Abstract
Vegetables are particularly rich sources of micronutrients and phytochemicals such as polyphenols and vitamins. These plant-derived bioactive compounds provide antitumor and antioxidant properties due to their capacity to interact with reactive oxygen species (ROS). The objective of this study was to determine the effect of iodine biofortification (potassium iodate/KIO3/, 5-iodosalicylic acid/5-ISA/, and 3,5-diiodosalicylic acid/3,5-diISA/) on the antioxidant activity of lettuce (Lactuca sativa L. capitata) cv. ‘Melodion’. In this work, HPLC analysis was used to identify polyphenolic compounds while the antioxidant activity of iodine-enriched vegetables was determined by using DPPH, ABTS and FRAP methods. The content of the water-soluble vitamins was analyzed by using the LC-MS/MS technique. The impact of extracts from iodine-biofortified lettuce on production of reactive oxygen species (ROS) in gastrointestinal cancer cells was also evaluated. The results from this research indicate that application of iodine compounds improves the antioxidant potential of lettuce by increasing the concentration of some vitamins, antioxidant enzymes and polyphenolic compounds in the enriched plants. Moreover, the study has shown that iodine-biofortified lettuce induces production of ROS in cancer cells, resulting in an anticancer effect by the induction of programmed cancer cell death.
Vegetables are particularly rich sources of micronutrients and phytochemicals such as polyphenols and vitamins.
1. Introduction
The right balance between antioxidant defense and the production of reactive oxygen species plays a crucial role in all living organisms. Disruption of this homeostasis may cause cellular damage and genome instability and induce carcinogenic processes.1,2 Depending on the type of cells, reactive oxygen species may affect them in different ways. In cancer cells, the higher level of ROS may cause a cytotoxic effect and lead to the cell cycle interruption that induces the programmed cell death pathway.3,4 The mechanism of oxidative damage is commonly used in chemotherapy of cancer in which chemotherapeutics agents induce ROS generation.5
Plants are regarded as a good source of natural exogenous antioxidants that can contribute to reduction of oxidative stress and anti-ageing effects by eliminating ROS.6 Therefore, it is necessary to maintain the appropriate level of fruit and vegetables in the daily diet to avoid dietary antioxidant deficiency and prevent chronic and degenerative illnesses. However, there is evidence that bioactive compounds derived from natural products also have the ability to reduce antioxidant potential in cancer cells and increase their sensitivity to oxidative stress.7,8
Currently, numerous studies have been conducted to understand the role of nuclear factor erythroid 2-related factor 2 (Nrf2) in cancer chemoprevention. Nrf2 is a critical transcription factor in human cells that controls oxidative stress by increasing the expression of genes involved in the antioxidant defense.9 The research showed that high concentration of natural phytochemicals may have anticancer properties by effective inhibiting of Nrf2 pathway in cancer cells.8
Polyphenols, carotenoids and vitamins belong to natural exogenous antioxidants that are present in food products. The most important vitamins with antioxidant activity are vitamin E and vitamin C that protect cells against reactive oxygen species and oxidative damage.10,11 The high antioxidant potential of plants results from the fact that they have enzymatic and non-enzymatic mechanisms, which play an important role in protecting cells from damage caused by the free radicals. The first line of defense by antioxidants is formed by enzymes, such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx) and glutathione reductase (GR). Non-enzymatic systems are composed of compounds of low molecular weight, such as phenolic acids and glutathione, as well as those that have high molecular weight, such as secondary products of plants.6
Lettuce is considered to be a frequently consumed salad vegetable that is rich in phytonutrients. The concentration of bioactive compounds and antioxidant activity in lettuce may depend on variation and species, but they may vary within the same species. Moreover, the growing conditions and genotype also have an impact on composition of biologically active compounds in plants.12,13
The objective of the study was to evaluate the influence of iodine biofortification (potassium iodate/KIO3/, 5-iodosalicylic acid/5-ISA/, and 3,5-diiodosalicylic acid/3,5-diISA/) on the antioxidant activity of lettuce (Lactuca sativa L. capitata) cv. ‘Melodion’. Furthermore, we determined the oxidative stress level in human gastrointestinal cancer cells treated with extracts from iodine-biofortified lettuce.
2. Materials and methods
2.1. Plant material and cultivation
The detailed description concerning the plant material and growning condition was described in our previous study.14 The hydroponic cultivation of lettuce L. sativa cv. ‘Melodion’ was conducted in an NFT (Nutrient Film Technique) system. The experiments were located in a greenhouse of University of Agriculture in Kraków (50°05′04.1′′N 19°57′02.1′′E). Seed sowing was performed at the beginning of March (13 March 2018 and 4 March 2019). Seeds were sown into 112-cell propagation trays with 32 × 32 × 40 mm sized cells filled with peat substrate mixed with sand (1 : 1 v/v). Seedlings of 4–5 true leaves were transplanted into the NFT system (10 April 2018 and 2 April 2019). Seedlings were placed into holes (spaced 25 cm apart) of styrofoam slabs filling NFT beds. The nutrient solution contained macro- and micronutrients [concentrations of elements and types of fertilizers used for the preparation of the medium see Sularz et al.14]; EC of nutrient solution of all treatments in all the experiments was 1.75 mS cm−1 and the pH of all nutrient solutions was adjusted to 5.70 with the use of 38% nitric acid.
The distinguishing factor of experiment was the chemical form of iodine applied to nutrient solution in NFT systems: (1) control (non-biofortified lettuce); (2) KIO3, (3) 5-iodosalicylic acid (5-ISA), (4) 3.5-diiodosalicylic acid (3.5-diISA). Inorganic (KIO3) and organic forms of iodine, i.e., 5-ISA and 3.5-diISA (all puriss p.a., Sigma-Aldrich Co. LLC, St. Louis, MO, USA) were applied once in a concentration of 10 μM calculated per molar mass of a whole compound. The application of these compounds in to nutrient solution was started when the plants were in the rosette phase (18 April, 2018 and 12 April, 2019). The iodine used in base nutrient solutions (control) was iodide I− (25.52 μg I·dm−3) and iodate IO3− (0.29 μg I·dm−3). The content of iodine was natural (from water and dissolved fertilizers). The selection of dose and type of applied iodine compounds was based on the previous studies of our team performed on lettuce and tomato.15–17
The experiment was conducted in randomized block design with four repetitions within one NFT set. Plants were cultivated in four replications of 15 plants (60 plants per treatments). The plants were harvested at the stage of head production by plants – 15 May 2018 and 7 May 2019. The average head weight of lettuce head was measured during the plants harvest – see Sularz et al.14 For all further described chemical analyses the lettuce heads were cut in half and mixed in order to obtain a representative sample of all leaves (old and young) from all heads in each treatment.
2.2. Determination of water-soluble vitamins (B1, B2, B3, B5, B6, B7, B9, PP)
The content of the following water-soluble vitamins was analyzed using the LC-MS/MS technique: B1 (thiamine hydrochloride), B2 (riboflavin), B3 (nicotinic acid), B5 (pantothenic acid), B6 (pyridoxine hydrochloride), B7 (D-biotin), B9 (folic acid) and PP (nicotinamide). To 7 mL polypropylene tube: 0.075 g air-dried, grounded plant samples were weighted and 5 mL of extractant was added. Extractant was mixture of ACN/H2O/100 mM NH4Ac (90/5/5, v/v/v), pH 5.8. After mixing, samples were incubated for 15 min in room temperature in ultrasonic bath, mixed thoroughly and centrifuged for 15 min at 4500 rpm. The supernatants were filtered through a 0.22 μm syringe filter. Content of this water soluble vitamins was determined in these extracts using LC-MS/MS.18 Mass spectrometer with electrospray ionization (4500 Qtrap, Sciex) was coupled to liquid chromatography (Ultimate 3000, Thermo Scientific). This method employed HILIC column (Phenomenex Luna 3 μm HILIC 200 Å, 100 mm × 2 mm) with precolumn (Phenomenex Security Guard Cartriges, 4 × 2.0 mm, HILIC). The column temperature was set to 25 °C. Acetonitrile (A), demineralized water (B) and 100 mM NH4Ac (C) were used as eluents. The analysis lasted 20 min. To separate vitamins multi-step gradient at 0.3 mL min−1 (0–8 min and 17–20 min) and 0.5 mL min−1 (8–16 min) was used. Gradient was 0% B (0–8 min), 0–35% B (8–15 min), 35–0% B (15–15.2 min), 0% B (15.2–20 min) with constant 10% of mobile phase C. Electrospray ionization (ESI) in positive ion mode was used. Scheduled MRM mode was used for quantitative studies.18 The LC-MS/MS system was controlled using Analyst 1.7 with HotFix 3 software, which was also used for data processing.
Determination of vitamins B was based on calibration of standard solutions with growing concentrations. Standard solutions was analysed before analysis of samples. Then using concentrations and the area of peaks the calibration line was made. Area of each vitamin in sample was then related to its linear function. To calculate amount of each vitamin B following formula was used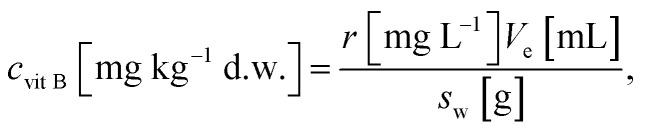 where: r – is result in mg L−1 from calibration, Ve – is volume of extractant, Sw – is sample weight (dry).
where: r – is result in mg L−1 from calibration, Ve – is volume of extractant, Sw – is sample weight (dry).
2.3. Determination of content of l-ascorbic acid
The content of ascorbic acid (AA) and dehydroascorbic acid (DHA) in fresh leaf samples was analyzed by Beckman PA 800 Plus capillary electrophoresis (CE) system with DAD detection according to method described in the earlier publication.19 The content of AA in leaves was analyzed after the homogenisation of 20 g samples in 80 cm3 of 2% oxalate acid (puriss p.a., Avantor Performance Materials) and further centrifugation for 15 min at 4500 rpm, 5 °C. The supernatants were collected, further centrifuged for 10 min at 10 000 rpm, and analyzed by capillary electrophoresis system with diode array detector (DAD) detection. Capillaries of 50 μm i.d. and 365 μm o.d. and those of a total length of 50 cm (40 cm to detector) were used. A negative power supply of 25 kV was applied. The running buffer solution containing 30 mM NaH2PO4 (puriss p.a., Avantor Performance Materials), 15 mM Na2B4O7 (puriss p.a., Sigma-Aldrich) and 0.2 mM cetyltrimethylammonium bromide (CTAB) (puriss p.a., Sigma-Aldrich) (pH 8.80).19
Determination of vitamin C was based on calibration of standard solutions with growing concentrations. Standard solutions was analysed before analysis of samples. Then using concentrations and the area of peaks the calibration line was made. Area of vitamin C in sample was the related to its linear function. To calculate amount of nine vitamins following formulas was used:
To quantify amount of vitamin C: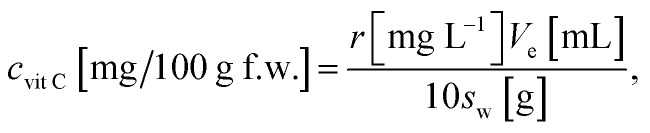 where: r – is result in mg L−1 from calibration, Ve – is volume of extractant, Sw – is sample weight (fresh).
where: r – is result in mg L−1 from calibration, Ve – is volume of extractant, Sw – is sample weight (fresh).
2.4. Determination of antioxidant activity and total polyphenols content
Ethanolic extracts for analyses of contents of total polyphenols, and for assays of the antioxidative activity against ABTS, DPPH and FRAP radicals were prepared by weighing portions of lettuce and pouring them with 80% ethanol, followed by refluxing for 15 min. After cooling, the samples were filtered. Thus prepared extract was used for analyses with the spectrophotometric method using a Hitachi U-2900 UV-VIS spectrophotometer (Hitachi, Tokyo, Japan).
The anti-free-radical activity of lettuce was determined using a free DPPH radical (1,1-diphenyl-2-picrylhydrazyl) and ABTS radical (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)).20 Absorbance was measured 10 min after the free radical solution had been added to the sample, at wavelengths of 516 nm for DPPH and 734 nm for ABTS. The antioxidative activity of lettuce was expressed in w μM Trolox per g of lettuce.
The analysis of the antioxidant activity by the FRAP method was performed according to Benzie.20 TPTZ solution was added to the lettuce ethanol extract and incubated at 37 °C for 10 minutes. After this time, the samples were cooled and the absorbance was measured at 516 nm. The results are expressed as μmol Fe2+ per g of lettuce.
The total polyphenols content was determined with the method involving the use of Folin–Ciocalteau reagent.20 The reagent and 25% sodium carbonate were added to the sample, which was then mixed using a Labnet vortex mixer (Edison, USA). The mixed samples were left in a dark place, at room temperature for 60 min. Afterwards, absorbance was measured at a wavelength of 675 nm. The total content of polyphenols was read out from a standard curve plotted for (+)-catechin.
2.5. Determination of enzyme activity
The extracts for catalase (CAT) and guaiacol peroxidase (POX) activity determination were prepared from 1 g of fresh leaf tissue homogenized with 100 mM phosphate buffer (pH 7.5) containing 1 mM EDTA and 1% PVP-40. Homogenates were centrifuged for 15 min at 4500 rpm, 5 °C. Supernatants were collected, further centrifuged for 10 min at 10 000 rpm. Supernatants after second centrifugation were intended for further analysis. The analytical procedure described in the earlier publication Halka et al. was used.21
To determine the activity of polyphenyl oxidase (PPO) and peroxidase (PER), a sample of lettuce was ground with 0.2 mol dm−3 phosphate buffer at pH 7. The appropriate sample was transferred to a volumetric flask and filled with 0.05 mol dm−3 phosphate buffer and left for 2 hours in the refrigerator.
In order to determine the PPO activity, an aliquot of the extract was mixed with a catechol solution (0.07 mol dm−3 in a 0.05 mol dm−3 phosphate buffer solution) and the increase in absorbance was measured at 420 nm. The PER determination was carried out by adding phosphate buffer to the lettuce extract and simultaneously a solution of H2O2 (3 mmol dm−3) and p-phenyldiamine (1 g/100 g) and then measuring the increase in absorbance at 485 nm. Measurements of the changes in absorbance were carried out in 3 minutes in both cases. The enzyme activity was determined from the rectilinear section of the curve.
2.6. Determination of polyphenols content
Samples for polyphenols content determination with the HPLC method were prepared according to the procedure described in Klimczak et al. in own modification.20 NaOH (2 mol dm−3) was added (1 : 1, v/v) to the lettuce extracts prepared as described above and the sample was mixed using a Labnet vortex mixer (Edison, USA) and left in a dark place for 4 h (room temperature). Then, it was neutralized to pH from 2.1 to 2.6 with HCl (2 mol dm−3) using a pH-meter (Metrohm, Herisau, Switzerland) and transferred quantitatively to a measuring flask with 1% l-ascorbic acid dissolved in methanol. Before the chromatographic analysis, the samples were centrifuged in an MPW – 260R centrifuge (Warsaw, Poland) (RCF 30 065g, 20 min, 4 °C) and filtered through a PTFE-L filter with pore diameter of 0.22 μm. Before injection onto the column, the samples were stored at 4 °C.
The chromatographic analysis was carried out in the HPLC Dionex UltiMate 3000 system with DAD detector (Thermo Scientific, Germering, Germany), using a Cosmosil 5C18-MS-II column (250 × 4.6 mm ID, 5 μm) (Nacalai Tesque, INC., Kyoto, Japan). Two eluents were used as the mobile phase: A – 2% (v/v) an aqueous solution of acetic acid, and B – 100% methanol. The flow rate of the mobile phase was 1 mL min−1 throughout the analysis, which lasted 50 min and was performed in the following system of eluents: eluent A – 0 min 95%; 10 min 70%; 25 min 50%; 35 min 30%; and 40 min 95%. Identification of polyphenolic compounds present in lettuce was made by comparing the retention times and UV-vis spectra of the obtained peaks with the chromatograms obtained for individual standard substances.
2.7. Preparation of plant extracts
Ethanolic extracts for cell culture studies were performed by using lyophilized plant leaf material. 0.7 g freeze-dried lettuce was extracted by shaking in the water bath rotary shaker (2 h, 30 °C) with 100 mL of 70% ethanol. Next extracts were filtrated through a filter paper and evaporated in the rotary evaporator (40 ± 2 °C) to remove ethanol from plant extracts. The prepared extracts were stored at −20 °C until determination of oxidative stress in human cancer cell lines.
2.8. Cell cultures and treatments
This research was conducted with use of human gastric adenocarcinoma cell line AGS (ATCC® CRL-1739™), human colorectal adenocarcinoma cell line HT-29 (ATCC® HTB-38™) and human normal colon epithelial cell line CCD 841 CoN (ATCC® CRL-1790™). Cells were cultured under controlled conditions (atmosphere: air 95%; CO2 5%; temperature 37 °C) and in appropriate medium with the addition of 10% FBS as per the ATCC protocol. To conduct the oxidative stress analysis, cancer cells were seeded in a density of 1 × 105 cells per well in 12-well plates. The cells were subsequently incubated with medium containing extracts from iodine-biofortified lettuce and synthetic iodosalicylic acids. As a negative control, cells cultivated only in complete growth medium were used. Menadione (200 μM for 3 hours) was used as a positive control to induce ROS in cells.
2.9. Determination of oxidative stress
The level of oxidative stress was measured by using the Muse® Oxidative Stress Kit (Merck Millipore, Catalog number MCH100111) which enables the quantitative measurements of cellular populations undergoing oxidative stress. This assay is based on dihydroethidium (DHE) which allows to detect intracellular reactive oxygen species (ROS) in cellular populations. The measures of oxidative stress were performed using the Guava Muse Cell Analyzer, according to the manufacturer's instructions.
2.10. Statistical analysis
The statistical analysis was performed by using Statistica 13.1 PL programme. All experiments were performed in at least in three replications. Results are shown as mean ± standard deviation (SD). All of the data of plant analysis were examined using analysis of variance (ANOVA). Statistically significant differences were assessed by the post hoc Duncan's test. p values less than 0.05 were considered as statistically significant. Statistical analysis of oxidative stress in gastrointestical cells was conducted using an independent samples t-test. p values below 0.01 were regarded as statistically significant.
3. Results
3.1. The content of vitamins
Table 1 shows the content of vitamins in lettuce biofortified with iodine compounds. The results for the vitamins B and PP were expressed in dry weight of lettuce. The result for the vitamin C was presented in fresh weight of plants. Application of iodosalicylic acids caused a significant effect on changes the content of certain vitamins in biofortified lettuce leaves. The highest content of vitamin C and B3 was noted in plants fertilized with 3,5-diISA. There was no statistically significant difference in the content of thiamine hydrochloride, riboflavin and D-biotin in lettuce. The highest level of vitamin PP and B5 was observed in plants biofortified with KIO3. Control and KIO3 fortified plants were characterized by higher content of B6 and B9 vitamins in comparison to the 5-ISA and 3,5-diISA treatments.
The content of vitamins in lettucea.
| Combinations | Vitamin C (mg/100 g f.w.) | Vitamin B3 (mg kg−1 d.w.) | Vitamin B7 (mg kg−1 d.w.) | Vitamin PP (mg kg−1 d.w.) | Vitamin B5 (mg kg−1 d.w.) | Vitamin B6 (mg kg−1 d.w.) | Vitamin B2 (mg kg−1 d.w.) | Vitamin B9 (mg kg−1 d.w.) | Vitamin B1 (mg kg−1 d.w.) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 20.45 ± 2.52 ab | 3.35 ± 0.50a | 0.10 ± 0.02a | 7.31 ± 1.71a | 5.24 ± 0.47a | 0.32 ± 0.06b | 5.41 ± 0.74a | 0.98 ± 0.30b | 4.50 ± 0.31a |
| KIO3 | 16.90 ± 3.03a | 4.88 ± 0.29 ab | 0.08 ± 0.01a | 13.95 ± 0.81b | 5.97 ± 0.55b | 0.38 ± 0.02b | 5.40 ± 0.13a | 0.76 ± 0.16 ab | 5.30 ± 0.24a |
| 5-ISA | 28.50 ± 3.25bc | 3.18 ± 0.17a | 0.10 ± 0.02a | 8.51 ± 0.58a | 4.86 ± 0.09a | 0.20 ± 0.01a | 5.72 ± 0.16a | 0.49 ± 0.10a | 4.42 ± 0.20a |
| 3,5-diISA | 35.58 ± 4.64c | 5.22 ± 0.26c | 0.08 ± 0.02a | 7.85 ± 0.30a | 5.29 ± 0.19a | 0.14 ± 0.01a | 4.16 ± 0.18a | 0.46 ± 0.09a | 4.47 ± 0.25a |
Results are shown as mean for 2018–2019 ± standard deviation (SD); n = 8, means followed by the same letter (a, b, c) are not significantly different (p < 0.05), d.w. dry weight; f.w. fresh weight.
3.2. The antioxidant activity and the content of total polyphenols
A statistically significant influence of iodine enrichment on antioxidant activity in lettuce leaves was observed. The antioxidant capacity of plants biofortified with iodine was evaluated by using three assays: DPPH, ABTS and FRAP (Table 2). In comparison to control and other treatments, the highest antioxidant activity was noticed in lettuce biofortified with 3,5-diISA determined by the ABTS assay. DPPH and FRAP assays also showed that fertilization with 3,5-diISA caused the statistically significant increase of antioxidant potential in tested plants. The total polyphenol content of the tested lettuce samples ranged from 6.07–12.98 mg/100 g f.w. The statistically significant higher concentration of total polyphenols was noted in plants after fortification with 3,5-diISA. However, there was no difference in the content of these compounds between control and lettuce after KIO3 and 5-ISA treatment.
The antioxidant activity and the content of total polyphenolsa.
| Combinations | DPPH (μmol Trolox g−1 f.w.) | ABTS (μmol Trolox g−1 f.w.) | FRAP (μmol Fe2+ g−1 f.w.) | Total polyphenols (mg/100 g f.w.) |
|---|---|---|---|---|
| Control | 2.22 ± 0.20a | 18.84 ± 1.66a | 1.56 ± 0.13a | 6.66 ± 0.34a |
| KIO3 | 2.29 ± 0.30a | 17.19 ± 1.17a | 1.50 ± 0.13a | 6.07 ± 0.33a |
| 5-ISA | 3.30 ± 0.24a | 20.44 ± 1.19a | 2.14 ± 0.14a | 7.69 ± 0.41a |
| 3,5-diISA | 7.36 ± 0.41b | 32.83 ± 1.49b | 3.40 ± 0.17b | 12.98 ± 0.45b |
Results are shown as means for 2018–2019 ± standard deviation (SD); n = 8, means followed by the same letter (a, b) are not significantly different (p < 0.05), f.w. fresh weight.
3.3. Activity of enzymes
Activity of antioxidant enzymes in lettuce biofortified with iodine is shown in Table 3. Iodine fertilization had statistically significant influence on increase in activity of selected enzymes in the tested samples. Plants biofortified with 5-ISA was characterized by the highest activity of catalase as compared to non-enriched lettuce and other combinations. The statistically significant increase of peroxidase activity was observed in lettuce after 3.5-diISA treatment. The highest activity of polyphenyl oxidase was noted in lettuce after application KIO3. Application of 5-ISA caused decrease activity of this enzyme compared to control and KIO3-fortified plants. In comparison to the other combinations, the highest activity of guaiacol peroxidase was noticed in control lettuce leaves.
Activity of enzymesa.
| Combinations | Catalase activity (U mg−1 protein) | Guaiacol peroxidase activity (U mg−1 protein) | Polyphenyl oxidase (ΔE420nm min−1 g−1) | Peroxidase (ΔE485nm min−1 g−1) |
|---|---|---|---|---|
| Control | 0.0435 ± 0.0201a | 0.0126 ± 0.0017b | 6.4304 ± 0.0675b | 13.557 ± 0.1804a |
| KIO3 | 0.0838 ± 0.0258a | 0.0033 ± 0.0017a | 7.5057 ± 0.0919c | 13.624 ± 0.6928a |
| 5-ISA | 0.1253 ± 0.0434b | 0.0034 ± 0.0010a | 5.2687 ± 0.2514a | 13.092 ± 0.4896a |
| 3,5-diISA | 0.0680 ± 0.0385a | 0.0029 ± 0.0006a | 5.9611 ± 0.2013 ab | 14.684 ± 0.6331b |
Results are shown as means for 2018–2019 ± standard deviation (SD); n = 8, means followed by the same letter (a, b) are not significantly different (p < 0.05).
3.4. The content of polyphenolic compounds
The results concerning concentration of polyphenolic compounds in tested plants are shown in Table 4. Treatment with iodosalicylic acids had a statistically significant impact on concentration of polyphenolic compounds in lettuce leaves. After the fertilization of iodosalicylic acids the content of chlorogenic acid, sinapic acid, p-coumaric acid, ferulic acid, hippuric acid, protocatechuic acid and 3-hydroxybenzoic acid was statistically increased. In comparison to control and other combinations, the highest concentration of these compounds was found in lettuce after 3,5-diISA treatment. Application of 5-ISA caused accumulation higher amount of ferulic acid and 3-hydroxybenzoic acid as compared to control and other treatments. However, the significantly lowest concentration of protocatechuic acid was noticed in plants fortified with this form of iodine.
The concentration of polyphenolic compoundsa.
| Combinations | Chlorogenic acid (mg/100 g f.w.) | Sinapic acid (mg/100 g f.w.) | p-Coumaric acid (mg/100 g f.w.) | Ferulic acid (mg/100 g f.w.) | Hippuric acid (mg/100 g f.w.) | Protocatechuic acid (mg/100 g f.w.) | 3-Hydroxybenzoic acid (mg/100 g f.w.) |
|---|---|---|---|---|---|---|---|
| Control | 183.69 ± 19.06a | 1.80 ± 0.13 ab | 2.72 ± 1.28 ab | 17.13 ± 1.88a | 9.18 ± 1.13a | 26.34 ± 1.70a | 2.41 ± 0.44a |
| KIO3 | 163.37 ± 19.64a | 1.59 ± 0.17a | 2.26 ± 0.18a | 15.08 ± 0.79a | 8.37 ± 0.98a | 25.35 ± 1.95a | 3.11 ± 0.39a |
| 5-ISA | 204.61 ± 34.75a | 2.16 ± 0.27b | 3.63 ± 0.40b | 34.13 ± 3.62b | 11.25 ± 1.42a | 18.21 ± 1.48b | 5.44 ± 2.19b |
| 3,5-diISA | 406.49 ± 34.03b | 3.41 ± 0.20c | 5.78 ± 0.33c | 106.56 ± 8.76c | 14.57 ± 0.70b | 21.09 ± 1.00c | 5.49 ± 0.86b |
Results are shown as means for 2018–2019 ± standard deviation (SD); n = 8, means followed by the same letter (a, b, c) are not significantly different (p < 0.05), f.w. fresh weight.
3.5. The effect of extracts from iodine-biofortified lettuce on ROS level in human cancer and normal cell lines
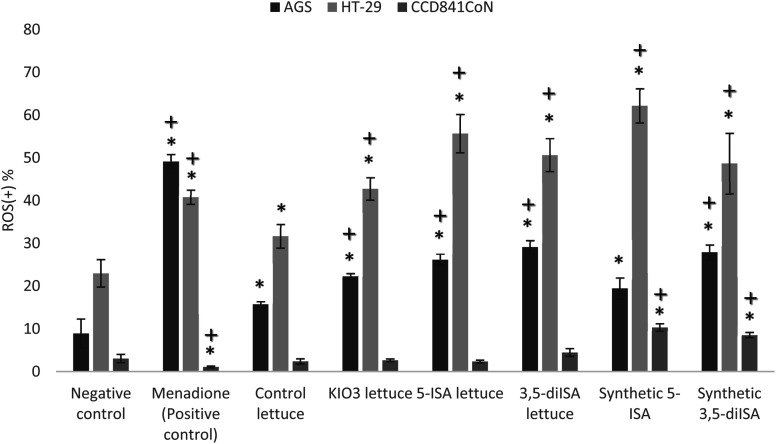
Fig. 1 and 2 show ROS level in gastrointestinal cancer cells and normal colon epithelial cell after 24 h exposure to extracts from iodine-biofortified lettuce. Results obtained from the Muse® Oxidative Stress Assay shows that treatments with 1000 μg mL−1 extracts from iodine-biofortified lettuce caused an increase endogenous ROS level in both tested cell lines. In comparison to untreated control cells, the statistically significant higher ROS level was observed in gastric cancer cell line (AGS) as well as colon cancer cell line (HT-29) after treatment with fortified-lettuce extracts. In AGS cells, the highest ROS level was observed in the 3,5-diISA-treated group where a population of cells exhibiting ROS activity was 29.1%. Whereas, in HT-29 cells, incubation with iodosalicylates caused increased in the ROS level to 55.69% and 50.65% after 5-ISA and 3,5-diISA treatment, respectively. The application of synthetic forms of these compounds had also significant effect on increase in cell population undergoing oxidative stress. Treatment with synthetic form of 5-ISA resulted in statistically significant increase of intracellular superoxide radical levels to 19.48% in AGS and 62.19% in HT-29. An average percentage of AGS and HT-29 cells that produce ROS after synthetic 3,5-diISA treatment was respectively 27.93% and 48.68%. Our results showed that application of iodine fortified lettuce had no impact on increasing the ROS level in normal colon epithelial cells (CCD 841 CoN). However, incubation with synthetic forms of iodosalicylates caused increase the ROS levels compared to the control cells as well as control lettuce.
Fig. 1. The effect of extracts from iodine-biofortified lettuce (1000 μg mL−1) on ROS level in human gastric adenocarcinoma cell line (AGS), colorectal adenocarcinoma cell line (HT-29) and normal colon epithelial cell line (CCD 841 CoN). * vs. negative control (NC) when p < 0.01; + vs. control lettuce when p < 0.01. Cells were treated for 24 h with 1000 μg mL−1 extract from non-enriched lettuce (control lettuce), or KIO3-fortified lettuce, or 5-ISA-fortified lettuce, or 3,5-diISA-fortified lettuce, or synthetic 5-ISA, or synthetic 3,5-diISA, or 200 μM menadione as positive control.
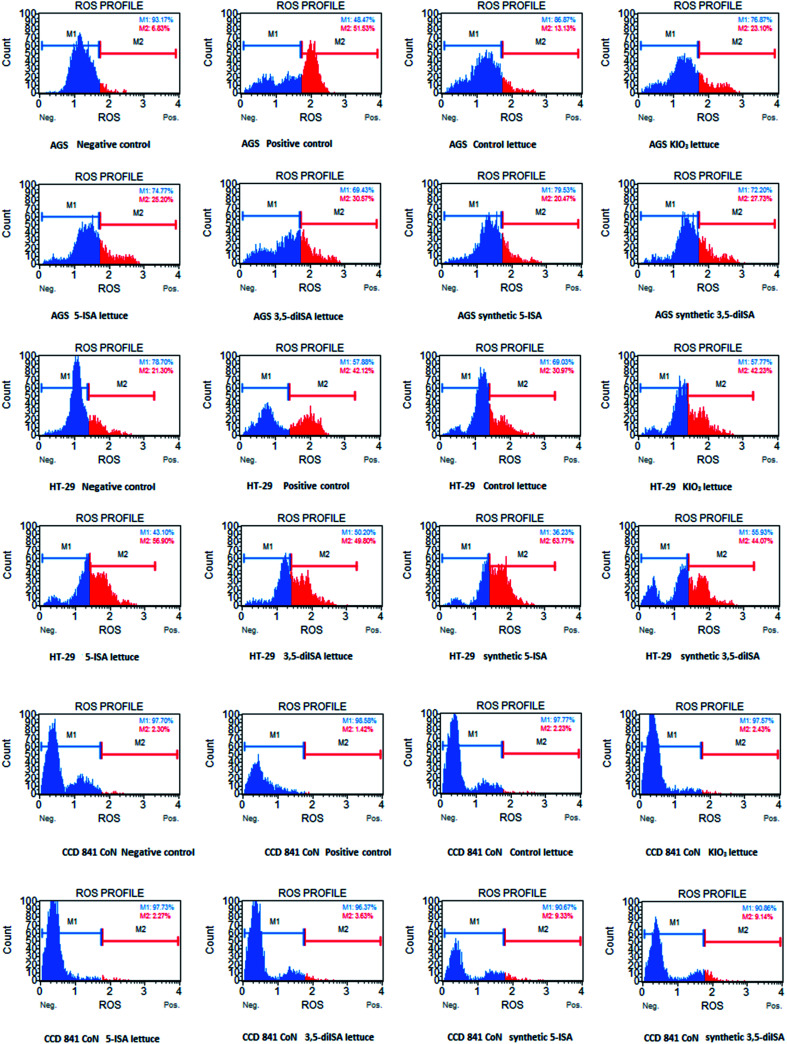
Fig. 2. Representative plots from Muse® Oxidative Stress Assay. The above plots show the histograms of gated cells with two markers providing data on two cellular populations: marked with blue color: ROS(−) and marked with red color: ROS(+) cells. Cells were treated for 24 h with 1000 μg mL−1 extract from non-enriched lettuce (control lettuce), or KIO3-fortified lettuce, or 5-ISA-fortified lettuce, or 3,5-diISA-fortified lettuce, or synthetic 5-ISA, or synthetic 3,5-diISA, or 200 μM menadione as positive control.
4. Discussion
One of the greatest challenges facing health policy today is the aging process of the population. Therefore, a lot of attention is being given to search for methods of prevention of non-communicable diseases. It is considered that a high intake of plant foods can reduce the risk of developing chronic diseases such as cancers, type 2 diabetes, cardiovascular or musculoskeletal disorders and consequently lowers the number of early deaths around the world. Beneficial effects of a plant-based diet on humans are related to high antioxidant potentials of some vegetables and fruit. Our previous study has shown that biofortification of vegetables allows the production of plants of high nutritional value.14 Lettuce fertilized with iodosalicylates was characterized by higher amounts of selected macro- and micronutrients (Mg, Na, Ca, B, Zn). Additionally, fortification with iodine compounds led to changes in the concentration of sugars, fatty acids, dietary fiber and nitrates in leaves.14 In this research, we focused on an important issue – antioxidant activity of lettuce biofortified with iodosalicylic acids.
Antioxidant activity of plants is quite varied and depends on the content of vitamins, polyphenols and carotenoids, which are natural exogenous antioxidants. These compounds are regarded as powerful reducing agents that have the ability to scavenge free radicals. Besides that, they can enhance the effect induced by endogenous antioxidants and protect from damage in repair systems.22 Vitamins are crucial organic compounds with high antioxidant potentials. While vitamins C and E are among the best known antioxidant compounds, vitamins from group B should also be taken into consideration. There are studies that confirm that a shortage of some types of B vitamins in the diet may lead to increased lipid peroxidation levels and enhancement of oxidative stress.23 The present research demonstrated that fertilization of lettuce with iodine compounds resulted in positive changes in levels of most of the analyzed vitamins. In comparison to non-enriched plants, fortified lettuce was characterized by higher level of vitamin C and selected vitamins B. Vitamin C belongs to a group of compounds that cannot be synthesized by the human body, therefore its level in an organism depends on dietary consumption. To avoid the prevalence of deficiency and hypovitaminosis of vitamin C, it is necessary to provide the appropriate amounts of fruits and vegetables every day, because these products are regarded as one of the richest source of this vitamin. Despite the fact that at physiological levels vitamin C functions as an antioxidant, in higher concentration it promotes the formation of hydrogen peroxide and production of reactive oxygen species that consequently can have a cytotoxic effect against cancer cells. Interestingly vitamin C, by influencing the DNA demethylation and histone demethylation processes, plays an important role in epigenetic regulation in the human genome. And that is why a large number of studies have been being conducted to learn about the importance of vitamin C in combination therapy for cancer treatment for many years.24 Physiological significance of vitamin C is extremely broad and not limited to antioxidant activity. At the same time it acts as a cofactor for many enzymes participating in human and plan metabolism. Besides being beneficial for human health, it is associated with replenishment of micronutrient deficiencies. Increasing the level of vitamin C in crop plants can also contribute to improving their resistance to different types of stress and to extend the shelf life of these products.24
In our study we showed that after fortification with iodine compounds, the content of vitamin C was in the range of 16.90–35.58 mg/100 g f.w. with the highest level in plants after fortification with the organic form of iodine i.e. 3,5-diISA. Different studies have shown that iodine induces the accumulation of ascorbic acid in plants. Weng et al. who have studied vitamin C content in water spinach, claimed that the concentration of ascorbic acid was higher after uptake of I−, whereas application of IO3− and CH2ICOO− resulted in a decrease compared to the untreated plants.25 Blasco et al. reported that application of the inorganic form of iodine increase the level of l-ascorbic acid (AA) and dehydroascorbic acid (DHA) in lettuce leaves compared to control samples.26 They demonstrated that the content of AA increased with increasing concentrations of I− and IO3− and the higher was at a dose of 80 μM of I−. Similar results were obtained in the study of Halka et al., which also confirmed that fertilization with KI increases AA in tomato roots and leaves.21 Moreover, addition of an organic form of iodine (3,5-diISA) into the nutrient solution caused increases in the level of AA in tomato leaves although this effect was not proven in roots.21 In comparison to the results obtained by the majority of the above-mentioned authors, we did not confirm that the application of iodine in the form of potassium iodate (KIO3) has significant impact on concentration of vitamin C in lettuce leaves.
In this research we also analyzed the effect of iodine fortification on the concentration of B vitamins in lettuce. After application of iodine, a statistically significant increase was found in the level of vitamins B3, B5 and PP. Currently, many studies are focused on learning the importance of B vitamins in the immune response and inflammation. Inadequate intake of these vitamins can lead to compromising humoral and cellular immune response, because of important contribution of B vitamins to protein biosynthesis. In addition, deficiencies of some of the B vitamins may contribute to the generation of oxidative stress in cells as a result of increasing homocysteine concentration.27 In the available literature, there is little information concerning effect of iodine fortification on vitamin B content. We showed that fertilization with different forms of iodine has no impact on vitamins B1, B2 and B7 concentration. Additionally, after KIO3 treatment, there was no statistically significant differences in the content of vitamins B6 and B9 in comparison with control plants. Nevertheless, we have demonstrated increasing concentrations of nicotinic acid (B3) and nicotinamide (PP). The highest content of vitamin B3 and PP was observed in lettuce after the application of 3,5-diISA and KIO3, respectively. Vitamins B3 and PP, also known as “niacin”, are precursors of two coenzymes nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP).28 NAD and NADP, through participation in the redox and non-redox reactions, are involved in maintaining energy metabolism in cells. These play a role in regulation of crucial cellular signaling and biological functions such as cell cycle progression, repair of damaged genetic material or programmed cell death. Furthermore, a high level of NAD prevents the generation of reactive oxygen species and increases survival of cells.28 In addition, because of anti-inflammatory effect and ability to inhibit neutrophil infiltration, niacin strengthens the immune system.29 Therefore, the increased level of vitamin B3 in 3,5-diISA-fortified plants, which was demonstrated in our study, may be important in adjunct therapy for patients with virus infections, as for example COVID-19. Moreover, application of KIO3 resulted in an increase of nicotinamide concentration that have suppressive effects in different types of cancers.28
There are many methods used to measure antioxidant potential in plants and food samples, which have a different mechanisms of action and antioxidant defense system. Among the widely used methods to quantify antioxidant capacity are the DPPH radical scavenging assay, ferric reducing antioxidant power (FRAP) assay and the Trolox equivalent antioxidant capacity (TEAC or ABTS) assay. It is recommended that a minimum set of three tests be carried out to obtain credible results of antioxidant potential of tested samples.30 In the present study, the antioxidant activity of biofortified lettuce was determined by using three methods: DPPH, ABTS and FRAP. The results obtained from all performed tests showed that the highest free radical scavenging activity was detected in the extract from lettuce biofortified with 3,5-diISA. Values from DPPH and the FRAP assays were lower than the ABTS test. The antioxidant capacity in lettuce fertilized with inorganic forms of iodine was no significantly different with respect to control plants. Different results were revealed by Blasco et al., who found an increased radical scavenging activity of fortified lettuce after fertilization with I−.31 They evaluated antioxidant activity by FRAP, TEAC and a reducing power test (Fe3+-TPTZ) and showed considerable differences between dose and forms of the applied iodine in all conducted tests. Their study demonstrates that plants fertilized with I− at a dose from 10 to 240 μM has a higher level of antioxidant activity than non-enriched lettuce which was evaluated in the FRAP test.31 Interesting data were presented by Krzepiłko et al., who analyzed the effect of KI biofortification on total antioxidant capacity in lettuce and radish seedlings.32 Their results, using the DPPH test, suggest that the level of antioxidant capacity in lettuce seedlings should be reduced after fertilization of plants with higher doses of KI (0.375–1.5 mg per Petri dishes).32 This research has proved that the total polyphenol content was higher in lettuce after fertilization with 5-ISA. Different result was obtained by Blasco et al., who found that application of inorganic form of iodine (I− and IO3−) had a significant impact on the concentration of total phenolic in lettuce plants.31 Kiferle et al. results also showed that iodine treatment (KI and KIO3) significantly increases total polyphenols as well as the antioxidant potential in basil plants (Ocimum basilicum L.).33
According to the current state of knowledge, ROS are formed in plants through normal metabolic processes and act as cell signaling molecules that are involved in physiological process such as growth, development and response to biotic and abiotic stress factors. However, excessive amounts of ROS in plants can cause oxidation damage of the genetic material and cell death.34 In the available literature there are data confirming the importance of antioxidant enzymes in active plant defense mechanisms against abiotic stress factors, such as salinity, toxic metals, cold, drought or deficiency of some macro- and micronutrients, that may increase the ROS level in cells.35 There are studies that reveal increased activity of antioxidant enzymes in plants under environmental stress which indicates their crucial role in maintaining the appropriate level of ROS inside plant cells.36 Positive changes in activity of antioxidant enzymes after application of iodine compounds were found by many researchers. Blasco et al., who also studied activity of antioxidant enzymes in iodine-fortified lettuce, showed that application of 80 μM of iodide (I−) had the effect of increasing CAT and l-galactono dehydrogenase activities, but they also observed a reduction in the biomass of tested plants.26 However, their study has proved that fertilization with iodate (IO3−) caused increases in the ROS detoxifying enzymes (SOD, APX and CAT) in the absence of phytotoxic effects in lettuce.26 Other authors who investigated the effect of application of IO3− on improving the activity of antioxidants in soybean seeds under cadmium stress obtained similar results.37 They showed an increased level of antioxidant enzymes (SOD, APX and GR) in plants treated with different doses (20, 40 80 μM) of iodine. Interesting findings were presented in a study by Leyva et al., in which it was proven that the fortification of lettuce with iodine (IO3−) may contribute to bridging the harmful effects caused by salt stress.38 Their results showed that IO3− fortified plants were characterized by enhanced activities of SOD, APX and GR that allowed the plants to avoid damage caused by excessive generation of free radicals and oxidative stress.38
The results of the present study were consistent with other authors. We determined that biofortification with iodine increases the activity of some enzymes in lettuce leaves, which may indicate an ability to induce the response of the antioxidant system in plants. Compared to control plants, the application of 5-ISA caused the significant increase of CAT activity, however this effect was not observed after fertilization of lettuce with 3,5-diISA. However, little research has been conducted to explain how the organic form of iodine effects the antioxidant potential in vegetables. In contrast with our results, Halka et al. demonstrated that application of iodosalicylates (5-ISA and 3,5-diISA) resulted in reduction of CAT activity in young tomato plants as compared to the control group.39 They also showed that the activity of POX in tomato leaves was dependent on the form and concentration of iodine.39 A similar study was performed by Medrano-Macias et al., who also investigated antioxidant activity in tomato seedlings treated with iodide (I−) and potassium iodate (IO3−) at a dose of 1 μM daily and 100 μM twice per week.40 Their study revealed that there were no statistically significant differences between the CAT activity of plants cultivated in the control and iodine combinations.40
A large amount of phenolic compounds in lettuce makes it is a vegetable with a powerful antioxidant activity. Phenolic acids that are plant-derived natural antioxidants are regarded as one of the most important groups of secondary metabolites in plants. These compounds have a broad spectrum of biological activity, including anti-inflammatory, antidepressant, cytotoxic, anticancer and primarily antioxidant activities.41 Our findings demonstrated that biofortification of lettuce with the organic form of iodine has an impact on the concentration of polyphenolic compounds. We found that fertilization of plants with 3,5-diISA resulted in an increase in the level of all identified polyphenolic compounds, as compared to control and other treatments. High-performance liquid chromatography (HPLC) analysis revealed the presence of the 7 compounds – chlorogenic acid, sinapic acid, p-coumaric acid, ferulic acid, hippuric acid, protocatechuic acid and 3-hydroxybenzoic acids – in the plant extracts. The dominant compound in iodine-fortified lettuce was chlorogenic acid in the range of 163.37–406.49 mg/100 g f.w. This compound is an ester of caffeic and quinic acids that is mainly found in tea, coffee, fruit and vegetable juice. Most of the health benefits from consumption of these beverages is the result of the cardioprotective effect and their ability to mitigate oxidative stress, which are related to high concentrations of chlorogenic acid in plant materials.42 There are other research studies that confirm that the addition of certain mineral elements enhances the level of the polyphenol synthesis in plants. Interesting results were obtained in the investigation of Wulanjari et al., who examined the impact of mineral elements (Si, B, Ca, K and I) as foliar treatments in arabica coffee leaves for improving plant disease resistance.43 Their research demonstrated that after application of iodine the average of polyphenol content in coffee leaves was over 2.5 times higher than the average of control plants. This is important, because an increased level of polyphenols in plants contributes to strengthening the cell walls that are crucial for protection against penetration of microorganisms.43 Skoczylas et al. found that juices made from carrot fortified with an inorganic form of iodine was characterized by a significant increase in the concentration of ferulic, as well as caffeic and salicylic acids, in comparison to the juice obtained from non-enriched vegetables.20 However, they showed that fertilization with iodine has a negative impact on the content of catechin in carrot juice.20 Kiferle et al. who evaluated the impact of KIO3 and KI treatments on phenolic production in basil plants (Ocimum basilicum L.) noted that there is a positive correlation between iodine biofortification and polyphenolic compounds, whereas this effect was dose-dependent.33 The highest concentration of cinnamic and rosmarinic acid was observed in plants after application of 10 mM of iodine compounds.33 Blasco et al., in a study that concerned hydroponically grown lettuce, determined that application of 20 and 40 μM of IO3− caused an increased level of hydroxycinnamic acids, resulting in increased tolerance to salinity stress and have health-promoting properties.44
Currently, a lot of attention is being given to the role of dietary phytochemicals and their role in cancer prevention as well as therapy. An inverse relationship between consumption of foods rich in phytochemicals and incidence was observed in the case of the most frequent types of cancer, such as prostate, colon, breast or lung cancers. Plant-derived compounds may regulate the cellular processes such as cell cycle, proliferation or apoptosis that are strictly involved in the process of oncogenesis. Moreover, phytochemicals take a part in activation or inactivation of proto- and anti-oncogenes and elimination of free radicals.45 One of the most important causes of the development of cancer is redox homeostasis disorders that are induced by rapid growth of reactive oxygen species (ROS). Oxidative stress occurs if the endogenous antioxidants are not able to reduce an elevated level of ROS. It is believed that through DNA damage and interference with other cellular structures, oxidative stress contributes to the generation of cancer cells.45 However, more and more scientific research indicates that plant polyphenols may act as pro-oxidants and through ROS production lead to programmed cell death.46
In the present study, we evaluated the effects of extracts from iodine-biofortified lettuce on the level of intracellular reactive oxygen species in gastrointestinal human cancer cells. This experiment demonstrated that treatment with iodine-fortified lettuce extracts and synthetic forms of the iodosalicylic acids increased ROS production in HT-29 and AGS cells, which may suggest the potential role of iodine compounds in antitumor therapy. We proved that iodine-enriched lettuce extracts increased the ROS level of cancer cells more effectively than extracts obtained from non-enriched plants. Interestingly, we provided that iodine-enriched lettuce extracts have no impact on increasing the ROS level in normal colon epithelial cell line (CCD 841 CoN). Therefore, our results indicate that depending on type of cells these extracts may act as anti- or pro-oxidant factor.
There are several possible mechanisms of increasing the ROS level in cancer cells. It is possible to hypothesize that iodine-enriched lettuce extracts exerts pro-oxidative effect through the inhibition of antioxidant enzymes activity in cancer cells.8 Cancer cells are characterized by lower level of antioxidants, therefore they may generate higher level of ROS compared with properly functioning cells.8,47,48 Moreover, disruption of mitochondrial homeostasis in cancer cells also lead to overproduction of ROS and oxidative damage of cellular components. Excessive intracellular reactive oxygen species generation and ability to induction oxidative damage may be used to anticancer therapy.47 Examples include chemotherapeutics such as cisplatin that induce programmed cell death by disrupt mitochondrial structure.49 As demonstrated in the studies, cisplatin has ability to formation of DNA adducts by binding to genetic material and through increasing of reactive oxygen species it may activate apoptosis signaling pathways in cancer cells.50,51 According to the research, iodine treatment contributes to the reduction of proliferation in cancer cells. The antiproliferative effect is mainly caused by the formation of iodinated derivatives of arachidonic acid, such as 6-iodo-5-hydroxy-8,11,14-eicosatrienoic acid (6-iodolactone; 6-IL).52 Aceves et al. reported that apoptotic effect of these compounds may result from the fact that 6-IL is a specific ligand of PPAR (peroxisome proliferator-activated receptor).52 Iodine treatment has the effect of increasing PPARγ expression. PPARγ are a specific ligand-dependent transcription factors that regulate the cell cycle and apoptosis process. There are studies that confirm a positive role of PPARγ ligands for treatment and prevention of cancer.53
Our results are consistent with previous reports, which indicate that bioactive compounds present in plant extracts affect cancer cells as pro-oxidants.54 As we have shown earlier, iodine-fortified lettuce was characterized by the highest concentration of some polyphenolic compounds. Thus, our findings suggest that pro-oxidant action of iodine-fortified plants can result from increasing the accumulation of bioactive compounds in plants. Other interesting results were obtained by Hou et al., who studied the effect on chlorogenic acid (CGA) on generation of ROS in human colon cancer lines: HT-29 and HCT116.55 The authors of this study observed that after CGA treatment of colon cancer cells, the generation of ROS was enhanced, which suggests that high doses of CGA may protect against carcinogenesis through the induction of DNA damage and inhibiting the viability of cancer cells. The researchers suggested that the mechanism of the reduction of viability may stem from the arrest of cells in the S phase of the cell cycle and the inactivation of the ERK signaling pathway that are responsible for proliferation and differentiation of cells.55,56 D'Angelo et al., who studied pro-oxidant effect in human breast cancer cells, also reported that polyphenol extracts obtained from the Italian apple fruits (Annurca) may lead to increased generation of ROS and thus inhibit proliferation and cell cycle progression in MCF-7 cells.57 Comparable results were observed in the research of Wihadmadyatami et al., who studied the effect of ethanolic extract of Ocimum sanctum in the human lung adenocarcinoma cell line.58 They showed that anticancer activity of extracts is associated with significant increased levels of ROS. Moreover, they found that the expression level of caspase-3 was increased in cells after treatment with plant extracts, which may indicate that bioactive compounds of plant origin are involved in programmed cell death.58
We hypothesize that the content of antioxidant compounds in lettuce is dependent on the form of applied iodine and that plants biofortified with iodosalicylic acids have the higher antioxidant potential. Additionally, treatment of gastrointestinal cancer cells with the extracts from iodine-enriched lettuce causes changes in level of reactive oxygen species (ROS).
5. Conclusions
This study showed that fortification of vegetables with an organic form of iodine is an effective method to increase antioxidant potential of crop plants. Iodine-fortified plants were characterized by an increased level of some vitamins and polyphenolic compounds. We also showed that the fertilization of lettuce with iodine has a positive effect on activity of antioxidant enzymes, which through diminishing ROS generation plays a principal protective and preventive role against chronic diseases. However, many studies have been concentrated on understanding ROS interaction with cellular components and explaining the mechanism of action of bioactive compounds in cancer cells. The induction of oxidative stress through increased accumulation of ROS in cancer cells can be used in anticancer therapies. In this research we demonstrated that iodine-biofortified lettuce may act as pro-oxidant by increasing ROS production in gastrointestinal cancer cell lines (AGS and HT-29) and therefore can contribute to induce programmed cell death, also called apoptosis. Thus, the obtained results indicate the need to conduct further studies to confirm the ability of extracts from iodine-biofortified lettuce to regulate the expression of genes and proteins that are involved in proliferation, cell cycle and apoptotic process in cancer cells.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This research was financed by the National Science Centre, Poland (Grant no. UMO-2017/25/B/NZ9/00312).
References
- Birben E. Sahiner U. M. Sackesen C. Erzurum S. Kalayci O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljsak B. Šuput D. Milisav I. Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxid. Med. Cell. Longevity. 2013;29:1–11. doi: 10.1155/2013/956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snezhkina A. V. Kudryavtseva A. V. Kardymon O. L. Savvateeva M. V. Melnikova N. V. Krasnov G. S. Dmitriev A. A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longevity. 2019:1–17. doi: 10.1155/2019/6175804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco-Torres G. Baltiérrez-Hoyos R. Andrade-Jorge E. Villa-Treviño S. Trujillo-Ferrara J. G. Vásquez-Garzón V. R. Cytotoxicity, Oxidative Stress, Cell Cycle Arrest, and Mitochondrial Apoptosis after Combined Treatment of Hepatocarcinoma Cells with Maleic Anhydride Derivatives and Quercetin. Oxid. Med. Cell. Longevity. 2017:1–16. doi: 10.1155/2017/2734976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sá Junior P. L. Câmara D. A. D. Porcacchia A. S. Fonseca P. M. M. Jorge S. D. Araldi R. P. Ferreira A. K. The Roles of ROS in Cancer Heterogeneity and Therapy. Oxid. Med. Cell. Longevity. 2017:1–12. doi: 10.1155/2017/2467940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasote D. M. Katyare S. S. Hegde M. V. Bae H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015;11:982–991. doi: 10.7150/ijbs.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior R. L. Wu X. Diet Antioxidant Capacity: Relationships to Oxidative Stress and Health. Am. J. Biomed. Sci. 2013:126–139. doi: 10.5099/aj130200126. [DOI] [Google Scholar]
- Sznarkowska A. Kostecka A. Meller K. Bielawski K. P. Inhibition of cancer antioxidant defense by natural compounds. Oncotarget. 2017;8:15996–16016. doi: 10.18632/oncotarget.13723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F. Ru X. Wen T. NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020;21:1–23. doi: 10.3390/ijms21134777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Senousey H. K. Chen B. Wang J. Y. Atta A. M. Mohamed F. R. Nie Q. H. Effects of dietary vitamin C, vitamin E, and alpha-lipoic acid supplementation on the antioxidant defense system and immune-related gene expression in broilers exposed to oxidative stress by dexamethasone. Poult. Sci. 2018;97:30–38. doi: 10.3382/ps/pex298. [DOI] [PubMed] [Google Scholar]
- Lourenço S. C. Moldão-Martins M. Alves V. D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules. 2019;24:14–16. doi: 10.3390/molecules24224132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-López U. Pinzino C. Quartacci M. F. Ranieri A. Sgherri C. Phenolic Composition and Related Antioxidant Properties in Differently Colored Lettuces: A Study by Electron Paramagnetic Resonance (EPR) Kinetics. J. Agric. Food Chem. 2014;62:12001–12007. doi: 10.1021/jf503260v. [DOI] [PubMed] [Google Scholar]
- Shetty A. A. Madadum S. Managanvi K. Vegetables as Sources of Antioxidants. J. Food Nutr. Disord. 2013;2:1–5. [Google Scholar]
- Sularz O. Smoleń S. Koronowicz A. Kowalska I. Leszczyńska T. Chemical Composition of Lettuce (Lactuca sativa L.) Biofortified with Iodine by KIO3, 5-Iodo-, and 3.5-Diiodosalicylic Acid in a Hydroponic Cultivation. Agronomy. 2020;10:1–17. doi: 10.3390/agronomy10071022. [DOI] [Google Scholar]
- Halka M. Klimek-Chodacka M. Smoleń S. Baranski R. Ledwożyw-Smoleń I. Sady W. Organic iodine supply affects tomato plants differently than inorganic iodine. Physiol. Plant. 2018;164:290–306. doi: 10.1111/ppl.12733. [DOI] [PubMed] [Google Scholar]
- Halka M. Smoleń S. Czernicka M. Klimek-Chodacka M. Pitala J. Tutaj K. Iodine biofortification through expression of HMT, SAMT and S3H genes in Solanum lycopersicum L. Plant Physiol. Biochem. 2019;144:35–48. doi: 10.1016/j.plaphy.2019.09.028. [DOI] [PubMed] [Google Scholar]
- Smoleń S. Kowalska I. Halka M. Ledwozyw-Smoleń I. Grzanka M. Skoczylas Ł. Czernicka M. Pitala J. Selected aspects of iodate and iodosalicylate metabolism in lettuce including the activity of vanadium dependent haloperoxidases as affected by exogenous vanadium. Agronomy. 2020;10(1):1–21. doi: 10.3390/agronomy10010001. [DOI] [Google Scholar]
- Santos J. Mendiola J. A. Oliveira M. B. P. P. Ibáñez E. Herrero M. Sequential determination of fat- and water-soluble vitamins in green leafy vegetables during storage. J. Chromatogr. A. 2012;1261:179–188. doi: 10.1016/j.chroma.2012.04.067. [DOI] [PubMed] [Google Scholar]
- Smoleń S. Kowalska I. Czernicka M. Halka M. Kęska K. Sady W. Iodine and selenium biofortification with additional application of salicylic acid affects yield, selected molecular parameters and chemical composition of lettuce plants (Lactuca sativa L. var. capitata) Front. Plant Sci. 2016;7:1–16. doi: 10.3389/fpls.2016.01553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoczylas Ł. Tabaszewska M. Smoleń S. Słupski J. Liszka-Skoczylas M. Baranski R. Carrots (Daucus carota L.) biofortified with iodine and selenium as a raw material for the production of juice with additional nutritional functions. Agronomy. 2020;10:1–17. doi: 10.3390/agronomy10091360. [DOI] [Google Scholar]
- Halka M. Smoleń S. Ledwozyw-Smoleń I. Sady W. Iodosalicylates and iodobenzoates supplied to tomato plants affect the antioxidative and sugar metabolism differently than potassium iodide. Folia Hortic. Sin. 2019;31:385–400. doi: 10.2478/fhort-2019-0031. [DOI] [Google Scholar]
- Salehi B. Azzini E. Zucca P. Varoni E. M. Kumar N. V. A. Dini L. Panzarini E. Rajkovic J. Fokou P. V. T. Peluso I. Mishra A. P. Nigam M. El Rayess Y. El Beyrouthy M. Setzer W. N. Polito L. Iriti M. Sureda A. Quetglas-Llabrés M. M. Martorell M. Martins N. Sharifi-Rad M. Estevinho L. M. Sharifi-Rad J. Plant-derived bioactives and oxidative stress-related disorders: a key trend towards healthy aging and longevity promotion. Appl. Sci. 2020;10(3):947. doi: 10.3390/app10030947. [DOI] [Google Scholar]
- Hsu C. C. Cheng C. H. Hsu C. L. Lee W. J. Huang S. C. Huang Y. C. Role of vitamin B6 status on antioxidant defenses, glutathione, and related enzyme activities in mice with homocysteine-induced oxidative stress. Food Nutr. Res. 2015;59:1–7. doi: 10.3402/fnr.v59.25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gorkom G. N. Y. Lookermans E. L. Van Elssen C. H. M. J. Bos G. M. J. The effect of vitamin C (Ascorbic acid) in the treatment of patients with cancer: a systematic review. Nutrients. 2019;11:977. doi: 10.3390/nu11050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng H. X. Yan A. L. Hong C. L. Xie L. L. Qin Y. C. Cheng C. Q. Uptake of different species of iodine by water spinach and its effect to growth. Biol. Trace Elem. Res. 2008;124:184–194. doi: 10.1007/s12011-008-8137-4. [DOI] [PubMed] [Google Scholar]
- Blasco B. Ríos J. J. Leyva R. Cervilla L. M. Sánchez-Rodríguez E. Rubio-Wilhelmi M. M. Rosales M. A. Ruiz J. M. Romero L. Does iodine biofortification affect oxidative metabolism in lettuce plants. Biol. Trace Elem. Res. 2011;142:831–842. doi: 10.1007/s12011-010-8816-9. [DOI] [PubMed] [Google Scholar]
- Mikkelsen K. Prakash M. D. Kuol N. Nurgali K. Stojanovska L. Apostolopoulos V. Anti-tumor effects of vitamin B2, B6 and B9 in promonocytic lymphoma cells. Int. J. Mol. Sci. 2019;20:3763. doi: 10.3390/ijms20153763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikas I. P. Paschou S. A. Ryu H. S. The role of nicotinamide in cancer chemoprevention and therapy. Biomolecules. 2020;10:1–20. doi: 10.3390/biom10030477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hira S. Jack F. Mikkelsen K. Al Dhaheri A. S. Ali H. I. Platat C. Ismail L. C. Stojanovska L. Apostolopoulos V. Be well: a potential role for vitamin B in COVID-19. Maturitas. 2021;144:108–111. doi: 10.1016/j.maturitas.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeer N. B. Montesano D. Albrizio S. Zengin G. Mahomoodally M. F. The versatility of antioxidant assays in food science and safety—chemistry, applications, strengths, and limitations. Antioxidants. 2020;9:1–39. doi: 10.3390/antiox9080709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco B. Rios J. J. Cervilla L. M. Sánchez-Rodrigez E. Ruiz J. M. Romero L. Iodine biofortification and antioxidant capacity of lettuce: potential benefits for cultivation and human health. Ann. Appl. Biol. 2008;152:289–299. doi: 10.1111/j.1744-7348.2008.00217.x. [DOI] [Google Scholar]
- Krzepiłko A. Zych-Wężyk I. Molas J. Skwaryło-Bednarz B. Święciło A. Skowrońska M. The effect of iodine biofortification on selected biological quality parameters of lettuce and radish seedlings. Acta Sci. Pol. Hortorum Cultus. 2016;15:3–16. [Google Scholar]
- Kiferle C. Ascrizzi R. Martinelli M. Gonzali S. Mariotti L. Pistelli L. Flamini G. Perata P. Effect of Iodine treatments on Ocimum basilicum L.: biofortification, phenolics production and essential oil composition. PLoS One. 2019;14:1–23. doi: 10.1371/journal.pone.0226559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. Ullah F. Zhou D. X. Yi M. Zhao Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019;10:1–10. doi: 10.3389/fpls.2019.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel Ç. K. Bor M. Ryser P. Interspecific diversity in root antioxidative enzyme activities reflect root turnover strategies and preferred habitats in wetland graminoids. BMC Ecol. Evol. 2014;4:841–850. doi: 10.1002/ece3.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszár J. Lantos E. Tari I. Madoşă E. Wodala B. Vashegyi Á. Horváth F. Pécsváradi A. Szabó M. Bartha B. Gallé Á. Lazăr A. Coradini G. Staicu M. Postelnicu S. Mihacea S. Nedelea G. Erdei L. Antioxidant enzyme activities in Allium species and their cultivars under water stress. Plant, Soil Environ. 2007;53:517–523. doi: 10.17221/2192-PSE. [DOI] [Google Scholar]
- Gupta N. Bajpai M. Majumdar R. Mishra P. Response of iodine on antioxidant levels of Glycine max L. Grown under Cd2+ stress. Adv. Biol. Res. 2015;9:40–48. [Google Scholar]
- Leyva R. Sánchez-Rodríguez E. Ríos J. J. Rubio-Wilhelmi M. M. Romero L. Ruiz J. M. Blasco B. Beneficial effects of exogenous iodine in lettuce plants subjected to salinity stress. Plant Sci. 2011;181:195–202. doi: 10.1016/j.plantsci.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Halka M. Smoleń S. Ledwożyw-Smoleń I. Sady W. Comparison of Effects of Potassium Iodide and Iodosalicylates on the Antioxidant Potential and Iodine Accumulation in Young Tomato Plants. J. Plant Growth Regul. 2019;39:282–295. doi: 10.1007/s00344-019-09981-2. [DOI] [Google Scholar]
- Medrano-Macías J. Leija-Martínez P. Juárez-Maldonado A. Rocha-Estrada A. Benavides-Mendoza A. Effect of iodine application on antioxidants in tomato seedlings. Rev. Chapingo, Ser. Hortic. 2016;22:133–143. doi: 10.5154/r.rchsh.2015.12.025. [DOI] [Google Scholar]
- Ghasemzadeh A. Ghasemzadeh N. Flavonoids and phenolic acids: role and biochemical activity in plants and human. J. Med. Plants Res. 2011;5:6697–6703. [Google Scholar]
- Naveed M. Hejazi V. Abbas M. Kamboh A. A. Khan G. J. Shumzaid M. Ahmad F. Babazadeh D. FangFang X. Modarresi-Ghazani F. WenHua L. XiaoHui Z. Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomed. Pharmacother. 2018;97:67–74. doi: 10.1016/j.biopha.2017.10.064. [DOI] [PubMed] [Google Scholar]
- Wulanjari D. Wijaya K. A. Rosyady M. G. Wafa A. Polyphenol Content and Enhancing Plant resistance of Lowland Arabica Coffee. E3S Web Conf. 2020;142:1–3. [Google Scholar]
- Blasco B. Leyva R. Romero L. Ruiz J. M. Iodine effects on phenolic metabolism in lettuce plants under salt stress. J. Agric. Food Chem. 2013;61:2591–2596. doi: 10.1021/jf303917n. [DOI] [PubMed] [Google Scholar]
- Chikara S. Nagaprashantha L. D. Singhal J. Horne D. Awasthi S. Singhal S. S. Oxidative stress and dietary phytochemicals: role in cancer chemoprevention and treatment. Cancer Lett. 2018;413:122–134. doi: 10.1016/j.canlet.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Eghbaliferiz S. Iranshahi M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phyther. Res. 2016:1379–1391. doi: 10.1002/ptr.5643. [DOI] [PubMed] [Google Scholar]
- Zaidieh T. Smith J. R. Ball K. E. An Q. ROS as a novel indicator to predict anticancer drug efficacy. BMC Cancer. 2019;19:1–14. doi: 10.1186/s12885-019-6438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J. Song M. H. Oh J. W. Keum Y. S. Saini R. K. Pro-oxidant Actions of Carotenoids in Triggering Apoptosis of Cancer Cells: A Review of Emerging Evidence. Antioxidants. 2020;9:1–17. doi: 10.3390/antiox9060532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabas K. Milner R. Lurie D. Adin C. Cisplatin: a review of toxicities and therapeutic applications. Vet. Comp. Oncol. 2008;6:1–18. doi: 10.1111/j.1476-5829.2007.00142.x. [DOI] [PubMed] [Google Scholar]
- Marullo R. Werner E. Degtyareva N. Moore B. Altavilla G. Ramalingam S. S. Doetsch P. W. Cisplatin Induces a Mitochondrial-ROS Response That Contributes to Cytotoxicity Depending on Mitochondrial Redox Status and Bioenergetic Functions. PLoS One. 2013;8:1–15. doi: 10.1371/journal.pone.0081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. M. Kim H. K. Shim W. Anwar M. A. Kwon J. W. Kwon H. K. Kim H. J. Jeong H. Kim H. M. Hwang D. Kim H. S. Choi S. Mechanism of Cisplatin-Induced Cytotoxicity Is Correlated to Impaired Metabolism Due to Mitochondrial ROS Generation. PLoS One. 2015;10:1–21. doi: 10.1371/journal.pone.0135083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aceves C. García-Solís P. Arroyo-Helguera O. Vega-Riveroll L. Delgado G. Anguiano B. Antineoplastic effect of iodine in mammary cancer: participation of 6-iodolactone (6-IL) and peroxisome proliferator-activated receptors (PPAR) Mol. Cancer. 2009;8:1–9. doi: 10.1186/1476-4598-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T. Kojima K. Yoshiura K. Hiraishi H. Terano A. Characteristics of the peroxisome proliferator activated receptor γ (PPARγ) ligand induced apoptosis in colon cancer cells. Gut. 2002;50:658–664. doi: 10.1136/gut.50.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procházková D. Boušová I. Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82:513–523. doi: 10.1016/j.fitote.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Hou N. Liu N. Han J. Yan Y. Li J. Chlorogenic acid induces reactive oxygen species generation and inhibits the viability of human colon cancer cells. Anticancer Drugs. 2017;28:59–65. doi: 10.1097/CAD.0000000000000430. [DOI] [PubMed] [Google Scholar]
- Sun Y. Liu W. Z. Liu T. Feng X. Yang N. Zhou H. F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduction. 2015;35:600–604. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- D'Angelo S. Martino E. Ilisso C. P. Bagarolo M. L. Porcelli M. Cacciapuoti G. Pro-oxidant and pro-apoptotic activity of polyphenol extract from Annurca apple and its underlying mechanisms in human breast cancer cells. Int. J. Oncol. 2017;51:939–948. doi: 10.3892/ijo.2017.4088. [DOI] [PubMed] [Google Scholar]
- Wihadmadyatami H. Karnati S. Hening P. Tjahjono Y. Rizal Maharjanti F. Kusindarta D. L. Triyono T. Supriatno Ethanolic extract Ocimum sanctum Linn. induces an apoptosis in human lung adenocarcinoma (A549) cells. Heliyon. 2019;5:e02772. doi: 10.1016/j.heliyon.2019.e02772. [DOI] [PMC free article] [PubMed] [Google Scholar]