Key Points
NPM1 non–exon 12 mutations and rearrangements in AML lead to NPM1c+, indicating this event is critical for leukemogenesis.
Immunohistochemistry, next-generation sequencing, and fluorescence in situ hybridization can detect rare NPM1 mutations and rearrangements.
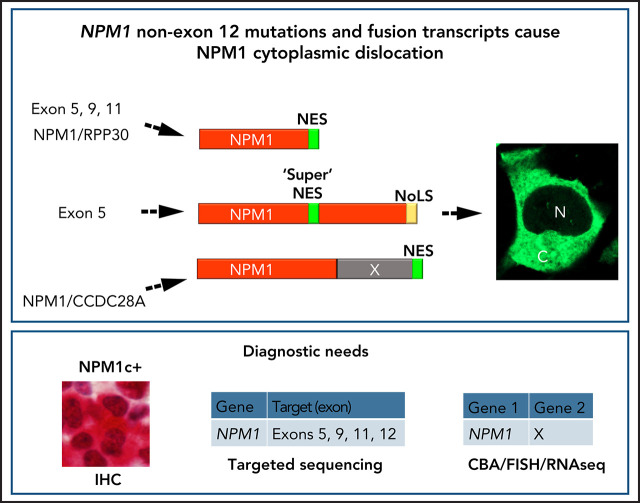
Visual Abstract
Abstract
Nucleophosmin (NPM1) mutations in acute myeloid leukemia (AML) affect exon 12, but also sporadically affect exons 9 and 11, causing changes at the protein C-terminal end (tryptophan loss, nuclear export signal [NES] motif creation) that lead to aberrant cytoplasmic NPM1 (NPM1c+), detectable by immunohistochemistry. Combining immunohistochemistry and molecular analyses in 929 patients with AML, we found non–exon 12 NPM1 mutations in 5 (1.3%) of 387 NPM1c+ cases. Besides mutations in exons 9 (n = 1) and 11 (n = 1), novel exon 5 mutations were discovered (n = 3). Another exon 5 mutation was identified in an additional 141 patients with AML selected for wild-type NPM1 exon 12. Three NPM1 rearrangements (NPM1/RPP30, NPM1/SETBP1, NPM1/CCDC28A) were detected and characterized among 13 979 AML samples screened by cytogenetic/fluorescence in situ hybridization and RNA sequencing. Functional studies demonstrated that in AML cases, new NPM1 proteins harbored an efficient extra NES, either newly created or already present in the fusion partner, ensuring its cytoplasmic accumulation. Our findings support NPM1 cytoplasmic relocation as critical for leukemogenesis and reinforce the role of immunohistochemistry in predicting AML-associated NPM1 genetic lesions. This study highlights the need to develop new assays for molecular diagnosis and monitoring of NPM1-mutated AML.
Introduction
The nucleophosmin (NPM1) gene, encoding for a multifunctional shuttling protein located in the nucleolus,1 is mutated in approximately one-third of acute myeloid leukemias (AMLs).2 NPM1 mutations affect the last exon (exon 12) almost exclusively and cause changes at the C-terminus end (loss of tryptophans and creation of a nuclear export signal [NES] motif) leading to the aberrant dislocation of mutant NPM1 (and wild-type [WT] NPM1) in the cytoplasm of AML cells (NPM1c+).3-5 This event is easily detectable by immunohistochemistry (IHC) in paraffin-embedded biopsies.6,7 Rare NPM1 mutations involving exons 98 and 11,9 as well as the NPM1/MLF1 fusion generated by t(3;5)(q25;q34)10 and NPM1/HAUS1 fusion created by t(5;18)(q35;q21),11 also lead to cytoplasmic NPM1, indicating that this event is critical for leukemogenesis.12,13 We report novel NPM1 mutations and fusion transcripts in AML and discuss their functional significance and the best approach to identify them.
Study design
From 2005 to 2019, 929 unselected AML patient samples afferring to Perugia (PG patients) from different Italian centers were studied by IHC in paraffin-embedded bone marrow (BM) biopsies, western blot (WB) with anti-NPM1 antibodies (supplemental Figure 1), and NPM1 exon 12 Sanger sequencing6,14 (screening A). On IHC in BM biopsies, 387 (41.6%) of 929 samples were NPM1c+. NPM1c+ cases that were exon 12 WT and/or negative by WB with antibodies against NPM1 exon 12 mutants14 were regarded as discrepant and further investigated. We also screened an additional 141 patients with AML from the Munich Leukemia Laboratory (MLL) who were selected based on the following criteria: (1) normal/intermediate karyotype, (2) no NPM1 exon 12 mutations, and (3) absence of genetic alterations mutually exclusive with NPM1 mutations.15 All 141 cases were investigated by targeted sequencing for NPM1 exons 1 to 12 by a next-generation sequencing (NGS) TruSeq custom panel (Illumina; screening B).
Finally, we characterized 3 new NPM1 rearrangements found within 13 979 AML patient samples analyzed by chromosome banding analysis in routine diagnostics, followed by fluorescence in situ hybridization and RNA sequencing16 (screening C). BM biopsies were not available from patients included in screening B and C cohorts, because this examination is not routinely performed in some centers. The study was conducted in accordance with the Declaration of Helsinki and received local institutional review board approval.
The newly identified NPM1 mutants and fusion transcripts were cloned into pEGFP-C1 vectors and further studied functionally by ectopic expression in murine fibroblast NIH-3T3 cells.3,12 Details are given in the supplemental Methods.
Results and discussion
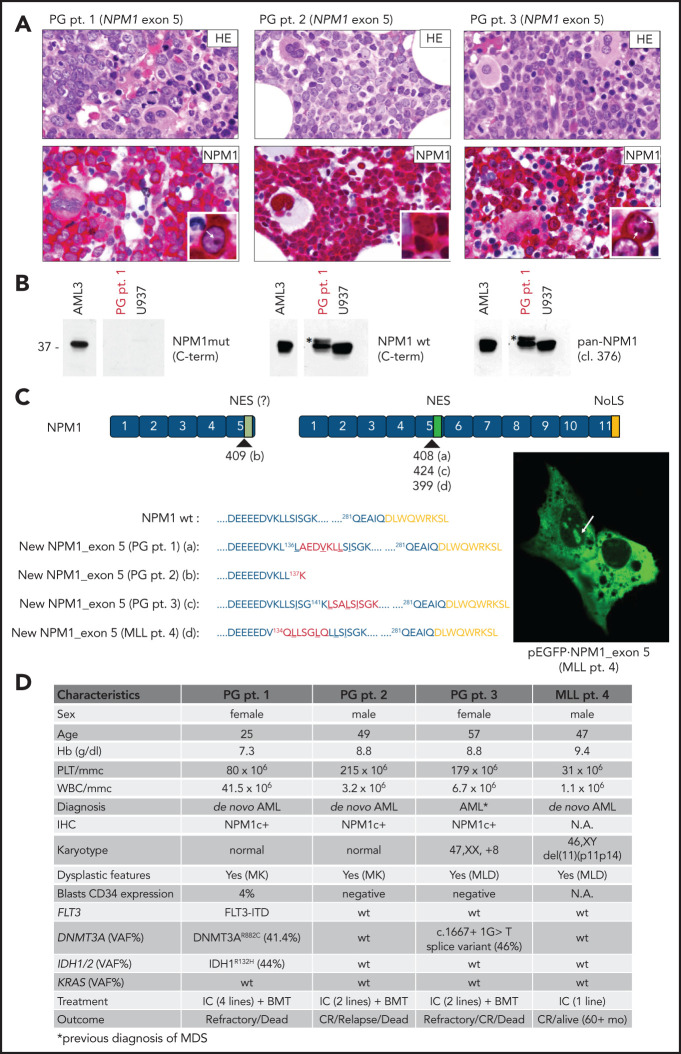
Screening A identified a diagnostic discrepancy in 5 (1.3%) of 387 NPM1c+ AML cases (Figure 1A-B; data not shown). Sanger sequencing and/or targeted sequencing of the NPM1 coding sequence detected an exon 11 mutation in 1 patient (data not shown),9,17 involvement of exon 98 in another patient (supplemental Figure 2), and 3 novel mutations involving exon 5 in the other 3 cases (PG patients 1, 2, and 3; Figure 1C). Specifically, PG patient 1 showed a 21-nucleotide in-frame insertion at c408-409 (F,5'-GCGGAGGATGTGAAACTCTTA) producing a new mutant NPM1 protein, 7 aa longer than WT (p.L136_137insAEDVKLL), whereas PG patient 2 displayed an 18-nucleotide out-of-frame insertion at c409-410 (F,5'-AATGATCTGTCACTTCTG), with the creation of a new stop codon leading to a truncated protein of 137 aa (p.S137_K137fs*). In PG patient 3, the exon 5 mutation consisted of a 27-nucleotide in-frame insertion at c424-425 (F,5'-TTTCTGCCTTAAGTATATCTGGAAAGC), with the production of a new NPM1 mutant protein 9 aa longer than WT (p.K141_142insLSALSISGK; Figure 1C).
Figure 1.
Novel NPM1 gene mutations involving exon 5. (A) IHC staining of BM trephine from PG patients 1, 2, and 3 showing diffuse infiltration by leukemic blasts (hematoxylin and eosin [HE], upper) with aberrant cytoplasmic positivity for NPM1 (NPM1, lower). Besides cytoplamic staining, nucleoli staining is shown in the inset (white arrows) for PG patients 1 and 3. NPM1 staining: mouse monoclonal clone 376 anti-NPM1 N-terminus by antialkaline phosphatase technique with hematoxylin counterstaining. Images were collected using an Olympus B61 microscope with a UPlanApo 40×/0.85 U (40× magnification) and UPlan FI 100×/1.3 NA oil (×100 original magnification) objective for the inset, Camedia 4040 (Dp_soft version 3.2), and Adobe Photoshop CC 2019. (B) WB analysis of total protein extracts from PG patient 1 showing the reactivity pattern with the different anti-NPM1 antibodies (supplemental Figure 1). Specifically, although the anti-NPM1 mutant did not show positivity (left), the anti-NPM WT recognized, besides the known WT NPM1 protein at 37 kDa, a band at a slightly higher molecular weight (middle; asterisk). This was also recognized by clone 376, indicating it was NPM1 (pan-NPM1; right; asterisk). (C) Graphical representation and predicted protein sequence of the new NPM1 exon 5 mutants. Nucleotides insertion points are indicated for each mutation (a,b,c,d) according to the NPM1 complementary DNA transcript ENST00000296930. The newly acquired amino acids (aa) are highlighted in red, and the predicted NES motif is underlined. Mutants from PG patient 1, PG patient 3, and MLL patient 4 retain the C-terminus of the WT NPM1 (nucleolar localization signal [NoLS]; yellow). Representative image of NIH-3T3 overexpressing the new GFP-NPM1 exon 5 fusion protein from MLL patient 4 showing the aberrant localization in the cytoplasm and, concomitantly, in the nucleoli (right; white arrow). Images were acquired using a Zeiss LSM 800 confocal microscope (Carl Zeiss) with a 488-nm (for eGFP) laser line for excitation and 63×/1.4 oil Plan-Apochromat objective (×63 original magnification). (D) Table illustrating the most relevant characteristics of patients with AML carrying NPM1 exon 5 mutations. BMT, BM allogeneic transplantation; CR, complete remission; Hb, hemoglobin; IC, standard intensive chemotherapy; MK, megakaryocytes; MLD, multilineage dysplasia; NA, not available; PLT, platelets; pt., patient; VAF, variant allele frequency; WBC, white blood cells.
Screening B identified a fourth exon 5 mutation in another patient (MLL patient 4; Figure 1C). In this case, 2 in-frame insertions/duplications, each consisting of 9 nucleotides, occurred at c399-400 (F,5'-CAACTCTTA) and c400-401 (F,5'-GTGGGCTGC), leading to a new NPM1 mutant protein 6 aa longer than WT (p.K134_Q134insLLSGLQ; Figure 1C).
Notably, with the exception of PG patient 2, all other patients with NPM1 mutants retained the C-terminus of NPM1 WT, including the NoLS (Figure 1A-C; supplemental Figures 3 and 4). This is a unique finding, because all NPM1 mutant cases reported so far were found to carry a disrupted NoLS resulting from loss of both tryptophans 288 and 290 or 288 alone.3,12 Despite retaining NoLS, these exon 5 mutants showed cytoplasmic localization both by IHC (Figure 1A) and when transfected as GFP-NPM1 mutants in NIH-3T3 (supplemental Figures 3 and 4). Cytoplasmic dislocation was promoted by an extra NES with a high score18 (super NES) that was able to counteract the NoLS driving the mutant to the nucleolus (Figure 1C). This was also confirmed by the pRev(1.4)-eGFP NES efficiency assay, as previously described12 (supplemental Figure 5). NES-dependent cytoplasmic localization was proven by relocation into the nucleus/nucleoli upon exportin-1 inhibition by leptomycin B (supplemental Figure 6). In addition to cytoplasmic localization, all new mutant samples except for PG patient 2 also retained a nucleolar localization both in transfected cells (Figure 1C; supplemental Figures 3E and 4E) and IHC (PG patients 1 and 3; Figure 1A), likely to have resulted from the intact NoLS.
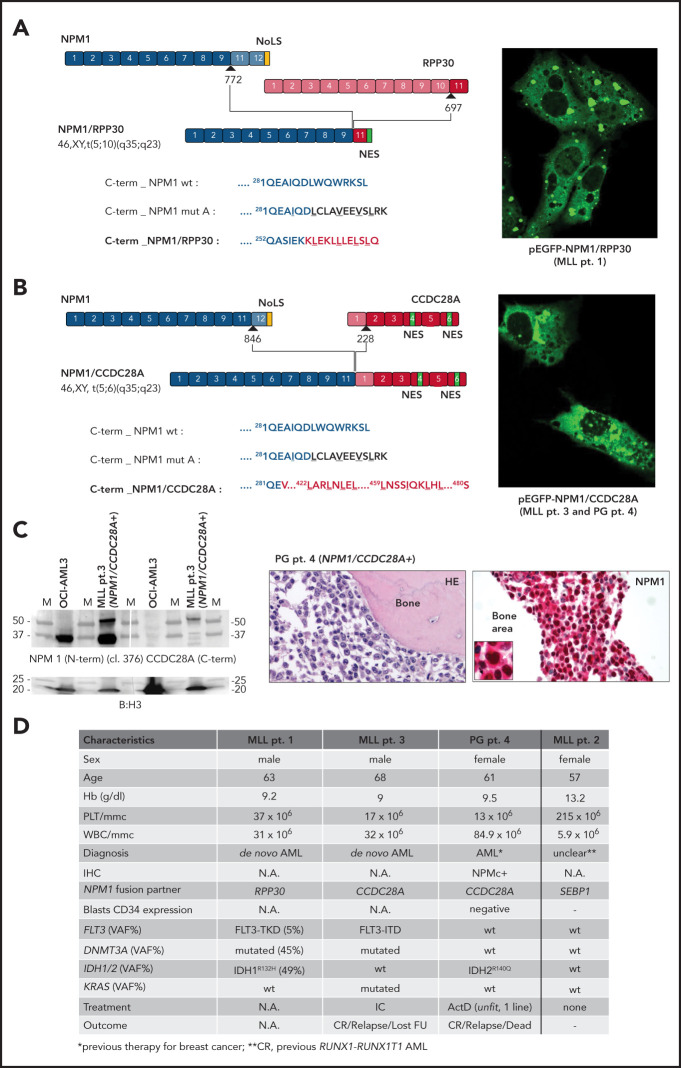
In screening C, 3 new NPM1 translocations were identified: t(5;10)(q35;q23), t(5;18)(q34;q12), and t(5;6)(q35;q23), generating the NPM1/RPP30 (MLL patient 1), NPM1/SETBP1 (MLL patient 2), and NPM1/CCDC28A (MLL patient 3) fusion transcripts, respectively (Figure 2A-D). NPM1/CCDC28A was also retrospectively detected in 1 of the Italian patients, who at screening A showed a diagnostic discrepancy for NPM1 between IHC and molecular analysis (PG patient 4; Figure 2C-D; supplemental Figure 7). This fusion was recently reported but not further characterized in a pediatric patient,19 confirming its recurrence in AML. The encoded fusions were predicted to be out of frame for NPM1/RPP30 but in frame for both NPM1/SETBP1 and NPM1/CCDC28A (Figure 2A-B; supplemental Figure 8). The NPM1/CCDC28A fusion protein was shown to be expressed by WB in MLL patient 1 and located in the cytoplasm of AML cells by IHC in PG patient 4 (Figure 2C). In all cases, NPM1 was involved as an upstream gene so that, in the derived protein, the original C-terminus of NPM1 containing the NoLS was lost (Figure 2A-B; supplemental Figure 8). Strikingly, with the exception of MLL patient 2 (carrying NPM1/SETBP1, with previous RUNX1/RUNX1T1 AML, and in complete molecular remission at time of analysis), a C-terminal NES, either newly created (in NPM1/RPP30) or present in the partner protein (in NPM1/CCDC28A), was added to the original NPM1 protein sequence (Figure 2A-B; supplemental Figures 8 and 9). Accordingly, the corresponding GFP-NPM fusion proteins (supplemental Figure 10) showed an NES-dependent cytoplasmic localization only for NPM1/RPP30 and NPM1/CCDC28A (Figure 2; supplemental Figure 6). The NPM1/SETBP1 transcript in MLL patient 2 indeed spontaneously disappeared within 1.2 years, calling into doubt its pathogenetic role in AML.
Figure 2.
Novel NPM1 gene fusion transcripts. (A) NPM1/RPP30 rearrangement. In MLL patient 1, NPM1 was rearranged with the RPP30 gene at the end of exon 9 (breakpoint at position 772 of NPM1 complementary DNA [cDNA] based on transcript ENST00000296930), and RPP30 was rearranged with NPM1 at exon 11 (breakpoint at position 697 in RPP30 cDNA ENST00000371703.7). The encoded fusion was predicted to be out of frame. The new fusion protein is 269 aa long, with a predicted molecular weight (MW) of 29.6 kDa. The predicted protein sequence of the C-terminus of the new fusion protein (red) is shown as compared with the C-terminus of either NPM1 WT or mutant A. The newly acquired NES domain is underlined (LLLL). (B) NPM1/CCDC28A rearrangement. In MLL patient 3 and PG patient 4, NPM1 was rearranged with the CCDC28A gene at the end of exon 11 (breakpoint at position 846 of NPM1 cDNA based on transcript ENST00000296930), and CCDC28A was rearranged with NPM1 at the beginning of exon 2 (breakpoint at position 228 in CCDC28A cDNA ENST00000611852.4). The encoded fusion was predicted to be in frame. The new fusion protein is 480 aa long, with a predicted MW of 53.1 kDa. Two NES domains at the C-terminus of the new fusion protein (red) are underlined (LLLL, LILL). (A-B) Representative images of NIH-3T3 overexpressing the new GFP-NPM1 fusion protein NPM1/RPP30 (MLL patient 1) (A) and NPM1/CCDC28A (MLL patient 3 and PG patient 4) (B) showing the aberrant localization in the cytoplasm (right). Of note, the nucleoli are also positive because of the loss of NoLS. Images were acquired using a Zeiss LSM 800 confocal microscope (Carl Zeiss) with a 488-nm (for eGFP) laser line for excitation and 63×/1.4 oil Plan-Apochromat objective (×63 original magnification). (C) Left, WB analysis with either the anti-NPM1 antibody (clone 376) recognizing the N-terminus of NPM1 (upper left) or the anti-CCDC28A antibody raised against the C-terminal part of CCDC28A protein (upper right) on the total protein extract from MLL patient 3. The AML sample carrying the NPM1/CCDC28A fusion transcript shows a band recognized by both antibodies just above 50 kDa, corresponding to the predicted 53-kDa MW of the new fusion protein. OCI-AML3 protein extract was used as control for the clone 376 anti-NPM1 antibody (band at ∼37 kDa). Samples were loaded in duplicate. The white vertical line indicates where the membrane was cut. Blotting for histone H3 was used as control for protein loading (lower subpanel). Right, BM trephine from PG patient 4 carrying the NPM1/CCDC28A fusion transcript showing diffuse BM infiltration by leukemic blasts (hematoxylin eosin [HE]; left) with cytoplasmic NPM1 (inset for details; right). NPM1 staining: mouse monoclonal anti-NPM1 clone 376 by antialkaline phosphatase technique with hematoxylin counterstaining. Images were collected using an Olympus B61 microscope and a UPlanApo 40×/0.85 (×40 original magnification) and UPlan FI 100×/1.3 NA oil (×100 original magnification) objective for the inset, Camedia 4040 (Dp_soft version 3.2), and Adobe Photoshop CC 2019. (D) Table illustrating the most relevant characteristics of patients carrying the new NPM1 fusion transcripts. Dash indicates not applicable. ActD, actinomycin D at 12.5 mg/kg per day for 5 days25; CR, complete remission; FU, follow-up; Hb, hemoglobin; IC, standard intensive chemotherapy; NA, not available; PLT, platelets; VAF, variant allele frequency; WBC, white blood cells.
In conclusion, we report novel NPM1 mutations and fusion transcripts in AML and demonstrate that, similarly to exon 12 mutations, they act to delocalize NPM1 in the cytoplasm, further supporting our original hypothesis that this event is critical for leukemogenesis.4,12,13 Other biological similarities with AML mutated at NPM1 exon 12 include de novo occurrence, CD34 negativity, morphological pattern (multilineage dysplasia,20 increased megakaryocytes21), and type of associated mutations22 (Figures 1D and 2D).
Our findings also raise the question of how to recognize these rare variants.17,23 As diagnostic workflow for AML at presentation, we recommend that, in addition to standard molecular analyses according to European LeukemiaNet24 2017, a BM biopsy is also performed. If IHC with a monoclonal antibody against the NPM1 N-terminus clearly shows cytoplasmic positivity for NPM1 but no NPM1 exon 12 mutations are detected by conventional molecular assays, all other NPM1 exons should be sequenced by NGS. If no rare NPM1 mutations are detected, fluorescence in situ hybridization/RNA sequencing will allow identification of NPM1-containing fusion transcripts. For centers not performing BM biopsy or IHC at diagnosis, the only way to detect these rare NPM1 mutations is to perform targeted NGS in all AMLs, by including in the analysis NPM1 exons other than 12. This should be feasible, because NGS, although recommended by European LeukemiaNet 2017 only in the context of clinical trials, is now increasingly used, especially in Western countries.
Because of their rarity (∼1%), the clinical impact of these novel NPM1 genetic alterations remains unclear, and its assessment will require specific analysis in large clinical trials. Moreover, identification of these very rare NPM1 mutations/rearrangements would allow minimal residual disease monitoring by designing patient-specific quantitative reverse transcription polymerase chain reaction assays.17
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Claudia Tibidò and Laura Pinacoli for secretarial assistance.
This work was supported by Associazione Italiana per la Ricerca sul Cancro 2016 Investigator Grant 18568 (B.F.), European Research Council (ERC) 2016 advanced grant 740230 (B.F.), and ERC 2016 Consolidator Grant 725725 (M.P.M.). This article is dedicated to the memory of Roberta Pacini.
Footnotes
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.P.M. and B.F. conceived the study; R.R., A.V., V.M.P., G.M., R.C., E.V., L.B., C.Q., F. Mezzasoma, I.G., and F. Milano performed the experiments and analyzed the data; O.S., V.C., F.A., G.S., C.V., F.D.R., O.A., G.A., A.R., and F.F. provided patient samples; G.M. and S.A. carried out histological analyses, with technical support from R.P., A.T., and B.B.; E.T. and P.S. supervised molecular analyses; M.M., T.H., and C.H. identified the new translocations and provided samples; M.P.M. and B.F. wrote the manuscript; and all authors contributed to writing and approval of the manuscript.
Conflict-of-interest disclosure: B.F. licensed a patent on NPM1 mutants (102004901256449). B.F. and M.P.M. declare honoraria from Rasna Therapeutics, Inc., for scientific advisor activities. M.P.M. also declares honoraria/consultancy and scientific advisory board activities for AbbVie, Amgen, Celgene, Janssen, Novartis, Pfizer, and Jazz Pharmaceuticals. V.M.P. declares consultancy and scientific advisory board activities for Novartis. L.B. and C.V. declare consultancy and scientific advisory board activities for AbbVie. F.D.R. declares honoraria/consultancy and scientific advisory board activities for Jazz Pharmaceuticals, Bristol-Myers Squibb, Janssen, and Novartis. O.A. declares honoraria/consultancy and scientific advisory board activities for Janssen, Novartis, Celgene, and Amgen. A.R. declares honoraria/consultancy and scientific advisory board activities for Novartis, Amgen, Pfizer, Gilead, Celgene/Bristol-Myers Squibb, Sanofi, Jazz Pharmaceuticals, TEVA, Italfarmaco, Omeros, and Coimmune. P.S. declares honoraria/consultancy and scientific advisory board activities for AbbVie, Janssen, Novartis, AstraZeneca, and Incyte. T.H., C.H., and M.M. declare part ownership of Munich Leukemia Laboratory. The remaining authors declare no competing financial interests.
Correspondence: Brunangelo Falini, Centro di Ricerche Emato-Oncologiche (CREO), University of Perugia, Ospedale S Maria della Misericordia, S Andrea delle Fratte 06132 Perugia, Italy; e-mail: brunangelo.falini@unipg.it; and Maria Paola Martelli, Centro di Ricerche Emato-Oncologiche (CREO), University of Perugia, Ospedale S Maria della Misericordia, S Andrea delle Fratte 06132 Perugia, Italy; e-mail: maria.martelli@unipg.it.
REFERENCES
- 1.Grisendi S, Mecucci C, Falini B, Pandolfi PP. Nucleophosmin and cancer. Nat Rev Cancer. 2006;6(7):493-505. [DOI] [PubMed] [Google Scholar]
- 2.Falini B, Mecucci C, Tiacci E, et al. ; GIMEMA Acute Leukemia Working Party . Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352(3):254-266. [DOI] [PubMed] [Google Scholar]
- 3.Falini B, Bolli N, Shan J, et al. Both carboxy-terminus NES motif and mutated tryptophan(s) are crucial for aberrant nuclear export of nucleophosmin leukemic mutants in NPMc+ AML. Blood. 2006;107(11):4514-4523. [DOI] [PubMed] [Google Scholar]
- 4.Falini B, Nicoletti I, Martelli MF, Mecucci C. Acute myeloid leukemia carrying cytoplasmic/mutated nucleophosmin (NPMc+ AML): biologic and clinical features. Blood. 2007;109(3):874-885. [DOI] [PubMed] [Google Scholar]
- 5.Falini B, Brunetti L, Sportoletti P, Martelli MP. NPM1-mutated acute myeloid leukemia: from bench to bedside. Blood. 2020;136(15):1707-1721. [DOI] [PubMed] [Google Scholar]
- 6.Falini B, Martelli MP, Bolli N, et al. Immunohistochemistry predicts nucleophosmin (NPM) mutations in acute myeloid leukemia. Blood. 2006;108(6):1999-2005. [DOI] [PubMed] [Google Scholar]
- 7.Falini B, Lenze D, Hasserjian R, et al. Cytoplasmic mutated nucleophosmin (NPM) defines the molecular status of a significant fraction of myeloid sarcomas. Leukemia. 2007;21(7):1566-1570. [DOI] [PubMed] [Google Scholar]
- 8.Mariano AR, Colombo E, Luzi L, et al. Cytoplasmic localization of NPM in myeloid leukemias is dictated by gain-of-function mutations that create a functional nuclear export signal. Oncogene. 2006;25(31): 4376-4380. [DOI] [PubMed] [Google Scholar]
- 9.Albiero E, Madeo D, Bolli N, et al. Identification and functional characterization of a cytoplasmic nucleophosmin leukaemic mutant generated by a novel exon-11 NPM1 mutation. Leukemia. 2007;21(5):1099-1103. [DOI] [PubMed] [Google Scholar]
- 10.Falini B, Bigerna B, Pucciarini A, et al. Aberrant subcellular expression of nucleophosmin and NPM-MLF1 fusion protein in acute myeloid leukaemia carrying t(3;5): a comparison with NPMc+ AML [published correction appears in Leukemia. 2006;20:1330]. Leukemia. 2006;20(2): 368-371. [DOI] [PubMed] [Google Scholar]
- 11.Campregher PV, de Oliveira Pereira W, Lisboa B, et al. A novel mechanism of NPM1 cytoplasmic localization in acute myeloid leukemia: the recurrent gene fusion NPM1-HAUS1. Haematologica. 2016;101(7): e287-e290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolli N, Nicoletti I, De Marco MF, et al. Born to be exported: COOH-terminal nuclear export signals of different strength ensure cytoplasmic accumulation of nucleophosmin leukemic mutants. Cancer Res. 2007;67(13):6230-6237. [DOI] [PubMed] [Google Scholar]
- 13.Brunetti L, Gundry MC, Sorcini D, et al. Mutant NPM1 maintains the leukemic state through HOX expression. Cancer Cell. 2018;34(3):499-512.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martelli MP, Manes N, Liso A, et al. A western blot assay for detecting mutant nucleophosmin (NPM1) proteins in acute myeloid leukaemia. Leukemia. 2008;22(12):2285-2288. [DOI] [PubMed] [Google Scholar]
- 15.Falini B, Mecucci C, Saglio G, et al. NPM1 mutations and cytoplasmic nucleophosmin are mutually exclusive of recurrent genetic abnormalities: a comparative analysis of 2562 patients with acute myeloid leukemia. Haematologica. 2008;93(3): 439-442. [DOI] [PubMed] [Google Scholar]
- 16.Stengel A, Nadarajah N, Haferlach T, et al. Detection of recurrent and of novel fusion transcripts in myeloid malignancies by targeted RNA sequencing. Leukemia. 2018;32(5):1229-1238. [DOI] [PubMed] [Google Scholar]
- 17.Falini B, Sciabolacci S, Falini L, Brunetti L, Martelli MP. Diagnostic and therapeutic pitfalls in NPM1-mutated AML: notes from the field [published online ahead of print 20 April 2021]. Leukemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu D, Grishin NV, Chook YM. NESdb: a database of NES-containing CRM1 cargoes. Mol Biol Cell. 2012;23(18):3673-3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiba N, Yoshida K, Hara Y, et al. Transcriptome analysis offers a comprehensive illustration of the genetic background of pediatric acute myeloid leukemia. Blood Adv. 2019;3(20):3157-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasqualucci L, Liso A, Martelli MP, et al. Mutated nucleophosmin detects clonal multilineage involvement in acute myeloid leukemia: impact on WHO classification. Blood. 2006;108(13):4146-4155. [DOI] [PubMed] [Google Scholar]
- 21.Sportoletti P, Varasano E, Rossi R, et al. The human NPM1 mutation A perturbs megakaryopoiesis in a conditional mouse model. Blood. 2013;121(17):3447-3458. [DOI] [PubMed] [Google Scholar]
- 22.Ley TJ, Miller C, Ding L, et al. ; Cancer Genome Atlas Research Network . Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falini B, Brunetti L, Martelli MP. How I diagnose and treat NPM1-mutated AML. Blood. 2021;137(5):589-599. [DOI] [PubMed] [Google Scholar]
- 24.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017; 129(4):424-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gionfriddo I, Brunetti L, Mezzasoma F, et al. Dactinomycin induces complete remission associated with nucleolar stress response in relapsed/refractory NPM1-mutated AML. Leukemia. 2021;35(9):2552-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.