Abstract
Background
Disparities in access to anti-SARS-CoV-2 monoclonal antibodies have not been well characterized.
Objective
We sought to explore the impact of race/ethnicity as a social construct on monoclonal antibody delivery.
Design/Patients
Following implementation of a centralized infusion program at a large academic healthcare system, we reviewed a random sample of high-risk ambulatory adult patients with COVID-19 referred for monoclonal antibody therapy.
Main Measures
We examined the relationship between treatment delivery, race/ethnicity, and other demographics using descriptive statistics, binary logistic regression, and spatial analysis.
Key Results
There was no significant difference in racial composition between patients who did (n = 25) and patients who did not (n = 378) decline treatment (p = 0.638). Of patients who did not decline treatment, 64.8% identified as White, 14.8% as Hispanic/Latinx, and 11.1% as Black. Only 44.6% of Hispanic/Latinx and 31.0% of Black patients received treatment compared to 64.1% of White patients (OR 0.45, 95% CI 0.25–0.81, p = 0.008, and OR 0.25, 95% CI 0.12–0.50, p < 0.001, respectively). In multivariable analysis including age, race, insurance status, non-English primary language, county Social Vulnerability Index, illness severity, and total number of comorbidities, associations between receiving treatment and Hispanic/Latinx or Black race were no longer statistically significant (AOR 1.32, 95% CI 0.69–2.53, p = 0.400, and AOR 1.34, 95% CI 0.64–2.80, p = 0.439, respectively). However, patients who were uninsured or whose primary language was not English were less likely to receive treatment (AOR 0.16, 95% CI 0.03–0.88, p = 0.035, and AOR 0.37, 95% CI 0.15–0.90, p = 0.028, respectively). Spatial analysis suggested decreased monoclonal antibody delivery to Cook County patients residing in socially vulnerable communities.
Conclusions
High-risk ambulatory patients with COVID-19 who identified as Hispanic/Latinx or Black were less likely to receive monoclonal antibody therapy in univariate analysis, a finding not explained by patient refusal. Multivariable and spatial analyses suggested insurance status, language, and social vulnerability contributed to racial disparities.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-022-07603-4.
KEY WORDS: COVID-19, SARS-CoV-2, monoclonal antibody, racial/ethnic disparities, Social Vulnerability Index
INTRODUCTION
To date, the U.S. Food and Drug Administration (FDA) has granted Emergency Use Authorization (EUA) for the following anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) monoclonal antibodies to use in mild to moderate coronavirus disease 2019 (COVID-19): bamlanivimab (LY-CoV555, Eli Lilly), bamlanivimab (LY-CoV555, Eli Lilly)/etesevimab (LY-CoV016, Eli Lilly), casirivimab (REGN10933, Regeneron)/imdevimab (REGN10987, Regeneron), sotrovimab (VIR-7831, GlaxoSmithKline/Vir Technology), and bebtelovimab (LY-CoV1404, Eli Lilly) .1–3 (Due to resistance in circulating variants, access is currently limited to bebtelovimab.) Monoclonal antibodies have been associated with decreased viral load, hospitalization, and mortality in high-risk ambulatory patients.4–10 Prior to increased demand in response to rising Delta and then Omicron variant infections (as well as vaccine misinformation and hesitancy), monoclonal antibodies had been underutilized due to low referral rates and limited healthcare capacity for ambulatory infusions including space and staffing.11–14 Local access to monoclonal antibodies in underserved communities has not been well characterized.
Between October 2020 and January 2021, the federal government purchased 950,000 doses of bamlanivimab (modified later to include etesevimab) and over 2.9 million doses of casirivimab/imdevimab.15–17 By March 2, 2021, it had allocated 946,613 and delivered 761,614 doses of monoclonal antibodies.18 Under the U.S. Department of Health and Human Services (HHS) Special Projects for Equitable and Efficient Distribution (SPEED), 30,000 doses were allocated and distributed to underserved communities via dialysis centers, federally qualified health centers, long-term care facility pharmacies, home infusion providers, and correction facilities.19 With the suspension of federal allocation from February 2021 to September 13, 2021, during which providers ordered directly from the distributor, AmerisourceBergen, SPEED became defunct.
During the COVID-19 pandemic, Black, Hispanic/Latinx, and American Indian/Alaska Native communities have experienced significantly greater cumulative risk of infection, hospitalization, and death, exacerbating existing health disparities.20,21 Race/ethnicity serves as a surrogate for “complex connotations that reflect culture, history, socioeconomics and political status.”22 Aligned with this concept, we use race/ethnicity as a social, not biologic, construct reflecting the impact of structural determinants of health leading to healthcare disparities and, for the purposes of this study, focus on disparities associated with racial segregation at the neighborhood population level. Chicago is one of the most racially segregated metropolitan areas in the U.S.A. (Appendix Figure 5), with corresponding gaps in life expectancy, COVID-19 infection, and other health outcomes between its Central/North Side and West/South Side regions.23 When exploring this relationship, the Social Vulnerability Index (SVI) is often used as a more holistic descriptor of populations at risk for disparities during emergency health crises. SVI utilizes U.S. Census data to identify vulnerable communities based on factors affecting socioeconomic status, household composition/disability, minority status/language, and housing/transportation (Appendix Figure 6).24 SVI has been associated with COVID-19 incidence and mortality.25–27
In our initial analysis of bamlanivimab use to prevent hospitalization in high-risk ambulatory patients with COVID-19, we noted that Black and Hispanic/Latinx patients were less likely than White patients to receive monoclonal antibodies,7 a finding since reproduced in single-center and national retrospective studies.28,29 Another study reported that non-Hispanic White patients were more likely to accept monoclonal antibodies.30 In this study, we examine the impact of race/ethnicity as a social construct on monoclonal antibody delivery to high-risk ambulatory patients with COVID-19.
MATERIALS AND METHODS
Study Design
In the midst of the second wave of the pandemic in Illinois, our healthcare system, which comprises 10 hospitals and over 200 inpatient and outpatient sites in the greater Chicago, Illinois, area, initiated monoclonal antibody administration on November 20, 2020, using an electronic health record (EHR)–based referral order process. At the time of study, bamlanivimab was the only monoclonal antibody available in our system; its EUA was subsequently revoked due to the development of resistant variants.31,32 The ordering clinician was expected to provide informed consent using EUA patient information available in English and Spanish. Using over-the-phone interpretation as needed, a centralized call team made three attempts to reach each patient and scheduled appointments at designated outpatient infusion centers. Certain emergency departments were also able to administer treatment to qualifying patients at the time of presentation. Referral orders expired after 10 days. Referral volumes never exceeded capacity.
We reviewed a random selection of patients from the 482 non-canceled referral orders placed November 19, 2020 to January 19, 2021. Ambulatory adult patients were included if they had documented COVID-19, met clinical criteria as high risk or were healthcare workers, and were referred for treatment under EUA. Patients were excluded if they reported more than 14 days of symptoms prior to testing or received infusion at an outside healthcare system. Primary analysis focused on factors associated with monoclonal antibody delivery (defined as documented infusion within 10 days of referral order) in patients who did not explicitly decline treatment per call team notes. A secondary analysis compared patients who did and did not decline treatment. Additional data extracted from the EHR included age, gender, race, ethnicity, primary language spoken, insurance status, ZIP code, city, county, state, illness severity at time of first healthcare contact, and total number of comorbidities (TNC). (Definitions of severity, TNC, and high-risk factors are available in Appendix.) SVI scores from 2018 (available by county or census tract but not ZIP code) were assigned based on county of residence and ranged from 0 (lower vulnerability) to 1 (highest vulnerability).33
EHR fields limited self-identification of race to White, Black/African American, Asian, American Indian/Alaska Native (AIAN), Native Hawaiian/Pacific Islander (NHPI), other, declined, or unable to answer, and ethnicity to Hispanic/Latino, not Hispanic/Latino, declined, or unable to answer. Without an option to identify race as Latino/Latinx, many patients are misclassified as White, resulting in underreporting of disparities in COVID-19 cases and mortality.34 To be consistent with similar studies on racial disparities, we used a composite of self-reported race and ethnicity to define race categories as non-Hispanic White, Hispanic/Latinx, non-Hispanic Black, non-Hispanic Asian, non-Hispanic AIAN, non-Hispanic NHPI, non-Hispanic other/unable to answer, and non-Hispanic declined/missing data.35 We acknowledge the inadequate representation of Middle Eastern and biracial/multiracial identities.
Data Management and Statistical Analysis
Study data were obtained via the Northwestern Medicine Enterprise Data Warehouse and EHR chart review, and managed using Research Electronic Data Capture (REDCap) electronic data capture tools.36,37 Chi-square, t, and Mann-Whitney U tests were used to compare categorical and continuous values for patients who did and did not receive, and did and did not decline, monoclonal antibody therapy. Binary logistic regression was used for multivariable analysis modeling odds of receiving treatment. Statistical software R 4.0.3 and Stata/SE 16.0 were used for data analysis. Statistical significance was defined as p ≤ 0.05.
Spatial Analysis
In order to explore geographic trends in monoclonal antibody delivery (delivery rate = patients who received treatment / all patients referred for treatment who did not decline, % delivered = delivery rate * 100) via geographic information system mapping, we focused on 181 patients residing in Cook County, Illinois. ZIP codes were geographically represented by ZIP Code Tabulation Area (ZCTA) data provided as a shapefile by census.gov based on 2020 Census data. ZCTAs are built from census tracts and represent the most frequently occurring five-digit U.S. Postal Service ZIP code found within a given area. We grouped ZIP codes into twelve regions of Cook County to analyze data at a coarser geographic level (Appendix Figure 7 and Appendix Table 3). To provide additional context, we mapped census tract SVI scores from 2018 and healthcare system COVID-19 cases during the study period. Regional SVI scores were determined using average census tract SVI scores weighted by census tract population. Geographic analyses were performed with the GeoPandas package using Python 3.8.
Ethical Considerations
This study was approved by the Northwestern University IRB prior to any data collection or analysis.
RESULTS
We reviewed 419 high-risk ambulatory adult patients with COVID-19 referred for monoclonal antibody therapy during the study period (86.9% of referrals). Sixteen patients failed to meet inclusion or met exclusion criteria. Twenty-five patients explicitly declined treatment based on review of call team documentation. Of the 378 patients who did not decline, 64.8% identified as White, 14.8% Hispanic/Latinx, and 11.1% Black. Only 44.6% of Hispanic/Latinx and 31.0% of Black patients received treatment compared to 64.1% of White patients (odds ratio [OR] 0.45, 95% confidence interval [CI] 0.25–0.81, p = 0.008, and OR 0.25, 95% CI 0.12–0.50, p < 0.001, respectively) (Table 1). Patients who were uninsured were less likely to receive treatment compared to those with private insurance (OR 0.12, 95% CI 0.02–0.45, p = 0.006). Age, total number of comorbidities, and mild or undefined illness severity were associated with increased odds of receiving treatment (OR 1.02, 95% CI 1.01–1.04, p = 0.001; OR 1.28, 95% CI 1.08–1.54, p = 0.007; OR 3.89, 95% CI 1.27–14.50, p = 0.025; OR 6.67, 95% CI 1.60–33.00, p = 0.013; respectively). Language was not statistically significantly associated with monoclonal antibody delivery.
Table 1.
Baseline Characteristics of 378 Patients Referred for Monoclonal Antibody Therapy Who Did Not Decline
| Characteristics | Study population (n = 378) |
Monoclonal antibody (n = 218) |
No monoclonal antibody (n = 160) |
OR (95% CI) |
p Value |
|---|---|---|---|---|---|
| Age in years, mean (SD) | 62.37 (14.99) | 64.52 (13.71) | 59.45 (16.17) |
1.02 (1.01–1.04) |
0.001 |
| Race/ethnicity | |||||
| White | 245 (64.8%) | 157 (72.0%) | 88 (55.0%) | Ref | |
| Hispanic/Latinx | 56 (14.8%) | 25 (11.5%) | 31 (19.4%) |
0.45 (0.25–0.81) |
0.008 |
| Black | 42 (11.1%) | 13 (6.0%) | 29 (18.1%) |
0.25 (0.12–0.50) |
< 0.001 |
| Asian | 14 (3.7%) | 9 (4.1%) | 5 (3.1%) |
1.01 (0.34–3.37) |
> 0.900 |
| American Indian/Alaska Native | 2 (0.5%) | 0 (0%) | 2 (1.3%) | 0.00 | > 0.900 |
| Native Hawaiian/Pacific Islander | 1 (0.3%) | 0 (0%) | 1 (0.6%) | 0.00 | > 0.900 |
| Other | 14 (3.7%) | 10 (4.6%) | 4 (2.5%) |
1.40 (0.45–5.23) |
0.600 |
| Declined | 4 (1.1%) | 4 (1.8%) | 0 (0%) | 0.00 | > 0.900 |
| Insurance status | |||||
| Private | 160 (42.3%) | 94 (43.1%) | 66 (41.2%) | Ref | |
| Medicare | 178 (47.1%) | 109 (50%) | 69 (43.1%) |
1.11 (0.72–1.72) |
0.600 |
| Medicaid | 25 (6.6%) | 13 (6.0%) | 12 (7.5%) |
0.76 (0.32–1.79) |
0.500 |
| Uninsured | 14 (3.7%) | 2 (0.9%) | 12 (7.5%) |
0.12 (0.02–0.45) |
0.006 |
| Primary language spoken | |||||
| English | 347 (91.8%) | 207 (95.0%) | 140 (87.5%) | Ref | |
| Spanish | 27 (7.1%) | 11 (5.0%) | 16 (10.0%) |
0.46 (0.20–1.02) |
0.060 |
| Other | 4 (1.1%) | 0 (0%) | 4 (2.5%) | 0.00 | > 0.90 |
| County Social Vulnerability Index score, mean (SD) | 0.57 (0.27) | 0.58 (0.27) | 0.55 (0.26) |
1.67 (0.77–3.59) |
0.193 |
| Severity | |||||
| Asymptomatic | 14 (3.7%) | 4 (1.8%) | 10 (6.2%) | Ref | |
| Mild | 266 (70.4%) | 162 (74.3%) | 104 (65.0%) |
3.89 (1.27–14.50) |
0.025 |
| Moderate | 73 (19.3%) | 36 (16.5%) | 37 (23.1%) |
2.43 (0.74–9.52) |
0.200 |
| Severe | 3 (0.8%) | 0 (0%) | 3 (1.9%) | 0.00 | > 0.900 |
| Critical | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Unable to determine | 22 (5.8%) | 16 (7.3%) | 6 (3.8%) |
6.67 (1.60–33.00) |
0.013 |
| Total number of comorbidities, mean (SD) | 1.76 (1.19) | 1.90 (1.19) | 1.56 (1.18) |
1.28 (1.07–1.54) |
0.007 |
Of the 160 patients who did not decline yet did not receive treatment, 130 patients (70.2%) had expired/canceled infusion appointments for undocumented reasons, 12 (5.9%) had symptoms for more than 10 days at time of referral, 8 (4.3%) presented to infusion appointment with severe illness/hypoxia on pre-administration assessment, 6 (2.9%) had a test more than 5 days old at time of referral, and 4 (2.2%) had severe disease at time of first healthcare contact. Compared to patients who did not decline, patients who declined treatment had a higher average county SVI score (0.70 vs. 0.57, p = 0.017), a lower average TNC (1.36 vs. 1.76, p = 0.101), and no significant difference in racial composition (p = 0.638) (Table 2).
Table 2.
Comparison of 25 Patients Who Did and 378 patients Who Did Not Decline Monoclonal Antibody Therapy
| Characteristics | Declined (n = 25) |
Did not decline (n = 378) |
p Value |
|---|---|---|---|
| Age in years, mean (SD) | 60.80 (18.75) | 62.37 (14.99) | 0.618 |
| Race/ethnicity | |||
| White | 13 (52.0%) | 245 (64.8%) | 0.638 |
| Hispanic/Latinx | 5 (20.0%) | 56 (14.8%) | |
| Black | 4 (16.0%) | 42 (11.1%) | |
| All others | 3 (12.0%) | 35 (9.3%) | |
| Insurance status | |||
| Private | 15 (60.0%) | 160 (42.3%) | 0.419 |
| Medicare | 8 (32.0%) | 178 (47.1%) | |
| Medicaid | 2 (8.0%) | 25 (6.6%) | |
| Uninsured | 0 (0%) | 14 (3.7%) | |
| Primary language spoken | |||
| English | 22 (88.0%) | 347 (91.8%) | 0.593 |
| Spanish | 3 (12.0%) | 27 (7.1%) | |
| Other | 0 (0%) | 4 (1.1%) | |
| County Social Vulnerability Index score, mean (SD) | 0.70 (0.17) | 0.57 (0.27) | 0.017 |
| Severity | |||
| Asymptomatic | 1 (4.0%) | 14 (3.7%) | 0.191 |
| Mild | 21 (84.0%) | 266 (70.4%) | |
| Moderate | 2 (8.0%) | 73 (19.3%) | |
| Severe | 1 (4.0%) | 3 (0.8%) | |
| Critical | 0 | 0 | |
| Unable to determine | 0 (0%) | 22 (5.8%) | |
| Total number of comorbidities, mean (SD) | 1.36 (0.86) | 1.76 (1.19) | 0.101 |
In multivariable analysis including age, race, insurance status, non-English primary language, county SVI, illness severity, and TNC, associations between receiving treatment and Hispanic/Latinx or Black race did not retain statistical significance (adjusted OR [AOR] 1.32, 95% CI 0.69–2.53, p = 0.400, and AOR 1.34, 95% CI 0.64–2.80, p = 0.439, respectively). However, patients who were uninsured or whose primary language was not English were less likely to receive treatment compared to privately insured and English-speaking patients (AOR 0.16, 95% CI 0.03–0.88, p = 0.035, and AOR 0.37, 95% CI 0.15–0.90, p = 0.028), respectively. Patients who were older or who had more comorbidities remained more likely to receive treatment (AOR 1.03, 95% CI 1.01–1.05, p = 0.004, and AOR 1.34, 95% CI 1.10–1.64, p = 0.004, respectively). Patients with mild, moderate, or undefined illness severity were also more likely to receive treatment compared to asymptomatic patients (AOR 6.24, 95% CI 1.78–21.85, p = 0.004; AOR 4.46, 95% CI 1.18–16.88, p = 0.028; and AOR 8.73, 95% CI 1.85–41.09, p = 0.006; respectively).
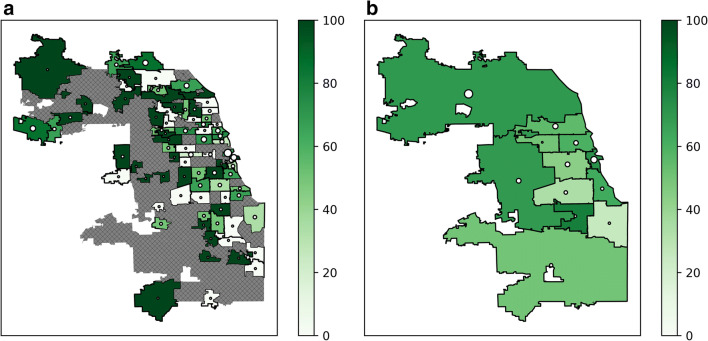
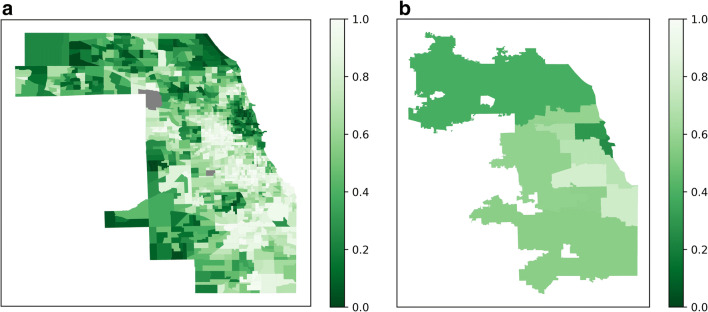
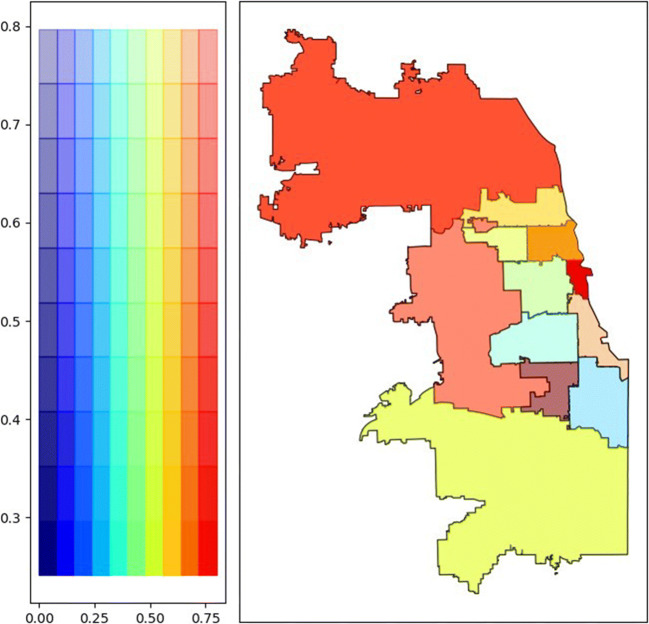
Patients reported addresses in 173 unique ZIP codes, including fourteen out of state. More referrals were generated from the northern suburbs of Cook County and Chicago’s Central region, and higher rates of monoclonal antibody delivery were seen in the northern and western suburbs of Cook County and Chicago’s Central and North Side regions, compared to Chicago’s West, Southwest, and Far South Sides (Fig. 1 and Appendix Table 4). We were unable to calculate global and local Moran’s I statistics for spatial autocorrelation due to multiple ZIP codes without referrals. Higher social vulnerability exists in Chicago’s West, South, and especially Southwest and Far South Sides (Fig. 2). A bivariate chloropleth map of regional monoclonal antibody delivery and SVI revealed lower monoclonal antibody delivery rates in the more socially vulnerable West, Southwest, and Far South Sides (Fig. 3).
Figure 1.
Healthcare system monoclonal antibody delivery. Geospatial representation of the percentage of healthcare system monoclonal antibody referrals administered by ZIP code (a) and region (b) of Cook County, Illinois. Hatch marks represent ZIP codes without referrals. Dots represent and are proportional to the total number of patients referred for monoclonal antibody from each ZIP code or region.
Figure 2.
CDC/ATSDR Social Vulnerability Index. Overall Social Vulnerability Index by ZIP code (a) and region (b) of Cook County, Illinois. SVI scores range from 0 (lower vulnerability) to 1 (highest vulnerability).
Figure 3.
Healthcare system monoclonal antibody delivery and CDC/ATSDR Social Vulnerability Index. Bivariate chloropleth map of monoclonal antibody delivery and SVI by region. Legend (left) ascribes heat map color scales to the monoclonal antibody delivery rate (X-axis) and SVI score (Y-axis). Regions with the highest delivery rates and lowest social vulnerability are shown in dark red and regions with the lowest delivery rates and highest social vulnerability in light blue.
During the study period, 31,066 healthcare system patients (62.8% White, 16.1% Hispanic/Latinx, 7.3% Black) residing in Cook County were diagnosed with COVID-19. More healthcare system cases were seen in the Central region and northwestern suburbs (Fig. 4). For reference, our healthcare system patient population is approximately 42.3% White, 10.7% Hispanic/Latinx, and 6.7% Black (race/ethnicity for 37.4% of patients is unknown or not reported); the racial/ethnic demographics of Cook County, Illinois, are 42.0% White, 25.6% Hispanic/Latinx, and 23.8% Black.38
Figure 4.
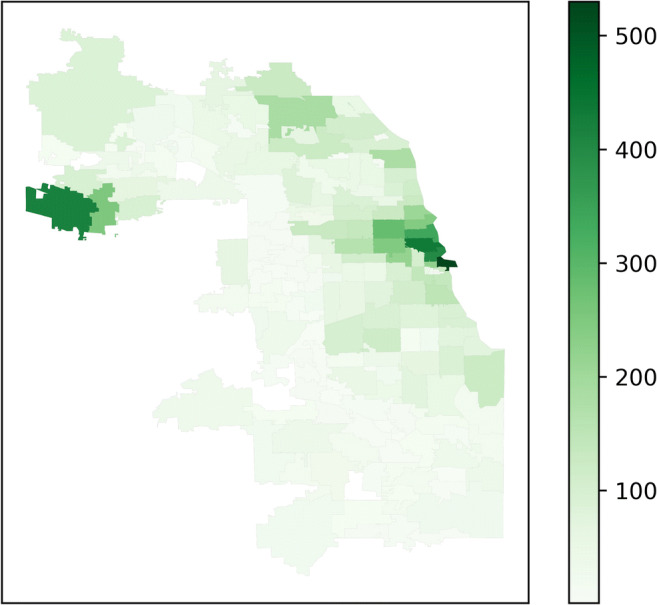
Healthcare system COVID-19 cases. Total number of healthcare system patients residing in Cook County, Illinois, by ZIP code diagnosed with COVID-19 during the study period.
DISCUSSION
Using the framework of race as a social construct representing structural determinants of health, we assessed disparities in monoclonal antibody delivery among high-risk ambulatory patients with COVID-19 through combined statistical and spatial analyses. In this single-center assessment of patients referred for monoclonal antibodies, Hispanic/Latinx and Black patients were less likely to receive monoclonal antibody therapy compared to White patients. After controlling for potential confounders, associations between receiving treatment and race were no longer statistically significant; instead, patients who were uninsured or whose primary language was not English were less likely to receive treatment.
Disparities in monoclonal antibody receipt were not clearly attributable to treatment refusal as patients who declined treatment were excluded from primary analysis. Of the patients who did not decline yet did not receive treatment, 18.8% no longer met clinical criteria for bamlanivimab under EUA due to illness duration or severity but were included in analysis to reflect real-world conditions. However, the remaining 81.2% had expired or canceled infusion appointments for undocumented reasons. Patients were not billed for monoclonal antibodies purchased and distributed through HHS, but we were unable to obtain information regarding the possibility of out-of-pocket infusion administration or other medication costs. None of the fourteen uninsured patients declined treatment, however, so it is unclear if cost was a concern. It is also notable that EUA patient fact sheets were available only in English and Spanish. Uninsured status and non-English primary language, however, do not encompass all of the barriers to accessing treatment, especially while ill (e.g., lack of reliable and affordable transit, paid time off, and social support); we attempted to capture some of these factors through spatial analysis and SVI.
Spatial analysis demonstrated more referrals for ZIP codes with more COVID-19 cases within our healthcare system. In contrast to previously published work validating the co-localization of spatial clusters of high COVID-19 cases and high social vulnerability in Chicago, in our healthcare system, high COVID-19 cases appeared to co-localize with lower social vulnerability, suggesting that our patient population was not representative of Cook County.25 Even so, lower monoclonal antibody delivery rates mirrored the higher social vulnerability in Chicago’s West, Southwest, and Far South Sides. For example, one out of six referrals was completed in ZIP code 60651, compared to eight of eight referrals in ZIP code 60611. ZCTA 60651 includes areas of the Austin and Humboldt Park neighborhoods in Chicago’s West Side; in the 2015–2019 American Community Survey, 52.7% of residents were Black and 42.9% were of Hispanic or Latino ethnicity.39 ZCTA 60611 comprises the Streeterville neighborhood in downtown Chicago; in the same survey, 73.8% of residents were White.
While our healthcare system developed a monoclonal antibody infusion program with a simple referral process and centralized appointment scheduling, delivery of this potentially life-saving treatment to socially vulnerable communities was not optimized. Our findings reinforce that unequal healthcare resource delivery reflects longstanding structural inequities at the neighborhood population level. In Chicago, factors contributing to social vulnerability follow boundaries marked by racial segregation. As such, populations at the highest risk of hospitalization and death are not afforded the best available treatments to prevent these complications of COVID-19.
Our study was limited by potential selection bias, retrospective chart review, and sample size. While monoclonal antibody referral pattern was outside the scope of this study, potentially disproportionate referrals across racial/ethnic groups could bias the racial disparities in receiving treatment. The total population of healthcare system patients eligible for monoclonal antibodies (i.e., mild to moderate cases) during the study period was not known as determination of illness severity was not uniformly captured. In addition, our sample could not account for refinements in the program as it matured. Adjustments for potential confounders were limited to demographic data available in the EHR; notably, we lacked factors contributing to socioeconomic status such as household income, education level, and healthcare worker status. Although comparison of patients who did and did not decline treatment revealed no statistically significant differences in race, insurance status, or language, relatively few patients declined treatment and we could not account for treatment hesitancy or disparate informed consent. We were unable to discern whether each patient fit the SVI demographic associated with their county apart from age, minority status, and language. Furthermore, use of county rather than census tract SVI in statistical modeling likely underestimated disparities. Geocoding, or converting addresses into geographic coordinates, should be considered for future studies. Finally, spatial statistics were limited by small sample size.
Our study’s strengths and limitations highlight the need for more nuanced characterization of disparities impacting monoclonal antibody delivery. While some challenges in drug allocation and distribution to states/territories, medical centers, and finally to patients reflect the difficulties of implementing a mass infusion program,40 our findings suggest that racial disparities in receiving treatment occur at the intersection of social determinants of health, which are defined by the physical space in which one lives.
In a pandemic that has compounded pre-existing racial inequities, examination of resource delivery offers not only insight into, but also solutions to, barriers in accessing care. Healthcare systems should strive to offer multilingual informed consent and scheduling, evening and weekend appointments, and transportation assistance. Larger medical centers can partner with community health centers to provide medication, infusion training, and on-site pharmacy. Mobile units can be deployed to rural communities and healthcare deserts to bring treatments to the community, rather than burdening vulnerable patients with the task of overcoming healthcare access hurdles.41 Allocation models including a prioritized or reserve system for socially vulnerable communities should be further explored.42 Finally, it is time to reprioritize universal healthcare and address the underlying structural inequities that have defined and continue to plague our nation’s fragmented healthcare system.
CONCLUSION
High-risk ambulatory patients with COVID-19 who identified as Hispanic/Latinx or Black were less likely to receive monoclonal antibody therapy in univariate analysis, a finding not explained by patient refusal. Multivariable and spatial analyses suggested insurance status, language, and social vulnerability contributed to racial disparities.
Supplementary Information
(DOCX 1102 kb)
Acknowledgements
We thank David H. Cooke, MD (Northwestern Memorial HealthCare); Cynthia Barnard, PhD MBA MSJS (Northwestern Memorial HealthCare); Amy Leonard, RN (Northwestern Medicine); Julia Bongiorno, PMP (Northwestern Medicine); Michael J. Postelnick, RPh BCPS (Northwestern Medicine); John Bailitz, MD (Department of Medicine, Northwestern University Feinberg School of Medicine); and Michael G. Ison, MD MS for their leadership in developing and implementing the monoclonal antibody infusion program across the Northwestern Medicine healthcare system.
Funding
Research reported in this publication was supported, in part, by the Northwestern University Feinberg School of Medicine Division of Infectious Diseases Emerging Infectious Diseases grant. EW is currently and RNK was previously (2019–2021) supported by the National Institute of Health (grant number T32 AI095207). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declarations
Conflict of Interest
V. Stosor reports research support, paid to Northwestern University, from Eli Lilly and Company. M. G. Ison reports research support, paid to Northwestern University, from GlaxoSmithKline and Regeneron.
Other Disclosures
M. P. Angarone reports DKBMed paid honoraria for lectures on COVID-19; AbbVie DSMB and consulting fees; and Allergan speakers’ bureau (last 2019).
V. Stosor reports Med Learning Group paid honoraria.
M. G. Ison reports research support, paid to Northwestern University, from Pulmocide; he is a paid consultant for Adagio, ADMA Biologics, AlloVir, Cidara, Genentech/Roche, Janssen, Shionogi, Takeda, and Viracor Eurofins; he is also a paid member of DSMBs from AlloVir, CSL Behring, Janssen, Merck, Sequiris, Takeda, and Talaris; he receives payment from UpToDate for chapters on viral respiratory illnesses.
C. J. Achenbach reports Abivax DSMB.
K. L. Gates reports paid honoraria from GlaxoSmithKline for lectures on COPD, Pri-Med for lectures on COVID-19, and ACP.
Footnotes
Key Points: High-risk ambulatory patients with COVID-19 who identified as Hispanic/Latinx or Black were less likely to receive monoclonal antibody therapy compared to White patients. Insurance status, language, and social vulnerability may explain racial disparities.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
En-Ling Wu, Email: enling.wu@northwestern.edu.
Khalilah L. Gates, Email: k-gates@northwestern.edu.
References
- 1.Eli Lilly and Company. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. U.S. Food and Drug Administration; 2021 [cited 2021 Oct 1]. Available from: https://www.fda.gov/media/145802/download.
- 2.Regeneron Pharmaceuticals, Inc. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of REGEN-COVTM (casirivimab and imdevimab). U.S. Food and Drug Administration; 2021 [cited 2021 Oct 1]. Available from: https://www.fda.gov/media/145611/download.
- 3.GlaxoSmithKline. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Sotrovimab. U.S. Food and Drug Administration; 2021 [cited 2021 Oct 1]. Available from: https://www.fda.gov/media/149534/download.
- 4.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen P, Nirula A, Heller B, et al. SARS-CoV-2 Neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar RN, Wu EL, Stosor V, Moore WJ, Achenbach C, Ison MG, Angarone MP. Real-world experience of bamlanivimab for COVID-19: a case-control study. Clin Infect Dis. 2021:ciab305. [DOI] [PMC free article] [PubMed]
- 8.Bariola JR, McCreary EK, Wadas RJ, et al. Impact of bamlanivimab monoclonal antibody treatment on hospitalization and mortality among nonhospitalized adults with severe acute respiratory syndrome coronavirus 2 infection. Open Forum Infect Dis. 2021;8(7):ofab254. doi: 10.1093/ofid/ofab254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rainwater-Lovett K, Redd JT, Stewart MA, et al. Real-world effect of monoclonal antibody treatment in COVID-19 patients in a diverse population in the United States. Open Forum Infect Dis. 2021;8(8):ofab398. doi: 10.1093/ofid/ofab398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganesh R, Pawlowski CF, O'Horo JC, et al. Intravenous bamlanivimab use associates with reduced hospitalization in high-risk patients with mild to moderate COVID-19. J Clin Invest. 2021;19:151697. doi: 10.1172/JCI151697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bratcher-Bowman N. Rapid Expert Consultation on Allocating COVID-19 Monoclonal Antibody Therapies and Other Novel Therapeutics. Washington, DC: The National Academies Press; 2021 [cited 2021 Oct 1]. Available from: https://www.nap.edu/read/26063/chapter/1.
- 12.Anderson TS, O'Donoghue AL, Dechen T, et al. Uptake of outpatient monoclonal antibody treatments for COVID-19 in the United States: a cross-sectional analysis. J Gen Intern Med. 2021;36(12):3922–3924. doi: 10.1007/s11606-021-07109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernstein L, McGinley L. Monoclonal antibodies are free and effective against covid-19 but few people are getting them. The Washington Post [Internet]. 2021 20 [cited 2021 Oct 1]. Available from: https://www.washingtonpost.com/health/covid-monoclonal-abbott/2021/08/19/a39a0b5e-0029-11ec-a664-4f6de3e17ff0_story.html.
- 14.Bernstein L. Biden administration moves to stave off shortages of monoclonal antibodies. The Washington Post [Internet]. 2021 14 [cited 2021 Oct 1]. Available from: https://www.washingtonpost.com/health/2021/09/14/monoclonal-antibodies-shortage/.
- 15.Eli Lilly and Company News Release. Lilly announces 650,000 additional doses of neutralizing antibody bamlanivimab (LY-CoV555) purchased by U.S. government to treat COVID-19 [Internet]. Eli Lilly and Company; 2020 2 [cited 2021 Oct 1]. Available from: https://investor.lilly.com/news-releases/news-release-details/lilly-announces-650000-additional-doses-neutralizing-antibody.
- 16.Eli Lilly and Company News Release. Lilly modified COVID-19 purchase agreement for bamlanivimab alone with the U.S. government and is focusing on supply of bamlanivimab and etesevimab together [Internet]. Eli Lilly and Company; 2021 12 [cited 2021 Oct 1]. Available from: https://investor.lilly.com/news-releases/news-release-details/lilly-modified-covid-19-purchase-agreement-bamlanivimab-alone-us.
- 17.PRNewswire. Regeneron announces U.S. government agreement to purchase additional COVID-19 antibody cocktail doses [Internet]. Tarrytown, NY: Regeneron Pharmaceuticals 2021 [cited 2021 Oct 1]. Available from: https://investor.regeneron.com/index.php/news-releases/news-release-details/regeneron-announces-us-government-agreement-purchase-additional.
- 18.Public Health Emergency. Allocation of Bamlanivimab by Jurisdiction [Internet]. U.S. Department of Health & Human Services; 2021 [cited 2021 Oct 1]. Available from: https://www.phe.gov/emergency/events/COVID19/investigation-MCM/Bamlanivimab/Pages/allocation.aspx.
- 19.Pfundt T. Special Projects for Equitable and Efficient Distribution (SPEED) of COVID-19 Outpatient Therapeutics [Internet]. U.S. Department of Health & Human Services; 2021 [cited 2021 Oct 1]. Available from: https://files.asprtracie.hhs.gov/documents/healthcare-operations-speaker-series%2D%2Dspeed.pdf.
- 20.Dorn AV, Cooney RE, Sabin ML. COVID-19 exacerbating inequalities in the US. Lancet. 2020;395(10232):1243–1244. doi: 10.1016/S0140-6736(20)30893-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Center for Immunization and Respiratory Diseases, Division of Viral Diseases. Risk for COVID-19 Infection, Hospitalization, and Death by Race/Ethnicity [Internet]. Centers for Disease Control and Infection; 2021 16 [cited 2021 Oct 1]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html.
- 22.Collins FS. What we do and don’t know about ‘race’, ‘ethnicity’, genetics and health at the dawn of the genome era. Nat Genet. 2004;36(11 Suppl):S13–5. doi: 10.1038/ng1436. [DOI] [PubMed] [Google Scholar]
- 23.NYU Research. Large Life Expectancy Gaps in U.S. Cities Linked to Racial & Ethnic Segregation by Neighborhood [Internet]. NYU Langone Health; 2019 5 [cited 2021 Oct 1]. Available from: https://nyulangone.org/news/large-life-expectancy-gaps-us-cities-linked-racial-ethnic-segregation-neighborhood.
- 24.Agency for Toxic Substances and Disease Registry. CDC/ATSDR Social Vulnerability Index [Internet]. Centers for Disease Control; 2021 [cited 2021 Oct 1]. Available from https://www.atsdr.cdc.gov/placeandhealth/svi/fact_sheet/fact_sheet.html.
- 25.Bilal U, Tabb LP, Barber S, Diez Roux AV. Spatial inequities in COVID-19 testing, positivity, confirmed cases, and mortality in 3 U.S. cities: an ecological study. Ann Intern Med. 2021;174(7):936–944. doi: 10.7326/M20-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dasgupta S, Bowen VB, Leidner A, et al. Association between social vulnerability and a county’s risk for becoming a COVID-19 hotspot - United States, June 1-July 25, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1535–1541. doi: 10.15585/mmwr.mm6942a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SJ, Bostwick W. Social vulnerability and racial inequality in COVID-19 deaths in Chicago. Health Educ Behav. 2020;47(4):509–513. doi: 10.1177/1090198120929677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein EJ, Hardesty A, Vieira K, et al. Use of anti-spike monoclonal antibodies in kidney transplant recipients with COVID-19: efficacy, ethnic and racial disparities. Am J Transplant. 2021 :10.1111/ajt.16843. [DOI] [PMC free article] [PubMed]
- 29.Wiltz JL, Feehan AK, Molinari NM, et al. Racial and ethnic disparities in receipt of medications for treatment of COVID-19 - United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2022;71(3):96–102. doi: 10.15585/mmwr.mm7103e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bierle DM, Ganesh R, Wilker CG, et al. Influence of social and cultural factors on the decision to consent for monoclonal antibody treatment among high-risk patients with mild-moderate COVID-19. J Prim Care Community Health. 2021;12:21501327211019282. doi: 10.1177/21501327211019282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eli Lilly and Company. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bebtelovimab. U.S. Food 5 and Drug Administration; 2022 [cited 2022 Apr 19]. Available from: https://www.fda.gov/media/156152/download.
- 32.FDA Newsroom. Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Monoclonal Antibody Bamlanivimab. U.S. Food and Drug Administration; 2021 Apr 16 [cited 2021 Oct 1]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab.
- 33.CDC/ATSDR Social Vulnerability Index 2018 Database of Illinois [Internet]. Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry/Geospatial Research, Analysis, and Services Program; 2020 [modified 2021 Jun 22; cited 2021 Oct 1]. Available from: https://www.atsdr.cdc.gov/placeandhealth/svi/documentation/SVI_documentation_2018.html.
- 34.Zamudio MI. How Many Chicago Latinos Have Died From COVID-19? There Are No Up-To-Date Numbers. WBEZ Chicago [Internet]. 2020 Apr 15 [cited 2021 Oct 1]. Available from: https://www.wbez.org/stories/how-many-chicago-latinos-have-died-from-covid-19-there-are-no-up-to-date-numbers/8835a044-05bd-4503-985a-3bb11cf45df7.
- 35.Yoon P, Hall J, Fuld J, et al. Alternative methods for grouping race and ethnicity to monitor COVID-19 outcomes and vaccination coverage. MMWR Morb Mortal Wkly Rep. 2021;70(32):1075–1080. doi: 10.15585/mmwr.mm7032a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.32. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.QuickFacts, Cook County, Illinois [Internet]. U.S. Census Bureau; 2021. Available from: https://www.census.gov/quickfacts/fact/table/cookcountyillinois/PST045221#.
- 39.2015-2019 American Community Survey 5-Year Data Profile Demographic and Housing Estimates [Internet]. U.S. Census Bureau; 2019. Available from: https://www.census.gov/acs/www/data/data-tables-and-tools/data-profiles/.
- 40.AMA Webinar. FDA experts discuss COVID-19 therapeutic clinical trials [Internet]. American Medical Association; 2021 [cited 2021 Oct 1]. Available from: https://www.ama-assn.org/delivering-care/public-health/fda-experts-discuss-covid-19-therapeutic-clinical-trials.
- 41.Tulledge-Scheitel S, Bell SJ, Larsen JJ, et al. A mobile unit overcomes the challenges to monoclonal antibody infusion for COVID-19 in skilled care facilities. J Am Geriatr Soc. 2021;69(4):868–873. doi: 10.1111/jgs.17090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubin E, Dryden-Peterson SL, Hammond SP, et al. A novel approach to equitable distribution of scarce therapeutics: institutional experience implementing a reserve system for allocation of COVID-19 monoclonal antibodies. Chest. 2021;160(6):2324–2331. doi: 10.1016/j.chest.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 1102 kb)