Abstract
The in vitro susceptibilities of 184 erythromycin-resistant streptococci to a novel ketolide, telithromycin (HMR 3647), were tested. These clinical isolates included 111 Streptococcus pyogenes, 18 group C streptococcus, 18 group G streptococcus, and 37 Streptococcus pneumoniae strains. The MICs for all but eight S. pyogenes strains were ≤0.5 μg/ml, indicating that telithromycin is active in vitro against erythromycin-resistant Streptococcus strains. All strains for which MICs were ≥1 μg/ml had an erm(B) resistance gene and six strains for which MICs were ≥4 μg/ml had a constitutive erm(B) gene (MIC range, 4 to 64 μg/ml). Interestingly, for S. pneumoniae strains with a constitutive erm(B) gene, MICs were ≤0.25 μg/ml (MIC range, ≤0.008 to 0.25 μg/ml). Our in vitro data show that for S. pyogenes strains which constitutively express the erm(B) methylase gene, MICs are so high that the strains might be clinically resistant to telithromycin.
Ketolides represent a new generation of macrolides, in which a 3-keto group replaces l-cladinose in the lactone ring. Ketolides have shown to be more active in vitro than other macrolides against various gram-positive bacteria such as Enterococcus species (6, 24), Staphylococcus aureus (6, 13), and Streptococcus species, including erythromycin-resistant Streptococcus pneumoniae and Streptococcus pyogenes strains (6, 16, 18). On the other hand, methicillin-resistant and some erythromycin-resistant S. aureus strains, as well as some Staphylococcus epidermidis strains (1, 18), seem to be resistant to ketolides (1, 6, 13).
In streptococci, there are two well-characterized macrolide resistance mechanisms: target site modification and active drug efflux. Target site modification is mediated by methylases encoded by the erm (erythromycin ribosome methylation) genes (21, 26). Methylation of A2058 of the peptidyl transferase loop of 23S rRNA causes resistance to macrolides as well as to lincosamides and streptogramin B antibiotics (the MLSB resistance phenotype) (26). The expression of the erm genes can be either constitutive or inducible (27). In streptococci, erm genes are carried on both the chromosome and plasmids (2, 21) and are associated with conjugative transposons (25). The active-efflux mechanism, encoded by the mef (macrolide efflux) genes, is more specific and causes resistance only to 14- and 15-member-ring macrolides (the M resistance phenotype) (3, 22). Expression of mef genes is constitutive (C. Arpin, M. H. Canron, P. Noury, and C. Quentin, Letter, J. Antimicrob. Chemother. 44:133–134, 1999). The mef genes are chromosomal (11, 17) and, at least in the case of S. pyogenes, can be transferred by conjugation (11).
The present work was carried out to study the activity of a novel ketolide, telithromycin (HMR 3647), against Streptococcus species with known macrolide resistance determinants. In addition, its activity against nine S. pneumoniae strains with an unknown macrolide resistance mechanism was tested.
MATERIALS AND METHODS
Bacterial strains.
Altogether 184 erythromycin-resistant streptococcal strains (MIC, ≥1 μg/ml) selected by the erythromycin resistance mechanism and 51 erythromycin-susceptible streptococcal strains (MIC, ≤0.25 μg/ml) were analyzed. These strains included 131 S. pyogenes, 28 group C streptococcus (GCS), 29 group G streptococcus (GGS), and 47 S. pneumoniae strains (Table 1). Erythromycin-resistant S. pyogenes strains were collected from the United States, Argentina, and various European countries between 1986 and 1997. Erythromycin-susceptible S. pyogenes strains were collected from Finland as described previously (9, 11). The GCS and GGS strains were collected in the North Karelian region in Finland between 1992 and 1995 (12). S. pneumoniae strains were obtained from microbiological laboratories situated all over Finland between 1994 and 1998. Each laboratory identified the strains using their own standard microbiology techniques and sent the strains to the Antimicrobial Research Laboratory of the National Public Health Institute, Turku, Finland, where the identification was further confirmed based on colony morphology and hemolysis on Blood Agar Base (Oxoid Ltd., Basingstoke, Hampshire, England) plates supplemented with 7.5% sheep blood. For S. pyogenes, GCS, and GGS strains the Streptex test (Murex Biotech Ltd., Kent, England) was also used, and for S. pneumoniae, the Optochin Disc (Oxoid Ltd.) or Slidex Pneumo-Kit (bioMérieux SA, Marcy l'Etoile, France) was also used. Two control strains, S. pyogenes ATCC 10389 and S. pneumoniae ATCC 49619, were tested together with the studied strains.
TABLE 1.
Streptococcus strains characterized according to their macrolide resistance determinants and MICs
| Organism | Genea | n | MIC (μg/ml)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Telithromycin (HMR 3647)
|
Erythromycin
|
Clindamycin
|
|||||||||
| Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | |||
| S. pyogenes | S | 20 | 0.008–0.063 | 0.016 | 0.063 | ≤0.031–0.25 | 0.063 | 0.125 | ≤0.031–0.125 | 0.063 | 0.063 |
| erm(TRi) | 42 | 0.031–0.25 | 0.063 | 0.063 | 0.5–>64 | 4 | 8 | 0.063–>64 | 0.125 | 0.25 | |
| erm(TRc) | 1 | 0.25 | >64 | >64 | |||||||
| erm(Bi) | 2 | 0.5–1 | >64 | 0.25 | |||||||
| erm(Bc) | 6 | 4–64 | 8 | 64 | >64 | >64 | >64 | >64 | >64 | >64 | |
| mef(A) | 58 | 0.063–0.5 | 0.5 | 0.5 | 4–16 | 8 | 8 | ≤0.031–0.125 | 0.063 | 0.063 | |
| erm(TRi) + mef(A) | 1 | 0.25 | 8 | 0.063 | |||||||
| erm(Bi) + mef(A) | 1 | 4 | >64 | 0.25 | |||||||
| bsc | S | 10 | ≤0.008–0.25 | 0.016 | 0.031 | ≤0.031–0.125 | 0.063 | 0.063 | ≤0.031–0.125 | 0.125 | 0.125 |
| mef(A) | 18 | 0.031–0.5 | 0.25 | 0.5 | 1–8 | 4 | 8 | 0.063–0.125 | 0.125 | 0.125 | |
| bsg | S | 11 | ≤0.008–0.031 | 0.031 | 0.031 | 0.063–0.25 | 0.063 | 0.063 | ≤0.031–0.25 | 0.063 | 0.125 |
| erm(TRi) | 18 | 0.031–0.063 | 0.063 | 0.063 | 1–>64 | 4 | 64 | 0.063–0.5 | 0.125 | 0.5 | |
| S. pneumoniae | S | 10 | ≤0.008–0.031 | ≤0.008 | 0.031 | ≤0.031–0.063 | ≤0.03 | ≤0.031 | ≤0.031–0.063 | 0.063 | 0.063 |
| erm(Bc) | 20 | ≤0.008–0.25 | 0.063 | 0.25 | 64–>64 | >64 | >64 | 0.25–>64 | >64 | >64 | |
| mef(A) | 8 | 0.063–0.5 | 0.125 | 0.5 | 1–4 | 2 | 4 | 0.063–0.125 | 0.063 | 0.125 | |
| Novel phenotypeb | 9 | 0.031–0.125 | 0.063 | 0.125 | 64–>64 | >64 | >64 | 0.5–4 | 1 | 4 | |
| MIC (μg/ml)
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Azithromycin
|
Clarithromycin
|
Spiramycin
|
Quinupristin-dalfopristin (RP59500)
|
||||||||
| Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% |
| 0.063–0.5 | 0.125 | 0.25 | ≤0.031–0.125 | 0.031 | 0.063 | 0.25–1 | 0.25 | 0.5 | 0.25–1 | 0.5 | 1 |
| 0.5–>64 | 16 | 32 | 0.25–>64 | 2 | 2 | 0.5–64 | 1 | 8 | 0.25–1 | 0.5 | 0.5 |
| >64 | >64 | 64 | 0.25 | ||||||||
| >64 | >64 | >64 | 0.5 | ||||||||
| >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | 0.5–1 | 0.5 | 1 |
| 1–16 | 4 | 8 | 1–8 | 4 | 8 | 0.125–2 | 0.25 | 0.5 | 0.25–1 | 0.5 | 0.5 |
| 8 | 8 | 0.5 | 0.25 | ||||||||
| >64 | >64 | >64 | 0.5 | ||||||||
| 0.063–0.25 | 0.125 | 0.25 | ≤0.031–0.063 | 0.031 | 0.063 | 0.125–0.5 | 0.25 | 0.5 | 0.5–2 | 1 | 2 |
| 1–4 | 4 | 4 | 0.25–4 | 4 | 4 | 0.125–1 | 0.5 | 1 | 0.5–4 | 1 | 2 |
| 0.125–1 | 0.125 | 0.125 | ≤0.031–0.25 | ≤0.031 | 0.063 | 0.125–1 | 0.5 | 0.5 | 0.5–1 | 1 | 1 |
| 4–>64 | 16 | >64 | 0.5–>64 | 1 | 8 | 0.5–4 | 2 | 4 | 1–2 | 1 | 1 |
| ≤0.031–0.125 | 0.125 | 0.125 | ≤0.031 | ≤0.031 | ≤0.031 | 0.125–0.25 | 0.125 | 0.25 | 0.5–2 | 1 | 2 |
| >64 | >64 | >64 | 8–>64 | >64 | >64 | 16–>64 | >64 | >64 | 0.5–4 | 2 | 4 |
| 1–4 | 2 | 4 | 1–4 | 2 | 4 | 0.125–0.25 | 0.125 | 0.25 | 0.5–1 | 1 | 1 |
| 32–>64 | 64 | >64 | 16–>64 | >64 | >64 | >64 | >64 | >64 | 2–4 | 4 | 4 |
i, inducible; c, constitutive; S, erythromycin-susceptible strain; erm = erythromycin ribosome methylation genes; mef, macrolide eflux genes.
See text for details.
MIC testing.
MIC testing was done using the agar dilution technique. The bacteria were cultured for 20 h in air at 35°C on Mueller-Hinton II (Becton Dickinson Microbiology Systems, Cockeysville, Md.) agar plates supplemented with 5% sheep blood. The antibiotics used were telithromycin (HMR3647), erythromycin, azithromycin, clarithromycin (Hoechst Marion Roussel [Aventis Pharma], Romainville Cedex, France), quinupristin-dalfopristin (30/70) (RP59500), spiramycin (Rhône-Poulenc Rorer, Vitrysur-Seine, France), and clindamycin (Sigma-Aldrich Chemie, Gmbh, Steinheim, Germany). If available, NCCLS MIC breakpoints were used (15); otherwise, interpretation of MIC results was done based on the distribution of MICs.
Phenotyping.
The double-disk method with erythromycin (diffusible content, 78 μg) and clindamycin (diffusible content, 25 μg) (Neo-sensitabs; A/S Rosco, Taastrup, Denmark) disks was used for classification of macrolide resistance phenotypes. The disks were placed 15 to 20 mm apart on Mueller-Hinton II agar plates supplemented with 5% sheep blood. Bacteria were cultured for 20 h in 5% CO2 at 35°C. After incubation, blunting of the clindamycin zone of inhibition proximal to the erythromycin disk was considered to indicate inducible MLSB resistance (20). In addition to strains with well-characterized macrolide resistance types (the MLSB and M phenotypes), nine S. pneumoniae strains with a novel resistance phenotype were included in the analyses. In these nine strains no known macrolide resistance gene has been found by PCR. These strains are resistant to erythromycin, azithromycin, and clarithromycin, and the spiramycin and clindamycin MICs for these strains are elevated compared to those for strains with the M resistance phenotype, although MICs of clindamycin are not as high as for strains with a constitutive erm(B) resistance gene. Resistance to clindamycin is not inducible (unpublished observations). A similar phenotype for S. pneumoniae has been previously described for clinical isolates (8) and recently also for laboratory strains carrying mutations in 23S rRNA or ribosomal protein L4 (23).
Resistance gene determinations and clonality studies.
Macrolide resistance genes were determined using PCR as described previously (11). The clonality of six S. pyogenes strains was studied using serotyping (14), Vir typing (5), and random amplified polymorphic DNA analysis (19).
RESULTS AND DISCUSSION
In this study, the in vitro activities of telithromycin and six other antibiotics against 184 erythromycin-resistant and 51 erythromycin-susceptible Streptococcus strains were studied (Table 1 and Fig. 1). In general, telithromycin was more active than 14- and 15-member-ring macrolides (erythromycin, azithromycin, and clarithromycin) against erythromycin-resistant Streptococcus strains. MICs of telithromycin were ≤0.5 μg/ml for all but eight S. pyogenes strains (see below). Quinupristin-dalfopristin, a mixture of streptogramin A and B antibiotics, showed activity similar to that of telithromycin, although MICs were somewhat higher (MIC at which 90% of the isolates are inhibited [MIC90], 0.5 to 4 μg/ml). Clindamycin (a lincosamide) was active against strains with an inducible erm methylase gene (MIC90, 0.063 to 0,5 μg/ml) and was even more active than telithromycin against strains with a mef gene (MIC90, 0.063 to 0.125 μg/ml). The clindamycin MICs for strains with a constitutive erm methylase gene were high (MIC90, >64 μg/ml). Also, S. pneumoniae strains with the novel resistance phenotype were intermediate or resistant to clindamycin (MIC90, 4 μg/ml). Spiramycin (a 16-member-ring macrolide) showed activity similar to that of telithromycin against strains with a mef resistance gene. However, the spiramycin MICs for all strains with an erm methylase gene (inducible or constitutive) and for S. pneumoniae strains with the novel resistance phenotype were elevated (MIC90, 4 to >64 μg/ml).
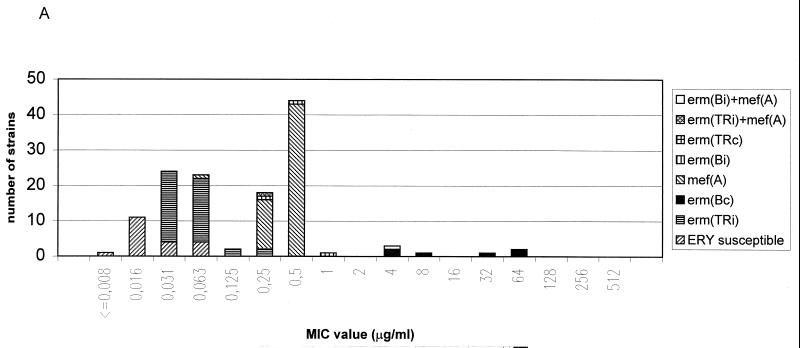
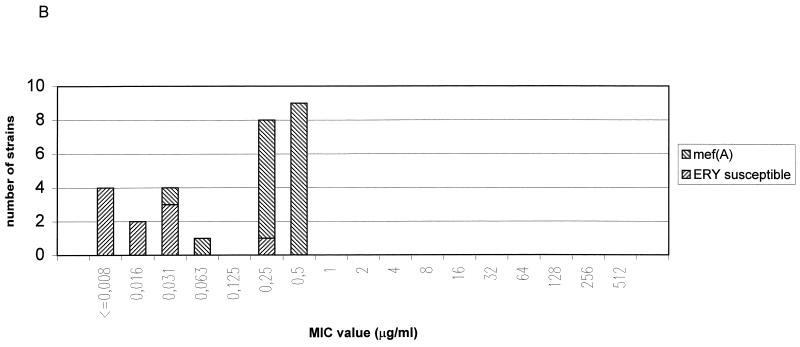
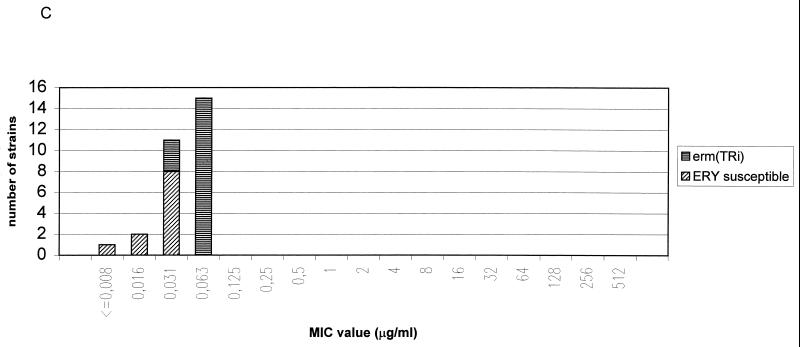
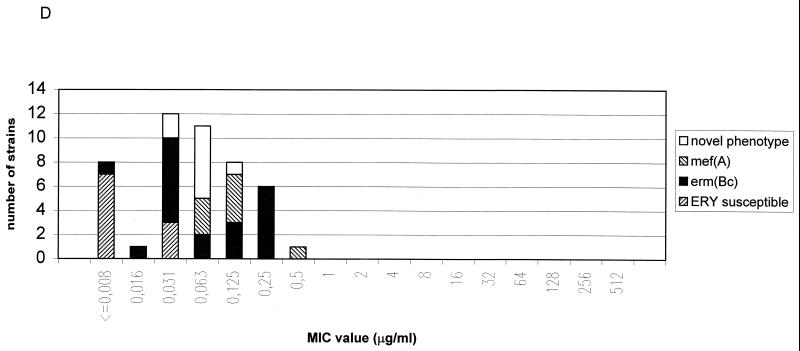
FIG. 1.
Distribution of macrolide resistance genes of S. pyogenes (A), GCS (B), GGS (C), and S. pneumoniae (D) strains according to telithromycin MICs Abbreviations: ERY, erythromycin; c, constitutive; i, inducible; erm, erythromycin ribosome methylation genes; mef, macrolide efflux genes. See text for details of the novel resistance phenotype.
The eight S. pyogenes strains for which the telithromycin MICs were elevated included one strain with an inducible erm(B) gene (MIC, 1 μg/ml), one strain with two resistance genes [an inducible erm(B) gene and a mef(A) gene; MIC, 4 μg/ml], and, most importantly, six strains with a constitutive erm(B) gene (MIC90, 64 μg/ml). These six S. pyogenes strains belonged to six different serotypes (T12M22 opacity factor positive, T12M12 opacity factor negative, T1M1 opacity factor negative, T12M66 opacity factor positive, T6M6 opacity factor negative, and T28R28 opacity factor positive), six different Vir types, and six different random amplified polymorphic DNA types, indicating that the strains were not of the same clonal origin. In contrast to the case for S. pyogenes strains, telithromycin MICs for gene S. pneumoniae strains with the constitutive erm(B) were low (MIC90, 0.25 μg/ml). This finding is concordant with a previous study where telithromycin MICs for S. pneumoniae strains with the constitutive MLSB phenotype of erythromycin resistance were low (MIC90, 0.25 μg/ml) (16). Similar to the case for S. pyogenes, telithromycin MICs for constitutively erythromycin-resistant S. aureus strains have been shown to be elevated (6). Telithromycin MICs for S. pyogenes and GGS strains which had an inducible erm(TR) gene were low (MIC90, 0.063 μg/ml). In contrast to the case for the constitutive erm(B) gene, constitutive expression of the erm(TR) gene in one S. pyogenes strain did not increase the telithromycin MIC.
An interesting question is why the Erm(B) methylase does not protect S. pneumoniae as well as it protects S. pyogenes against telithromycin. The Erm(B) methylases of S. pneumoniae and S. pyogenes are nearly identical (99% similarity at the amino acid level [21]), so it is unlikely that the differences in the erm(B) genes can explain differences in susceptibility to telithromycin. Structural differences in the 23S rRNA molecules or other ribosomal components are more likely explanations. Ketolides as well as other macrolides interact with the peptidyl transferase loop in the V domain of the 23S rRNA. For example, both telithromycin and erythromycin protect positions A2058, A2059, and G2505 of the 23S rRNA against chemical modification (7, 28). It has also been shown that hairpin 35 in domain II of the 23S rRNA constitutes a part of the binding site of macrolides and ketolides (7, 28). Telithromycin protects against chemical modification, and erythromycin enhances chemical modification, of residue A752 in hairpin 35 (7). Actually, using different ketolide derivatives it has been shown that interaction with hairpin 35 is essential for the antimicrobial activity of ketolides (4). Methylation of A2058 of the peptidyl transferase loop in the V domain confers resistance to MLSB antibiotics by inhibiting the binding of the antibiotics to the ribosomes. However, as we have shown here with S. pneumoniae and as has been shown with several other bacteria (6, 18), this methylation does not protect against telithromycin. It has been proposed that although methylation of A2058 weakens the binding of macrolides and ketolides to the ribosomes by interfering with the interaction between the antibiotic and the residues on the peptidyl transferase loop, it does not prevent the strong interaction of ketolides with hairpin 35. This interaction therefore is probably enough to bind the antibiotic to the ribosome and to prevent protein synthesis (28). The same is true with mutations in the peptidyl transferase loop, which can inhibit binding of macrolides but not ketolides to the ribosomes if ketolides have alkyl-aryl 11/12 lactone ring extensions, which can make contact with hairpin 35 and the drug (4). It might be that in S. pyogenes the interaction of telithromycin with hairpin 35 is weaker than that in S. pneumoniae and because of that, telithromycin can not bind to the ribosomes of S. pyogenes if A2058 is methylated. Structural differences in the ribosomes of the two bacteria may thus explain the differences in the interactions between telithromycin and hairpin 35.
Quite recently, Tait-Kamradt et al. (23) demonstrated that in S. pneumoniae mutations in the 23S rRNA or ribosomal protein L4 can cause resistance to macrolides. Mutations in the pepdityl transferase loop of the 23S rRNA caused phenotypes that were similar but not identical to those described in this work for S. pneumoniae strains without any known macrolide resistance genes. The type, number, and positions of mutations in the peptidyl transferase loop affect the phenotype, so it is possible that the strains we describe here also carry resistance-causing mutations in 23S rRNA molecules.
Telithromycin is a novel ketolide that belongs to the macrolide family of antibiotics. Our work in addition to several other studies, indicates that the new ketolides are active in vitro against various gram-positive bacteria, including strains that are resistant to other macrolides (6, 16). Telithromycin MICs were high only for S. pyogenes strains with the constitutive erm(B) resistance gene. In recent years these strains have comprised about 10% of all erythromycin-resistant S. pyogenes strains in Finland (11) and several other countries (10). Although the presence of a constitutive erm(B) gene in S. pyogenes varies in different countries (10), it seems that telithromycin has good in vitro activity against most S. pyogenes strains.
ACKNOWLEDGMENTS
We thank Tuula Randell and Anna-Liisa Lumiaho for their excellent technical assistance. We are grateful to the Finnish Study Group for Antimicrobial Resistance and to Androulla Efstratiou, Emilio Pérez-Trallero, Antoaneta Detcheva, Michael Jacobs. Horacio Lopardo, J. P. Garrahan, Dianella Savoia, Claes Schalén. Eva Tzelepi, and Pietro Varaldo for the bacterial strains used in this study. We also thank André Bryskier (Aventis Pharma Inc.) for supplying telithromycin.
This work was supported by a grant from Aventis Pharma Inc.
REFERENCES
- 1.Boswell F J. The in-vitro activity of HMR 3647, a new ketolide antimicrobial agent. J Antimicrob Chemother. 1998;42:703–709. doi: 10.1093/jac/42.6.703. [DOI] [PubMed] [Google Scholar]
- 2.Ceglowski P, Alonso J C. Gene organization of the Streptococcus pyogenes plasmid pDB101: sequence analysis of the orf eta-copS region. Gene. 1994;145:33–39. doi: 10.1016/0378-1119(94)90319-0. [DOI] [PubMed] [Google Scholar]
- 3.Clancy J, Petitpas J, Dib-Hajj F, Yuan W, Cronan M, Kamath A V, et al. Molecular cloning and functional analysis of a novel macrolide-resistance determinant, mefA, from Streptococcus pyogenes. Mol Microbiol. 1996;22:867–879. doi: 10.1046/j.1365-2958.1996.01521.x. [DOI] [PubMed] [Google Scholar]
- 4.Douthwaite S, Hansen L H, Mauvais P. Macrolide-ketolide inhibition of MLS-resistant ribosomes is improved by alternative drug interaction with domain II of 23S rRNA. Mol Microbiol. 2000;36:183–193. doi: 10.1046/j.1365-2958.2000.01841.x. [DOI] [PubMed] [Google Scholar]
- 5.Gardiner D, Hartas J, Currie B, Mathews J D, Kemp D J, Sriprakash K S. Vir typing: a long-PCR typing method for group A streptococci. PCR Methods Appl. 1995;4:288–293. doi: 10.1101/gr.4.5.288. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton-Miller J M T, Shah S. Comparative in-vitro activity of ketolide HMR 3647 and four macrolides against gram-positive cocci of known erythromycin susceptibility status. J Antimicrob Chemother. 1998;41:649–653. doi: 10.1093/jac/41.6.649. [DOI] [PubMed] [Google Scholar]
- 7.Hansen L H, Mauvais P, Douthwaite S. The macrolide-ketolide antibiotic binding site is formed by structures in domains II and V of 23S ribosomal RNA. Mol Microbiol. 1999;31:623–631. doi: 10.1046/j.1365-2958.1999.01202.x. [DOI] [PubMed] [Google Scholar]
- 8.Johnston N J, de Azavedo J C, Kellner J D, Low D E. Prevalence and characterization of the mechanisms of macrolide, lincosamide, and streptogramin resistance in isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1998;42:2425–2426. doi: 10.1128/aac.42.9.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kataja J, Huovinen P, Muotiala A, Vuopio-Varkila J, Efstratiou A, Hallas G, et al. Clonal spread of group A streptococcus with the new type of erythromycin resistance. J Infect Dis. 1998;177:786–789. doi: 10.1086/517809. [DOI] [PubMed] [Google Scholar]
- 10.Kataja J, Huovinen P, Seppala H. Erythromycin resistance genes in group A streptococci of different geographical origins. J Antimicrob Chemother. 2000;46:789–792. doi: 10.1093/jac/46.5.789. [DOI] [PubMed] [Google Scholar]
- 11.Kataja J, Huovinen P, Skurnik M, Seppälä H. Erythromycin resistance genes in group A streptococci in Finland. Antimicrob Agents Chemother. 1999;43:48–52. doi: 10.1128/aac.43.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kataja J, Seppälä H, Skurnik M, Sarkkinen H, Huovinen P. Different erythromycin resistance mechanisms in group C and group G streptococci. Antimicrob Agents Chemother. 1998;42:1493–1494. doi: 10.1128/aac.42.6.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malathum K, Coque T M, Singh K S, Murray B E. In vitro activities of two ketolides, HMR 3647 and HMR 3004, against gram-positive bacteria. Antimicrob Agents Chemother. 1999;43:930–936. doi: 10.1128/aac.43.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muotiala A, Seppälä H, Huovinen P, Vuopio-Varkila J. Molecular comparison of group A streptococci of T1M1 serotype from invasive and noninvasive infections in Finland. J Infect Dis. 1997;175:392–399. doi: 10.1093/infdis/175.2.392. [DOI] [PubMed] [Google Scholar]
- 15.NCCLS. Performance standards for antimicrobial susceptibility testing; ninth informational supplement, M100–S9. Vol. 19. Wayne, Pa: NCCLS; 1999. [Google Scholar]
- 16.Reinert R R, Bryskier A, Lütticken R. In vitro activities of the new ketolide antibiotics HMR 3004 and HMR 3647 against Streptococcus pneumoniae in Germany. Antimicrob Agents Chemother. 1998;42:1509–1511. doi: 10.1128/aac.42.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santagati M, Iannelli F, Oggioni M R, Stefani S, Pozzi G. Characterization of a genetic element carrying the macrolide efflux gene mef(A) in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2000;44:2585–2587. doi: 10.1128/aac.44.9.2585-2587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schülin T, Wennersten C B, Moellering R C, Jr, Eliopoulos G M. In-vitro activity of the new ketolide antibiotic HMR 3647 against gram-positive bacteria. J Antimicrob Chemother. 1998;42:297–301. doi: 10.1093/jac/42.3.297. [DOI] [PubMed] [Google Scholar]
- 19.Seppälä H, He Q, österblad M, Huovinen P. Typing of group A streptococci by random amplified polymorphic DNA analysis. J Clin Microbiol. 1994;32:1945–1948. doi: 10.1128/jcm.32.8.1945-1948.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seppälä H, Nissinen A, Yu Q, Huovinen P. Three different phenotypes of erythromycin-resistant Streptococcus pyogenes in Finland. J Antimicrob Chemother. 1993;32:885–891. doi: 10.1093/jac/32.6.885. [DOI] [PubMed] [Google Scholar]
- 21.Seppälä H, Skurnik M, Soini H, Roberts M C, Huovinen P. A novel erythromycin resistance methylase gene (ermTR) in Streptococcus pyogenes. Antimicrob Agents Chemother. 1998;42:257–262. doi: 10.1128/aac.42.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutcliffe J, Tait-Kamradt A, Wondrack L. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob Agents Chemother. 1996;40:1817–1824. doi: 10.1128/aac.40.8.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tait-Kamradt A, Davies T, Cronan M, Jacobs M R, Appelbaum P C, Sutcliffe J. Mutations in 23S rRNA and ribosomal protein L4 account for resistance in pneumococcal strains selected in vitro by macrolide passage. Antimicrob Agents Chemother. 2000;44:2118–2125. doi: 10.1128/aac.44.8.2118-2125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torres C, Zarazaga M, Tenorio C, Portillo A, Saenz Y, Ruiz F, et al. In vitro activity of the new ketolide HMR3647 in comparison with those of macrolides and pristinamycins against Enterococcus spp. Antimicrob Agents Chemother. 1998;42:3279–3281. doi: 10.1128/aac.42.12.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trieu-Cuot P, Poyart-Salmeron C, Carlier C, Courvalin P. Nucleotide sequence of the erythromycin resistance gene of the conjugative transposon Tn1545. Nucleic Acids Res. 1990;18:3660. doi: 10.1093/nar/18.12.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weisblum B. Erythromycin resistance by ribosome modification. Antimicrob Agents Chemother. 1995;39:577–585. doi: 10.1128/AAC.39.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weisblum B. Insights into erythromycin action from studies of its activity as inducer of resistance. Antimicrob Agents Chemother. 1995;39:797–805. doi: 10.1128/aac.39.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong L, Shah S, Mauvais P, Mankin A S. A ketolide resistance mutation in domain II of 23S rRNA reveals the proximity of hairpin 35 to the peptidyl transferase centre. Mol Microbiol. 1999;31:633–639. doi: 10.1046/j.1365-2958.1999.01203.x. [DOI] [PubMed] [Google Scholar]