Abstract
The role of moxifloxacin and levofloxacin pharmacokinetics (PK) in antimicrobial efficacy and in the selection of fluoroquinolone-resistant Streptococcus pneumoniae strains was investigated using the rabbit tissue cage abscess model. A rabbit tissue cage was created by insertion of sterile Wiffle balls in the dorsal cervical area. Animals orally received a range of moxifloxacin or levofloxacin doses that simulate human PK for 7 days 48 h after the Wiffle balls were inoculated with fluoroquinolone-sensitive S. pneumoniae (107 CFU). Abscess fluid was collected on a daily basis over 14 days to measure bacterial density and MICs. Moxifloxacin regimens produced a range of area under the concentration-time curve (AUC)/MIC ratios ranging from 9.2 to 444 and peak/MIC ratios ranging from 1.3 to 102. Levofloxacin doses produced AUC/MIC ratios of 5.1 to 85.5 and peak/MIC ratio of 0.9 to 14.8. Moxifloxacin at 6.5, 26, and 42 mg/kg reduced the bacterial log CFU per milliliter in abscess fluid (percentage of that in a sterile animal) by 4.2 ± 2.2 (20%), 5.8 ± 0.4 (100%), and 5.4 ± 0.4 (100%), respectively, over the dosing period. Levofloxacin at 5.5, 22, and 32 mg/kg reduced the log CFU per milliliter in abscess fluid (percentage of that in a sterile animal) by 2.8 ± 0.7 (20%), 5.1 ± 1.3 (80%), and 4.6 ± 1.3 (60%), respectively. Moxifloxacin has a greater bactericidal rate as determined by regression of log CFU versus time data. The AUC/MIC and peak/MIC ratios correlated with the efficacy of both drugs (P < 0.05). Resistance to either drug did not develop with any of the doses as assessed by a change in the MIC. In conclusion, data derived from this study show that moxifloxacin and levofloxacin exhibit rapid bactericidal activity against S. pneumoniae in vivo, and moxifloxacin exhibits enhanced bactericidal activity compared to levofloxacin, with AUC/MIC and peak/MIC ratios correlated with antimicrobial efficacy for both drugs. The development of fluoroquinolone-resistant S. pneumoniae was not observed with either drug in this model.
Streptococcus pneumoniae is a common pathogen in adult community-acquired respiratory tract infections (RTIs) such as pneumonia, acute sinusitis, and exacerbation of chronic bronchitis. The high prevalence of RTIs and the increasing emergence of antibiotic-resistant bacterial pathogens, including S. pneumoniae have become increasing problems in numerous clinical settings (8, 13, 14). This necessitates the development of new, more potent antimicrobial agents. Moxifloxacin is a new 8-methoxy fluoroquinolone with improved activity against gram-positive bacteria, including penicillin-resistant pneumococci (1, 2). Levofloxacin, a semisynthetic fluoroquinolone, is indicated for a variety of community-acquired RTIs, especially with penicillin-resistant S. pneumoniae (4, 6).
In this study, we evaluated S. pneumoniae responses to a range of different drug exposures, including the moxifloxacin and levofloxacin exposure that simulates human pharmacokinetics in vivo. In addition, the impact of the pharmacokinetics of moxifloxacin and levofloxacin on their antimicrobial efficacy was studied.
MATERIALS AND METHODS
Microorganism.
SP18 is a penicillin-intermediate S. pneumoniae strain which was isolated from a previously hospitalized patient. The test organism was frozen in skim milk medium (Becton Dickinson, Cockeysville, Md.) at −80°C and subcultured twice onto Trypticase soy agar with 5% sheep blood (Becton Dickinson) before use in all experiments. The levofloxacin and moxifloxacin MICs for this bacterial isolate are 1 and 0.25 μg/ml, respectively.
Antimicrobial test agents.
Analytical grade standard moxifloxacin obtained from Bayer Corporation, West Haven, Conn., was used for all of the in vitro testing and in vivo studies. Analytical grade standard levofloxacin obtained from the R. W. Johnson Pharmaceutical Research Institute, Spring House, Pa., was used for in vitro testing. For all of the in vivo studies, commercially available levofloxacin (Levaquin) injection solution was obtained from Ortho-McNeil Pharmaceutical Inc., Raritan, N.J.
Surgical preparation and animal care.
Female New Zealand White rabbits, weighing between 3.5 and 4.2 kg, were quarantined for at least 7 days before surgery. Animals were housed one per cage in our institutional animal care facility and allowed food and water ad libitum. All care and the experiments described herein were approved by and performed in accordance with guidelines of the Hartford Hospital Institutional Animal Care and Use Committee. Animals were anesthetized with ketamine-xylazine, and two sterile golf practice Wiffle balls were surgically implanted in the dorsal cervical area. The rabbits were given a single 0.1-mg/kg dose of buprenophine intramuscularly every 12 h (Q12h) for 48 h postoperation for the reduction of pain and also a single 84,000-IU dose of Bicilline intramuscularly for the prevention of infection after surgery. The rabbits were given a 4-week recovery period before further manipulation.
Animal infection.
Prior to the infection experiment, a 0.5-ml sample of abscess fluid was removed from the Wiffle ball to determine if the abscess fluid was sterile. Rabbits with bacteria present in the abscess fluid before inoculation of S. pneumoniae were excluded from the study. No rabbits were excluded from this study due to a prior infection within the Wiffle ball. The Wiffle balls were infected by injection of 2 ml of an S. pneumoniae (1.5 × 107 CFU/ml) saline suspension directly into the Wiffle balls. The rabbits remained infected without treatment for 2 days.
Treatment.
Antimicrobial agents were given through a no. 16 French urethral catheter passed orally into the stomach of each rabbit at 48 h after the bacterial infection. Three doses (5.5, 22, and 32 mg/kg) of levofloxacin and three doses (6.5, 26, and 42 mg/kg) of moxifloxacin were given once daily for 7 days to create a range of different drug exposures. Untreated rabbits were given saline following the same dosing schedule as controls. Five rabbits were used for each of the study aims.
Bacterial density assessment.
A 0.5-ml sample of abscess fluid was removed from the Wiffle ball every morning prior to the daily drug administration during the treatment period. At the end of the treatment period, the sampling was continued once a day for an additional 7 days. The abscess fluid samples were subjected to serial 10-fold dilutions (1:100 to 1:107) with chilled, sterile 0.9% NaCl and were plated onto blood agar plates for overnight incubation at 37°C for manual colony counts and subsequent MIC and minimum bactericidal concentration (MBC) determinations. The detection limit was 50 CFU/ml.
Susceptibility testing of recovered organism.
The emergence of resistance under the dosing regimens studied was assessed by measuring the MICs and MBCs for S. pneumoniae recovered from abscess fluid over the 7-day treatment period and the 7-day posttreatment observation period. The MICs and MBCs of both moxifloxacin and levofloxacin for the test strain were determined in duplicate using the broth microdilution technique established by the National Committee for Clinical Laboratory Standards (7). Briefly, the MIC of each antimicrobial agent was determined in Mueller-Hinton broth supplemented with 5% sheep blood with an inoculum of 106 CFU/ml, and the testing agent was added in twofold dilutions. Microtiter plates were incubated at 37°C for 20 h. The MICs were the lowest concentrations where no visible growth was observed. The MBCs were determined by plating 5 μl from each microtiter well without visible growth and 5 μl from controls on 5% blood agar plate for another 24 h of incubation. The MBCs were the lowest concentration that reduced the number of CFU of the inoculum by at least 99.9%.
Pharmacokinetic sampling.
Blood samples were collected via the ear marginal vein at 0, 1, 2, 6, 12, and 24 h after the fifth dose. Serum samples were separated by centrifugation at 8,000 × g for 15 min and frozen at −80°C until analyzed. Additional abscess fluid sample (0.5 ml) was also collected from the inserted wiffle ball with a syringe at 0, 2, 6, 12, and 24 h after dosing. Samples were frozen −80°C until drug analysis.
Drug analysis.
Moxifloxacin concentrations in serum were analyzed by a validated high-performance liquid chromatographic (HPLC) method. Equipment included a pump (model 515; Waters, Milford, Mass.), an autosampler (WISP 717 plus; Waters), a fluorescence detector (model 980; Applied Biosystems, Foster City, Calif.), and a chromatography data system (EZChrome Elite; Scientific Software, San Ramon, Calif.). Chromatographic separation was performed with a reverse-phase HPLC column (Nucleosil 100 C18; 4.6 by 250 mm; Alltech, Deerfield, Ill.). The mobile phase consisted of 20% HPLC grade acetonitrile and 80% sodium phosphate buffer (0.01 M, vol/vol) with 0.01 M tetrabutylammonium hydrogen sulfate. The mobile phase flow rate was 1.4 ml/min, and the excitation and emission wavelengths were 290 and 418 nm, respectively.
Moxifloxacin samples were allowed to thaw at room temperature prior to analysis. Ciprofloxacin (internal standard) solution was added to all of the unknowns, as well as the standards. Protein precipitation was accomplished by adding 3 volumes of acetonitrile to the samples, vortexing them for 30 s, and centrifuging them at 3,000 × g for 10 min. A 50-μl volume of 0.2 N NaOH was added to the resultant supernatant, it was vortexed, and 2 ml of dichloromethane was added. The mixture was vortexed for 30 s, and the aqueous layer was separated by centrifugation at 3,000 × g for 10 min and injected into the HPLC column. The standard curve ranged from 0.1 to 2.0 μg/ml, and the limit of quantitation was 0.1 μg/ml. The inter- and intra-assay coefficients of variation were <10%.
The HPLC equipment used for the levofloxacin assay was the same as that listed above, except that the excitation wavelength was set at 290 nm for the fluorescence detector. Chromatographic separation was performed with a reverse-phase HPLC column (Nucleosil 100 C18, 4.6 by 250 mm; Alltech). The mobile phase consisted of 13% HPLC grade acetonitrile and 87% sodium phosphate buffer (0.01 M, vol/vol) with 0.01 M tetrabutylammonium hydrogen sulfate. The mobile-phase flow rate was 1.4 ml/min.
The levofloxacin sample extraction procedure was the same as the procedure used for the moxifloxacin assay, except that lemofloxacin was used as the internal standard. The standard curve ranged from 0.1 to 5.0 μg/ml, and the limit of quantitation was 0.1 μg/ml. The inter- and intra-assay coefficients of variation were <10%.
Pharmacokinetic analysis.
The time course of moxifloxacin and levofloxacin concentrations in serum and the abscess fluid profile were analyzed for each rabbit using the noncompartmental method. Cmax, the maximum concentration measured in serum or abscess fluid, was taken directly from the concentration-time profiles. The elimination half-life in serum or abscess fluid was estimated by the expression −ln2/β, where β is the slope of the elimination regression line. AUC0–24, the area under the concentration-time curve from 0 to 24 h, was calculated by the trapezoidal rule.
Pharmacodynamic analysis.
The efficacy of moxifloxacin and levofloxacin, defined as the bacterial killing rate, was calculated as the decrease in bacterial numbers over 7 days of treatment compared with the pretreatment bacterial levels. For each rabbit, the rate at which the log CFU per milliliter decreased over the 7-day dosing period was regarded as the drug efficacy and was estimated as the slope of a linear regression of the log CFU per milliliter versus time plot. The dose-response effect of each antibiotic was investigated by fitting the AUC/MIC ratio versus efficacy and peak/MIC ratio versus efficacy curves with a sigmoid Emax model.
Statistical analysis.
All results are provided as means ± standard deviations. Spearman's rank correlation coefficient was calculated to evaluate the relationship between drug efficacy and the AUC/MIC ratio and the peak/MIC ratio in serum and in abscess fluid. The sigmoid Emax model was used to further characterize the relationships between the above-mentioned variables. A P value of <0.05 was considered statistically significant.
RESULTS
Fluroquinolone concentrations in infected rabbits.
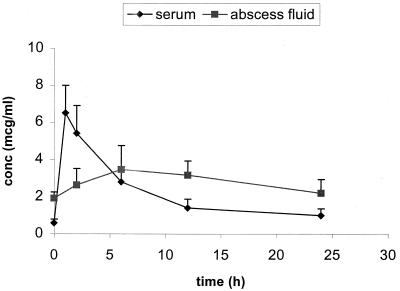
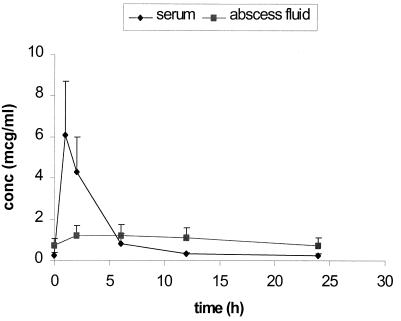
Steady-state serum and abscess fluid Cmax and AUC0–24 for the infected rabbits are summarized in Table 1. The time to maximum drug concentration in serum of both fluroquinolones was approximately 1 h. The estimates of drug half-life in serum were 8.0 ± 2.5 and 5.8 ± 2.5 h for moxifloxacin and levofloxacin, respectively. The penetration and elimination pharmacokinetics of moxifloxacin and levofloxacin in abscess fluid were considerably dampened compared with the pharmacokinetics in serum. The mean Cmax in abscess fluid/Cmax in serum ratios were 0.4 and 0.2 for moxifloxacin and levofloxacin, respectively. The time to maximum concentration of both fluroquinolones in abscess fluid was approximately 6 h. The drug penetration ratios calculated by the AUC0–24 in abscess fluid/AUC0–24 in serum were 113 ± 37 and 84 ± 20 for moxifloxacin and levofloxacin, respectively. Two typical concentration-time profiles for moxifloxacin at 26 mg/kg and levofloxacin at 22 mg/kg are presented in Fig. 1 and 2, respectively.
TABLE 1.
Multiple-dose pharmacokinetics of moxifloxacin and levofloxacin in abscess fluid and serum of five S. pneumoniae-infected rabbitsa
| Drug and dose (mg/kg) | Serum
|
Abscess fluid
|
||
|---|---|---|---|---|
| AUC (μg · h/ml) | Cmax (μg/ml) | AUC (μg · h/ml) | Cmax (μg/ml) | |
| Moxifloxacin | ||||
| 6.5 | 9.2 ± 5.8 | 1.7 ± 0.8 | 11.5 ± 6.0 | 0.7 ± 0.4 |
| 26 | 53.1 ± 12.8 | 6.5 ± 1.5 | 68.9 ± 19.4 | 3.5 ± 1.3 |
| 42 | 96.0 ± 16.8 | 15.5 ± 6.1 | 71.4 ± 22.7 | 4.2 ± 2.2 |
| Levofloxacin | ||||
| 5.5 | 7.8 ± 4.2 | 1.6 ± 1.0 | 5.2 ± 2.3 | 0.3 ± 0.1 |
| 22 | 25.3 ± 9.8 | 6.1 ± 2.6 | 24.8 ± 10.2 | 1.2 ± 0.6 |
| 32 | 70.1 ± 17.7 | 10.6 ± 3.8 | 52.5 ± 23.1 | 2.9 ± 1.3 |
The values shown are means and standard deviations.
FIG. 1.
Steady-state concentration (conc)-time profile of moxifloxacin (26 mg/kg Q24h) in S. pneumoniae-infected rabbits (n = 5). mcg, micrograms.
FIG. 2.
Steady-state concentration (conc)-time profile of levofloxacin (22 mg/kg Q24h) in S. pneumoniae-infected rabbits (n = 5). mcg, micrograms.
Bacterial density change within the Wiffle ball.
The efficacy of each fluroquinolone was assessed by evaluating the number of viable bacteria in the abscess over the 7-day dosing period. The bacterial density in the abscess fluid of untreated controls remained at approximately 107 CFU/ml, which is comparable to the beginning bacterial load. Table 2 summarizes the percentage of animals with sterile abscess fluid over the 7-day treatment period. Moxifloxacin treatment of the S. pneumoniae-infected rabbits resulted in a rapid, dose-dependent reduction in the number of S. pneumoniae organisms in the abscess fluid. Moxifloxacin at 6.5 mg/kg reduced the log CFU per milliliter in abscess fluid by 4.2 ± 2.2 over the 7 days of dosing, and only one of five rabbits had viable counts below the detection limit. Five of five rabbits receiving moxifloxacin at 26 mg/kg had viable counts below the detection limit after six doses, with a reduction in bacterial log CFU per milliliter in abscess fluid of 5.8 ± 0.4. After four doses of moxifloxacin at 42 mg/kg, five of five rabbits had viable counts below the detection limit, with a reduction in the log CFU per milliliter in abscess fluid of 5.4 ± 0.4. By comparison, levofloxacin at 5.5, 22, and 32 mg/kg had reduced the log CFU per milliliter in abscess fluid by 2.8 ± 0.7, 5.1 ± 1.3, and 4.6 ± 1.3, respectively, over the 7 days of dosing. One of five rabbits administered levofloxacin at 5.5 mg/kg, four of five rabbits given levofloxacin at 22 mg/kg, and three of five rabbits given levofloxacin at 32 mg/kg had viable counts below the detection limit after 7 doses, respectively. For all of the animals with viable counts below the detection limit, no regrowth was detected during the 7-day posttreatment period.
TABLE 2.
Percentages of rabbits with abscess fluid bacterial densities under the detection limita at various time points
| Drug and dose (mg/kg) | % of rabbits (n = 5) with sterile abscess fluid at:
|
|||
|---|---|---|---|---|
| Day 4 | Day 5 | Day 6 | Day 7 | |
| None (control) | 0 | 0 | 0 | 0 |
| Moxifloxacin | ||||
| 6.5 | 0 | 20 | 20 | 20 |
| 26 | 80 | 80 | 100 | 100 |
| 42 | 100 | 100 | 100 | 100 |
| Levofloxacin | ||||
| 5.5 | 0 | 20 | 20 | 20 |
| 22 | 40 | 40 | 40 | 80 |
| 32 | 40 | 60 | 60 | 60 |
50 CFU/ml.
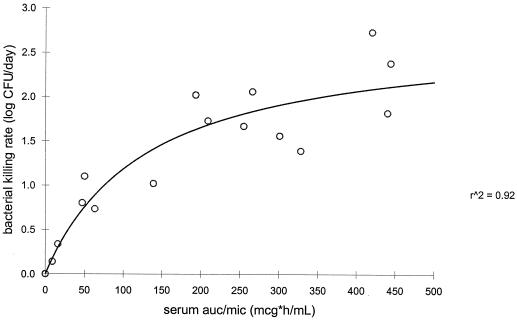
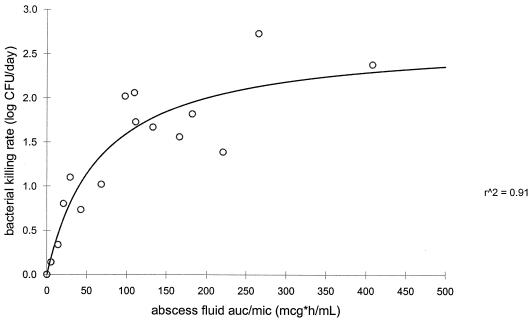
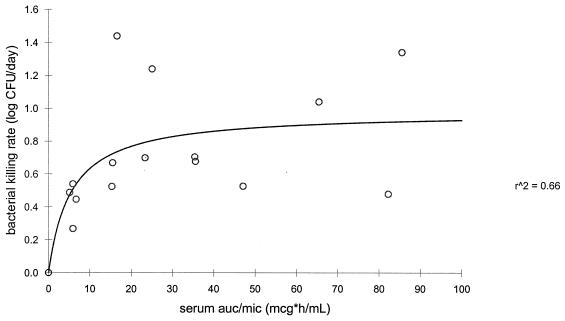
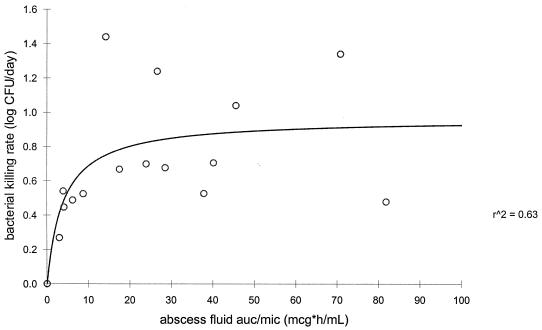
The rate of bacterial killing was calculated by linear fitting of the log CFU per milliliter versus time curve for each rabbit. Regression analysis showed a significant correlation between the bacterial killing rate and the AUC/MIC ratio in serum and abscess fluid and between the serum and abscess fluid peak/MIC ratios for both fluroquinolones (P < 0.05). Figures 3 and 4 represent the sigmoid-Emax model evaluation of the moxifloxacin AUC0–24 in serum/MIC ratio and the AUC0–24 in abscess fluid/MIC ratio as a function of the rate of bacterial killing, respectively. Figures 5 and 6 represent the sigmoid-Emax relationship between the rate of bacterial killing and the AUC0–24 in serum/MIC ratio and the AUC0–24 in abscess fluid/MIC ratio of levofloxacin, respectively. An Emax of 2.7 ± 0.4 log CFU/day was observed for moxifloxacin under the drug exposure regimen studied. By comparison, an Emax of 1.0 ± 0.2 log CFU/day was observed for levofloxacin under the drug exposure regimen studied.
FIG. 3.
Sigmoid-Emax model evaluating the bacterial killing rate as a function of the serum AUC/MIC ratio of moxifloxacin. mcg, micrograms; r^2, r2.
FIG. 4.
Sigmoid-Emax model evaluating the bacterial killing rate as a function of the abscess fluid AUC/MIC ratio of moxifloxacin. mcg, micrograms; r^2, r2.
FIG. 5.
Sigmoid-Emax model evaluating the bacterial killing rate as a function of the serum AUC/MIC ratio of levofloxacin. mcg, micrograms; r^2, r2.
FIG. 6.
Sigmoid-Emax model evaluating the bacterial killing rate as a function of the abscess fluid AUC/MIC ratio of levofloxacin. mcg, micrograms; r^2, r2.
Susceptibility of organism recovered from the Wiffle ball.
The bacterial strain remained susceptible to both fluroquinolones throughout the study period, as the MIC and MBC of moxifloxacin were 0.25 and 0.5 μg/ml, respectively, compared to the MIC of 1 μg/ml and the MBC of 2 μg/ml for levofloxacin.
DISCUSSION
The isolation of S. pneumoniae strains resistant to fluroquinolones has been reported. The resistance mechanism suggests that the development of resistance is related to mutations in the genes parC, parE, and gyrA (5, 9, 12). In addition, mutations conferring resistance to fluroquinolones appear to occur in a stepwise process, with the first-step mutation usually conferring low-level resistance and subsequent mutations leading to higher levels of resistance (10). In view of the clinical problem of bacterial resistance to fluoroquinolone therapy, we studied the role of moxifloxacin and levofloxacin pharmacokinetics in the selection of resistance via phenotypic expression using the rabbit tissue cage model.
In the present study, we measured the MIC/MBC ratio on a daily basis throughout the entire study period for treatment regimens involving a range of drug exposures that simulate the pharmacokinetics in humans. The three moxifloxacin dosing regimens used produced a range of AUC/MIC and peak/MIC ratios of 9.2 to 444 and 1.3 to 102, respectively. By comparison, a range of AUC/MIC ratios of 5.1 to 85.5 and a range of peak/MIC ratios of 0.9 to 14.8 were achieved by the three levofloxacin dosing regimens used. Development of resistance, as assessed by MIC change, was not observed with either fluoroquinolone, independently of the drug exposure studied. S. pneumoniae remains sensitive to both drugs, with moxifloxacin and levofloxacin MIC/MBC ratio of 0.25/0.5 and 1/2, respectively. Using a rat granuloma pouch model, Dalhoff et al. studied the emergence of resistance to moxifloxacin in ciprofloxacin-susceptible Staphylococca aureus and in ciprofloxacin- and methicillin-resistant S. aureus, as well as in S. pneumoniae. They did not observed any development of resistance despite suboptimal moxifloxacin dosing (A. Dalhoff, M. Heidtmann, S. Obertegger, et al., 8th Int. Congr. Infect. Dis., poster 47.003, 1998). In addition, several reports have shown that the mutation rate for resistance to moxifloxacin was very low (3, 11; A. Pong, K. S. Thompson, E. S. Moland, et al., 37th Intersci. Conf. Antimcrob. Agents Chemother., poster C-85, 1997). Data derived from the study of Drugeon et al. have shown a very low potential of levofloxacin to select for fluroquinolone-resistant S. pneumoniae in vitro and in vivo (4). Our data are in accordance with these reports, suggesting that moxifloxacin and levofloxacin have a low propensity to initiate and drive the development of drug-resistant S. pneumoniae. These observations are likely to be related to rapid bacterial killing activity, good drug penetration, and low spontaneous resistance mutation frequencies in the context of the starting inoculum used in this study.
In the present study, moxifloxacin at 26 mg/kg produced a mean steady-state AUC of 53.1 ± 12.8 μg · h/ml, which is comparable to the AUC of 48 μg · h/ml observed in human: given 400 mg of moxifloxacin once daily. The mean AUC achieved with levofloxacin dosing at 32 mg/kg in the current study was 70.1 ± 17.7 μg · h/ml, which exceeds the target human AUC of 48 μg · h/ml after administration of 500 mg once daily. As shown in Table 2, five of five rabbits receiving moxifloxacin at 26 mg/kg had viable counts below the detection limit after six doses while only three of five rabbits given levofloxacin at 32 mg/kg had viable counts below the detection limit after seven doses. This observed greater bacterial killing rate with moxifloxacin dosing is likely to be related to its greater intrinsic bactericidal potency against the bacterial isolate studied, i.e., a MIC of 0.125 versus 1 μg of levofloxacin per ml.
Many of the in vitro and in vivo assessments of the activity of antibacterial agents have measured the logarithmic decrease in the bacterial CFU count after the exposure of bacteria to antibiotics for a certain period of time (e.g., 24 h). These endpoint measurements are, by nature, discrete and may not provide information on the dynamic interaction between a drug and bacteria over time. In order to assess the potential time-dependent killing rate over the entire treatment period, we calculated the bacterial killing rate from the log CFU per milliliter versus time curve by linear regression assuming the first-order killing exerted by antibiotics. Furthermore, we delineated the relationship between the bacterial killing rate and drug exposure by nonlinear regression using the sigmoid Emax model. Regression analysis showed a good correlation between the bacterial killing rate and the serum AUC/MIC ratio, the serum peak/MIC ratio, the abscess fluid AUC/MIC ratio, and the abscess fluid peak/MIC ratio for moxifloxacin with a correlation coefficient of approximately 0.9. We did, however, observe a large levofloxacin response variability in the current study, leading to a correlation coefficient of only 0.6 despite significant correlation (P < 0.05). The levofloxacin response variability is certainly a reflection of the complexity of the in vivo system and difficult to explain, but we think it might also be related, at least in part, to the larger variability in pharmacokinetics observed in this study.
We have to point out two issues related to this unique animal model. First, we assessed drug exposure by determining steady-state pharmacokinetics in the current investigation. The underlying assumption was that intraindividual variance in pharmacokinetics is relatively small throughout the course of treatment; in another words, the impact of the evolving microbiological status of the host over the treatment period on the drug pharmacokinetic disposition is considered to be constant. This assumption apparently needs to be verified. Second, the anaerobic characteristics of the abscess model might play a role in the interaction among a drug, bacteria, and the host defense system. Further investigation of this potential influence is warranted.
In conclusion, data derived from this study show that moxifloxacin and levofloxacin exhibit rapid, concentration-dependent killing of S. pneumoniae in vivo. Drug exposure (AUC/MIC and peak/MIC ratios) correlated closely with the antimicrobial efficacy of both drugs. In the context of the treatment regimens studied, which conferred a range of drug exposures that simulate the pharmacokinetics in humans, the antimicrobial effect of moxifloxacin appears to be greater than that of levofloxacin and the potential to select for fluoroquinolone-resistant S. pneumoniae is very low for both agents in this animal model.
ACKNOWLEDGMENT
This work was supported by a grant from the Bayer Corporation.
REFERENCES
- 1.Aldridge K E, Ashcraft D. Comparison of the in vitro activities of BAY 12-8039, a new quinolone, and other antimicrobials against clinically important anaerobes. Antimicrob Agents Chemother. 1997;41:709–711. doi: 10.1128/aac.41.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauernfeind A. Comparison of the antimicrobial activities of the quinolones BAY 12-8039, gatifloxacin (AM 1155), trovafloxacin, clinafloxacin, levofloxacin and ciprofloxacin. J Antimicrob Chemother. 1997;40:639–651. doi: 10.1093/jac/40.5.639. [DOI] [PubMed] [Google Scholar]
- 3.Dalhoff A, Petersen U, Endermann R. In vitro activity of BAY12–8039, a new fluoroquinolone. Chemotherapy. 1996;42:410–425. doi: 10.1159/000239474. [DOI] [PubMed] [Google Scholar]
- 4.Drugeon H B, Juvin M E, Bryskier A. Relative potential for selection of fluoroquinolone-resistant Streptococcus pneumoniae strains by levofloxacin: comparison with ciprofloxacin, sparfloxacin and ofloxacin. J Antimicrob Chemother. 1999;43(Suppl. C):55–59. doi: 10.1093/jac/43.suppl_3.55. [DOI] [PubMed] [Google Scholar]
- 5.Janoir C, Zeller V, Kitzis M-D, Moreau N J, Gutmann L. High-level fluoroquinolone resistance in Streptococcus pneumoniae requires mutations in parC and gyrA. Antimicrob Agents Chemother. 1996;40:2760–2764. doi: 10.1128/aac.40.12.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lister P D, Sanders C C. Pharmacodynamics of levofloxacin and ciprofloxacin against Streptococcus pneumoniae. J Antimicrob Chemother. 1999;43:79–86. doi: 10.1093/jac/43.1.79. [DOI] [PubMed] [Google Scholar]
- 7.National Committee for Clinical laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically fourth edition. Approved standards. Document M7–A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 8.Niederman M S. Introduction: disease management of pulmonary infections. Chest. 1998;113(Suppl.):165S. [PubMed] [Google Scholar]
- 9.Pan X-S, Ambler J, Mehtar S, Fisher L M. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40:2321–2326. doi: 10.1128/aac.40.10.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perichon B, Tankovic J, Courvalin P. Characterization of a mutation in the parE gene that confers fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:1166–1167. doi: 10.1128/aac.41.5.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souli M, Wennersten C B, Eliopoulos G M. In vitro activity of BAY12–8039, a new fluoroquinolone, against species representative of respiratory tract pathogens. Int J Antimicrob Agents. 1998;10:23–30. doi: 10.1016/s0924-8579(98)00020-x. [DOI] [PubMed] [Google Scholar]
- 12.Tankovic J, Perickon B, Duval J, Courvalin P. Contribution of mutations in gyrA and parC genes to fluoroquinolone resistance of mutants of Streptococcus pneumoniae obtained in vivo and in vitro. Antimicrob Agents Chemother. 1996;40:2505–2510. doi: 10.1128/aac.40.11.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomasz A. Antibiotic resistance in Streptococcus pneumoniae. Clin Infect Dis. 1997;24:S85–S88. doi: 10.1093/clinids/24.supplement_1.s85. [DOI] [PubMed] [Google Scholar]
- 14.Wise R, Brenwaid N, Gill M, Fraise A. Streptococcus pneumoniae resistance to fluoroquinolone. Lancet. 1996;348:1660. doi: 10.1016/s0140-6736(05)65724-8. [DOI] [PubMed] [Google Scholar]