Abstract
This study was conducted in order to (i) determine the effect of food, orange juice, or antacids on the absorption of a single oral 500-mg dose of ethionamide (ETA) in healthy volunteers, including an assessment of bioequivalence, and (ii) determine ETA population pharmacokinetic (PK) parameters. The pharmacokinetics of ETA in serum was determined for 12 healthy males and females in a randomized, four-period crossover study. Volunteers received single 500-mg doses of ETA either on an empty stomach (reference) or with food, orange juice, or antacids. Serum samples were collected for 48 h and assayed by high-performance liquid chromatography. Data were analyzed by noncompartmental and population methods. Mean test/reference ratios and 90% confidence intervals were determined. No statistically significant differences were seen in the maximum concentration of ETA (Cmax), time to maximum concentration (Tmax), or area under the concentration-time curve from 0 h to infinity (AUC0–∞) between the four treatments (P > 0.05 by analysis of variance). The least-squares mean ratios (with confidence intervals in parentheses) for Cmax were 105% (81.2 to 135%) after orange juice, 94% (72.8 to 121%) after food, and 88% (68.4 to 114%) after antacids. The least-squares mean ratios (with confidence intervals is in parentheses) for AUC0–∞ were 91% (72.7 to 115%) after orange juice, 96% (76.4 to 121%) after food, and 95% (75.5 to 120%) after antacids. The mean Tmax was slightly prolonged following antacid or food administration (2.3 to 2.6 h) compared to administration on an empty stomach or with juice (1.7 to 1.9 h). The median population PK parameters were as follows: Ka = 0.37 to 0.48 h−1, V/F = 2.0 to 2.8 liters/kg, CL/F = 56.5 to 72.2 liters/h, and terminal half-life = 1.7 to 2.1 h, where Ka is the absorption rate constant, V is the volume of distribution, and CL is clearance. The PK behavior of ETA was not significantly modified by the different conditions studied. Mean ratios for AUC ranged from 0.91 to 0.96 for the orange juice, food, and antacid treatments, indicating a minimal effect on relative bioavailability. ETA can, therefore, be administered with food if tolerance is an issue.
Ethionamide (ETA) is a bacteriostatic, isonicotinic acid-derived antituberculosis agent (10, 28). It is used in combination for the treatment of clinical tuberculosis that has failed to respond to adequate first-line therapy (1). Very limited information exists in the literature regarding the pharmacokinetics (PK) of ETA in healthy volunteers or in patients with tuberculosis. ETA is often administered with meals to reduce gastrointestinal intolerance (1). However, to our knowledge, the effects of food, as well as those of antacids or acidic beverages on the PK of ETA have not been evaluated in a crossover study. We have previously demonstrated that food has minimal effect on the absorption of ethambutol and pyrazinamide, while antacids should be avoided near the time of ethambutol dosing (17, 18). Similarly, we have found that rifampin and isoniazid should be given on an empty stomach whenever possible (20, 21). It is important to determine the conditions that may impair or promote the achievement of adequate serum ETA concentrations. Low, concentrations in plasma may prevent the complete eradication of Mycobacterium tuberculosis, leading to therapeutic failure and the development of drug resistance. This study was conducted in order to (i) determine the effect of food, orange juice, or antacids on the absorption of a single 500-mg oral dose of ETA in healthy volunteers, including an assessment of bioequivalence, and (ii) determine ETA population PK parameters.
MATERIALS AND METHODS
We conducted a four-period, randomized crossover study of ETA. The study protocol followed the guidelines of the Helsinki Declaration of 1975 and its amendments and was approved by the institutional review board at the University of Arizona, Tucson. Written informed consent was obtained from each subject before the study. Sixteen healthy volunteers were scheduled to participate. Subjects were eligible if they were 18 years of age or older and were considered in good health, based on medical history, physical examination, routine serum chemistries, complete blood count with platelets, and urinalysis.
Subjects were excluded if they had histories of kidney disease or an estimated creatinine clearance of <50 ml/min, liver or cardiovascular diseases, or a hematocrit of <36% at screening (7). They also were excluded if any conditions known to interfere with the absorption of drugs were present or if they had known positive human immunodeficiency virus (HIV) serology, AIDS, or a history of hypersensitivity to ETA, clofazimine, cycloserine, para-aminosalicylic acid, or pyridoxine. They were also excluded if they weighed >130% of ideal body weight, were pregnant or nursing, or had donated blood within 30 days prior to the study. The subjects did not take any other prescription or nonprescription drugs for 1 week before the study or during the entire study period.
Experimental design.
Sixteen subjects were randomized in four blocks of four subjects each. This study employed a four-period, randomized crossover design. All subjects received single oral doses of 500 mg of ETA (Wyeth-Ayerst, Philadelphia, Pa), 200 mg of clofazimine (Novartis Pharmaceuticals, East Hanover, N.J.), 500 mg of cycloserine (Dura Pharmaceuticals, San Diego, Calif.), 6 g of para-aminosalicylic acid granules (Jacobus Pharmaceuticals, Princeton, N.J.), and 100 mg of pyridoxine (Goldline Laboratories, Inc., Fort Lauderdale, Fla.) on four different occasions, each separated by at least 2 weeks. This single-dose drug regimen was administered under four different conditions: on an empty stomach (reference), with orange juice, with a high-fat meal, and with antacids. The subjects were housed at the study center from 1 h before to 24 h after the dosing and returned for the 36- and 48-h blood collections. The subjects were instructed to fast overnight prior to each treatment visit. With the exception of study medications and food items included in the study methods, the subjects continued to fast until 4 h after study medication dosing. The subjects were allowed to drink water ad libitum. The orange juice treatment (240 ml of Minute Maid from concentrate) was administered at the same time as study medications. The high-fat meal consisted of two scrambled eggs, two pieces of toast with two teaspoons of butter, six ounces of hashed brown potatoes, two strips of bacon, and eight ounces of whole milk. Subjects began eating the high-fat meal 15 min prior to dosing and were instructed to complete the meal within 30 min. The meal was interrupted for dosing. The antacid treatment consisted of 15 ml of maximum-strength Mylanta (400 mg of aluminum hydroxide, 400 mg of magnesium hydroxide, and 40 mg of simethicone per 5 ml) administered 9 h before dosing, at the same time as dosing, and immediately after meals and at bedtime on the dosing day and the following day.
Sample collection.
A 20-gauge venous catheter was inserted into a forearm vein for the collection of blood samples and was maintained patent with a dilute heparin solution (10 U/ml). Two millimeters of blood was withdrawn and discarded prior to collection of each blood sample. A total of 18 12-ml blood samples were collected in vacuum tubes containing heparin sodium before dosing and at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, 14, 24, 36, and 48 h after the doses. Samples were centrifuged at 2,500 to 3,000 × g for 10 min. The plasma was then harvested and frozen at −70°C until assay. A baseline urine sample was collected within 30 min of dosing. Subsequently, all urine was collected from 0 to 24 h. Samples were kept refrigerated during the period of collection. The total volume of the 24-h urine was measured at the end of the collection period, and 10-ml aliquots were frozen at −70°C until assay.
Sample analysis.
Plasma ETA concentrations were determined using a validated high-performance liquid chromatography (HPLC) assay. The standard curve for plasma ETA concentrations ranged from 0.2 to 10 μg/ml. The absolute recovery of ETA from plasma was 91%. The within-day precision (coefficient of variation [CV]) of validation quality control (QC) samples was 0.36 to 6.39%, and the overall validation precision was 0.81 to 4.66%. The urine method was similar to that for plasma with an additional 1:1 dilution postextraction. The assay error pattern was determined from QC samples assayed over the course of the study. The assay error pattern was determined by fitting a first-order polynomial to the plot of the assay standard deviations (y) versus the means (x), producing the formula y = 0.014707 + 0.12393x. The specificity of the HPLC assay for ethionamide was determined by testing spiked samples for approximately 90 other drugs, including the other study drugs. No interferences with the measurement of ETA by clofazimine, cycloserine, para-aminosalicylic acid, or pyridoxine were observed.
Safety analysis.
Health assessment, including vital signs, physical examination, and clinical laboratory testing (described above), was performed. Subjects were interviewed at the beginning and end of each study period and were monitored throughout the confinement period to determine any adverse events potentially related to study medications or procedures.
PK analysis.
PK analyses were performed by using both noncompartmental and population methods (9). Maximum concentrations of ETA in serum (Cmax) and times to these concentrations (Tmax) were determined by visual inspection of the plasma concentration-time profiles. At each time point (t), (Ct/Cmax) × 100% per individual was calculated, and the maximum, median, and minimum values across all subjects were determined. These percentages can provide some guidance regarding sampling times that can be used clinically.
The area under the concentration-time curve from 0 h to infinity (AUC0–∞) was calculated by the linear trapezoidal rule using the AUC from 0 h to the last measured concentration (Clast) plus Clast/Kel where tlast is the time of the last measured concentration and Kel is the terminal elimination rate constant. The elimination of ETA was described by monoexponential decay in plasma ETA concentrations. Therefore, concentrations in plasma following ETA oral administration under all conditions were fit using a linear one-compartment PK model with first-order absorption and elimination processes.
Individual PK parameter estimates were first obtained for each ETA treatment using an iterative two-stage maximum a posteriori probability Bayesian population algorithm (IT2B). These estimates were then used as initial estimates to perform a population PK analysis for each ETA treatment group using a nonparametric expectation maximization (NPEM2) analysis (USCPACK software) (user manual, Laboratory of Applied Pharmacokinetics, University of Southern California, Los Angeles). The four ETA treatment groups were thereafter pooled together because similar results were obtained with all four, and a population PK analysis was reinitiated using the method previously described. Fitted PK parameters included the absorption rate constant (Ka), volume of distribution (V/F), and oral clearance (CL/F). The elimination rate constant (kel) was determined by dividing CL/F by V/F. The absorption (t1/2abs) and elimination (t1/2) half-lives were calculated as 0.693/Ka and 0.693/kel, respectively.
The amount of ETA recovered in the urine was calculated as the measured volume of urine multiplied by the corresponding ETA concentration. The percent dose recovered was calculated as total recovery divided by dose multiplied by 100%.
Statistical analysis.
Statistical analyses were performed by using JMP, version 3.2.6 (SAS Institute, Cary, N.C.). PK parameters for the four ETA treatments were compared by using an analysis of variance (ANOVA) including effects due to treatment, period, sequence, and subject nested in sequence. If the overall test for differences was statistically significant with α ≤0.05, pairwise differences between treatments and reference were tested by constructing linear contrasts. The comparisons of interest were between fasting ETA (reference treatment) and the test treatments. Cmax and AUC0–∞ were analyzed after logarithmic transformation. Geometric least-squares (LS) means were calculated for each ETA treatment. Calculation of 90% confidence intervals for the ratio of test to reference treatment geometric LS means was conducted for Cmax and AUC0–∞.
RESULTS
Twelve subjects completed all four ETA treatments. Their characteristics are described in Table 1. Four subjects (two white females, one Hispanic female, and one white male; ages, 29 to 43 years), withdrew from the study due to adverse events following completion of one to three treatment periods, and their data were not included in the analysis. Overall, only one subject experienced no adverse effects. The most commonly reported adverse effects were gastrointestinal in nature, including nausea, vomiting, and diarrhea. For the majority, these side effects occurred in the morning and improved within 2 to 4 h after lunch. Two subjects had persistent nausea and emesis for up to 12 h after dosing. Headache, dizziness, weakness, and difficulty concentrating were the central nervous system symptoms most frequently noted by study subjects. Gastrointestinal effects were more prevalent when ETA and the other medications were taken with orange juice.
TABLE 1.
ETA population demographics
| Characteristica | Mean ± SD |
|---|---|
| Sex | |
| Female | 6 |
| Male | 6 |
| Age (yr) | 36 ± 8 |
| Race | |
| Hispanic | 3 |
| Indian | 2 |
| White | 7 |
| Ht (cm) | 174.3 ± 7 |
| Wt (kg) | 78 ± 9 |
| SCR (mg/dl) | 0.88 ± 0.15 |
| CLCR (ml/min) | 103.7 ± 18 |
SCR, serum creatinine; CLCR, creatinine clearance (Cockroft and Gault equation) (7).
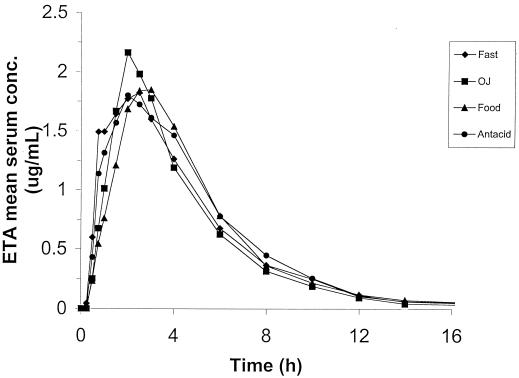
The absorption PK parameters following a single 500-mg dose of ETA are reported in Table 2 for the four different treatments. There was substantial intersubject variability in these parameters, as shown by the wide ranges and the fairly large variability (CV = 36 to 65%). Weight normalizing led to modestly increased variability for Cmax and AUC0–∞. The percentage of AUC extrapolated to infinity accounted for less than 5% of the total AUC0–∞ for all treatments (range, 2.3 to 3.5%). Compared with that for the fasting state, the mean Cmax was increased by orange juice (9%) and slightly decreased by antacids (−4%), and no effect was shown with food. The absorption of ETA was delayed by the concurrent ingestion of orange juice (12%), food (53%), or antacids (35%). The mean extent of absorption for ETA was slightly reduced by orange juice (−4%) and slightly or increased by antacid (4%) but not affected by food. The ANOVA for Cmax, AUC0–∞, and Tmax did not show significant differences between ETA treatments. This is supported by Fig. 1, where nearly superimposed plasma concentration curves were observed for the four different ETA treatments. The effect of sequence for Cmax and AUC0–∞ did reach statistical significance. Table 3 provides the 90% confidence intervals for the ratio (test/reference, as a percentage) of geometric LS means of Cmax and AUC0–∞ Table 4 shows a summary of concentrations relative to individual Cmax values at each time point (0.25 to 4 h). The median percentage of Cmax attained was highest between 2 and 3 h (range, 84 to 87% of Cmax), indicating that samples obtained between 2 and 3 h postdosing are more likely to capture the maximal concentration than samples taken at other times.
TABLE 2.
PK parameters for ETA administered in the fasting state, with a high-fat meal, with orange juice, or with an aluminum-magnesium antacid
| ETA treatment (n = 12) |
Cmax (μg/ml)
|
Tmax (h)
|
AUC0–∞ (μg · h/ml)
|
|||
|---|---|---|---|---|---|---|
| Mean (CV)a | Range | Mean (CV)a | Range | Mean (CV)a | Range | |
| Fasting | 2.3 (59) | 0.99–6.1 | 1.7 (51) | 0.75–3.0 | 10.0 (36) | 5.4–17 |
| Orange juice | 2.5 (45) | 0.47–5.2 | 1.9 (39) | 0.50–3.0 | 9.6 (39) | 2.7–16 |
| Food | 2.3 (44) | 0.76–4.2 | 2.6 (36) | 0.75–4.0 | 10.0 (38) | 4.1–18 |
| Antacids | 2.2 (65) | 0.68–6.2 | 2.3 (44) | 0.75–4.0 | 10.4 (50) | 3.1–19 |
CV is expressed as a percentage.
FIG. 1.
Mean serum concentration-versus-time profiles for oral ETA administered to 12 healthy volunteers under fasting conditions or with orange juice, food, or antacids over 16 h.
TABLE 3.
Mean ratios (treatment/reference) and 90% confidence intervals for ETA bioequivalence following administration with orange juice, a high-fat meal, or an aluminum-magnesium antacid compared to fasting
| Parameter | Treatment | Mean ratioa | 90% CIb |
|---|---|---|---|
| Cmax | Orange juice | 105 | 81.2–135 |
| High-fat meal | 94 | 72.8–121 | |
| Antacids | 88 | 68.4–114 | |
| AUC0–∞ | Orange juice | 91 | 72.7–115 |
| High-fat meal | 96 | 76.4–121 | |
| Antacids | 95 | 75.5–120 |
Ratio of geometric LS means.
CI, confidence interval.
TABLE 4.
Concentrations of ETA in serum following fasting administration of ETA expressed as a percentage of individual Cmax over a period of 0.25 to 4 h
| Time postdosing (h) | Concn of ETA in plasma (% of Cmax)
|
||
|---|---|---|---|
| Highest | Median | Lowest | |
| 0.25 | 23 | 0 | 0 |
| 0.50 | 78 | 23 | 2 |
| 0.75 | 100 | 59 | 6 |
| 1.00 | 100 | 71 | 14 |
| 1.50 | 94 | 70 | 51 |
| 2.00 | 100 | 87 | 51 |
| 2.50 | 100 | 87 | 47 |
| 3.00 | 100 | 84 | 36 |
| 4.00 | 76 | 64 | 33 |
The fasting treatment (n = 12) population ETA median PK parameter estimates (with CVs in parentheses) were as follows: Ka, 0.48 h−1 (136%), t1/2abs, 1.4 h (40%), V/F, 2.4 liters/kg (30%), CL/F 64.5 liters/h (24%), kel, 0.39 h−1, (26%), and t1/2, 1.8 h (37%). The other treatment parameter values, as well as the overall pooled (n = 48) population parameter estimates, were very similar to the fasting treatment values. The only statistically significant difference was observed for the PK parameter V/F (ANOVA, P = 0.045), which was largest for the food and antacid treatments. No analysis was performed on the urinary data because the concentrations of ETA in urine were below the detection limit.
Simulation of eight daily doses of ETA at 500 mg per dose showed that the 24 h concentration for all days was below the limit of quantitation (<0.20 μg/ml), so no accumulation would be expected. Simulation of 16 doses of ETA at 500 mg per dose given every 12 h showed that the Cmax on day 1 was approximately 95% of the steady-state value. The third-dose (day 2) Cmax was >99% of the steady-state value, with little change thereafter. Therefore, samples collected as early as day 2 of treatment would provide a reasonable estimate of the steady-state Cmax (Table 4).
DISCUSSION
ETA is known to be poorly tolerated (15, 24, 28). Gradually increasing the dose and dividing the total daily dose into two or three daily doses are routine strategies to prevent or minimize gastrointestinal disturbances associated with this drug (1). Enteric-coated ETA tablets have been developed in an attempt to improve tolerability; however, no significant reduction in gastrointestinal symptoms has been found. Moreover, the enteric-coated tablet was associated with lower and more variable concentrations in plasma (10, 28). Since multiple medications were administered in this study, it is difficult to incriminate ETA as the causal agent of the untoward reactions. Clofazimine and para-aminosalicylic acid may also cause gastrointestinal intolerance (14, 22, 23).
The PK parameters observed in this study are consistent with prior reports. Mean Cmax values ranging from 2.2 to 2.6 μg/ml, with corresponding average Tmax values of 1.5 to 3 h, have been reported following a single 500-mg dose of ETA taken on an empty stomach (10–13, 19) or with fruit juice (26). For AUC0–∞, we have previously found a similar value of 10.3 μg · h/ml (19). No studies of the PK behavior of ETA administered with food or antacids compared with that for administration to fasting subjects have been published.
Relatively high interindividual variability was seen in Cmax, Tmax, and AUC0–∞. The CVs for Cmax ranged from 44 to 65% (59% for the fasting treatment) in the present study. In a previous study, the CV for Cmax was 36.6% for 12 healthy adult male volunteers (19). The variability of AUC0–∞ ranged from 36 to 50% (36% for the fasting treatment) in this study compared to 22.2% in the earlier study (19). Considerable variability has also been observed in the individual plasma ETA concentrations of samples drawn at 1, 3 and 5 h postdosing for 20 subjects treated for pulmonary tuberculosis (26). ETA may undergo first-pass metabolism that could contribute to this variability (5, 11, 13, 25). The wide range of body weights in our subjects also might have contributed to the variability. However, correction for body weight modestly increased the variability in the results, suggesting that this is not an important variable. Although they were not statistically significant in our analyses, we cannot rule out gender and age differences in PK that might have contributed to the higher variability. ETA may be administered with food, orange juice, or antacids or on an empty stomach, since no significant differences in the Cmax and AUC0–∞ were found between the four treatments.
The effect of sequence did reach statistical significance for Cmax and AUC0–∞. The presence of carryover is unlikely, since the washout period of 14 days was more than sufficient to allow the complete elimination of ETA between study periods. The elimination half-life of ETA has been estimated to be less than 3 h (11–13, 19). In addition, the predose ETA concentrations in plasma were below the detection limit for all subjects in all four periods. Similarly, samples drawn after 16 h did not exhibit any detectable ETA concentrations. Finally, ETA has not been shown to inhibit or induce hepatic microsomal enzymes. The likelihood of finding a significant sequence effect when one is using a four-treatment crossover design is considerable, even in the absence of a true sequence effect (8). Therefore, we do not consider this statistical result to be an important variable in the results.
Bioequivalence was assessed using standard equations. The interpretation of our bioequivalence results appears to be limited by the statistical power of the study. The ratios of geometric LS means (percent test/reference) for Cmax and AUC0–∞ were close to 100%, and no statistical differences were found for these two parameters between the four ETA treatments (ANOVA, P > 0.05). However, the data failed to meet the Food and Drug Administration (FDA) bioequivalence criteria. This is the typical situation encountered with highly variable drugs, those exhibiting a CV greater than 30% (2, 3, 6, 16, 25). With a 40% intrasubject CV, 70 subjects would have been required to perform the procedure involving two one-sided tests, far more subjects than we could have enrolled (4, 6, 27). Although strict criteria for bioequivalence were not met in this study for ETA in the presence of a high-fat meal, orange juice, or an antacid, the results show very small effects on mean bioavailability. It appears safe to administer ETA in the presence of a high-fat meal, orange juice, or aluminum-magnesium antacids as required to achieve patient adherence to the regimen.
ETA population PK estimates are relatively similar to those previously reported (11–13, 19). The oral clearance values are slightly greater than the 51.2 liters/h that we have previously reported (19). We have reported a similar value of 2.8 liters/kg for the volume of distribution after oral administration of ETA, while Jenner and colleagues have reported a smaller value of approximately 1.1 liters/kg following intravenous administration (12, 19). Bioavailability is a major factor accounting for the larger values obtained for this parameter after oral administration of ETA.
The absence of detectable concentrations of ETA in the urine is consistent with the extensive metabolism of ETA, presumably occurring via the liver. So far, six metabolites have been identified, including the active sulfoxide (5, 11, 13). Small fractions, ranging from 0.15 to 0.18%, of an ETA dose of 500 mg, were excreted unchanged in the urine, while an average of 1.2% was eliminated by this route as sulfoxide (11, 13). The measurable amounts of ETA in the urine, although very small, contrast with our results. The use of a lower detection limit, 0.01 μg/ml compared to our limit of 0.2 μg/ml, to determine urine ETA concentrations with a similar HPLC assay may have contributed to this difference (13). Measurement of concentrations of ETA metabolites in plasma and urine was beyond the scope of this analysis. The fecal excretion of ETA has been negligible, with a reported value of less than 0.1% (13).
The PK behavior of ETA was not significantly altered by the different conditions studied. ETA can, therefore, be given with food, orange juice, or antacids or on an empty stomach, as needed, to improve tolerability for the patient.
ACKNOWLEDGMENT
This study was supported, in part, by NIH grant 1 RO1 AI37845.
REFERENCES
- 1.American Thoracic Society. Treatment of tuberculosis infection in adults and children. Am J Crit Care Med. 1994;149:1359–1374. doi: 10.1164/ajrccm.149.5.8173779. [DOI] [PubMed] [Google Scholar]
- 2.Balthasar J P. Bioequivalence and bioequivalence testing. Am J Pharm Educ. 1999;63:194–198. [Google Scholar]
- 3.Benet L Z. Understanding bioequivalence testing. Transplant Proc. 1999;31(Suppl. 3A):7S–9S. doi: 10.1016/s0041-1345(99)00104-9. [DOI] [PubMed] [Google Scholar]
- 4.Benet L Z, Goyan J E. Bioequivalence and narrow therapeutic index drugs. Pharmacotherapy. 1995;15:433–440. [PubMed] [Google Scholar]
- 5.Bieder A, Brunel P, Mazeau L. Identification de trois nouveaux metabolites de l'ethionamide: chromatographie, spectrophotometrie, polarographie. Ann Pharm Fr. 1966;24:493–500. [PubMed] [Google Scholar]
- 6.Boddy A W, Snikeris F C, Kringle R O, Wei G C G, Oppermann J A, Midha K K. An approach for widening the bioequivalence acceptance limits in the case of highly variable drugs. Pharm Res. 1995;12:1865–1868. doi: 10.1023/a:1016219317744. [DOI] [PubMed] [Google Scholar]
- 7.Cockroft D W, Gault M H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;10:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 8.Food and Drug Administration. Bioavailability and bioequivalence requirements. Fed Regist. 1992;57:17997–18001. [Google Scholar]
- 9.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York, N.Y: Marcel Dekker; 1982. [Google Scholar]
- 10.Gronroos J A, Toivanen A. Blood ethionamide levels after administration of enteric-coated and uncoated tablets. Curr Ther Res. 1964;6:105–114. [PubMed] [Google Scholar]
- 11.Jenner P J, Ellard G A. High performance liquid chromatographic determination of ethionamide and prothionamide in body fluids. J Chromatogr. 1981;222:245–251. doi: 10.1016/s0378-4347(00)80269-8. [DOI] [PubMed] [Google Scholar]
- 12.Jenner P J, Smith S E. Plasma levels of ethionamide and prothionamide in a volunteer following intravenous and oral dosages. Lepr Rev. 1987;58:31–37. doi: 10.5935/0305-7518.19870004. [DOI] [PubMed] [Google Scholar]
- 13.Jenner P J, Ellard G A, Gruer P J K, Aber V R. A comparison of the blood levels and urinary excretion of ethionamide and prothionamide in man. J Antimicrob Chemother. 1984;13:267–277. doi: 10.1093/jac/13.3.267. [DOI] [PubMed] [Google Scholar]
- 14.Lal S, Garg B R, Hameedulla A. Gastro-intestinal side effects of clofazimine. Lepr India. 1981;53:285–288. [PubMed] [Google Scholar]
- 15.Lees A W. Ethionamide and isoniazid in previously untreated cases of pulmonary tuberculosis. Dis Chest. 1964;45:247–250. doi: 10.1378/chest.45.3.247. [DOI] [PubMed] [Google Scholar]
- 16.Midha K K, Ormsby E D, Hubbard J W, McKay G, Hawes E M, Gavalas L, McGilveray I J. Logarithmic transformation in bioequivalence: application with two formulations of perphenazine. J Pharm Sci. 1993;82:138–144. doi: 10.1002/jps.2600820205. [DOI] [PubMed] [Google Scholar]
- 17.Peloquin C A, Bulpitt A E, Jaresko G S, Jelliffe R W, James G T, Nix D E. Pharmacokinetics of pyrazinamide under fasting conditions, with food, and with antacids. Pharmacotherapy. 1998;18:1205–1211. [PubMed] [Google Scholar]
- 18.Peloquin C A, Bulpitt A E, Jaresko G S, Jelliffe R W, Childs J M, Nix D E. Pharmacokinetics of ethambutol under fasting conditions, with food, and with antacids. Antimicrob Agents Chemother. 1999;43:568–572. doi: 10.1128/aac.43.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peloquin C A, James G T, McCarthy E, Goble M. Pharmacokinetic evaluation of ethionamide suppositories. Pharmacotherapy. 1991;11:359–363. [PubMed] [Google Scholar]
- 20.Peloquin C A, Namdar R, Nix D E. Pharmacokinetics of isoniazid under fasting conditions, with food, and with antacids. Int J Tuber Lung Dis. 1999;3:703–710. [PubMed] [Google Scholar]
- 21.Peloquin C A, Namdar R, Singleton M D, Nix D E. Pharmacokinetics of rifampin under fasting conditions, with food, and with antacids. Chest. 1999;115:12–18. doi: 10.1378/chest.115.1.12. [DOI] [PubMed] [Google Scholar]
- 22.Peloquin C A, Berning S E, Huitt G A, Childs J M, Singleton M D, Gordon T J. Once-daily and twice-daily dosing of p-aminosalicylic acid granules. Am J Respir Crit Care Med. 1999;159:932–934. doi: 10.1164/ajrccm.159.3.9807131. [DOI] [PubMed] [Google Scholar]
- 23.Ramu G, Iyer C G S. Side effects of clofazimine therapy. Lepr India. 1976;48(Suppl.):722–731. [PubMed] [Google Scholar]
- 24.Schwartz W S. Comparison of ethionamide with isoniazid in original treatment cases of pulmonary tuberculosis. Am Rev Respir Dis. 1966;93:685–692. doi: 10.1164/arrd.1966.93.5.685. [DOI] [PubMed] [Google Scholar]
- 25.Shah V P, Yacobi A, Barr W H, et al. Evaluation of orally administered highly variable drugs and drug formulations. Pharm Res. 1996;13:1590–1594. doi: 10.1023/a:1016468018478. [DOI] [PubMed] [Google Scholar]
- 26.Tiitinen H. Isoniazid and ethionamide serum levels and inactivation in Finnish subjects. Scand J Respir Dis. 1969;50:110–124. [PubMed] [Google Scholar]
- 27.Tsang Y C, Pop R, Gordon P, Hems J, Spino M. High variability in drug pharmacokinetics complicates determination of bioequivalence: experience with verapamil. Pharm Res. 1996;13:846–850. doi: 10.1023/a:1016040825844. [DOI] [PubMed] [Google Scholar]
- 28.Weinstein H J, Hallett W Y, Sarauw A S. The absorption and toxicity of ethionamide. Am Rev Respir Dis. 1962;86:576–578. doi: 10.1164/arrd.1962.86.4.576. [DOI] [PubMed] [Google Scholar]