Abstract
Bladder cancer (BC) is among the top ten most common cancer types globally. Muscle invasive BC has a high incidence of metastasis. Metastatic BC has a poor prognosis and limited treatment options. Here, we present a middle-aged man with oligometastatic BC, which was treated with palliative chemotherapy. He had significant clinical improvement. However, interim 18F-Fluorodeoxyglucose positron emission tomography/computed tomography demonstrates a rapid disease progression extensive metastasis.
Keywords: Fluorodeoxyglucose positron emission tomography/computed tomography, metastatic disease, progressive disease, treatment response evaluation, urinary bladder carcinoma
A 55-year-old male presented with painless hematuria for two months. He had no associated fever or weight loss. Contrast-enhanced computed tomography of the abdomen and pelvic showed a mass in the UB. A transurethral biopsy confirmed muscle-invasive bladder cancer (MIBC). The pretreatment fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) revealed aUB mass with nontracer avid sub-centimeter size pelvis lymph node (LN). Because of suspicious metastatic lung nodules, he received four cycles of carboplatin and gemcitabine-based palliative chemotherapy. His chemotherapies were uneventful, and hematuria subsided. The patient was referred for a follow-up interim FDG PET/CT scan to evaluate treatment response. It showed rapid progression of the metastatic disease burden [Figures 1 and 2].
Figure 1.
(a) Maximum intensity projection images of baseline 18-F fluorodeoxyglucose positron emission tomography/computed tomography revealed fluorodeoxyglucose avid lesion in the urinary bladder (black arrow) and few nodules in the lungs (blue arrow). (b) Fused whole-body coronal images show fluorodeoxyglucose avid thickening of urinary bladder (black arrow). (c and d) Follow-up maximum intensity projection and fused coronal images reveal residual thickening in the urinary bladder. Apart from that, multiple areas of uptake are noted in retroperitoneal lymph nodes (blue arrow), mediastinal lymph node, lung (blue arrow), and liver (red arrow)
Figure 2.
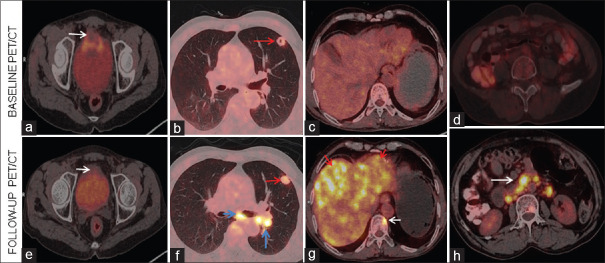
Baseline (a-d) trans-axial images fused positron emission tomography/computed tomography show mass involving the anterosuperior wall of urinary bladder (white arrow). A faintly fluorodeoxyglucose avid nodule is noted in the upper lobe of the left lung (red arrow). The liver and retroperitoneum are unremarkable. Follow-up fluorodeoxyglucose positron emission tomography/computed tomography images (e-h) reveal residual primary (white arrow in e) lesion of the urinary bladder. Fluorodeoxyglucose avid nodule in the upper lobe of the left lung (red arrow in f) with supraclavicular and mediastinal lymph nodes (blue arrows) are seen. Extensive fluorodeoxyglucose avid (red arrows in g) hepatic lesions are noted with a bone lesion in the D8 vertebra (white arrow in g). Multiple fluorodeoxyglucose avid (white arrow, h) retroperitoneal lymph nodes are noted
Bladder cancer (BC) is the ninth most common cancer in developed countries with poor prognosis and outcome. It is mainly due to delayed diagnosis and limited treatment possibilities. It arises from the urothelial cell lining.[1] The main risk factor for BC is tobacco smoking.[2] At the time of diagnosis, approximately 75% of these have non-MIBC, with the remaining 25% having MIBCor metastatic disease. Early diagnosis and multimodality therapy could result in optimal outcomes. However, metastatic disease is generally incurable, with a dismal 5-year overall survival rate of 15%.[3] Tumors in the advanced T category and atypical histologic features metastasize earlier. It commonly metastasis to the LNs, bones, lungs, liver, and peritoneum.[4]
The metastatic disease had limited treatment options for decades. Cisplatin-based chemotherapy remained a mainstay of treatment. However, with recent advancements, various new therapeutic agents are available. The use of immune checkpoint inhibitors (ICIs) directed at programmed cell-death protein 1 has led to unprecedented survival advantages in selected patients. Atezolizumab and pembrolizumab (both ICIs) may be given in platinum-ineligible advanced UC patients. Recently erdafitinib, a pan-FGFR inhibitor, is approved for advanced metastatic UC.[5]
FDG/PET is increasingly used in the initial metastatic workup and treatment response evaluation. The pooled sensitivity and specificity of FDG PET/CT for staging or restaging are 82% and 89%, respectively.[6] It is found more accurate than CT in the presurgery stage. Following neoadjuvant chemotherapy in MIBC, FDG-PET/CT demonstrated 75% sensitivity and 90% specificity in identifying a complete pathological response.[7] To access the response of first-line systemic chemotherapy (cisplatin and gemcitabine), FDG PET/CT outperformed CT interpretation alone based on Response Criteria in Solid Tumors RECIST criteria.[8] FDGPET/CT predicts progression-free survival and overall survival after two cycles of palliative chemotherapy in metastatic BC.[9] FDG PET/CT helps to detect recurrence and change in treatment decisions in approximately 40% of the patients.[10] Rapid asymptomatic progression of the metastatic BC is uncommon and needs to be reported. This case highlights the value of interim PET-CT to evaluate the treatment response in metastatic BC.
Declaration of patient consent
The authors certify that they have obtained allappropriate patient consent forms. In the form the patient (s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Višnjar T, Romih R, Zupančič D. Lectins as possible tools for improved urinary bladder cancer management. Glycobiology. 2019;29:355–65. doi: 10.1093/glycob/cwz001. [DOI] [PubMed] [Google Scholar]
- 2.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–41. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 4.Shinagare AB, Ramaiya NH, Jagannathan JP, Fennessy FM, Taplin ME, Van den Abbeele AD. Metastatic pattern of bladder cancer: Correlation with the characteristics of the primary tumor. AJR Am J Roentgenol. 2011;196:117–22. doi: 10.2214/AJR.10.5036. [DOI] [PubMed] [Google Scholar]
- 5.Nadal R, Bellmunt J. Management of metastatic bladder cancer. Cancer Treat Rev. 2019;76:10–21. doi: 10.1016/j.ctrv.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Lu YY, Chen JH, Liang JA, Wang HY, Lin CC, Lin WY, et al. Clinical value of FDG PET or PET/CT in urinary bladder cancer: A systemic review and meta-analysis. Eur J Radiol. 2012;81:2411–6. doi: 10.1016/j.ejrad.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Soubra A, Gencturk M, Froelich J, Balaji P, Gupta S, Jha G, et al. FDG-PET/CT for assessing the response to neoadjuvant chemotherapy in bladder cancer patients. Clin Genitourin Cancer. 2018;16:360–4. doi: 10.1016/j.clgc.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Öztürk H. Comparing RECIST with EORTC criteria in metastatic bladder cancer. J Cancer Res Clin Oncol. 2016;142:187–94. doi: 10.1007/s00432-015-2022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giannatempo P, Alessi A, Miceli R, Raggi D, Farè E, Nicolai N, et al. Interim fluorine-18 fluorodeoxyglucose positron emission tomography for early metabolic assessment of therapeutic response to chemotherapy for metastatic transitional cell carcinoma. Clin Genitourin Cancer. 2014;12:433–9. doi: 10.1016/j.clgc.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Alongi P, Caobelli F, Gentile R, Stefano A, Russo G, Albano D, et al. Recurrent bladder carcinoma: Clinical and prognostic role of 18 F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2017;44:224–33. doi: 10.1007/s00259-016-3500-8. [DOI] [PubMed] [Google Scholar]