Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a rare, life-threatening inflammatory syndrome of severe immune system activation. It is a diagnostic challenge with high morbidity and mortality. We present a case of HLH due to anaplasmosis infection. A 54-year-old man with chronic obstructive pulmonary disease presented with fever, nausea, vomiting, dyspnea, and arthralgias for 6 days. He had a rapidly progressive clinical decline requiring intubation for acute respiratory failure and dialysis for acute renal failure. He tested positive for anaplasmosis. His workup met criteria for HLH. He was treated with doxycycline and a steroid taper with clinical improvement allowing for extubation and renal recovery. Patients with persistent fevers, hepatosplenomegaly, cytopenias, and hyperferritinemia should be worked up for HLH.
Keywords: Anaplasma, hemophagocytic, HLH, lymphohistiocytosis
Hemophagocytic lymphohistiocytosis (HLH) encompasses a variety of nonmalignant, life-threatening disorders.1 It is further classified as either primary (genetic) or secondary (acquired) based on the underlying etiology.1,2 Secondary HLH is often associated with a trigger that results in severe inflammatory states.1,3 We present a rare case of anaplasmosis-induced HLH.
Case Report
A 54-year-old man with a history of chronic obstructive pulmonary disease presented with nausea, vomiting, and dyspnea for 6 days. Physical exam was significant for respiratory distress and bilateral lower extremity petechiae. Laboratory investigations showed a white blood cell count of 2.8 K/μL, hemoglobin of 8.4 g/dL, platelet count of 17 K/μL, creatinine of 8.7 mg/dL, triglyceride level of 521 mg/dL, and ferritin level >7500 ng/mL. A peripheral smear revealed pancytopenia with marked thrombocytopenia. Chest x-ray was unrevealing. Further workup showed an antinuclear antibody titer of 1:320 with a homogenous pattern, soluble interleukin-2 receptor (sCD25) level of 36,628 pg/mL, Anaplasma phagocytophilum detected by polymerase chain reaction (PCR) with an IgM titer of >1:320 and IgG titer of 1:64, Epstein-Barr virus (EBV) not detected by PCR with an IgM titer of 33.8 U/mL and IgG titer of 194 U/mL, Varicella zoster (VZV) IgM titer of 1.34 U and IgG titer of 2134 U, and Parvovirus B-19 IgM titer of 1.08 U. Bone marrow biopsy revealed hemophagocytic histocytes with varying numbers of erythroid precursors and platelets.
His hospital course was complicated by respiratory failure requiring intubation. He developed acute renal failure due to acute tubular necrosis as evidenced by diffuse granular, muddy brown casts on urine microscopy requiring renal replacement therapy. He completed a 10-day course of doxycycline and a 14-day course of dexamethasone 20 mg daily. He was extubated and no longer required renal replacement therapy. He was discharged on a steroid taper following the HLH-94 protocol. On outpatient follow-up 2 weeks after discharge, laboratory investigations revealed improvement in ferritin to 1470 ng/mL and sCD25 to 1013 pg/mL.
Discussion
HLH is a rare, life-threatening syndrome that is a diagnostic challenge. HLH results from overstimulation of the immune system leading to systemic inflammation, cytokine storm, and multiorgan failure.3,4 The pathogenesis for secondary HLH is not well defined.2 The current understanding of the pathophysiology includes an overactivation of CD8+ T lymphocytes and macrophages that attack the liver, bone marrow, and central nervous system.5 Patients with HLH syndrome experience a cytokine storm resulting in elevations in interleukin-2, interleukin-6, tumor necrosis factor-alpha, interferon-gamma, and other inflammatory mediators such as prostaglandins.2 This results in massive overactivation of antigen-presenting cells and CD8+ T cells, leading to end-organ damage.2
Clinically, HLH is characterized by persistent fevers, hepatosplenomegaly, cytopenias, and hyperferritinemia.1,2,6 The diagnosis of HLH can be made if either a molecular diagnosis consistent with HLH is obtained or at least five of eight criteria are met from the HLH-2004 guidelines (Table 1).7,8 The H-score is a new diagnostic test used to identify suspected reactive HLH in adults. This score may have been more appropriate to use in our patient; however, it would not have changed his management.
Table 1.
HLH-2004 diagnostic criteria
| Criteria | Patient meeting criterion |
|---|---|
| Fever (≥38.5°C) | No |
| Splenomegaly | No |
| Cytopenias (≥2) | Yes: hemoglobin 8.4 g/dL, platelets 17 K/μL |
| Hypertriglyceridemia | Yes: 521 mg/dL |
| Hemophagocytosis | Yes: in bone marrow |
| Low or absent NK cell activity | – |
| Ferritin >500 ng/mL | Yes: >7500 |
| Elevated soluble CD25 | Yes: 36,628 U/mL |
| Elevated CXCL9 | – |
Known triggers for secondary HLH include infections, malignancies (primarily lymphoma), and autoimmune disorders, among others.7 Reports from East Asia have found rickettsial diseases resulting in HLH.9 Limited reports have identified anaplasmosis-induced HLH.10–12 Although our patient had elevated antibody titers for other potential etiologies including EBV, VZV, and parvovirus, the titers of the antibodies against these viruses were very low. We suspect that the formation of these antibodies was secondary to the significant inflammatory response that results from HLH. Our patient had a negative EBV PCR test and a positive A. phagocytophilum PCR test confirming the diagnosis of human granulocytic anaplasmosis.
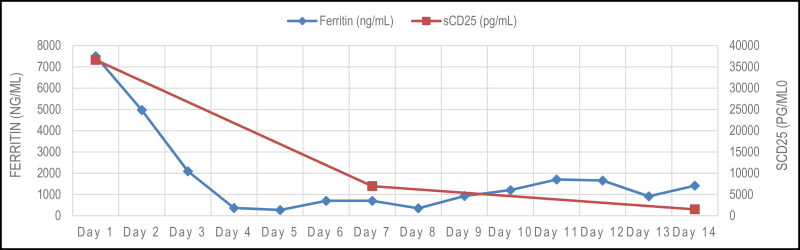
The HLH-94 protocol is the gold standard of treatment for HLH in both adult and pediatric patients. The patient’s clinical improvement and downtrend in his ferritin and sCD25 after treatment with doxycycline and steroids (Figure 1) per the HLH-94 protocol supports the diagnosis of anaplasmosis-induced HLH. The standard HLH-94 protocol includes treatment with etoposide in combination with dexamethasone.12 The decision to not treat the patient with etoposide or anakinra was made after discussion with nephrology, given the patient’s acute renal failure. HLH-2004 added cyclosporine A up front in addition to dexamethasone and etoposide; however, this was not explored due to the patient’s impaired kidney function.13 In prior cases of anaplasmosis-induced HLH, symptoms and laboratory markers improved with doxycycline alone.10,11 It is unclear why our patient suffered such severe disease. It may be the anaplasmosis that predisposed him to such severity. Follow-up in the outpatient setting revealed further improvement in the inflammatory markers ferritin, lactate dehydrogenase, and sCD25.
Figure 1.
The trend of ferritin and sCD25 levels after initiation of dexamethasone and doxycycline treatment.
Morbidity and mortality in patients with HLH continue to be very high. The incidence of shock ranges from 50% to 80%.6 The incidence of acute respiratory failure requiring mechanical ventilation varies from 58% to 100%.6 The incidence of acute renal failure requiring renal replacement therapy has been reported to be as high as 59%.6 Anaplasmosis rarely causes renal failure and is unlikely to have caused the renal failure in our patient.14 Therefore, physicians need to consider the diagnosis of HLH in hospitalized patients who worsen despite treatment for the purported diagnosis. This case report further supports Anaplasmosis as an etiology of secondary HLH. Anaplasmosis should be considered as a potential trigger in patients with HLH due to unknown etiology and in populations with a high incidence of tickborne disease.
References
- 1.Filipovich A, McClain K, Grom A.. Histiocytic disorders: recent insights into pathophysiology and practical guidelines. Biol Blood Marrow Transplant. 2010;16(1 Suppl):S82–S89. doi: 10.1016/j.bbmt.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Kleynberg RL, Schiller GJ.. Secondary hemophagocytic lymphohistiocytosis in adults: an update on diagnosis and therapy. Clin Adv Hematol Oncol. 2012;10(11):726–732. [PubMed] [Google Scholar]
- 3.Hayden A, Park S, Giustini D, Lee AYY, Chen LYC.. Hemophagocytic syndromes (HPSs) including hemophagocytic lymphohistiocytosis (HLH) in adults: a systematic scoping review. Blood Rev. 2016;30(6):411–420. doi: 10.1016/j.blre.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Buyse S, Teixeira L, Galicier L, Mariotte E, Lemiale V, Seguin A, Bertheau P.. Critical care management of patients with hemophagocytic lymphohistiocytosis. Intensive Care Med. 2010;36(10):1695–1702. doi: 10.1007/s00134-010-1936-z. [DOI] [PubMed] [Google Scholar]
- 5.Jameson J, Fauci A, Kasper D, Hauser S.. Primary immune deficiency diseases. In: Harrison’s Principles of Internal Medicine. 20th ed. McGraw Hill; 2021. [Google Scholar]
- 6.Kapoor S, Morgan CK, Siddique MA, Guntupalli K.. Intensive care unit complications and outcomes of adult patients with hemophagocytic lymphohistiocytosis: a retrospective study of 16 cases. World J Crit Care Med. 2018;7(6):73–83. doi: 10.5492/wjccm.v7.i6.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.La Rosée P, Horne AC, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465–2477. doi: 10.1182/blood.2018894618. [DOI] [PubMed] [Google Scholar]
- 8.Henter J-I, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 9.Yi J, Kim KH, Ko MK, Lee EY, Choi SJ, Oh MD.. Human granulocytic anaplasmosis as a cause of febrile illness in Korea since at least 2006. Am J Trop Med Hyg. 2017;96(4):777–782. doi: 10.4269/ajtmh.16-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocco JM, Mallarino-Haeger C, McCurry D, Shah N.. Severe anaplasmosis represents a treatable cause of secondary hemophagocytic lymphohistiocytosis: two cases and review of literature. Ticks Tick Borne Dis. 2020;11(5):101468. doi: 10.1016/j.ttbdis.2020.101468. [DOI] [PubMed] [Google Scholar]
- 11.Johnson C, Davis M, Law A, Sulpher J.. Shared risk factors for cardiovascular disease and cancer: implications for preventive health and clinical care in oncology patients. Can J Cardiol. 2016;32(7):900–907. doi: 10.1016/j.cjca.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Dumler JS, Barat NC, Barat CE, Bakken JS.. Human granulocytic anaplasmosis and macrophage activation. Clin Infect Dis. 2007;45(2):199–204. doi: 10.1086/518834. [DOI] [PubMed] [Google Scholar]
- 13.Bergsten E, Horne AC, Aricó M, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood. 2017;130(25):2728–2738. doi: 10.1182/blood-2017-06-788349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trottestam H, Horne AC, Aricò M, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. 2011;118(17):4577–4584. doi: 10.1182/blood-2011-06-356261. [DOI] [PMC free article] [PubMed] [Google Scholar]