Abstract
Background
The role of branched-chain amino acids (BCAA) in improving muscle mass in cirrhosis is presently debatable.
Aims
To evaluate the role of BCAA in improving muscle mass in a double-blind randomized placebo-controlled trial in patients with cirrhosis having sarcopenia.
Methods
Consecutive patients with cirrhosis with Child–Pugh score < 10 and sarcopenia were randomized to receive either 12 g/day of BCAA orally or a placebo (1:1) for 6 months in addition to a home-based exercise program (30 min/day), dietary counselling and standard medical therapy. Sarcopenia was defined according to gender-specific axial skeletal muscle index (SMI) cut-offs. The primary endpoint was a change in muscle mass based on CT scan (SMI) after 6 months of supplementation.
Results
Sixty patients [mean age 41.6 ± 9.9 years; males (66.6%) of predominantly viral (40%) and alcohol-related (31.7%) cirrhosis] were randomized. Baseline clinical and demographic characters were similar except MELD score (10.2 ± 2.8 vs. 12.2 ± 3.5, p = 0.02) and calorie intake (1838.1 kcal ± 631.5 vs. 2217.5 kcal ± 707.3, p = 0.03), both being higher in the placebo arm. After adjusting for both baseline confounders, baseline SMI and protein intake, the change in SMI at 6 months was similar in both groups [mean adjusted difference (MAD) + 0.84, CI − 2.9; + 1.2, p = 0.42] by intention-to-treat analysis. The secondary outcomes including change in handgrip strength (p = 0.65), 6-m gait speed (p = 0.20), 6-min walk distance (p = 0.39) were similar in both arms. Four patients had minor adverse events in each arm.
Conclusion
Addition of BCAA to exercise, dietary counselling and standard medical therapy did not improve muscle mass in patients with cirrhosis having sarcopenia. (CTRI/2019/05/019269).
Trial registration number
CTRI/2019/05/019269 (Clinical Trials Registry of India).
Supplementary Information
The online version contains supplementary material available at 10.1007/s12072-022-10334-7.
Keywords: End-stage liver disease, Malnutrition, Quality of life, Leucine, Exercise
Introduction
Sarcopenia is a well-recognised complication of cirrhosis [1]. It is associated with an increased risk of complications, poor quality of life, higher transplant waitlist mortality and post-transplant mortality [2–4]. Treatment options are limited and focused on deficiency replacement rather than targeting the underlying cause [5, 6]. The pathogenesis of sarcopenia in cirrhosis is multifactorial. Malnutrition secondary to ascites, delayed gastric emptying, reduced appetite and reduced palatability of a low sodium diet lead to reduced intake of food and imbalance between muscle synthesis and degradation [5, 7]. Key mediators at the cellular level which alter muscle protein synthesis are ammonia, myostatin, growth hormone and others [8, 9]. The three key pathways involved in muscle balance are mTOR pathway, autophagy and the ubiquitin–proteasome pathway [5].
Branched-chain amino acids (BCAA) including leucine, valine and isoleucine are unique as they escape hepatic uptake and are primarily utilised by the skeletal muscle [10]. In muscle, BCAA serves as both energy substrate and precursors for other amino acids and play important roles in promoting protein synthesis. BCAA has been shown to play a role in reducing muscle breakdown and promoting protein synthesis by regulating the mTOR pathway [11]. Synthesis is further supported by providing essential amino acids and maintenance of the glutamate pool which is usually deficient in cirrhosis due to excess ammonia detoxification [12].
Few studies have been conducted to assess the role of BCAA on muscle improvement, however, the results are conflicting [13–15]. Other clinical effects of BCAA demonstrated in studies include improvement in quality of life, reduced fatigue, improvement in hepatic encephalopathy and also reduction in tumorigenesis [15–17].
We hypothesized that BCAA treatment can improve muscle mass in patients with cirrhosis having sarcopenia. We conducted a double-blind randomized placebo-controlled trial of supplementation of oral BCAA in patients with cirrhosis to examine the effects on muscle mass as compared to placebo and other associated parameters were examined.
Materials and methods
This was an investigator-initiated double-blind, 2-arm parallel design, randomized placebo-controlled trial conducted at a tertiary care hospital in New Delhi, India from 1 May 2019 to 14 September 2020. The institutional ethics committee (IECPG-213/27.03.2019) approved the study. Informed consent was obtained from all individual participants in the study. The study was conducted as per the Indian Council of Medical Research and Good Clinical Practice guidelines. The trial was registered with the Clinical Trials Registry of India (CTRI/2019/05/019269). All authors approved the final manuscript.
Patients
Patients with cirrhosis attending our gastroenterology clinic and having sarcopenia on imaging irrespective of the etiology of liver disease were screened for inclusion in this study. Cirrhosis was diagnosed by a combination of clinical features, biochemical, radiological or histological (in those with available liver biopsy) features [18]. Supportive radiological features included nodular outline of the liver with signs of portal hypertension.
Patients with age < 18 years or > 60 years, overt hepatic encephalopathy (HE), hepatocellular carcinoma, gastrointestinal bleeding within the past 6 months, concomitant sepsis, concurrent steroid use, pregnancy, acute-on-chronic liver failure (ACLF) [19] or Child–Pugh class C were excluded from the study.
Sarcopenia was defined by low muscle mass, as indicated by a skeletal muscle index (SMI) of < 50 cm2/m2 for men and < 39 cm2/m2 for women [4]. Muscle mass was assessed by measuring skeletal muscle area (SMA) in a transverse CT image in the venous phase at the mid-body of the third lumbar (L3) vertebral level. Skeletal muscle area (SMA) included the sum of areas of paraspinal, psoas, transverses abdominis, internal oblique, external oblique, and rectus abdominis muscle at the L3 vertebral level (Fig. 1). The area ranging from (− 29) Hounsfield unit (HU) to 150 HU was considered as muscle area. The images were analysed using 3D slicer software version 4.10.2 (http://www.slicer.org), which is an image computing platform used for quantitative imaging [20]. Two independent observers did SMI calculation and if there was more than 5% difference then we resolved it by mutual consensus. It was then normalized for the height by dividing it by the squared height and expressed as SMI in cm2/m2 [21].
Fig. 1.
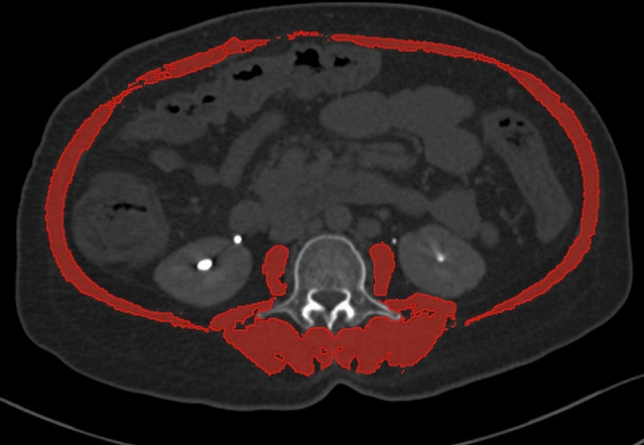
Computed tomography scan at third lumbar vertebra showing the calculation of skeletal muscle area (red highlighted area)
Randomization and masking
Based on computer-generated random numbers, generated by the statistician, eligible patients were randomized by simple randomization to receive either BCAA or a matching placebo in a 1:1 ratio. A double-blind procedure was followed to ensure minimum bias and both care provider and patient were blinded. BCAA and placebo were provided in identical packaging as a white opaque sachet (Supplementary Fig. 1). Boxes with 3 months dose BCAA/placebo were prepared in advance according to the computer-generated numbers and codes were kept with an investigator not involved in the distribution of intervention. To minimise the risk of bias, the investigators involved in randomization and drug administration did not participate in collecting the outcome data and analysis of the results.
Study intervention
All enrolled patients received either orally administered BCAA or a matching placebo for a period of 6 months. Each sachet contained 10 g of powder of which 6 g was BCAA and the remaining were preservatives and orange flavouring to increase palatability. The main components of the placebo were maltodextrin and sucralose. The detailed composition of both sachets is available in Supplementary Tables 1 and 2. The sachets were provided to the patients for 3 months at a time during the initial and 3-month visit. The morning dose is a part of breakfast and second dose is a late evening snack after a meal. Empty and non-consumed sachets were kept and returned at each visit to accurately assess compliance. All other treatment was given at the discretion of the clinicians. All patients were continued on standard medical therapy under which included etiology-specific management, diuretics and salt restriction for ascites, beta blockers and endotherapy for gastrointestinal bleed, lactulose and rifaximin for hepatic encephalopathy whenever indicated. (Supplementary text).
Outcome measures and their assessment
The primary objective of the study was to assess the change in SMI (which reflects muscle mass) at the end of 6 months of intervention. The secondary objectives included assessment of change in muscle strength, muscle performance, quality of life, ammonia levels and serum myostatin levels. Adverse events were also monitored.
Muscle strength was assessed by handgrip strength (HGS), which was measured in the non-dominant hand using a digital hand dynamometer (DHD-1, Saehan Corporation, South Korea) according to standard protocols [22].
Muscle performance was assessed by two methods. A 6-m gait speed was assessed by making the patient walk at a usual pace. This was done thrice with a gap of 30 s after each attempt and the mean of the readings was calculated. Six-minute walk distance was also measured, according to the recommendations of the American thoracic society, to calculate the six-minute walk distance (6MWD) [23].
Quality of life was assessed at baseline and at 6 months by a validated questionnaire in the form of a chronic liver disease questionnaire (CLDQ) [24, 25]. It is a validated questionnaire which assesses the physical, psychological and social aspects of health in patients with chronic liver disease in a quantitative measure. Details are provided in supplementary text.
Serum myostatin was measured at baseline and at 6 months using commercially available kits [R&D systems, Minneapolis, USA] based on solid-state sandwich enzyme-linked immunosorbent assay (ELISA) technique. Plasma ammonia was measured using commercially available kits (Randox Laboratories Ltd, UK) by an enzymatic ultraviolet-based technique.
Dietary assessment and counselling
All patients were seen by a dietician at the time of recruitment. A pretested open-ended semi-quantitative food frequency pro forma was used to collect nutrient and dietary information on seven food groups and miscellaneous food items from each patient at baseline and at 6 months. Details for assessment are available in the Supplementary text. After calculation, a 25–30 kcal/day diet along with 1–1.2 g/kg/day of protein intake was advised for all patients.
Exercise counselling
A home-based exercise of approximately 30–45 min per day was recommended to all patients. It included 5–10 min of resistance training, 5 min of balance exercise and 15–30 min of brisk walking. The exercises were demonstrated to the patients and a paper-based visual aid was provided to them for reference. (Supplementary Fig. 2) The patient was given a checklist with a daily monitoring chart to record whether they completed the exercise regimen for that day. This checklist was collected at each visit and adherence was reinforced. However, poor adherence (< 80% compliance) to exercise was not considered as part of protocol violation.
Sample size estimation and statistical analysis
Based on the results of previous studies, we assumed a change in SMI (mean ± standard error) of 5 ± 3% in the placebo arm and 10 ± 7% in the BCAA arm [26–28]. Assuming a false positivity (α) rate of 5% and power of 80%, for a confidence interval (CI) of 95%, the calculated sample size for the primary outcome was 50. To allow for a 20% dropout rate, we aimed to recruit 60 patients.
Descriptive statistics were summarized as mean ± standard deviation (SD) or median with interquartile range (IQR). Comparison of the continuous baseline characters was based on the Student’s t test or Wilcoxon rank-sum test, depending on the normalcy of distribution. Categorical data were represented as frequency (percentage) and compared using the χ2 test or Fischer’s exact test.
Primary and secondary objectives were compared between the BCAA and placebo arms and reported with 95% confidence interval (CI). We conducted the primary analysis with the intention to treat population (ITT), defined as all the patients who underwent randomization. In the per-protocol population, we included those who completed the duration of follow-up and had no major protocol violations. Those who reported adherence of > 80% for BCAA were included in the per-protocol analysis.
For the primary analysis, we evaluated the outcomes in the two arms using analysis of covariance (ANCOVA) after adjusting for baseline SMI, MELD score, baseline calorie intake and protein intake. Mean adjusted difference (MAD) with 95% confidence intervals was computed. Serum myostatin and plasma ammonia were not adjusted for comparison.
For the secondary analysis, we evaluated the change in muscle mass in the per-protocol population and subgroups classified by Child–Pugh class, gender, alcoholic etiology of cirrhosis and using the cut-off for sarcopenia studied in the Asian population (SMI of < 36.5 cm2/m2 for men and < 30.2 cm2/m2 for women) [29]. Missing data were handled using the last observation carried forward method. All analysis was performed using the Stata software version 15.1 (STATA, College Station, TX). Profile diagram and Kaplan–Meier curve were drawn using Rstudio.
Flow of study
At enrolment, all patients underwent baseline hematological, biochemical investigations and invasive tests when necessary to assess the severity and etiology of liver disease. Tests to evaluate the outcome measures were done for all patients. Patients were then randomised and assigned intervention. Compliance to intervention was ensured and assessed telephonically fortnightly. All patients were followed up after baseline visit at 3 months, 6 months and in between as needed. At the end of 6 months of follow-up, all evaluation necessary for outcome assessment was repeated.
Results
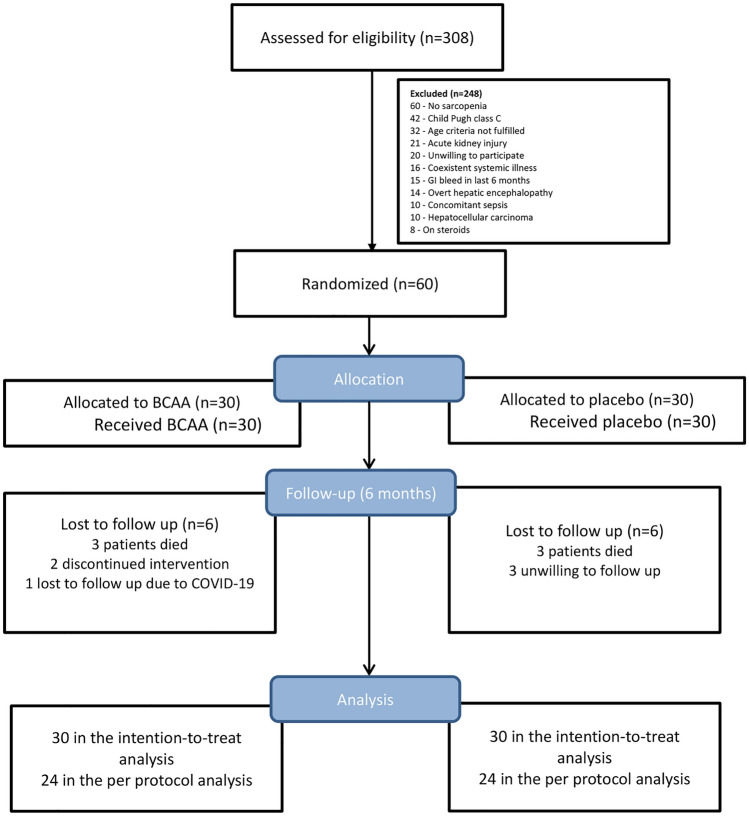
A total of 308 patients with cirrhosis were screened for eligibility from May 2019 to March 2020 and sixty patients (30 patients in each arm after randomization) were included and followed for a period of 6 months. All patients were included for ITT analysis and forty-eight were included in the per-protocol analysis. Reasons for the exclusion and loss of follow-up are presented in the CONSORT diagram (Fig. 2).
Fig. 2.
CONSORT diagram showing the flow of study and number of patients at each step
Baseline parameters are tabulated in Table 1. The mean age of the patients included in the trial was 41.6 ± 9.9 years with no significant difference between both groups (p = 0.663). Patients were predominantly males (66.6%), with no significant difference between two arms (BCAA—63.3% vs. placebo—70%, p = 0.584). The most common etiology of cirrhosis was viral (40%) followed by alcohol-related (31.7%). Patients in the placebo arm had a higher MELD score [10.2 ± 2.8 (BCAA arm) vs. 12.2 ± 3.5 (placebo), p = 0.015] but a comparable Child–Pugh score (BCAA—6.9 ± 1.5 vs. placebo—7.5 ± 1.6, p = 0.123). Most patients belonged to Child class B (66.6%) with no significant difference among both arms (p = 0.273). Clinical decompensation like ascites, past gastrointestinal bleed and past history of encephalopathy were comparable between both arms (Table 1). Baseline laboratory parameters including bilirubin, albumin, INR were also similar in both arms. On dietary estimation, the mean calorie intake (kcal/day) was significantly higher in placebo arm compared to those administered BCAA [1838.1 ± 631.5 (BCAA) vs. 2217.5 ± 707.3 (placebo), p = 0.03] (Table 1); but baseline protein consumption (g/day) (70.0 ± 27.2 (BCAA) vs. 69.6 ± 21.0 (placebo), p = 0.13) was similar in both arms.
Table 1.
Comparison of baseline characters between both arms
| BCAA (n = 30)# | Placebo (n = 30)# | p value | |
|---|---|---|---|
| Age (years) | 42.26 ± 10.07 | 40.83 ± 9.80 | 0.663 |
| Gender | 0.584 | ||
|
Males Females |
19 (63.3%) 11 (36.7%) |
21 (70%) 9 (30%) |
|
| Alcohol consumption | 10 (33.3%) | 12 (40%) | 0.592 |
| Diabetes mellitus | 6 (20%) | 3 (10%) | 0.472 |
| Endoscopy findings | 0.053 | ||
|
No varices Low risk varices High risk varices Eradicated varices |
8 17 1 4 |
1 21 4 4 |
|
| Hypertension | 6 (20%) | 1 (3.3%) | 0.103 |
| Etiology | 0.434 | ||
|
Alcohol Hepatitis C Hepatitis B NASH related Autoimmune Cryptogenic |
8 (26.7%) 7 (23.3%) 3 (10%) 5 (16.6%) 1 (3.3%) 6 (20.0%) |
11 (36.7%) 7 (23.3%) 7 (23.3%) 2 (6.7%) 0 3 (10.0%) |
|
| History of decompensation | |||
|
GI bleed Ascites Encephalopathy |
9 (30%) 21 (70%) 4 (13.3%) |
10 (33.3%) 23 (76.7%) 3 (10%) |
0.781 0.559 0.688 |
| Platelet count (× 109/L) | 107 (27–283) | 70 (30–270) | 0.103 |
| Bilirubin (mg/dL) | 1.2 (0.5–4.1) | 1.4 (0.5–4.9) | 0.461 |
| Albumin (g/dL) | 3.58 ± 0.66 | 3.29 ± 0.62 | 0.094 |
| INR | 1.3 ± 0.2 | 1.4 ± 0.3 | 0.070 |
| Creatinine (mg/dL) | 0.8 (0.6–1.4) | 0.8 (0.5–1.9) | 0.817 |
| Plasma ammonia (µmol/L) | 71 (64.5–84.3) | 75 (66–142) | 0.222 |
| BMI (kg/m2) | 23.8 ± 5.2 | 23.5 ± 5.4 | 0.798 |
| Calorie intake (kcal/day) | 1838.1 ± 631.5 | 2217.48 ± 707.3 | 0.033 |
| Carbohydrate intake (g/day) | 182.4 ± 61.3 | 204.8 ± 65.1 | 0.114 |
| Protein intake (g/day) | 60.9 ± 27.2 | 69.6 ± 21.0 | 0.135 |
| Child class | 0.273 | ||
|
A B |
12 (40%) 18 (60%) |
9 (30%) 21 (70%) |
|
| CTP score | 6.9 ± 1.5 | 7.5 ± 1.6 | 0.123 |
| MELD | 10.2 ± 2.8 | 12.2 ± 3.5 | 0.015 |
BCAA branched-chain amino acids, BMI body mass index, CTP Child–Pugh-Turcotte, INR international normalised ratio, MELD model for end stage of liver disease, NASH non-alcoholic steatohepatitis
#Values expressed as mean ± standard deviation, median (interquartile range) or n (%)
A dropout rate of 10% was observed in our study (n = 6) and a further 10% succumbed to the illness during follow-up. Of these, one patient succumbed to COVID-19 illness and two could not attend the clinic at the stipulated time due to travel restrictions during COVID-19 pandemic. Eighty-eight percent (n = 53) patients completed the first follow-up at 3 months of therapy. Eighty percent of the patients had a > 80% adherence to the BCAA/placebo and complied with the study protocol till 6 months. The number of patients with good adherence to exercise (> 80%) were similar in both arms (BCAA-60% vs. placebo-63.3%, p = 0.791).
Assessment of change in muscle mass (primary outcome)
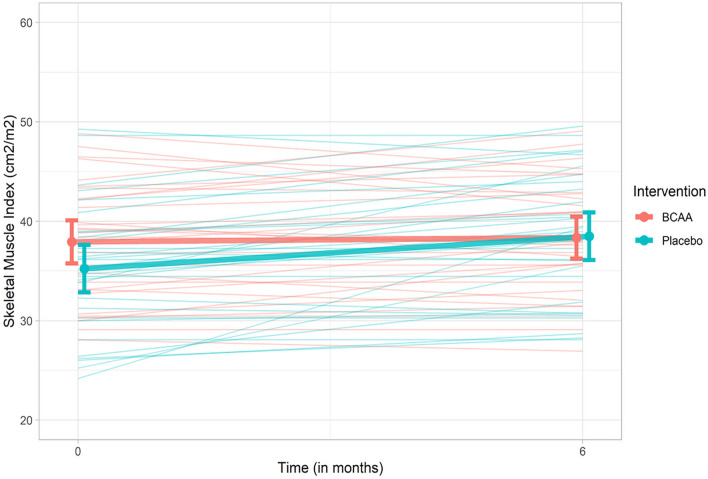
The baseline measures of sarcopenia were similar in both arms [mean SMI (cm2/m2) 37.9 ± 5.9 (BCAA) vs. 35.2 ± 6.5 (placebo), p = 0.105). (Table 2) After the end of follow-up, the mean SMI (cm2/m2) was similar in both arms (38.9 ± 6.0 (BCAA) vs. 38.0 ± 6.4 (placebo), p = 0.551). Change in individual SMI of patients is depicted in Fig. 3. ANCOVA test was used to compare the change in SMI in both arms after adjusting for both confounders (MELD score and calorie intake), baseline SMI as well as protein intake. The mean adjusted difference (MAD) of SMI between both arms was not significant [− 0.84 (− 2.90 to 1.2), p = 0.418]. Similar result was obtained in the per-protocol analysis [− 0.73 (− 3.28 to 1.82), p = 0.567] (Table 3).
Table 2.
A comparison of outcome measures in BCAA and placebo arm at baseline and at end of follow up (6 months)
| Baseline | At 6 months of follow up | |||||
|---|---|---|---|---|---|---|
| BCAA (n = 30)* | Placebo (n = 30)* | p value | BCAA (n = 30)* | Placebo (n = 30)* | p value | |
| Skeletal muscle index (cm2/m2) | 37.92 ± 5.94 | 35.23 ± 6.54 | 0.105 | 38.93 ± 5.97 | 37.97 ± 6.43 | 0.551 |
| Hand grip strength (kg) | 22.17 ± 7.29 | 22.82 ± 7.39 | 0.734 | 23.35 ± 7.07 | 24.04 ± 7.73 | 0.723 |
| 6 m gait speed (m/s) | 0.96 ± 0.20 | 1.04 ± 0.26 | 0.143 | 1.03 ± 0.21 | 1.15 ± 0.20 | 0.037 |
| 6-min walk distance (m) | 307.3 ± 40.55 | 326.17 ± 55.03 | 0.144 | 342.3 ± 57.97 | 344.16 ± 64.40 | 0.218 |
| CLDQ (overall score) | 4.56 ± 1.18 | 4.61 ± 1.15 | 0.846 | 4.55 ± 1.17 | 4.61 ± 1.15 | 0.553 |
BCAA branched-chain amino acids, CLDQ chronic liver disease questionnaire
*Values represented as mean ± standard deviation
Fig. 3.
Profile diagram showing individual changes in SMI over 6 months as well as median change in each group
Table 3.
Comparison of change in SMI between both arms after adjusting for MELD score, calorie intake and protein intake
| Change in skeletal muscle index (SMI)a | Mean adjusted difference (95% CI)a | p value |
|---|---|---|
| Intention to treat analysis (n = 60) | − 0.84 (− 2.90, + 1.22) | 0.418 |
| Per-protocol analysis (n = 48) | − 0.73 (− 3.28, + 1.82) | 0.567 |
| Sub-group analysis | ||
| Males (n = 40) | − 2.20 (− 5.04, + 0.64) | 0.124 |
| Child–Pugh-Turcotte B (n = 40) | − 0.65 (− 3.32, + 2.02) | 0.622 |
| Asian cut-off (n = 25) | − 1.95 (− 6.29, + 2.38) | 0.357 |
| Alcohol (n = 22) | − 0.92 (− 5.37, + 3.53) | 0.667 |
aAfter adjusting for baseline SMI, MELD score, calorie intake and protein intake
Subgroup analysis was performed for primary outcome based on the male gender, alcoholic etiology of cirrhosis and Child–Pugh class B. In each of the subgroup analyses, change in SMI was similar in both arms (Table 3). Asian specific cut-offs have also been proposed in one study which are < 36.5 cm2/m2 for men and < 30.2 cm2/m2 for women [29]. Based on this, n = 25(41.7%) of patients had sarcopenia and subgroup analysis did not reveal any difference in change in SMI between both groups (p = 0.357).
Secondary outcomes
The change in muscle strength by HGD [0.49 (− 1.68 to 2.67), p = 0.652], 6 m gait speed [− 0.07 (− 0.17 to 0.04), p = 0.201] and 6-min walk distance [− 11.0 (− 36.4 to 14.4), p = 0.387] were also similar in both arms (Table 4). The median changes in serum myostatin (pg/ml) [0.7 (− 0.11 to 2.84) for BCAA arm vs. − 0.08 (− 0.22 to 4.71) in placebo arm, p = 0.913] and plasma ammonia levels (µmol/L) [0 (− 3 to + 1.5) in BCAA and – 1 (− 8 to + 6) in placebo arm, p = 0.728)] were similar in both arms. There was no difference in overall quality of life score (p = 0.553) or different domains of quality of life between both arms.
Table 4.
Comparison of change in secondary outcome measures between both arms
| Mean adjusted difference (95% CI)a | p value | |
|---|---|---|
| Change in muscle strength (HGS) | 0.494 (− 1.68, + 2.67) | 0.652 |
| Change in 6 m gait speed | − 0.068 (− 0.174, + 0.037) | 0.201 |
| Change in 6-min walk distance | − 11.03 (− 36.43, + 14.36) | 0.387 |
HGS hand grip strength
aAfter adjusting for baseline SMI, MELD score, calorie intake and protein intake
All patients (n = 60) were also analysed as a single group to assess the effect of exercise and dietary counselling as there was no intergroup difference based on BCAA. The SMI showed a mean improvement of 1.87 cm2/m2 (5.11%, p = < 0.001) from baseline. Similarly, all other measures of sarcopenia also showed an improvement. Handgrip improved by a mean of 1.2 kg (5.31%, p = 0.010), 6-min walk distance improved by 17.5 m (5.52%, p = < 0.001) and 6 m gait speed improved by 0.09 m/s (9%, p = 0.003).
A total of eight patients (four in each arm) experienced adverse events during treatment which were not explained by their ongoing standard treatment. Two patients in the placebo arm had worsening diabetes, dyspepsia occurred in three patients in BCAA arm and one in the placebo arm. Vomiting which was self-limiting occurred in one patient each in the BCAA and placebo arm. The two patients who had worsening diabetes had to stop the intervention within two months of initiation. There were no serious adverse events in any of the arms.
Assessment of parameters on follow-up
Clinical and laboratory parameters at the end of follow-up were similar in both arms. At the end of follow-up, the mean protein intake in the BCAA arm was 64.2 ± 27.1 g/day and was 73.0 ± 26.9 g/day in the placebo arm. This did not include the protein intake by BCAA. After taking this into account, the intake was statistically similar in both arms (p = 0.224).
A total of six patients (three patients in each arm) succumbed to the illness during the study period. The causes of death included sepsis precipitating acute decompensation in three patients and one patient each having severe alcoholic hepatitis, autoimmune hepatitis flare and disseminated tuberculosis. On Kaplan–Meier survival analysis there was no difference in both arms in terms of survival (5% vs. 5%, p = 0.96). (Supplementary Fig. 3) However, this study was not designed to study this outcome individually.
Discussion
In this randomized, double-blind placebo-controlled trial we found that contrary to our hypothesis, the addition of BCAA to home-based exercise and dietary counselling did not result in significant improvement in functional measures of sarcopenia in patients with cirrhosis. There was no intergroup difference in SMI after adjusting for baseline SMI, diet and MELD score. Other surrogate measures like change in muscle strength, muscle performance, quality of life also showed no difference in both arms.
The mean age of our cohort was 41.6 years which is lower than most cirrhosis studies. This is likely because we excluded patients above 60 to avoid confounding by age-related sarcopenia and Indian studies have shown a lower mean age than western countries [30]. Most clinical and laboratory parameters were similar at baseline; however, calorie intake and MELD were higher in the placebo arm. As the cohort was randomised, this was likely due to chance association. To take this into account, outcomes other than plasma ammonia and serum myostatin were adjusted for both the confounders, as well as protein intake which was similar in both arms. At the time of recruitment, all patients received similar dietary counselling and there was no difference in calorie or protein intake at the end of follow-up.
The rationale for use of BCAA is well supported by mechanistic studies. A landmark experiment in muscle tissue from patients with cirrhosis and rats demonstrated that leucine supplementation increased muscle protein content by affecting mTORC1 signalling and activation of the eIF2 alpha kinase [31]. In another experiment, the same group was able to show that over 7 h following BCAA administration, autophagy, whole-body muscle breakdown and mTOR signalling were reduced in patients with cirrhosis [32]. Studies have shown improved mid-arm muscle circumference in the BCAA arm and reduced number of hospitalisations [15, 33].
Recent studies focussed on muscle assessment and combination of BCAA with exercise have failed to demonstrate such improvements. Nishida et al., in a cohort study showed that home-based exercise with BCAA improved aerobic capacity but failed to improve muscle quality [14]. Kitajima et al. used CT scan to assess the effect of BCAA on muscle area index and intramuscular adipose tissue in patients with cirrhosis. No change in muscle parameters was found in the entire cohort but the subgroup of patients who had amelioration of hypoalbuminemia showed some improvement [13]. In their first study Hiraoka et al. showed that walking exercise with BCAA in cirrhosis for 3 months improved number of daily steps and muscle strength [34] However, recently they published that combination of exercise, BCAA and l-carnitine was unable to change muscle mass in cirrhosis [35]. To summarise, the current available evidence shows mixed results and the studies have a few limitations. They were uncontrolled, non-randomized and had a small sample size. The studies were also of heterogeneous in the method of measurement of sarcopenia. Another recent randomized controlled trial has shown improvement in SMI with the use of BCAA [36]. However, the groups in that study differ in terms of age with no adjustment for age-related sarcopenia. So we feel that the results need to be re-confirmed before accepting. In our study, we have tried to overcome the limitations using the gold standard, CT scan in a randomized study and targeted a subgroup of cirrhosis that had sarcopenia at baseline. The duration of the intervention is also longer in our study as compared to prior studies so as to evaluate the lasting effects of BCAA.
The reason for the lack of efficacy of BCAA can only be postulated. It is possible that because of the multifactorial pathogenesis of sarcopenia in cirrhosis, targeting a single pathway by BCAA could not improve muscle parameters. This is supported by serum myostatin and plasma ammonia levels which remained unchanged in both arms. Another proposed mechanism that could play a role is a ceiling effect of uptake of essential amino acids required for muscle formation. This is a rate-limiting step in catabolic states and can be increased only up to a certain extent. It is possible that patients with cirrhosis with adequate dietary intake are at this ceiling and maybe the reason why dietary BCAA may not be enough to lead to additional increased muscle formation [37]. Paradoxically, the muscle protective effect of BCAA has also been shown experimentally to reduce the availability of essential amino acids leading to a decline in muscle synthesis [38, 39].
Interestingly, there was an improvement in mean values of SMI and other outcomes when both groups were considered as a single group. The SMI showed a mean improvement by 1.87 cm2/m2 (5.11%, p = < 0.001) from baseline. Although this study was not designed so, it could suggest that regular exercise with adequate protein intake improves muscle mass and performance measures. This also lends support to the fact that the duration of intervention and sample size was adequate to detect a change with effective therapy. Another interesting finding was the lack of adverse events with BCAA. Previous studies have reported poor palatability to BCAA and therefore reduced compliance [28]. Flavouring of the protein likely led to better adherence and tolerability in our study.
BCAA has been proposed to improve net ammonia removal by muscles by supplying substrate for Kreb’s cycle. However, a slight increase in plasma ammonia levels has been observed, possibly due to the extra muscular metabolism of glutamine [12]. We found similar values in both arms and no significant intra-group difference. Similarly, serum myostatin was similar in both arms. Serum myostatin has been shown to correlate with muscle myostatin levels, which is a major inhibitory regulator of muscle formation. Although BCAA has been postulated to alter the mTOR signalling, myostatin expression remains unchanged [32].
The cut-off for sarcopenia that was used had been derived from the Caucasian population which has been validated and shown to be associated with higher mortality [4]. There has been data from India and Japan which has defined a population-specific cut-off for sarcopenia in cirrhosis which is lower than the Western cut-off [29, 40]. When the subgroup of patients who fulfilled only the Asian cut-off were analysed, there was no difference in outcomes.
Ours is a pragmatic study especially in the current on-going pandemic, as three additional patients were lost to follow-up (1 due to death by COVID-19, and two due to inaccessibility to the hospital on stipulated time, others due to various reasons). The study is not without limitations. It is a single centre study and the number of patients lost to follow-up was higher than expected. However, due to unusual circumstances during the pandemic, this could not be controlled for. In addition, considering that both the per-protocol and ITT analysis did not show any trend towards difference, it is unlikely that a larger sample size or prolonged duration of therapy would change the results. Secondly, as discussed, there was a difference in the baseline MELD and calorie intake between both groups however we have adjusted for these variables while evaluating the primary and secondary outcomes. Thirdly, although we did assess exercise adherence, the overall adherence to exercise (65%) was lower than that for BCAA. However, similar and even lower adherence for home-based exercise regimens have been reported in previous larger trials and the higher adherence in our study could be due to telephonic reminders and visual aid provided to the patient [41]. Another limitation is that we included only those of Child A and B in our study and excluded Child C patients. This was because the transplant-free survival of Chlid C cirrhotics would be further lower and lead to a higher loss to follow-up, so we proceeded to test this hypothesis in compensated and early decompensated cirrhotics.
To conclude, this trial demonstrates that the addition of BCAA to exercise and adequate dietary intake does not improve muscle mass, functional measures of sarcopenia or quality of life in sarcopenic patients with compensated and early decompensated cirrhosis. Further trials may be useful to assess if any subgroup of patients may benefit, especially Child C patients as they have the highest short-term mortality and prevalence of sarcopenia. With the current evidence, however, routine use of BCAA for the improvement of sarcopenia in patients with cirrhosis cannot be recommended.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Supplementary Figure 1: Unlabelled opaque sachet in which branched-chain amino acid and placebo were provided (TIF 260 KB)
Supplementary file2 Supplementary Figure 2: Visual aid given to patients after exercise demonstration (TIFF 539 KB)
Supplementary file3 Supplementary Figure 3: Kaplan-Meier curve comparing survival between both groups at 6 months (TIF 359 KB)
Author contributions
All authors contributed to the study concept and design. Material preparation, data collection and analysis were performed by SM, AA, SQ, SA, NS, RMP and KSM. The first draft of the manuscript was written by SM and reviewed and edited by SS, DG, NS and AS. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No funding was obtained for this study.
Availability of data and material
Due to its propriety nature, supporting data and main data sets are not available openly and can be provided on request to the corresponding author [AS].
Code availability
Not applicable.
Declarations
Conflict of interest
We would like to thank Waterley pharmaceuticals Pvt. Ltd. for donating the BCAA and placebo for the conduct of the study. However, they had no participation in the study design, the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. Srikant Mohta, Abhinav Anand, Sanchit Sharma, Sumaira Qamar, Samagra Agarwal, Deepak Gunjan, K. S. Madhusudhan, R. M. Pandey and Anoop Saraya declare that they have no conflict of interest.
Ethics approval
Approved by independent Institute Ethics committee (IECPG-213/27.03.2019).
Clinical trial registration
Trial is registered in Clinical Trial Registry of India (CTRI/2019/05/019269).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Animal research ethics
Not applicable.
Consent for publication
Consent for publication was obtained from all participants included in the study.
Plant reproducibility
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Srikant Mohta, Email: srikantmohta27491@gmail.com.
Abhinav Anand, Email: abhinav.anand28@gmail.com.
Sanchit Sharma, Email: dr.sanchit_sharma@rediffmail.com.
Sumaira Qamar, Email: sumeira.masudi@gmail.com.
Samagra Agarwal, Email: samagra.agarwal@gmail.com.
Deepak Gunjan, Email: drdg_01@rediffmail.com.
Namrata Singh, Email: namratamohil@gmail.com.
Kumble Seetarama Madhusudhan, Email: drmadhuks@gmail.com.
Ravindra Mohan Pandey, Email: rmpandey@yahoo.com.
Anoop Saraya, Email: ansaraya@yahoo.com.
References
- 1.Dasarathy S. Consilience in sarcopenia of cirrhosis. J Cachexia Sarcopenia Muscle. 2012;3(4):225–237. doi: 10.1007/s13539-012-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krell RW, Kaul DR, Martin AR, et al. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl. 2013;19(12):1396–1402. doi: 10.1002/lt.23752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montano-Loza AJ, Meza-Junco J, Prado CMM, et al. Muscle wasting is associated with mortality in patients. Clin Gastroenterol Hepatol. 2012;10(2):166–173. doi: 10.1016/j.cgh.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Carey EJ, Lai JC, Wang CW, et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl. 2017;23(5):625–633. doi: 10.1002/lt.24750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65(6):1232–1244. doi: 10.1016/j.jhep.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naseer M, Turse EP, Syed A, Dailey FE, Zatreh M, Tahan V. Interventions to improve sarcopenia in cirrhosis: a systematic review. World J Clin Cases. 2019;7(2):156–170. doi: 10.12998/wjcc.v7.i2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders J, Brian A, Wright M, Stroud M. Malnutrition and nutrition support in patients with liver disease. Frontline Gastroenterol. 2010;1(2):105–111. doi: 10.1136/fg.2009.000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holecek M. Ammonia and amino acid profiles in liver cirrhosis: effects of variables leading to hepatic encephalopathy. Nutrition. 2015;31(1):14–20. doi: 10.1016/j.nut.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Qiu J, Tsien C, Thapalaya S, et al. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am J Physiol Endocrinol Metab. 2012;303(8):E983–993. doi: 10.1152/ajpendo.00183.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pere MC, Baudelin A, Briggs K, Teng C, Gilbert M. Hepatic amino acid metabolism during hyperinsulinemia and hyperaminoacidemia in conscious rabbit during late pregnancy. Metabolism. 1994;43(9):1079–1085. doi: 10.1016/0026-0495(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 11.Nishitani S, Ijichi C, Takehana K, Fujitani S, Sonaka I. Pharmacological activities of branched-chain amino acids: specificity of tissue and signal transduction. Biochem Biophys Res Commun. 2004;313(2):387–389. doi: 10.1016/j.bbrc.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Dam G, Ott P, Aagaard NK, Vilstrup H. Branched-chain amino acids and muscle ammonia detoxification in cirrhosis. Metab Brain Dis. 2013;28(2):217–220. doi: 10.1007/s11011-013-9377-3. [DOI] [PubMed] [Google Scholar]
- 13.Kitajima Y, Takahashi H, Akiyama T, et al. Supplementation with branched-chain amino acids ameliorates hypoalbuminemia, prevents sarcopenia, and reduces fat accumulation in the skeletal muscles of patients with liver cirrhosis. J Gastroenterol. 2018;53(3):427–437. doi: 10.1007/s00535-017-1370-x. [DOI] [PubMed] [Google Scholar]
- 14.Nishida Y, Ide Y, Okada M, et al. Effects of home-based exercise and branched-chain amino acid supplementation on aerobic capacity and glycemic control in patients with cirrhosis. Hepatol Res. 2017;47(3):E193–E200. doi: 10.1111/hepr.12748. [DOI] [PubMed] [Google Scholar]
- 15.Marchesini G, Bianchi G, Merli M, et al. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124(7):1792–1801. doi: 10.1016/S0016-5085(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 16.Nakaya Y, Okita K, Suzuki K, et al. BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition. 2007;23(2):113–120. doi: 10.1016/j.nut.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi Y, Hiroshima Y, Matsuo K, et al. A randomized clinical trial of preoperative administration of branched-chain amino acids to prevent postoperative ascites in patients with liver resection for hepatocellular carcinoma. Ann Surg Oncol. 2016;23(11):3727–3735. doi: 10.1245/s10434-016-5348-3. [DOI] [PubMed] [Google Scholar]
- 18.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371(9615):838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarin SK, Kumar A, Almeida JA, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL) Hepatol Int. 2008;3(1):269–282. doi: 10.1007/s12072-008-9106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging. 2012;30(9):1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Portal D, Hofstetter L, Eshed I, et al. L3 skeletal muscle index (L3SMI) is a surrogate marker of sarcopenia and frailty in non-small cell lung cancer patients. Cancer Manag Res. 2019;11:2579–2588. doi: 10.2147/CMAR.S195869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sousa-Santos AR, Amaral TF. Differences in handgrip strength protocols to identify sarcopenia and frailty—a systematic review. BMC Geriatr. 2017;17(1):238. doi: 10.1186/s12877-017-0625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Statement ATS. Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 24.Younossi ZM, Guyatt G, Kiwi M, Boparai N, King D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45(2):295–300. doi: 10.1136/gut.45.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamin J, Mani K, Saraya A, Joshi YK. Translation and validation of the Hindi version of chronic liver disease questionnaire (CLDQ) for the assessment of health related quality of life in patient with chronic liver disease in India. Trop Gastroenterol. 2017;37(2):112–122. doi: 10.7869/tg.335. [DOI] [PubMed] [Google Scholar]
- 26.Román E, Torrades MT, Nadal MJ, et al. Randomized pilot study: effects of an exercise programme and leucine supplementation in patients with cirrhosis. Dig Dis Sci. 2014;59(8):1966–1975. doi: 10.1007/s10620-014-3086-6. [DOI] [PubMed] [Google Scholar]
- 27.Aamann L, Dam G, Borre M, et al. Resistance training increases muscle strength and muscle size in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2020;18(5):1179–1187.e6. doi: 10.1016/j.cgh.2019.07.058. [DOI] [PubMed] [Google Scholar]
- 28.Singh Tejavath A, Mathur A, Nathiya D, et al. Impact of branched chain amino acid on muscle mass, muscle strength, physical performance, combined survival, and maintenance of liver function changes in laboratory and prognostic markers on sarcopenic patients with liver cirrhosis (BCAAS study): a randomized clinical trial. Front Nutr. 2021;8:619. doi: 10.3389/fnut.2021.715795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benjamin J, Shasthry V, Kaal CR, et al. Characterization of body composition and definition of sarcopenia in patients with alcoholic cirrhosis: a computed tomography based study. Liver Int. 2017;37(11):1668–1674. doi: 10.1111/liv.13509. [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee PS, Vishnubhatla S, Amarapurkar DN, et al. Etiology and mode of presentation of chronic liver diseases in India: a multi centric study. PLoS ONE. 2017;12(10):e0187033. doi: 10.1371/journal.pone.0187033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davuluri G, Krokowski D, Guan BJ, et al. Metabolic adaptation of skeletal muscle to hyperammonemia drives the beneficial effects of l-leucine in cirrhosis. J Hepatol. 2016;65(5):929–937. doi: 10.1016/j.jhep.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsien C, Davuluri G, Singh D, et al. Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology. 2015;61(6):2018–2029. doi: 10.1002/hep.27717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Les I, Doval E, García-Martínez R, et al. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am J Gastroenterol. 2011;106(6):1081–1088. doi: 10.1038/ajg.2011.9. [DOI] [PubMed] [Google Scholar]
- 34.Hiraoka A, Michitaka K, Kiguchi D, et al. Efficacy of branched-chain amino acid supplementation and walking exercise for preventing sarcopenia in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2017;29(12):1416–1423. doi: 10.1097/MEG.0000000000000986. [DOI] [PubMed] [Google Scholar]
- 35.Hiraoka A, Kiguchi D, Ninomiya T, et al. Can l-carnitine supplementation and exercise improve muscle complications in patients with liver cirrhosis who receive branched-chain amino acid supplementation? Eur J Gastroenterol Hepatol. 2019;31(7):878–884. doi: 10.1097/MEG.0000000000001368. [DOI] [PubMed] [Google Scholar]
- 36.Hernández-Conde M, Llop E, Gómez-Pimpollo L, et al. Adding branched-chain amino acids to an enhanced standard-of-care treatment improves muscle mass of cirrhotic patients with sarcopenia: a placebo-controlled trial. Am J Gastroenterol ACG. 2021 doi: 10.14309/ajg.0000000000001301. [DOI] [PubMed] [Google Scholar]
- 37.Wolfe RR. Branched-chain amino acids and muscle protein synthesis in humans: myth or reality? J Int Soc Sports Nutr. 2017;14:30. doi: 10.1186/s12970-017-0184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Louard RJ, Barrett EJ, Gelfand RA. Effect of infused branched-chain amino acids on muscle and whole-body amino acid metabolism in man. Clin Sci (Lond) 1990;79(5):457–466. doi: 10.1042/cs0790457. [DOI] [PubMed] [Google Scholar]
- 39.Louard RJ, Barrett EJ, Gelfand RA. Overnight branched-chain amino acid infusion causes sustained suppression of muscle proteolysis. Metabolism. 1995;44(4):424–429. doi: 10.1016/0026-0495(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 40.Hamaguchi Y, Kaido T, Okumura S, et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition. 2016;32(11–12):1200–1205. doi: 10.1016/j.nut.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Lai JC, Dodge JL, Kappus MR, et al. A multicenter pilot randomized clinical trial of a home-based exercise program for patients with cirrhosis: the strength training intervention (STRIVE) Am J Gastroenterol ACG. 2021 doi: 10.14309/ajg.0000000000001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Supplementary Figure 1: Unlabelled opaque sachet in which branched-chain amino acid and placebo were provided (TIF 260 KB)
Supplementary file2 Supplementary Figure 2: Visual aid given to patients after exercise demonstration (TIFF 539 KB)
Supplementary file3 Supplementary Figure 3: Kaplan-Meier curve comparing survival between both groups at 6 months (TIF 359 KB)
Data Availability Statement
Due to its propriety nature, supporting data and main data sets are not available openly and can be provided on request to the corresponding author [AS].
Not applicable.