioAnalyzer 2100 and RNA NanoChip assay (Agilent Technologies).
The relative mRNA expression was determined using NanoString nCounter XT Assay (NanoString Technologies, Seattle, WA) for 32 genes (Table 3) as described in the study of Coleman et al. (2018a). These genes were chosen based on their participation in the RvD1 metabolic pathway, inflammatory response pathway, Met cycle, DNA methylation, FA uptake and release, FA synthesis and transcription factors, and housekeeping genes. Treatment’s effect was evaluated for the abundance of mRNA of the housekeeping genes; there were no treatment effects on abundances of any of the five mRNA transcripts of genes of the tissues. Thus, the five housekeeping target genes were used to normalize the data.
Table 3.
Name and GenBank accession number of the genes evaluated on lamb liver samples during the finishing period1
| Gene2 | Accession number |
|---|---|
| Resolvin metabolic pathway | |
| ALOX15 | XM_027975516.1 |
| ALOX15B | XM_027974935.1 |
| ALOX5 | XM_015104505.1 |
| ALOX5AP | XM_012184625.2 |
| COX-2 | NM_001009432.1 |
| Inflammatory response | |
| TNF | NM_001024860.1 |
| IL1B | NM_001009465.2 |
| IL6 | NM_001009392.1 |
| Met cycle | |
| MAT1A | XM_012105414.2 |
| AHCY | XM_004014507.2 |
| DNA methylation | |
| DNMT1 | NM_001009473.1 |
| DNMT2 | XM_004014246.3 |
| DNMT3A | XM_012166008.2 |
| DNMT3B | XM_012189044.2 |
| Lipid metabolism | |
| DGAT1 | NM_001110164.1 |
| FATP1 | XM_015095580.1 |
| FABP4 | NM_001114667.1 |
| FADS1 | XM_012101996.2 |
| FADS2 | XM_015103138.1 |
| ELOVL2 | XM_012101293.2 |
| FFAR1 | XM_015095580.1 |
| FFAR4 | XM_015100194.1 |
| FASN | NM_001123003.1 |
| SCD | NM_001009254.1 |
| PPARA | XM_012175774.2 |
| PPARD | XM_004018768.3 |
| PPARG | NM_001100921.1 |
| Housekeeping | |
| APOB | XM_012175938.1 |
| TBP | XM_015097549.1 |
| PPIB | XM_004010536.3 |
| PGK1 | NM_001142516.1 |
| POLR1B | XM_004005912.3 |
ALOX15, arachidonate 15-lipoxygenase; ALOX15B, 15-lipoxygenase-2; ALOX5, arachidonate 5-lipoxygenase; ALOX5AP, arachidonate 5-lipoxygenase activating protein; COX-2, prostaglandin-endoperoxide synthase 2; TNF, tumor necrosis factor-alpha; IL1B, interleukin 1 Beta; IL6, interleukin 6; MAT1A, methionine adenosyltransferase 1A; AHCY, adenosylhomocysteinase; DNMT1, DNA methyltransferase 1; DNMT2, DNA methyltransferase 2; DNMT3A, DNA methyltransferase 3 Alpha; DNMT3B, DNA methyltransferase 3 Beta; DGAT1, diacylglycerol O-acyltransferase 1; FATP1, fatty acid transport protein 1; FABP4, fatty acid-binding protein 4; FADS1, delta-5-desaturase; FADS2, delta-6-desaturase; ELOVL2, ELOVL fatty acid elongase 2; FFAR1, free fatty acid receptor 1; FFAR4, free fatty acid receptor 4; FASN, fatty acid synthase; SCD, stearoyl-CoA desaturase; PPARA, peroxisome proliferator activated receptor alpha; PPARD, peroxisome proliferator activated receptor delta; PPARG, peroxisome proliferator activated receptor gamma; APOB, apolipoprotein B; TBP, TATA-box-binding protein; PPIB, cyclophilin B; PGK1, phosphoglycerate kinase 1; POLR1B, RNA polymerase I subunit B.
Statistical analyses
The number of experimental units needed per treatment was estimated using previous data, considering the variation in mRNA expression of reported genes from ruminant fetal programming studies (Coleman et al., 2018a; Batistel et al., 2019). Ewe performance data (BW, average daily gain [ADG], and BCS) and plasma glucose and NEFA concentration were analyzed as a randomized complete block design using the MIXED procedure of SAS (9.4, SAS Institute, Cary, NC, USA). The model tested the fixed effects of FA supplementation, Met supplementation, their interaction, and the random effect of pen within each block. Pen was the experimental unit. Initial BW and BCS were used as covariates for changes in dam BW and BCS, respectively.
Lamb plasma RvD1 and FA concentration at birth data were analyzed as a randomized complete block design using the MIXED procedure (9.4, SAS Institute, Cary, NC, USA). The model tested the fixed effects of dam treatment (FA supplementation, Met supplementation, and their interaction), and the random effect of the pen within each block. Lamb sex was added as the second block criteria for the plasma RVD1 concentration.
Lamb BW, ADG, and DMI during the finishing period, and plasma concentration of hormones and metabolites data, were analyzed as a randomized complete block design with repeated measures using the MIXED procedure (9.4, SAS Institute, Cary, NC, USA). The model tested the fixed effects of dam treatments (main effects and interactions), lamb sex, time, the interactions between the aforementioned, and the random effects of pen or lamb (if only one lamb was sampled, i.e., GTT and carcass data) within each block. Pen (or lamb, if only one animal per pen was sampled) was considered the experimental unit. The day was included as a repeated measurement. For lamb data before weaning, type of birth (single or multiple) was included as a covariate and removed if it was not significant (P > 0.1). Different covariance structures were compared (compound symmetry, unstructured, autoregressive, and variance components) for the repeated measurements. The compound symmetry structure was used based on the lowest Akaike information criterion. To determine the denominator degrees of freedom for tests of fixed effects, the Kenward Rogers degrees-of-freedom approximation was used.
Least square means (LSMEANS) and SEM were determined using the LSMEANS statement in the MIXED procedure. Significance was set at P ≤ 0.05; marginal significance was determined at P > 0.05 and P ≤ 0.10. The SLICE option of SAS was used for mean separation when the P-value for an interaction with time was ≤0.10 and discussed as different if the P-value of the comparison was ≤0.05. In the case of significant difference on main effect, double, or triple interaction, the PDIFF option of SAS was used for mean separation.
Results
Ewe BW, BCS, and plasma metabolites
The different treatments fed to ewes during the last 50 d of gestation had no observed effect (P > 0.20) on the dam’s BW, ADG, BCS, or plasma glucose concentration (Supplementary Table 1). There was, however, a marginal significant effect (P = 0.08) for the interaction of treatments for plasma NEFA concentration, where ewes supplemented with both nutrients had lesser plasma NEFA concentration when compared with the ewes in the other three treatments.
Lamb growth performance until weaning, plasma metabolites, and plasma FA profile and RvD1 at birth
Lamb sex or sex by treatments interaction did not have any effect (P ≥ 0.33) on lamb’s BW or plasma glucose concentration during the preweaning period (Table 4). A dam’s treatment by time interaction was observed (FS × MS × Time; P = 0.02) for offspring BW during the preweaning period. At birth and at 30 d of age, all lambs showed similar BW (P ≥ 0.35). At 60 d of age (weaning), lambs born to ewes in the FS-MS treatment were lighter than lambs born from MS and FS (P < 0.01). Lambs born from NS ewes had a similar weight compared with the other three treatments (P ≥ 0.11).
Table 4.
Effect of maternal supplementation with a source of omega-3 PUFA and Met during the last 50 d of gestation on lamb body weight (BW) and plasma metabolites during the preweaning period1
| Item | Treatments | SEM | P-value2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NS | MS | FS | FS-MS | FS | MS | Time | Sex | FS × MS | MS × Time | FS × Sex | FS × MS × Time | FS × MS × Sex | ||
| Lambs (pens) | 18 (6) | 18 (6) | 18 (6) | 18 (6) | ||||||||||
| BW, kg3,4 | ||||||||||||||
| Birth (day 0) | 5.62 | 5.72 | 5.47 | 5.66 | 1.28 | 0.64 | 0.99 | <0.01 | 0.33 | 0.12 | 0.67 | 0.49 | 0.02 | 0.62 |
| Day 30 | 16.42 | 17.17 | 17.13 | 16.85 | ||||||||||
| Day 60 | 26.83a,b | 28.09a | 27.62a | 25.82b | ||||||||||
| Glucose, mg/dL3 | ||||||||||||||
| Birth (day 0) | 158.71a | 116.05c | 121.41b | 104.06d | 13.76 | 0.18 | 0.09 | 0.55 | 0.75 | 0.38 | 0.05 | 0.66 | 0.53 | 0.33 |
| Day 60 | 122.17a | 119.19b | 118.88c | 122.24a | ||||||||||
| NEFA, µEq/L3 | ||||||||||||||
| Birth (day 0) F | 992.59b | 789.43c | 711.23c | 1,094.89a | 137.77 | 0.19 | 0.87 | <0.01 | 0.03 | 0.09 | 0.43 | 0.08 | 0.33 | 0.06 |
| Birth (day 0) M | 819.22b | 849.38b | 548.26d | 553.68d | ||||||||||
| Day 60 F | 389.91a | 292.47d | 366.66b | 381.63a | 81.97 | |||||||||
| Day 60 M | 365.96b | 344.66b | 359.12b | 318.00c | ||||||||||
Data are presented as a least square means ± SEM.
FS, lipid effect of FA supplementation in the dam diet; MS, methionine effect of ME supplementation in the dam; FS × MS= lipid and methionine effect of FS-MS supplementation in the dam.
Lamb’s sex did not have an effect.
Interactions not presented had a P-value > 0.32.
–cValues with different superscripts differ with a P-value ≤ 0.05.
Abbreviations: F, female; FS, fatty acid; FS-MS, fatty acids and methionine supplementation; M, male; MS, NEFA, nonesterified fatty acids; NS, basal diet with no supplementations.
Regarding the plasma glucose concentration of the lambs during the preweaning period, there was a dam’s Met supplementation by time interaction (P = 0.05), where, at birth, lambs born to ewes in the Met-supplemented ewes (MS and FS-MS treatments) showed a lesser plasma glucose concentration compared with lambs born to ewes not supplemented with Met (Table 4). On the other hand, at 60 d of birth, lambs born to Met-supplemented ewes (MS and FS-MS) had a greater plasma glucose concentration compared with lambs born to ewes in the FS treatment and similar plasma glucose concentration compared with lambs born to ewes in the NS treatment. Plasma NEFA concentration demonstrated a time effect (P < 0.01) and a marginal significant effect for a treatment by sex interaction (FS × MS × Sex; P = 0.06). At birth, lambs born to ewes in the four dietary treatments showed a greater (P < 0.01) plasma NEFA concentration compared with the lambs NEFA concentration at weaning. Moreover, ewe lambs and wethers presented an opposite concentration in plasma NEFA during the preweaning period, where ewe lambs born to FS-MS ewes showed a greater plasma NEFA concentration compared with weathers born to ewes in the same treatment, and ewe lambs born to MS ewes had a lesser plasma NEFA concentration compared with weathers born from ewes in the same treatment (Table 4).
Two FS × MS interactions were observed for plasma FAs profile at lambing (Table 5). Lambs born from Met-supplemented ewes (FS-MS and MS) showed a lesser (P = 0.01) C18:1 t6-8 plasma concentration than the ones born to ewes in the other two treatments. Furthermore, lambs born to ewes supplemented with both nutrients during late gestation showed a marginal significant effect (P = 0.08) for plasma conjugated linoleic acid (CLA) concentration when compared with lambs in the other three treatments (Table 5). On the other hand, a greater (P ≤ 0.03) docosanoic (C22:0), DHA, total n-3, and total EPA and DHA plasma concentration was observed; also a marginal significant effect (P ≤ 0.1) for a greater trans vaccenic acid (C18:1 t11), and EPA plasma concentration was observed in lambs born to FA supplemented ewes (FS and FS-MS) when compared with lambs born from ewes in the other two treatments (Table 5). In addition, a lesser (P ≤ 0.02) plasma concentration of C18:1 c12 and C18:1 c15 was observed in lambs born to FS dams when compared with lambs born in the other three treatments. Moreover, lambs born to ewes supplemented with Met during late gestation showed greater (P ≤ 0.04) plasma concentration of C8:0, C10:0, C14:0, and C18:1 c15. Also, lambs born to Met supplemented ewes reported a marginal significant effect (P ≤ 0.09) for a greater plasma concentration of C12:0 and C16:0 in comparison with lambs born from dams not supplemented with methionine during late gestation (Table 5).
Table 5.
Effect of maternal supplementation during the last 50 of gestation on lambs’ plasma fatty acid concentration (% total fatty acid methyl esters) at birth1
| Item | Treatment2 | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| NS | MS | FS | FS-MS | FS | MS | FS × MS | ||
| C4:0 | 0.19 | 0.11 | 0.07 | — | 0.08 | 0.18 | 0.37 | 0.94 |
| C6:0 | 0.26 | 0.42 | 0.17 | 0.26 | 0.16 | 0.32 | 0.34 | 0.77 |
| C8:0 | — | 0.04 | 0.01 | 0.06 | 0.02 | 0.42 | 0.04 | 0.78 |
| C10:0 | 0.34 | 0.67 | 0.30 | 0.74 | 0.14 | 0.90 | <0.01 | 0.62 |
| C12:0 | 0.78 | 1.21 | 0.57 | 0.93 | 0.25 | 0.24 | 0.07 | 0.87 |
| C14:0 | 5.32 | 7.97 | 4.31 | 7.17 | 1.47 | 0.45 | 0.03 | 0.93 |
| C15:0 iso | 0.17 | 0.16 | 0.21 | 0.16 | 0.07 | 0.77 | 0.63 | 0.79 |
| C15:0 ante | 0.27 | 0.31 | 0.14 | 0.26 | 0.08 | 0.22 | 0.25 | 0.54 |
| C15:0 | 0.76 | 0.75 | 0.72 | 0.72 | 0.07 | 0.58 | 0.96 | 0.90 |
| C16:0 iso | 0.14 | 0.17 | 0.20 | 0.19 | 0.06 | 0.55 | 0.89 | 0.75 |
| C16:0 | 28.21 | 30.38 | 26.33 | 30.32 | 2.06 | 0.57 | 0.09 | 0.59 |
| C17 iso | 1.36 | 0.85 | 1.02 | 0.96 | 0.24 | 0.56 | 0.17 | 0.27 |
| C16:1 & C17:0 ante | 3.37 | 2.84 | 3.22 | 3.28 | 0.46 | 0.70 | 0.53 | 0.43 |
| C17:0 | 0.70 | 0.56 | 0.58 | 0.53 | 0.06 | 0.24 | 0.12 | 0.45 |
| C18:0 | 10.90 | 9.62 | 10.58 | 9.10 | 1.18 | 0.68 | 0.17 | 0.91 |
| C18:1 t6-8 | 0.85a | 0.46c | 0.56b | 0.50c | 0.11 | 0.21 | 0.03 | 0.10 |
| C18:1 t9 | 0.15 | 0.22 | 0.26 | 0.18 | 0.05 | 0.43 | 0.87 | 0.12 |
| C18:1 t10 | 1.31 | 1.67 | 2.76 | 2.18 | 0.74 | 0.12 | 0.86 | 0.44 |
| C18:1 t11 | 0.62 | 0.75 | 1.73 | 0.89 | 0.43 | 0.10 | 0.36 | 0.21 |
| C18:1 t12 | 0.66 | 0.52 | 0.55 | 0.49 | 0.17 | 0.62 | 0.51 | 0.79 |
| C18:1 c9 | 31.62 | 27.34 | 26.59 | 27.49 | 3.77 | 0.43 | 0.58 | 0.40 |
| C18:1 c11 | 2.27 | 1.91 | 2.33 | 1.97 | 0.50 | 0.87 | 0.38 | 0.99 |
| C18:1 c12 | 0.27b | 0.36a | 0.12c | 0.17c | 0.10 | 0.05 | 0.36 | 0.81 |
| C18:1 c13 | — | 0.05 | 0.04 | 0.04 | 0.03 | 0.68 | 0.42 | 0.45 |
| C18:1 c15 | 0.04b | 0.11a | - | 0.04b | 0.02 | 0.02 | 0.02 | 0.47 |
| C18:1 c16 | 0.27 | 0.21 | 0.31 | 0.28 | 0.10 | 0.48 | 0.85 | 0.59 |
| C18:2 c9, c12 | 4.41 | 5.59 | 9.97 | 5.52 | 3.82 | 0.38 | 0.60 | 0.37 |
| C18:3 | 0.15 | 0.23 | 0.22 | 0.21 | 0.09 | 0.72 | 0.63 | 0.57 |
| C20:0 | 0.10 | 0.20 | 0.19 | 0.23 | 0.07 | 0.38 | 0.32 | 0.66 |
| C20:1 | 0.20 | 0.31 | 0.45 | 0.39 | 0.16 | 0.26 | 0.85 | 0.57 |
| C18:2 c9, t11 | 0.47 | 0.76 | 0.75 | 0.88 | 0.22 | 0.31 | 0.29 | 0.68 |
| CLA3 other | 0.11 | 0.03 | — | 0.15 | 0.07 | 0.99 | 0.59 | 0.08 |
| C22:0 | 0.49c | 0.32d | 1.01a | 0.66b | 0.23 | 0.05 | 0.23 | 0.65 |
| C20:3 n-6 | 0.05 | 0.12 | 0.21 | 0.14 | 0.07 | 0.15 | 0.95 | 0.25 |
| C22:1 | 1.62 | 1.32 | 1.48 | 1.28 | 0.33 | 0.77 | 0.43 | 0.87 |
| C20:5 n-3 | 0.23 | 0.11 | 0.56 | 0.32 | 0.18 | 0.08 | 0.25 | 0.69 |
| C24:0 | 0.12 | 0.08 | 0.21 | 0.13 | 0.08 | 0.27 | 0.36 | 0.76 |
| C22:6 n-3 | 0.49d | 0.32c | 1.01a | 0.67b | 0.23 | 0.05 | 0.23 | 0.65 |
| Total MUFA | 40.15 | 34.54 | 34.70 | 35.05 | 4.45 | 0.49 | 0.47 | 0.41 |
| Total PUFA | 5.33 | 6.38 | 11.92 | 6.85 | 4.15 | 0.30 | 0.55 | 0.37 |
| Total n-33 | 0.87c | 0.68d | 1.78a | 1.21b | 0.37 | 0.03 | 0.24 | 0.57 |
| Total n-63 | 4.47 | 5.71 | 10.18 | 5.65 | 3.87 | 0.37 | 0.60 | 0.36 |
| Total EPA and DHA3 | 0.72c | 0.44d | 1.55a | 0.98b | 0.38 | 0.05 | 0.20 | 0.65 |
| n-6/n-3 | 6.12 | 6.55 | 4.38 | 5.45 | 2.85 | 0.51 | 0.72 | 0.88 |
Data are presented as a least square means ± SEM.
Treatments of ewes supplemented with FS, fatty acid supplementation—dams were fed to meet sheep requirements of nutrients (NRC, 2007) and supplemented with 10 g/kg of Ca salts of polyunsaturated fatty acids containing EPA and DHA (FA; StrataG113, Virtus Nutrition LLC, Corcoran, CA); MS, methionine supplementation—dams were fed to meet sheep requirements of nutrients (NRC, 2007) and supplemented with 1 g/kg (dry matter basis) of rumen-protected methionine (Smartamine M, Adisseo, Alpharetta, GA); FS-MS, supplementation with fatty acids and methionine.
CLA, conjugated linoleic acid; n-3, omega-3; n-6, omega-6; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
–cValues with different superscripts differ with a P-value ≤ 0.05.
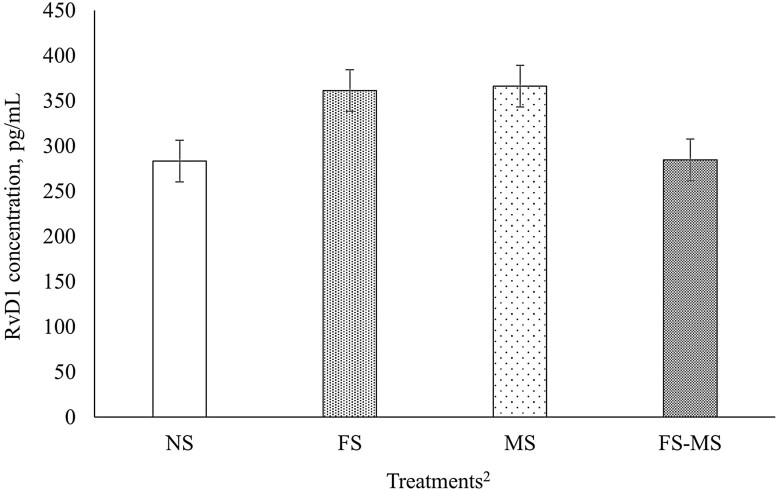
At birth, an FS × MS interaction (P = 0.09) was observed for plasma RvD1 concentration, and lambs born to ewes in the NS and FS-MS treatments showed a lesser plasma concentration of RvD1 when compared with those born from the FS or MS treatments (Figure 1).
Figure 1.
Effect of maternal supplementation with a source of omega-3 and Met during the last 50 d of gestation on plasma Resolvin (RvD1) concentrations at birth in male and female lambs.1 1Data are presented as a least square mean of FS × MS (P = 0.09; SEM = 56.87). No difference (P ≥ 0.12) for FS, MS, Sex, or FS × MS × Sex. 2FS, lipid effect of FA supplementation in the dam diet; MS, methionine effect of ME supplementation in the dam; FS × MS, lipid and methionine effect of FS-MS supplementation in the dam.
Lamb growth performance, plasma metabolites, and liver mRNA expression during the finishing period
No two-, three-, or four-way interactions (P ≥ 0.12) were observed on lamb growth variables. There were a sex and a time effect (P < 0.01) and a marginal significant effect (P = 0.09) for MS in lamb BW during the finishing period. Wethers were heavier than ewe lambs (P < 0.01; Table 6). Moreover, there was a marginal significant effect (P = 0.09) for BW and Met supplementation; lambs born to Met-supplemented (MS and FS-MS) ewes were lighter than lambs born to ewes in the NS and FS treatments during the whole 54-d period after weaning (Table 6). Offspring ADG, feed intake, gain-to-feed ratio, plasma glucose, and NEFA concentration were not affected (P ≥ 0.17) by maternal supplementation or sex of the lamb during the finishing period (Table 6). There was, however, a marginal significant effect for an FS × Sex interaction (P = 0.08), where wethers born to ewes supplemented with a source of omega-3 (FS, and FS-MS) had greater plasma NEFA concentration than ewe lambs born to dams in the same treatments.
Table 6.
Effect of maternal supplementation with a source of omega-3 PUFA and Met during the last 50 d of gestation on body weight (BW) and plasma metabolites during the finishing period in male (M) and female (F) lambs1
| Treatment1 | NS | MS | FS | FS-MS | SEM | P-value* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lamb sex | F | M | F | M | F | M | F | M | FS | MS | Sex | Time | |
| Lambs (pens) | 9 (3) | 9 (3) | 9 (3) | 9 (3) | 9 (3) | 9 (3) | 9 (3) | 9 (3) | |||||
| BW, kg2 | 3.65 | 0.46 | 0.09 | <0.01 | <0.01 | ||||||||
| fd 0 | 32.84 | 32.06 | 30.57 | 33.49 | 31.93 | 34.45 | 29.64 | 32.69 | |||||
| fd 28 | 41.02 | 40.45 | 38.37 | 42.33 | 39.24 | 42.86 | 37.63 | 40.47 | |||||
| fd 54 | 48.56 | 48.33 | 46.65 | 50.15 | 46.82 | 50.29 | 44.88 | 47.90 | |||||
| ADG, kg/d3 | 0.07 | 0.17 | 0.99 | 0.39 | 0.83 | ||||||||
| FP1 | 0.29 | 0.30 | 0.28 | 0.32 | 0.26 | 0.30 | 0.28 | 0.28 | |||||
| FP2 | 0.29 | 0.29 | 0.32 | 0.30 | 0.29 | 0.29 | 0.28 | 0.28 | |||||
| DMI, kg3 | 0.25 | 0.28 | 0.89 | 0.19 | <0.01 | ||||||||
| FP1 | 2.73 | 2.70 | 2.56 | 2.89 | 2.59 | 2.88 | 2.45 | 2.79 | |||||
| FP2 | 3.25 | 3.02 | 3.22 | 3.36 | 2.98 | 3.08 | 2.67 | 3.14 | |||||
| G:F3 | 0.02 | 0.93 | 0.54 | 0.48 | <0.01 | ||||||||
| FP1 | 0.24 | 0.24 | 0.24 | 0.24 | 0.22 | 0.23 | 0.26 | 0.22 | |||||
| FP2 | 0.19 | 0.21 | 0.21 | 0.20 | 0.21 | 0.20 | 0.22 | 0.20 | |||||
| Glucose, mg/dL4 | 68.75 | 61.71 | 67.57 | 62.28 | 69.96 | 66.94 | 65.81 | 63.79 | 8.61 | 0.80 | 0.74 | 0.47 | — |
| NEFA, µEq/L4,5 | 102.34 | 134.13 | 101.80 | 119.18 | 154.16 | 99.11 | 159.40 | 100.10 | 33.74 | 0.56 | 0.93 | 0.49 | — |
Treatments of ewes supplemented with Ca salts of MUFA (PDS; EnerGII, Virtus Nutrition LLC, Corcoran, CA., USA.), PUFA (EDS; Strata G113, Virtus Nutrition LLC, Corcoran, CA., USA.), or no supplementation (NF) during the last 50 d of gestation.
Body weight measure at the starting of the finishing period (fd 0), 28 days into the finishing period (fd 28), and 54 days from the start of the finishing period (fd 54). Interactions not presented had a P-value ≥0.12.
FP1 consists of the sampling between the initial body weight of the finishing period (fd 0) and fd 28 of the finishing period. FP2 consists of the sampling between day 28 and 54 sampling of the finishing period. Interactions not presented had a P-value ≥ 0.13.
There were no FS × MS, or FS × MS × Sex interactions (P ≥ 0.76). FS, lipid effect of FA supplementation in the dam diet; MS, methionine effect of ME supplementation in the dam; FS × MS, lipid and methionine effect of FS-MS supplementation in the dam.
There was a tendency for an FS × Sex interaction (P = 0.08).
Values with different superscripts differ with a P ≤ 0.05.
There were no treatments, treatments by sex, or treatments by day interactions in any of the studied parameters during the finishing period (P ≥ 0.12). There were no three- or four-way interactions either (P ≥ 0.31).
No treatments interactions (P ≥ 0.12; Table 7 and Supplementary Table 2) were observed with regard to the mRNA expression; however, an FS effect was observed on the mRNA expression of genes involved in the RvD1 metabolic pathway (Table 7). Animals born to dams that consumed a source of omega-3 (FS and FS-MS) showed an increase (P = 0.05) in the relative expression of COX-2 mRNA and a marginal significant increase (P = 0.09) in the relative expression of ALOX15. There was no effect (P ≥ 0.49) of Met nor an interaction of both nutrients in the relative expression of COX-2 mRNA or ALOX15 observed (Table 7). For the genes involved in lipid metabolism, only FABP4 was affected by dam Met supplementation; lambs born to ewes supplemented with methionine (MS and FS-MS) showed an increase (P = 0.03) in FABP4 mRNA expression when compared with lambs born to ewes that were not supplemented with Met (NS and FS) during late gestation (Table 7). The liver mRNA expression of the offspring of the studied genes involved in the inflammatory response pathways, Met cycle, and methylation of DNA was not affected (P ≥ 0.18) by maternal supplementation during late gestation (Supplementary Table 2).
Table 7.
Effects of maternal supplementation with a source of omega-3 PUFA and Met on lamb liver mRNA expression during the finishing period1
| Gene | Treatment | SEM | P-value2 | |||||
|---|---|---|---|---|---|---|---|---|
| NS | MS | FS | FS-MS | FS | MS | FS × MS | ||
| Resolvin metabolic pathway | ||||||||
| ALOX15 | 3.16c | 3.57c | 5.45b | 7.43a | 1.75 | 0.09 | 0.49 | 0.65 |
| ALOX15B | 5.63 | 7.09 | 5.01 | 5.99 | 0.98 | 0.38 | 0.22 | 0.80 |
| ALOX5 | 23.07 | 24.67 | 25.45 | 21.50 | 3.68 | 0.91 | 0.75 | 0.45 |
| ALOX5AP | 11.22 | 11.38 | 10.90 | 9.25 | 2.04 | 0.55 | 0.71 | 0.66 |
| COX-2 | 50.22c | 60.71b | 74.18a | 74.58a | 9.32 | 0.05 | 0.56 | 0.59 |
| Lipid metabolism | ||||||||
| DGAT1 | 433.02 | 436.50 | 452.75 | 424.52 | 23.76 | 0.90 | 0.60 | 0.50 |
| FATP1 | 18.84 | 19.09 | 21.38 | 18.65 | 3.50 | 0.76 | 0.67 | 0.72 |
| FABP4 | 9.25c | 11.18b | 5.90d | 12.69a | 1.91 | 0.63 | 0.03 | 0.21 |
| FADS1 | 1247.47 | 1,906.15 | 1,433.16 | 1,146.04 | 352.27 | 0.42 | 0.60 | 0.19 |
| FADS2 | 3,434.60 | 4,753.34 | 3,519.57 | 2,925.68 | 900.91 | 0.34 | 0.69 | 0.29 |
| ELOVL2 | 226.15 | 204.22 | 303.62 | 301.95 | 52.95 | 0.11 | 0.82 | 0.83 |
| FFAR1 | 4.57 | 6.11 | 6.84 | 5.53 | 1.06 | 0.43 | 0.91 | 0.19 |
| FFAR4 | 4.65 | 6.52 | 7.49 | 6.55 | 1.03 | 0.17 | 0.65 | 0.18 |
| FASN | 31.92 | 33.64 | 32.29 | 26.95 | 4.08 | 0.44 | 0.66 | 0.39 |
| SCD | 1,727.01 | 3,046.23 | 2,041.41 | 1,307.79 | 816.64 | 0.38 | 0.72 | 0.22 |
| PPARA | 1,742.33 | 1,720.01 | 1,637.96 | 1,567.80 | 162.92 | 0.43 | 0.78 | 0.88 |
| PPARD | 26.16 | 32.45 | 33.23 | 29.68 | 3.00 | 0.47 | 0.65 | 0.11 |
| PPARG | 13.72 | 16.48 | 18.93 | 14.79 | 2.13 | 0.41 | 0.75 | 0.12 |
Data are presented as a least square means ± SEM. The data on the genes involved in the inflammatory response and DNA methylation are presented in Supplementary Material.
FS, lipid effect of FA supplementation in the dam diet; MS, methionine effect of ME supplementation in the dam; FS × MS, lipid and methionine effect of FS-MS supplementation in the dam.
Values with different superscripts differ with a P-value ≤ 0.05.
Abbreviations: FS, fatty acid; FS-MS, fatty acids and methionine supplementation; MS, methionine supplementation; Met, methionine; NEFA, nonesterified fatty acids; NS, basal diet with no supplementation; PUFA, polyunsaturated fatty acids.
Treatment or a treatment by sex interaction was not observed (P ≥ 0.15) for lamb carcass characteristics (Table 8). Nonetheless, a marginal significant effect (P = 0.09) for maternal FA supplementation was observed for REA (Table 8); lambs born to ewes supplemented with a source of omega-3 (FS and FS-MS) have a smaller REA when compared with lambs born to dams in the other two treatments. Additionally, lamb’s sex (P ≤ 0.05) affected offspring’s HCW and REA. Wethers had heavier HCW and a greater REA than ewe lambs (Table 8). The other carcass characteristics studied in the current experiment such as dressing percentage, BFT, BWT, and marbling were not affected (P ≥ 0.15) by dam supplementation. Lamb sex had no effect (P ≥ 0.15) on the dressing percentage, BFT, BWT, and marbling (Table 8).
Table 8.
Effect of maternal supplementation during the last 50 d of gestation on male (M) and female (F) lambs’ carcass characteristics1
| Treatment2 | NS | MS | FS | FS-MS | SEM | P-value3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lamb sex | F | M | F | M | F | M | F | M | FS | MS | FS × MS | Sex | FS × MS × Sex | |
| Item | ||||||||||||||
| Hot carcass weight, kg | 20.02b | 20.52a | 18.96b | 21.17a | 19.02b | 21.25a | 19.39b | 19.88a | 3.37 | 0.59 | 0.52 | 0.87 | 0.05 | 0.15 |
| Dressing, % | 0.52 | 0.53 | 0.54 | 0.55 | 0.53 | 0.54 | 0.55 | 0.54 | 0.02 | 0.50 | 0.29 | 0.77 | 0.47 | 0.83 |
| Back fat thickness, cm | 0.64 | 0.43 | 0.56 | 0.58 | 0.64 | 0.51 | 0.61 | 0.51 | 0.05 | 0.82 | 0.93 | 0.78 | 0.26 | 0.58 |
| Body wall thickness, cm | 2.18 | 1.85 | 2.13 | 2.10 | 2.13 | 2.08 | 2.36 | 2.01 | 0.09 | 0.54 | 0.49 | 0.90 | 0.15 | 0.27 |
| Ribeye area, cm 2 | 16.77c | 18.26a | 16.13c | 18.90a | 14.52d | 17.42b | 16.26c | 17.87b | 0.16 | 0.09 | 0.35 | 0.35 | < 0.01 | 0.26 |
| Marbling score 4 | 10.00 | 10.33 | 9.67 | 9.33 | 9.67 | 10.50 | 10.33 | 10.00 | 0.47 | 0.41 | 0.41 | 0.29 | 0.72 | 0.72 |
Data are presented as a least square means ± SEM.
FS, lipid effect of FA supplementation in the dam diet; MS, methionine effect of ME supplementation in the dam; FS × MS, lipid and methionine effect of FS-MS supplementation in the dam.
There were no FS × Sex or MS × Sex for any of the studied carcass characteristics (P ≥ 0.19).
Marbling score is based on a scale: 9 = slight and 10 = small.
Values with different superscripts differ with a P-value ≤ 0.05.
Abbreviations: F, female; FS, fatty acid; FS-MS, fatty acids, and methionine supplementation; M, male; MS, methionine supplementation; NEFA, nonesterified fatty acids; NS, basal diet with no supplementations.
Glucose tolerance test
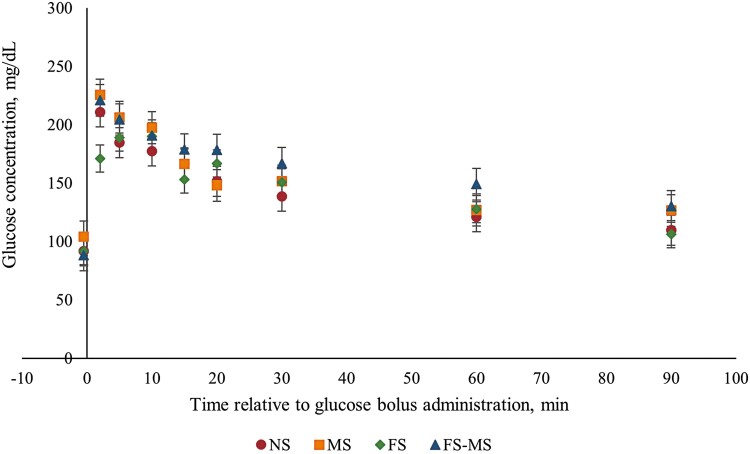
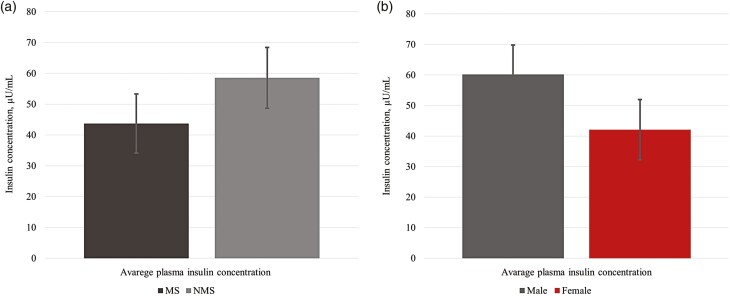
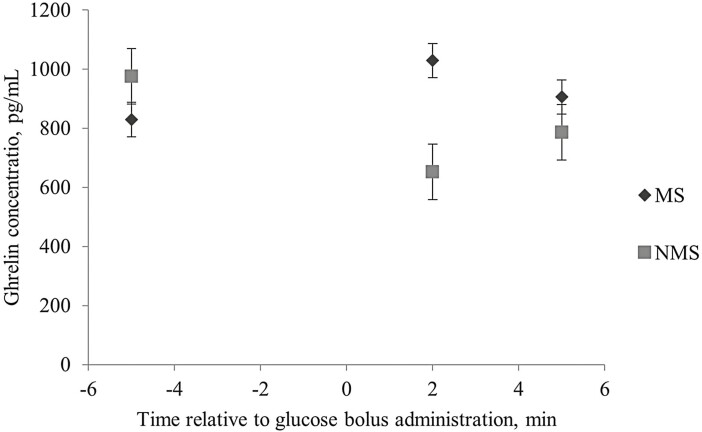
No four-way interactions (P ≥ 0.18) or single effects (P ≥ 0.20) were observed for plasma glucose concentration (Supplementary Figure 1). A marginal significant FS × MS × Time interaction was observed during the GTT (P = 0.07; Figure 2). Animals born from FS ewes showed a lesser plasma glucose concentration compared with lambs born to Met-supplemented ewes (MS and FS-MS) at 2 min after bolus administration. This was maintained until 20 min after bolus administration where lambs born to FS dams showed greater or similar plasma glucose concentration than lambs born to MS ewes but lower glucose concentration than lambs born to FS-MS ewes. However, at the end of the test, lambs born to FS ewes showed lesser glucose concentration compared with lambs born to Met-supplemented ewes. Moreover, a sex by time interaction (P < 0.01) was observed; ewe lambs had a greater plasma glucose concentration than weathers at minute 2 relative to the glucose bolus administration in the GTT (Supplementary Figure 1). No two-, three-, or four-way interactions were observed (P ≥ 0.14) for insulin plasma concentration during the GTT. A marginal significant effect (P = 0.06) for maternal Met supplementation (MS and FS-MS) during late gestation demonstrated a decrease in the mean lamb insulin concentration during the GTT (Figure 3a). At the same time when a lamb’s sex effect was observed (P = 0.02), wethers showed a greater plasma insulin concentration when compared with ewe lambs (Figure 3b). No three- or four-way interaction or single effect was observed for plasma ghrelin concentration (P ≥ 0.38). A marginal significant Met by time interaction (P = 0.07) was observed for lamb plasma ghrelin concentration during the GTT (Figure 4). Lambs born to ewes that were supplemented with Met (MS and FS-MS) during late gestation reported a greater ghrelin concentration at 2 and 5 min relative to the glucose bolus administration, and the opposite was observed at 5 min before the bolus administration where lambs born to Met-supplemented ewes showed a lesser plasma ghrelin concentration when compared with lambs born to ewes that were not supplemented with Met during late gestation (Figure 4).
Figure 2.
Treatment by time interaction on plasma glucose concentration during the glucose tolerance test on finishing lambs born from dams fed a source of n-3 polyunsaturated fatty acids and methionine during the last 50 d of gestation. Data are presented as the least square mean of FS × MS × Time (P = 0.07; SEM = 15.05). There were no observed differences (P ≥ 0.18) for the 4-way interaction or main effects. A sex by time interaction (P < 0.01) was observed (Supplementary Figure 1). NS, no fatty acid or methionine supplementation; FS, lipid effect of FA supplementation in the dam diet; MS, methionine effect of ME supplementation in the dam; FS × MS, lipid and methionine effect of FS-MS supplementation in the dam.
Figure 3.
Effect of maternal methionine supplementation during the last 50 d of gestation on plasma insulin concentration during the glucose tolerance test in finishing lambs (a). Due to the lack of observed difference (P ≥ 0.14) for FS, FS × MS, FS × MS × Time, and Sex × Time, the data are presented as the least square mean of MS vs. not Met supplementation (NMS) (P = 0.06; SEM = 9.59). Effect of offspring sex on plasma insulin concentrations during the glucose tolerance test (b). Due to the lack of observed difference (P ≥ 0.14) for FS, FS × MS, FS × MS × Time, and Sex × Time, the data are presented as the least square mean of Sex (P = 0.02; SEM = 9.85). FS, lipid effect of FA supplementation in the dam diet; MS, methionine effect of ME supplementation in the dam; FS × MS, lipid and methionine effect of FS-MS supplementation in the dam.
Figure 4.
Effect of maternal methionine supplementation during the last 50 d of gestation on plasma ghrelin concentrations during the glucose tolerance test in finishing lambs. Due to the lack of observed difference (P ≥ 0.36) for FS, MS, Time, Sex, and the interactions not presented, the data are presented as the least square mean of MS × Time (P = 0.07; SEM = 134.25). FS, lipid effect of FA supplementation in the dam diet; MS, methionine effect of ME supplementation in the dam; FS × MS, lipid and methionine effect of FS-MS supplementation in the dam.
Discussion
Ewe BW, BCS, and plasma metabolites
Our results on ewe BW and BCS are in accordance with other authors who treated ewes with a source of omega-3 (Coleman et al., 2018b, Sheibani et al., 2018; Nickles et al., 2019) or Met (Waterman et al., 2012; Osorio et al., 2013) during late gestation. We, therefore, did not expect the ewes BW or BCS to change when these nutrients were fed during the 50-d study.
Plasma maternal metabolites were recorded at the beginning and termination of the study to evaluate the effect of the treatments fed on the nutritional state of the late gestating ewe. Previous studies where a source of omega-3 PUFA (Coleman et al., 2018b) or Met (Jacometo et al., 2016) were fed during late gestation did not report any differences in dam’s plasma glucose or NEFA concentrations. However, in a study conducted by Zhou et al. (2016) where dairy cows were supplemented with a different methyl donor (Choline), supplementation during late gestation led to an increase in glucose and insulin concentrations with a decreased glucose:insulin ratio when compared with the control treatment, suggesting an improvement in the metabolic energy status of the dams that received dietary methyl donors during the last third of gestation. In contrast with the available literature, a tendency for a decrease in plasma NEFA concentration was reported in the current experiment when both nutrients were supplemented during late gestation. Our data suggest that the observed diminution in circulating NEFA concentration in ewes supplemented with both nutrients during late gestation may be because of a redistribution in the usage of nutrients in tissues where there is not as much FA mobilization. To our knowledge, this is the first study conducted in ruminants that evaluates the effects of supplementation with omega-3 PUFA and Met during late gestation. Hence, the dietary supply of these two nutrients on a dam’s performance and energy metabolism needs further investigation in order to corroborate our findings and the effects on these parameters during the peripartal and postpartal periods.
Lamb growth performance until weaning, plasma metabolites, and plasma FA profile and resolvin D1 at birth
An increase in offspring weight during the preweaning period has been reported when a source of omega-3 PUFAs (Santos et al., 2013; Garcia et al., 2014) or Met (Batistel et al., 2017; Alharthi et al., 2018) was supplemented to a dam during late gestation. To our knowledge, however, no data are available that report how the interaction of these two nutrients supplied during late gestation can affect an offspring’s growth and immune metabolic markers. Nonetheless, offspring postnatal growth (Acosta et al., 2017; Marquez et al., 2017; Nickles et al., 2019; Rosa-Velazquez et al., 2020, 2021) after maternal dietary supplementation with these nutrients has been linked to changes in plasma concentration of metabolites involved in energy metabolism (Nickles et al., 2019; Rosa-Velazquez et al., 2021), molecules with anti-inflammatory activity such as haptoglobin and RvD1 (Marques et al., 2017, and Rosa-Velazquez et al., 2020, respectively), and genes associated with the inflammatory response in a dams’ follicular cells (Acosta et al., 2017). Therefore, we were expecting an increase in the BW of lambs born to ewes fed with omega-3 or Met, and a positive interaction between these two nutrients during the preweaning period. Contrary to what was hypothesized, in the current study, lambs born to dams treated with omega-3 and Met during late gestation showed similar preweaning growth compared with lambs born to dams that were not supplemented. On the other hand, the greatest increase in preweaning weight of lambs born to dams supplemented with Met indicates that the supplementation of RPM during late gestation could have had a better effect on the development of the offspring in utero. These results differ from those published by Rosa-Velazquez et al. (2020) who reported no changes in fetal BW when dams were supplemented during the last 45 d of gestation with the same treatment diets that were fed in the current experiment. The differences observed in the current experiment with what was reported by Rosa-Velazquez et al. (2020) could be related to the time when offspring BW was recorded, Rosa-Velazquez et al. (2020) only recorded the fetal weight, while in the current experiment, the differences in offspring BW were more prominent at weaning. Our data indicate that the changes observed in BW of lambs born from Met supplementation could be related to postnatal changes in energy metabolism. Interestingly, lambs born to dams supplemented with just lipids or Met during late gestation showed a heavier weight at 60 d of age compared with the lambs from ewes supplemented with both nutrients (lipids and MET). This difference in BW was maintained until weaning. The observed changes in offspring’s BW during this period (preweaning period) could be related to the reported changes in plasma metabolites and RvD1. Offspring BW and glucose plasma concentration at weaning were greater in lambs born to Met-supplemented ewes. As for the changes observed in the plasma NEFA concentration, our results suggest that the reported diminution in NEFA at weaning on lambs born to ewes supplemented with both nutrients during late gestation could possibly be due to a reduction in FA mobilization. This reduction in FA mobilization could be cause perhaps by a decrease in the concentration of these nutrients in their diet, more specifically from the consumption of maternal milk since maternal supplementation was terminated at lambing. Another factor that could be affecting offspring preweaning weight is the reported increase in RvD1 concentration at the birth of lambs born to dams that were supplemented with a source of omega-3 PUFAs or RPM. Our results are in accordance with previous studies (Rosa-Velazquez et al., 2021) where an observed increase in plasma RvD1 concentration at birth was positively associated with changes in offspring BW during the finishing period when omega-3 was supplemented during late gestation and where a reduction in mRNA abundance of genes involved in inflammation was reported positively associated with a greater birth weight when RPM was supplied to neonatal claves (Abdelmegeid et al., 2017). Our results suggest that supplementation with a source of omega-3 PUFAs or RPM during late gestation could improve offspring response to an inflammatory challenge by programming them in the prenatal period to have a more specific and shorter inflammatory response (Roque-Jimenez et al., 2021), thus allowing these lambs to use the conserved energy for their growth and development.
Studies conducted in other mammal models reported that maternal circulating FAs are the principal source of offspring prenatal FAs (Campbell et al. 1997). In non-ruminants, FAs consumed during pregnancy can be transported through the placenta reaching the offspring during the prenatal period (Kabaran and Besler, 2015). In ruminants, a study conducted in sheep reported that supplementation with saturated FAs and MUFAs in the first trimester of gestation increased free FAs receptors in the cotyledons (maternal part of the placenta) and caruncles (the fetal part of the placenta) (Roque-Jimenez et al., 2020). Moreover, studies conducted in bovines, where dams were supplemented with a source rich of EPA and DHA during late gestation, reported an increase in plasma DHA (Moallem and Zachut, 2012) and EPA, and lesser linoleic and oleic acid (Shao et al., 2020), in newborn calves before colostrum consumption. Likewise, an increase in long-chain PUFAs in the fetal circulation has been reported during the last third of gestation (Cetin et al., 2009). Concurring to previous studies (Mollaem et al., 2012; Or-Rashid et al., 2012; Rosa-Velazquez et al., 2021), the differences in lamb plasma FAs reported in the present study reflected the FA profiles of the Ca salts consumed by the dam during late gestation. As expected, a greater plasma long-chain PUFA (CLA, DHA, EPA, docosanoic acid, and C18:1 trans isomers) concentration was found at birth in lambs born to dams that were supplemented with a source of omega-3 FAs (FS and FS-MS). The lesser plasma concentration of cis isomers and the greater trans isomers of C18:1 reported in lambs born to FS ewes could be because of a change in the dams ruminal FA biohydrogenation. Dietary DHA and EPA can inhibit rumen biohydrogenation, which leads to the formation of trans isomers (Bauman and Griinari, 2003), possibly modifying maternal circulating FA concentration, and then transferring these FAs to the offspring at the end of gestation or via colostrum. Studies conducted in non-ruminants have reported an increase in linoleic acid in liver tissue in a murine model (Sugiyama et al., 1998), and greater total omega-3 and total long-chain omega-3 FA in the breast muscle of broilers (Khan et al., 2021) in subjects that received a dietary supplementation with Met (Sugiyama et al., 1998) or Met + source of omega-3 (Khan et al., 2021) when compared to their cohorts that were supplemented with a lower level of Met (Sugiyama et al., 1998) or only supplemented with a source of omega-3 (Khan et al., 2021). To our knowledge, this is the first research conducted in ruminants that studies offspring plasma FA profile after maternal Met dietary supplementation during gestation. Contrary to what was expected based on previous literature, lambs born to dams that were fed a diet with Met showed a greater plasma concentration of medium- and long-chain saturated FAs. The reasons for these results are uncertain, but according to what was observed in previous studies (Sugiyama et al., 1998) in non-ruminants, dietary Met supply during late gestation could be affecting maternal lipid metabolism, hence modifying the FAs that are transferred to the offspring during the prenatal period.
Lamb growth performance, plasma metabolites, liver mRNA expression, and GTT during the finishing period
There is evidence that maternal dietary supply of omega-3 PUFAs (Coleman et al., 2018a; Nickles et al., 2019) or RPM (Osorio et al., 2014; Batistel et al., 2019) can induce epigenetic changes that may modify offspring immune response and energy metabolism markers that subsequently alter offspring’s growth and development during the postnatal period. Thus, the maternal supply of these nutrients in late gestation may impact offspring metabolism and physiology modifying offspring growth and health in the long run. More specifically, offspring postweaning weight (Batistel et al., 2017) and ADG were increased (Alharthi et al., 2018) after RPM was supplemented during late gestation. Likewise, maternal supplementation with a source of omega-3 PUFAs during the last third of gestation could increase BW and HCW during the growing period (Marquez et al., 2017; Carranza-Martin et al., 2018; Jolazadeh et al., 2019; Nickles et al., 2019; Rosa-Velazquez et al., 2021). Interestingly, and contrary to the available literature in sheep, in the study conducted by Rosa-Velazquez et al. (2021), offspring BW and HCW were affected by omega-3 supplementation and offspring sex. Wethers born to omega-3-supplemented ewes tended to have greater BW and HCW when compared with the other treatment and control lambs. Something similar was observed in the current experiment on offspring BW and HCW—wethers born to supplemented dams tended to be heavier than females. Also, our data indicate that supplementing dams during late gestation with Met could have a greater positive effect on offspring BW than if they are supplemented with both nutrients during this period. It is important to note that these changes in offspring growth during the finishing period were not related to an increase in DM intake, suggesting that the modification in metabolism (increase in plasma NEFA concentration during the finishing period) and immune response (changes in molecules involved in the inflammatory response) observed in our study could have driven the increase in lambs finishing period BW. Sex divergent effects were reported in the mRNA abundance of metabolites involved in DNA methylation, and inflammation when RPM or a source of omega-3 was supplied during late gestation. Transcriptional changes have been observed in DNMT; DNMTA and DNMT3B were upregulated in the female placenta of lambs born to RPM-supplemented dams (Batistel et al., 2019). The maternal supply of RPM has been also associated with a decreased mRNA expression of TNF and IL-1B in their offspring, suggesting that maternal supplementation with this nutrient could attenuate offspring proinflammatory response (Jacometo et al., 2018). In the current experiment, maternal omega-3 PUFA supplementation increased the relative abundance of enzymes (COX-2 and ALOX15) involved in the formation of RvD1 as reported in previous studies in sheep fetal liver (Rosa-Velazquez et al., 2020). These findings reinstate the modulatory effect of omega-3 PUFA on the immunometabolism of the offspring. The relative mRNA expression of FABP4, the only gene involved in the FA metabolism, is affected by maternal Met supplementation during late gestation. FA-binding protein 4 encodes the FA-binding protein found in adipocytes, binds to long-chain FAs, and can participate in the uptake, transport, and metabolism of FA (Thompson et al., 2017). In placental tissue, FABP4 actively transports DHA and arachidonic acid (Duttaroy, 2016). The increase in FABP4 liver mRNA expression in lambs born from dams supplemented with Met could indicate an increase in mobilization and metabolism of long-chain FAs in the liver.
Glucose tolerance test
Ewe omega-3 PUFAs dietary supply can modify offspring energy metabolism by modulating the glucose–insulin system by increasing a lamb’s plasma glucose concentration during the finishing period (Nickles et al., 2019, Rosa-Velazquez et al., 2021). These changes in offspring energy metabolism were positively associated with an increase in the lambs finishing BW (Nickles et al., 2019; Rosa-Velazquez et al., 2021). On the other hand, cow supplementation with RPM during late gestation upregulated placental glucose transporters (Batistel et al., 2017). Contrary to Nickles et al.’s (2019) findings, maternal supplementation with a source of omega-3 (FS) fed during late gestation decreased plasma glucose concentration when compared with lambs that were born to ewes supplemented with RPM (MS and FS-MS). Overall, lambs that were born to Met-supplemented ewes showed a greater glucose plasma concentration during the GTT. Nonetheless, our results suggest that the plasma glucose concentration of the offspring can be affected by their sex possibly because wethers greater muscle mass, when compared with ewe lambs muscle mass, could lead to a greater capacity to decrease plasma glucose. The increase in glucose and insulin plasma concentrations in lambs born to Met-supplemented ewes could be because of an increase in glucose transporters, thus increasing offspring circulating glucose. However, further investigation is required to elucidate the effect of maternal Met supplementation during late gestation on the liver glucose transporters of the offspring. On the other hand, our results in the offspring plasma insulin concentration suggest that supplementation with Met during late gestation can improve insulin resistance, and that insulin sensitivity can be affected by the sex of the offspring. Based on the previous studies (Nickles et al., 2019), we were expecting a decrease in plasma ghrelin concentration in lambs born to ewes fed a source of omega-3 during late gestation. Similarly, to what was expected, lambs born to ewes supplemented with omega-3 PUFA (FS) showed a lesser plasma ghrelin concentration at 2 and 5 min after the glucose bolus administration during the GTT; however, plasma ghrelin concentration was greater before administration, suggesting that an increase in the influx of circulatory glucose can affect offspring plasma ghrelin concentration in offspring born to FA-supplemented ewes.
Conclusion
Contrary to what was expected, the interaction of omega-3 and Met when fed to ewes during the last third of gestation did not show a synergistic effect on postnatal growth and metabolism of the offspring when compared with lambs born to ewes supplemented with either omega-3 or Met. On the other hand, maternal supplementation during late gestation with omega-3 or Met has an effect on offspring development, increasing offspring growth with a concomitant change in RvD1 and energy metabolism markers (glucose and insulin concentrations). Moreover, maternal Met dietary supply during late gestation could modulate offspring glucose–insulin system in a sex-dependent manner by modifying their insulin sensitivity, which may affect lambs finishing BW.
Supplementary Material
Acknowledgments
This material was partially presented at the 2020 Midwest American Society of Animal Science and American Dairy Science Association Joint Meeting. We are grateful to P. Dieter and the Ohio Agricultural Research and Development Center beef and sheep team for their assistance with animal care, feeding, and sampling. Salary support was provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center, The Ohio State University. This experiment was partially supported by the United States Department of Agriculture (USDA) National Institute of Food and Agriculture, Hatch Project OHO01461 number 1018667, and by the College of Food, Agricultural, and Environmental Sciences (CFAES) seeds grant matched by Adisseo and Virtus Nutrition.
Glossary
Abbreviations
- ADF
acid detergent fiber
- AHCY
adenosylhomocysteinase
- ALOX5
arachidonate 5-lipoxygenase
- ALOX5AP
arachidonate 5-lipoxygenase activating protein
- ALOX15
arachidonate 15-lipoxygenase
- ALOX15B
15-lipoxygenase-2
- APOB
apolipoprotein B
- BCS
body condition score
- BW
body weight
- COX-2
prostaglandin-endoperoxide synthase 2
- CP
crude protein
- DGAT1
diacylglycerol o-acyltransferase 1
- DHA
docosahexaenoic acid
- DM
dry matter
- DNMT
DNA methyltransferase
- DNMT1
DNA methyltransferase 1
- DNMT2
DNA methyltransferase 2
- DNMT3A
DNA methyltransferase 3 Alpha
- DNMT3B
DNA methyltransferase 3 Beta
- EPA
eicosapentaenoic acid
- FA
fatty acids
- FS
fatty acid supplementation
- FS-MS
fatty acid and methionine supplementation
- HCW
hot carcass weight
- IL1B
interleukin 1 Beta
- IL6
interleukin 6
- Met
methionine
- MUFA
monounsaturated fatty acids
- MS
methionine supplementation
- NDF
neutral detergent fiber
- NEFA
nonesterified fatty acids
- NEm
Net energy for maintenance
- NMS
no methionine supplementation
- NS
basal diet
- PUFA
polyunsaturated fatty acids
- RPM
rumen protected methionine
- RvD1
resolvin D1
- SAM
S-adenosylmethionine
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Abdelmegeid, M., Vailati-Riboni M., Alharthi A., Batistel F., and Loor J.. . 2017. Supplemental methionine, choline, or taurine alter in vitro gene network expression of polymorphonuclear leukocytes from neonatal Holstein calves. J. Dairy Sci. 201(100):3155–3165. doi: 10.3168/jds.2016-12025 [DOI] [PubMed] [Google Scholar]
- Acosta, D. A. V., Rivevelli M. I., Skenandore C., Zhou Z., Keisler D. H., and Luchini D.. . 2017. Effects of rumen-protected methionine and choline supplementation on steroidogenic potential of the first postpartum dominant follicle and expression of immune mediators in Holstein cows. Theriogenology. 96:1–9. doi: 10.1016/j.theriogenology.2017.03.022 [DOI] [PubMed] [Google Scholar]
- Alharthi, A. S., Batistel F., Abdelmegeid M. K., Lascano G., Parys C., Helmbrecht A., Trevisi E., and Loor J.. . 2018. Maternal supply of methionine during late pregnancy rate of Holstein calf development in utero and postnatal growth to a greater extent than colostrum source. J. Anim. Sci. Biotech. 9:83. doi: 10.1186/s40104-018-0298-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC. 1990. Official methods of analysis. 16th ed. Richmond (VA): Association of Official Analytical Chemists. [Google Scholar]
- Batistel, F., Alharthi A. S., Wang L., Parys C., Pan Y. X., and Cardoso C.. . 2017. Placentome nutrient transporters and mammalian target of rapamycin signaling proteins are altered by the methionine supply during late gestation in dairy cows and are associated with newborn birth weight. J. Nutr. 47(9):1640–1647. doi: 10.3945/jn.117.251876 [DOI] [PubMed] [Google Scholar]
- Batistel, F., Alharthi A. S., Yambao R. R., Elolimy A. A., Pan X. X., and Parys C.. . 2019. Methionine supply during late gestation triggers offspring sex-specific divergent changes in metabolic and epigenetic signatures in bovine placenta. J. Nutr. 149:6–17. doi: 10.1093/jn/nxy240 [DOI] [PubMed] [Google Scholar]
- Bauman, D. E., and Griinari J. M.. . 2003. Nutritional regulation of milk fat synthesis. Annu. Rev. Nutr. 23:203–227. doi: 10.1146/annurev.nutr.23.011702.073408 [DOI] [PubMed] [Google Scholar]
- Benson, D. A., Karsch-Mizrachi I., Lipman D. J., Ostell J., and Wheeler D. L.. . 2005. GenBank: update. Nucleic Acids Res. 33(Database Issue):D34–D38. doi: 10.1093/nar/gki063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandão, A. P., Cooke R. F., Schubach K. M., Rett B., Souza O. A., and Schachtschneider C. L.. . 2020. Supplementing Ca salts of soybean oil to late-gestating beef cows: impacts on performance and physiological responses of the offspring. J. Anim. Sci. 98(8):S22–S26. doi: 10.1093/tas/txaa090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, F. M., Clohessy A. M., Gordon M. J., Page K. R., and Dutta-Roy A. K.. . 1997. Uptake of long chain fatty acids by human placental choriocarcinoma (BeWo) cells: role of plasma membrane fatty acid-binding protein. J. Lipid Res. 38:2558–2568. doi: 10.1016/S0022-2275(20)30040-7 [DOI] [PubMed] [Google Scholar]
- Carranza-Martin, A. C., Coleman D. N., Garcia L. G., Furnus C. C., and Relling A. E.. . 2018. Prepartum fatty acid supplementation in sheep. III. Effect of eicosapentaenoic acid and docosahexaenoic acid during finishing on performance, hypothalamus gene expression, and muscle fatty acids composition in lambs. J. Anim. Sci. 96:5300–5310. doi: 10.1093/jas/sky360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin, I., Alvino G., and Cardellicchio M.. . 2009. Long chain fatty acids and dietary fats in fetal nutrition. J. Physiol. 587(14):3441–3451. doi: 10.1113/jphysiol.2009.173062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, D. N., Murphy K. D., and Relling A. E.. . 2018a. Prepartum fatty acid supplementation in sheep II. Supplementation of eicosapentaenoic acid and docosahexaenoic acid during late gestation alters the fatty acid profile of plasma, colostrum, milk, and adipose tissue, and increases lipogenic gene expression of adipose tissue. J. Anim. Sci. 96:1181–1204. doi: 10.1093/jas/skx013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, D. N., Rivera-Acevedo K. C., and Relling A. E.. . 2018b. Prepartum fatty acid supplementation in sheep I. Eicosapentaenoic and docosahexaenoic acid supplementation do not modify ewe and lamb metabolic status and performance through weaning. J. Anim. Sci. 96:364–374. doi: 10.1093/jas/skx012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doreau, M., Rearte D., Portelli J., and Peyraud J. L.. . 2007. Fatty acid ruminal metabolism and digestibility in cows fed perennial ryegrass. Eur. J. Lipid. Sci. Tech. 109:790–798. doi: 10.1002/ejlt.200700003 [DOI] [Google Scholar]
- Duttaroy, A. K. 2016. Docosahexaenoic acid supports feto-placental growth and protects cardiovascular and cognitive function: a mini review. Eur. J. Lipid Sci. Technol. 118(10):S1439–S1449. doi: 10.1002/ejlt.201500496 [DOI] [Google Scholar]
- FEDNA. 2010. Tablas FEDNA de composición y valor nutritivo de alimentos para la fabricación de piensos compuestos. 3rd ed. Madrid (Spain): Fundación Española para el Desarrollo de la Nutrición Animal. [Google Scholar]
- Folch, J., Lees M., and Sloane Stanley G. H.. . 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226:497–509. doi: 10.1016/s0021-9258(18)64849-5 [DOI] [PubMed] [Google Scholar]
- Garcia, M., Greco L. F., Favoreto M. G., Marsola R. S., Wang D., Shin J. H., Block E., Thatcher W. W., Santos J. E. P., and Staples C. R.. . 2014. Effect of supplementing essential fatty acids to pregnant nonlactating Holstein cows and their preweaned calves on calf performance, immune response, and health. J. Dairy Sci. 97:5045–5064. doi: 10.3168/jds.2013-7473 [DOI] [PubMed] [Google Scholar]
- Jacometo, C. B., Alharthi A. S., Zhou Z., Luchini D., and Loor J. J.. . 2018. Maternal supply of methionine during late pregnancy is associated with changes in immune function and abundance of microRNA and mRNA in Holstein calf polymorphonuclear leukocytes. J. Dairy Sci. 101:8146–8158. doi: 10.3168/jds.2018-14428 [DOI] [PubMed] [Google Scholar]
- Jacometo, C. B., Zhou Z., Luchini D., Trevisi E., Corrêa M. N., and Loor J. J.. . 2016. Maternal rumen-protected methionine supplementation and its effect on blood and liver biomarkers of energy metabolism, inflammation, and oxidative stress in neonatal Holstein calves. J. Dairy Sci. 99:6753–6763. doi: 10.3168/jds.2016-11018 [DOI] [PubMed] [Google Scholar]
- Jolazadeh, A. R., Mohammadabadi T., Dehghan-Banadaky M., Chaji M., and Garcia M.. . 2019. Effect of supplementation fat during the last 3 weeks of uterine life and the preweaning period on performance, ruminal fermentation, blood metabolites, passive immunity, and health of the newborn calf. Br. J. Nutr. 122:1346–1358. doi: 10.1017/S0007114519002174 [DOI] [PubMed] [Google Scholar]
- Kabaran, S., and Besler H. T.. . 2015. Do fatty acids affect fetal programming? J. Health Popul. Nutr. 13:33–14. doi: 10.1186/s41043-015-0018-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, I. A., Parker N. B., Lohr C. V., and Cherian G.. . 2021. Docosahexaenoic acid (22:6 n-3)-rich microalgae along with methionine supplementation in broiler chickens: effects on production performance, breast muscle quality attributes, lipid profile, and incidence of white striping and myopathy. Poult. Sci. 100(2):865–874. doi: 10.1016/j.psj.2020.10.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C., Batistel F., Osorio J. S., Drackley J. K., Luchini D., and Loor J.. . 2016. Peripartal rumen-protected methionine supplementation to higher energy diets elicits positive effects on blood neutrophil gene networks, performance, and liver lipid content in dairy cows. J. Anim. Sci. Biotechnol. 9:7–18. doi: 10.1186/s40104-016-0077-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques, R. S., Cooke R. F., Rodrigues M. C., Brandão A. P., Schubach K. M., Lippolis K. D., Moriel P., Perry G. A., Lock A., and Bohnert D. W.. . 2017. Effects of supplementing calcium salts of polyunsaturated fatty acids to late-gestating beef cows on performance and physiological responses of the offspring. J. Anim. Sci. 95:5347–5357. doi: 10.2527/jas2017.1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moallem, U., and Zachut M.. . 2012. Short Communication: The effects of supplementation of various n-3 fatty acids to late-pregnant dairy cows on plasma fatty acid composition of the newborn calves. J. Dairy Sci. 95:4055–4058. doi: 10.3168/jds.2012-5457 [DOI] [PubMed] [Google Scholar]
- Nickles, K. R., Hamer L., Coleman D. N., and Relling A. E.. . 2019. Supplementation with eicosapentaenoic and docosahexaenoic acids in late gestation in ewes changes adipose tissue gene expression in the ewe and growth and plasma concentration of ghrelin in the offspring. J. Anim. Sci. 97:2631–2643. doi: 10.1093/jas/skz141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC. 2007. Nutrient requirements of small ruminants: sheep, goats, cervids, and new world camelids. Washington (DC): National Academies Press. [Google Scholar]
- Ordway, R. S., Boucher S. E., Whitehouse N. L., Schwab C. G., and Sloan B. K.. . 2009. Effects of providing two forms of supplemental methionine to periparturient Holstein dairy cows on feed intake and lactational performance. J. Dairy Sci. 92(10):5154–5166. doi: 10.3168/jds.2009-2259 [DOI] [PubMed] [Google Scholar]
- Or-Rashid, M. M., Fisher R., Karrow N., AlZahal O., and McBride B. W.. . 2012. Plasma fatty acid profile of gestating ewes supplemented with fishmeal. Am. J. Anim. Vet. Sci. 7:67–74. doi: 10.3844/ajavsp.2012.67.74 [DOI] [PubMed] [Google Scholar]
- Osorio, J. S., Drackley J. P., Luchini D., and Loor J. J.. . 2014. Smartamine M and MetaSmart supplementation during the peripartal period alter hepatic expression of gene networks in 1-carbon metabolism, inflammation, oxidative stress, and the growth hormone–insulin-like growth factor 1 axis pathways. J. Dairy Sci. 97:7451–7464. doi: 10.3168/jds.2014-8680 [DOI] [PubMed] [Google Scholar]
- Osorio, J. S., Ji P., Drackley J. K., Luchini D., and Loor J. J.. . 2013. Supplemental smartamine M or MetaSmart during the transition period benefits postpartal cow performance and blood neutrophil function. J. Dairy Sci. 96(10):6248–6263. doi: 10.3168/jds.2012-5790 [DOI] [PubMed] [Google Scholar]
- Relling, A. E., Pate J. L., Reynolds C. K., and Loerch S. C.. . 2010. Effect of feed restriction and supplemental dietary fat on gut peptide and hypothalamic neuropeptide messenger ribonucleic acid concentrations in growing wethers. J. Anim. Sci. 88:737–748. doi: 10.2527/jas.2009-2316 [DOI] [PubMed] [Google Scholar]
- Roque-Jimenez, J. A., Oviedo-Ojeda M. F., Whalin M., Lee-Rangel H. A., and Relling A. E.. . 2020. Eicosapentaenoic and docosahexaenoic acid supplementation during early gestation modified relative abundance on placenta and fetal liver tissue mRNA and concentration pattern of fatty acids in fetal liver and fetal central nervous system of sheep. PLoS One. 15:e035217. doi: 10.1371/journal.pone.0235217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque-Jimenez, J. A., Velazquez M., Pinos Rodriguez J. M., Vicente-Martinez J. G., Mendoza-Cervantes G., Flore-Primo A., Lee-Rangel H. A., and Relling A. E.. . 2021. Role of long chain fatty acids in developmental programming in ruminants. Animals. 11:762. doi: 10.3390/ani11030762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa-Velazquez, M., Batistel F., Pinos-Rodriguez J. M., and Relling A. E.. . 2020. Effects of maternal dietary omega-3 polyunsaturated fatty acids and methionine during late gestation on fetal growth, DNA methylation, and mRNA relative expression of genes associated with the inflammatory response, lipid metabolism and DNA methylation in placenta and offspring’s liver in sheep. J. Anim. Sci. Biotechnol. 11(1):111. doi: 10.1186/s40104-020-00513-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa-Velazquez, M., Jaborek J. R., Pinos-Rodriguez J. M., and Relling A. E.. . 2021. Maternal supply of fatty acids during late gestation on offspring’s growth, metabolism, and carcass characteristics in sheep. Animals. 11:719. doi: 10.3390/ani11030719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel, A. J. F., Doney J. M., and Gunn R.. . 1969. Subjective assessment of body fat in live sheep. J. Agri. Sci. 72:451–454. doi: 10.1017/S0021859600024874 [DOI] [Google Scholar]
- Santos, J. E. P., Greco L. F., Garcia M., Thatcher W. W., and Staples C. R.. . 2013. The role of specific fatty acids on dairy cattle performance and fertility. 24th Annual Ruminant Nutrition Symposium, February 5–6, 2013. Gainesville (FL).
- Shao, T., Ireland F. A., McCann J. C., and Shike D. W.. . 2020. 225 Effects of late gestation calcium salts of fatty acids supplementation to beef cows on offspring pre-weaning growth performance and gene expression. J. Anim. Sci. 98:136. doi: 10.1093/jas/skaa054.236 [DOI] [Google Scholar]
- Sheibani, S., Mohammadi-Sangcheshmeh A., Alamouti A. A., Khadem A. A., and Norouzian M. A.. . 2018. Metabolic and molecular responses to calcium soap of fish oil fed to ewes during peripartal period. Cell Mol. Biol. 63(10):4–10. doi: 10.14715/cmb/2017.63.10.2 [DOI] [PubMed] [Google Scholar]
- Sugiyama, K., Kumazawa A., Zhou H., and Saeki S.. . 1998. Dietary methionine level affects linoleic acid metabolism through phosphatidylethanolamine n-methylation in rats. Lipids. 33(3):235–242. doi: 10.1007/s11745-998-0201-2 [DOI] [PubMed] [Google Scholar]
- Thompson, K. J., Austin R. G., Nazari S. S., Gersin K. S., Lannitti D. A., and McKillop I. H.. . 2017. Altered fatty acid-binding protein 4 (FABP4) expression and function in human and animal models of hepatocellular carcinoma. Liver Int. 38(6):1074–1083. doi: 10.1111/liv.13639 [DOI] [PubMed] [Google Scholar]
- Van den Veyver, I. B. 2001. Genetic effects of methylation diets. Annu. Rev. Nutr. 22:255–282. doi: 10.1146/annurev.nutr.22.010402.102932 [DOI] [PubMed] [Google Scholar]
- Van Soest, P. J., Robertson J. B., and Lewis B. A.. . 1991. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]
- Waterman, R. C., Ujazdowski V. L., and Petersen M. K.. . 2012. Effects of rumen-protected methionine on plasma amino acid concentrations during a period of weight loss for late gestating beef heifers. Amino Acids 43(5):2165–2177. doi: 10.1007/s00726-012-1301-3 [DOI] [PubMed] [Google Scholar]
- Weiss, W. P., and Wyatt D. J.. . 2003. Effect of dietary fat and vitamin E on α-tocopherol in milk from dairy cows. J. Dairy Sci. 86(11):3582–3591. doi: 10.3168/jds.S0022-0302(03)73964-2 [DOI] [PubMed] [Google Scholar]
- Whitehouse, N. L. 2016. Using the plasma free amino acid dose response method to determine metabolizable protein concentrations of lysine and methionine in rumen protected supplements [doctoral dissertation]. Durham (NH): University of Hampshire Scholars’ Repository. University of Hampshire. Available from https://scholars.unh.edu/cgi/viewcontent.cgi?article=2382&context=dissertation [accessed June 9, 2020]. [Google Scholar]
- Zhou, Z., Vailati-Riboni M., Trevisi E., Drackley J. K., Luchini D. N., and Loor J. J.. . 2016. Better postpartal performance in dairy cow supplemented with rumen-protected methionine compared with choline during the peripartal period. J. Dairy Sci. 99:8716–8732. doi: 10.3168/jds.2015-10525 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.