Abstract
Botanical carnivory is a novel feeding strategy associated with numerous physiological and morphological adaptations. However, the benefits of these novel carnivorous traits are rarely tested. We used field observations, lab experiments, and a semi-natural experiment to test prey capture function of the marginal spikes on snap traps of the Venus flytrap (Dionaea muscipula). Our field and laboratory results suggested inefficient capture success: fewer than 1 in 4 prey encounters led to prey capture. Removing the marginal spikes decreased the rate of prey capture success for moderate-sized cricket prey by 90%, but this effect disappeared for larger prey. The nonlinear benefit of spikes suggests that they provide a better cage for capturing more abundant insects of moderate and small sizes, but may also provide a foothold for rare large prey to escape. Our observations support Darwin’s hypothesis that the marginal spikes form a ‘horrid prison’ that increases prey capture success for moderate-sized prey, but the decreasing benefit for larger prey is unexpected and previously undocumented. Thus, we find surprising complexity in the adaptive landscape for one of the most wonderful evolutionary innovations among all plants. These findings enrich understanding of the evolution and diversification of novel trap morphology in carnivorous plants.
Keywords: snap-trap, carnivorous plant, prey capture performance, novelty, key innovation, exaptation
Introduction
The origins of novel structures remain an important and poorly understood problem in evolutionary biology (Mayr 1960; Mozcek 2008). Novel traits are often key innovations providing new ecological opportunities (Maia et al. 2013; Stroud and Losos 2016; Wainwright et al. 2012; Martin and Wainwright 2013). Despite the importance of these traits, our understanding of the adaptive value of novel structures is often assumed and rarely tested directly. Frequently, this is because it is difficult or impossible to manipulate the trait without impairing organismal function in an unintended way; however, many carnivorous plant traits do not present this obstacle.
Botanical carnivory is a novel feeding strategy that has evolved at least nine separate times in over 700 species of angiosperms, typically in areas with severely limited nitrogen and phosphorus (Ellison 2006; Givnish 2015; Givnish et al. 1984; Król et al. 2012, Roberts and Oosting 1958). Pitfall traps evolved independently at least 6 times and sticky traps 5 times. However, snap traps have most likely evolved only once in the ancestral lineage leading to the aquatic waterwheel (Aldrovandra vesiculosa) and Venus flytrap (Dionaea muscipula), which is sister to the sundews (Drosera spp.) and within the Caryophyllales (Cameron 2002, Givnish 2015, Walker et al. 2017). Multiple hypotheses have been proposed for why snap traps evolved including the ability to capture larger prey, capture prey more quickly, or more completely digest prey (Darwin 1875; Gibson and Waller 2009). However, these hypotheses have rarely been tested except for a few field studies documenting the size and diversity of arthropod prey (Jones 1923; Gibson 1991; Hutchens and Luken 2015; Youngsteadt et al. 2018).
The marginal spikes found in Dionaea are modified trichomes that extend from the margin of the trap lobes. These spikes are homologous to the trichomes of sundews, but do not exude any sticky resin and have lost the mucus glands in these spikes (Gibson and Waller 2009). Darwin was the first to document evidence for carnivory in flytraps and sundews in a series of careful experiments and proposed that the marginal spikes of flytraps enhance prey capture success by providing a cage-like structure around the top of the trap that contains the prey (Darwin 1875; Gibson and Waller 2009). Darwin (1875) also hypothesized that while small insects will be able to escape between the spikes, a moderately sized insect will be “pushed back again into its horrid prison with closing walls” (page 312), and large, strong insects will be able to free themselves. Determining the function of the marginal spikes is important for understanding the rarity of mechanical snap traps.
Traits that enhance prey capture ability are expected to be strongly selected for given the benefits of additional nutrients and the energetic and opportunity costs associated with a triggered trap missing its intended prey. The marginal spikes provide a novel function that potentially increases prey capture rate and minimizes the costs associated with a failed trap closing event. Nutrients from insect prey increase the growth rate of Venus flytraps (Darwin 1878; Roberts and Oosting 1958) at a cost of lower photosynthetic efficiency of carnivorous plants compared to other plants (Ellison and Gotelli 2009; Pavlovic et al. 2009). The traps are triggered by an action potential when specialized trigger hairs are stimulated (Volkov et al. 2008, 2009) and close as quickly as 100 milliseconds forming a cage around the prey item (Poppinga et al. 2013). If the trap fails to capture an insect, it takes between two and three days for the trap to reopen, during which time it is unable to be used for prey capture. Beyond the energy expended to close a trap and the opportunity cost of a miss, there is a cost associated with declining trap performance and trap death. Traps that have closed and reopened have lower subsequent trap closure speeds and trap gape angle (Stuhlman 1948). Additionally, after a few closings, traps rapidly die.
We measured prey capture efficiency, trap closure time, and the effect of marginal spikes using field observations of wild Venus flytraps, laboratory experiments, and a semi-natural experiment. By testing the prey capture ability of plants with intact spikes and ones with the spikes clipped off, we assessed the novel function of the marginal spike cage for prey capture.
Methods:
Field Data Collection
The Green Swamp Preserve, NC, USA is one of the last remaining eastern pine savannah habitats containing endemic flytraps. To estimate prey capture rates, we identified individual plants (n = 14) and recorded the number of traps that fell into four categories: alive and closed, dead and closed, alive and open, and dead and open. All closed traps (n = 100) had their length, defined here as the widest point of the lobes on the long axis, recorded with digital calipers. We used a flashlight to illuminate the trap from behind making anything inside the trap visible as a silhouette. If the trap contained something it was assigned a value of 1 for “catch” and if it contained nothing it was assigned a 0 for “miss”. We also noted when a trap was closed on another trap or contained debris inside such as sticks or grass (these were considered a miss; n = 7). Both logistic regression and a generalized linear mixed-effects model (package lme4; Bates et al. 2015) in R using RStudio (R Statistical Programming Group 2018; RStudio Team 2015) were used to determine if trap length had a significant effect on prey capture rate in the field.
Laboratory prey capture experiments
Plants used in lab experiments were tissue-cultured and purchased from commercial suppliers (bugbitingplants.com; stores.ebay.com/joelscarnivorousplants/). The plants were maintained in 40 liter terraria under high-output fluorescent lighting (14-hour daylight cycle) with 8 cm pots submerged in 1-4 cm of reverse osmosis water at all times. Throughout the duration of the experiments, the plants were kept at ambient temperatures under the lights, ranging from 35° C during the day to 22° C at night), and 50 – 90% humidity, similar to natural conditions in the field during summer months. Crickets were purchased from Petsmart and kept in 4-liter plastic containers with shelter, water, and a complete diet (Fluker’s cricket food).
To assess the adaptive role of marginal spikes, we set up prey capture arenas (Fig. 1). Each arena consisted of one plant in a petri dish of distilled water, one cricket of known length (range: 0.7 cm – 2.3 cm) and mass (range: 0.026 g – 0.420 g), cricket food, and a ramp from the dry bottom of the arena to the plant. The relationship between prey mass and catch rate was plotted to ensure the relationship was linear and account for non-isometric power scaling in cricket hind legs. Only healthy crickets with all six legs were used for prey capture trials. Orthopterans make up approximately 10% of flytrap prey in the wild (Ellison and Gotelli 2009; CHM pers. obs.), and this may represent an underestimate of how often they visit plants in the wild because they may be more likely to escape than less powerful prey like ants or small beetles. The crickets used in this study ranged between 7 mm and 23 mm, which is within the natural distribution of orthopteran prey sizes in the Green Swamp in which very large individuals were observed (reaching at least 54mm; CHM pers. obs.). All closed traps were initially marked with a permanent marker. We checked the plants for closed traps after three days and after one week. Every closed, empty trap was recorded as a 0 for “miss” and every closed trap that contained prey was recorded as a 1 for “catch”. Following one unmanipulated trial with the spikes intact, we used scissors to clip the spikes from every trap on the plant (Fig. 1). The plants were then allowed to recover for a week until the traps reopened. After the traps reopened, we placed each plant through a second trial with a new cricket. We performed 51 prey capture trials (34 plants total, 17 used only for unmanipulated trials, and 17 used once before and after spike removal). Only 1 trial resulted in no traps triggered over the full week. We also set up control trials (n = 5) with a newly dead cricket placed on the bottom of the tank and negative controls with no cricket at all (n = 2) to ensure that any experimental trap closures were triggered by the cricket and not spontaneous.
Figure 1.
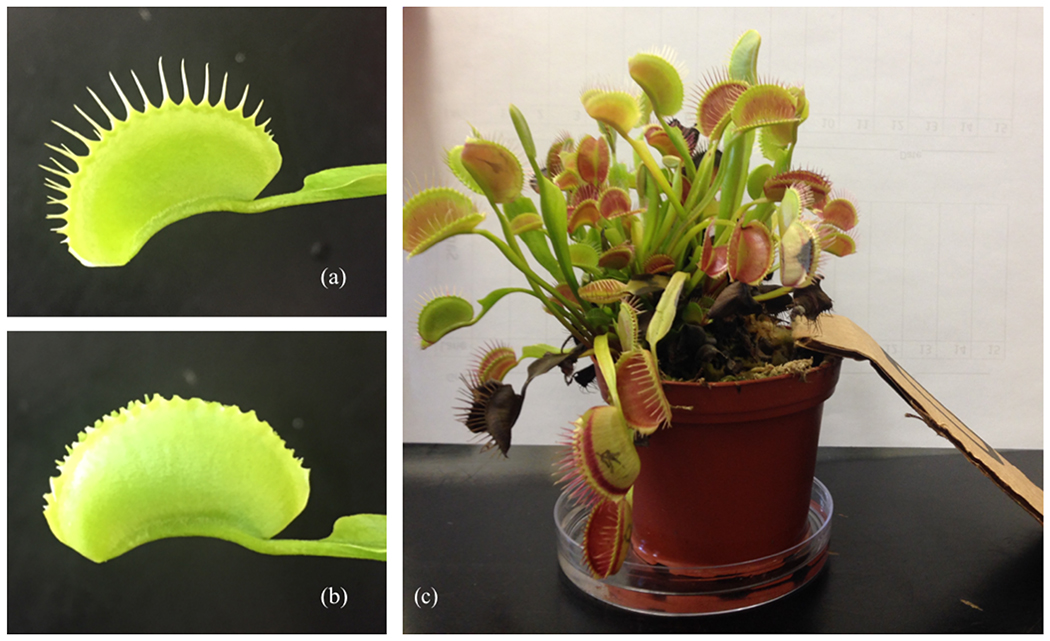
(a) Intact trap; (b) trap with the marginal spikes removed; (c) representative prey capture arena containing one plant, one cricket, a ramp, and a petri dish of water.
To analyze the relationship between prey mass, treatment, trap length, and prey capture success we used multiple logistic regression models in R and generalized linear mixed-effect models (GLMMs) using the lme4 package (Bates et al. 2015). Plant ID was included as a random effect to account for variation in plant-level performance in addition to the fixed effects of treatment, prey mass, and trap length with the binomial response variable of prey capture success for each closed trap during the observation period. For the GLMMs, we used Akaike information criteria with correction for small sample size (AICc) to compare models. We chose prey capture success as our proxy for performance and fitness due to the evidence that the growth rate of flytraps is greatly enhanced by ingesting insect prey (Schulze et al. 2001). We visualized changes in the performance landscape due to removing marginal spikes by estimating thin-plate splines for the binomial prey capture success data for trials with and without spikes. We fit splines by generalized cross-validation using the Tps function in the Fields package (Nychka et al. 2015) in R (R Core Team 2018).
Semi-Natural Experiment
To expand upon data from the laboratory prey capture experiments, we planted 22 flytraps in the North Carolina Botanical Garden, with half the traps on each plant with intact marginal spikes and the other half with the spikes removed. Traps were randomly chosen for removal of spikes and allowed to reopen in laboratory terraria before placement in the field. Plants were kept in an open, forested area of the gardens in standing water with ramps for terrestrial arthropod access for a period of 4 weeks. Catch data was collected after each week. Catch data and trap length data was recorded in the same way as the laboratory experiments and all captured prey items had their length recorded in the laboratory. Identification of captured prey was recorded if possible given the amount of digestion. The effect of the marginal spikes and trap length on prey capture were assessed using a GLMM and results from the GLMM were combined with results from laboratory experiments using Fisher’s method.
Trap Closure Time
We measured trap closure time as a function of number of previous trap closures in order to characterize the effect of using plants for manipulated trials following control trials. Trap closure times were measured for ten traps on each of seven tissue-cultured plants (not previously used for prey capture experiments). Measurements of closing speed were taken on the first closure for all traps, and then recorded for each subsequent closure until the trap spontaneously died (maximum of four closings per trap). Trigger hairs on each trap were stimulated with a toothpick and high-speed video was recorded at 960 frames per second using a Sony DSC-RX 10 camera. The video sequences were then imported into Adobe Photoshop CC and converted into an image sequence to obtain the total duration of trap closure. The number of frames from first movement until the marginal spikes began to overlap was used to determine trap closure time.
Results:
Field Prey Capture Rates
In the Green Swamp, only 24% of closed wild flytraps contained prey. This number represents a high-end estimate because anything inside the plants was counted as a catch, despite the possibility that the object was a piece of debris instead of an insect or spider. Of the 98 closed traps recorded, 8 were closed around obvious plant debris, and 2 contained identifiable prey (1 ant and 1 spider). 55% ± 5% (mean +/− SE) of wild flytraps were open and alive, therefore able to capture prey. The percentage of closed traps that contained prey ranged from 0% to 50% for any individual plant. Five plants had a success rate of 0%, five were between 0-33%, and four had a success rate between 34-50%.
Laboratory Prey Capture Rates
Similarly in the lab, only 16.5% of flytraps successfully captured prey out of all closed traps among unmanipulated plants. Only 5.8% of flytraps with marginal spikes removed on these same plants successfully captured prey. Tissue damage due to clipping marginal spikes quickly healed and clipped traps reopened within 4 days; thus, this disparity does not appear to be due to any deleterious effect of tissue damage. Furthermore, no differences in trap closing speeds, health, or growth rates of manipulated traps were apparent. Indeed, marginal teeth began to regrow within approximately one week after removal, suggesting that we underestimated the effect of spike removal on prey capture since spikes were partially regrown by the end of each trial.
Removing marginal spikes reduced the odds of prey capture by 90% relative to unmanipulated traps from the same plant while controlling for prey mass and trap length (effect of manipulation: P = 0.002; linear mixed-effect model relative to model without treatment variable: ΔAICc = 11). At large prey sizes and large trap lengths the beneficial effects of marginal spikes on prey capture disappeared (note that spline SE crosses at large prey and trap sizes; Fig. 2). Removal of the spikes also leads to a depression of the prey-capture landscape, particularly at small prey and trap sizes (Fig. 3).
Figure 2.
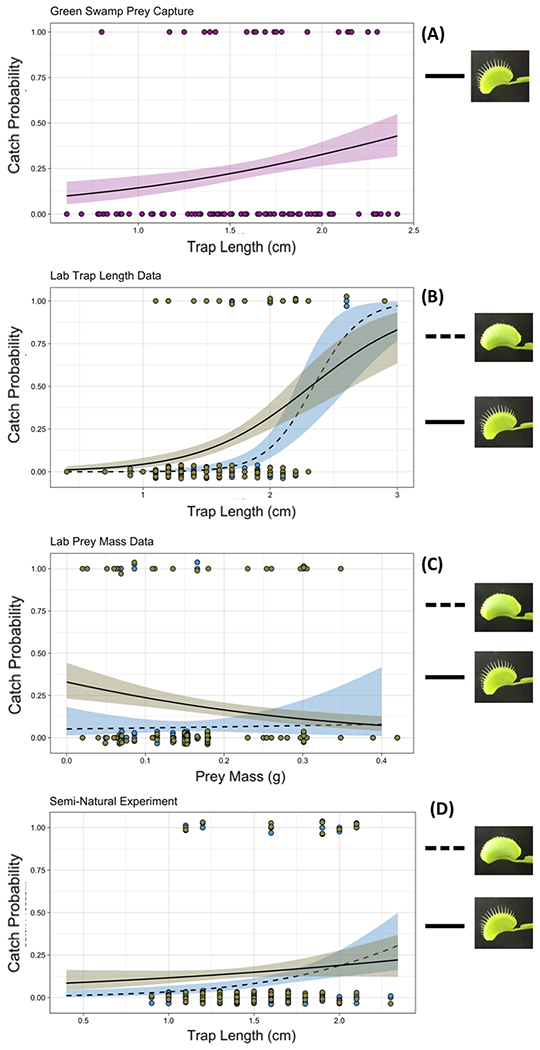
(A) Prey capture success of wild plants in the Green Swamp Preserve, NC as a function of trap length (measured to the nearest 0.01 cm). (B) Prey capture success of laboratory plants as a function of trap length (measured to the nearest 0.1 cm) (C) Prey capture success of laboratory plants as a function of prey mass. (D) Prey capture success of plants kept in the North Carolina Botanical Garden as a function of trap length (measured to the nearest 0.1 cm). Lines of best fit were estimated using logistic regression with shaded areas corresponding to ± 1 SE. Each point represents one successful (1) or unsuccessful (0) capture by a flytrap, often resulting in multiple failed captures per cricket mass.
Figure 3.
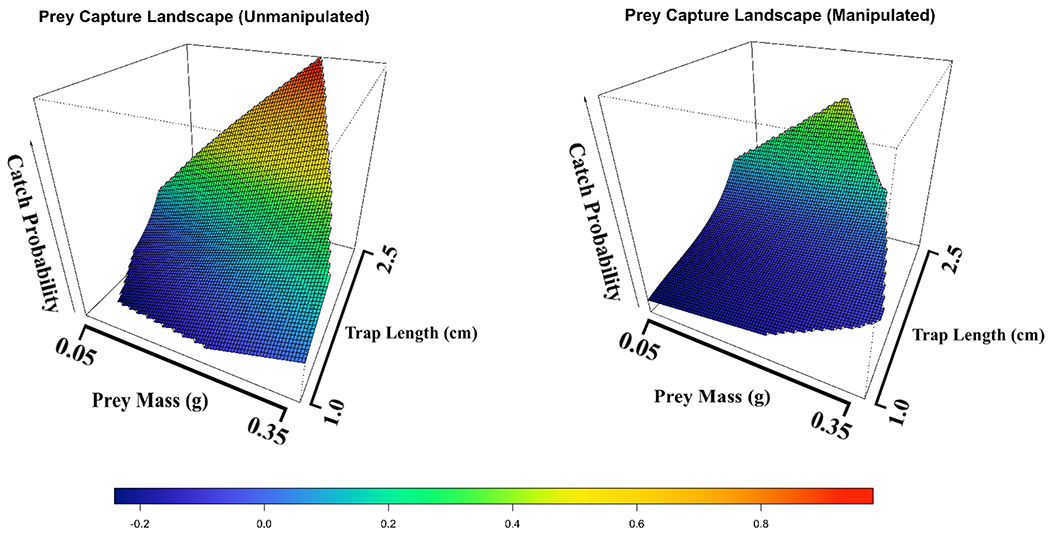
Prey capture performance landscapes for intact plants (left) and manipulated plants (right). Catch probability is on the z axis and represented by the heat colors relative to insect prey mass and trap length plotted in the x-y plane. The performance landscape for plants with marginal spikes removed (B) is greatly depressed at small trap sizes, but is similar at large trap/prey sizes.
Effect of Prey Mass and Trap Length
A linear mixed effect model with prey mass included provided a far better fit to the data than one without (ΔAICc = 15). In the full model, prey mass was a significant predictor of prey capture success (P = 0.0004), with every 0.1 g increase in prey mass corresponding to a 73% decrease in prey capture performance (Fig. 2).
Larger trap size also increased the probability of successful prey capture after controlling for prey size, with every 1 cm increase in trap length increasing the odds of prey capture by 2.9-fold (Table 1). Larger trap size increased prey capture success for both manipulated and non-manipulated plants (Fig 3; logistic regression; manipulated: P = 0.020; non-manipulated: P = 0.003). A linear mixed effect model including trap length provided a much better fit to the data than one without (ΔAICc = 31). For the data from the Green Swamp, a logistic model that assessed each trap as independent found a marginally significant relationship between trap length and prey capture success (P = 0.066). This association was diminished when considering the effect of individual plant ID within a generalized linear-mixed effect model (P = 0.097).
Table 1.
Generalized linear mixed-effect model showing the effect of removing the marginal spikes (manipulation), trap length, and prey mass on prey capture performance (generalized linear mixed model with plant ID included as a random effect). Significant P-values are bolded.
| model term | estimate ± SE | P | df residual |
|---|---|---|---|
| manipulation | −2.32 ± 0.75 | 0.002 | 154 |
| trap length | 4.74 ± 1.08 | 1e−5 | 154 |
| prey mass | −13.36 ± 3.80 | 4e−4 | 154 |
Semi-Natural Experiment
Plants that were kept in the NC Botanical Garden had a prey capture success rate of 13.3% and 9.2% for intact and manipulated plants, respectively. This is the same general trend as in laboratory plants. Furthermore, the spline SE crosses at larger trap sizes, indicating the effect is strongest for moderate-sized prey. However, the effect of manipulation was not significant (P = 0.14; Figure 2). This is likely due to reduced statistical power from numerous trap closures that were triggered by an atypical spring snowfall in 2018. We cannot discern the exact number of closures caused by the snow, but this result in excessive misses (closed, empty traps) following the snowfall. We used Fisher’s method to test the significance of the marginal spikes and trap length given both the laboratory and semi-natural data and found a more significant effect of these variables on prey capture performance (Pspikes = 0.003; Plength = 10e−5).
Trap Closure Time
The average trap closure time was 283 ± 29 ms (mean ± SE) for the first closure, 383 ± 43 ms for the second, 528 ± 62 ms for closure three, and the few that survived to four closures took 772 ± 374 ms (Figure 4; 1-way ANOVA, P = 10−16) Only 38 of the 50 traps survived the second closure, 25 of those 38 made it to the third closure, and 3 traps survived the full four weeks.
Figure 4.
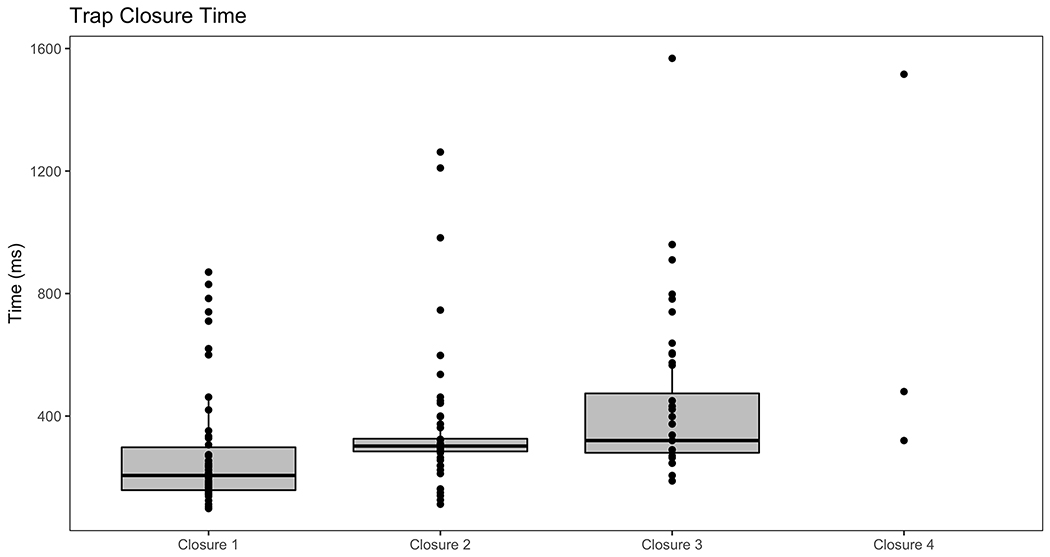
Trap closure times for the first, second, third, and fourth closures on a single trap measured by high-speed video (ANOVA P = 10−16). Data was included for all surviving traps at each level.
Discussion
We provide the first direct test of how prey capture performance is affected by the presence of marginal spikes, trichomes which provide a novel function in Venus flytraps by forming what Darwin described as a “horrid prison”. We found that the marginal spikes are adaptive for prey capture of small and medium sized insects, but not larger insects. In controlled laboratory prey capture trials, 16.5% of trap closures resulted in successful prey capture whereas only 5.8% of trap closures successfully captured prey when marginal spikes were removed (Fig. 2b–c). It is unlikely that this difference is slower closing speeds in the later experimental trials because the difference in trap closure speed from the first to the second closure is 100 ms (Fig. 4), 1/2 of the amount of time it takes a cricket to initiate a jump in response to a stimulus (Tauber and Camhi 1995), and few traps were triggered during both trials. We also found similarly low prey capture rates in the Green Swamp Preserve, NC (Fig. 2), one of few remaining natural habitats of the Venus flytrap, and in semi-natural experiments in the NC Botanical Garden, Chapel Hill, NC (Fig. 2d). Furthermore, only about half of the wild traps were open, alive, and available to catch prey. Given the documented tradeoff between photosynthetic efficiency and carnivory and costs associated with maintaining traps (Ellison and Gotelli 2009; Pavlovic et al. 2009), it is possible that the nutrients acquired from a relatively small number of traps are sufficient to maintain the plant. In support of this hypothesis, other carnivorous plants (Sarracenia purpurea and Darlingtonia californica) sustain themselves with prey capture rates as low as 2% for ants and wasps (Newell and Nastase 1998; Dixon et al., 2005). Alternatively, prey capture rates for tropical pitcher plants (Nepenthes rafflesian) may reach 100% for ants (Bauer et al. 2008). Given that most Venus flytraps fall within this range for pitfall traps (7.9% for traps between 1cm - 2cm, 52.9% for traps > 2cm), additional factors beyond increasing prey capture rates may underlie the origins of mechanical snap traps.
The relatively inefficient prey capture rates found in this experiment are similar to the findings of Bauer et al. (2015) (comparable to Gibson and Waller 2009). They found that inefficient prey capture by pitcher plants allows for recruitment of more prey, which in turn, led to more total insects being captured by the traps. It is possible that the same phenomenon may hold true for more complex traps like that of Dionaea. Adaptive inefficiency could also explain why only half of the traps on the plant are open and available for prey capture.
Dionaea has a generalist trap that is less specialized than other carnivorous plants such as Brocchinia, Nepenthes, or Utricularia (Ellison and Gotelli 2009). Because flytraps do not appear to be specialized for certain insects we must consider the total range of available insect prey when assessing the adaptive role of the marginal spikes. Orthopteran prey used here had an average size of 15.2 mm, which is close to the predicted and experimental prey sizes for peak snap trap returns (Gibson and Waller 2009). Models generated from empirical data even show substantial returns for up to 30 mm prey. In the Green Swamp preserve there are large prey items including arachnids and orthopterans that exceed 30 mm (personal observations). Thus, the range of prey sizes included here (7 mm - 23 mm) is within the range of available insects (< 2 mm -> 50mm). The dramatic difference in prey capture rate of orthopteran prey with the spikes cut off versus intact likely means that the marginal spikes allow the plant to more fully take advantage of the available prey. This holds especially true for medium-sized traps. Medium-sized traps experience both the most rapid decline in capture rate for medium-sized prey and gain the most from having the marginal spikes intact.
Surprisingly, the effect of removing the marginal spikes for medium-sized traps on prey capture success nearly disappears for larger traps in both laboratory experiments and semi-natural field experiments. We observed a possible mechanistic explanation for this counterintuitive result. Crickets are often climbing on the marginal spikes of large traps, and when they trigger them they are able to push against the marginal spikes to pry themselves free. In contrast, when a cricket triggers a large trap with no spikes, it has nothing to use to free itself. Marginal spikes appear to provide leverage for larger insect prey to escape. It is also important to note that the crickets did not appear to use their powerful femurs to pry the trap open, although it is still possible that this occurred but was obscured by the trap lobes. There is also a possible physical explanation for the diminishing benefit of the marginal spikes at large trap sizes. Stuhlman (1948) speculated that friction between the marginal spikes may slow down trap closure. Because the contact area over which friction matters is proportional to the length squared, we would expect disproportionally larger frictional forces as the length of marginal spikes increases on larger traps.
We demonstrated that the novel marginal spikes, forming a ‘horrid prison’, are an adaptation for prey capture with nonlinear effects at larger prey/trap sizes. Furthermore, this system lends itself to tractable experimental work carried out by undergraduate researchers. This project was carried out entirely during a one-semester course-based undergraduate research experience (CURE; Bangera and Brownell 2014) followed by one semester of independent study for three students to perform follow-up experiments. Characterizing the role of adaptive traits aids our understanding of selective forces underlying the diversity of trap types and the rarity of snaptraps, offering insights into the origins of one of the most wonderful evolutionary innovations among all plants.
Acknowledgements
We thank Joseph McGirr, Christine Liloia, John Randall, and Michael Dunn for logistical assistance and helpful discussion that benefited this work. This work was supported by the Quality Enhancement Program at the University of North Carolina at Chapel Hill, which funded this course-based undergraduate research experience (CURE). Kelly Hogan kindly facilitated and encouraged the development of this course. We also thank Blaire Steinwand and John Bruno for helpful feedback on course design.
Footnotes
Data accessibility
All data and R code used for this study is deposited in the Dryad Digital Repository: doi:10.5061/dryad.h8401kn.
References
- Bangera G, and Brownwell SE. 2014. Course-based undergraduate research experiences can make scientific research more inclusive. CBE—Life Sciences Education 13:602–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, and Walker S. 2015. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 67:1–48. [Google Scholar]
- Bauer U, Bohn HF, and Federle W. 2008. Harmless nectar source or deadly trap: Nepenthes pitchers are activated by rain, condensation and nectar. Proceedings of the Royal Society B: Biological Sciences 275:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer U, Federle W, Seidel H, Grafe TU, and Ioannou CC. 2015. How to catch more prey with less effective traps: explaining the evolution of temporarily inactive traps in carnivorous pitcher plants. Proceedings of the Royal Society of London B: Biological Sciences, 282:2014–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron KM, 2002. Molecular Evidence for the Common Origin of Snap-Traps Among Carnivorous Plants. American Journal of Botany 89:1503–1509. [DOI] [PubMed] [Google Scholar]
- Darwin Charles. Insectivorous Plants. 1875. Pages 286–320 in The Complete Works of Charles Darwin Online. Retrieved 2009-08-26.
- Darwin F, 1878. Experiments on the Nutrition of Drosera rotundifolia. Botanical Journal of the Linnean Society, 17:17–31. [Google Scholar]
- Dixon PM, Ellison AM, and J Gotelli N. 2005. Improving the precision of estimates of the frequency of rare events. Ecology 86:1114–1123. [Google Scholar]
- Ellison AM Nutrient Limitation and Stoichiometry of Carnivorous Plants. 2006. Harvard University. [DOI] [PubMed] [Google Scholar]
- Ellison DM, and Gotelli NJ. 2009. Energetics and the evolution of carnivorous plants—Darwin’s ‘Most Wonderful plants in the world. Experiment Botany 60:19–42. [DOI] [PubMed] [Google Scholar]
- Gibson TC, 1991. Differential escape of insect from carnivorous plant traps. American Midland Naturalist 125:55–62. [Google Scholar]
- Gibson TC, and Waller DM. 2009. Evolving Darwin’s ‘most wonderful’ plant: Ecological Steps to a Snap-trap. New Phytologist 183:575–587. [DOI] [PubMed] [Google Scholar]
- Givnish TJ, Burkhardt EL, Happel RE, and Weintraub JD. 1984. Carnivory in the bromeliad Brocchinia reducta, with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient-poor habitats. American Naturalist 124:479–497. [Google Scholar]
- Givnish TJ, 2015. New evidence on the origin of carnivorous plants. Proceedings of the National Academy of Sciences 112:10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchens JJ, and Luken JO. 2009. Prey Capture in the Venus Flytrap: Collection or Selection. Botany 87:1007–1010. [Google Scholar]
- Jones FM, 1923. The most wonderful plant in the world. Natural History 23:589–596. [Google Scholar]
- Król E, Płachno BJ, Adamec L, Stolarz M, Dziubińska H, and Trębacz K. 2012. Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’. Annals of Botany 109:47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luken JO 2005. Dionaea muscipula (Venus flytrap) establishment, release, and response of associated species in mowed patches on the rims of Carolina bays. Restoration Ecology 13:678–684. [Google Scholar]
- Maia R, Rubenstein DR, and Shawkey MD. 2013. Key ornamental innovations facilitate diversification in an avian radiation. Proceedings of the National Academy of Sciences 110:10687–10692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CH, and Wainwright PC. 2013. Multiple fitness peaks on the adaptive landscape drive adaptive radiation in the wild. Science 339:208–211. [DOI] [PubMed] [Google Scholar]
- Mayr E, 1960. The emergence of evolutionary novelties. Evolution after Darwin, 1:349–380. [Google Scholar]
- Newell SJ, Nastase AJ. 1998. Efficiency of insect capture by Sarracenia purpurea (Sarraceniaceae), the northern pitcher plant. American Journal of Botany 85:88–91. [PubMed] [Google Scholar]
- Nychka D, Furrer R, Paige J, Sain S. 2015. “fields: Tools for spatial data.”, R package version 9.0
- Pavlovič A, and Saganová M. 2015. A novel insight into the cost–benefit model for the evolution of botanical carnivory. Annals of botany 115:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppinga S, Masselter T, Speck T. 2013. Faster than their prey: new insights into the rapid movements of active carnivorous plants traps. BioEssays 35:649–657. [DOI] [PubMed] [Google Scholar]
- R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [Google Scholar]
- RStudio Team (2015). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA: URL http://www.rstudio.com/. [Google Scholar]
- Roberts PR, and Oosting HJ. 1955. Responses of Venus flytrap (Dionaea muscipula) to factors involved in its endemism. Ecological Monographs 28:193–218. [Google Scholar]
- Schulze W, Schulze ED, Schulze I, and Oren R. 2001. Quantification of insect nitrogen utilization by the venus flytrap Dionaea muscipula catching prey with highly variable isotope signatures. Journal of Experimental Botany 52:1041–1049. [DOI] [PubMed] [Google Scholar]
- Stroud JT, and Losos JB. 2016. Ecological opportunity and adaptive radiation. Annual Review of Ecology, Evolution, and Systematics 47:507–532. [Google Scholar]
- Stuhlman O, 1948. A physical analysis of the opening and closing movements of the lobes of Venus’ fly-trap. Bulletin of the Torrey Botanical Club 1:22–44. [Google Scholar]
- Tauber ERAN, and Camhi J. 1995. The wind-evoked escape behavior of the cricket Gryllus bimaculatus: integration of behavioral elements. Journal of Experimental Biology 198:1895–1907. [DOI] [PubMed] [Google Scholar]
- Volkov AG, Adesina T, Markin VS, and Jovanov E. 2008. Kinetics and Mechanism of Dionaea muscipula Trap Closing. American Society of Plant Biologists 146:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov AG, Carrell H, and Markin VS. 2009. Biologically Closed Electrical Circuits in Venus Flytrap. Plant Physiology 149:1661–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright PC, Smith WL, Price SA, Tang KL, Sparks JS, Ferry LA, Kuhn KL, Eytan RI, and Near TJ. 2012. The evolution of pharyngognathy: a phylogenetic and functional appraisal of the pharyngeal jaw key innovation in labroid fishes and beyond. Systematic Biology 61:1001–1027. [DOI] [PubMed] [Google Scholar]
- Walker JF, Yang Y, Moore MJ, Mikenas J, Timoneda A, Brockington SF, and Smith SA. 2017. Widespread paleopolyploidy, gene tree conflict, and recalcitrant relationships among the carnivorous Caryophyllales. American journal of botany 104:858–867. [DOI] [PubMed] [Google Scholar]
- Wickham H ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag; New York, 2009. [Google Scholar]
- Youngsteadt E, Irwin RE, Fowler A, Bertone MA, Giacomini SJ, Kunz M, Suiter D, and Sorenson CE. 2018. Venus Flytrap Rarely Traps Its Pollinators. The American Naturalist 191:539–546. [DOI] [PubMed] [Google Scholar]


