Abstract
Background
Whilst myocarditis or myocardial injury due to severe acute respiratory syndrome coronavirus 2 infection is commonly reported, profound primary cardiac dysfunction requiring mechanical circulatory support, with the development of fulminant myocarditis prior to respiratory failure, is rarely described. The endomyocardial biopsy (EMB) findings in these patients is seldom reported, the findings are varied, and effective treatment unknown.
Case summary
A 39-year-old female with no significant past medical history and confirmed Delta variant coronavirus disease 2019 (COVID-19) infection (Day 3), presented with a 1 day history of diarrhoea, vomiting, and abdominal pain. The patient denied respiratory symptoms and chest X-ray was clear. Lactate level was 6.3, initial troponin T 118 ng/L. Despite resuscitation, the patient significantly deteriorated in the emergency department, resulting in pulseless electrical activity arrest requiring veno-arterial extra-corporeal membrane oxygenation cardiopulmonary resuscitation. Over the following 36 h, cardiac function deteriorated to near-complete left ventricular (LV) standstill. Coronary angiography revealed normal coronary arteries with slow flow. Endomyocardial biopsy showed diffuse interstitial macrophage infiltrate and small vessel thromboses. Left ventricular function did not improve over the following 7 days, and despite treatment with tocilizumab, high-dose steroids, and intravenous immunoglobulin, she eventually died due to disease-related complications.
Discussion
Primary cardiac dysfunction secondary to COVID-19 infection is rarely reported. Little is known about the incidence, natural history, and pathophysiology of fulminant COVID-19 myocarditis. We present the most severe case of cardiac dysfunction due to COVID-19 reported in a young patient without respiratory compromise who never recovered from any cardiac function.
Keywords: Case report, COVID-19, Fulminant myocarditis, Myocardial injury, Mechanical circulatory support, Endomyocardial biopsy
Learning points.
Coronavirus disease 2019 may present with primary cardiac dysfunction, prior to significant respiratory symptoms.
Early risk stratification of patients remains challenging and troponins may remain low despite significant myocardial oedema.
Endomyocardial biopsy should be considered in cases of cardiac dysfunction.
Introduction
The effects of coronavirus disease 2019 (COVID-19) on the cardiovascular system are multifaceted with inflammatory-mediated vasoplegia, myocardial ischaemia, arrythmias, and myocarditis all reported.1,2 Whilst myocarditis and/or myocardial injury in patients with COVID-19 is reported with an incidence between 2% and 20%,3,4 there exists very few reports of patients presenting with primary severe cardiac dysfunction and even fewer requiring mechanical circulatory support (MCS) prior to respiratory failure.
We present the case of a previously healthy 39-year-old female with known COVID-19 who rapidly progressed to cardiac arrest within hours of presentation, requiring veno-arterial extra-corporeal membrane oxygenation (V-A ECMO) support, with subsequent development of fulminant myocarditis and who died due to her disease.
Timeline
| Day | Events |
|---|---|
| 4 days prior to presentation | Close contact with a coronavirus disease 2019 (COVID-19) positive patient. |
| 3 days prior to presentation | Positive polymerase chain reaction for COVID-19. |
| Day 0 | Presented to emergency department with diarrhoea, vomiting and abdominal pain. Progressed to pulseless electrical activity cardiac arrest with extra-corporeal membrane oxygenation (ECMO)–cardiopulmonary resuscitation (CPR). Distal perfusion cannulae placed. |
| Day 1 | Treatment with intravenous immunoglobulin (3 days), methylprednisolone (3 days), and tocilizumab. Pericardial tamponade requiring insertion of pericardial drain. Complete loss of left ventricular (LV) ejection requiring insertion of LV venting cannula. |
| Day 3 | Coronary angiogram and endomyocardial biopsy performed via right common femoral artery (CFA). Development of ischaemic left lower leg requiring digital subtraction angiography (DSA) and fasciotomy [extra-corporeal membrane oxygenation (ECMO) return cannulae with distal perfusion cannulae in left CFA]. |
| Day 4 | Insertion of additional access and return ECMO cannulae. |
| Day 5–6 | Worsening myocardial oedema, rising troponin, and creatinine kinase (CK), no improvement in LV function. |
| Day 7 | Development of ischaemic right lower leg (without ECMO cannulae) requiring DSA and fasciotomy. Ischaemia persisted and amputation deemed not possible. |
| Day 8 | Multi-disciplinary team and family discussions. |
| Day 9 | Decannulated and patient died. |
Case presentation
A 39-year-old previously healthy woman was brought in by ambulance to the emergency department (ED) with a 1-day history of profuse diarrhoea, vomiting, abdominal pain, and no respiratory symptoms. Nasopharyngeal swab polymerase chain reaction (PCR) for severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) was positive 3 days earlier, with genomic sequencing identifying the Delta variant. Her only past medical history was laparotomy with ovarian cystectomy, she was of Japanese ethnicity, there was no significant family history of cardiac pathology, and she was not vaccinated against COVID-19. On paramedic arrival, the patient was hypotensive with systolic blood pressure (SBP) 70 mmHg, heart rate (HR) 120, oxygen saturations 100% on room air, and a temperature of 36.9. She received 500 mL bolus of Hartmann’s solution and was transported to ED. At triage, she was alert but looking pale and lethargic, with initial vital signs demonstrating a HR of 98 b.p.m. in sinus rhythm, blood pressure 82/69 mmHg, respiratory rate 18 b.p.m., oxygen saturations of 98% on room air, and a temperature of 36.6°C. Physical examination revealed normal heart sounds with no murmurs, no pericardial rub, warm peripheries, a capillary refill of <3 s, and no peripheral oedema. Jugular venous pressure was not visualized. There were no rales and the abdomen was soft and non-tender.
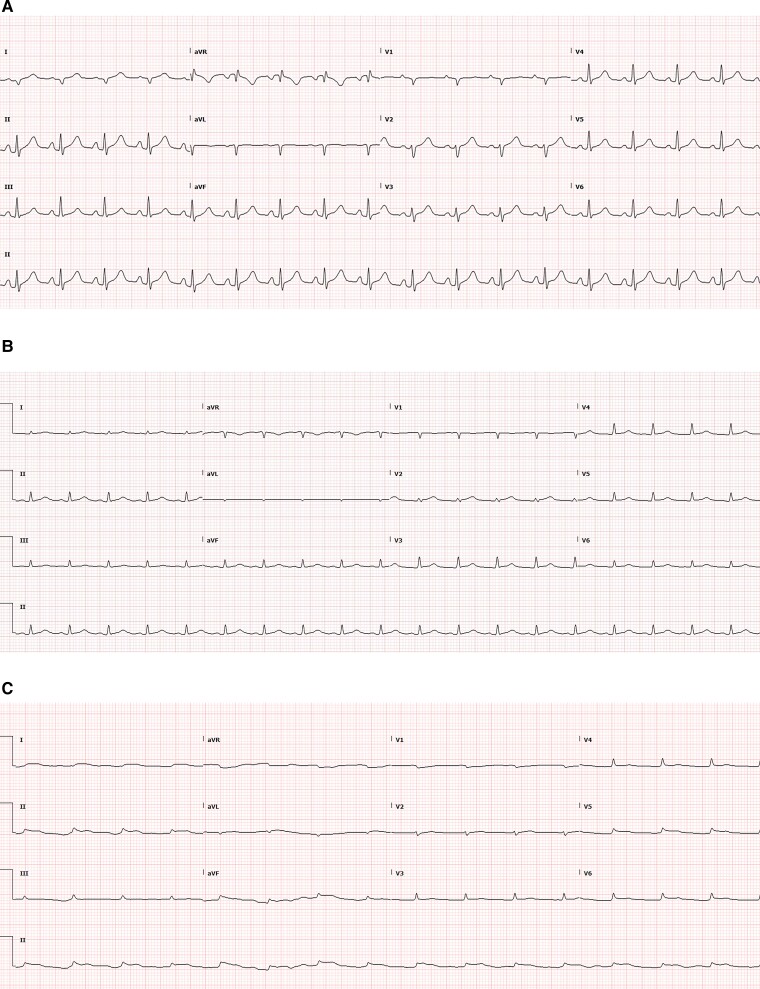
Electrocardiogram (ECG) demonstrated normal sinus rhythm (102 b.p.m.) without ST changes (Figure 1A). Initial high-sensitivity Troponin T was 118 ng/L (normal <14 ng/L), C-reactive protein (CRP) 1.6 (Table 1). Venous blood gas revealed pH 7.24, bicarbonate 22, lactate 6.3, and base excess (BE) -6. Resuscitation was commenced with 1 L of 0.9% saline.
Figure 1.
Electrocardiograms (A) electrocardiogram on admission demonstrating sinus tachycardia with normal QRS and ST segments. (B) Electrocardiogram on Day 2 admission demonstrating low-voltage QRS segments. (C) Electrocardiogram on Day 4 admission demonstrating a progression of myocardial oedema, with low voltage and a broadening of the QRS.
Table 1.
| Normal Values | Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | |
|---|---|---|---|---|---|---|---|---|---|---|
| hsTroponin (ng/L) | <14 | 118 | 931 | 1452 | 3805 | 3452 | 7715 | 9830 | 13 408 | 10 790 |
| NT pro-BNP (ng/L) | <125 | 6543 | 23 616 | 11 517 | ||||||
| Lactate (mmol/L) | <1.9 | 6.1 (venous) | 11.2 | 9.7 | 4.1 | 3.9 | 2.8 | 2.6 | 2.3 | 2.4 |
| CRP (mg/L) | <5 | 1.6 | 1.4 | 5.6 | 7.1 | 18.6 | 45.1 | 117.3 | ||
| D-dimer (mg/L) | <0.25 | >10 | 5.64 | 3.98 | 1.52 | 2.23 | 3.51 | 3.22 | 1.88 | |
| LDH (U/L) | <250 | 306 | 771 | 643 | 604 | 553 | 2005 | 4341 | 5473 | 5913 |
| Ferritin (g/L) | 30–150 | 175 | 3522 | 1151 | 183 | 287 | 357 | |||
| CK (U/L) | 30–150 | 186 | 14 689 | 64 171 | 136 720 | 153 260 | ||||
| Hb (g/L) | 120–150 | 158 | 101 | 105 | 92 | 91 | 79 | 79 | 77 | 75 |
| Platelets (×109/L) | 150–400 | 134 | 99 | 75 | 59 | 67 | 60 | 81 | 93 | 64 |
| WCC (×109/L) | 4–10 | 7.8 | 11.6 | 20.3 | 19.9 | 9.1 | 21.8 | 30.5 | 30.6 | 29.8 |
| K (mmol/L) | 3.5–5.2 | 4.4 | 4.1 | 4.7 | 5.8 | 6.5 | 5.6 | 4.0 | 4.3 | 4.8 |
There was a gradual deterioration during the 4 h in the ED. Repeat blood gas revealed a worsening lactatemia (lactate 7.2), and a second 1 L 0.9% saline, followed by 1 L Hartmann’s solution, was administered for ongoing hypotension (SBP 80–90 mmHg). At ∼4 h after ED presentation, she became critically unwell with profound shock (SBP 53 mmHg), reduced level of consciousness, delayed capillary refill, and a temperature of 31°C. The patient became acutely bradycardic and then suffered a pulseless electrical activity cardiac arrest.
Cardiopulmonary resuscitation (CPR) was commenced, and three rounds of adrenaline (1 mg IV) were administered without a sustained return of spontaneous circulation. The ECMO team arrived within 25 min and V-A ECMO [bi-femoral 23 French (Fr) access, 15 Fr return cannulae (Maquet)] was established 16 min later. The time from arrest to V-A ECMO flow was 42 min; 7 Fr distal perfusion cannulae was inserted post establishment of ECMO flows. Inotropic and vasopressor support with dobutamine, noradrenaline, and vasopressin were commenced. Extra-corporeal membrane oxygenation flows were maintained at ∼3.7 litres per minute (LPM), with left ventricular (LV) ejection present and a pulse pressure of 10–15 mmHg. Bivalirudin was commenced targeting a therapeutic range of activated partial thromboplastin time 50–70 s.
Computed tomography (CT) of the chest reported bilateral patchy consolidation and ground glass opacification in the lower lobes. There were no significant findings on CT of the abdomen and brain.
On trans-thoracic echocardiogram (TTE), there was a severely reduced biventricular function, normal cardiac chamber size, and a globally thickened, bright myocardium (measuring 16 mm in the septum and inferolateral wall) with a small-to-moderate-sized pericardial effusion (see Supplementary material online, Video 1). Owing to increasing inotropic and vasopressor requirement with significant pulsus paradoxus, urgent pericardiocentesis was performed, which resulted in immediate improvement in native cardiac output. Continuous renal replacement therapy, hydrocortisone 50 mg 6-hourly, and broad-spectrum antibiotics (piperacillin/tazobactam and vancomycin) were commenced.
On Day 1 admission, troponin levels remained modestly raised (Table 1), CRP was normal, and ferritin elevated. Left ventricular function declined to near ventricular standstill (see Supplementary material online, Video 1) with a reduction in pulse pressure. Surgical LV venting cannula (via mini-thoracotomy and right super pulmonary vein) was performed. Empirical treatment with methylprednisolone 1 g (3 days), intravenous immunoglobulin (IVIG) 1 g/kg (2 days), and Tocilizumab was completed.
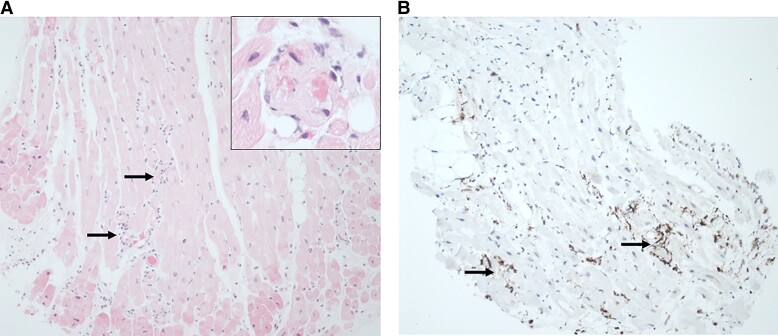
Coronary angiogram and endomyocardial biopsy (EMB) were performed, demonstrating severe slow flow with no coronary lesions (see Supplementary material online, Video 2). Endomyocardial biopsy returned the following day, showing a subtle mild interstitial infiltrate consisting mostly of CD68+ macrophages along with a lesser number of CD3+ T cells. Microthrombi were identified, with the degradation products present suggestive of a sub-acute microangiopathic process (Figure 2). No fibrosis nor myocardial necrosis was present. No viral particles were seen on electron microscopy. With regard to other causes of myocarditis, the EMB did not show interstitial fibrosis, iron accumulation, evidence of sarcoid or amyloid, or changes consistent with hypertrophic obstructive cardiomyopathy. A number of investigations for other causes of myocarditis were also performed, including, extended viral PCR panel, serology for coxsackie and echovirus, human immunodeficiency virus (HIV)1/2 and hepatitis C antibodies, and autoimmune and immunological screens. All were negative.
Figure 2.
Endomyocardial biopsy slides (A) mild diffuse interstitial infiltrate of macrophages (arrows) and multiple small vessel thrombi (inset) but no evidence of lymphocytic myocarditis. (B) An immunohistochemical stain for CD68 highlights the diffuse macrophage infiltration (arrows).
On Day 3, digital subtraction angiography (DSA) of the left leg demonstrated severe vasospasm of the anterior and posterior tibial arteries but patent flow into the foot requiring four-compartment fasciotomies.
Over the following days, a widening and flattening of the QRS on ECG (Figure 1B and C) was noted. Serial TTE demonstrated no recovery of LV function with persistent near-complete cardiac standstill. Troponin rose to a peak of 13 408 g/L on Day 7. Profound anasarca developed with capillary leak. Systemic perfusion was maintained by ECMO flows of ∼4.0 LPM with high but stable inotropic and vasopressor requirements.
On Day 7 of admission, the patient developed critical ischaemia of the right leg from vasospasm in the proximal tibial vessels. Subsequent DSA revealed no distal flow and ultimately an unsalvageable distal right leg. There was extensive multidisciplinary discussion with the family and then consensus reached that this was not a survivable situation. The goals of care were transitioned to that of comfort and dignity and the patient died 9 days after arriving to hospital.
Discussion
We describe the case of a 39-year-old previously healthy female who presented without respiratory symptoms, in shock, who then subsequently developed fulminant COVID-19 myocarditis. After 8 days on V-A ECMO, treatment with IVIG and methylprednisolone, cardiac function did not recover from near-complete cardiac standstill.
The severity of cardiac dysfunction due to fulminant COVID-19 myocarditis in this case has not been reported. A very small number of case reports describe patients with severe cardiac dysfunction requiring MCS; however, they all a demonstrated recovery of cardiac function within 7 days.5–8 Two of those patients suffered cardiac arrest prior to the initiation of MCS; however, both had alternative reasons to arrest (cardiac tamponade7 and during insertion of pulmonary artery catheter6).
The isolated cardiac dysfunction without significant respiratory symptoms is also unique to this case. Despite significant oedema on echocardiography and LV dysfunction, initial troponins were remarkably low and rose to levels more consistent with myocarditis throughout her admission. Given that COVID-19 infection is associated with microthrombi (as was found on our patient’s EMB), it is possible that the myocardial oedema seen on echocardiography and slow coronary flow was a result of profound microvascular dysfunction from thrombi deposition, and myocyte disruption was minimal, resulting in the unexpectedly low initial troponins.
We have found no other cases of patients requiring MCS who did not have respiratory symptoms prior to developing cardiogenic shock. One case report claims to present the first patient with fulminant COVID-19 myocarditis without respiratory failure,8 although we note that the patient presented with a 4-day history of cough and required supplemental oxygen prior to developing cardiogenic shock. That patient’s cardiac function recovered within 7 days, without treatment with IVIG or steroids. Our patient’s CT chest demonstrated changes consistent with COVID-19 pneumonitis; however, they never had respiratory symptoms or required oxygen.
The pathophysiological aetiology of fulminant COVID-19 myocarditis is not established.11 The histopathological pattern of diffuse interstitial macrophage infiltrate and small vessel thromboses seen in patients with COVID-19 associated myocardial injury12,13 was also demonstrated on EMB in our case. This pattern was seen in case reports demonstrating SARS-COV-2 viral particles in the myocardium on PCR5,14; however, it was notably distinct from the dense lymphocytic infiltrate of a typical viral myocarditis. The general consensus in the limited literature is that the underlying mechanism of cardiac dysfunction is likely due to the viral-induced inflammatory response rather than direct viral-mediated myocyte destruction, possibly supported by an absence of viral particles on electron microscopy in our case.
An important discussion point is whether myocarditis was, in fact, due to COVID-19, or an alternate cause of myocarditis, or another pathology. Whilst other causes of myocarditis are possible, given the clinical course, a lack of exposure to, or symptoms suggestive of, other causes and the additional testing that was completed makes another cause other than COVID-19 very unlikely. In particular, the EMB demonstrated features consistent with published autopsy findings in COVID-19, and a lack of classical findings of other causes of myocarditis. These results, along with the positive findings mentioned previously, suggest no alternate cause for myocarditis in this case.
To our knowledge, this is the first reported case of primary cardiac dysfunction due to COVID-19 myocarditis, without respiratory compromise, in a patient who never recovered from any cardiac function, despite MCS with V-A ECMO. Early data suggest that the symptomatology of COVID-19 is changing15 and that rates of myocarditis are increasing.16 Our hope is that the information in this report may provide new insights into this ever-evolving disease.
Supplementary Material
Lead author biography

Alistair Thomson is an ICU trainee at Royal Prince Alfred Hospital, Sydney. He has a particular interest in cardiothoracic intensive care and anaesthesia.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for
Consent: Informed written consent was obtained from the patient’s family as well as the locally appointed ethics committee for the submission and publication of this case report, including images and the associated text.
Conflict of interest: None declared.
Funding: Dr Mark Dennis is supported by a Post-Doctoral Scholarship (Ref: 105849) from the National Heart Foundation of Australia.
References
- 1. Chow J, Alhussaini A, Calvillo-Argüelles O, Billia F, Luk A. Cardiovascular collapse in COVID-19 infection: the role of venoarterial extracorporeal membrane oxygenation (VA-ECMO). CJC Open 2020;2:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pellicori P, Doolub G, Wong CM, Lee KS, Mangion K, Ahmad M, Berry C, Squire I, Lambiase PD, Lyon A, McConnachie A, Taylor RS, Cleland JG. COVID-19 and its cardiovascular effects: a systematic review of prevalence studies. Cochrane Database Syst Rev 2021;2021:CD013879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Halushka M, Vander Heide R. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol 2021;50:107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bozkurt B, Kamat I, Hotez P. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021;144:471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Albert CL, Carmano-Rubio AE, Weiss AJ, Procop GG, Starling RC, Rodriguez ER. The enemy within: sudden-onset reversible cardiogenic chock with biopsy-proven cardiac myocyte infection by severe acute respiratory syndrome coronavirus 2. Circulation 2020;142:1865–1870. [DOI] [PubMed] [Google Scholar]
- 6. Irabien-Ortiz A, Carreras-Mora J, Pamies J, Montiel J, Tauron M. Fulminant myocarditis due to COVID-19. Rev Esp Cardiol 2020;73:503–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sampaio PRN, Ferreira RM, de Albuquerque FN, Colafranceschi AS, Almeida ACP, Nunes MAV, Mansur Filho J, Lima RAC. Rescue venoarterial extracorporeal membrane oxygenation after cardiac arrest in COVID-19 myopericarditis: a case report. Cardiovasc Revasc Med 2021;28:57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Papageorgiou J-M, Almroth H, Tornudd M, van der Wal H, Varelogianni G, Sederholm Lawesson S. Fulminant myocarditis in a COVID-19 positive patient treated with mechanical circulatory support – a case report. Eur Heart J Case Rep 2021;5:ytaa523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bernal-Torres W, Herrera-Escandon A, Hurtado-Rivera M, Plata-Mosquera CA. COVID-19 fulminant myocarditis: a case report. Eur Heart J Case Rep 2020;4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paul JF, Charles P, Richaud C, Caussin C, Diakov C. Myocarditis revealing COVID-19 infection in a young patitent. Eur Heart J - Cardiovasc Imaging 2020;21:776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mele D, Flamigni F, Rapezzi C, Ferrari R. Myocarditis in COVID-19 patients: current problems. Intern Emerg Med 2021;16:1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, Aubry M-C, Bois MC, Lin PT, Maleszewski JJ, Stone JR. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J 2020;41:3827–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fox SE, Falgout L, Vander Heide RS. COVID-19 myocarditis: quantitative analysis of the inflammatory infiltrate and a proposed mechanism. Cardiovasc Pathol 2021;54:107361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 2020;22:911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spector T. ZOE COVID study. What are the new top 5 symptoms? London, UK. Updated 2021 July. https://covid.joinzoe.com/post/new-top-5-covid-symptoms [Google Scholar]
- 16. Szarpak L, Jaguszewski M, Pruc M, Rafique Z. Myocardial injury: a future challenge for long-COVID-19 complications. Eur. Heart J. - Qual. Care Clin. Outcomes 2021;7:618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.