Abstract
Occupational exposures to flame retardants (FRs), a class of suspected endocrine-disrupting compounds, are of health concern for firefighters. We sought to characterize exposure to FR compounds and evaluate their association with thyroid hormone levels, a biomarker of early effect, in female firefighters and office workers in San Francisco. In a cross-sectional study, we measured replacement organophosphate and organohalogen FRs in spot urine samples from firefighters (N = 86) and office workers (N = 84), as well as total thyroxine (T4) and thyroid-stimulating hormone in plasma for 84 firefighters and 81 office workers. Median bis(1,3-dichloro-2-propyl)phosphate (BDCPP) levels were 5 times higher in firefighters than office workers. Among firefighters, a doubling of BDCPP was associated with a 2.88% decrease (95% confidence interval −5.28, −0.42) in T4. We did not observe significant associations between FRs and T4 among office workers. In the full group, intermediate body mass index and a college education were associated with higher FR levels. The inverse association observed between FRs and T4 coupled with the lack of studies on women workers and evidence of adverse health effects from FR exposure—including endocrine disruption and breast cancer risk—warrant further research on occupational exposures and identification of opportunities for exposure reduction.
Keywords: flame retardants; thyroid hormone; firefighters; endocrine disruption; bis(1,3-dichloro-2-propyl)phosphate
Short abstract
This study measures exposure to flame-retardant chemicals—which are suspected endocrine-disrupting compounds and potential breast carcinogens—in women firefighters and office workers from San Francisco and evaluates the association between flame-retardant exposure and thyroid hormone levels.
Introduction
Occupational exposures in firefighting have been well-documented among male firefighters yet understudied among women. Common exposures include polyaromatic hydrocarbons, formaldehyde, benzene, dioxins, diesel, per- and poly-fluoroalkyl substances (PFASs), and flame retardants (FRs) including organohalogen FRs, such as polybrominated diphenyl ethers (PBDEs), and organophosphate FRs (OPFRs).1−11 While exposure sources among men and women in firefighting are expected to be similar, there may be differences in metabolism or susceptibility for certain chemicals that could alter the health risks of exposure. A recent paper on PFAS exposure among women firefighters of the Women Workers Biomonitoring Collaborative (WWBC) study found that women firefighters had elevated levels of some PFAS chemicals compared to women office workers but lower levels than previous studies on male firefighters.11
FRs are of particular interest for firefighters because they have been found in firefighting gear12 and in fire station dust.9,10 Additionally, firefighter biomonitoring and studies of fire station dust samples have found elevated levels of brominated and organohalogen FRs compared to homes and offices including the organophosphate tris(1,3-dichloro-isopropyl)-phosphate (TDCPP).7,9,10
Exposure to FRs is common in the general population despite the phase out of mandatory FR use in furniture in 2013. California’s furniture flammability standard required furniture filling such as polyurethane foam to withstand an open flame test without igniting, which led to widespread use of FRs in furniture-filling materials sold across the country.13−15 Because FRs are often added to products post-production and are not chemically bound to fabric and foams, they can leach out of materials and contaminate air and dust.13,16 Concerns about toxicity, bioaccumulation, and long half-life of PBDEs led to an increase in the use of replacement FRs in the early 2000s, including OPFRs, in furniture, fabrics, and electronics.17 In addition to polyurethane foam, OPFRs are commonly used in fabric, electronics, and engine lubricants and as plasticizers.17−19 Due to OPFR use in fabrics and the detection of PBDEs in firefighter gear,12 OPFRs are likely used in firefighter clothing and protective gear. The half-lives of OPFRs and tetrabromobisphenol A are not well-known, although they are generally considered to be shorter than those of PBDEs—potentially on the order of hours or days.20,21 While direct strong evidence is still lacking in human studies, exposure to OPFRs has been associated with endocrine disruption relevant to breast cancer22,23 as well as breast tumor development in animal studies and limited human studies.22,24,25
This paper focuses on thyroid hormone disruption as a biomarker of early effect of potential relevance for breast cancer and other adverse health outcomes. Prior studies have found that women firefighters have a higher incidence of and mortality from breast cancer than the general (nonfirefighter) population.26−28 Additionally, thyroid dysfunction is associated with increased cancer risk, including breast cancer, in epidemiology studies.29−31 Methodological challenges from the low numbers of women in the fire service and in most fire departments, as well as the long latency period between exposure and onset of disease, make it difficult to assess cancer risk of firefighting among women. However, understanding women firefighters’ exposure to chemicals linked to cancer and biomarkers of effect, such as thyroid hormone disruption, can inform timely prevention efforts. In addition to concerns about cancer, organophosphate and brominated FRs have also been associated with altered thyroid hormone levels both in vitro and in vivo (Table S1).32−36
Thyroid Hormone Disruption
Circulating thyroid-stimulating hormone (TSH) and thyroid hormone, including thyroxine (T4), may be altered by exposure to environmental chemicals including OPFRs,23,25,34,37 and thyroid hormones may be relevant to diverse downstream adverse health impacts such as cardiovascular disease and cancer.23,38,39 Thyroid hormones have multiple forms and include total T4, free T4, and triiodothyronine (T3) in both the free and bound forms. Total T4 is the sum of bound and free T4 and is important for maintaining homeostasis and for transporting thyroid hormones to target tissues. Alterations to total T4 may also be an indicator of biological perturbations.37
Studies of zebrafish and chicken embryos show that OPFRs can disrupt thyroid hormone homeostasis and decrease thyroid hormone levels.35,40 Human studies suggest that exposure to OPFRs can influence thyroid hormone levels, measured by total and free T4, although research remains limited and the direction of the association is mixed.33,41,42
While biomonitoring studies show that firefighters have higher body burdens of FR chemicals than the general United States population,7,12,43 very little research has investigated the extent and health implications of exposure among women firefighters due to the limited number of women in most fire departments. Assessing thyroid hormone disruption associated with FR exposures enables identification of early biological perturbations of potential relevance to thyroid dysfunction or disease44 and long-term adverse health outcomes such as cancer.39
The San Francisco Fire Department (SFFD) has one of the largest urban forces of women firefighters in the U.S.—approximately 15% of firefighters in the SFFD are women. The WWBC is a community-based participatory research study with women firefighters from the SFFD. Our cross-sectional study sought to characterize exposures to OPFR metabolites and replacement organohalogen FRs (henceforth referred to together as FRs) among women firefighters and office workers, identify predictors of exposure, and assess the association between FR exposures and total T4 and TSH.
Materials and Methods
Study design and participant recruitment protocols have been described elsewhere.4,11 Briefly, participant recruitment, interviews, and sample collections took place between 2014 and 2015. Participants were employees of the City and County of San Francisco or the SFFD. Firefighter collaborators and researchers actively recruited study participants through the Fire Department, as well as firefighter advocacy organizations including the San Francisco Firefighters Cancer Prevention Foundation, United Fire Service Women, and the International Association of Firefighters Union Local 798. Office workers, who were not first responder employees of the City and County of San Francisco, were recruited by listserv emails, tabling at employee events, and presentations by research staff and firefighter collaborators. Potential participants were eligible to enroll if they were 18 years or older and nonsmokers. Firefighters were required to have worked in the SFFD for a minimum of 5 years and be on “active duty” (i.e., currently assigned to a fire station at the time of recruitment). There was no requirement for duration of employment for the office workers. Participants were consented into the study following protocols approved by the Institutional Review Board of the University of California, Berkeley (# 2013-07-5512).
Data Collection
All participants completed an in-person exposure assessment interview. Research staff collected demographic and basic health information including body mass index (BMI) and the use of hormone replacement medications (relevant interview questions can be found in the Supporting Information). We also asked about possible sources of FR exposure including diet (types and quantities of different foods and food packaging and preparation) and occupational activities (time sitting at a computer and office furnishings for office workers, fire suppression activities, firehouse activities, and use of personal protective equipment for firefighters). A subset of participants (N = 66) gave researchers permission to access their departmental firefighting history records, from which we abstracted the number of fires fought in the 7 days and month prior to the sample collection date to investigate if a recent firefighting activity was associated with FR levels. We chose to look at this time frame because the half-lives for OFPRs vary and are not well-described.21
A trained phlebotomist collected blood in ethylenediaminetetraacetic acid-treated lavender top tubes. Urine specimens were collected by participants in 60 mL polypropylene biospecimen cups. Biospecimens were put on ice and transferred to the laboratory at the University of California, San Francisco, where research staff processed samples within 3 h of their collection. Blood collection tubes were spun at 3000 rpm for 10 min and plasma was aliquoted into 1.1 mL cryovial tubes. Urine samples were aliquoted into 3.5 mL cryovial tubes. All samples were stored at −80 °C until analysis.
Laboratory Analysis
FR Analysis
We measured FRs in spot urine samples from study participants. We selected compounds that would likely be detected in urine and are potentially in consumer products such as furniture and electronics17 and possibly in firefighter gear. We measured the following organophosphate metabolites: bis(1,3-dichloro-2-propyl)phosphate (BDCPP), bis(2-chloroethyl)phosphate (BCEP), dibutyl phosphate (DBuP), dibenzyl phosphate, di-p-cresyl phosphate (DpCP), and di-o-cresyl phosphate and 2 brominated compounds: tetrabromobisphenol A (TBBPA) and the metabolite 2,3,4,5-tetrabromobenzoic acid.
Quantification of the eight analytes was performed using liquid-chromatography-tandem mass spectrometry (LC–MS/MS) on an Agilent LC 1260 instrument (Agilent Technologies, Santa Clara, CA)—AB Sciex 5500 system (Sciex, Redwood City, CA). To account for urine dilution, we quantified levels of creatinine in participants’ urine samples (details of the LC–MS/MS method can be found in the Supporting Information).
T4 and TSH Measurement
To assess potential biological perturbations associated with FR exposures, we measured TSH and total T4 in plasma because environmental chemicals have been shown to interact with thyroid hormone transport proteins and facilitate metabolism and excretion of the thyroid hormone from the body.45 TSH and total T4 levels were measured using enzyme-linked immunosorbent assay (antibodies-online, cat. no. ABIN2773773) following the manufacturer’s protocol.46 Room-temperature calibrators, controls, and samples (25 μL for T4 and 50 μL for TSH) were loaded onto streptavidin-coated wells followed by the addition of the biotinylated antibody for T4 or TSH. Standard curves were constructed in duplicate with calibrators supplied in the kit. The reaction was incubated at room temperature for 1 h and then washed three times. Substrate solution was added and stopped after 15 min. Absorbance at 450 nm was read immediately using a microplate reader. The T4 and TSH concentrations of each sample, run in duplicate, were obtained from the standard curve. According to the manufacturer’s instructions, samples below the limit of detection (LOD) were re-run with a 30 min development time.
Statistical Analysis
The goal of this analysis was twofold: first, to characterize exposure to FRs and identify potential predictors of exposure among firefighters and office workers, and second, to assess the relationship between exposure to FRs and T4 and TSH.
Because of the evidence for skewed distributions
for FRs, T4, and TSH, we used nonparametric approaches
to test differences between groups (permutation, Wilcoxon) and natural
log-transformed creatinine values which improved normality for their
use in linear models.47 We used two different
linear regression models based on whether FR levels were being evaluated
as the outcome or exposure and to account for FR levels below the
LOD. In bivariate analyses looking at the relationship between covariates
(e.g., food consumption or position in the fire department)
and FR levels as the outcome, we used the maximum likelihood estimation
(MLE) model from the NADA package in R, which accounts for levels
below the LOD without the need for substitution, when the chemical
measurement is the outcome.48 To assess
the relationship between FRs (exposure) and thyroid hormone (outcome),
we used ordinary least squares (OLS) regression models and operationalized
levels below the LOD in the following ways: we included all LC–MS/MS
reported values (even if those values were below the LOD) and substituted 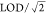 for any remaining nondetect
values.
for any remaining nondetect
values.
First, we treated FRs as the outcome to evaluate predictors of measured levels and assess differences between firefighters and office workers. We calculated summary statistics for FRs including the geometric mean (GM), geometric standard deviation (GSD), and distribution percentiles for each group. In the full group, we assessed the impact of variables collected from the exposure assessment interview on FR levels as the outcome using MLE regression. We limited analyses to FRs with a detection frequency (DF) of 70% or higher in at least one group (firefighters or office workers) and applied separate MLE regression models on each FR chemical (continuous outcome) controlling for occupation and creatinine. We analyzed the relationship between FR levels and the following variables: age, race/ethnicity, BMI, and educational attainment controlling for occupation and creatinine. We also assessed the relationship between FR exposures and eating certain foods and packaged foods based on prior literature suggesting an association with FR exposures.49
We then limited the analysis to firefighters to explore specific occupational activities that might be associated with FR levels such as the participant’s assigned position in the fire department (i.e., firefighter, officer, or driver), the frequency of using a self-contained breathing apparatus (SCBA) during fire suppression, salvage, and overhaul, and the frequency of showering or washing up after a fire event, if the participant was on duty during the 24 h prior to sample collection or had been off duty for more than 24 h. We also assessed the number of fires a participant fought in the week and month prior to the sample collection to see if fighting a recent fire event was associated with FR levels. We used MLE regression and exponentiated the β coefficients and 95% confidence interval (CI) to find the proportional change in GM for each unit increase or category change versus the referent.
Next, we assessed the association between FRs (exposure) and TSH and total T4 (outcome) using OLS regression and substitution for FR values below the LOD. We ran separate models for each FR chemical and for the outcomes, TSH and total T4. We considered variables to adjust for in our models if they demonstrated a statistically significant association (p-value < 0.05) with the exposure (at least one FR) and the outcome (TSH or T4) in our data or if the previous literature identified an association (Figure S1). Although age was not associated with FRs in our data, it has been associated with thyroid hormone levels in other studies50 and therefore we included it as a covariate in regression models. We did not control for BMI in regression models because studies indicate that thyroid hormone disruption may be associated with increased BMI,51−53 implying that it may be a collider for which adjustment could induce a spurious association. Since neither race/ethnicity nor education were associated with TSH or T4 in our data (data not shown), we did not include them in final models. We excluded the participants without thyroid hormone measurements or who reported taking thyroid hormone replacement medications.
Final models were adjusted for age and log(creatinine). Due to large differences in DFs between firefighters and office workers, we stratified the analysis by occupation. We used continuous FR values when the DF ≥ 70% and we categorized FR values when DF < 70%. FRs were categorized into the following groups: <LOD and ≥LOD (for FRs with a DF of 25 to 50%) and <LOD, LOD to 50th percentile, and >50th percentile (for FRs with DF between 50 and 70%). Compounds with DF below 25% were excluded from the multivariate analysis.
Results from the OLS regression models with continuous exposure and outcome variables were converted to the percent change in the outcome for a twofold increase in FR exposure with the formula: (2β – 1) × 100. From OLS regression models with categorical FR exposure and continuous outcomes, we calculated the percent change in the outcome for each category compared to the referent (<LOD) with the formula: (eβ – 1) × 100.
Analyses were conducted using R version 3.6.1 and R-studio version 1.2.1335.54,55
Results
FR Levels and Predictors of Exposure
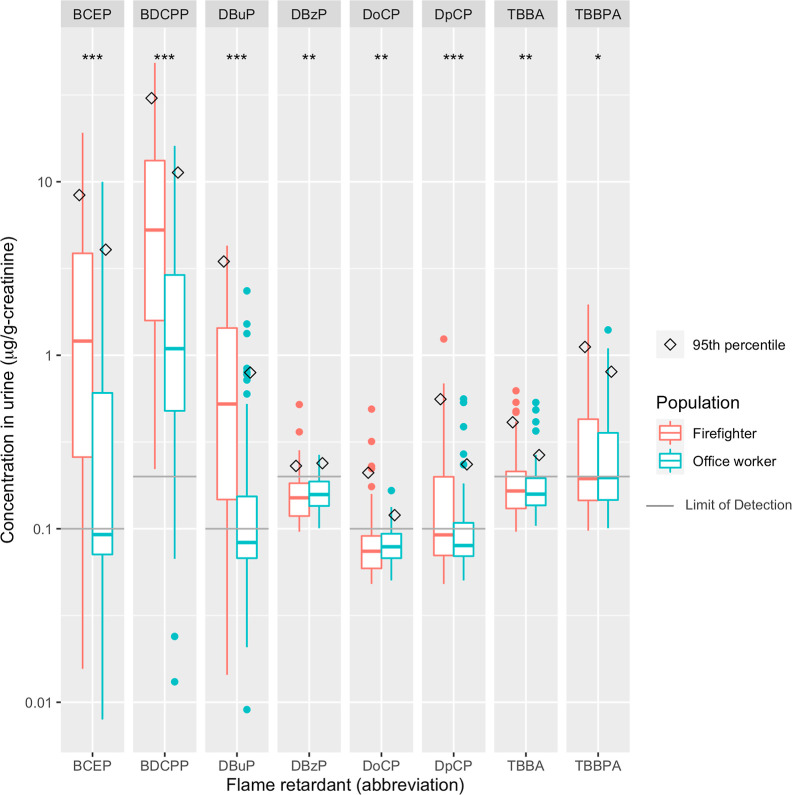
Firefighters had both higher DFs and higher average levels of FR metabolites compared to office workers for all FRs, with the largest median differences observed for DBuP, BDCPP, and BCEP (Table 1). Figure 1 shows the distribution [median, interquartile range (IQR), and 95th percentile] of each FR detected. BDCPP levels in firefighters were 5 times higher than in office workers. When compared to National Health and Nutrition Examination Survey (NHANES) adult women, firefighters had higher levels of DBuP, BDCPP, and BCEP, while office workers had similar BDCPP levels but lower levels of DBuP and BCEP compared to NHANES women (Figure S2).
Table 1. Descriptive Statistics of FRs Measured in Firefighters (N = 86) and Office Workers (N = 84)a.
| percentiles |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| chemical measured | parent compound | group | LOD | DF % | GM (GSD) | 25th | 50th | 75th | 95th |
| BDCPP: bis(1,3-dichloro-2-propyl)phosphate | tris(1,3-dichloro-2-propyl)phosphate (TDCIPP) | firefighter | 0.2 | 100 | 4.08 (4.53) | 1.30 | 5.37 | 13.3 | 32.22 |
| office worker | 0.2 | 90 | 0.96 (3.99) | <LOD | 0.92 | 2.34 | 8.72 | ||
| DBuP: di-n-butyl phosphate | tri-n-butyl phosphate | firefighter | 0.1 | 83 | 0.41 (3.92) | 0.15 | 0.50 | 1.26 | 3.04 |
| office worker | 0.1 | 29 | <LOD | <LOD | <LOD | 0.13 | 0.56 | ||
| BCEP: bis(2-chloroethyl)phosphate | tris(2-chloroethyl)phosphate (TCEP) | firefighter | 0.1 | 78 | 0.85 (5.74) | 0.23 | 1.22 | 3.98 | 8.51 |
| office worker | 0.1 | 39 | <LOD | <LOD | <LOD | 0.48 | 3.31 | ||
| DpCP: di-p-cresyl phosphate | tri-p-cresyl phosphate | firefighter | 0.1 | 41 | <LOD | <LOD | <LOD | 0.23 | 0.45 |
| office worker | 0.1 | 17 | <LOD | <LOD | <LOD | <LOD | 0.20 | ||
| DoCP: di-o-cresyl phosphate | tri-o-cresyl phosphate | firefighter | 0.1 | 9 | <LOD | <LOD | <LOD | <LOD | 0.15 |
| office worker | 0.1 | 1 | <LOD | <LOD | <LOD | <LOD | <LOD | ||
| BDzP: dibenzyl phosphate | tri-benzyl phosphate | firefighter | 0.2 | 7 | <LOD | <LOD | <LOD | <LOD | 0.22 |
| office worker | 0.2 | 0 | <LOD | <LOD | <LOD | <LOD | <LOD | ||
| TBBPA: tetrabromobisphenol-A | NA | firefighter | 0.2 | 45 | <LOD | <LOD | <LOD | 0.41 | 0.96 |
| office worker | 0.2 | 42 | <LOD | <LOD | <LOD | 0.29 | 0.64 | ||
| TBBA: 2,3,4,5-tetrabromobenzoic acid | 2-ethylhexyl-2,3,4,5-tetrabromobenzoate | firefighter | 0.2 | 24 | <LOD | <LOD | <LOD | <LOD | 0.42 |
| office worker | 0.2 | 8 | <LOD | <LOD | <LOD | <LOD | 0.24 | ||
Abbreviations: LOD = limit of detection; DF = detection frequency; GM = geometric mean; and GSD = geometric standard deviation. Additional information on sources and toxicology highlights is included in Table S1.
Figure 1.
Distribution (median,
IQR, and 95th percentile) of FRs (μg/g-creatinine)
in urine from 86 firefighters and 84 office workers of the Women Firefighters
Biomonitoring Collaborative (WFBC) (2014–15). We substituted
values below the LOD with 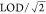 . Significance stars
represent the p-value from the permutation test of
the difference in the
average chemical level between firefighters and office workers: ***
<0.001; ** <0.05; and * <0.1.
. Significance stars
represent the p-value from the permutation test of
the difference in the
average chemical level between firefighters and office workers: ***
<0.001; ** <0.05; and * <0.1.
We applied MLE regression models to assess the relationship between descriptive variables and FR levels, controlling for occupation and log(creatinine) (Table 2). We limited the analysis to BDCPP, BCEP, and DBuP since the DF was greater than 70% in at least one group. We found that having a BMI of 25.0 to 29.0 as compared to 18.5 to 24.9 was associated with increased levels of BDCPP, BCEP, and DBuP. Likewise, completing a bachelor’s degree or higher was associated with elevated FRs compared to those who completed some college or less. Race and ethnicity were not associated with most OPFRs except for BDCPP, where black participants had a GM 2.52 times (95% CI: 1.10, 5.75) higher than white participants. We did not find an association between food consumption and eating packaged foods with FRs in either firefighters or office workers (Table S2).
Table 2. Demographic Variables for Firefighters and Office Workers and Their Association with FR Levelsa.
| descriptive
statistics |
MLE model results |
||||
|---|---|---|---|---|---|
| mean (±SD)
or N (%) |
exponentiated
β (95% CI) |
||||
| variable | office workers (N = 84) | firefighters (N = 86) | BDCPP | BCEP | DBuP |
| age (years) | 48.3 (±10.5) | 47.5 (±4.6) | 1.00 (0.97, 1.03) | 0.99 (0.94, 1.04) | 1.00 (0.96, 1.04) |
| time lived in CA (years) | 35.5 (±14.5) | 40.0 (±10.1) | 0.99 (0.97, 1.01) | 1.00 (0.97, 1.04) | 1.01 (0.99, 1.04) |
| U.S. born | 62 (73.8%) | 77 (89.5%) | 0.80 (0.44, 1.46) | 0.69 (0.24, 2.02) | 0.90 (0.40, 2.02) |
| BMIc,d | |||||
| 18.5–24.9 | 43 (51.2%) | 33 (38.4%) | reference | reference | reference |
| 25.0–29.0 | 23 (27.4%) | 35 (40.7%) | 1.71 (1.02, 2.84) | 2.70 (1.06, 6.84) | 2.45 (1.28, 4.68) |
| >30 | 16 (19.0%) | 13 (15.1%) | 0.84 (0.44, 1.58) | 0.75 (0.23, 2.50) | 1.55 (0.68, 3.54) |
| race/ethnicity | |||||
| white | 37 (44.0%) | 40 (46.5%) | reference | reference | reference |
| black | 5 (6.0%) | 9 (10.5%) | 2.52 (1.10, 5.75) | 0.76 (0.17, 3.46) | 0.73 (0.25, 2.17) |
| latina | 13 (15.5%) | 19 (22.1%) | 0.56 (0.31, 1.03) | 0.89 (0.30, 2.67) | 0.82 (0.37, 1.79) |
| asian | 19 (22.6%) | 11 (12.8%) | 1.02 (0.55, 1.88) | 1.60 (0.52, 4.93) | 1.15 (0.50, 2.63) |
| other/multi | 10 (11.9%) | 7 (8.1%) | 0.66 (0.31, 1.42) | 0.43 (0.10, 1.89) | 0.29 (0.09, 0.89) |
| education | |||||
| some college or less | 15 (17.9%) | 48 (55.8%) | reference | reference | reference |
| bachelors or greater | 69 (82.1%) | 38 (44.2%) | 1.65 (1.00, 2.73) | 3.63 (1.49, 8.84) | 2.24 (1.17, 4.31) |
Modelb results show the proportional change in GM (95% CI) of urinary FR concentration (ng/mL) (DF > 70%) for each unit increase or category increase compared to the reference.
Maximum likelihood estimation (MLE) regression models adjusted for occupation (firefighter or office worker) and log(creatinine).
CDC guidelines for BMI classification: normal weight 18.5–24.9; overweight 25.0–29.9, obese >30; and BMI units: kg/m2, it is important to note that muscular women may be inappropriately categorized as “overweight” or “obese” based on these guidelines.
Seven participants declined to answer height/weight questions.
We then limited the analysis to firefighters to assess occupational specific activities in relation to FR levels (Table 3). We observed that FR levels were higher in firefighters who were on duty at the time of the sample collection [BDCPP exp(β) (95% CI) = 1.98 (0.92, 4.26) and BCEP (2.76 (0.91, 8.38))] and we found modest associations by firefighters’ assigned role; officers and firefighters showed slightly elevated levels of BDCPP and BCEP compared to drivers. SCBA use in general was associated with lower levels of FRs except for use during exterior fire suppression which was associated with higher mean BCEP compared to those firefighters who answered that they sometimes used SCBA. Firefighters assigned to the San Francisco International Airport fire stations had elevated levels of BDCPP, BCEP, and DBuP compared to firefighters assigned to other SFFD stations. Fighting a fire within the 24 h and 7 days prior to the sample collection was also modestly associated with BDCPP and BCEP levels. The number of hours spent in vehicles per week for both home and work and the number of years worked with the SFFD were not associated with FR levels.
Table 3. Association between Firefighter Activities or Characteristics and Urinary FR Metabolite Levels (ng/mL, DF > 70%) in Firefightersa.
| variable | N | BDCPP | BCEP | DBuP |
|---|---|---|---|---|
| years worked with SFFD | 86 | 0.93 (0.87, 1.00) | 0.89 (0.80, 1.00) | 0.92 (0.85, 0.99) |
| hours spent in vehicle per week | 86 | 1.01 (0.98, 1.04) | 1.04 (1.00, 1.09) | 1.01 (0.98, 1.05) |
| categorical variables (“no” as referent) | ||||
| on duty at sample collection (yes) | 21 | 1.98 (0.92, 4.26) | 2.76 (0.91, 8.38) | 0.98 (0.41, 2.33) |
| assigned to airport (yes) | 14 | 1.51 (0.66, 3.44) | 1.73 (0.50, 5.99) | 1.73 (0.69, 4.33) |
| fire in last 24 h (yes) | 15 | 1.31 (0.58, 2.94) | 1.52 (0.45, 5.17) | 0.98 (0.40, 2.43) |
| fire last 7 days (yes)c | 18 | 1.32 (0.61, 2.89) | 1.86 (0.59, 5.86) | 0.75 (0.30, 1.90) |
| fire last month (yes)c | 44 | 1.24 (0.59, 2.57) | 1.21 (0.40, 3.66) | 0.95 (0.39, 2.30) |
| SCBA use with:d | ||||
| interior fire suppression (always vs often or less) | 60 | 0.75 (0.39, 1.47) | 1.26 (0.45, 3.48) | 0.95 (0.45, 2.02) |
| exterior fire suppression (often/always vs sometimes or less) | 32 | 0.90 (0.48, 1.70) | 2.52 (0.92, 6.88) | 1.01 (0.49, 2.08) |
| salvage and overhaul (often/always vs often or less) | 26 | 0.51 (0.27, 0.99) | 0.78 (0.28, 2.16) | 0.50 (0.24, 1.05) |
| position in SFFD | ||||
| driver | 21 | reference | reference | reference |
| firefighter | 40 | 1.40 (0.65, 3.01) | 1.31 (0.41, 4.23) | 1.19 (0.50, 2.83) |
| officer | 25 | 1.62 (0.71, 3.73) | 1.88 (0.53, 6.72) | 1.40 (0.55, 3.59) |
Model results show the adjustedb proportional change in GM of urine FRs metabolites by unit increase or category change from the referent of each variable.
Maximum likelihood estimation (MLE) models limited to firefighters and adjusted for log(creatinine).
N = 66 firefighters who consented to giving researchers access to their SFFD fire history records.
Responses collected as “never, rarely, sometimes, often, always” and combined due to the low frequency in response categories. DF = detection frequency.
FR Exposure and Thyroid Hormone Levels
T4 and TSH were slightly negatively correlated with each other (Spearman correlation coefficient: −0.13 and p-value = 0.1). We found that most participants were within the reference range (i.e., the range of levels that are considered normal)50,56 for both TSH and T4 (Table 4). Six percent (N = 11) of participants had TSH levels outside (below or above) of the reference range and 16 participants (9%) had T4 levels outside the reference range (12 were below the reference level), with firefighters making up the majority of those either below or above the reference range.
Table 4. Geometric Mean, Geometric Standard Deviation, and Percentile Distributions for TSH and Total Thyroxine (T4) in Firefighters (N = 84) and Office Workers (N = 81)a.
| percentiles |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| reference rangeb | group | min | max | GM (GSD) | 0.25 | 0.50 | 0.75 | 0.95 | |
| TSH | 0.4 to 4.0 mIU/L | firefighter | 0.17 | 11.4 | 1.27 (2.04) | 0.82 | 1.38 | 1.95 | 3.36 |
| office worker | 0.03 | 4.99 | 1.25 (2.09) | 0.88 | 1.36 | 1.96 | 3.36 | ||
| T4 | 4.6 to 12 μg/dL | firefighter | 3.69 | 18.7 | 6.08 (1.32) | 5.14 | 5.83 | 7.09 | 9.17 |
| office worker | 4.38 | 20.58 | 6.76 (1.28) | 5.9 | 6.64 | 7.44 | 9.32 | ||
Abbreviations: GM = geometric mean, GSD = geometric standard deviation, and IU = international unit.
Chiovato et al. 2019; Hollowell et al. 2002.
The final number analyzed for thyroid hormone disruption was N = 165 (84 firefighters and 81 office workers who had T4 and TSH measurements and who reported that they were not taking thyroid hormone medications). Average TSH levels were not significantly different between firefighters and office workers (permutation test p-value = 0.6); however, average T4 levels were borderline different by occupation (permutation test p-value = 0.06).
We applied OLS regression models to assess the association between FR levels and T4 and TSH controlling for log(creatinine) and age. FR levels were not associated with TSH in our models (Table S3); however, we observed a relationship between several FR metabolites and T4 levels. BDCPP, which we defined as continuous (DF > 70%) in OLS regression models, was negatively associated with T4 (Table 5). In the full group, a twofold increase in BDCPP levels was associated with a decrease of 1.95% (95% CI: 3.57, 0.29) in T4, and in models limited to firefighters, a twofold increase in BDCCP was associated with a T4 decrease of 2.88% (95% CI: 5.28, 0.42), controlling for age and log(creatinine). The percent change among office workers was smaller (0.23%, 95% CI: −2.49, 3.03) and not significant.
Table 5. Percent Change (95% CI) in T4 Levels for Each Doubling of BDCPP in Adjusteda OLS Regression Modelsb.
| model | percent change (95% CI) |
|---|---|
| full group (N = 165) | –1.95 (−3.57, −0.29) |
| firefighters only (N = 84) | –2.88 (−5.28, −0.42) |
| office workers only (N = 81) | 0.23 (−2.49, 3.026) |
Models adjusted for age and log(creatinine).
Values below LOD replaced with LOD/sqrt(2).
Due to large differences in DF for DBuP, BCEP, TBBPA, and DpCP between firefighters and office workers, we ran separate OLS regression models by occupation for each FR as a predictor and T4 as a continuous outcome. For office workers, we categorized DBuP, BCEP, TBBPA, and DpCP into <LOD and ≥LOD. Among firefighters, we categorized TBBPA and DpCP as <LOD and ≥LOD and categorized DBuP and BCEP as <LOD, LOD to 50%, and >50%. Because the DF for DBuP and BCEP was >70% for firefighters, we also ran OLS regression models with the continuous DBuP and BCEP. Although there was evidence of lower T4 levels among firefighters for DBuP, the associations between DBuP or BCEP and T4 were not statistically significant for either firefighters or office workers (Table S4).
Discussion
To our knowledge, this is the first study to measure exposures to replacement FRs and their association with thyroid function among women firefighters compared to office workers. Our results align with prior reports that firefighters have higher levels of FRs compared to nonfirefighters and contribute further evidence that FRs may affect thyroid function in humans.
FR Levels and Predictors of Exposure
FRs were detected in all firefighters and most office workers tested. Women firefighters had higher DFs and higher average levels of FRs compared to women office workers. BDCPP, BCEP, and DBuP were detected more frequently and at higher concentrations among firefighters compared to office workers. BDCPP is a metabolite of TDCPP, an FR that is commonly detected in household dust and in furniture foam,57 which may help explain why office workers also had a high DF of this compound. The office workers in our study had similar median levels of BDCPP but higher median levels of BCEP and DBuP compared to adult women (age 18+) from the 2013–2014 NHANES, which included both workers and nonworkers and is a nationally representative sample of the US population.58
Few demographic variables were associated with FRs. Age, for example, was not associated with FRs in our study possibly because of the limited age range of our participants (95% of participants were between the ages of 40 and 55 for firefighters and 32 and 64 for office workers). The positive relationship we observed between OPFR exposures and BMI is consistent with other studies.51 While black participants had higher levels of BDCPP compared to white participants when controlling for age and occupation, this relationship may in fact reflect other unknown exposures and be a function of the limited numbers of black participants in both the firefighter and office worker groups.
California’s flammability standard and the changes implemented in 2013 may have affected the exposures in our study population. Concerns about the bioaccumulative properties of brominated FRs and the updated regulation TB177-2013 led to a shift away from PBDE FRs in furniture. Indeed, studies have observed a decrease in PBDEs in people and the environment, while levels of OPFRs and other replacement FRs are increasing in dust and human biomonitoring studies.16,58−60 Additionally, furniture and electronics in homes and offices can be a reservoir for both legacy and replacement FRs; many of these products are not frequently replaced and the chemicals applied to them are relatively stable, enabling these compounds to persist in homes and offices for decades.59 The elevated levels of organophosphate and brominated FRs in furniture, homes, and offices may also be an important exposure source among firefighters as well as the office workers in our study. Similarly, FR-containing furniture may add to the toxic burden of fighting fires and translate to higher exposures among firefighters when they respond to fires. Studies have shown that fighting fires can contaminate firefighter gear, trucks, engines, and equipment, bringing chemical exposures indoors.10,61−63
Fire station dust and fire-fighting gear are also potentially important sources of FRs among firefighters. The increased levels of OPFRs found in the women firefighters compared to office workers are consistent with other studies measuring elevated levels of OPFRs in fire station dust in the U.S. and abroad. Shen et al. (2018) found higher levels of TDCPP, tri-n-butyl phosphate, and tris(2-chloroisopropyl)phosphate, in dust collected in 2015 from fire stations across the U.S., compared to levels measured in homes and other occupational settings.10 Similarly, a study of Australian fire station dust found higher median OPFR levels in fire stations than from dust samples collected in homes and offices.63 Firefighter gear is also likely to be a source of exposure to FRs. A 2016 study of firefighter gear found that some new hoods and gloves had detectable levels of brominated FRs, but to our knowledge, studies have not analyzed firefighter gear for OPFRs. Nevertheless, firefighter gear may contain FR compounds that can contribute to exposures from dust accumulated on the gear from fires and calls.12,64,65 A recent study of firefighting gear found that fighting fires may contaminate the hoods firefighters wear under their protective equipment with OPFRs.62,64,65 Additionally, laundering may have a limited impact on reducing exposures and, in some cases, may cross-contaminate gear.62
We were unable to identify specific sources of FRs or why firefighters had higher levels of exposure than office workers. While durable consumer goods such as couches and mattresses may contribute to levels in the general population and fire station dust may be an important source in firefighters, this does not fully explain where the FR exposures are coming from and why the levels are higher in firefighters. Future studies are needed to elucidate potential sources of exposure to legacy and replacement FRs which would facilitate the development and promotion of effective exposure prevention strategies.
FR Exposure and Thyroid Hormone Levels
Our study contributes to the limited literature on thyroid hormone disruption associated with OPFR exposure among women workers. In addition to demonstrating endocrine disruption, changes in thyroid hormone levels can also be a useful biomarker of biological perturbations relevant to downstream health effects including breast cancer.30,31 Our results showing associations between FRs with decreased T4 were primarily observed among firefighters, particularly for BDCPP, which had the highest DF of the FRs we measured. The broader range and much higher levels of exposure among the firefighters in our study may have allowed us to observe an association between FR exposure on T4 that we could not among office workers who had lower exposure levels. Our findings support evidence of an association between T4 levels and TDCPP, one that has also been identified in studies conducted in vitro, in vivo, and in limited human studies.33,35,40
In vitro studies provide evidence for the mechanistic plausibility of thyroid hormone disruption from OPFR exposure; however, the mechanism of effect is not yet understood. Hill et al. (2018) tested the effect of organophosphate triesters on the binding of T4 and transthyretin (TTR)—one of the many transport proteins for T4. They found that TDCPP and other organophosphate tri- and di-esters increased the binding affinity of T4 with TTR. They hypothesized that organophosphate compounds may bind to the surface of TTR, creating a conformational change allowing more T4 binding and increasing the delivery of T4 to target cells resulting in lower circulating levels of T4 and disrupting T4 homeostasis.32 Our study provides evidence that exposure to TDCPP, and to a lesser extent DBuP, is associated with lower thyroid hormone levels. Although we observed small changes in total T4 levels, small disruptions to thyroid hormones can have important adverse downstream health effects45 even within normal ranges or subclinical levels.66 Thyroid dysfunction affects up to 5% of the population and is more likely to affect women than men.50 Identifying environmental chemical exposures that may be associated with biological changes, such as thyroid disruption could be relevant to adverse health outcomes such as cardiovascular disease,38 thyroid disease,44 brain development of offspring during gestation, and long-term adverse health outcomes such as cancer.39
Limitations
This was a cross-sectional study of the association between FR exposure and measures of thyroid function, which precludes making inferences regarding causality. Because we did not quantify free T4, T3, or thyroid peroxidase antibody, we were not able to assess clinical outcomes in our participants. While the ratio of free T4 to TSH is useful for clinical diagnosis of hypo- or hyperthyroidism, changes in total T4 can provide important insights on early biological perturbations that may be associated with environmental exposures.
Thyroid disease and medications—including thyroid hormone replacement medications—may alter thyroid hormone levels. We asked participants “Do you take any hormones other than birth control, such as Premarin? If so what?” Although some participants disclosed taking thyroid medications or having thyroid problems, the questionnaire may not have accurately captured all the participants with thyroid dysfunction or those who take thyroid hormone replacements nor did we ask about other medications that may affect thyroid hormone levels. Therefore, we may not have excluded everyone with artificial or abnormal thyroid hormone levels due to illness or medication use rather than chemical exposure. This could over- or under-estimate thyroid hormone levels depending on the thyroid problem, making it difficult to predict in what direction this could affect our results.
Another potential limitation is that we used spot urine samples when measuring FR levels, potentially increasing the variability of the urine concentration and of chemicals measured in our samples. Studies describing the stability of OPFRs over time vary;67,68 however, the variability would be nondifferential between firefighters and office workers and not likely to affect the differences we observed in their chemical levels nor the FR’s relationship with potential covariates. In addition, samples were collected in the morning for most participants, and we measured and adjusted for creatinine to account for urine dilution, reducing some of this variability.47
Finally, there may be residual confounding from co-exposures that we were not able to measure. Our sample size was modest and may have limited statistical power to detect associations between FR levels and exposure sources, as well as associations between FR exposure and thyroid hormone disruption, especially at the lower exposure levels we observed in office workers.
This is the first study to measure FR exposure in a study of women firefighters and office workers. Most participants had detectable levels of at least one OPFR, and both DFs and levels were much higher among women firefighters than in women office workers, especially for BDCPP, DBuP, and BCEP. Median levels of the carcinogen metabolite BDCPP were 5 times higher in firefighters than for office workers. Additionally, exposure to BDCPP, and to a smaller extent DBuP, was associated with decreased levels of total T4, particularly among firefighters. The observed thyroid hormone disruption may indicate potential biological perturbations resulting from occupational exposure to these compounds. Further research is needed to understand exposure sources in occupational settings and fully characterize potential health impacts of these replacement FRs.
Acknowledgments
The authors thank all the WWBC [formerly known as the WFBC] participants for their contribution to the study. This work is supported by the California Breast Cancer Research Program #19BB-2900 and #23BB-1700 & 1701 & 1702 (J.T., R.G., C.C., M.M., C.A., R.R., H.B., V.B., and R.M.-F.), the National Institute of Environmental Health Sciences R01ES027051 (RMF), the National Institute for Occupational Safety and Health, Targeted Research Training Program T42 OH008429 (J.T.), the San Francisco Firefighter Cancer Prevention Foundation (H.B.), and the International Association of Firefighters-Local 798. We thank Anthony Stefani, Emily O’Rourke, Nancy Carmona, Karen Kerr, Julie Mau, Natasha Parks, Lisa Holdcroft, San Francisco Fire Chief Jeanine Nicholson, former San Francisco Fire Chief Joanne Hayes-White, Sharyle Patton, Connie Engel, Nancy Buermeyer, and Lauren Baehner for their contributions to the study.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.1c05140.
FR compounds, their metabolites, potential sources, uses, thyroid effects, and listing on California’s proposition 65 list of carcinogens and reproductive and developmental toxicants; model results of urinary FR metabolite levels and foods eaten by firefighters and office workers; model results of the association between FRs and TSH in the full both firefighters and office workers in adjusted OLS regression models; Percent change (95% CI) in total T4 for each category increase in FR compared to levels below the LOD for firefighters and office workers from adjusted OLS regression models; directed acyclic graph of variables considered for inclusion for linear regression models of FR exposure and thyroid hormone levels; median and 95th percentiles of FR metabolites measured in urine (μg/g-creatinine) from 2014 to 2015 WFBC firefighters and office workers compared to 2013–2014 NHANES adult women (ages 18 to 65) for compounds with at least 70% DF among firefighters of the WFBC; additional information on laboratory methods and QA/QC for FR quantification in urine; and select questions from study questionnaire (PDF)
The authors declare no competing financial interest.
Notes
R.R. and V.B. are employed at the Silent Spring Institute, a scientific research organization dedicated to studying environmental factors in women’s health. The institute is a 501(c)3 public charity funded by federal grants and contracts, foundation grants, and private donations, including from breast cancer organizations. H.B. is a former president and member of United Fire Service Women, a 501(c)3 public charity dedicated to supporting the welfare of women in the SFFD.
Supplementary Material
References
- Caux C.; O’Brien C.; Viau C. Determination of Firefighter Exposure to Polycyclic Aromatic Hydrocarbons and Benzene during Fire Fighting Using Measurement of Biological Indicators. Appl. Occup. Environ. Hyg. 2002, 17, 379–386. 10.1080/10473220252864987. [DOI] [PubMed] [Google Scholar]
- Dobraca D.; Israel L.; McNeel S.; Voss R.; Wang M.; Gajek R.; Park J.-S.; Harwani S.; Barley F.; She J.; Das R. Biomonitoring in California Firefighters: Metals and Perfluorinated Chemicals. J. Occup. Environ. Med. 2015, 57, 88–97. 10.1097/JOM.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fent K. W.; Eisenberg J.; Snawder J.; Sammons D.; Pleil J. D.; Stiegel M. A.; Mueller C.; Horn G. P.; Dalton J. Systemic Exposure to PAHs and Benzene in Firefighters Suppressing Controlled Structure Fires. Ann. Occup. Hyg. 2014, 58, 830–845. 10.1093/annhyg/meu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashow R.; Bessonneau V.; Gerona R. R.; Wang A.; Trowbridge J.; Lin T.; Buren H.; Rudel R. A.; Morello-Frosch R. Integrating Exposure Knowledge and Serum Suspect Screening as a New Approach to Biomonitoring: An Application in Firefighters and Office Workers. Environ. Sci. Technol. 2020, 54, 4344–4355. 10.1021/acs.est.9b04579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C.; Sun Y.; Islam A.; Qian Y.; Ducatman A. Perfluoroalkyl Acids Including Perfluorooctane Sulfonate and Perfluorohexane Sulfonate in Firefighters. J. Occup. Environ. Med. 2011, 53, 324–328. 10.1097/JOM.0b013e31820d1314. [DOI] [PubMed] [Google Scholar]
- Laitinen J. A.; Koponen J.; Koikkalainen J.; Kiviranta H. Firefighters’ Exposure to Perfluoroalkyl Acids and 2-Butoxyethanol Present in Firefighting Foams. Toxicol. Lett. 2014, 231, 227–232. 10.1016/j.toxlet.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Park J.-S.; Voss R. W.; McNeel S.; Wu N.; Guo T.; Wang Y.; Israel L.; Das R.; Petreas M. High Exposure of California Firefighters to Polybrominated Diphenyl Ethers. Environ. Sci. Technol. 2015, 49, 2948–2958. 10.1021/es5055918. [DOI] [PubMed] [Google Scholar]
- Shaw S. D.; Berger M. L.; Harris J. H.; Yun S. H.; Wu Q.; Liao C.; Blum A.; Stefani A.; Kannan K. Persistent Organic Pollutants Including Polychlorinated and Polybrominated Dibenzo-p-Dioxins and Dibenzofurans in Firefighters from Northern California. Chemosphere 2013, 91, 1386–1394. 10.1016/j.chemosphere.2012.12.070. [DOI] [PubMed] [Google Scholar]
- Shen B.; Whitehead T. P.; McNeel S.; Brown F. R.; Dhaliwal J.; Das R.; Israel L.; Park J.-S.; Petreas M. High Levels of Polybrominated Diphenyl Ethers in Vacuum Cleaner Dust from California Fire Stations. Environ. Sci. Technol. 2015, 49, 4988–4994. 10.1021/es505463g. [DOI] [PubMed] [Google Scholar]
- Shen B.; Whitehead T. P.; Gill R.; Dhaliwal J.; Brown F. R.; Petreas M.; Patton S.; Hammond S. K. Organophosphate Flame Retardants in Dust Collected from United States Fire Stations. Environ. Int. 2018, 112, 41–48. 10.1016/j.envint.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Trowbridge J.; Gerona R. R.; Lin T.; Rudel R. A.; Bessonneau V.; Buren H.; Morello-Frosch R. Exposure to Perfluoroalkyl Substances in a Cohort of Women Firefighters and Office Workers in San Francisco. Environ. Sci. Technol. 2020, 54, 3363–3374. 10.1021/acs.est.9b05490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander B. M.; Baxter C. S. Flame-Retardant Contamination of Firefighter Personal Protective Clothing - A Potential Health Risk for Firefighters. J. Occup. Environ. Hyg. 2016, 13, D148–D155. 10.1080/15459624.2016.1183016. [DOI] [PubMed] [Google Scholar]
- Stapleton H. M.; Sharma S.; Getzinger G.; Ferguson P. L.; Gabriel M.; Webster T. F.; Blum A. Novel and High Volume Use Flame Retardants in US Couches Reflective of the 2005 PentaBDE Phase Out. Environ. Sci. Technol. 2012, 46, 13432–13439. 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson R. E.; Van den Eede N.; Covaci A.; Perovich L. J.; Brody J. G.; Rudel R. A. Urinary Biomonitoring of Phosphate Flame Retardants: Levels in California Adults and Recommendations for Future Studies. Environ. Sci. Technol. 2014, 48, 13625–13633. 10.1021/es503445c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castorina R.; Bradman A.; Stapleton H. M.; Butt C.; Avery D.; Harley K. G.; Gunier R. B.; Holland N.; Eskenazi B. Current-Use Flame Retardants: Maternal Exposure and Neurodevelopment in Children of the CHAMACOS Cohort. Chemosphere 2017, 189, 574–580. 10.1016/j.chemosphere.2017.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson R. E.; Perovich L. J.; Covaci A.; Van den Eede N.; Ionas A. C.; Dirtu A. C.; Brody J. G.; Rudel R. A. After the PBDE Phase-out: A Broad Suite of Flame Retardants in Repeat House Dust Samples from California. Environ. Sci. Technol. 2012, 46, 13056–13066. 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen I.; de Boer J. Phosphorus Flame Retardants: Properties, Production, Environmental Occurrence, Toxicity and Analysis. Chemosphere 2012, 88, 1119–1153. 10.1016/j.chemosphere.2012.03.067. [DOI] [PubMed] [Google Scholar]
- Covaci A.; Harrad S.; Abdallah M. A.-E.; Ali N.; Law R. J.; Herzke D.; de Wit C. A. Novel Brominated Flame Retardants: A Review of Their Analysis, Environmental Fate and Behaviour. Environ. Int. 2011, 37, 532–556. 10.1016/j.envint.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Van den Eede N.; Dirtu A. C.; Ali N.; Neels H.; Covaci A. Multi-Residue Method for the Determination of Brominated and Organophosphate Flame Retardants in Indoor Dust. Talanta 2012, 89, 292–300. 10.1016/j.talanta.2011.12.031. [DOI] [PubMed] [Google Scholar]
- Nomeir A. A.; Kato S.; Matthews H. B. The Metabolism and Disposition of Tris(1,3-Dichloro-2-Propyl) Phosphate (Fyrol FR-2) in the Rat. Toxicol. Appl. Pharmacol. 1981, 57, 401–413. 10.1016/0041-008x(81)90238-6. [DOI] [PubMed] [Google Scholar]
- Carignan C. C.; McClean M. D.; Cooper E. M.; Watkins D. J.; Fraser A. J.; Heiger-Bernays W.; Stapleton H. M.; Webster T. F. Predictors of Tris(1,3-Dichloro-2-Propyl) Phosphate Metabolite in the Urine of Office Workers. Environ. Int. 2013, 55, 56–61. 10.1016/j.envint.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel R. A.; Fenton S. E.; Ackerman J. M.; Euling S. Y.; Makris S. L. Environmental Exposures and Mammary Gland Development: State of the Science, Public Health Implications, and Research Recommendations. Environ. Health Perspect. 2011, 119, 1053–1061. 10.1289/ehp.1002864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore A. C.; Chappell V. A.; Fenton S. E.; Flaws J. A.; Nadal A.; Prins G. S.; Toppari J.; Zoeller R. T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers K. M.; Udesky J. O.; Rudel R. A.; Brody J. G. Environmental Chemicals and Breast Cancer: An Updated Review of Epidemiological Literature Informed by Biological Mechanisms. Environ. Res. 2018, 160, 152–182. 10.1016/j.envres.2017.08.045. [DOI] [PubMed] [Google Scholar]
- Rudel R. A.; Ackerman J. M.; Attfield K. R.; Brody J. G. New Exposure Biomarkers as Tools for Breast Cancer Epidemiology, Biomonitoring, and Prevention: A Systematic Approach Based on Animal Evidence. Environ. Health Perspect. 2014, 122, 881–895. 10.1289/ehp.1307455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. J.; Koru-Sengul T.; Hernandez M. N.; Caban-Martinez A. J.; McClure L. A.; Mackinnon J. A.; Kobetz E. N. Cancer Risk among Career Male and Female Florida Firefighters: Evidence from the Florida Firefighter Cancer Registry (1981-2014). Am. J. Ind. Med. 2020, 63, 285–299. 10.1002/ajim.23086. [DOI] [PubMed] [Google Scholar]
- Ma F.; Fleming L. E.; Lee D. J.; Trapido E.; Gerace T. A. Cancer Incidence in Florida Professional Firefighters, 1981 to 1999. J. Occup. Environ. Med. 2006, 48, 883–888. 10.1097/01.jom.0000235862.12518.04. [DOI] [PubMed] [Google Scholar]
- Ma F.; Fleming L. E.; Lee D. J.; Trapido E.; Gerace T. A.; Lai H.; Lai S. Mortality in Florida Professional Firefighters, 1972 to 1999. Am. J. Ind. Med. 2005, 47, 509–517. 10.1002/ajim.20160. [DOI] [PubMed] [Google Scholar]
- Angelousi A.; Diamanti-Kandarakis E.; Zapanti E.; Nonni A.; Ktenas E.; Mantzou A.; Kontzoglou K.; Kouraklis G. Is There an Association between Thyroid Function Abnormalities and Breast Cancer?. Arch. Endocrinol. Metab. 2017, 61, 54–61. 10.1590/2359-3997000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. Y.; Chang Y.; Lee K. H.; Yun J. S.; Park Y. L.; Park C. H.; Ahn J.; Shin H.; Ryu S. Serum Concentration of Thyroid Hormones in Abnormal and Euthyroid Ranges and Breast Cancer Risk: A Cohort Study. Int. J. Cancer 2019, 145, 3257–3266. 10.1002/ijc.32283. [DOI] [PubMed] [Google Scholar]
- Khan S. R.; Chaker L.; Ruiter R.; Aerts J. G. J. V.; Hofman A.; Dehghan A.; Franco O. H.; Stricker B. H. C.; Peeters R. P. Thyroid Function and Cancer Risk: The Rotterdam Study. J. Clin. Endocrinol. Metab. 2016, 101, 5030. 10.1210/jc.2016-2104. [DOI] [PubMed] [Google Scholar]
- Hill K. L.; Hamers T.; Kamstra J. H.; Willmore W. G.; Letcher R. J. Organophosphate Triesters and Selected Metabolites Enhance Binding of Thyroxine to Human Transthyretin in Vitro. Toxicol. Lett. 2018, 285, 87–93. 10.1016/j.toxlet.2017.12.030. [DOI] [PubMed] [Google Scholar]
- Meeker J. D.; Stapleton H. M. House Dust Concentrations of Organophosphate Flame Retardants in Relation to Hormone Levels and Semen Quality Parameters. Environ. Health Perspect. 2010, 118, 318–323. 10.1289/ehp.0901332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishaw L. V.; Macaulay L. J.; Roberts S. C.; Stapleton H. M. Exposures, Mechanisms, and Impacts of Endocrine-Active Flame Retardants. Curr. Opin. Pharmacol. 2014, 19, 125–133. 10.1016/j.coph.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat A.; Crump D.; Chiu S.; Williams K. L.; Letcher R. J.; Gauthier L. T.; Kennedy S. W. In Ovo Effects of Two Organophosphate Flame Retardants--TCPP and TDCPP--on Pipping Success, Development, MRNA Expression, and Thyroid Hormone Levels in Chicken Embryos. Toxicol. Sci. 2013, 134, 92–102. 10.1093/toxsci/kft100. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Starling A. P.; Haug L. S.; Eggesbo M.; Becher G.; Thomsen C.; Travlos G.; King D.; Hoppin J. A.; Rogan W. J.; Longnecker M. P. Association between Perfluoroalkyl Substances and Thyroid Stimulating Hormone among Pregnant Women: A Cross-Sectional Study. Environ. Health 2013, 12, 76. 10.1186/1476-069X-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E.; Bourguignon J.-P.; Giudice L. C.; Hauser R.; Prins G. S.; Soto A. M.; Zoeller R. T.; Gore A. C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodondi N.; Aujesky D.; Vittinghoff E.; Cornuz J.; Bauer D. C. Subclinical Hypothyroidism and the Risk of Coronary Heart Disease: A Meta-Analysis. Am. J. Med. 2006, 119, 541–551. 10.1016/j.amjmed.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Krashin E.; Piekiełko-Witkowska A.; Ellis M.; Ashur-Fabian O. Thyroid Hormones and Cancer: A Comprehensive Review of Preclinical and Clinical Studies. Front. Endocrinol. 2019, 10, 59. 10.3389/fendo.2019.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Liang K.; Liu J.; Yang L.; Guo Y.; Liu C.; Zhou B. Exposure of Zebrafish Embryos/Larvae to TDCPP Alters Concentrations of Thyroid Hormones and Transcriptions of Genes Involved in the Hypothalamic-Pituitary-Thyroid Axis. Aquat. Toxicol. 2013, 126, 207–213. 10.1016/j.aquatox.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Gravel S.; Lavoué J.; Bakhiyi B.; Lavoie J.; Roberge B.; Patry L.; Bouchard M. F.; Verner M.-A.; Zayed J.; Labrèche F. Multi-Exposures to Suspected Endocrine Disruptors in Electronic Waste Recycling Workers: Associations with Thyroid and Reproductive Hormones. Int. J. Hyg. Environ. Health 2020, 225, 113445. 10.1016/j.ijheh.2019.113445. [DOI] [PubMed] [Google Scholar]
- Preston E. V.; McClean M. D.; Claus Henn B.; Stapleton H. M.; Braverman L. E.; Pearce E. N.; Makey C. M.; Webster T. F. Associations between Urinary Diphenyl Phosphate and Thyroid Function. Environ. Int. 2017, 101, 158–164. 10.1016/j.envint.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown F. R.; Whitehead T. P.; Park J.-S.; Metayer C.; Petreas M. X. Levels of Non-Polybrominated Diphenyl Ether Brominated Flame Retardants in Residential House Dust Samples and Fire Station Dust Samples in California. Environ. Res. 2014, 135, 9–14. 10.1016/j.envres.2014.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E. M.; Jemal A.; Chen A. Increasing Incidence of Thyroid Cancer: Is Diagnostic Scrutiny the Sole Explanation?. Future Oncol. 2010, 6, 185–188. 10.2217/fon.09.161. [DOI] [PubMed] [Google Scholar]
- Boas M.; Feldt-Rasmussen U.; Main K. M. Thyroid Effects of Endocrine Disrupting Chemicals. Mol. Cell. Endocrinol. 2012, 355, 240–248. 10.1016/j.mce.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Antibodies-online . T3, T4, TSH ELISA Kit—Product Details. https://www.antibodies-online.com/kit/2773773/T3,+T4,+TSH+ELISA+Kit/ (accessed February 25, 2020).
- Barr D. B.; Wilder L. C.; Caudill S. P.; Gonzalez A. J.; Needham L. L.; Pirkle J. L. Urinary Creatinine Concentrations in the U.S. Population: Implications for Urinary Biologic Monitoring Measurements. Environ. Health Perspect. 2005, 113, 192–200. 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsel D. R. More than Obvious: Better Methods for Interpreting Nondetect Data. Environ. Sci. Technol. 2005, 39, 419A–423A. 10.1021/es053368a. [DOI] [PubMed] [Google Scholar]
- Kim H.; Rebholz C. M.; Wong E.; Buckley J. P. Urinary Organophosphate Ester Concentrations in Relation to Ultra-Processed Food Consumption in the General US Population. Environ. Res. 2020, 182, 109070. 10.1016/j.envres.2019.109070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollowell J. G.; Staehling N. W.; Flanders W. D.; Hannon W. H.; Gunter E. W.; Spencer C. A.; Braverman L. E. Serum TSH, T(4), and Thyroid Antibodies in the United States Population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 2002, 87, 489–499. 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- Boyle M.; Buckley J. P.; Quirós-Alcalá L. Associations between Urinary Organophosphate Ester Metabolites and Measures of Adiposity among U.S. Children and Adults: NHANES 2013-2014. Environ. Int. 2019, 127, 754–763. 10.1016/j.envint.2019.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen N.; Laurberg P.; Rasmussen L. B.; Bülow I.; Perrild H.; Ovesen L.; Jørgensen T. Small Differences in Thyroid Function May Be Important for Body Mass Index and the Occurrence of Obesity in the Population. J. Clin. Endocrinol. Metab. 2005, 90, 4019–4024. 10.1210/jc.2004-2225. [DOI] [PubMed] [Google Scholar]
- Vail G. M.; Walley S. N.; Yasrebi A.; Maeng A.; Conde K. M.; Roepke T. A. The Interactions of Diet-Induced Obesity and Organophosphate Flame Retardant Exposure on Energy Homeostasis in Adult Male and Female Mice. J. Toxicol. Environ. Health, Part A 2020, 83, 438–455. 10.1080/15287394.2020.1777235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudio Team . RStudio: Integrated Development for R; RStudio Inc.: Boston, MA, 2016.
- R Core Team . R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015.
- Chiovato L.; Magri F.; Carlé A. Hypothyroidism in Context: Where We’ve Been and Where We’re Going. Adv. Ther. 2019, 36, 47–58. 10.1007/s12325-019-01080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel S. C.; Hoffman K.; Lorenzo A. M.; Chen A.; Phillips A. L.; Butt C. M.; Sosa J. A.; Webster T. F.; Stapleton H. M. Associations Between Flame Retardant Applications in Furniture Foam, House Dust Levels, and Residents’ Serum Levels. Environ. Int. 2017, 107, 181–189. 10.1016/j.envint.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ospina M.; Jayatilaka N. K.; Wong L.-Y.; Restrepo P.; Calafat A. M. Exposure to Organophosphate Flame Retardant Chemicals in the U.S. General Population: Data from the 2013-2014 National Health and Nutrition Examination Survey. Environ. Int. 2018, 110, 32–41. 10.1016/j.envint.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota A. R.; Linderholm L.; Park J.-S.; Petreas M.; Guo T.; Privalsky M. L.; Zoeller R. T.; Woodruff T. J. Temporal Comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the Serum of Second Trimester Pregnant Women Recruited from San Francisco General Hospital, California. Environ. Sci. Technol. 2013, 47, 11776–11784. 10.1021/es402204y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M.; Allen J. G.; Kelly S. M.; Konstantinov A.; Klosterhaus S.; Watkins D.; McClean M. D.; Webster T. F. Alternate and New Brominated Flame Retardants Detected in U.S. House Dust. Environ. Sci. Technol. 2008, 42, 6910–6916. 10.1021/es801070p. [DOI] [PubMed] [Google Scholar]
- Fent K. W.; Evans D. E.; Booher D.; Pleil J. D.; Stiegel M. A.; Horn G. P.; Dalton J. Volatile Organic Compounds Off-Gassing from Firefighters’ Personal Protective Equipment Ensembles after Use. J. Occup. Environ. Hyg. 2015, 12, 404–414. 10.1080/15459624.2015.1025135. [DOI] [PubMed] [Google Scholar]
- Mayer A. C.; Fent K. W.; Bertke S.; Horn G. P.; Smith D. L.; Kerber S.; La Guardia M. J. Firefighter Hood Contamination: Efficiency of Laundering to Remove PAHs and FRs. J. Occup. Environ. Hyg. 2019, 16, 129–140. 10.1080/15459624.2018.1540877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks A. P. W.; Engelsman M.; He C.; Wang X.; Mueller J. F. The Occurrence of PAHs and Flame-Retardants in Air and Dust from Australian Fire Stations. J. Occup. Environ. Hyg. 2020, 17, 73–84. 10.1080/15459624.2019.1699246. [DOI] [PubMed] [Google Scholar]
- Mayer A. C.; Horn G. P.; Fent K. W.; Bertke S. J.; Kerber S.; Kesler R. M.; Newman H.; Smith D. L. Impact of Select PPE Design Elements and Repeated Laundering in Firefighter Protection from Smoke Exposure. J. Occup. Environ. Hyg. 2020, 17, 505–514. 10.1080/15459624.2020.1811869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fent K. W.; LaGuardia M.; Luellen D.; McCormick S.; Mayer A.; Chen I.-C.; Kerber S.; Smith D.; Horn G. P. Flame Retardants, Dioxins, and Furans in Air and on Firefighters’ Protective Ensembles during Controlled Residential Firefighting. Environ. Int. 2020, 140, 105756. 10.1016/j.envint.2020.105756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. N.; Razvi S.; Pearce S. H.; Dayan C. M. Clinical Review: A Review of the Clinical Consequences of Variation in Thyroid Function within the Reference Range. J. Clin. Endocrinol. Metab. 2013, 98, 3562–3571. 10.1210/jc.2013-1315. [DOI] [PubMed] [Google Scholar]
- Wang L.-M.; Luo D.; Li X.; Hu L.-Q.; Chen J.-X.; Tu Z.-Z.; Sun B.; Chen H.-G.; Liu L.; Yu M.; Li Y.-P.; Pan A.; Messerlian C.; Mei S.-R.; Wang Y.-X. Temporal Variability of Organophosphate Flame Retardant Metabolites in Spot, First Morning, and 24-h Urine Samples among Healthy Adults. Environ. Res. 2021, 196, 110373. 10.1016/j.envres.2020.110373. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Li W.; Martínez-Moral M. P.; Sun H.; Kannan K. Metabolites of Organophosphate Esters in Urine from the United States: Concentrations, Temporal Variability, and Exposure Assessment. Environ. Int. 2019, 122, 213–221. 10.1016/j.envint.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.