Abstract
Introduction
Catheter ablations for cardiac arrhythmias are conventionally performed under fluoroscopic guidance. To guide these procedures, zero/minimal fluoroscopy (Z/MF) approaches have become available, using three-dimensional electroanatomical mapping systems. Our aim was to conduct a meta-analysis comparing these two different methods for the treatment of paroxysmal supraventricular tachycardia (SVT).
Methods
Electronic databases were searched and systematically reviewed for studies comparing procedural parameters and outcomes of conventional, fluoroscopy-guided vs. Z/MF approaches in patients undergoing electrophysiology (EP) procedures for SVTs. The random-effects model was used to derive mean difference (MD) and risk ratios (RRs) with 95% confidence interval (CI).
Results
Twenty-four studies involving 9,074 patients met our inclusion criteria. There was no difference between the groups in terms of acute success rate (RR = 1.00, 95% CI, 0.99–1.01; p = 0.97) and long-term success rate (RR: 1.01, 95% CI, 1.00–1.03; p = 0.13). Compared to the conventional method, zero-and-minimal fluoroscopy (Z/MF) ablation significantly reduced fluoroscopic time [MD: −1.58 min (95% CI, −2.21 to −0.96 min; p < 0.01)] and ablation time [MD: −25.23 s (95% CI: −42.04 to −8.43 s; p < 0.01)]. No difference could be detected between the two groups in terms of the procedure time [MD: 3.06 min (95% CI: −0.97 to 7.08; p = 0.14)] and the number of ablation applications [MD: 0.13 (95% CI: −0.86 to 1.11; p = 0.80)]. The complication rate was 1.59% in the entire study population and did not differ among the groups (RR: 0.68, 95% CI: 0.45–1.05; p = 0.08).
Conclusions
The Z/MF approach for the catheter ablation of SVTs is a feasible method that reduces radiation exposure and ablation time without compromising the acute and long-term success or complication rates.
Keywords: meta-analysis, paroxysmal supraventricular tachycardia, catheter ablation, zero fluoroscopy, zero fluoroscopy ablation
Introduction
Catheter ablation has evolved as the standard treatment method for paroxysmal supraventricular tachycardia (SVT) owing to its low complications and high success rate (1). These procedures are conventionally performed using a fluoroscopy-guided approach, exposing both patients and medical staff with a potentially dangerous amount of ionizing radiation. Prolonged exposure to radiation may increase the chance of dermatitis, cataracts, and congenital defects, and. it can increase the risk of cancer in the exposed individuals (2).
Although this risk can be reduced by applying various forms of radiation protection described by the as low as reasonably achievable (ALARA) principle (3), based on recent publications, radiation protection is still not optimal in cardiac electrophysiology (EP) (4).
A notable development can be observed in terms of the three-dimensional (3D) electroanatomical mapping (EAM) systems of the past decade. The EAM systems can significantly reduce radiation dose and fluoroscopy time during procedures, and early studies showed that the zero-and-minimal fluoroscopy (Z/MF) approach during EP procedures is a safe and effective method. A previous meta-analysis including 2,261 patients from 10 trials published in 2016 showed reduced fluoroscopic and ablation time using Z/MF ablation for the treatment of cardiac arrhythmias, whereas there was no difference in procedure time and acute and long-term success rates compared to conventional, fluoroscopy-guided ablation procedures (5). Following this meta-analysis, further important studies–including prospective, randomized, and multicenter trials–have been published by comparing these two different strategies.
To gain further insight into the low fluoroscopy approach to catheter ablation, we aimed to study the subgroup of patients with supraventricular arrhythmias and we conducted a meta-analysis to compare the safety, efficacy, and procedural parameters between patients with SVT who underwent catheter ablation procedures either with Z/MF or with fluoroscopy guidance.
Methods
Search Strategy
Electronic databases [PubMed, Excerpta Medica Database (EMBASE), Cochrane Central Register of Controlled Trials (CENTRAL)] were searched for relevant articles between January of 2000 and July of 2021. The search string was “zero-fluoroscopy or near-zero fluoroscopy or fluoroless or non-fluoroscopic” and “electrophysiology or electrophysiological” and “catheter ablation” and “or supraventricular or supraventricular tachycardia or paroxysmal supraventricular tachycardia”. We extended the search with the reference list of the relevant studies. Duplicates and review publications were excluded. We performed the analyses according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines (6).
In this meta-analysis, we included studies that accomplished the following criteria: (1) patients who underwent EP study and/or catheter ablation for paroxysmal SVT, atrioventricular nodal reentrant tachycardia (AVNRT), atrioventricular reentrant tachycardia (AVRT), atrial tachycardia (AT), or cavotricuspid isthmus-dependent atrial flutter (AFL); (2) patients having at least 1 Z/MF -only and one conventional fluoroscopy-only arm; (3) randomized or non-randomized prospective studies and retrospective studies enrolling consecutive patients; and (4) studies written in English. Case reports, letters, abstracts, conference presentations, and ablation of atrial fibrillation or left atrial macroreentrant tachycardia were excluded.
Zero fluoroscopy was defined as no radiation and was used during the procedure. Under “minimal fluoroscopy”, we meant those cases in which, although the operator planned to follow zero-fluoroscopy strategy, the limited use of radiation became necessary during the procedure.
Data Acquisition and Statistical Analysis
Study selections and data acquisition were performed independently by two reviewers (D.D. and P.K.). Disagreements were resolved by consensus.
Endpoints of Interest
The primary outcome of the study was the acute success rate. Secondary outcomes included procedural parameters: “skin-to-skin procedure time” (minutes); “ablation time”, that is the sum of ablation time during the entire procedure (seconds); “application number”, which means the sum of the radiofrequency delivery; and “total fluoroscopy time” (minutes), “fluoroscopy dose” (mGy), and “fluoroscopy exposure” [dose area product (DAP), cGy/cm2]. Complications and long-term success rate were also analyzed.
Statistical Analysis
We performed the analyses according to the PRISMA guidelines using the dmetar 0.0.9, meta 4.15-1, and metaphor 2.4-0 packages with R statistical software 4.0.3 (6, 7). Pooled treatment effects as mean difference (MD) on continuous data and risk ratios (RRs) for binary end-points were compared with their 95% confidence intervals (CIs). The significance of the pooled estimates was determined by the Z-test, and p < 0.05 was considered as statistically significant. We quantified the possibility of heterogeneity between studies and the proportion of inter-study variability by Cochran's Q-statistic and I2 statistics, respectively. The latter describes the percentage of total variation across studies due to heterogeneity rather than due to chance. Values of I2 < 25% were considered as low and values of I2 > 75% were considered as high. The choice of the random-effects model was made based on the consideration that the true effect of low-dose fluoroscopy strategy may vary from study to study influenced by heterogeneity of the included trials. The random-effects model provides more conservative and robust results and accounts better for inter-study differences, however, it also tends to have a higher impact of small study bias. Thus, statistical inference was based on the results of random-effects model analyses. However, we also present results of fixed-effect modeling as a sensitivity exercise. In the random-effects models, the DerSimonian–Laird tau 2 estimator was used to estimate the variance of the distribution of true effect sizes and account for inter-study variability. To assess the stability of acquired effect estimates, a leave-one-out sensitivity analysis was applied. Quality assessment was performed with Cochrane's tool for assessing bias, wherein studies are scored as high, low, or unclear risk of bias in five domains: selection, performance, detection, attrition, and reporting. Funnel plot was drawn to assess publication bias, and asymmetry was assessed by visual estimation and by Egger's linear regression test. In case of any of these suggested substantial asymmetry, Duval and Tweedie's trim-and-fill procedure was applied. With imputing missing studies into the funnel plot until symmetry is reached again, this helps to estimate what the actual effect size would be had the “missing” small studies been published.
Results
Study Characteristics
Twenty-four studies involving 9,074 patients were analyzed (Figure 1) (8–31). Among the 24 included studies, three were randomized controlled studies, whereas the rest were observational trials. The main characteristics of the trials are summarized in Table 1. The EnSite NavX system was used in 18, the EnSite Precision System in 3, the EnSite Velocity System in 3, the CARTO System in 8, the Rhythmia System in 1, and the MediGuide System in one studies. The mean length of the follow-up period varied between 42 and 1,584 days.
Figure 1.
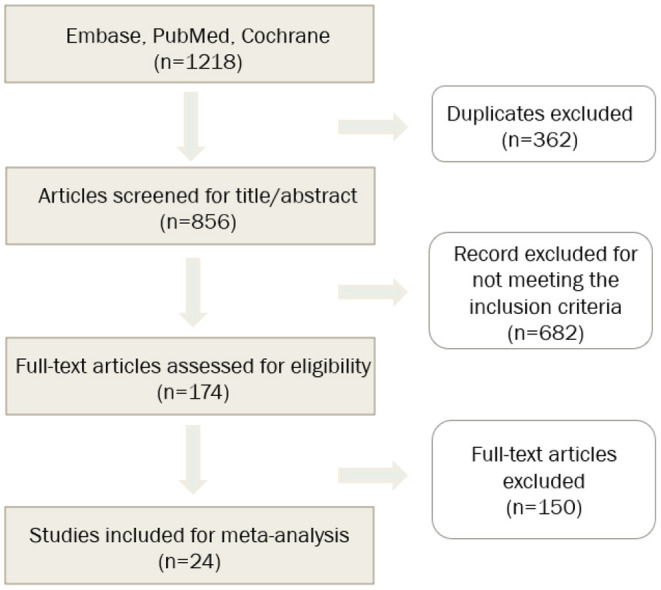
Flowchart of study selection.
Table 1.
Study and patients' characteristics of the included trials.
| References | Design | Patient number | Number of EAM systems | Procedure type | Operators | Operators' experience with EAM systems | EAM system | Use of ICE | Sex (male/female number) | Median follow-up (days) | Mean age | Mean BMI or weight | ZF success |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Earley et al. (8) | Single center, prospective randomized | 96 | 45 | AVNRT, AVRT, AFL, Other | 2 | NA | EnSite NavX | NA | 53/43 | 42 | 52 ± 15; 47 ± 16 | NA | 100% |
| Smith and Clark (10) | Single center, retrospective, non-randomized | 60 | 30 | AVNRT, WPW, concealed pathway | NA | NA | EnSite NavX | NA | 25/35 | 90 | 12.6 ± 4.35 | 21.4; 18.4 (BMI) | 80% |
| Álvarez et al. (9) | Single center, prospective, non-randomized | 100 | 50 | AVNRT | NA | NA | EnSite NavX | NA | 20/80 | 180 | 59.15 ± 15 | NA | 98% |
| Kwong et al. (11) | Single center, retrospective, non-randomized | 388 | 318 | AVRT, AVNRT | NA | NA | EnSite NavX | NA | 219/167 | NA | 11.9 ± 4.2 12.2 ± 3.7 | 47 ± 19.6 53.1 ± 22.4 (kg) |
NA |
| Stec et al. (12) | Multicenter, prospective, non-randomized | 902 | 188 | AVNRT, WPW/AVRT, AFL, AT | NA | NO | EnSite NavX | NO | 413/489 | 240 ± 156; 330 ± 171 | 45 ± 21; 52 ± 18 | NA | 95% |
| Casella et al. (13) | Multicenter, prospective, randomized | 262 | 134 | AVNRT, Right AP, Left AP, AFL, AT | NA | YES | EnSite NavX | NA | 110/152 | 360 ± 132 | 36.3 ± 10.4 35.4 ± 10.4 | 24.4+ 4.4 23.5+ 4.4 (BMI) |
72% |
| Schoene et al. (14) |
Single center, prospective, randomized | 40 | 20 | AFL | 2 | YES | MediGuide | NA | 34/6 | 180 | 65.2 ± 12 | 28.8 ± 4 (BMI) |
NA |
| Romero et al. (15) | Single center, prospective, non-randomized | 779 | 255 | AT, AVNRT, WPW, AFL | NA | NA | EnSite NavX, CARTO | NA | 440/332 | NA | 52 ± 19 | NA | NA |
| Giaccardi et al. (16) | Multicenter, retrospective, non-randomized | 442 | 297 | AT, AVNRT, AVRT, AFL | 3 | NO | EnSite Velocity | NA | 104/338 | NA | 59 ± 19; 58 ± 19 | NA | NA |
| Seizer et al. (17) | Single center, retrospective, non-randomized | 184 | 91 | AVNRT, WPW, AT, AFL | NA | NA | EnSite NavX and Velocity | NO | 87/97 | 389 ± 217 | 52.1 ± 19.1; 36.0 ± 22.1 | 79.4 ± 20.4; 70.5 ± 21.3 (kg) |
100% |
| See et al. (18) | Single center, prospective, non-randomized | 200 | 79 | AVNRT, AVRT | NA | NA | EnSite NavX, CARTO | NA | 110/90 | 360 | 39.5 ± 16.3; 43.4 ± 17.9 | NA | NA |
| Nagaraju et al. (19) |
Single center, retrospective | 83 | 63 | AVNRT, AVRT | 1 | NO | CARTO | YES (only for transseptal puncture) | 46/37 | 148 (ZF) 329 (F) |
13.7; 16.9 | NA | 54% |
| Marini et al. (20) | Single center, retrospective, non-randomized | 93 | 57 | AVNRT, AVRT, AT, EPS, VT | NA | NA | EnSite NavX, CARTO | NA | 57/26 | 720 | NA | 65 (55–70); 57 (54–60) (kg) |
NA |
| Swissa et al. (21) | Single center, prospective, non-randomized | 139 | 64 | AVNRT | 2 | NA | EnSite NavX | NA | 68/71 | 360 | 12.8 ± 3.5 (4.3–17.8); 12.9 ± 3.8 (5–17.9) | 19.5 ± 1.9 (15.7–22.1); 20.1 ± 4.1 (12.4–30.5) (BMI) |
NA |
| Walsh et al. (22) | Single center, retrospective, non-randomized | 92 | 50 | AT, AVNRT, AVRT, EPS | 1 | NO | EnSite Precision | YES (only for transseptal puncture) | 55/37 | 147 | 56 (36-69); 66 (49–74) | NA | 94% |
| Tseng et al. (23) | Single center, retrospective, non-randomized | 109 | 41 | AVNRT, AT | NA | NA | EnSite Precision | NA | 56/47 | 321 | 12.5; 12 | 53.1; 46.1 (kg) |
100% |
| Pires et al. (24) | Single center, prospective, randomized | 23 | 12 | SVT, AFL, RVOT, AT | NA | NA | EnSite NavX | NA | 9/14 | NA | 48.5 ± 1.6; 46.3 ± 16.6 | NA | 100% |
| Dengke et al. (25) | Single center, retrospective, non-randomized | 227 | 112 | left-AVRT | NA | NO | EnSite NavX | NA | 135/92 | 90 | 50.2 ± 18.9 55.6 ± 17.9 | NA | NA |
| Ceresnak et al. (26) | Multicenter, retrospective, non-randomized | 651 | 366 | AVRT | NA | NA | EnSite NavX, CARTO | NO | 378/273 | 42 ± 36 | 13.0 ± 4.0 | 54.3 ± 23.3 (kg) |
NA |
| Cauti et al. (29) | Single center | 20 | 10 | AVNRT, AT, AVRT, AFL | 4 | NA | Rhythmia | NA | NA | 180 | 58 ± 12 | NA | 80% |
| Chen et al. (28) | Multicenter, prospective, non-randomized | 3,060 | 1,020 | AVNRT, AVRT | NA | NA | EnSite NavX | YES | 1,367/1,693 | 291 ± 120 | 45.3 ± 5.4 | 63.8 ± 11.7 (kg) |
99.3% |
| Fadhle et al. (27) | Single center, prospective, non-randomized | 300 | 200 | AVNRT, AVRT | 4 | NO | EnSite NavX, CARTO | NA | 118/282 | 360 | 45.3 ± 15.4 | 63.8 ± 11.7 (kg) |
99.5% |
| Di Cori et al. (30) | Single center, retrospective, non-randomized | 206 | 93 | EPS, AVNRT, AVRT, AT, AFL | NA | NA | CARTO, EnSite NavX/Velocity/Precision | NA | 107/99 | 360 | 53 ± 19 | 26 ± 3.4; 25 ± 3.5 |
58% |
| Bergonti et al. (31) |
Single center, retrospective, non-randomized | 618 | 206 | AVNRT, AVRT | NA | NA | EnSite NavX, CARTO | NA | 247/371 | 1,584 | 38 ± 15 | NA | 67.5% |
Abl, ablation; AFL, atrial flutter; AP, accessory pathway; AT, atrial tachycardia; AVNRT, atrioventricular nodal reentrant tachycardia; AVRT, atrioventricular reentrant tachycardia; BMI, body mass index; EPS, electrophysiology study; NA, not available; RVOT, right ventricular outflow tract-ventricular tachycardia; WPW, Wolff–Parkinson–White syndrome.
Efficacy and Safety Outcome Events
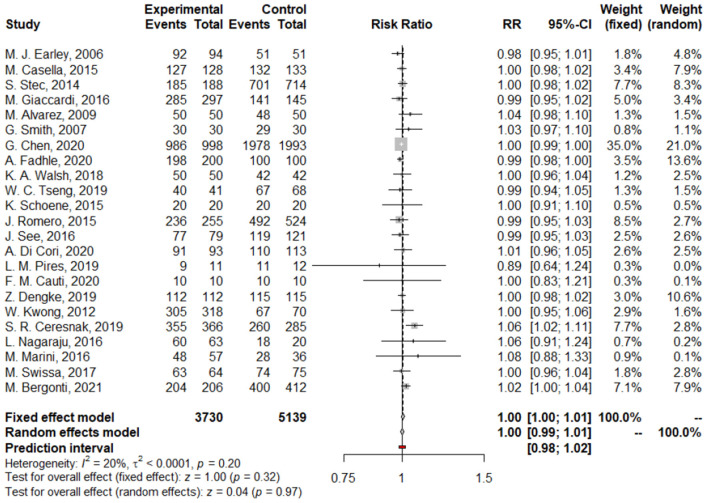
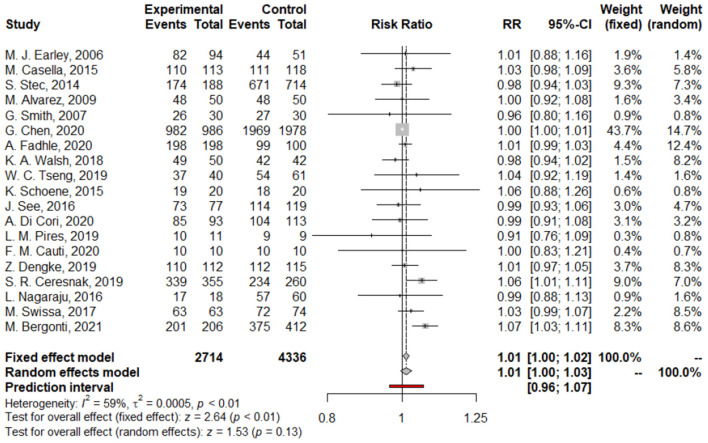
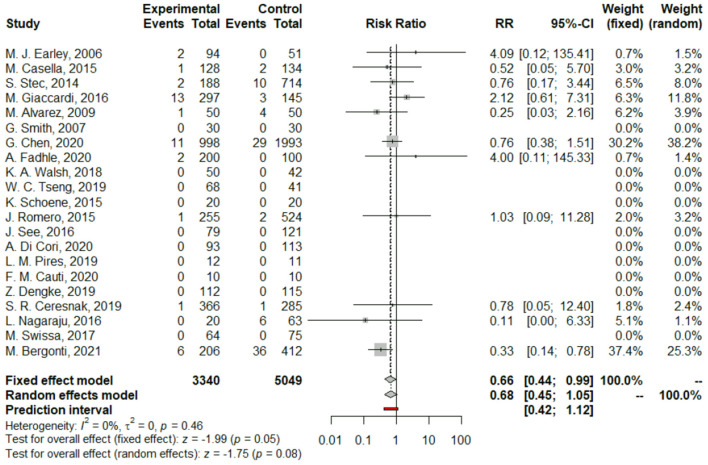
We found no difference between Z/MF and conventional ablation procedures in acute success rate (97.4 vs. 97.55%; RR: 1.00, 95% CI: 0.99–1.01, p = 0.97; Figure 2) and long-term success rate (97.02 vs. 96.17%; RR: 1.01, 95% CI: 1.00–1.03, p = 0.13; Figure 3). Complication rate was 1.59% in the entire study population and did not differ among the groups (RR: 0.68, 95% CI: 0.45–1.05, p = 0.08; Figure 4).
Figure 2.
Forest plots of acute success rate.
Figure 3.
Forest plots of long-term success rate.
Figure 4.
Forest plots of complications.
We performed a leave-one-out analysis, which showed similarly no difference between the groups for acute success rate (Supplementary Figure S1).
Procedural Parameters
Compared to the conventional method, the Z/MF approach significantly reduced fluoroscopic time [MD: −1.58 min (95% CI, −2.21 to −0.96 min; p < 0.01)], fluoroscopy dose [MD: −10.95 mGy (95% CI: −18.43 to −3.46 mGy)], and radiation exposure [DAP; MD: −52.39 cGy/cm2 (95% CI: −65.38 to −39.40 cGy/cm2)]. Ablation time was shorter with the Z/MF method [MD: −25.23 s (95% CI: −42.04 to −8.43 s; p < 0.01)], whereas no difference could be detected between the two groups in terms of the number of ablation applications [MD: 0.13 (95% CI: −0.86 to 1.11; p = 0.80)] and procedure time [MD: 3.06 min (95% CI: −0.97 to 7.08 min; p = 0.14)] (Table 2).
Table 2.
Summary of outcomes of secondary endpoints.
| Outcome | Number of | Number of | Mean difference | Test for | Heterogeneity |
|---|---|---|---|---|---|
| studies | patients | (95% CI) | overall effect | ||
| Ablation time | 7 | 4,750 | −25.23 s (−42.04; −8.43) | p < 0.01 | I2 = 40%; p < 0.12 |
| Ablation application number | 8 | 4,098 | 0.13 min (−0.86; 1.11) | p = 0.80 | I2 = 71%; p < 0.01 |
| Fluoroscopy time | 17 | 7,326 | −1.58 min (−2.21; −0.96) | p < 0.01 | I2 = 98%; p < 0.01 |
| Fluoroscopy dose | 5 | 1,154 | −10.95 mGy (−18.43; −3.46) | p < 0.01 | I2 = 97%; p < 0.01 |
| DAP | 5 | 1,651 | −52.39 cGy/cm2 (−65.38; −39.40) | p < 0.01 | I2 = 100%; p = 0 |
| Procedure time | 15 | 7,290 | 3.06 min (−0.97; 7.08) | p = 0.14 | I2 = 91%; p < 0.01 |
DAP, dose area product.
Funnel plot analyses and Egger's regression test showed no sign of possible publication bias (Supplementary Figure S1).
Discussion
Our meta-analysis of 24 studies with 9,074 patients who underwent EP intervention due to paroxysmal SVT demonstrates that Z/MF ablation can significantly reduce radiation exposure, fluoroscopy, and ablation time. Compared to the fluoroscopy-guided ablation, the use of the Z/MF method proved to have no impact on the procedure time, the risk of complications, the acute or long-term success rate, and the number of ablation applications.
Medical exposure is the highest manmade source of radiation, representing a mean effective dose of 1–3 mSv per person per year (32). Radiation increases the life-time risk of cataract, dermatitis, and cancer via stochastic and deterministic effects (33–35).
Over the past decades, the reduction of the ionizing radiation during EP procedures has become a center of interest. Intraoperative mapping systems enable the visualization of the real-time anatomy of vessels and chambers of the heart, and the movement of the catheters. Owing to the fact that the use of EAM systems does not affect the procedure safety and efficacy, their use has become the most common method to achieve zero- or limited fluoroscopic guidance during cardiac ablation (36).
Preferring ZF guidance to traditional fluoroscopic approach is extremely important in high-risk populations, particularly in pregnant women and children (37, 38). According to the latest guideline of the European Society of Cardiology (ESC), fluoroless catheter ablation should be performed in pregnant women with drug-refractory or poorly tolerated SVT (1).
A previous meta-analysis of 2016, with the inclusion of 2,261 patients, compared the Z/MF and fluoroscopic approaches during ablation of cardiac arrhythmias (5). In correspondence with our recent findings, this meta-analysis also showed a significant reduction of fluoroscopy and ablation time, whereas the procedure time, ablation time, complications, and acute and long-term success rates were similar between the two groups. However, we had the opportunity to involve significantly more patients, which strengthens the generalizability of these results to SVTs.
Procedural Parameters
Theoretically, the use of EAM systems may reduce the procedure length due to 3D visualization and allows easier return to a desired place with the catheters. On the other hand, the creation of an EAM systems requires several minutes contrary to the conventional, fluoroscopy-guided method. In our analysis (in which atrial fibrillation ablation procedures were not included), we found no difference in terms of the procedure time between the groups; however, a significant heterogeneity was detected among the 18 studies in this regard: some studies found longer procedure time (9–12, 15, 16, 22, 28, 30), whereas other trials showed significantly reduced procedure time, with the Z/MF method (13, 17, 18, 21, 23, 25). This may be attributed to the heterogeneity of the performed ablation procedures, including AT, AVNRT, AVRT, and AFL, and the different methods of performance of these interventions among different centers. This fact may also explain that ablation time was found shorter with the use of EAM systems, despite the fact that similar amount of ablation applications occurred in the groups. We found much more favorable results in terms of procedural parameters in the Z/MF group; these findings were consistent between the studies included.
Acute Success
Acute success rate was above 97% in the entire study population. We found no difference between the groups. Among 23 trials reporting acute success rate, comparing the fluoroscopy-only and the Z/MF-only approaches, Ceresnak et al. (26) demonstrated a significant difference analyzing children with the Wolff–Parkinson–White syndrome (26). In this multicenter retrospective trial, the use of EAM systems improved the acute success rate; however, the rate of the procedures utilizing cryoenergy was higher in the fluoroscopy-only group, and cryoablation was associated with decreased success rate on multivariable analysis (26).
Complications
Complication rate was low (1.59%) and did not differ significantly among the groups. No significant heterogeneity was detected among 21 studies reporting complications. Interestingly, a retrospective observational trial by Bergonti et al. (31) found higher rate of complications in the conventional arm compared to the Z/MF approach (8.73 vs. 2.91%). This difference mainly comprised late complications (i.e., advance AV block and need for pacemaker implantation). According to the authors, these results may be explained by the fact that, with EAM systems, the proximity of the His bundle area can be safely monitored all along the procedure.
Long-Term Success
Eighteen trials included in our analysis reported on long-term success results, and only two of them, including patients with AVNRT and AVRT, found difference between conventional and Z/MF ablation procedures, namely the Z/MF approach, which was associated with a lower recurrence rate (26, 31). Surprisingly, Bergonti et al. (31) reported 8.98% recurrence rate during the 52-month follow-up in the conventional arm (31), which is much higher than the literature data (1). This difference may be explained by the fact that recurrence was defined as “experience recurrence of arrhythmias” even without electrocardiography (ECG) documentation. Nevertheless, our analysis showed no difference in long-term success rate between Z/MF and conventional, fluoroscopy-guided methods.
To analyze the potential impact of this trial to the results of the meta-analysis and its heterogeneity, in-depth analyses were carried out (Supplementary Figures S3–S5). Based on these, the study of Bergonti et al. (31) was identified to be among the five studies being the potential source of the data heterogeneity; however, the impact on the results was negligible. Moreover, in leave-one-out analyses, the omission of this study did not impact our results (Supplementary Figure S2).
To acquire the skills to be able to properly apply the Z/MF-guiding technique, the operators have to complete a learning curve, which comprises 20 procedures in the case of SVTs (39). Besides the operators' experience, the type of the arrhythmia and the center volume may also have an effect on the success of zero-fluoroscopy strategy (40). The most challenging part of the total fluoroless ablation procedure is the transseptal puncture; however, this step can be guided by intracardiac echocardiography (ICE). In addition, the combination of EAM systems and ICE provides an even more accurate approach compared to the standard fluoroscopy views (41).
The use of EAM systems may increase EP procedure costs; however, Casella el al. (13) found that the additional cost of the Z/MF method is approximately equal to the extra costs associated with the increased cancer treatment and the reduction in the quality of life associated with conventional fluoroscopy-guided techniques (13). We believe that this higher cost should not be a barrier to improve the safety of both patients and medical staff in EP procedures.
Limitation
Some aspects of our study should also be discussed as they may serve as possible limitations. First, only three randomized studies were included, and the majority of data originate from observational studies. This may introduce potential biases and/or effects of unmeasured confounders. Important differences may also exist in patient demographics that might affect outcomes and are not accounted for in this analysis (e.g., body mass index, ethnicity, or gender). Second, a high degree of heterogeneity was observed (>50%) between the different study populations. The use of a random-effects model can help mitigate the potential effect of heterogeneity, and the high level of significance supports the validity of the results. Third, outcomes were not reported by all of the included studies, limiting further analysis of potential mechanisms. Finally, data regarding the operators performing the procedures and especially data on operators' previous experience with the Z/MF approach were also insufficient despite the probable impact of a learning curve effect.
Conclusion
In conclusion, our meta-analysis including 9,074 patients demonstrated that the Z/MF approach for the treatment of SVT is a feasible method that reduces fluoroscopy time, radiation exposure, and ablation duration but does not compromise the acute and long-term success or complication rates.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
PK, TS, and DD contributed to conception and design of the study. AK performed the statistical analysis. DD, PK, MV, and AK wrote sections of the manuscript. Tables and figures were designed by PK, DD, and KJ. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
MV reports consulting fees and/or nonfinancial support from Biotronik, Medtronic, and Pfizer, outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.856145/full#supplementary-material
References
- 1.Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomstrom-Lundqvist C, et al. 2019 ESC guidelines for themanagement of patients with supraventricular tachycardia. Eur Heart J. (2020) 41:655–720. 10.1093/eurheartj/ehz467 [DOI] [PubMed] [Google Scholar]
- 2.Houmsse M, Daoud EG. Radiation exposure: a silent complication of catheter ablation procedures. Hear Rhythm. (2012) 9:715–6. 10.1016/j.hrthm.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 3.Brodsky A. Principles and practices for keeping occupational. radiation exposures at medical institutions as low as reasonably achievable. (1993) 1−96. [Google Scholar]
- 4.Kleemann T, Brachmann J, Lewalter T, Andresen D, Willems S, Spitzer SG, et al. Development of radiation exposure in patients undergoing pulmonary vein isolation in Germany between 2007 and 2014: great potential to minimize radiation dosage. Clin Res Cardiol. (2016) 105:858–64. 10.1007/s00392-016-0994-9 [DOI] [PubMed] [Google Scholar]
- 5.Yang L, Sun G, Chen X, Chen G, Yang S, Guo P, et al. Meta-analysis of zero or near-zero fluoroscopy use during ablation of cardiac arrhythmias. Am J Cardiol. (2016) 118:1511–8. 10.1016/j.amjcard.2016.08.014 [DOI] [PubMed] [Google Scholar]
- 6.Silverwood V, Chew-Graham C, Shiers D. Improving the physical health of people with severe mental illness. InnovAiT Educ Inspir Gen Pract. (2019) 12:203–10. 10.1177/175573801882380027443217 [DOI] [Google Scholar]
- 7.Viechtbauer W. Conducting meta-analyses in R with the metafor. J Stat Softw. (2010) 36:1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 8.Earley MJ, Showkathali R, Alzetani M, Kistler PM, Gupta D, Abrams DJ, et al. Radiofrequency ablation of arrhythmias guided by non-fluoroscopic catheter location: a prospective randomized trial. Eur Heart J. (2006) 27:1223–9. 10.1093/eurheartj/ehi834 [DOI] [PubMed] [Google Scholar]
- 9.Álvarez M, Tercedor L, Almansa I, Ros N, Galdeano RS, Burillo F, et al. Safety and feasibility of catheter ablation for atrioventricular nodal re-entrant tachycardia without fluoroscopic guidance. Hear Rhythm. (2009) 6:1714–20. 10.1016/j.hrthm.2009.08.037 [DOI] [PubMed] [Google Scholar]
- 10.Smith G, Clark JM. Elimination of fluoroscopy use in a pediatric electrophysiology laboratory utilizing three-dimensional mapping. Pacing Clin Electrophysiol. (2007) 30:510–8. 10.1111/j.1540-8159.2007.00701.x [DOI] [PubMed] [Google Scholar]
- 11.Kwong W, Neilson AL, Chiu CC, Gross GJ, Hamilton RM, Soucie L, et al. The effect of NavX on fluoroscopy times in pediatric catheter ablation. J Interv Card Electrophysiol. (2012) 33:123–6. 10.1007/s10840-011-9604-y [DOI] [PubMed] [Google Scholar]
- 12.Stec S, Sledz J, Mazij M, Raś M, Ludwik B, Chrabaszcz M, et al. Feasibility of implementation of a “simplified, No-X-Ray, no-lead apron, two-catheter approach” for ablation of supraventricular arrhythmias in children and adults. J Cardiovasc Electrophysiol. (2014) 25:866–74. 10.1111/jce.12414 [DOI] [PubMed] [Google Scholar]
- 13.Casella M, Dello Russo A, Pelargonio G, Del Greco M, Zingarini G, Piacenti M, et al. Near zerO fluoroscopic exPosure during catheter ablAtion of supRavenTricular arrhYthmias: The NO-PARTY multicentre randomized trial. Europace. (2016) 18:1565–72. 10.1093/europace/euv344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoene K, Rolf S, Schloma D, John S, Arya A, Dinov B, et al. Ablation of typical atrial flutter using a non-fluoroscopic catheter tracking system vs. conventional fluoroscopy-results from a prospective randomized study. Europace. (2015) 17:1117–21. 10.1093/europace/euu398 [DOI] [PubMed] [Google Scholar]
- 15.Romero J, Lupercio F, Goodman-Meza D, Ruiz JC, Briceno DF, Fisher JD, et al. Electroanatomic mapping systems (CARTO/EnSite NavX) vs. conventional mapping for ablation procedures in a training program. J Interv Card Electrophysiol. (2016) 45:71–80. 10.1007/s10840-015-0073-6 [DOI] [PubMed] [Google Scholar]
- 16.Giaccardi M, Del Rosso A, Guarnaccia V, Ballo P, Mascia G, Chiodi L, et al. Near-zero x-ray in arrhythmia ablation using a 3-dimensional electroanatomic mapping system: a multicenter experience. Hear Rhythm. (2016) 13:150–6. 10.1016/j.hrthm.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 17.Seizer P, Bucher V, Frische C, Heinzmann D, Gramlich M, Müller I, et al. Effektivität und sicherheit der komplett strahlenfreien katheterablation für supraventrikuläre tachykardien: verwendung optionaler anpresskraftkontrolle bei der strahlenfreien katheterablation in klinischem routinesetting. Herz. (2016) 41:241–5. 10.1007/s00059-015-4358-4 [DOI] [PubMed] [Google Scholar]
- 18.See J, Amora JL, Lee S, Lim P, Teo WS, Tan BY, et al. Non-fluoroscopic navigation systems for radiofrequency catheter ablation for supraventricular tachycardia reduce ionising radiation exposure. Singapore Med J. (2016) 57:390–5. 10.11622/smedj.2016017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagaraju L, Menon D, Aziz PF. Use of 3D electroanatomical navigation (CARTO-3) to minimize or eliminate fluoroscopy use in the ablation of pediatric supraventricular tachyarrhythmias. Pacing Clin Electrophysiol. (2016) 39:574–80. 10.1111/pace.12830 [DOI] [PubMed] [Google Scholar]
- 20.Marini M, Del Greco M, Ravanelli D, Cima A, Coser A, Porcedda G, et al. The benefit of a general, systematic use of mapping systems during electrophysiological procedures in children and teenagers: the experience of an adult EP laboratory. Pediatr Cardiol. (2016) 37:802–9. 10.1007/s00246-016-1354-2 [DOI] [PubMed] [Google Scholar]
- 21.Swissa M, Birk E, Dagan T, Naimer SA, Fogelman M, Einbinder T, et al. Radiofrequency catheter ablation of atrioventricular node reentrant tachycardia in children with limited fluoroscopy. Int J Cardiol. (2017) 236:198–202. 10.1016/j.ijcard.2017.01.128 [DOI] [PubMed] [Google Scholar]
- 22.Walsh KA, Galvin J, Keaney J, Keelan E, Szeplaki G. First experience with zero-fluoroscopic ablation for supraventricular tachycardias using a novel impedance and magnetic-field-based mapping system. Clin Res Cardiol. (2018) 107:578–85. 10.1007/s00392-018-1220-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng WC, Wu MH, Lu CW, Wu KL, Wang JK, Lin MT, et al. Zero fluoroscopy during ablation of right-sided supraventricular tachycardia substrates in a pediatric population–initial experience in Taiwan. Acta Cardiol Sin. (2019) 35:476–83. 10.6515/ACS.201909_35(5).20190211A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pires LM, Leiria TLL, Kruse ML, de Lima GG. Non-fluoroscopic catheter ablation: a randomized trial. Indian Pacing Electrophysiol J. (2019) 19:189–94. 10.1016/j.ipej.2019.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dengke Z, Lan L, Xiangli S, Shubin J. Treatment of left accessory cardiac pathway conduction disorders using radiofrequency catheter ablation under the guidance of the Ensite NavX 3D mapping system: a retrospective study. Int J Cardiovasc Imaging. (2019) 35:387–92. 10.1007/s10554-018-1449-3 [DOI] [PubMed] [Google Scholar]
- 26.Ceresnak SR, Dubin AM, Kim JJ, Valdes SO, Fishberger SB, Shetty I, et al. Success rates in pediatric WPW ablation are improved with 3-dimensional mapping systems compared with fluoroscopy alone: a multicenter study. J Cardiovasc Electrophysiol. (2015) 26:412–6. 10.1111/jce.12623 [DOI] [PubMed] [Google Scholar]
- 27.Fadhle A, Hu M, Wang Y. The safety and efficacy of zero-fluoroscopy ablation versus conventional ablation in patients with supraventricular tachycardia. Kardiol Pol. (2020) 78:552–8. 10.33963/KP.15293 [DOI] [PubMed] [Google Scholar]
- 28.Chen G, Wang Y, Proietti R, Wang X, Ouyang F, Ma CS, et al. Zero-fluoroscopy approach for ablation of supraventricular tachycardia using the Ensite NavX system: a multicenter experience. BMC Cardiovasc Disord. (2020) 20:1–10. 10.1186/s12872-020-01344-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cauti FM, Rossi P, Iaia L, Bianchi S. A new mapping method with the RhythmiaTM navigation system reduces radiation exposure. Preliminary experience in SVT procedures. J Electrocardiol. (2020) 58:92–5. 10.1016/j.jelectrocard.2019.11.049 [DOI] [PubMed] [Google Scholar]
- 30.Di Cori A, Zucchelli G, Segreti L, Barletta V, Viani S, Paperini L, et al. Predictors of zero X ray procedures in supraventricular arrhythmias ablation. Int J Cardiovasc Imaging. (2020) 36:1599–607. 10.1007/s10554-020-01884-8 [DOI] [PubMed] [Google Scholar]
- 31.Bergonti M, Dello Russo A, Sicuso R, Ribatti V, Compagnucci P, Catto V, et al. Long-term outcomes of near-zero radiation ablation of paroxysmal supraventricular tachycardia: a comparison with fluoroscopy-guided approach. JACC Clin Electrophysiol. (2021) 7:1108–17. 10.1016/j.jacep.2021.02.017 [DOI] [PubMed] [Google Scholar]
- 32.Mettler FA, Bhargavan M, Faulkner K, Gilley DB, Gray JE, Ibbott GS, et al. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources-1950–2007. Radiology. (2009) 253:520–31. 10.1148/radiol.2532082010 [DOI] [PubMed] [Google Scholar]
- 33.Amis ES, Butler PF, Applegate KE, Birnbaum SB, Brateman LF, Hevezi JM, et al. American college of radiology white paper on radiation dose in medicine. J Am Coll Radiol. (2007) 4:272–84. 10.1016/j.jacr.2007.03.002 [DOI] [PubMed] [Google Scholar]
- 34.Picano E, Vano E. The radiation issue in cardiology: the time for action is now. Cardiovasc Ultrasound. (2011) 9:1–13. 10.1186/1476-7120-9-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roguin A, Goldstein J, Bar O, Goldstein JA. Brain and neck tumors among physicians performing interventional procedures. Am J Cardiol. (2013) 111:1368–72. 10.1016/j.amjcard.2012.12.060 [DOI] [PubMed] [Google Scholar]
- 36.Gaita F, Guerra PG, Battaglia A, Anselmino M. The dream of near-zero X-rays ablation comes true. Eur Heart J. (2016) 37:2749–55. 10.1093/eurheartj/ehw223 [DOI] [PubMed] [Google Scholar]
- 37.Driver K, Chisholm CA, Darby AE, Malhotra R, Dimarco JP, Ferguson JD. Catheter ablation of arrhythmia during pregnancy. J Cardiovasc Electrophysiol. (2015) 26:698–702. 10.1111/jce.12675 [DOI] [PubMed] [Google Scholar]
- 38.Drago F, Silvetti MS, Di Pino A, Grutter G, Bevilacqua M, Leibovich S. Exclusion of fluoroscopy during ablation treatment of right accessory pathway in children. J Cardiovasc Electrophysiol. (2002) 13:778–82. 10.1046/j.1540-8167.2002.00778.x [DOI] [PubMed] [Google Scholar]
- 39.Kochar A, Ahmed T, Donnellan E, Wazni O, Tchou P, Chung R. Operator learning curve and clinical outcomes of zero fluoroscopy catheter ablation of atrial fibrillation, supraventricular tachycardia, and ventricular arrhythmias. J Interv Card Electrophysiol. (2021) 61:165–70. 10.1007/s10840-020-00798-8 [DOI] [PubMed] [Google Scholar]
- 40.Pani A, Belotti G, Bonanno C, Bongiorni MG, Bottoni N, Brambilla R, et al. Predictors of zero X-ray ablation for supraventricular tachycardias in a nationwide multicenter experience. Circ Arrhythmia Electrophysiol. (2018) 11:1–11. 10.1161/CIRCEP.117.005592 [DOI] [PubMed] [Google Scholar]
- 41.Bartel T, Müller S, Biviano A, Hahn RT. Why is intracardiac echocardiography helpful? Benefits, costs, and how to learn. Eur Heart J. (2014) 35:69–76. 10.1093/eurheartj/eht411 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.