Abstract
Background
Chromogranin A (CgA) and its fragment vasostatin I (VS-I) are secreted in the blood by endocrine/neuroendocrine cells and regulate stress responses. Their involvement in Coronavirus 2019 disease (COVID-19) has not been investigated.
Methods
CgA and VS-I plasma concentrations were measured at hospital admission from March to May 2020 in 190 patients. 40 age- and sex-matched healthy volunteers served as controls. CgA and VS-I levels relationship with demographics, comorbidities and disease severity was assessed through Mann Whitney U test or Spearman correlation test. Cox regression analysis and Kaplan Meier survival curves were performed to investigate the impact of the CgA and VS-I levels on in-hospital mortality.
Results
Median CgA and VS-I levels were higher in patients than in healthy controls (CgA: 0.558 nM [interquartile range, IQR 0.358–1.046] vs 0.368 nM [IQR 0.288–0.490] respectively, p = 0.0017; VS-I: 0.357 nM [IQR 0.196–0.465] vs 0.144 nM [0.144–0.156] respectively, p<0.0001). Concentration of CgA, but not of VS-I, significantly increased in patients who died (n = 47) than in survivors (n = 143) (median 0.948 nM [IQR 0.514–1.754] vs 0.507 nM [IQR 0.343–0.785], p = 0.00026). Levels of CgA were independent predictors of in-hospital mortality (hazard ratio 1.28 [95% confidence interval 1.077–1.522], p = 0.005) when adjusted for age, number of comorbidities, respiratory insufficiency degree, C-reactive protein levels and time from symptom onset to sampling. Kaplan Meier curves revealed a significantly increased mortality rate in patients with CgA levels above 0.558 nM (median value, log rank test, p = 0.001).
Conclusion
Plasma CgA levels increase in COVID-19 patients and represent an early independent predictor of mortality.
Introduction
Chromogranin A (CgA) is a 439-residue-long protein member of the secretogranin family [1]. It is expressed and secreted by various normal and neoplastic endocrine/neuroendocrine cells and expressed by myocardial cells and immune cells [2–4]. Within the cells, CgA has a role in the regulation of calcium homeostasis and granule biogenesis [5]. Intracellular and secreted CgA is cleaved by proteases, including prohormone convertases, furin, cathepsin, plasmin and thrombin, to generate biologically active fragments [6] such as Vasostatin I (VS-I) [7], a 76-residue long polypeptide that regulates vascular homeostasis and heart function [8].
High plasma levels of CgA have been first described in patients with neuroendocrine tumors [9], but have been also found in patients affected by heart failure, arterial hypertension (HTN), renal failure, rheumatoid arthritis, giant cell arteritis, diabetes mellitus (DM), inflammatory bowel diseases and sepsis [10, 11]. Elevated CgA plasma levels are associated with mortality risk in patients with myocardial infarction, acute coronary syndrome and heart failure [12]. High levels of CgA and VS-1 have been also described in fatal cases of systemic inflammatory response syndrome [13, 14]. The secreted CgA fragments contributes to the host defense and are part of the acute phase response [15], yielding upon proteolytic processing moieties that have anti-microbial proprieties [16], activate neutrophils [17] regulate macrophage polarization [18] and monocyte chemotaxis [19].
Coronavirus disease 2019 (COVID-19) presents with various degrees of severity. Most patients are asymptomatic or experience symptoms that reflect upper or lower airway involvement. In other patients, a less effective innate immune response fails to limit viral replication, eventually resulting in acute respiratory distress syndrome, metabolic derangements and multiorgan failure [20–22]. Age and pre-existing comorbidities including cardiovascular and respiratory diseases, diabetes mellitus (DM) and hypertension (HTN) are risk factors for severe COVID-19 [23]. In turn, COVID-19 cardiovascular, neurological, renal, and vascular complications are associated with mortality [24, 25].
The aims of this study were to assess whether the release of CgA and VS-I in circulation is part of the early host response in COVID-19 and whether these molecules, measured at disease onset, might predict adverse outcomes.
Methods
Patients and study design
This retrospective and prospective study included one hundred ninety patients. The inclusion criteria were: age ≥18 years, confirmed SARS-CoV-2 infection, clinical and/or radiological signs of COVID-19 pneumonia, admission to the Emergency Department of San Raffaele University Hospital, Milan, from March 18 to May 5, 2020, blood sampling at hospital admission. No exclusion criteria were applied [26]. COVID-19 was diagnosed based on a positive real-time reverse-transcriptase polymerase chain reaction (RT-PCR) from a nasopharyngeal swab in the presence of clinical and/or radiologic findings of COVID-19 pneumonia. Blood samples were collected at hospital admission and stored in a dedicated institutional biobank [27]. Detailed demographic, laboratory and clinical data from all patients were recorded in a specific electronic case record form. Patients were prospectively followed until hospital discharge or death. All patients signed an informed consent. The study is compliant with the declaration of Helsinki, was approved by the Hospital Ethics Committee (protocol no. 34/int/2020) and registered on ClinicalTrials.gov (NCT04318366). Forty age- and sex-matched volunteers served as healthy controls (HC).
CgA and VS-I measurement
Plasma-EDTA samples were obtained from venous blood by double centrifugation, according with the Institutional Biobank procedures [27]. The samples were then immediately transferred at –80 °C and 24–72 hours later stored in liquid nitrogen until usage. Plasma were transferred to research laboratory the day of the analysis, thawed and inactivated using tri-(n-butyl) phosphate and Triton X-100 (Sigma) (0.3% and 1% respectively) for 2 hours [28, 29]. CgA and VS-I were measured by enzyme-linked immunosorbent assay as previously described [30]. Samples were diluted 1:10 for CgA assay and or 1:5 for VS-I assay.
Variables and outcome
The following variables were included: age, sex, selected pre-existing comorbidities (HTN, coronary artery disease [CAD], DM, chronic obstructive pulmonary disease [COPD], chronic kidney disease [CKD], active neoplasia), clinical and laboratory data at hospital admission (the ratio of arterial oxygen partial pressure [PaO2] in mmHg to fractional inspired oxygen [FiO2] expressed as a fraction [PaO2/FiO2], neutrophil to lymphocyte ratio [NLR], concentration of C-reactive protein [CRP] and of lactate dehydrogenase [LDH]). Time from symptom onset to blood sampling, rate of hospitalization, length of stay, therapy administered during hospital stay, transfer to the intensive care unit (ICU) and death were recorded. In-hospital mortality was used as primary outcome.
Statistical analysis
Absolute counts (percentage) and median (interquartile range [IQR]) were used to express categorical and continuous variables, respectively. The only exception was the number of comorbidities that was expressed as mean (standard deviation). Differences in categorical and continuous variables between groups were assessed using Chi-squared or Fisher test, as appropriate and Mann-Whitney U test, respectively. Spearman’s correlation test was used to investigate the relationships between continuous variables. Multivariable Cox regression analysis was performed to investigate the impact of CgA on the primary outcome when adjusting for confounders. Variables that showed substantial redundancy with other variables (i.e. CRP vs. NLR) were excluded from the multivariable regression analysis to prevent model overfitting. Similarly, chronic proton pump inhibitor (PPI) therapy, which is associated with high levels of CgA in plasma [31], was not included in the multivariable model. Statistical analyses were performed using R statistical package (version 4.0.0. R Foundation for Statistical Computing, Vienna, Austria), with a two-sided significance level set at p <0.05.
Results
Patient characteristics
Demographic, clinical and laboratory characteristics of the patients (n = 190) are summarized in Table 1. Blood samples were obtained at hospital admission (median [IQR] time from admission to blood draw was 1 [0–1] days). 185 patients (97.3%) had not received any COVID-19-related treatment prior to sample collection. 32 patients (17%) were on chronic PPI therapy. Most patients were males (64%) and median (IQR) age was 61.5 (49.9–72.1) years. More than half of patients had pre-existing comorbidities (52%), the most frequent being HTN (41%). 152 patients (80%) were hospitalized for a median (IQR) time of 15 (9–29) days. 41 (21%) patients were transferred to the intensive care unit (ICU) and 47 (25%) died. Survival time in patients who died was 12 (5–21) days. As expected, patients who died were older and with a higher burden of comorbidities. In addition, patients with a fatal outcome had lower levels of PaO2/FiO2 and higher levels of CRP, NLR and LDH at hospital admission (all p<0.001, Table 1). Steroids and LMWH were, as expected, administered more frequently during hospital stay in non-survivors due to a more severe disease burden (both p<0.05).
Table 1. General and disease characteristics of COVID-19 patients.
| Overall (n = 190) | Dead (n = 47) | Alive (n = 143) | P value | |
|---|---|---|---|---|
| Age (years) | 61.5 (49.9–72.1) | 72.6 (62.6–79.6) | 57.7 (48.5–67.2) | <0.0001 |
| Male sex | 122 (64) | 30 (63) | 92 (64) | >0.99 |
| Comorbidities | ||||
| ≥1 comorbidity | 98 (52) | 35 (74) | 63 (44) | 0.00056 |
| Number of comorbidities | 0.9 (0.008) | 1.5 (0.18) | 0.7 (0.082) | <0.0001 |
| HTN | 78 (41) | 27 (57) | 51 (36) | 0.014 |
| COPD | 10 (5) | 6 (13) | 4 (3) | 0.023 |
| CAD | 22 (11) | 12 (25) | 10 (7) | 0.0015 |
| DM | 39 (20) | 15 (32) | 24 (17) | 0.043 |
| CKD | 17 (9) | 9 (19) | 8 (6) | 0.011 |
| Active neoplasia | 6 (3) | 3 (6) | 3 (2) | 0.32 |
| Time from symptom onset to sampling (days) | 8 (4–11) | 5 (2–8) | 8 (5–11) | <0.0001 |
| At hospital admission | ||||
| PaO2/FiO2 | 278.5 (190.5–334.6) | 159.1 (79.2–266.6) | 304.5 (238.1–348.0) | <0.0001 |
| NLR | 5.3 (3.5–8.5) | 9.9 (5.4–13.5) | 4.8 (3.2–7.2) | <0.0001 |
| CRP (mg/dL) | 78.8 (30.3–153.3) | 151.1 (79.4–213.6) | 68.7 (20.4–125.6) | <0.0001 |
| LDH (U/L) | 383 (275–493.5) | 440 (365–627) | 354 (271.5–466.5) | 0.00040 |
| Hospitalization | 152 (80) | 46 (98) | 106 (74) | <0.0001 |
| Length of stay (days)† | 12 (4–22) | 12 (3–24) | 12 (3–24) | 0.44 |
| ICU transfer | 41 (22) | 22 (47) | 19 (13) | <0.0001 |
| Death | 47 (25) | 47 (100) | 0 | - |
| Therapy during hospitalization | ||||
| Steroids | 43 (23) | 22 (47) | 21 (15) | <0.0001 |
| LMWH | 86 (45) | 25 (53) | 61 (43) | <0.020 |
Categorical variables were expressed as count (percentage), while continuous variables as median (interquartile range), with the exception of the number of comorbidities which was expressed as mean (standard deviation).
† Calculated as the time from hospital admission to death or discharge.
Abbreviations: PaO2/FiO2, ratio of arterial oxygen partial pressure to fractional inspired oxygen; NLR, neutrophil to lymphocyte ratio; ICU, intensive care unit, LMWH, low molecular weight heparin
CgA and VS-I plasma levels in COVID-19
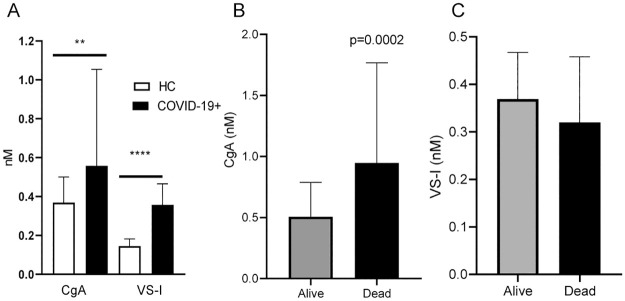
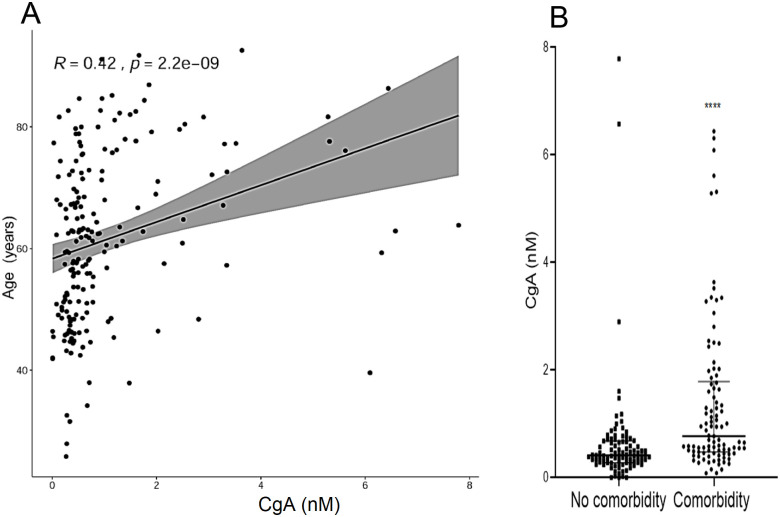
Plasma levels of CgA were significantly higher in COVID-19 patients compared with HC (0.558 nM [0.358–1.046] vs 0.368 nM [0.288–0.490] respectively, p = 0.0017, Fig 1, panel A). Similarly, VS-I plasma levels were more elevated in patients than in HC (0.357 [0.196–0.465] nM vs 0.144 [0.144–0.156] nM respectively, p<0.0001, Fig 1, panel A). The CgA and V76 plasma levels were similar in male and female patients (0.5190 [0.351–0.926] nM and 0.6705 [0.36975–1.439] nM for CgA and 0.3505 [0.185–0.45125] nM and 0.3660 [0.2505–0.492] nM for V76). CgA levels at admission correlated with age (R 0.42, p<0.0001, Fig 2, panel A) and were higher in patients with at least a single pre-existing comorbidity compared with non-comorbid patients (0.778 [0.483–1.761] nM vs 0.419 [0.281–0.675] nM respectively, p<0.0001, Fig 2, panel B). CgA or VS-I plasma levels did not differ between females and males (not shown). CgA correlated with the degree of hypoxia, as quantified by PaO2/FiO2 (R -0.20, p = 0.0057) and with CRP (R 0.30, p<0.0001), NLR (R 0.21. p = 0.0062) and LDH levels (R 0.17, p = 0.017). No significant correlation was observed between VS-I concentration and age, comorbidities, CRP or LDH levels.
Fig 1.
Panel A: CgA and VS-I plasma levels in age- and sex-matched healthy controls (HC) and COVID-19 patients at hospital admission. Panel B: CgA plasma levels in COVID-19 patients with favorable outcome (Alive) or who died (Dead). Panel C: VS-I in Alive or Dead patients. ** p<0.001.
Fig 2.
Panel A: Correlation of CgA plasma levels with age. Panel B: CgA plasma levels in patients with or without comorbidities. *** <0.0001.
CgA and VS-I levels in survivors and non-survivors
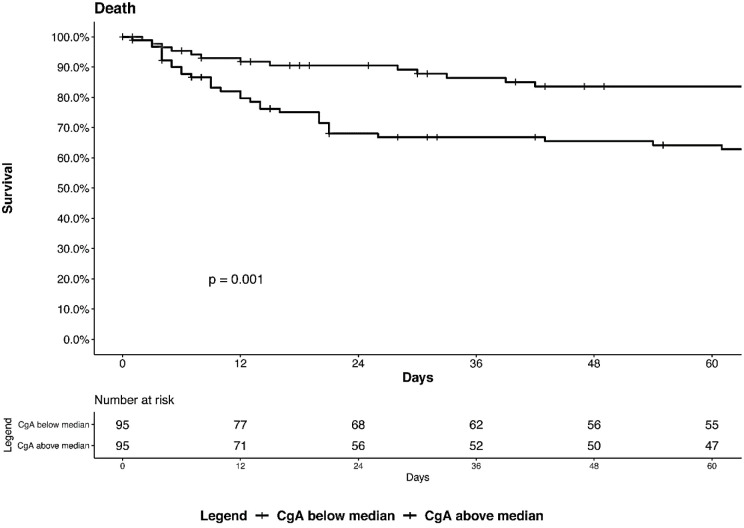
Patients who died had higher plasma levels of CgA at admission than COVID-19 survivors (0.948 [0.514–1.754] nM vs 0.507 [0.343–0.785] nM respectively, p = 0.00026) (Fig 1, panel B). In contrast, VS-I plasma levels were similar in survivors and patients who died (p>0.05, Fig 1, panel B). At multivariable cox regression analysis, CgA plasma levels at admission were independent predictors of in-hospital mortality when adjusting for age, number of comorbidities, degree of respiratory dysfunction (PaO2/FiO2), systemic inflammation as reflected by CRP levels at admission and time from symptom onset to sampling (Table 2). Kaplan Meier survival analysis confirmed that patients with levels of CgA above the median value (0.557 nM) at admission had a higher risk of mortality (log rank test, p = 0.001, Fig 3).
Table 2. Multivariable Cox regression analysis predicting death.
| HR | 95% CI | P value | |
|---|---|---|---|
| CgA (nM) | 1.23 | 1.02–1.47 | 0.025 |
| Age (years) | 1.02 | 0.99–1.05 | 0.20 |
| Number of comorbidities | 1.34 | 1.01–1.76 | 0.039 |
| PaO2/FiO2 | 0.99 | 0.99–0.99 | 0.018 |
| CRP (mg/dL) | 1.00 | 0.99–1.01 | 0.19 |
| Time from symptom onset to sampling (days) | 0.88 | 0.81–0.95 | 0.002 |
Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval; CgA, chromogranin A; PaO2/FiO2, ratio of arterial oxygen partial pressure to fractional inspired oxygen; CRP, C-reactive protein
Fig 3. Kaplan-Meier survival curves in patients with CgA levels below (low) or above (high) the median value of 0.558 nM.
Log rank test, p = 0.001.
Discussion
To our knowledge, this is the first report on CgA and VS-1 levels in COVID-19 and the association of this event with clinical outcomes, as evidenced by in-hospital mortality. The host response to SARS-CoV-2 in patients is substantial, with inflammation, vascular activation and coagulopathy playing major roles in the outcome. Neuroendocrine activation is less studied. The observation that CgA accumulates in patients with COVID-19 and that this occurs preferentially in those that eventually die offers a tool to disentangle the immune/neuroendocrine connection in the host response to SARS-CoV-2.
Age and comorbidities are important risk factors for adverse outcome in COVID-19 [26, 32]. We found a correlation between plasma CgA levels and age, and observed that the concentration of CgA was higher in comorbid patients. Nevertheless, CgA was a significant predictor of mortality independently of age and comorbidities. These results are consistent with the association between CgA levels and outcomes in patients with systemic inflammatory response syndrome [13]. Plasma levels of VS-I are increased in critically ill patients [14]. In contrast VS-I plasma levels in COVID-19 patients, although higher than in HC, were similar in survivors and non-survivors. However, CgA and VS-I exert different physiopathological roles, especially in the context of the cardiovascular and immune systems [33]. VS-1 is a product of CgA N-terminal proteolytic processing. Several enzymes are involved, depending on the site of action and on the pathophysiological conditions [5, 6]. We found that in COVID-19 patients the extent of CgA release into the blood predicts clinical outcome. In contrast, the proteolytic processing of CgA yielding VS-I did not change in patients with severe outcome, suggesting that the molecular machinery involved in the generation of VS-I is similarly regulated in COVID-19 patients regardless of disease progression.
CgA levels correlated with hypoxia and with systemic inflammation. Increased values of CgA have been previously described in inflammatory and autoimmune disorders. CgA in turn controls the response to cytokines of the endothelium [34]. CgA influences the vascular remodeling in stress conditions, directly and through the generation of bioactive fragments [35, 36]. In particular, full length CgA induces in endothelial cells the protease nexin-1, an antiangiogenic protein [37, 38]. Nexin-1 is also an inhibitor of plasmin and thrombin [39]. This inhibition might prevent the cleavage of CgA by these enzymes, thereby preserving its activity. SARS-CoV-2 infection and consequent inflammation induce endothelial damage, platelet activation, thrombosis, microangiopathy and neo-angiogenesis in response to tissue injury [40, 41]. Platelet derived microparticles [42] and high levels of angiopoietin-2, follistatin and PAI-1, markers of endothelial injury, increase the risk of mortality [43], and signs of intussusceptive angiogenesis, a proposed mechanism for vessel generation in late stages of chronic lung injury, have been found in lung biopsies of COVID-19 patients [44]. Thus, CgA accumulation in patients with severe COVID-19 might affect the microvascular response to the SARS-CoV-2 infection, possibly influencing the clinical outcome.
We did not identify the origin and mechanisms of CgA production in COVID-19 patients. Potential mechanisms include an enhanced stress-induced sympathetic tone and neuroendocrine activation, leading to enhanced secretion of CgA. Several other mechanisms might contribute to the size of the circulating pool of CgA, including reduced clearance of the molecule itself, viral-induced changes in neurosecretory cells activity and injury, and, at least for those patients under PPI treatment, drug-induced production of CgA by enterochromaffin-like cells or damage of secretory cells [45].
We included 40 age- and sex-matched healthy controls. The relatively small size of samples from healthy controls, which were collected prior to the pandemic outbreak, was forced by the rapid spread of SARS-CoV-2 infection and the systematic vaccination against SARS-CoV-2, events that may represent potential bias for data interpretation.
CgA plasma levels are not gender-dependent [46], but daily fluctuations in CgA levels may occur [47]. Circulating CgA levels may be also altered by treatment with PPI [31, 48]. In our cohort, a minority of patients were on chronic PPI therapy at the time of blood withdrawal. Whether the observed increase in CgA levels in non-survivors is at least in part related to PPI therapy remains uncertain. However, the finding of higher levels of CgA also in patients not receiving PPI compared with HC and the relatively low prevalence of PPI therapy in the cohort imply that additional mechanisms other than PPI therapy are responsible for CgA overexpression. Starting from 2020, several signals have been identified as players in the natural history of the disease. Accordingly, potential biomarkers and predictors of clinical outcome have been proposed (e.g. see [49–54]) with several characteristics of blood cells, inflammatory signals and pathways, biomarkers of innate and acquired immunity, altered cell metabolism and coagulation providing valuable information for dissecting patients heterogeneity [49]. Our results demonstrate for the first time the involvement of CgA, a prototype member of the granin glycoprotein family produced and released by a wide range of different cells throughout the body in the response to SARS-CoV-2 infection. Further studies are needed to identify the role played by CgA and the possibility that the signal can be used for early stratification of patients based on the risk of adverse outcome.
Conclusions
This study provides the first evidence of an elevation of CgA and its fragment VS-I in COVID-19 patients suggesting a neuroendocrine activation in these patients. CgA levels (but not VS-I) predicts the risk of death independently of other risk factors for adverse outcome. CgA plasma level at hospital admission could therefore represent a tool for the early identification of patients at increased risk of unfavorable disease evolution and therefore needing a more intense management.
Acknowledgments
The authors wish to thank the Institutional Biobank whose support made it possible to carry out this study in a very difficult situation for the Institution and for the country. Authors also thank the Bio Angels for COVID-BioB Study Group composed of Luigi De Filippo (lead author for this group, defilippo.luigi@hsr.it), Nicola Farina, Marco Battista, Domenico Grosso, Francesca Gorgoni, Carlo Di Biase, Alessio Grazioli Moretti, Lucio Granata, Filippo Bonaldi, Giulia Bettinelli, Elena Delmastro, Damiano Salvato, Chiara Maggioni, Giulia Magni, Monica Avino, Paolo Betti, Romina Bucci, Iulia Dumoa, Simona Bossolasco and Federica Morselli (All the group members are affiliate to Vita-Salute San Raffaele University, Milan, Italy).
Abbreviations
- 95% CI
95% confidence interval
- CAD
coronary artery disease
- CgA
chromogranin A
- COVID-19
Coronavirus disease 2019
- CRP
C-reactive protein
- DM
diabetes mellitus
- FiO2
fractional inspired oxygen
- HTN
arterial hypertension
- ICU
intensive care unit
- IQR
interquartile range
- LDH
lactate dehydrogenase
- NLR
neutrophil to lymphocyte ratio
- PaO2
arterial oxygen partial pressure
- PPI
proton-pump inhibitors
- VS-I
vasostatin I
Data Availability
All relevant data are within the paper.
Funding Statement
The study was supported by the Italian Ministero della Salute (COVID-2020-12371617) and by COVID-19 donations. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Blaschko H. Comline R. S. Schneider F. H. Silver M. Smith A. D.: Secretion of a chromaffin granule protein, chromogranin, from the adrenal gland after splanchnic stimulation. Nature 1967, 215(5096):58–59. doi: 10.1038/215058a0 [DOI] [PubMed] [Google Scholar]
- 2.Tasiemski A. Hammad H., Vandenbulcke F. Breton C. Bilfinger T., Pestel J. et al. Presence of chromogranin-derived antimicrobial peptides in plasma during coronary artery bypass surgery and evidence of an immune origin of these peptides. Blood 2002, 100(2):553–559. doi: 10.1182/blood.v100.2.553 [DOI] [PubMed] [Google Scholar]
- 3.Taupenot L., Harper KL, O’Connor DT: The chromogranin-secretogranin family. N Engl J Med 2003, 348(12):1134–1149. doi: 10.1056/NEJMra021405 [DOI] [PubMed] [Google Scholar]
- 4.Pieroni M. Corti A. Tota B. Curnis F. Angelone T. Colombo B., et al. Myocardial production of chromogranin A in human heart: a new regulatory peptide of cardiac function. Eur Heart J 2007, 28(9):1117–1127. doi: 10.1093/eurheartj/ehm022 [DOI] [PubMed] [Google Scholar]
- 5.D’Amico M. A. Ghinassi B. Izzicupo P., Manzoli L. Di Baldassarre A. et al. Biological function and clinical relevance of chromogranin A and derived peptides. Endocr Connect 2014, 3(2):R45–R54. doi: 10.1530/EC-14-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corti A, Marcucci F, Bachetti T. Circulating chromogranin A and its fragments as diagnostic and prognostic disease markers. Pflugers Arch 2018, 470(1):199–210. doi: 10.1007/s00424-017-2030-y [DOI] [PubMed] [Google Scholar]
- 7.Aardal S, Helle KB. The vasoinhibitory activity of bovine chromogranin A fragment (vasostatin) and its independence of extracellular calcium in isolated segments of human blood vessels. Regul Pept 1992, 41(1):9–18. doi: 10.1016/0167-0115(92)90509-s [DOI] [PubMed] [Google Scholar]
- 8.Helle KB: The chromogranin A-derived peptides vasostatin-I and catestatin as regulatory peptides for cardiovascular functions. Cardiovasc Res 2010, 85(1):9–16. doi: 10.1093/cvr/cvp266 [DOI] [PubMed] [Google Scholar]
- 9.O’Connor DT, Bernstein KN. Radioimmunoassay of chromogranin A in plasma as a measure of exocytotic sympathoadrenal activity in normal subjects and patients with pheochromocytoma. N Engl J Med 1984, 311(12):764–770. doi: 10.1056/NEJM198409203111204 [DOI] [PubMed] [Google Scholar]
- 10.Helle KB, Corti A, Metz-Boutigue MH, Tota B. The endocrine role for chromogranin A: a prohormone for peptides with regulatory properties. Cell Mol Life Sci 2007, 64(22):2863–2886. doi: 10.1007/s00018-007-7254-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tombetti E. Colombo B. Di Chio M. C. Sartorelli S. Papa M. Salerno A. et al. Chromogranin-A production and fragmentation in patients with Takayasu arteritis. Arthritis Res Ther 2016, 18:187. doi: 10.1186/s13075-016-1082-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahata SK, Corti A. Chromogranin A and its fragments in cardiovascular, immunometabolic, and cancer regulation. Ann N Y Acad Sci 2019, 1455(1):34–58. doi: 10.1111/nyas.14249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang D., Lavaux T., Sapin R., Lavigne T. Castelain V. Aunis D. et al. Serum concentration of chromogranin A at admission: an early biomarker of severity in critically ill patients. Ann Med 2009, 41(1):38–44. doi: 10.1080/07853890802199791 [DOI] [PubMed] [Google Scholar]
- 14.Schneider F, Bach C, Chung H, Crippa L, Lavaux T, Bollaert PE, et al. Vasostatin-I, a chromogranin A-derived peptide, in non-selected critically ill patients: distribution, kinetics, and prognostic significance. Intensive Care Med 2012, 38(9):1514–1522. Epub 02012 Jun 00116. doi: 10.1007/s00134-012-2611-3 [DOI] [PubMed] [Google Scholar]
- 15.Helle K. B. Metz-Boutigue M. H. Cerra M. C. Angelone T. et al. Chromogranins: from discovery to current times. Pflugers Arch 2018, 470(1):143–154. doi: 10.1007/s00424-017-2027-6 [DOI] [PubMed] [Google Scholar]
- 16.Lugardon K. Raffner R. Goumon Y. Corti A. Delmas A., Bulet P. et al. Antibacterial and antifungal activities of vasostatin-1, the N-terminal fragment of chromogranin A. J Biol Chem 2000, 275(15):10745–10753. doi: 10.1074/jbc.275.15.10745 [DOI] [PubMed] [Google Scholar]
- 17.Zhang D., Shooshtarizadeh P. Laventie B. J. Colin D. A. Chich J. F. Vidic J. et al. Two chromogranin a-derived peptides induce calcium entry in human neutrophils by calmodulin-regulated calcium independent phospholipase A2. PLoS One 2009, 4(2):e4501. doi: 10.1371/journal.pone.0004501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eissa N. Hussein H. Tshikudi D. M. Hendy G. N. Bernstein C. N. Ghia J. E Interdependence between Chromogranin-A, Alternatively Activated Macrophages, Tight Junction Proteins and the Epithelial Functions. A Human and In-Vivo/In-Vitro Descriptive Study. Int J Mol Sci 2020, 21(21). doi: 10.3390/ijms21217976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egger M. Beer A. G., Theurl M. Schgoer W., Hotter B. Tatarczyk T. et al. Monocyte migration: a novel effect and signaling pathways of catestatin. Eur J Pharmacol 2008, 598(1–3):104–111. doi: 10.1016/j.ejphar.2008.09.016 [DOI] [PubMed] [Google Scholar]
- 20.Bulow Anderberg S. Luther T. Berglund M. Larsson R. Rubertsson S. Lipcsey M., et al. Increased levels of plasma cytokines and correlations to organ failure and 30-day mortality in critically ill Covid-19 patients. Cytokine 2021, 138:155389. doi: 10.1016/j.cyto.2020.155389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian W. Jiang W. Yao J. Nicholson C. J. Li R. H. Sigurslid H. H. et al. Predictors of mortality in hospitalized COVID-19 patients: A systematic review and meta-analysis. J Med Virol 2020, 92(10):1875–1883. doi: 10.1002/jmv.26050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farina N. Ramirez G. A. De Lorenzo R. Di Filippo L. Conte C. Ciceri F., et al. COVID-19: Pharmacology and kinetics of viral clearance. Pharmacol Res 2020, 161:105114. doi: 10.1016/j.phrs.2020.105114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venturini S, et al. Classification and analysis of outcome predictors in non-critically ill COVID-19 patients. Intern Med J 2021, 51(4):506–514. doi: 10.1111/imj.15140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maguire D., Richards C. Woods M. Dolan R. Wilson Veitch J. Sim W. M. J. et al. The systemic inflammatory response and clinicopathological characteristics in patients admitted to hospital with COVID-19 infection: Comparison of 2 consecutive cohorts. PLoS One 2021, 16(5):e0251924. doi: 10.1371/journal.pone.0251924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polidoro R. B. Hagan R. S. de Santis Santiago R. Schmidt N. W. Overview: Systemic Inflammatory Response Derived From Lung Injury Caused by SARS-CoV-2 Infection Explains Severe Outcomes in COVID-19. Front Immunol 2020, 11:1626. doi: 10.3389/fimmu.2020.01626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciceri F. Castagna A. Rovere-Querini P. De Cobelli F. Ruggeri A. Galli L., et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol 2020, 217:108509. doi: 10.1016/j.clim.2020.108509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rovere-Querini P. Tresoldi C. Conte C. Ruggeri A. Ghezzi S. De Lorenzo R., et al. Biobanking for COVID-19 research. Panminerva Med 2020. doi: 10.23736/S0031-0808.20.04168-3 [DOI] [PubMed] [Google Scholar]
- 28.De Lorenzo R. Lore N. I. Finardi A. Mandelli A. Cirillo D. M. Tresoldi C., et al. Blood neurofilament light chain and total tau levels at admission predict death in COVID-19 patients. J Neurol 2021. doi: 10.1007/s00415-021-10595-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darnell ME, Taylor DR. Evaluation of inactivation methods for severe acute respiratory syndrome coronavirus in noncellular blood products. Transfusion 2006, 46(10):1770–1777. doi: 10.1111/j.1537-2995.2006.00976.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corsello A, Di Filippo L, Massironi S, Sileo F, Dolcetta Capuzzo A, Gemma M, et al. Vasostatin-1: A novel circulating biomarker for ileal and pancreatic neuroendocrine neoplasms. PLoS One 2018, 13(5):e0196858. doi: 10.1371/journal.pone.0196858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giusti M, Sidoti M, Augeri C., Rabitti C, Minuto F. Effect of short-term treatment with low dosages of the proton-pump inhibitor omeprazole on serum chromogranin A levels in man. Eur J Endocrinol 2004, 150(3):299–303. doi: 10.1530/eje.0.1500299 [DOI] [PubMed] [Google Scholar]
- 32.Mokhtari T. Hassani F. Ghaffari N. Ebrahimi B. Yarahmadi A. Hassanzadeh G. COVID-19 and multiorgan failure: A narrative review on potential mechanisms. J Mol Histol 2020, 51(6):613–628. doi: 10.1007/s10735-020-09915-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loh Y. P. Cheng Y. Mahata S. K. Corti A. Tota B. Chromogranin A and derived peptides in health and disease. J Mol Neurosci 2012, 48(2):347–356. doi: 10.1007/s12031-012-9728-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Comite G. Rossi C. M. Marinosci A. Lolmede K. Baldissera E. Aiello P., et al. Circulating chromogranin A reveals extra-articular involvement in patients with rheumatoid arthritis and curbs TNF-alpha-elicited endothelial activation. J Leukoc Biol 2009, 85(1):81–87. doi: 10.1189/jlb.0608358 [DOI] [PubMed] [Google Scholar]
- 35.Crippa L. Bianco M. Colombo B. Gasparri A. M. Ferrero E. Loh Y. P., et al. A new chromogranin A-dependent angiogenic switch activated by thrombin. Blood 2013, 121(2):392–402. doi: 10.1182/blood-2012-05-430314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Theurl M. Schgoer W. Albrecht K. Jeschke J. Egger M. Beer A. G., et al. The neuropeptide catestatin acts as a novel angiogenic cytokine via a basic fibroblast growth factor-dependent mechanism. Circ Res 2010, 107(11):1326–1335. doi: 10.1161/CIRCRESAHA.110.219493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selbonne S. Azibani F. Iatmanen S. Boulaftali Y. Richard B. Jandrot-Perrus M, et al. In vitro and in vivo antiangiogenic properties of the serpin protease nexin-1. Mol Cell Biol 2012, 32(8):1496–1505. doi: 10.1128/MCB.06554-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curnis F. Dallatomasina A. Bianco M. Gasparri A. Sacchi A. Colombo B., et al. Regulation of tumor growth by circulating full-length chromogranin A. Oncotarget 2016, 7(45):72716–72732. doi: 10.18632/oncotarget.12237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bouton M. C. Boulaftali Y. Richard B. Arocas V. Michel J. B. Jandrot-Perrus M., et al. Emerging role of serpinE2/protease nexin-1 in hemostasis and vascular biology. Blood 2012, 119(11):2452–2457. doi: 10.1182/blood-2011-10-387464 [DOI] [PubMed] [Google Scholar]
- 40.Castro P. Palomo M. Moreno-Castano A. B. Fernandez S. Torramade-Moix S. Pascual G., et al. Is the Endothelium the Missing Link in the Pathophysiology and Treatment of COVID-19 Complications? Cardiovasc Drugs Ther 2021. doi: 10.1007/s10557-021-07207-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramirez G. A. Calvisi S. L. De Lorenzo R. Da Prat V, Borio G. Gallina G., et al. A novel evidence-based algorithm to predict thromboembolism in patients with COVID-19: preliminary data from a single-centre cohort. Minerva Med 2021. doi: 10.23736/S0026-4806.21.07331-6 [DOI] [PubMed] [Google Scholar]
- 42.Maugeri N. De Lorenzo R. Clementi N. Diotti R. A. Criscuolo E. Godino C., et al. Unconventional CD147-dependent platelet activation elicited by SARS-CoV-2 in COVID-19. J Thromb Haemost 2021. doi: 10.1111/jth.15575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pine A. B. Meizlish M. L. Goshua G. Chang C. H. Zhang H. Bishai J, et al. Circulating markers of angiogenesis and endotheliopathy in COVID-19. Pulm Circ 2020, 10(4):2045894020966547. doi: 10.1177/2045894020966547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ackermann M. Verleden S. E. Kuehnel M. Haverich A. Welte T. Laenger F et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med 2020, 383(2):120–128. doi: 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muller J. A. Gross R. Conzelmann C. Kruger J. Merle U. Steinhart J., et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab 2021, 3(2):149–165. doi: 10.1038/s42255-021-00347-1 [DOI] [PubMed] [Google Scholar]
- 46.Dittadi R, Meo S, Gion M. Biological variation of plasma chromogranin A. Clin Chem Lab Med 2004, 42(1):109–110. doi: 10.1515/CCLM.2004.022 [DOI] [PubMed] [Google Scholar]
- 47.Takiyyuddin M. A. Neumann H. P. Cervenka J. H. Kennedy B. Dinh T. Q. Ziegler M. G., et al. Ultradian variations of chromogranin A in humans. Am J Physiol 1991, 261(4 Pt 2):R939–944. doi: 10.1152/ajpregu.1991.261.4.R939 [DOI] [PubMed] [Google Scholar]
- 48.Waldum H. L. Arnestad J. S. Brenna E. Eide I. Syversen U. Sandvik A. K, Marked increase in gastric acid secretory capacity after omeprazole treatment. Gut 1996, 39(5):649–65 doi: 10.1136/gut.39.5.649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.COvid-19 Multi-omics Blood ATlas (COMBAT) Consortium, A blood atlas of COVID-19 defines hallmarks of disease severity and specificity. Cell, 2022. 185(5): 916–938 e58. doi: 10.1016/j.cell.2022.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gorog D. A. Storey R. F. Gurbel P. A. Tantry U. S. Berger J. S. Chan M. Y., et al., Current and novel biomarkers of thrombotic risk in COVID-19: a Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat Rev Cardiol. 2022. Jan 13:1–21. doi: 10.1038/s41569-021-00665-7 Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Musa A. LaBere B. Habiballah S. Nguyen A. A. Chou J., Advances in clinical outcomes: What we have learned during the COVID-19 pandemic. The Journal of allergy and clinical immunology, 2022. 149(2): 569–578. doi: 10.1016/j.jaci.2021.12.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wanhella K.J. and Fernandez-Patron C., Biomarkers of ageing and frailty may predict COVID-19 severity. Ageing research reviews, 2022. 73: 101513. doi: 10.1016/j.arr.2021.101513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lore N. I. De Lorenzo R. Rancoita P. M. V. Cugnata F. Agresti A. Benedetti F., et al. CXCL10 levels at hospital admission predict COVID-19 outcome: hierarchical assessment of 53 putative inflammatory biomarkers in an observational study. Molecular medicine, 2021. 27(1): 129. doi: 10.1186/s10020-021-00390-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ackermann M., Anders H.J. Bilyy Rostyslav, B Gary L. De Lorenzo R. et al., Patients with COVID-19: in the dark-NETs of neutrophils. Cell Death & Differentiation, 2021. 28(11): 3125–3139. doi: 10.1038/s41418-021-00805-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.