Abstract
Sulfonamide resistance in Streptococcus pneumoniae is due to changes in the chromosomal folP (sulA) gene coding for dihydropteroate synthase (DHPS). The first reported laboratory-selected sulfonamide-resistant S. pneumoniae isolate had a 6-bp repetition, the sul-d mutation, leading to a repetition of the amino acids Ile66 and Glu67 in the gene product DHPS. More recently, clinical isolates showing this and other repetitions have been reported. WA-5, a clinical isolate from Washington State, contains a 6-bp repetition in the folP gene, identical to the sul-d mutation. The repetition was deleted by site-directed mutagenesis. Enzyme kinetic measurements showed that the deletion was associated with a 35-fold difference in Ki for sulfathiazole but changed the Km for p-aminobenzoic acid only 2.5-fold and did not significantly change the Km for 2-amino-4-hydroxy-6-hydroxymethyl-7,8-dihydropteridine pyrophosphate. The enzyme characteristics of the deletion variant were identical to those of DHPS from a sulfonamide-susceptible strain. DHPS from clinical isolates with repetitions of Ser61 had very similar enzyme characteristics to the DHPS from WA-5. The results confirm that the repetitions are sufficient for development of a resistant enzyme and suggest that the fitness cost to the organism of developing resistance may be very low.
Streptococcus pneumoniae is a major cause of morbidity and mortality worldwide. It is the leading cause of community-acquired pneumonia, otitis media, meningitis, and bacteremia. It has been estimated that S. pneumoniae is responsible for more than 1 million deaths per year in children from developing countries. Antibiotic-resistant and multidrug-resistant S. pneumoniae strains have increased during the past 20 years, with multidrug-resistant strains, those that are resistant to two or more classes of antibiotic, currently limited to a few major serotypes, including 6B, 9V, 19F, and 23F (6, 7, 17).
Trimethoprim-sulfamethoxazole (SXT) has been used in treatment of a range of S. pneumoniae diseases, especially in children, because it is inexpensive and generally effective. Many of the multidrug-resistant strains of S. pneumoniae are resistant to SXT, with high rates of resistance described worldwide, including South Africa, parts of Europe, and Alaska in North America (11, 12). Trimethoprim (TMP) interacts with the dihydrofolate reductase, and sulfonamides inhibit the dihydropteroate synthase (DHPS). Both enzymes are in a single bacterial pathway leading to formation of tetrahydrofolate. Resistance to both TMP and sulfamethoxazole have been examined in a limited number of S. pneumoniae isolates. With TMP resistance, single or multiple amino acid substitutions have been identified in the dihydrofolate reductase (1). In contrast, in a laboratory-derived sulfonamide-resistant (Sulr) S. pneumoniae, an insertion of 6 bp in the folP gene resulting in duplication of amino acids Ile66 and Glu67 in the gene product DHPS was identified (8). The gene encoding DHPS in S. pneumoniae was initially designated sulA. We propose here that the designation folP should be used as in other bacteria (5, 16) in order to facilitate comparisons of the genomes between different organisms. More recently, Maskell et al. examined six Sulr clinical isolates and found 3- or 6-bp duplications in the folP gene. Transformation experiments showed that the duplications are sufficient for conferring high-level Sulr (11). However, no report has addressed the effects these mutations have on the kinetics of the DHPS enzyme, which could have consequences for the fitness of resistant mutants in competition with Suls pneumococci.
In this study, we have examined 11 S. pneumoniae isolates from Washington State, including 5 that have previously been shown to be part of a multidrug-resistant clone group (10). These strains included three of the four serogroups that are most frequently multidrug resistant. We included in the study two strains with single and double Ser61 repetitions from a previous study (11). We found a number of different duplications in these 13 isolates and examined the DHPS kinetic parameters of three of them. We mutagenized the folP gene of one Sulr strain with an Ile66-Glu67 repetition to yield a susceptible strain, demonstrating that the duplicated amino acids were sufficient to account for Sulr in this S. pneumoniae isolate.
MATERIALS AND METHODS
Bacteria.
We examined 11 SXT-resistant S. pneumoniae isolates collected from patients aged 6 months to 83 years across Washington State from October 1995 to April 1997. Six isolates (serogroups 6, 19, and 23) were collected during a statewide surveillance study (6), and five isolates (serotypes 19A and 19F) were members of a multidrug-resistant pneumococcal clone group described previously (10) (Table 1). Two isolates from a previous study, PN93/720 and J94/76, were also investigated (11)
TABLE 1.
Characteristics of S. pneumoniae isolates
| Isolate | Location | Date (mo/yr) | Specimen | Serogroup | folP gene change | Repeat | Reference(s) |
|---|---|---|---|---|---|---|---|
| WA-5 | Renton, WA | 10/95 | Blood | 6 | 6-bp insertion; sul-d | I66E67 | 6, 9 |
| WA-5 mutant | Laboratory mutant | Δsul-d | None | This work | |||
| WA-33 | Renton, WA | ?/95b | Blood | 19 | 6-bp insertion | S61S62 | 6, 9 |
| WA-45a | Tacoma, WA | 12/95 | Sputum | 19F | 3-bp insertion | S61 | 10 |
| WA-54a | Tacoma, WA | 12/95 | Sputum | 19A | 3-bp insertion | S61 | 10 |
| WA-127 | Puyallup, WA | 2/96 | Blood | 23 | 3-bp insertion | S61 | 6, 9 |
| WA-133 | Everett, WA | 2/96 | Blood | 6 | 6-bp insertion | S62Y63 | 6, 9 |
| WA-152 | Spokane, WA | 10/95 | Sinus | 23 | 6-bp insertion | S62Y63 | 6, 9 |
| WA-159 | Spokane, WA | 12/95 | Middle ear fluid | 19F | 3-bp insertion | Y63 | 10 |
| WA-187 | Seattle, WA | 4/96 | Blood | 19F | 6-bp insertion | S61S62 | 6, 9 |
| WA-970195a | Tacoma, WA | 2/97 | Blood | 19F | 3-bp insertion | S61 | 10 |
| WA-263a | Spokane, WA | 2/97 | Blood | 19F | 3-bp insertion | S61 | 10 |
| PN93/720 | 3-bp insertion | S61 | 11 | ||||
| CP1015 | Wild type | None | 11 | ||||
| J94/76 | 6-bp insertion | S61S62 | 11 |
These isolates were identified as members of a multidrug-resistant S. pneumoniae clone group found in Washington State (10).
Month unknown.
PCR amplification and cloning.
The folP gene from isolate WA-5 was amplified by PCR using primers pneumo 1 and pneumo 2 (Table 2) and cloned into pUC18 using the Sure Clone ligation kit (Amersham-Pharmacia Biotech, Uppsala, Sweden). For the other strains primers pneumo 7 and pneumo 8 were used and ligated in pUC18 (18) using the enzymes EcoRI and XbaI. PCRs were performed in a Perkin-Elmer model 480 thermocycler in PCR buffer containing MgCl2 at a final concentration of 1.5 mM, nucleotides at a concentration of 200 μM, primers at 1 μM each, and 2 U of of Vent-polymerase (New England Biolabs, Beverly, Mass.) for a 25-μl reaction mixture. A total of 25 to 30 cycles of denaturation at 94°C for 1 min, annealing at 52°C for 1 min, and extension at 74°C for 2 min were run. Resulting PCR products were separated on 1% agarose gels, and fragments of the appropriate size (900 bp) were excised from the gel and extracted with the QIAquick Gel Extraction kit (Qiagen, Valencia, Calif.). Transformations into host strains DH5α and C600ΔfolP::Kmr (5) were usually done by electroporation (3).
TABLE 2.
Oligonucleotide primers used for cloning, sequence determination, and mutation procedures
| Primer | Sequence | Restriction sitea |
|---|---|---|
| pneumo 1 | 5′-GATGAATGCATCGTGTCCATC-3′ | |
| pneumo 2 | 5′-CCGTCCGGTAGTTAGCAATCC-3′ | |
| pneumo 3 | 5′-CAAGGCACTCCAGCAGGCTCG-3′ | |
| pneumo 4 | 5′-CCCTGTCTCGCAGCGATACTAG-3′ | |
| pneumo 5 | 5′-CTGCTGGTGCCGATCTAGTC-3′ | |
| pneumo 6 | 5′-GTCAGACCAAAGCCAATTCC-3′ | |
| pneumo 7 | 5′-TGA GAA TTC ATG TCA AGT AAA GCC AAT-3′ | EcoRI |
| pneumo 8 | 5′-TGA TCT AGA TTA TTT ATA TTG TTT TAA-3′ | XbaI |
| pneumo-Δ | 5′-ACA ACA CGT TGG ATT TCC TCT TCT ATC TCA ATA TAA CTA C-3′ |
Sites of restriction enzyme cutting are underlined in the corresponding sequences.
Site-directed mutagenesis.
Mutagenesis to delete the 6-bp repetition was performed by the PCR-based megaprimer method (14). In the first PCR step, the mutagenesis primer, pneumo-Δ, was used together with primer pneumo 7 to create the megaprimer. The PCR products were separated on a 1% agarose gel, and products with a size of 200 bp were excised and purified as described above. The purified product was used as a primer in the second PCR together with primer pneumo 8 to amplify the complete gene. Conditions for PCR were as described above. The final PCR product was cloned into pUC18 and introduced into Escherichia coli strains DH5α and C600ΔfolP::Kmr by electroporation.
Nucleotide sequence determinations.
The nucleotide sequences of the folP genes from WA-5 and the derived deletion mutant were determined using the Autoread sequencing kit and read using the ALF Express (Amersham-Pharmacia Biotech) apparatus. The sources for sequencing were genes cloned in pUC18, and the universal and reverse primers included in the kit were used. All other sequence determinations were done by a cycle sequencing method using 33P-labeled terminators (Amersham-Pharmacia Biotech). Primers pneumo 1 to pneumo 6 (Table 2) were used.
Preparation of cell extracts.
Cultures of C600ΔfolP::Kmr harboring the respective plasmids with cloned folP genes were grown in 800-ml batches of Luria-Bertani medium to a density of 5 × 108 cells/ml. Cells were pelleted by centrifugation at 3,000 × g for 5 min and resuspended in 3 ml of 0.1 M potassium phosphate buffer, pH 7.0, containing 1 mM dithiothreitol (Sigma, St. Louis, Mo.). The resuspended cells were disrupted twice by sonication for 30 s and were centrifuged at 15,000 × g for 30 min. The enzyme was partially purified by gel filtration and ion-exchange chromatography as described earlier (15). Determination of DHPS activity and calculation of enzyme kinetic parameters were done as described earlier (5, 13). GraphPad Prism software was used for calculations of Km and Ki.
RESULTS
Nucleotide sequence determination of folP genes of Sulr pneumococcal isolates from Washington State.
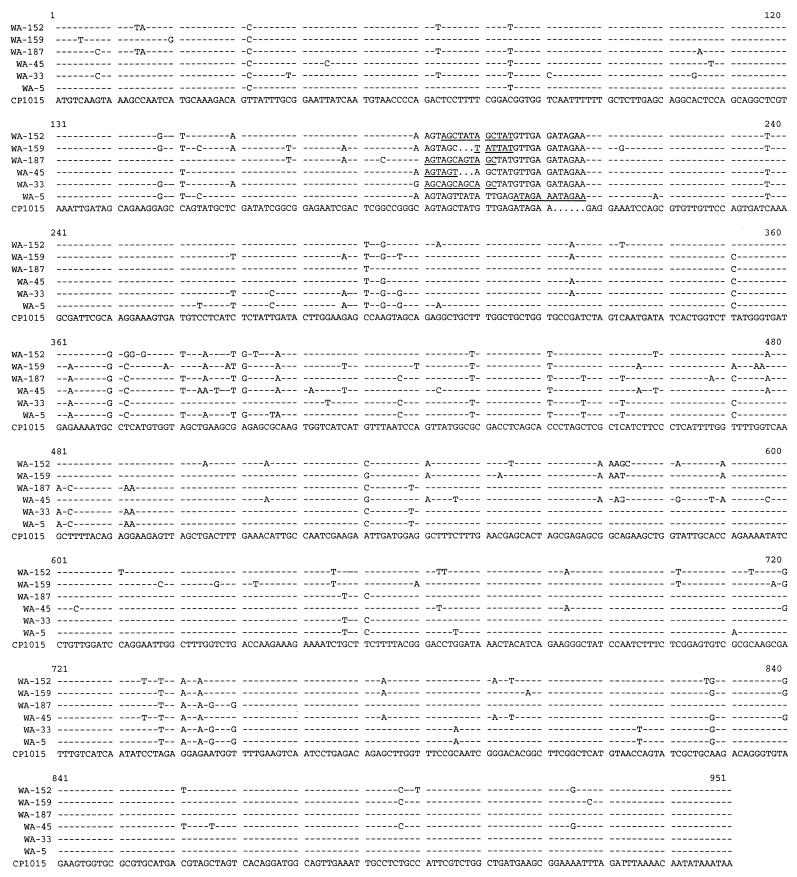
The nucleotide sequences of the folP genes from a total of 11 isolates from WA were determined (Fig. 1 and Table 1). Of these 11, 4 were previously found to be genetically related (10). All isolates had varying types of 3- or 6-bp repetitions resulting in insertions of one or two extra amino acids. This has previously been associated with Sulr (8, 11, 12). Two of the isolates, WA-133 and WA-152, had new variants of the 6-bp repetition with Ser62-Tyr63 as the repeated amino acids. Besides these insertions several other differences were found between the strains (Fig. 1). The 11 isolates fell into five different classes, with small differences between the classes. The five isolates with a single Ser61 repetition had identical DHPS sequences throughout. Similarly, the two isolates with Ser-Tyr insertions were identical in sequence, but the two isolates with double Ser insertions showed substantial variation in nucleotide sequence. The single isolate with a Tyr insertion had a distinctly different nucleotide sequence compared to the others. In all cases, many of the nucleotide differences were synonymous changes, and as a consequence the number of amino acid differences was less in each case, varying from 9 (WA-33) to 18 (WA-159) in total (Table 3). Isolate WA-33 had an identical amino acid sequence to CP1015 in some regions (e.g., from P122 to T170) where all other isolates showed several differences.
FIG. 1.
The nucleotide sequences of the folP genes from all Washington State isolates are compared. Only nucleotides that differ from those of CP1015 are shown. Isolate WA-133 had a sequence identical to that of WA-152. Isolates WA-54, WA-127, WA-970195, and WA-263 had sequences identical to that of WA-45.
TABLE 3.
Amino acid differences in DHPS predicted from nucleotide sequences
| Isolate | Repeat | Difference in amino acid from isolate CP1015
|
|||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S2 | S3 | A5 | V11 | L36 | D107 | P122 | H123 | V125 | E127 | A128 | R129 | Q131 | G155 | G157 | A159 | E163 | D167 | T170 | E175 | E178 | A179 | A189 | E190 | P195 | T202 | N247 | A274 | A287 | N307 | ||
| WA-152a | S62Y63 | V | A | N | A | E | K | V | K | K | D | T | N | Q | Q | L | V | D | |||||||||||||
| WA-159 | Y63 | L | A | N | A | I | K | D | G | K | N | N | T | N | Q | ||||||||||||||||
| WA-187 | S61S62 | T | V | A | I | A | K | G | K | S | T | K | D | V | S | ||||||||||||||||
| WA-45b | S61 | A | N | A | N | G | K | K | D | T | E | Q | V | D | |||||||||||||||||
| WA-33 | S61S62 | T | A | N | A | T | K | D | V | S | |||||||||||||||||||||
| WA-5 | I66E67 | A | A | K | G | K | T | K | D | V | S | ||||||||||||||||||||
Isolate WA-133 had an identical sequence.
Isolates WA-54, WA-127, WA-970195, and WA-263 had identical sequences.
The 6-bp repetition of isolate WA-5 results in high-level sulfonamide resistance in S. pneumoniae.
Isolate WA-5 had the same 6-bp repetition in the folP gene as originally described in the laboratory-selected mutant reported by Lopez et al. (8). In addition to the insertion, the WA-5 gene contained a number of nucleotide sequence differences throughout the length of the DHPS sequence compared to the reference Suls isolates R6 and CP1015 (8, 11). The number of amino acid differences was 10. It was therefore not clear whether the 2-amino-acid insertion by itself was responsible for the resistance of isolate WA-5. To examine this further, we deleted the 6-bp repetition by site-directed mutagenesis and compared the enzyme characteristics before and after removal of the 6 bp (Table 4). The change in the enzyme led to a 2.5-fold-lower Km for p-aminobenzoic acid (p-AB), no change to the Km for the pteridine substrate 2-amino-4-hydroxy-6-hydroxymethyl-7,8-dihydropteridine pyrophosphate (H2-pteridine), and a 35-fold reduction in Ki for sulfathiazole. The enzyme characteristics of the mutated WA-5 DHPS were compared with those of DHPS from the Suls reference strain CP1015 that was prepared in the same way as the WA-5 DHPS. The results from these two extracts were identical within experimental errors (Table 4), suggesting that the 6-bp insertion in WA-5 is both necessary and sufficient to confer clinically relevant Sulr and that the other mutations have no significant impact on the kinetic characteristics of DHPS.
TABLE 4.
Kinetic properties of DHPS enzymes from S. pneumoniae strains with different repeats of amino acids
| Source of DHPS | Amino acid repeat | Mean Km ± SD (μM) for:
|
Mean Ki for STZa ± SD (μM) | |
|---|---|---|---|---|
| p-AB | Pteridine | |||
| WA-5, Sulr | Ile66-Glu67 | 2.0 ± 0.18 | 5.3 ± 1.6 | 18 ± 4 |
| WA-5 Mut, Suls | None | 0.8 ± 0.12 | 5.5 ± 1.0 | 0.43 ± 0.16 |
| CP1015, Suls | None | 0.73 ± 0.04 | 4.5 ± 0.4 | 0.54 ± 0.19 |
| Pn93/720, Sulr | Ser61 | 2.6 ± 0.23 | 5.5 ± 1.0 | 7.5 ± 1.20 |
| J94/76, Sulr | Ser61-Ser62 | 1.3 ± 0.22 | 9.7 ± 0.5 | 12.3 ± 2.1 |
STZ, sulfathiazole.
Effects of Ser61 repetitions.
In a majority of the isolates studied here (Fig. 1) and previously (11, 12), Ser61 or Ser61-Ser62 are repeated, leading to a DHPS with either three or four sequential Ser residues. Transformation experiments reported earlier (11) showed that duplications of serine were sufficient to confer Sulr in pneumococci. To compare the effects on the activity of DHPS of the serine repetitions with the Ile-Glu repetitions in WA-5, DHPS from one isolate with a single Ser repetition and one with a double Ser repetition were analyzed with respect to enzyme kinetics. No large differences in Km for p-AB or H2-pteridine could be detected between the different variants of the enzyme (Table 4). The Km values for p-AB were two to four times higher than those for wild-type DHPS, and the only isolate with a twofold-higher Km for H2-pteridine was J94/76 with the double Ser repetition. The variation in Ki for sulfathiazole was more pronounced. Isolate WA-5 had the highest Ki, 18 μM; J94/76 was intermediate, with a Ki of 12.3 μM; and P93/720 showed the lowest Ki, 7.5 μM, which is still 15 times above the Ki of wild-type Suls DHPS. The main effect of the repetitions is thus an increased Ki for sulfathiazole which explains the resistance, while the relatively slight changes in Km for both substrates suggest a limited effect on enzyme function.
DISCUSSION
A large number of clinical isolates of Sulr S. pneumoniae harbor 3- or 6-bp repeats in the region coding for amino acids 58 to 67 of the folP gene, which encodes the drug target DHPS. The collection of isolates from Washington analyzed here all carried variants of these repeats. The repetition of codons for Ser62-Tyr63 is described for the first time. Isolates with a single Ser repeat were most common, but the majority of these belong to a clone that is widely spread in the area. One of the isolates, WA-5, carried the same insertion that was seen in the laboratory isolate first sequenced by Lopez et al. (8); other examples of this insertion were reported by Padayachee and Klugman (12). As with other resistant isolates described previously, the folP genes in the Washington isolates contain a number of mutations in other areas of the gene in addition to the insertions.
For three enzymes with insertions representing duplications of Ile66-Glu67, Ser61, and Ser61-Ser62, in addition to other mutations, the Ki for sulfathiazole was found to be more than 10fold higher than for DHPS from the control susceptible strain, CP1015. This was associated with a small rise in Km for p-AB (up to 3.5-fold), and in the case of the Ser61-Ser62 duplication, a 2-fold rise in Km for pteridine. The effects of the different duplications are apparently rather similar. In the WA-5 mutant enzyme, in which the Ile66-Glu67 was deleted but other mutations were retained, this created an enzyme that acted like the Suls enzyme. This suggests that the other mutations found in WA-5 have little or no effect on enzyme function and that the duplication alone is both sufficient and necessary for the generation of Sulr. The same is likely to be true for other resistant isolates which share many of the same mutations. The relatively small changes in Km for natural substrates contrasts with what we have found earlier for Neisseria meningitidis, where a 2-amino-acid insertion does change the Km for both substrates substantially and where other mutations are necessary for stable resistance (5, 13). This difference may partially explain why Sulr S. pneumoniae isolates are common and more varied in sequence. To explain how these amino acid repetitions can have the effect of substantially reducing inhibitor binding while not severely affecting substrate binding will require more-precise characterization of substrate-enzyme interaction. A recent publication describes substrate binding to DHPS from Mycobacterium tuberculosis and clearly shows that the region of the enzyme we study here is involved in forming a pocket for binding of p-AB (2).
The demonstration within isolates from Washington of a duplication leading to resistance that has not previously been described emphasizes that resistance is likely to have arisen independently on several occasions. The duplications appear to occur easily, and it is possible that the region contains a hot spot for replication errors. One cannot exclude the possibility that pneumococci have acquired resistant folP genes from related streptococci, in a manner analogous to the generation of penicillin resistance through mosaic penicillin binding proteins (4). We have earlier shown that the development of a resistant DHPS directly in N. meningitidis is unlikely and that the resistance determinant in this case has been acquired by transformation. Both in N. meningitidis and in Streptococcus pyogenes there are more-extensive differences in amino acid sequence between DHPS from resistant and susceptible strains, respectively (5, 13, 16). However, the data presented here for S. pneumoniae are most consistent with several independent replication errors which duplicate one or two amino acids in a particular region of the DHPS. These duplications do not seem to lead to negative consequences for DHPS function, and the ability of the S. pneumoniae to mutate to Sulr should be considered prior to any further development of DHPS inhibitors.
ACKNOWLEDGMENTS
We thank Kristina Lundberg for technical assistance in sequence determinations and Elisabeth Richter for performing some of the DHPS assays.
This work was supported by a grant from the Swedish Medical Research Council to Göte Swedberg (K2000-16X-000172-36B).
REFERENCES
- 1.Adrian P V, Klugman K P. Mutations in the dihydrofolate reductase gene of trimethoprim-resistant isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:2406–2413. doi: 10.1128/aac.41.11.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baca A M, Sirawaraporn R, Turley S, Sirawaraporn W, Hol W G J. Crystal structure of Mycobacterium tuberculosis 6-hydroxymethyl-7,8-dihydropteroate synthase in complex with pterin monophosphate: new insight into the enzymatic mechanism and sulfa-drug action. J Mol Biol. 2000;302:1193–1212. doi: 10.1006/jmbi.2000.4094. [DOI] [PubMed] [Google Scholar]
- 3.Dower W J, Miller J F, Ragsdale C W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988;16:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dowson C G, Coffey T J, Spratt B G. Origin and molecular epidemiology of penicillin-binding-protein-mediated resistance to β-lactam antibiotics. Trends Microbiol. 1994;2:361–366. doi: 10.1016/0966-842x(94)90612-2. [DOI] [PubMed] [Google Scholar]
- 5.Fermér C, Swedberg G. Adaptation to sulfonamide resistance in Neisseria meningitidis may have required compensatory changes to retain enzyme function: kinetic analysis of dihydropteroate synthases from N. meningitidis expressed in a knockout mutant of Escherichia coli. J Bacteriol. 1997;179:831–837. doi: 10.1128/jb.179.3.831-837.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frick P A, Black D J, Duchin J S, Deliganis S, McKee W M, Fritsche T R. Prevalence of antimicrobial drug-resistant Streptococcus pneumoniae in Washington State. West J Med. 1998;169:364–369. [PMC free article] [PubMed] [Google Scholar]
- 7.Klugman K P. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990;3:171–196. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez P, Espinosa M, Greenberg B, Lacks S A. Sulfonamide resistance in Streptococcus pneumoniae: DNA sequence of the gene encoding dihydropteroate synthase and characterization of the enzyme. J Bacteriol. 1987;169:4320–4326. doi: 10.1128/jb.169.9.4320-4326.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luna V A, Roberts M C. In-vitro activities of 11 antibiotics against 75 strains of Streptococcus pneumoniae with reduced susceptibilities to penicillin isolated from patients in Washington state. J Antimicrob Chemother. 1999;44:578–580. doi: 10.1093/jac/44.4.578. [DOI] [PubMed] [Google Scholar]
- 10.Luna V A, Jernigan D B, Tice A, Kellner J D, Roberts M C. A novel multiresistant Streptococcus pneumoniae serogroup 19 clone from Washington State identified by pulsed field gel electrophoresis and restriction fragment length patterns. J Clin Microbiol. 2000;38:1575–1580. doi: 10.1128/jcm.38.4.1575-1580.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maskell J P, Sefton A M, Hall L M C. Mechanism of sulfonamide resistance in clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:2121–2126. doi: 10.1128/aac.41.10.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padayachee T, Klugman K P. Novel expansions of the gene encoding dihydropteroate synthase in trimethoprim-sulfamethoxazole-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:2225–2230. doi: 10.1128/aac.43.9.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qvarnström Y, Swedberg G. Additive effects of a two-amino-acid insertion and a single-amino-acid substitution in dihydropteroate synthase for the development of sulphonamide-resistant Neisseria meningitidis. Microbiology. 2000;146:1151–1156. doi: 10.1099/00221287-146-5-1151. [DOI] [PubMed] [Google Scholar]
- 14.Sarkar G, Sommer S S. The “megaprimer” method of site-directed mutagenesis. BioTechniques. 1990;8:404–407. [PubMed] [Google Scholar]
- 15.Swedberg G, Sköld O. Characterization of different plasmid-borne dihydropteroate synthases mediating bacterial resistance to sulfonamides. J Bacteriol. 1980;142:1–7. doi: 10.1128/jb.142.1.1-7.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swedberg G, Ringertz S, Sköld O. Sulfonamide resistance in Streptococcus pyogenes is associated with differences in the amino acid sequence of its chromosomal dihydropteroate synthase. Antimicrob Agents Chemother. 1998;42:1062–1067. doi: 10.1128/aac.42.5.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomasz A. Antibiotic resistance in Streptococcus pneumoniae. Clin Infect Dis. 1997;24(Suppl. 1):S85–S88. doi: 10.1093/clinids/24.supplement_1.s85. [DOI] [PubMed] [Google Scholar]
- 18.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]