Abstract
Myocardial injury occurs in 20% to 30% of hospitalized patients with COVID-19 infection, and cardiovascular complications contribute to approximately 40% of all COVID-19–related deaths. Most cases of myocarditis related to COVID-19 infection occur in the acute phase of infection and are self-limited. We describe a case of delayed-onset fulminant myocarditis that developed 5 weeks after mild COVID-19 infection leading to cardiogenic shock and the need for mechanical circulatory support. Our case illustrates how myocarditis can occur as a late complication of COVID-19 infection, even in those with a mild initial course.
Keywords: Acute heart failure, cardiogenic shock, COVID-19, myocarditis
The severe acute respiratory syndrome coronavirus (SARS-CoV-2) causing COVID-19 infection was initially detected in Wuhan, China, at the end of 2019 and has now caused a global pandemic resulting in infection of more than 60 million people across the world and loss of over 1 million lives.1,2 Myocardial injury as evidenced by elevated troponin appears to be a prominent feature of the disease, occurring in 20% to 30% of hospitalized patients, and cardiovascular complications contribute to approximately 40% of all COVID-19–related deaths.3 Despite this, clinical myocarditis during the acute phase of illness has been reported in only 1.4% to 7.2% of cases in autopsy studies.4 However, approximately 60% to 80% of patients who recover from COVID-19 were found to have cardiac magnetic resonance imaging evidence of myocarditis at a median of 70 days from infection.5 More importantly, most of these patients had minimal underlying cardiac disease and mild initial infection that did not require hospitalization.
Case Description
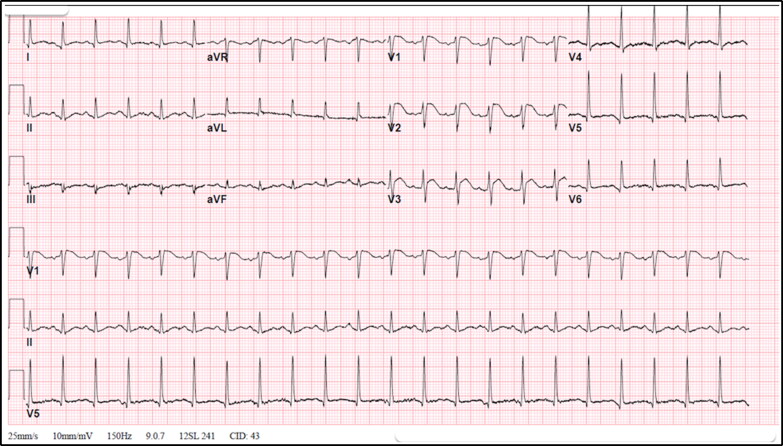
A 53-year-old man without any past medical history presented with fever and upper respiratory symptoms and was diagnosed with COVID-19 infection via polymerase chain reaction testing. Given his mild symptoms, he was managed conservatively and his symptoms resolved within 10 days. Five weeks later, he presented to the emergency department with chest pain, diaphoresis, intense fatigue, and body aches. His blood pressure was 77/46 mm Hg; heart rate, 132 beats/min; respiratory rate, 26 breaths/min; oxygen saturation, 90% on room air; and temperature, 38.9°C. Physical examination was unremarkable except for an S3 gallop. His white blood cell count was 25.1 k/μL; serum creatinine, 2.36 mg/dL; C-reactive protein, >375 mg/L; procalcitonin, 9.30 ng/mL; and lactic acid, 7.8 mmol/L. The initial troponin level was 2.83 ng/mL (normal <0.04 ng/mL), with a peak of 13.6 ng/mL, and N-terminal-pro-brain natriuretic peptide was 60,230 pg/mL. An electrocardiogram showed sinus tachycardia (133 beats/min) with ST elevation in V1 to V3 (Figure 1). Chest x-ray was unremarkable. He received an intravenous fluid bolus, antibiotics, and pressor support (norepinephrine). Transthoracic echocardiography revealed severe global hypokinesis of the left ventricle with a left ventricular ejection fraction of 25% (Supplemental Video 1). Emergent coronary angiogram revealed normal coronary arteries. The differential diagnosis at this point included acute myocarditis vs stress-induced cardiomyopathy following COVID-19 infection.
Figure 1.
Electrocardiogram showing sinus rhythm with ST segment elevation in V1, V2, and V3.
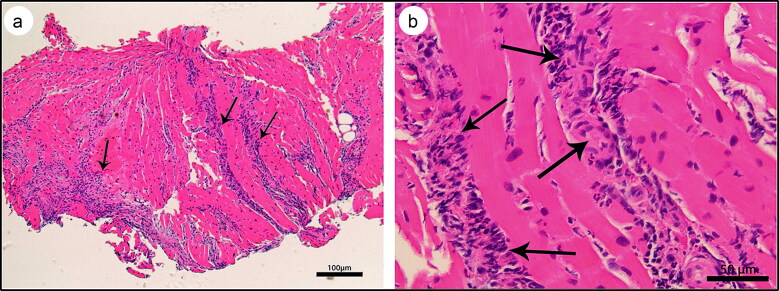
Despite vasopressor support, the patient’s hemodynamic status continued to deteriorate; thus, he underwent emergent right heart catheterization with endomyocardial biopsy. The cardiac hemodynamics were suggestive of combined right and left ventricular failure, and the patient underwent placement of a percutaneous transvalvular left ventricular support device (Impella CP). Despite left ventricular unloading with Impella, the patient continued to worsen with progressive multiorgan failure. He was thus placed on veno-arterial extracorporeal membrane oxygenation (ECMO) and was started on steroids and hemodialysis, with significant improvement in hemodynamics and ejection fraction over the next 5 days. The histopathology of endomyocardial biopsy showed diffuse interstitial and perivascular neutrophilic and lymphocytic infiltration with rare eosinophils and rare myocyte necrosis, suggesting fulminant myocarditis (Figure 2).
Figure 2.
Myocardial histology, hematoxylin and eosin stain, showing (a) acute myocarditis with predominant neutrophilic inflammation (arrows) (4×) and (b) bands of inflammatory infiltrate invading through the myocardium and consisting predominantly of neutrophils with rare lymphocytes and eosinophils (arrows) (20×).
Discussion
The three most notable aspects of our case are (1) the unusual delay between resolution of COVID-19 symptoms and onset of fulminant myocarditis; (2) the discordance between the severity of early illness and that of the late myocarditis; and (3) the rapid recovery of fulminant myocarditis with mechanical circulatory support and steroids.
The mechanism of cardiac injury in COVID-19 remains poorly understood. Proposed mechanisms of injury include direct infection via the angiotensin-converting enzyme 2 receptors, cytokine storm-related inflammation, autoimmune damage, coronary endothelial inflammation, or a combination of the above.3 Given the delay from initial COVID-19 symptoms and the mild initial infection, it is likely that autoimmune myocarditis was the predominant mechanism of myocyte injury in our patient. While viral SARS-CoV-2 RNA has been detected in human hearts of COVID-19 patients, viral particles appear to localize to the interstitium, and there has been no demonstration of direct infection of cardiomyocytes with the virus.6
All previously published cases of COVID-19–related myocarditis occurred within the first week after initial symptoms of SARS-CoV-2 infection, except two cases that reported delayed clinical myocarditis.7,8 While some cases of acute fulminant myocarditis were managed with intravenous immunoglobulins (IVIG), others were treated with steroids alone, which were indicated for concomitant acute lung injury. In our patient, we used steroids primarily for the myocarditis, as our patient did not have significant lung injury. The efficacy of IVIG and steroids in COVID-19 myocarditis is unclear. In prior reports, successful use of ECMO as a bridge to recovery has been described. However, in all these patients, severe myocarditis occurred early in the disease process during the viremic phase.9 In one recent systematic review of COVID-19–related myocarditis that included 14 case reports, steroids were used in 58%, IVIG in 25%, and mechanical circulatory support in 17% of patients, with the most common modality being ECMO.10
Severe myocarditis is a known complication of COVID-19 infection that can occur late, even after recovery from initial infection. The triggers for such delayed fulminant myocarditis are unclear and the true incidence of such presentations is unknown. Cardiac magnetic resonance imaging is a sensitive test for detecting subclinical myocarditis in patients without overt evidence of myocarditis. Aggressive medical therapy and mechanical support may improve outcomes in these patients.
Supplementary Material
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong E, Du H, Gardner L.. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akhmerov A, Marbán E.. COVID-19 and the heart. Circ Res. 2020;126(10):1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halushka MK, Vander Heide RS.. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50:107300. doi: 10.1016/j.carpath.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindner D, Fitzek A, Bräuninger H, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5(11):1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicol M, Cacoub L, Baudet M, et al. Delayed acute myocarditis and COVID-19-related multisystem inflammatory syndrome. ESC Heart Fail. 2020;7(6):4371–4376. doi: 10.1002/ehf2.13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnet M, Champagnac A, Lantelme P, Harbaoui B.. Endomyocardial biopsy findings in Kawasaki-like disease associated with SARS-CoV-2. Eur Heart J. 2020;41(39):3863–3864. doi: 10.1093/eurheartj/ehaa588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tavazzi G, Pellegrini C, Maurelli M, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawalha K, Abozenah M, Kadado AJ, et al. Systematic review of COVID-19 related myocarditis: insights on management and outcome. Cardiovasc Revasc Med. 2021;23:107–113. doi: 10.1016/j.carrev.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.